Abstract
Hypocretin/orexin (Hcrt) is a critical neurotransmitter for the maintenance of wakefulness and has been implicated in several other functions, including energy metabolism and reward. Using whole-cell patch-clamp recordings from transgenic mice in which enhanced green fluorescent protein was linked to the Hcrt promoter, we investigated GABAergic control of the Hcrt neurones in hypothalamic slices. Bath application of GABA or muscimol caused an early hyperpolarization mediated by Cl− and a late depolarization mediated by the efflux of bicarbonate. These GABAA receptor-mediated responses were blocked by picrotoxin and bicuculline. Under the GABAA blockade condition, GABA produced consistent hyperpolarization, decreased firing rate and input resistance. The selective GABAB agonist (R)-baclofen caused a similar response with an EC50 of 7.1 μm. The effects of (R)-baclofen were blocked by the GABAB antagonist CGP 52432 but persisted in the presence of tetrodotoxin, suggesting direct postsynaptic effects. The existence of GABAB modulation was supported by GABAB(1) subunit immunoreactivity on Hcrt cells colabelled with antisera to the Hcrt-2 peptide. Furthermore, GABAB receptor activation inhibited the presynaptic release of both glutamate and GABA. (R)-Baclofen depressed the amplitude of evoked excitatory postsynaptic currents (EPSCs) and inhibitory synaptic currents (IPSCs), and also decreased the frequency of both spontaneous and miniature EPSCs and IPSCs with a modest effect on their amplitudes. These data suggest that GABAB receptors modulate Hcrt neuronal activity via both pre- and postsynaptic mechanisms, which may underlie the promotion of non-rapid eye movement sleep and have implications for the use of GABAB agonists in the treatment of substance addiction through direct interaction with the Hcrt system.
The control of sleep and wakefulness is thought to be mediated through an interaction between a distributed set of sleep-promoting and wake-promoting systems in the brain. The sleep-promoting system is primarily GABAergic in nature, with the relevant cell bodies located in the ventrolateral and median preoptic area. In contrast, the monoaminergic systems in the brainstem and the cholinergic systems in both the brainstem and basal forebrain have been recognized as wakefulness-promoting systems for some time (Brown et al. 2001; Saper et al. 2001; Jones, 2003; McGinty & Szymusiak, 2003). More recently, the hypocretin/orexin (Hcrt) system of the hypothalamus (De Lecea et al. 1998; Sakurai et al. 1998) has been identified as a powerful wakefulness-promoting system (Kilduff & Peyron, 2000), initially as a result of its dysfunction in the sleep disorder narcolepsy both in animals (Chemelli et al. 1999; Lin et al. 1999; Hara et al. 2001) and humans (Nishino et al. 2000; Peyron et al. 2000; Thannickal et al. 2000). The Hcrt neurones project to (Peyron et al. 1998; Date et al. 1999) and excite the monoaminergic (Horvath et al. 1999; Bayer et al. 2001; Brown et al. 2001; Eriksson et al. 2001; Yamanaka et al. 2002) and cholinergic (Eggermann et al. 2001; Burlet et al. 2002; Wu et al. 2004) nuclei. Moreover, the Hcrt neurones have been shown to directly excite thalamocortical synapses (Lambe & Aghajanian, 2003), a mechanism by which Hcrt neurones excite the prefrontal cortex and play a role in attention and alertness. In turn, the Hcrt neurones are thought to receive inhibitory GABAergic input from the preoptic area (Sakurai et al. 2005; Saper et al. 2005). Thus, the transition from wakefulness to sleep is likely mediated by a GABAergic inhibition of the wake-promoting Hcrt cells, which results in disfacilitation of the wake-promoting monoaminergic and cholinergic systems.
Given the central role of the Hcrt system in the regulation of wakefulness, the afferent inputs to the Hcrt cells and the response of these neurones to various neuroactive substances have been the focus of considerable recent research. Anatomical studies have indicated afferent inputs to the Hcrt neurones from the amygdala, the cholinergic neurones and GABAergic neurones in the basal forebrain, and serotonin (5-HT) neurones in the median/paramedian raphe nuclei (Sakurai et al. 2005; Yoshida et al. 2005). The creation of mice in which enhanced green fluorescent protein (EGFP) is under control of the prepro-orexin promoter (Yamanaka et al. 2003b) has allowed visualization of the Hcrt/EGFP cells and thus facilitated in vitro physiological studies of these neurones and identification of the neurotransmitters and neuromodulators to which these cells respond. Using these mice, the Hcrt cells have been shown to be excited by glutamate through both NMDA and AMPA receptors (Li et al. 2002; Yamanaka et al. 2003a), by ghrelin (Yamanaka et al. 2003b), by glucagon-like peptide 1 (Acuna-Goycolea & van den Pol, 2004), and by cholecystokinin (CCK) through the CCK-A receptor (Tsujino et al. 2005). In contrast, Hcrt cells can be inhibited by 5-HT acting through the 5-HT1A receptor (Muraki et al. 2004), by noradrenaline through both α1 and α2 receptors (Li & van den Pol, 2005), by neuropeptide Y through the Y1 receptor (Fu et al. 2004), by leptin (Yamanaka et al. 2003b), by glucose (Yamanaka et al. 2003b; Burdakov et al. 2005), and by GABA through the GABAA receptor (Li et al. 2002; Yamanaka et al. 2003b). Thus, the Hcrt cells seem to be under tight regulation by a combination of fast-acting neurotransmitters and slower-acting neuropeptides (Horvath & Gao, 2005).
In the present study, we have focused on GABAergic inputs to the Hcrt cells since the GABAergic system has both fast neurotransmission, mediated through the GABAA receptors, and slower neurotransmission, mediated through GABAB receptors. As indicated above, previous studies have examined GABAA signalling in Hcrt cells, but the GABAB system has not been investigated to this point. In the present study, we demonstrate that Hcrt cells are inhibited by both GABAA and GABAB receptor activation, anatomically document the presence of GABAB receptors on Hcrt cells, and determine that GABAB receptors modulate neurotransmission onto Hcrt cells through both presynaptic and postsynaptic mechanisms. In contrast to the transient inhibition mediated through GABAA receptors, we suggest that the prolonged inhibition of Hcrt cells mediated by GABAB signalling may underlie longer-term changes in behaviour and physiology in which the Hcrt cells are involved, such as the transition from wakefulness to sleep. A preliminary report of these data has appeared (Xie et al. 2004).
Methods
Animals
All experimental procedures involving animals were approved by the Animal Care and Use Committee at SRI International and were in accordance with NIH guidelines and the University of Tsukuba Animal Resource Center. All efforts were made to minimize animal suffering or discomfort and to reduce the number of animals used.
Slice preparation
Mouse brain slice preparation containing the lateral hypothalamic area (LHA) and the recording conditions followed the procedures previously described (Yamanaka et al. 2003a,b). Male and female Hcrt/EGFP mice, 3–6 weeks old, in which the human prepro-orexin promoter drives expression of EGFP were used for experiments. Mice were deeply anaesthetized with methoxyflurane and then decapitated. Brains were isolated in ice-cold oxygenated high sucrose solution containing (mm): sucrose 220, KCl 2.5, MgCl2 6, CaCl2 1, NaHCO3 26, NaH2PO4 1.25, bubbled with 95% O2–5% CO2. Brain slices (250 μm thickness) were cut coronally with a microtome (VT-1000S, Leica, Germany). Slices containing the LHA area were then transferred to an incubation chamber where they were superfused with physiological bicarbonate solution containing (mm): NaCl 130, KCl 5, CaCl2 2.4, MgSO4 1.3, NaHCO3 20, KH2PO4 1.25, glucose 10, bubbled with 95% O2–5% CO2 at room temperature (RT; 22–24°C) for at least 1 h before recordings. The osmolarity of these external solutions was checked by a vapour pressure osmometer (Advanced Instruments, Norwood, MA, USA) and ranged between 290 and 310 mosmol l−1.
