Abstract
Some studies report that the positive relationship between L-type Ca2+ current (ICa−L) and frequency in cardiac myocytes is mainly due to a direct negative feedback of sarcoplasmic reticulum Ca2+ release on ICa−L inactivation while others provide evidence for activation of calmodulin kinase II (CaMKII). To further elucidate the role of endogenous CaMKII activity, the CaMKII inhibitory peptides, autocamtide-2 relating inhibitory peptide (AIP) and myristoylated AIP were applied using conventional and perforated patch-clamp methods. AIP inhibited the normal adaptive increase in ICa−L in response to abrupt increase in pacing frequency from 0.05 to 2 Hz. The positive ICa−L–frequency relationship was reversed by AIP and the inhibitory effect of AIP was significantly exaggerated at fast pacing rates. The onset of inactivation of ICa−L was not altered by AIP. After thapsigargin treatment, AIP slowed recovery from inactivation of ICa−L and this effect was exaggerated during fast pacing. Buffering of [Ca2+]i by BAPTA and EGTA accelerated recovery of ICa−L from inactivation, and BAPTA partly eliminated the effect of AIP on the recovery. We conclude that: (1) [Ca2+]i directly slows ICa−L recovery from inactivation; and (2) Ca2+-dependent endogenous CaMKII activity accelerates the ICa−L recovery. Thus, at fast heart rates, elevated [Ca2+]i activates endogenous CaMKII and compensates for its direct inhibitory effect on ICa−L recovery from inactivation. Dynamic activity of endogenous CaMKII enhances the positive ICa−L–frequency relationship.
The positive force–frequency relationship plays an important adaptative role in regulating heart function allowing appropriate response to the haemodynamic needs of the body. The positive relationship between the magnitude of L-type Ca2+ current (ICa−L) and frequency (use-dependent facilitation of ICa−L) is an important part of this adaptive response. The mechanism of use-dependent facilitation of ICa−L was generally believed to relate to activation of calmodulin kinase (CaMKII). Evidence supporting this mechanism includes: (1) the fact that facilitation is largely eliminated by CaMKII blockers including KN-62 (Yuan & Bers, 1994), KN-93 (Maier et al. 2003) and various inhibitory peptides including CaMKII-209-390, CaMKII-273-302 (Yuan & Bers, 1994), ICK (Xiao et al. 1994) and AC3-I (Wu et al. 2001); and (2) that treatment with constitutively active CaMKII resulted in increases in open probability and prolonged open times of the L-type Ca2+ channel (Dzhura et al. 2000). Alternatively, we and others (Delgado et al. 1999; Guo & Duff, 2003) have provided evidence that sarcoplasmic reticulum (SR) calcium release negatively feeds back on the calcium channel by causing inactivation as a major mechanism of use-dependent facilitation of ICa−L. In those studies, abrupt shortening of the stimulus cycle length significantly decreased SR Ca2+ release and thereby produced less inactivation, thus eliciting facilitation of ICa−L (Delgado et al. 1999; Guo & Duff, 2003). In the present study we clarify the role of endogenous CaMKII in use-dependent facilitation and more generally, in the frequency response of ICa−L in ventricular myocytes.
Recovery from inactivation plays an important role in the response of ICa−L to changes in pacing frequency. Slowing recovery from inactivation is expected to have an exaggerated effect on the ICa−L amplitude when pacing at high frequency. Vinogradova et al. 2000 reported that KN-93, as well as autocamtide-2 relating inhibitory peptide (AIP), slowed the recovery of ICa−L from inactivation in sinoatrial node cells of heart. In contrast, Yuan & Bers (1994) found that CaMK inhibitory peptide CaMKII-290-309 had no effect on ICa−L recovery in working cardiac myocytes. When applying the more selective, potent and cell membrane-permeable CaMKII inhibitory peptide (myristoylated AIP), we observed that increases in [Ca2+]i produced by high-frequency pacing slowed recovery from inactivation. These data indicate that the Ca2+-dependent activity of endogenous CaMKII accelerates recovery from inactivation and thus offsets the ‘direct’ effect of [Ca2+]i to slow recovery from inactivation.
Methods
Cell isolation
This investigation was performed in accordance with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication no. 85–23, revised 1996). The protocol was approved by the University of Calgary Animal Care Committee. Single ventricular myocytes were isolated from adult rat hearts using a Langendorff procedure similar to that used in our previous experiments (Guo & Duff, 2003). Adult, male, LBN rats (weight, 200–300 g) were deeply anaesthetized with CO2 and the heart was rapidly removed. The aorta was cannulated and retrograde perfusion was initiated with a standard Tyrode solution at 37°C. Perfusate was changed to a nominally Ca2+-free Tyrode solution for 5 min followed by an 8-min perfusion with Tyrode solution containing 10 μmol l−1 Ca2+ and 0.05 mg ml−1 collagenase (Yakult, Tokyo, Japan). The left ventricle was removed and minced. Pieces of ventricle were gently agitated for 10–30 min in a shaking bath in the Ca2+-free Tyrode solution containing 0.5 mg ml−1 collagenase, 0.1 mg ml−1 protease (type XII, Sigma, St Louis, MO, USA) and 1 mg ml−1 bovine serum albumin. Aliquots of minced tissue were then drawn off at 5-min intervals, placed into Tyrode solution containing 0.1 mmol l−1 Ca2+ and stored at room temperature (20°C to 22°C) until the myocytes were used. Single quiescent cardiomyocytes with smooth surfaces and clear cross-striation were used.