Whole-cell patch clamp recordings
Patch pipettes were prepared from borosilicate capillary glass (GC150-10, Harvard Apparatus, Holliston, MA, USA) with a micropipette puller (P-97, Sutter Instruments, Novato, CA, USA). The pipettes were routinely filled with a KCl-internal solution containing (mm): KCl 145, MgCl2 1, EGTA-Na3 1.1, Hepes 10, Na2ATP 2, Na2GTP 0.5, adjusted to pH 7.2 with KOH. Some experiments were conducted using a potassium methanesulphonate (K-MeSO4) internal solution containing (mm): K-MeSO4 144, Hepes 20, EGTA 0.2, NaCl 2.8, Na2ATP 2, Na2GTP 0.5, pH 7.2–7.4. Osmolarity of these internal solutions was between 280 and 290 mosmol l−1. Pipette resistance measured in the external solution was 3–10 MΩ. The series resistance during recording was 10–25 MΩ and was compensated only in current-clamp mode. The reference electrode was an Ag–AgCl pellet immersed in the bath solution.
For electrophysiological recording, a single slice was transferred to a recording chamber (RC-27 l, Warner Instruments LLC, Hamden, CT, USA) and was superfused with the physiological bicarbonate solution at RT. Some experiments were conducted in a Hepes external solution containing (mm): NaCl 150, KCl 2.5, CaCl2 2, MgCl2 2, NaH2PO4 1.3, Hepes 10, and glucose 10, pH 7.4 with NaOH. The liquid junction potential of the patch pipette with the KCl solution and perfused Hepes external solution was estimated to be 3–5 mV and was not corrected for in the data analysis.
Hcrt/EGFP neurones were visualized under an upright microscope (Leica DM LFSA, Leica Instruments) using both infrared-differential interference contrast (IR-DIC) microscopy and fluorescence microscopy. Infrared images were acquired via a charge-coupled device (CCD) camera optimized for infrared wavelengths (DAGE-MITI, Michigan City, IN, USA); fluorescence images were acquired using a digitizer system (Leica LEI-750D, Leica Instruments). Recording pipettes were advanced towards individual fluorescent cells in the slice under positive pressure and, on contact, tight seals between the pipette and the cell membrane on the order of 0.5–1.0 GΩ were made by negative pressure. The membrane patch was then ruptured by suction and membrane potential or current was monitored using an Axopatch 1D patch clamp amplifier (Molecular Devices, formerly Axon Instruments, Union City, CA, USA). Only cells in which the resting membrane potential was −40 mV or more negative and had an input resistance greater than 200 MΩ were included in this study.
In current-clamp mode, hyperpolarizing or depolarizing current pulses (20–30 pA, 800 ms durations) were applied to cells at 5 s intervals from the resting membrane potential of 60 mV adjusted with direct current (DC), unless specified otherwise. The membrane input resistance was estimated by dividing the electronic hyperpolarizing potential by the injected current.
Evoked excitatory and inhibitory postsynaptic currents (eEPSCs) and (eIPSCs) were recorded from Hcrt/EGFP neurones under voltage-clamp at −60 mV using a KCl internal solution. Under this recording condition, both eEPSCs and eIPSCs were recorded as inward currents. Electrical stimuli (100–200 μA, 0.1 ms, 0.1 Hz) were generated using bipolar stimulation electrodes (TM33A05, World Precision Instruments, Sarasota, FL, USA) placed within the LHA. Spontaneous excitatory postsynaptic currents (sEPSCs) were recorded using the KCl internal solution supplemented with the sodium channel blocker QX-314 (1 mm) to inhibit action potentials in the neurone and with bicuculline (BIC; 20 μm) and picrotoxin (PTX; 100 μm) added to the physiological bicarbonate solution to block GABAA receptor-mediated neurotransmission. Spontaneous inhibitory postsynaptic currents (sIPSCs) were recorded using the same pipette solution but in the presence of 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX, 20 μm) and dl-2-amino-5-phosphono-pentanoic acid (AP-5, 50 μm) to block AMPA and NMDA receptor-mediated neurotransmission. Miniature EPSCs were recorded using KCl internal solution in the presence of 1 μm TTX and BIC (30 μm) and PTX (100 μm). Miniature IPSCs were recorded using a KCl internal solution in the presence of 1 μm TTX, 20 μm 6,7-dinitroquinoxaline-2,3-dione (DNQX) and 50 μm AP-5. Most drug applications were via bath perfusion. In some experiments, local drug applications were employed through a thin polyethylene tube positioned near the cells recorded.
The output voltage signal was low pass filtered at 5 kHz and digitized at 10 kHz, while the output current signal was low pass filtered at 1 kHz and digitized at 5 kHz. Data were recorded on a computer through a Digidata 1322A A/D converter using pCLAMP software version 9.2 (Molecular Devices, formerly Axon Instruments, Union City, CA, USA). Frequencies and amplitudes of spontaneous and miniature synaptic events were measured using Mini Analysis Program version 6.0 (Synaptosoft, Inc., Decatur, GA, USA) from records before, during and after washout of the drug. Only those events with amplitudes > 10 pA were used. Electrophysiological traces were processed for presentation using Origin 7.5 (OriginLab Corp., Northampton, MA, USA) and Canvas 9.0 software (Deneba Systems, Miami, FL, USA).
Drugs
GABA, baclofen, tetrodotoxin (TTX), N-2,6-dimethyl-phenylcarbamoylmethyl-triethylammonium bromide (QX-314), 6-cyano-7nitroquinoxaline-2,3-dione (CNQX), 6,7-dinitroquinoxaline-2,3-dione (DNQX), dl-2-amino-5-phosphono-pentanoic acid (AP-5), BIC and PTX, and most laboratory reagents were purchased from Sigma (St Louis, MO, USA), and CGP 52432 was purchased from Tocris (UK). In the electrophysiological experiments, all drugs were made in stock solution (10–100 mm), freshly dissolved in external solution and applied either by bath application or by local application.
Immunohistochemistry
Adult male mice C57BL/6J (20–25 g, Charles-River, San Diego, CA, USA) were anaesthetized with sodium pentobarbital (50 mg kg−1, i.p.) and perfused sequentially with 10 ml chilled saline and 30 ml of chilled 4% paraformaldehyde in 0.1 m phosphate buffer (PB). The brains were removed and immersed in the same fixative solution for 4 h and were then transferred to 30% sucrose solution for 2 days at 4°C. The brains were quickly frozen in embedding solution with O.C.T. compound (Sakura Finetechnical Co. Ltd, Tokyo, Japan). To visualize hypocretin-2 (Hcrt-2) labelling, cryostat sections of the lateral hypothalamus (20 μm) were incubated overnight at 4°C in primary antisera (goat anti-orexin B, 1: 5000; Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA), followed by a 60 min incubation in a blocking solution (3% normal donkey serum in phosphate-buffered saline (PBS) with 0.3% Triton X-100 (3% NDST)), rinsed 3 times using a 1% NDST solution, and visualized following a 2-h incubation with a secondary antibody containing a red fluorophore (donkey antigoat Alexa 488, Molecular Probes, 1: 750 in PBS). To determine GABAB(1) subunit expression, the same sections were again incubated overnight at 4°C in primary antisera, this time directed against GABAB(1) subunit (guinea pig anti-GABAB(1), 1: 2500; Chemicon, Temecula, CA, USA), followed by a 60-min incubation in a blocking solution (3% normal goat serum in PBS with 0.3% Triton X-100 (3% NGST)) and three rinses with a 1% NGST solution. GABAB(1) subunit expression was visualized following a 2 h incubation in secondary antisera conjugated against a green fluorophore (Alexa 546 goat antiguinea pig, Molecular Probes, 1: 750 in PBS). Slides were air dried and cover-slipped using Fluoromount-G (Southern Biotech, Birmingham, AL, USA). Sections were observed using a light microscope (Leica DM5000B) equipped for fluorescence and with a CCD video camera operating with a computer-based anatomical mapping and image-analysis system (StereoInvestigator, Microbrightfield, Inc., Williston, VT, USA). Specificity of both antibodies was confirmed using a preadsorption with each respective peptide and observing no signal.
Statistical analysis
Data are presented as means ± s.e.m. and were analysed by two-way ANOVA followed by post hoc analysis of significance by Fisher's protected least significant difference test using the StatView 4.5 software package (Abacus Concepts, Berkeley, CA, USA). Probability (P) values less than 0.05 were considered statistically significant.
Results
The Hcrt/EGFP neurones were visually identified under fluorescence illumination and patch recordings of the same neurones were made using IR-DIC. Under control conditions in current clamp mode, Hcrt/EGFP neurones displayed spontaneous firing of action potentials at 0.5–3 Hz when the slices were perfused with the physiological bicarbonate external solution and recordings were made using a KMeSO4 internal solution. When recordings were made using a KCl internal solution, the spontaneous firing of action potentials was slightly increased (1–5 Hz), as some action potentials could be triggered by large spontaneous depolarizing synaptic events mediated by GABAA receptors. Action potential overshoots were usually greater than 40 mV and resting membrane potentials were slightly depolarized (−51.3 ± 3 mV; n = 58). These results are consistent with previously reported electrophysiological properties for Hcrt neurones (Li et al. 2002; Eggermann et al. 2003; Yamanaka et al. 2003a).
GABAA receptor-mediated modulation of Hcrt neuronal activity
To characterize inhibitory regulation of Hcrt neurones, our initial studies examined the effect of the major inhibitory neurotransmitter GABA on Hcrt neuronal firing and membrane potential in current clamp mode. The physiological bicarbonate external solution and KMeSO4 internal solution were initially used. The membrane potential of Hcrt neurones was held at −60 mV with DC injection and the input resistance was monitored with hyperpolarizing current pulses (−0.3 nA, 800 ms) delivered every 5 s. The GABAA selective agonist muscimol or GABA was then bath applied to the slices. The bath application of muscimol (30 μm) produced a biphasic response in the Hcrt neurones. The response consisted of an initial, short hyperpolarization (−4.5 ± 0.6 mV, n = 3) followed by a long depolarization (12.4 ± 4 mV) during the 1 min drug application (Fig. 1A). Associated with the biphasic membrane potential change, muscimol decreased input resistance by 85% and subsequently the firing of action potentials ceased. Similarly, 0.3 mm GABA also induced a biphasic membrane potential response: a decrease in input resistance and a decrease in the firing of action potentials (Figs 1B, n = 4). These GABA-induced effects rapidly reversed upon removal of GABA from the external solution and were completely blocked by the combination of the GABAA receptor antagonists, BIC (50 μm) and PTX (200 μm; data not shown).
Figure 1. Effects of GABA on membrane potential and firing of action potentials of Hcrt/EGFP neurones in mouse hypothalamic slices.
A, when superfused with the physiological bicarbonate solution, bath application of muscimol (30 μm) induced an early small membrane hyperpolarization followed by a later depolarization associated with a dramatic decrease in the input resistance. Consequently, spontaneous firing of action potentials was blocked in the Hcrt neurone. Calibration in A also applies to B and C. B, bath application of GABA (0.3 mm) also caused a biphasic change in membrane potential, decreased the input resistance and blocked all action potentials. C, when the external solution was changed to Hepes, bath application of GABA (0.3 mm) caused a monophasic hyperpolarization, decreased the input resistance and reduced action potentials. Three Hcrt/EGFP neurons were held at −60 mV with DC injection and hyperpolarizing current pulses (0.3 nA, 800 ms) were applied every 5 s throughout the experiment. The position of 0 mV is indicated by the dotted line and most single spike amplitudes were truncated due to limited temporal resolution. Recordings of these three cells used K-MeSO4 internal solution. D, bar graph summarizing the effect of 300 μm GABA on the membrane potential of Hcrt neurones when applied in the presence and absence of HCO3− in the external solution. ‘Early’ and ‘late’ refer to initial and subsequent responses induced by the bath application of GABA. Data are presented as means ± s.e.m. (n = 4 in bicarbonate and n = 3 in Hepes experiments).
Similar biphasic responses to GABA have been observed in other brain regions in which the depolarization component is thought to be dependent on HCO3− (Staley & Proctor, 1999; Isomura et al. 2003). Therefore, we removed HCO3− from the external solution and replaced it with Hepes. Under these conditions, bath-application of 0.3 mm GABA produced a consistent hyperpolarization (−11.4 ± 4.6 mV, n = 3) of the Hcrt neurones (Fig. 1C). These results are summarized in Fig. 1D.
GABAB receptor-mediated modulation of Hcrt neuronal activity
To distinguish GABAB receptor-mediated responses induced by GABA from GABAA receptor-mediated responses, a KCl internal solution and the physiological bicarbonate external solution were used. Under these conditions, bath application of 0.3 mm GABA produced a large depolarization of the Hcrt neurones (31.8 ± 4.6 mV, n = 3; Fig. 2A). However, in the presence of saturating concentrations of the GABAA receptor antagonists BIC (40 μm) and PTX (500 μm), bath application of 0.3 mm GABA produced little membrane potential change, though the input resistance was decreased by approximately 30% and firing of action potentials was completely blocked (Fig. 2B). When the GABA concentration was increased to 3 mm, a clear hyperpolarization (−4.7 ± 2 mV, n = 3) and a further decrease in the input resistance (50 ± 6% of control) was observed (Fig. 2C). The GABA-induced responses were blocked by the GABAB receptor antagonist CGP 52432 (data not shown). The above experiments were suggestive of the presence of functional GABAB receptors on Hcrt neurones.
Figure 2. GABAB receptor-mediated responses and protein expression in Hcrt neurones.