Patch-clamp recordings
Conventional and perforated whole-cell voltage-clamp methods were used. All the experiments were performed at room temperature. Data acquisition and analysis were carried out using pClAMP 8 (Axon Instruments, Inc., Union City, CA, USA) and SigmaPlot 9 (SPSS Inc., Chicago, IL, USA). An Axopatch 200B (Axon Instruments) amplifier was used. Access resistance was < 8 MΩ in conventional whole-cell experiments. The β-escin perforated patch method (Fan & Palade, 1998) was also used in the present study. This method has been reported to reduce the ICa−L rundown (Fan & Palade, 1998). In this experiment, 50 μmol l−1 β-escin was added into the pipette solution and the experiment was carried out only when the access resistance was less than 15 MΩ. In all the experiments, the series resistance was routinely compensated to > 80%.
To block K+ conductance, the extracellular solution was K+-free and a Cs+-containing pipette solution was used. Most of the Na+ current was blocked by 20 μmol l−1 TTX. Any residual Na+ current and/or T-type Ca2+ current was inactivated by a ramp prepulse, which first stepped from −80 to −60 mV followed by a ramp to −40 mV over 80 ms that was maintained at −40 mV for another 20 ms. ICa−L was recorded using a depolarization test pulse to 0 mV from a holding potential of −80 mV after the prepulse (Guo & Duff, 2003). The typical ICa−L recording pulse protocol was repeated at different frequencies or intervals according to the needs of experiments. The peak ICa−L at 0 mV was measured as the amplitude of ICa−L.
The time course of recovery from inactivation of ICa−L was measured using a conventional paired pulse protocol. The I1−I2 intervals (the intervals between the first pulse and the second pulse) were systematically varied and relative recovery ratio was calculated as:
where Ipeak is the peak current level at the test pulse and Ilate is the current level at the end of the test pulse. Most protocols were repeated every 10 s (0.1 Hz). To measure the time course of recovery of ICa−L when preceded by fast pacing, the 20 pulses at a frequency of 1 Hz were given before introduction of the first test paired pulse and five pulses at 1 Hz were given before each I1–I2 pair (see Fig. 3A for protocol). All the recovery time courses were measured under the perforated patch-clamp configuration to prevent current rundown.
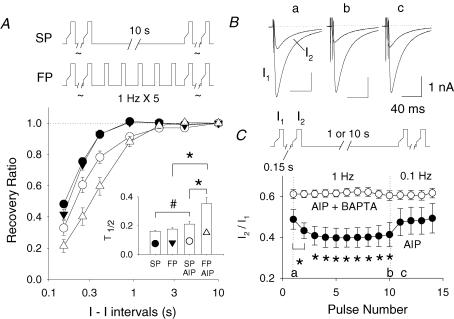
Figure 3. The effect of AIP is dependent on cycle length and [Ca2+]i.
A, the experimental protocols are shown at the top. Mean time courses of recovery from ICa−L inactivation measured during slow pacing (SP) and fast pacing (FP) conditions are shown with or without 2 μmol l−1 AIP treatment. See inset for symbol assignment. The inset shows the mean ICa−L recovery times, T0.5, of each groups (#P < 0.05; *P < 0.01). B, superimposed I1 and I2 traces of ICa−L before (a), during fast pacing at 1 Hz (b) and after pacing had slowed to 0.1 Hz (c). The currents were taken at the times marked in panel C (a–c). C, shows average dynamics of I2/I1 ratio during abrupt changes in pacing frequency. The experimental protocol is shown in the top panel. The I1–I2 interval was fixed at 0.15 s while the interpaired pulse interval was either 10 or 1 s. Two transitions were examined. After more than a 10-s rest, the interpulse interval was increased to 1 Hz for 10 pulses and then abruptly slowed to 0.1 Hz. The dynamics of the transitions of interpulse interval are plotted. *P < 0.01, when compared with the first I2/I1.
To allow comparison of the recovery time course between slow (0.1 Hz) and fast pacing (1 Hz), the recovery ratio during fast pacing was corrected using the following formula:
where recovery ratio (I10s/I1) is the recovery ratio calculated by traditional formula described above, when the I1–I2 interval was increased to 10 s during the fast pacing protocols. Although the β-escin perforated-patch technique was used, a small change of ICa−L with time (rundown or runup) still can be seen. We carefully monitored amplitude of all I1 values. If the amplitude of adjacent I1 (the I1 before and after every I2) was significantly altered, the data were discarded.
Solution and drugs
The Tyrode solution contained (mmol l−1): NaCl 145, KCl 5.4, CaCl2 1.8, MgCl2 1.0, Na2HPO4 1.0, Hepes 5.0 and glucose 5.5; pH adjusted to 7.4 with NaOH. Unless otherwise indicated, the extracellular superfusate contained 20 μmol l−1 TTX and was K+-free, with K+ being replaced by equimolar Cs+. The pipette solution contained (mmol l−1): CsCl 120, tetraethylammonium chloride 20, ATP-Mg 5, GTP-Na2 0.5, EGTA 0.2, Hepes 10; pH adjusted to 7.2 with CsOH. For the experiments, 5 mmol l−1 EGTA and 2 mmol l−1CaCl2, or 5 mmol l−1 BAPTA and 1.36 mmol l−1 CaCl2 (the calculated free [Ca2+] in the pipette solution was ∼100 nmol l−1 (Bers et al. 1994)) were added into pipette solutions according to the requirements.
EGTA was added to the pipette solution in some β-escin perforated patch-clamp experiments. EGTA passes through the β-escin channels (Fan & Palade 1998). The effect of EGTA dialysis was confirmed by the observation that cell shortening ceased when recording ICa−L. For the BAPTA experiments, the cells were incubated in 20 μmol l−1 BAPTA-AM for more than 1 h before the experiment, and 5 mmol l−1 BAPTA was added into the pipette solution during the experiment.
For AIP experiments, 2 μmol l−1 membrane permeable AIP (myristoylated AIP) was added to the recording solution and cells were preincubated with AIP in the Tyrode solution contain 1 mmol l−1 CaCl2 for more than 30 min before beginning the experiments. In a few cells, AIP was dialysed through the patch pipette. In this case, 100 μmol l−1 AIP (not myristoylated AIP) was added into the pipette solution. To enhance dialysis, a brief positive pressure was applied to the patch pipette during which the access resistance was less than 5 MΩ. No difference in AIP response was found when comparing membrane permeable AIP with dialysis and accordingly, we added the data sets together.