A, bath application of GABA (0.3 mm) caused consistent monophasic depolarization when recorded using KCl internal solution and standard external solution. B, in the presence of bicuculline (BIC, 40 μm) and picrotoxin (PTX, 500 μm), GABA (0.3 mm) did not obviously alter the membrane potential but decreased the input resistance (by 28%) in the same neurone as in A. C, under the same recording conditions as in B, a higher concentration of GABA (3 mm) produced hyperpolarization (−6 mV), decreased input resistance (by 32%) and blocked firing of action potentials in another Hcrt neurone. Three Hcrt neurons were all held at −60 mV with DC injection and hyperpolarizing current pulses (0.3 nA, 800 ms) were applied every 5 s throughout the experiment. Calibration in A also applies to B and C. The position of 0 mV is indicated by the dotted line and most single spike amplitudes were truncated due to limited temporal resolution. D, the GABAB(1) subunit is expressed in all Hcrt-2 (Hcrt)-positive cells, as shown by double-label immunoflourescence in the LHA. Left panel: Hcrt-2 immunoreactivity (red). Middle panel: GABAB(1)-immunoreactivity (green). Right panel: merged image. Arrows show GABAB(1)-immunoreactive cells which are positive for Hcrt-2 immunoreactivity; arrowheads indicate GABAB(1)-immunoreactive cells that do not colabel with Hcrt-2. Scale bar, 50 μm.
Since GABAB receptors had not been previously identified on mouse Hcrt neurones, we also conducted neuroanatomical studies to confirm the presence of these proteins in Hcrt cells. Using double immunofluoresence, we labelled LHA cells with antibodies targeting the Hcrt-2 peptide and the GABAB(1) subunit. We found many cells in the LHA to be GABAB(1)-immunoreactive (GABAB(1)-ir) and several neurones in the LHA that were both Hcrt-2-ir and GABAB(1)-ir (Fig. 2D). It appeared that every cell in the LHA that expressed Hcrt-2 was GABAB(1) positive, but not all GABAB(1)-ir cells were Hcrt-2-containing neurones (Fig. 2D). These results support the presence of a GABAB receptor on Hcrt cells.
To further characterize GABAB receptor-mediated modulation on Hcrt neuronal activity, we tested the effect of the GABAB selective agonist (R)-baclofen on Hcrt firing rate and membrane potential. Similar to the results observed when GABA was applied in the presence of GABAA receptor antagonists, bath application of 10 μm (R)-baclofen hyperpolarized Hcrt neurones (−5.6 ± 2 mV, n = 8) and decreased input resistance by 28 ± 5%, and consequently blocked the spontaneous firing of action potentials. In addition (R)-baclofen reduced membrane excitability in response to a depolarizing current injection (Fig. 3A, n = 4). The effect of (R)-baclofen on Hcrt membrane potential was concentration-dependent with an estimated half-maximal effective concentration (EC50) of 7.1 μm (Fig. 3B). This GABAB-mediated effect was reversible upon washout of (R)-baclofen. The ability of (R)-baclofen to reduce input resistance and firing rate of Hcrt neurones could be reversed by the addition of CGP 52432 (Fig. 3C). Lastly, in the presence of tetrodotoxin (TTX, 0.5 μm), spontaneous action potentials in Hcrt neurones were completely blocked, but local application of (R)-baclofen still produced hyperpolarization and decreased input resistance (Fig. 3D). After washout and recovery from the drug action, the patch recording was switched to voltage-clamp mode. When membrane potential was held at −60 mV (R)-baclofen (10 μm) induced an outward current in the same neurone. Both current- and voltage-clamp recordings in the presence of TTX indicate direct postsynaptic effects of baclofen on Hcrt neurones (Fig. 3D). The current–voltage relationship of neuronal responses in the presence and absence of baclofen indicates the reversal potential of baclofen-induced response is around −116 ± 6 mV, which is close to the K+ equilibrium potential (approximately −110 mV) under our experimental conditions (Fig. 3E). Taken together, these data suggest that the hyperpolarizing effect of GABA on Hcrt neurones observed in the presence of GABAA receptor blockade was due to a direct postsynaptic activation of GABAB receptors, presumably mediated by an inward rectifying potassium current.
Figure 3. Modulation of Hcrt/EGFP neuronal activity by the GABABR selective agonist (R)-baclofen.
A, bath application of (R)-baclofen (10 μm) hyperpolarized the Hcrt/EGFP neurones, decreased the input resistance, and suppressed the spontaneous firing of action potentials. Baclofen also depressed membrane excitability in response to depolarizing current injection (lower traces). B, (R)-baclofen (3–100 μm) caused membrane hyperpolarization in a concentration-dependent manner. The data were fitted using a dose–response curve fitting model (OriginPro 7.5) and yielded an estimated half-maximal effective concentration (EC50) of 7.1 μm. Values are means ± s.e.m. (n = 3–8). C, the baclofen-induced responses were blocked by the selective GABAB antagonist CGP 52432 (1 μm). The position of 0 mV is indicated by the dashed line and most single spike amplitudes were truncated due to limited temporal resolution. D, in the presence of TTX (0.5 μm), all spontaneous action potentials were blocked and local application of baclofen (10 μm) caused a hyperpolarization and a decrease in input resistance. After washout and recovery from the drug action under voltage-clamp mode (R)-baclofen (10 μm) induced an outward current in the same neurone at a holding potential of −60 mV. E, the current–voltage relationship of neuronal responses in the presence and absence of baclofen shows the reversal potential for baclofen-induced response around −115 mV, which is close to K+ equilibrium potential (calculated value of 108 mV) under our specific experimental conditions in which the external solution had 2 mm K+ and internal solution 145 mm K+ (n = 7).
Presynaptic GABAB receptor function in the hypothalamus
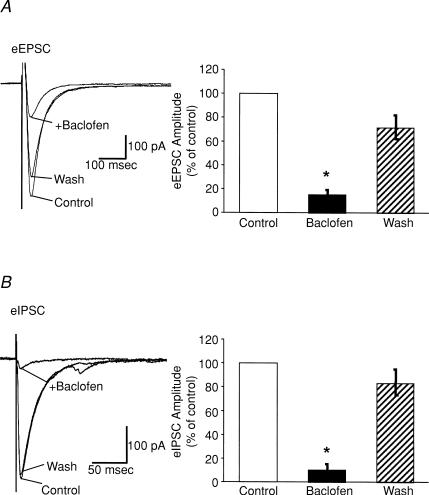
In most brain regions, GABAB receptors are also found presynaptically where they inhibit neurotransmitter release (Bowery et al. 2002; Bettler et al. 2004). To determine whether GABAB receptors also modulate neurotransmission onto Hcrt cells, we evaluated the effect of the GABAB agonist (R)-baclofen on evoked and spontaneous synaptic currents in voltage clamp mode. We first examined whether (R)-baclofen could alter currents elicited by the stimulation of neurotransmitter release. The neurones were voltage-clamped at −60 mV and synaptic currents were elicited every 10 s with local stimulation from a bipolar stimulating electrode placed within the LHA slice. EPSCs were isolated by pretreating the slices with PTX (100 μm). Under these conditions, bath application of (R)-baclofen (10 μm) reversibly depressed the evoked EPSC (eEPSC) amplitude to 18 ± 4% of control values (n = 5) (Fig. 4A). Similarly, when evoked inhibitory postsynaptic currents (eIPSCs) were pharmacologically isolated using CNQX (20 μm) and AP-5 (40 μm) to block AMPA/KA and NMDA receptors, respectively, (R)-baclofen (10 μm) reduced the amplitude of eIPSCs to 16 ± 5% of control values (Fig. 4B). These effects could be due to a decrease in the postsynaptic response to the neurotransmitters and/or to a change in the amount of neurotransmitter being released.
Figure 4. Modulation of evoked synaptic currents in Hcrt/EGFP neurones by (R)-baclofen.
A, eEPSCs were pharmacologically isolated by adding picrotoxin (100 μm) to the external solution to block inhibitory synaptic currents. Bath application of (R)-baclofen (10 μm) reversibly inhibited the amplitude of eEPSCs. The left panel shows average of five traces for an individual neuron under control conditions, in the presence of (R)-baclofen, and following washout of (R)-baclofen. The right panel shows a bar graph summarizing the effect of (R)-baclofen on eEPSC amplitude. The amplitude of eEPSCs was depressed to 18 ± 4% of control (n = 5, P < 0.001). All eEPSCs were recorded using KGlu as the pipette internal solution. B, eIPSCs were recorded in the presence of CNQX (20 μm) and AP-5 (40 μm) to block excitatory synaptic transmission. Bath application of (R)-baclofen (10 μm) also reversibly inhibited the amplitude of eIPSCs. The left panel shows average of five traces for an individual neuron under control conditions in the presence of (R)-baclofen and following washout of (R)-baclofen. The right panel shows a bar graph summarizing the effect of (R)-baclofen on eIPSC amplitude. The amplitude of eIPSCs was depressed to 16 ± 5% of control (n = 5, P < 0.001). All eIPSCs were recorded using KCl as the pipette internal solution. All cells recorded were voltage clamped at a holding potential of −60 mV and the bipolar stimulation electrode used to evoke synaptic currents was placed close to the recorded Hcrt/EGFP neuron in the LHA slice.
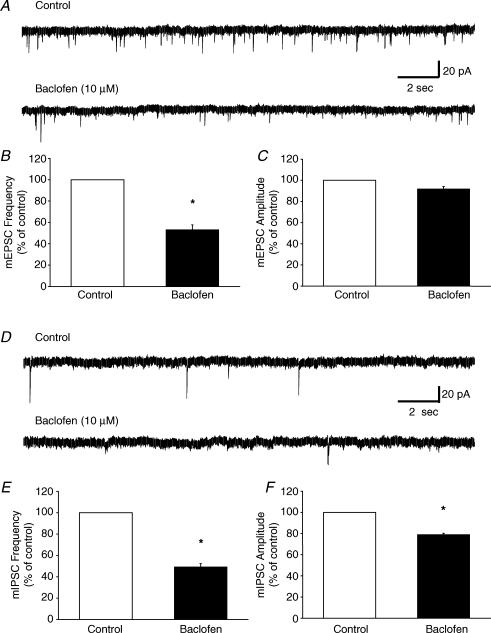
To further examine the possibility of a presynaptic function for GABAB receptors in modulating excitatory and inhibitory synaptic input to Hcrt neurones, we examined the effect of (R)-baclofen on spontaneous EPSCs and IPSCs. sEPSCs and sIPSCs were recorded in voltage clamp at a holding potential of −60 mV. Spontaneous excitatory events were recorded in the presence of BIC (20 μm) and PTX (100 μm). Under voltage clamp at a holding current of −60 mV, the mean frequency of sEPSCs was 3.8 ± 1.9 Hz (n = 7). Subsequent bath application of (R)-baclofen (10 μm) decreased the frequency of sEPSCs to 26 ± 6% of control values (n = 7). The decrease in sEPSC frequency was maintained throughout the duration of the (R)-baclofen application and fully recovered to baseline after washout of (R)-baclofen (Fig. 5A and B). The application of (R)-baclofen did not significantly inhibit the amplitude of these events (Fig. 5C). To isolate sIPSCs, the slices were pretreated with saturating concentrations of both the AMPA/KA and NMDA receptor antagonists CNQX (20 μm) and AP-5 (40 μm), respectively. Under these conditions, the mean frequency of sIPSCs recorded from Hcrt neurones was 4.5 ± 1.2 Hz (n = 7). Subsequent bath application of (R)-baclofen also significantly decreased the frequency of the inhibitory events to 20 ± 6% of control in all cells tested (n = 7, Fig. 5D and E). The effect of (R)-baclofen on sIPSC frequency was maintained during the (R)-baclofen application and returned to baseline upon washout of (R)-baclofen. The application of (R)-baclofen did not significantly inhibit the amplitude of these events (Fig. 5F). Taken together, these results suggest that GABAB receptors may act on presynaptic neurones to decrease the release of both excitatory and inhibitory neurotransmitters in the LHA.
Figure 5. Modulation of spontaneous EPSCs and IPSCs by GABAB receptor activation.
A, representative traces showing sEPSCs in control conditions, in the presence of (R)-baclofen, and following washout of (R)-baclofen. (R)-Baclofen reversibly decreased the frequency but not the amplitude of sEPSCs. B, bar graph summarizing the effect of (R)-baclofen on the frequency of sEPSCs. The mean frequency of sEPSCs was reversibly inhibited to 26 ± 6% of control (n = 7, P < 0.001). C, bar graph summarizing the effect of (R)-baclofen on the amplitude of sEPSCs. The mean amplitude of sEPSCs was not significantly altered (79.9 ± 15.4% of control; n = 4, P = 0.13). All sEPSCs were recorded in voltage clamp at a holding potential of −60 mV in the presence of bicuculline (20 μm) and picrotoxin (100 μm). D, representative traces showing sIPSCs in control conditions, in the presence of (R)-baclofen, and following washout of (R)-baclofen. (R)-Baclofen (10 μm) reversibly decreased the frequency but not the amplitude of sIPSCs. E, bar graph summarizing the effect of (R)-baclofen on the frequency of sIPSCs. The mean frequency of sIPSCs was reversibly inhibited to 20 ± 6% of control (n = 7, P < 0.001). F, bar graph summarizing the effect of (R)-baclofen on the amplitude of sIPSCs. The mean amplitude of sIPSCs was not significantly altered (79.8 ± 12.9% of control; n = 4, P = 0.17). sIPSCs were recorded under voltage clamp at a holding potential of −60 mV in the presence of CNQX (20 μm) and AP-5 (40 μm).
To determine if (R)-baclofen was acting directly at presynaptic terminals to decrease the probability of glutamate and GABA release, we further examined the effects of GABAB receptor activation on the frequency and amplitude of miniature EPSCs (mEPSCs) and IPSCs (mIPSCs). mEPSCs and mIPSCs were recorded in voltage clamp at a holding potential of −60 mV. mEPSCs were pharmacologically isolated by adding BIC (20 μm), PTX (100 μm) and TTX (1 μm) to the external solution. Under these conditions, the mean frequency of mEPSCs was 3.5 ± 0.4 Hz (n = 4). Bath application of (R)-baclofen (10 μm) significantly decreased the frequency of mEPSCs to 53.4 ± 4.6% of control (Fig. 6A and B). The amplitude of these events was not significantly altered by GABAB receptor activation (Fig. 6C). mIPSCs were isolated by adding DNQX (40 μm), AP-5 (40 μm) and TTX (1 μm) to the external solution. Under these conditions, the mean frequency of mIPSCs was 0.4 ± 0.1 Hz (n = 4). Bath application of (R)-baclofen (10 μm) decreased the frequency of mIPSCs to 49.3 ± 3% of control (Fig. 6D and E). Activation of GABAB receptors also caused a modest reduction in the amplitude of these events (Fig. 6F). These results suggest that GABAB receptors can presynaptically decrease the release of both glutamate and GABA onto Hcrt neurones in the LHA.