When required, cells were incubated in 1 μmol l−1 thapsigargin for more than 1 h before experiments. AIP, myristoylated AIP, KN-93, KN-92, TTX and thapsigargin were obtained from Calbiochem (CA, USA). All other chemicals were acquired from Sigma.
Data analysis
ICa−L inactivation time (T0.37) was measured as the time required for the current to decay to 0.37 of the peak amplitude. Fifty percent recovery time (T0.5) of ICa−L from inactivation was measured as the I1–I2 intervals required for I2 to recover to 50% of I1. Results are presented as means ± s.e.m. Data were analysed with paired and unpaired t tests.
Results
AIP eliminates facilitation of ICa−L but only at high-frequency pacing and without an effect on ICa−L inactivation
To evaluate the effects of CaMKII on facilitation, rat cardiac myocytes were incubated for > 30 min with membrane-permeable AIP (myristoylated AIP) (or AIP in the pipette) and compared with the vehicle control. Frequency-dependent changes in ICa−L were evaluated during abrupt increases in pacing rate from 0.05 Hz to a range of cycle lengths from 0.5 to 2 Hz. A significant increase in ICa−L was observed at abrupt increases in pacing rate. AIP inhibited facilitation but it was surprising to find that the extent of inhibition was cycle-length dependent (Fig. 1A and B). At a pacing frequency of 2 Hz, AIP completely eliminated ICa−L facilitation after an abrupt increase of pacing frequency. Moreover, a progressive decrease of the currents occurred during continued fast pacing, suggesting an accumulative effect. This progressive decrease rapidly recovered to the baseline level after the pacing frequency was slowed (Fig. 1B). When pacing at 1 and 0.5 Hz, the effects of AIP were substantially less (Fig. 1B). Similar changes were observed in all five experiments with AIP. Figure 1C shows the mean frequency-dependent effect of AIP on facilitation. At 2 Hz, a significant decrease was observed but non-significant trends were seen at pacing frequencies of 1 and 0.5 Hz (n = 7 in control, n = 5 with AIP). During prolonged fast pacing, AIP completely inhibited the positive ICa−L–frequency relationship; however, this was not evident during slower pacing frequencies. It is interesting that the extent of inhibition of facilitation progressively increased during application of multiple fast stimuli after blocking endogenous CaMKII.
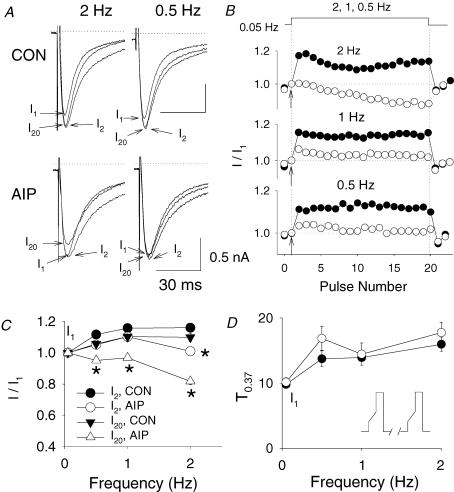
Figure 1. AIP inhibits the use-dependent facilitation of ICa−L in a frequency-dependent manner.
A, representative examples are shown for the first (I1), second (I2) and 20th ICa−L (I20) traces after the pacing frequencies were abruptly increased from 0.05 to 0.5 and 2 Hz in control (CON) and in the presence of 2 μmol l−1 AIP. ICa−L was induced by a depolarization to 0 mV from −80 mV after a ramp prepulse. Arrows indicate the peaks of currents. B, normalized ICa−L amplitude during the pacing frequency changes in control (•) and the presence of AIP (○) obtained from the experiments shown in A. All current amplitudes were normalized to I1 (arrows). C, average I2/I1– and I20/I1–frequency relationship of ICa−L in control (n = 7) and the presence of AIP (n = 5). D, mean inactivation time, T0.37, of I2 is plotted against the pacing frequency, showing control and with AIP. The experimental protocol is shown in the inset. *P < 0.01.
SR calcium release negatively feeds back on the calcium channel by producing and accelerating inactivation. Thapsigargin eliminates facilitation by decreasing the difference in the kinetics of onset of inactivation between the I1 and I2 pulses (Guo & Duff, 2003). In contrast, AIP inhibits facilitation at fast pacing frequency but without a change in the kinetics of inactivation between the I1 and I2 beats. The pattern of inactivation during AIP treatment was similar to control. During AIP treatment, while the magnitudes of I1 and I2 were similar, the kinetics of inactivation of the I2 pulse were still much slower than those of I1 (similar to control). AIP did not significantly change the inactivation time, T0.37, at all tested pacing frequencies (n = 5) compared to control (n = 7, Fig. 1D). This observation does not support the previous hypothesis that activation of CaMKII decreases ICa−L inactivation at high pacing frequency (Maier et al. 2003). It also makes it unlikely that AIP modulates the response of ICa−L to change in pacing frequency through its effects on SR Ca2+ storage and release.