Figure 6. Modulation of miniature EPSCs and IPSCs by GABAB receptor activation.
A, representative traces showing mEPSCs in control conditions and in the presence of (R)-baclofen. (R)-Baclofen (10 μm) decreased the frequency but not the amplitude of mEPSCs. B, bar graph summarizing the effect of (R)-baclofen on the frequency of mEPSCs. The mean frequency of mEPSCs was inhibited to 53.4 ± 4.6% of control (n = 4, P < 0.05). C, bar graph summarizing the effect of (R)-baclofen on the amplitude of mEPSCs. The mean amplitude of mEPSCs was not significantly altered (94.1 ± 1.3% of control; n = 4, P = 0.21). All mEPSCs were recorded in the presence of BIC (20 μm), PTX (100 μm) and TTX (1 μm) in voltage clamp at a holding potential of −60 mV. D, representative traces showing mIPSCs in control conditions and in the presence of (R)-baclofen. (R)-Baclofen (10 μm) decreased the frequency and amplitude of mIPSCs. E, bar graph summarizing the effect of (R)-baclofen on the frequency of mIPSCs. The mean frequency of mIPSCs was inhibited to 49.3 ± 3.0% of control (n = 4, P < 0.01). F, bar graph summarizing the effect of (R)-baclofen on the amplitude of mIPSCs. The mean amplitude of mIPSCs was decreased in the presence of (R)-baclofen (79.1 ± 1.18% of control; n = 4, P < 0.05). mIPSCs were recorded in the presence of DNQX (40 μm), AP-5 (20 μm) and TTX (1 μm). All miniature synaptic currents were recorded under voltage clamp at a holding potential of −60 mV.
Discussion
Hcrt neuron activity is modulated by both GABAA and GABAB receptors
Previous studies have demonstrated that LHA neurones in the developing nervous system can be depolarized by GABA (Obrietan & van den Pol, 1995). In the present study, we extend these observations by showing that GABAA receptor activation by GABA or muscimol produces a biphasic membrane potential change in Hcrt cells. Depolarizing responses to GABA are frequently reported in the developing nervous system (Obrietan & van den Pol, 1995; Chen et al. 1996; Wang et al. 2001) and, in the adult brain, in the presence of exogenous zinc or after intense stimulation (Xie & Smart, 1993; Isomura et al. 2003; Stein & Nicoll, 2003). The mechanisms proposed for GABA-mediated depolarization involve two GABAA channel permeant anions, Cl− and HCO3−. During development, the Cl− electrochemical equilibrium potential (ECl) is positive relative to resting membrane potential (Vrest) and activation of GABAA receptors results in a net efflux of Cl− and membrane depolarization (Lee et al. 2005). As development proceeds, a decrease in intracellular Cl− occurs (likely to be due to up-regulation of the neurone-specific potassium chloride cotransporter, KCC2; Lee et al. 2005), ECl becomes negative relative to Vrest, and GABAergic stimulation results in a net influx of Cl− and, thus, hyperpolarization. In contrast, in the mature brain, previous studies have shown that activation of dendritic GABAA receptors in CA1 hippocampal neurones causes a depolarization while somatic GABAA receptor activation causes a hyperpolarization (Stein & Nicoll, 2003). Although the mechanism underlying the depolarization is not fully understood, it is likely to involve permeability of GABAA channels to HCO3−. In the hippocampus, strong activation of dendritic GABAA receptors is proposed to cause the collapse of the hyperpolarizing chloride gradient, thereby unmasking the depolarizing HCO3− drive. Moreover, the depolarizing currents observed in the hippocampus in vitro have also been demonstrated in vivo, suggesting that they may play a role in physiological responses to GABAA receptor activation (Mercuri et al. 1991; Fan et al. 2005). In the present study, which was conducted on hypothalamic slices from postnatal 3- to 6-week-old mice that may not be completely developmentally mature, HCO3− flux appears to be the primary contributor to GABA-mediated depolarization (Fig. 1). Whether these depolarizing responses play a role in the response of Hcrt neurons to GABA in vivo is yet to be determined. The previous studies using local application of muscimol to the recorded cell body (Li et al. 2002; Eggermann et al. 2003; Yamanaka et al. 2003a) may preferentially activate somatic receptors, while the current study using bath application of GABA or muscimol would more likely activate both the somatic and dendritic GABAA receptors. In contrast, bath or local application of baclofen consistently induced hyperpolarization of Hcrt neurons.
Several lines of evidence indicate that GABAergic neurotransmission to the Hcrt neurones occurs through GABAB as well as through GABAA receptors. In the presence of the GABAA receptor blockers BIC and PTX, bath application of GABA hyperpolarized the Hcrt neurones and decreased the firing rate and the input resistance (Fig. 2). Similar results were obtained using the GABAB receptor agonist (R)-baclofen, and the effects of (R)-baclofen could be blocked by the GABAB agonist CGP 52432 (Fig. 3). Furthermore, the magnitude of membrane hyperpolarization was dependent upon the baclofen concentration (Fig. 3C). In support of these physiological observations, neurones in the LHA that were immunoreactive for Hcrt-2 also stained for the GABAB(1) subunit (Fig. 2C). GABAB(1)-ir and Hcrt-2-ir have also been reported to colocalize in the rat LHA (Backberg et al. 2003). In contrast to GABAA neurotransmission, which can be depolarizing under certain conditions as discussed above, GABAB receptor-mediated neurotransmission is consistently hyperpolarizing.
In addition to evidence for direct GABAB-mediated effects on Hcrt neurones, we also found evidence for GABAB-mediated modulation of both excitatory and inhibitory inputs to the Hcrt cells. (R)-Baclofen reduced the amplitude of evoked EPSCs and IPSCs (Fig. 4) and also decreased the frequency of spontaneous and miniature excitatory and inhibitory events, suggesting that activation of GABAB receptors at presynaptic terminals inhibits the release of both glutamate and GABA onto the Hcrt cells (Figs 5 and 6). These effects are similar to the role of GABAB receptors demonstrated in other brain regions (Doze et al. 1995; Vogt & Nicoll, 1999; Porter & Nieves, 2004).
As previously reported (Horvath & Gao, 2005), the frequency of miniature EPSCs was almost 10-fold higher than the frequency of miniature IPSCs, further indicating that there are fewer inhibitory synaptic inputs to Hcrt neurones than excitatory inputs. In the absence of TTX, the frequency of spontaneous IPSCs was almost equal to the frequency of spontaneous EPSCs, which suggests that the activity of presynaptic GABAergic inputs might compensate for the lower number of inhibitory synapses onto the Hcrt neurones. However, it appears that basal GABAergic tone does not significantly influence the spontaneous firing of Hcrt neurons in vitro since GABA receptor antagonists did not alter the firing rate of these neurones (data not shown). Thus, it is more likely that GABAergic inputs from remote brain regions influence the activity of Hcrt neurons.
Implications for control of sleep and wakefulness
The LHA both contains local GABAergic neurones and receives GABAergic input from other brain areas, including regions of the preoptic area that have been implicated in sleep promotion (Abrahamson et al. 2001; Gong et al. 2004; Sakurai et al. 2005). The α3 (Moragues et al. 2003; Backberg et al. 2004; Sergeeva et al. 2005) and ɛ (Moragues et al. 2003; Backberg et al. 2004; Sergeeva et al. 2005) GABAA receptor subunits have been localized on Hcrt neurones. In addition, GABAB(1) subunits have been previously identified in the rat LHA (Backberg et al. 2003) and are shown to be colocalized with Hcrt in the current study. Microdialysis delivery of the GABAA receptor antagonist, bicuculline, into the LHA of spontaneously sleeping rats resulted in a dose-dependent decrease in non-REM and REM sleep time, an increase in time awake, and an increased number of Fos-ir Hcrt neurones adjacent to the microdialysis probe (Alam et al. 2005). These results support the hypothesis that Hcrt neurones are subject to endogenous GABAergic inhibition mediated through GABAA receptors during sleep, but the role of GABAB receptors in sleep remains unclear.
Both clinical and basic studies suggest a role for GABAB receptors in the regulation of behavioural state. The GABAB agonist baclofen (25 mg) administered before sleep in a clinical study significantly prolonged total sleep time and reduced time spent awake after sleep onset (Finnimore et al. 1995). In aged rats, the GABAB antagonist CGP 35348 increased the duration of non-REM, REM and total sleep duration compared to saline-injected controls (Puigcerver et al. 1996). Blockade of GABAB receptors in the thalamus by microdialysis of CGP 35348 or another GABAB antagonist, 2-OH-saclofen, did not affect wakefulness or total sleep time in freely moving cats, but deep slow wave sleep and the mean power of slow waves (< 10 Hz) decreased while light slow wave sleep was increased (Juhasz et al. 1994). Microinjection of either the GABAA agonist muscimol or the GABAB agonist baclofen into the basal forebrain induced an increase in slow-wave sleep and an inhibition of wakefulness, but only muscimol caused a decrease in desynchronized sleep parameters (Manfridi et al. 2001). Microinjection of baclofen into the pedunculopontine tegmentum (PPT) suppressed spontaneous REM sleep in a dose-dependent manner (Ulloor et al. 2004). GABAB receptors have been implicated in presynaptic control of GABA release onto the histaminergic cells of the tuberomammillary nucleus (Stevens et al. 1999), which may provide a neural substrate for the effects observed in vivo. γ-Hydroxybutyrate (GHB) has long been known to increase slow wave sleep (stage 3 and 4) and total sleep time in both narcoleptic patients (Mamelak et al. 1977; Scharf et al. 1985; Scrima et al. 1989; Scrima et al. 1990) and healthy subjects (Van Cauter et al. 1997). Many investigations have concluded that the effects of exogenously applied GHB are mediated by GABAB receptors (Xie & Smart, 1992; Schuler et al. 2001; Kaupmann et al. 2003; Crunelli et al. 2005; Snead & Gibson, 2005). Together, these results point to an underappreciated role for GABAB receptors in sleep. Given the central role of Hcrt cells in maintenance of wakefulness, GABAB-mediated modulation of Hcrt neuron activity may also play a role.
Hcrt, GABAB receptors and other physiological functions
In addition to sleep/wakefulness, the Hcrt neurones have been implicated in neuroendocrine control (Van Den Pol et al. 1998; Samson et al. 2005), energy regulation (Hara et al. 2001; Yamanaka et al. 2003b; Sakurai, 2005) and various aspects of autonomic function (Samson et al. 2005). In some of these systems, Hcrt and GABAergic interactions have been well established. For example, Hcrt modulates release of both GABA and glutamate in the arcuate nucleus (van den Pol et al. 1998) and modulates GABAergic inputs to the dorsal medial nucleus of the vagus (Davis et al. 2003). A number of recent studies have implicated the Hcrt system in addictive behaviours for substances such as morphine and cocaine (Georgescu et al. 2003; Boutrel et al. 2005; Harris et al. 2005; Borgland et al. 2006). The apparent resistance of human narcoleptics, in whom the Hcrt neurons degenerate, to develop dependence on medications such as amphetamines has been known for some time (Guilleminault et al. 1974). In animal models, the Hcrt ligand knockout mouse fails to develop conditioned place preference to morphine (Narita et al. 2006). Since GABAB receptor agonists have shown potential for treatment of addictive disorders (Roberts, 2005), GABAB receptor-mediated modulation of Hcrt neuronal activity and synaptic inputs may be important in modifying addictive behaviours.
Acknowledgments
This work was supported by NIH RO1MH61755, R03NS050771 and RO1AG020584 and by a grant-in-aid for scientific research (S) and (B) and the 21st Century COE Program from the Ministry of Education, Culture, Sports, Science and Technology (MEXT) of Japan, Kanae Foundation.
References
- Abrahamson EE, Leak RK, Moore RY. The suprachiasmatic nucleus projects to posterior hypothalamic arousal systems. Neuroreport. 2001;12:435–440. doi: 10.1097/00001756-200102120-00048. [DOI] [PubMed] [Google Scholar]
- Acuna-Goycolea C, Van Den Pol A. Glucagon-like peptide 1 excites hypocretin/orexin neurons by direct and indirect mechanisms: implications for viscera-mediated arousal. J Neurosci. 2004;24:8141–8152. doi: 10.1523/JNEUROSCI.1607-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alam MN, Kumar S, Bashir T, Suntsova N, Methippara MM, Szymusiak R, McGinty D. GABA-mediated control of hypocretin- but not melanin-concentrating hormone-immunoreactive neurones during sleep in rats. J Physiol. 2005;563:569–582. doi: 10.1113/jphysiol.2004.076927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backberg M, Collin M, Ovesjo ML, Meister B. Chemical coding of GABAB receptor-immunoreactive neurones in hypothalamic regions regulating body weight. J Neuroendocrinol. 2003;15:1–14. doi: 10.1046/j.1365-2826.2003.00843.x. [DOI] [PubMed] [Google Scholar]
- Backberg M, Ultenius C, Fritschy JM, Meister B. Cellular localization of GABA receptor α subunit immunoreactivity in the rat hypothalamus: relationship with neurones containing orexigenic or anorexigenic peptides. J Neuroendocrinol. 2004;16:589–604. doi: 10.1111/j.1365-2826.2004.01207.x. [DOI] [PubMed] [Google Scholar]
- Bayer L, Eggermann E, Serafin M, Saint-Mleux B, Machard D, Jones B, Muhlethaler M. Orexins (hypocretins) directly excite tuberomammillary neurons. Eur J Neurosci. 2001;14:1571–1575. doi: 10.1046/j.0953-816x.2001.01777.x. [DOI] [PubMed] [Google Scholar]