AIP slows recovery from inactivation of ICa−L
To completely remove the contribution of SR Ca2+ release on the recovery of ICa−L (Guo & Duff, 2003), myocytes were pretreated for > 1 h with 1 μmol l−1 thapsigargin. Facilitation is not manifest after thapsigargin treatment. The magnitude of change in ICa−L was assessed during application of paired pulses at various I1–I2 coupling intervals (Fig. 2A). By 0.4 s, ICa−L had recovered almost to baseline (I1) under control conditions whereas in cells treated with AIP, the recovery had only reached about 70%. Figure 2B shows that AIP significantly prolonged the time course of recovery from inactivation. This slowing of recovery from inactivation occurs without any significant alteration of the time constant of onset of inactivation (Fig. 2C). To provide further evidence that slowing recovery from inactivation is due to inhibition of CaMKII, the effects of another CaMKII inhibitor, KN-93, were compared to the effects of its inactive analogue KN-92. Like AIP, 2 μmol l−1 KN-93 significantly slowed recovery from inactivation, compared to the response with 2 μmol l−1 KN-92 (Fig. 2D and E). It is interesting that KN-93 showed a much stronger inhibitory effect on the ICa−L recovery, which suggests that KN-93 was a more complete inhibitor of CaMKII than AIP, under our experimental conditions.
Figure 2. AIP slows recovery from inactivation of ICa−L.
A, after eliminating SR Ca2+ release by pretreatment with thapsigargin (1 μmol l−1), ICa−L at the I–I coupling intervals of 0.15, 0.4 and 2 s are superimposed. Only one representative I1 trace is shown. Note that the scales are different between left and right panels. Stimulation protocol is shown in the inset of B. B, shows mean time courses of recovery from ICa−L inactivation in either control (•, n = 7) or with AIP (○, n = 8) (#P < 0.05; *P < 0.01). C, mean onset of inactivation times of ICa−L, T0.37, are plotted against I–I intervals. D, shows the mean time courses of recovery from inactivation of ICa−L in the presence of either 2 μmol l−1 KN-92 (negative control, •, n = 5) or 2 μmol l−1 KN-93 (○, n = 3). The bars showing the error are too small to be seen (s.e.m. < 0.04). Ryanodine at 0.3 mmol l−1 was used instead of thapsigargin to block SR Ca2+ release. E, mean 50% recovery times (T0.5) of ICa−L, in control, and with AIP, KN-92 or KN-93 (#P < 0.05; *P < 0.01).
Figure 2E shows that AIP increased the 50% recovery time (T0.5) of ICa−L from 0.161 ± 0.005 s in control (n = 7) to 0.21 ± 0.018 s (n = 8). Similarly, KN-93 increased T0.5 from 0.133 ± 0.004 (in the presence of KN-92) to 0.400 ± 0.064 s (n = 3). These data indicate that CaMKII does significantly slow the ICa−L recovery from inactivation.
The effect of AIP is dependent on cycle length
As high-frequency activation of Ca2+ transients mediates progressive increases in CaMKII activity (De Koninck & Schulman, 1998), it seems plausible that the effects of AIP would be dependent on cycle length. After the contribution of SR Ca2+ stores and release had been eliminated by pretreatment with 1 μmol l−1 thapsigargin, the time course of recovery from inactivation of ICa−L was measured by application of paired pulses at various I1–I2 intervals. To assess the recovery of inactivation when pacing at high frequency, the impact of application of 20 pulses at a frequency of 1 Hz was evaluated. Figure 3A shows the time course of recovery from inactivation when preconditioned at a fast (1 Hz) compared with a slow (0.1 Hz) pacing frequency, with or without pretreatment with AIP. When no drug was present, preconditioning did not change recovery of ICa−L (Fig. 3A; slow pacing, •; fast pacing, ▾; n = 7 and 10, respectively) independent of whether preconditioned by fast or slow pacing; in fact, fast pacing trended to slow recovery. However, after blocking endogenous CaMKII with 2 μmol l−1 AIP, preconditioning at fast pacing rates significantly slowed recovery from inactivation (n = 11, 0.1 Hz, ^; 1.0 Hz, ▵ in Fig. 3A). The mean recovery time courses (T0.5) are shown in the inset of Fig. 3A.
To address whether changes in intracellular Ca2+ mediate the effects of the fast preconditioning pulse trains, we compared the effects of the preconditioning trains on I2/I1 recovery in the presence or absence of BAPTA. The protocol shown on top of Fig. 3C was used. Paired pulses at a fixed I1–I2 interval (0.15 s) were applied but the interpulse interval was either 10 or 1 s. Two transitions were examined. After more than a 10-s rest, the interpulse interval was increased to 1 s and then it was slowed to 10 s (protocol shown in inset to Fig. 3C). The dynamics of the effect of changes in interpulse interval during the transitions are plotted. During blockade of endogenous CaMKII with AIP alone, abrupt increases in pacing frequency decreased the I2/I1 ratios, which then returned to baseline when pacing frequency slowed (0.1 Hz; Fig. 3B and C). It is important to note that the decrease of I2/I1 ratio in response to fast pacing was completely eliminated by treatment with BAPTA. These data indicate that in the absence of endogenous CaMKII activity, fast pacing slows recovery from inactivation and that this effect is probably mediated by changes in [Ca2+]i.
Intracellular Ca2+ directly inhibits the recovery from inactivation of ICa−L
To provide further evidence that [Ca2+]i is an important determinant of recovery from inactivation, the effect of two Ca2+ chelators, BAPTA and EGTA, dialysed intracellularly, were evaluated. BAPTA chelates Ca2+ more rapidly than EGTA (Tsien, 1980). Cells were pretreated with thapsigargin to eliminate any contribution of SR Ca2+ stores or release on recovery from inactivation. Figure 4A shows that BAPTA had a biphasic effect: at short I1–I2 intervals (< 0.5 s), BAPTA accelerated recovery from inactivation; whereas at long coupling intervals (I1–I2 intervals longer than 0.5 s), it slowed recovery (Fig. 4A). Both effects were statistically significant (n = 10 in control, n = 7 with BAPTA). In contrast, EGTA, a slow Ca2+ chelator (Tsien, 1980), accelerated the recovery from inactivation at short I1–I2 coupling intervals and had no effect at longer intervals. These data indicate that BAPTA appears to have two effects, one acting directly and one acting through CaMKII.
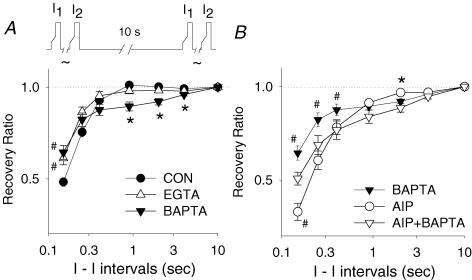
Figure 4. Intracellular Ca2+ directly inhibits the recovery from inactivation of ICa−L.
All cells were pretreated with thapsigargin (1 μmol l−1). A, recovery from inactivation under control conditions (n = 10) was compared to that in the presence of BAPTA (n = 7) and EGTA (n = 8). The loose standard slow pacing protocol was used (protocol shown in top panel). For statistical analysis, BAPTA and EGTA were compared to control. B, the recovery from inactivation in the presence of 2 μmol l−1 AIP plus BAPTA (n = 6) was compared to that in the presence of BAPTA alone (n = 7) or AIP alone (n = 10). *P < 0.01; #P < 0.05.
To further explore this duality, we assessed the effects of AIP (2 μmol l−1) in the presence of BAPTA (Fig. 4B). BAPTA was expected to chelate free Ca2+ rapidly and largely prevent the activation of CaMKII and for the most part eliminate the AIP effect. However, the ICa−L recovery in the presence of AIP plus BAPTA was significantly slower than that observed with BAPTA alone. These data suggests that BAPTA did not completely eliminate endogenous CaMKII activity.
Previous studies indicate that CaMKII maintains its activity long after a Ca2+ transient through autophosphorylation. The effect of BAPTA to slow recovery of ICa−L at longer coupling intervals may represent its ability to block CaMKII. If AIP could completely inhibit CaMKII activity, the effect of BAPTA on recovery at long coupling intervals would be expected to disappear. However, Fig. 4B shows that after blocking endogenous CaMKII with 2 μmol l−1 AIP, BAPTA still slowed the recovery at long coupling intervals (I1–I2 > 0.5 s, P < 0.01 at 2 s, BAPTA plus AIP) when comparing with AIP alone. Thus, assuming this is due to the effects of BAPTA on CaMKII activity, AIP may not be completely inhibiting CaMKII under these conditions.
Discussion
A novel Ca2+–CaMKII regulatory pathway is proposed by this study: intracellular Ca2+ directly inhibits the ICa−L recovery from inactivation, while Ca+-dependent CaMKII activity facilitates recovery from inactivation. Under physiological conditions of [Ca2+]i oscillations, endogenous CaMKII activity compensates for the direct inhibitory effect of [Ca2+]i on ICa−L recovery from inactivation. The presence of the new regulatory pathway is supported by the following evidence. (1) The CaMKII inhibitory peptide, AIP, eliminates the cycle length-dependent adaptive increase in ICa−L after abrupt increase in pacing frequency. This effect is exaggerated at fast pacing frequency. (2) After eliminating SR Ca2+ release, AIP, as well as KN-93, significantly slows ICa−L recovery from inactivation. (3) In the presence of thapsigargin, high-frequency pacing exaggerates the inhibitory effects of AIP on recovery from inactivation, and high-frequency pacing has no effect in the absence of AIP. (4) Buffering of intracellular Ca2+ substantially accelerates the ICa−L recovery from inactivation and eliminates the inhibitory effect of high-frequency pacing on recovery during AIP treatment.
ICa−L recovery is facilitated by endogenous CaMKII
Our previous study (Guo & Duff, 2003) showed that SR Ca2+ release acting through Ca2+-dependent inactivation has an important effect on the amplitude of ICa−L especially when two tightly coupled pulses are applied. Thus, when the SR function is intact, the recovery course of ICa−L inactivation will be dominantly affected by an SR Ca2+-related mechanism. Probably this is one of the main reasons that the effect of CaMKII on ICa−L recovery from inactivation has not been previously emphasized in working ventricular myocytes. In sinoatrial node cells, KN-93 as well as AIP slowed the ICa−L recovery (Vinogradova et al. 2000). A less developed SR Ca2+ store system in sinoatrial node cells probably favoured this observation. In working ventricular myocytes, some strong but less specific CaMK inhibitors, KN-62 and the less selective inhibitor, ruthenium red, have been reported to inhibit recovery from inactivation of ICa−L (Yuan & Bers, 1994; Sanchez et al. 2001; Netticadan et al. 1996). Probably due to the strong influence of the negative feedback of SR Ca2+ on ICa−L,Yuan & Bers (1994) could not reproduce these data using more selective inhibitory peptides of CaMKII in ventricular myocytes.
By eliminating the dominant regulatory factor of SR Ca2+ release (thapsigargin) and by using high-frequency pacing to increase endogenous CaMKII activity, we have shown clearly in this study a significant slowing of ICa−L recovery from inactivation by inhibition of endogenous CaMKII in the ventricular myocytes. It is important to note that this effect is Ca2+-dependent (Fig. 3B and C). AIP has its greatest effect during fast pacing, a condition that will increase endogenous CaMKII activity through elevated Ca2+ concentrations (De Koninck & Schulman, 1998).
In this study, KN-93 showed a much stronger inhibitory effect than AIP on ICa−L recovery. The difference may be explained by more complete inhibition of CaMKII by KN-93. AIP at 2 μmol l−1 probably did not completely inhibit endogenous CaMKII in our study. However, KN-93 inhibits many subtypes of calmodulin kinases, whereas AIP is more specific for CaMKII (Ishida et al. 1995). In Fig. 4B, at I1–I2 intervals of > 0.5 s., AIP plus BAPTA showed slower ICa−L recovery than did AIP alone. The cell permeability of myristoylated AIP may be the rate limiting step. Investigators have used a wide variety of myristoylated AIP concentrations from 1 to 20 μmol l−1 (Gailly, 1998; Tong et al. 2004; Vinogradova et al. 2000).
Intracellular Ca2+ directly inhibits ICa−L recovery
The present study shows that after inhibition of endogenous CaMKII activity, ICa−L recovery is significantly slowed by increasing [Ca2+]i at fast pacing rates (De Koninck & Schulman, 1998) and recovery is accelerated by chelation of Ca2+ by BAPTA and EGTA. This may reflect a direct effect of [Ca2+]i on the dynamics of dissociation of Ca2+ from the inactivation gate. Under physiological conditions, the direct inhibitory effect is largely overcome by the opposing effect of Ca2+-dependent activation of CaMKII. The recovery from the inactivation of ICa(v)1.2 expressed in HEK cells has been evaluated at various [Ca2+]i by Lacinova & Hofmann (2005). In that study, increased [Ca2+]i slowed recovery from the inactivation of ICa(v)1.2. CaMKII was not blocked or evaluated in their experiments. In HEK cells, the endogenous CaMKII activity will not be spatially or physiologically matched to the level of expression of Ca(v)1.2. Thus, the imbalance of endogenous CaMKII activity and ICa(v)1.2 expression in HEK cells would allow them to observe the direct effect of [Ca2+]i on recovery but would not allow them to uncover the complex regulatory pathway described herein.
Functional importance of accelerated recovery from inactivation by CaMKII activity
In the present study, we evaluated the effects of endogenous CaMKII in the presence of thapsigargin to eliminate SR Ca2+ release. However, under physiological conditions, SR Ca2+ release would be expected to contribute ∼90% of the systolic Ca2+ transient (Bers, 2001). Thus, it is reasonable to expect that under physiological conditions, the SR Ca2+ release would amplify both the direct and indirect regulatory mechanisms. The effects will be further exaggerated at fast pacing rates. Therefore, we propose that most of the effects of AIP on the frequency-dependent changes of ICa−L (Fig. 1) are attributable to the recovery from the inactivation mechanism.
Relationship to previous proposed regulatory mechanisms
Previous studies reported that CaMKII facilitates L-type Ca2+ channel activity by a direct interaction with the channel (Hudmon et al. 2005; Dzhura et al. 2000). After eliminating Ca2+-dependent inactivation of L-type channel by mutation, endogenous CaMKII activity was responsible for increases in the amplitude of ICa−L after abrupt increases in pacing frequency (Hudmon et al. 2005). Constitutively active CaMKII increased the open probability and prolonged the open times of the L-type Ca2+ channel when Ba2+ was used as a charge carrier (Dzhura et al. 2000). Thus Ca2+-dependent inactivation and recovery from inactivation of ICa−L were not involved in their observations. Theoretically, a direct interaction of Ca2+–CaMKII with the channel could accelerate recovery from inactivation. In the present study, we intermittently observed possible direct facilitation (recovery ratio of > 1 in the presence of thapsigargin) but the magnitude of this facilitation was quite small. At the point of its greatest magnitude, at an I–I interval of 0.9 s, the recovery ratios were 1.011 ± 0.010 and 1.006 ± 0.015, at slow and fast pacing rates, respectively (Fig. 3). Thus, it is unlikely that the direct facilitation mechanism of Ca2+–CaMKII could exclusively explain the effect of CaMKII on the recovery from inactivation observed in this study. After inhibition of CaMKII by AIP and KN-93, the T0.5 almost doubled (Figs 2 and 3). We believe that CaMKII accelerates the recovery from inactivation independent of its previously published direct mechanism.
Effects of BAPTA on recovery from inactivation
Binding of Ca2+–calmodulin to CaMKII activates it. BAPTA and EGTA chelate free Ca2+ and thus compete with CaMKII for Ca2+–calmodulin binding. BAPTA and EGTA inhibit endogenous CaMKII activity. In this study, BAPTA accelerates the recovery from inactivation at tight coupling intervals (I–I interval < 0.5 s; Fig. 4B) but slows the recovery at longer coupling intervals (I–I intervals > 0.5 s). This finding may be explained by a dual physiological effect of BAPTA: (1) it chelates intracellular Ca2+ thereby inhibiting its direct effect to slow recovery; and (2) it inhibits Ca2+-dependent activation of CaMKII activity thus producing the opposite effect. After Ca2+ activation, CaMKII autophosphorylates and maintains its activity even after [Ca2+]i decays (De Koninck & Schulman, 1998). The ability of BAPTA to slow the recovery at long coupling intervals may reflect its ability to eliminate the autophosphorylated CaMKII effect. In contrast, the ability of BAPTA to accelerate recovery from inactivation at tight coupling intervals may reflect inhibition of the direct effect of Ca2+.
Use-dependent facilitation can be eliminated by treatment with thapsigargin or ryanodine. Previous data (Wu et al. 1999; Anderson, 2004), have been interpreted to indicate that Ca2+ released by the SR rather than direct flux of Ca2+ entering through the pore of the L-type calcium channel activates CaMKII. However, recently it was suggested that the flux of Ca through the pore could activate CaMKII and thus modulate the function of L-type channel (Hudmon et al. 2005). Our study suggests that the CaMKII activity, which accelerates recovery from inactivation, is sensitive to local Ca2+ released from the inner mouth of the pore of the channel because BAPTA cannot completely eliminate the effect of AIP. Similarly, BAPTA cannot completely eliminate Ca2+-dependent inactivation of ICa−L (Kreiner & Lee, 2006). The Ca2+-binding site for the inactivation gate has been considered to be near or within the internal mouth of L-type Ca2+ channel (Naraghi & Neher, 1997).
Other studies also suggest a similar location for sites regulating CaMKII binding and sites mediating Ca2+-dependent inactivation. Binding of Ca2+–calmodulin to the C-terminal of the L-type Ca2+ channel has been proposed as a sensor for Ca2+-dependent inactivation (Alseikhan et al. 2002; Peterson et al. 1999; Pitt et al. 2001; Zuhlke et al. 1999, Zuhlke et al. 2000). Similarly, the binding site for CaMKII is proposed to exist in the C-terminal of the L-type Ca2+ channel (Hudmon et al. 2005). Dzhura et al. (2000) and Wu et al. 2001) have reported that calmodulin kinase and a calmodulin-binding ‘IQ’ domain both facilitate L-type Ca2+ current in rabbit ventricular myocytes via a similar mechanism. Cytoskeletal disrupting agents also prevent calmodulin kinase, IQ domain and voltage-dependent facilitation of L-type Ca2+ channels (Dzhura et al. 2002).
Kinetically, EGTA is a much slower Ca2+ buffer than BAPTA (∼100 times slower; Tsien, 1980). In our study, EGTA was not able to inhibit recovery at long coupling intervals (Fig. 4A). This suggests that EGTA may not effectively inhibit endogenous CaMKII activity. A similar difference between BAPTA and EGTA on their ability to inhibit endogenous CaMKII activity has been reported in cardiac sinoatrial node cells (Vinogradova et al. 2000). A possible explanation may relate to the relative affinity and kinetics of binding between endogenous CaMKII, EGTA and BAPTA-AM. Our data could be explained if: (1) CaMKII binds Ca2+ faster than EGTA but slower than BAPTA; (2) the subsarcolemmal compartment in proximity to the Ca2+ channel is more accessible to BAPTA than EGTA; or (3) insufficient EGTA entered the cell by dialysis. Against the latter possibility, EGTA effectively inhibited myocyte shortening. EGTA is traditionally and routinely added to the pipette solution for patch-clamp experiments when Ca2+ chelation is required. Different results may be obtained depending on the intracellular Ca2+ ion chelator used. Based on our study, if EGTA is used to chelate intracellular Ca2+, CaMKII activity may be maintained and recovery of ICa−L may be accelerated.
We propose a novel regulatory pathway which involves opposing effects of [Ca2+]i on recovery from inactivation; a direct effect to slow and a Ca2+-dependent CaMKII effect to accelerate recovery from inactivation. CaMKII is activated by [Ca2+]i and acts as a compensatory mechanism to respond to cycle length changes via phosphorylation and autophosphorylation of CaMKII. Any changes in CaMKII activity will change this balance and produce significant changes in ICa−L, [Ca2+]i and cellular function. Thus, this novel regulatory pathway is likely to be an important determinant of response to physiological and pathological stimuli.
Acknowledgments
This work was supported by the Canadian Institutes of Health Research and by the Heart and Stroke Foundation of Alberta.
References
- Alseikhan BA, DeMaria CD, Colecraft HM, Yue DT. Engineered calmodulins reveal the unexpected eminence of Ca2+ channel inactivation in controlling heart excitation. Proc Natl Acad Sci U S A. 2002;99:17185–17190. doi: 10.1073/pnas.262372999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson ME. Calmodulin kinase and L-type calcium channels; a recipe for arrhythmias? Trends Cardiovasc Med. 2004;14:152–161. doi: 10.1016/j.tcm.2004.02.005. [DOI] [PubMed] [Google Scholar]
- Bers DM. Control of cardiac contraction by ER and sarcolemmal Ca flux. In: Bers DM, editor. Excitation–Contraction Coupling and Cardiac Contractile Force. 2. Dordrecht: Kluwer Academic Publishers; 2001. pp. 3–39. [Google Scholar]
- Bers DM, Patton CW, Nuccitelli R. A practical guide to the preparation of Ca2+ buffers. Methods Cell Biol. 1994;40:3–29. doi: 10.1016/s0091-679x(08)61108-5. [DOI] [PubMed] [Google Scholar]
- De Koninck P, Schulman H. Sensitivity of CaM kinase II to the frequency of Ca2+ oscillations. Science. 1998;279:227–230. doi: 10.1126/science.279.5348.227. [DOI] [PubMed] [Google Scholar]
- Delgado C, Artiles A, Gomez AM, Vassort G. Frequency-dependent increase in cardiac Ca2+ current is due to reduced Ca2+ release by the sarcoplasmic reticulum. J Mol Cell Cardiol. 1999;31:1783–1793. doi: 10.1006/jmcc.1999.1023. [DOI] [PubMed] [Google Scholar]
- Dzhura I, Wu Y, Colbran RJ, Balser JR, Anderson ME. Calmodulin kinase determines calcium-dependent facilitation of L-type calcium channels. Nat Cell Biol. 2000;2:173–177. doi: 10.1038/35004052. [DOI] [PubMed] [Google Scholar]
- Dzhura I, Wu Y, Colbran RJ, Balser JR, Anderson ME. Cytoskeletal disrupting agents prevent calmodulin kinase, IQ domain and voltage-dependent facilitation of L-type Ca2+ channels. J Physiol. 2002;545:399–406. doi: 10.1113/jphysiol.2002.021881. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Fan JS, Palade P. Perforated patch recording with b-escin. Pflugers Arch. 1998;436:1021–1023. doi: 10.1007/pl00008086. [DOI] [PubMed] [Google Scholar]
- Gailly P. Ca2+ entry in CHO cells, after Ca2+ stores depletion, is mediated by arachidonic acid. Cell Calcium. 1998;24:293–304. doi: 10.1016/s0143-4160(98)90053-7. [DOI] [PubMed] [Google Scholar]
- Guo J, Duff HJ. Inactivation of ICa−L is the major determinant of use-dependent facilitation in rat cardiomyocytes. J Physiol. 2003;547:797–805. doi: 10.1113/jphysiol.2002.033340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudmon A, Schulman H, Kim J, Maltez JM, Tsien RW, Pitt GS. CaMKII tethers to L-type Ca2+ channels, establishing a local and dedicated integrator of Ca2+ signals for facilitation. J Cell Biol. 2005;171:537–547. doi: 10.1083/jcb.200505155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishida A, Kameshita I, Okuno S, Kitani T, Fujisawa H. A novel highly specific and potent inhibitor of calmodulin-dependent protein kinase II. Biochem Biophys Res Commun. 1995;212:806–812. doi: 10.1006/bbrc.1995.2040. [DOI] [PubMed] [Google Scholar]
- Kreiner L, Lee A. Endogenous and exogenous Ca2+buffers differentially modulate Ca2+-dependent inactivation of CaV2.1 Ca2+ channels. J Biol Chem. 2006;281:4691–4698. doi: 10.1074/jbc.M511971200. [DOI] [PubMed] [Google Scholar]
- Lacinova L, Hofmann F. Ca2+- and voltage-dependent inactivation of the expressed L-type Cav1.2 calcium channel. Arch Biochem Biophys. 2005;437:42–50. doi: 10.1016/j.abb.2005.02.025. [DOI] [PubMed] [Google Scholar]
- Maier LS, Zhang T, Chen L, DeSantiago J, Brown JH, Bers DM. Transgenic CaMKIIdeltaC overexpression uniquely alters cardiac myocyte Ca2+ handling: reduced SR Ca2+ load and activated SR Ca2+ release. Circ Res. 2003;92:904–911. doi: 10.1161/01.RES.0000069685.20258.F1. [DOI] [PubMed] [Google Scholar]
- Naraghi M, Neher E. Linearized buffered Ca2+ diffusion in microdomains and its implications for calculation of [Ca2+] at the mouth of a calcium channel. J Neurosci. 1997;17:6961–6973. doi: 10.1523/JNEUROSCI.17-18-06961.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netticadan T, Xu A, Narayanan N. Divergent effects of ruthenium red and ryanodine on Ca2+/calmodulin-dependent phosphorylation of the Ca2+ release channel (ryanodine receptor) in cardiac sarcoplasmic reticulum. Arch Biochem Biophys. 1996;333:368–376. doi: 10.1006/abbi.1996.0403. [DOI] [PubMed] [Google Scholar]
- Peterson BZ, DeMaria CD, Adelman JP, Yue DT. Calmodulin is the Ca2+ sensor for Ca2+-dependent inactivation of L-type calcium channels. Neuron. 1999;22:549–558. doi: 10.1016/s0896-6273(00)80709-6. [DOI] [PubMed] [Google Scholar]
- Pitt GS, Zuhlke RD, Hudmon A, Schulman H, Reuter H, Tsien RW. Molecular basis of calmodulin tethering and Ca2+-dependent inactivation of L-type Ca2+ channels. J Biol Chem. 2001;276:30794–30802. doi: 10.1074/jbc.M104959200. [DOI] [PubMed] [Google Scholar]
- Sanchez JA, Garcia MC, Sharma VK, Young KC, Matlib MA, Sheu SS. Mitochondria regulate inactivation of L-type Ca2+ channels in rat heart. J Physiol. 2001;536:387–396. doi: 10.1111/j.1469-7793.2001.0387c.xd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong CW, Gaffin RD, Zawieja DC, Muthuchamy M. Roles of phosphorylation of myosin binding protein-C and troponin I in mouse cardiac muscle twitch dynamics. J Physiol. 2004;558:927–941. doi: 10.1113/jphysiol.2004.062539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsien RY. New calcium indicators and buffers with high selectivity against magnesium and protons: design, synthesis, and properties of prototype structures. Biochemistry. 1980;19:2396–2404. doi: 10.1021/bi00552a018. [DOI] [PubMed] [Google Scholar]
- Vinogradova TM, Zhou YY, Bogdanov KY, Yang D, Kuschel M, Cheng H, et al. Sinoatrial node pacemaker activity requires Ca2+/calmodulin-dependent protein kinase II activation. Circ Res. 2000;87:760–767. doi: 10.1161/01.res.87.9.760. [DOI] [PubMed] [Google Scholar]
- Wu Y, Dzhura I, Colbran RJ, Anderson ME, Albat B. Calmodulin kinase and a calmodulin-binding ‘IQ’ domain facilitate L-type Ca2+ current in rabbit ventricular myocytes by a common mechanism. J Physiol. 2001;535:679–687. doi: 10.1111/j.1469-7793.2001.t01-1-00679.x. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Wu Y, MacMillan LB, McNeill RB, Colbran RJ, Anderson ME. CaM kinase augments cardiac L-type Ca2+ current: a cellular mechanism for long Q-T arrhythmias. Am J Physiol. 1999;276:H2168–H2178. doi: 10.1152/ajpheart.1999.276.6.H2168. [DOI] [PubMed] [Google Scholar]
- Xiao RP, Cheng H, Lederer WJ, Suzuki T, Lakatta EG. Dual regulation of Ca2+/calmodulin-dependent kinase II activity by membrane voltage and by calcium influx. Proc Natl Acad Sci U S A. 1994;91:9659–9663. doi: 10.1073/pnas.91.20.9659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan W, Bers DM. Ca-dependent facilitation of cardiac Ca current is due to Ca-calmodulin-dependent protein kinase. Am J Physiol. 1994;267:H982–H993. doi: 10.1152/ajpheart.1994.267.3.H982. [DOI] [PubMed] [Google Scholar]
- Zuhlke RD, Pitt GS, Deisseroth K, Tsien RW, Reuter H. Calmodulin supports both inactivation and facilitation of L-type calcium channels. Nature. 1999;399:159–162. doi: 10.1038/20200. [DOI] [PubMed] [Google Scholar]
- Zuhlke RD, Pitt GS, Tsien RW, Reuter H. Ca2+-sensitive inactivation and facilitation of L-type Ca2+ channels both depend on specific amino acid residues in a consensus calmodulin-binding motif in the (alpha) 1C subunit. J Biol Chem. 2000;275:21121–21129. doi: 10.1074/jbc.M002986200. [DOI] [PubMed] [Google Scholar]