- Bettler B, Kaupmann K, Mosbacher J, Gassmann M. Molecular structure and physiological functions of GABAB receptors. Physiol Rev. 2004;84:835–867. doi: 10.1152/physrev.00036.2003. [DOI] [PubMed] [Google Scholar]
- Borgland SL, Taha SA, Sarti F, Fields HL, Bonci A. Orexin A in the VTA is critical for the induction of synaptic plasticity and behavioral sensitization to cocaine. Neuron. 2006;49:589–601. doi: 10.1016/j.neuron.2006.01.016. [DOI] [PubMed] [Google Scholar]
- Boutrel B, Kenny PJ, Specio SE, Martin-Fardon R, Markou A, Koob GF, De Lecea L. Role for hypocretin in mediating stress-induced reinstatement of cocaine-seeking behavior. Proc Natl Acad Sci U S A. 2005;102:19168–19173. doi: 10.1073/pnas.0507480102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowery NG, Bettler B, Froestl W, Gallagher JP, Marshall F, Raiteri M, Bonner TI, Enna SJ. International Union of Pharmacology. XXXIII. Mammalian γ-aminobutyric acidB receptors: structure and function. Pharmacol Rev. 2002;54:247–264. doi: 10.1124/pr.54.2.247. [DOI] [PubMed] [Google Scholar]
- Brown RE, Stevens DR, Haas HL. The physiology of brain histamine. Prog Neurobiol. 2001;63:637–672. doi: 10.1016/s0301-0082(00)00039-3. [DOI] [PubMed] [Google Scholar]
- Burdakov D, Luckman SM, Verkhratsky A. Glucose-sensing neurons of the hypothalamus. Philos Trans R Soc Lond B Biol Sci. 2005;360:2227–2235. doi: 10.1098/rstb.2005.1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burlet S, Tyler CJ, Leonard CS. Direct and indirect excitation of laterodorsal tegmental neurons by hypocretin/orexin peptides: implications for wakefulness and narcolepsy. J Neurosci. 2002;22:2862–2872. doi: 10.1523/JNEUROSCI.22-07-02862.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chemelli RM, Willie JT, Sinton CM, Elmquist JK, Scammell T, Lee C, Richardson JA, Williams SC, Xiong Y, Kisanuki Y, Fitch TE, Nakazato M, Hammer RE, Saper CB, Yanagisawa M. Narcolepsy in orexin knockout mice: molecular genetics of sleep regulation. Cell. 1999;98:437–451. doi: 10.1016/s0092-8674(00)81973-x. [DOI] [PubMed] [Google Scholar]
- Chen G, Trombley PQ, Van Den Pol AN. Excitatory actions of GABA in developing rat hypothalamic neurones. J Physiol. 1996;494:451–464. doi: 10.1113/jphysiol.1996.sp021505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crunelli V, Emri Z, Leresche N. Unravelling the brain targets of γ-hydroxybutyric acid. Curr Opin Pharmacol. 2005;6:44–52. doi: 10.1016/j.coph.2005.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Date Y, Ueta Y, Yamashita H, Yamaguchi H, Matsukura S, Kangawa K, Sakurai T, Yanagisawa M, Nakazato M. Orexins, orexigenic hypothalamic peptides, interact with autonomic, neuroendocrine and neuroregulatory systems. Proc Natl Acad Sci U S A. 1999;96:748–753. doi: 10.1073/pnas.96.2.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis SF, Williams KW, Xu W, Glatzer NR, Smith BN. Selective enhancement of synaptic inhibition by hypocretin (orexin) in rat vagal motor neurons: implications for autonomic regulation. J Neurosci. 2003;23:3844–3854. doi: 10.1523/JNEUROSCI.23-09-03844.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Lecea L, Kilduff TS, Peyron C, Gao X-B, Foye PE, Danielson PE, Fukuhara C, Battenberg ELF, Gautvik VT, Bartlett FS, II, Frankel WN, Van Den Pol AN, Bloom FE, Gautvik KM, Sutcliffe JG. The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proc Natl Acad Sci U S A. 1998;95:322–327. doi: 10.1073/pnas.95.1.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doze VA, Cohen GA, Madison DV. Calcium channel involvement in GABAB receptor-mediated inhibition of GABA release in area CA1 of the rat hippocampus. J Neurophysiol. 1995;74:43–53. doi: 10.1152/jn.1995.74.1.43. [DOI] [PubMed] [Google Scholar]
- Eggermann E, Bayer L, Serafin M, Saint-Mleux B, Bernheim L, Machard D, Jones BE, Muhlethaler M. The wake-promoting hypocretin-orexin neurons are in an intrinsic state of membrane depolarization. J Neurosci. 2003;23:1557–1562. doi: 10.1523/JNEUROSCI.23-05-01557.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggermann E, Serafin M, Bayer L, Machard D, Saint-Mleux B, Jones BE, Muhlethaler M. Orexins/hypocretins excite basal forebrain cholinergic neurones. Neuroscience. 2001;108:177–181. doi: 10.1016/s0306-4522(01)00512-7. [DOI] [PubMed] [Google Scholar]
- Eriksson KS, Sergeeva O, Brown RS, Haas HL. Orexin/hypocretin excites the histaminergic neurons of the tuberomammillary nucleus. J Neurosci. 2001;21:9273–9279. doi: 10.1523/JNEUROSCI.21-23-09273.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Y, Zou B, Ruan Y, Pang Z, Xu ZC. In vivo demonstration of a late depolarizing postsynaptic potential in CA1 pyramidal neurons. J Neurophysiol. 2005;93:1326–1335. doi: 10.1152/jn.00734.2004. [DOI] [PubMed] [Google Scholar]
- Finnimore AJ, Roebuck M, Sajkov D, McEvoy RD. The effects of the GABA agonist, baclofen, on sleep and breathing. Eur Respir J. 1995;8:230–234. doi: 10.1183/09031936.95.08020230. [DOI] [PubMed] [Google Scholar]
- Fu LY, Acuna-Goycolea C, Van Den Pol AN. Neuropeptide Y inhibits hypocretin/orexin neurons by multiple presynaptic and postsynaptic mechanisms: tonic depression of the hypothalamic arousal system. J Neurosci. 2004;24:8741–8751. doi: 10.1523/JNEUROSCI.2268-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgescu D, Zachariou V, Barrot M, Mieda M, Willie JT, Eisch AJ, Yanagisawa M, Nestler EJ, DiLeone RJ. Involvement of the lateral hypothalamic peptide orexin in morphine dependence and withdrawal. J Neurosci. 2003;23:3106–3111. doi: 10.1523/JNEUROSCI.23-08-03106.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong H, McGinty D, Guzman-Marin R, Chew KT, Stewart D, Szymusiak R. Activation of c-fos in GABAergic neurones in the preoptic area during sleep and in response to sleep deprivation. J Physiol. 2004;556:935–946. doi: 10.1113/jphysiol.2003.056622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilleminault C, Carskadon M, Dement WC. On the treatment of rapid eye movement narcolepsy. Arch Neurol. 1974;30:90–93. doi: 10.1001/archneur.1974.00490310092014. [DOI] [PubMed] [Google Scholar]
- Hara J, Beuckmann CT, Nambu T, Willie JT, Chemelli RM, Sinton CM, Sugiyama F, Yagami K, Goto K, Yanagisawa M, Sakurai T. Genetic ablation of orexin neurons in mice results in narcolepsy, hypophagia, and obesity. Neuron. 2001;30:345–354. doi: 10.1016/s0896-6273(01)00293-8. [DOI] [PubMed] [Google Scholar]
- Harris GC, Wimmer M, Aston-Jones G. A role for lateral hypothalamic orexin neurons in reward seeking. Nature. 2005;437:556–559. doi: 10.1038/nature04071. [DOI] [PubMed] [Google Scholar]
- Horvath TL, Gao XB. Input organization and plasticity of hypocretin neurons: possible clues to obesity's association with insomnia. Cell Metab. 2005;1:279–286. doi: 10.1016/j.cmet.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Horvath TL, Peyron C, Diano S, Ivanov A, Aston-Jones G, Kilduff TS, Van Den Pol AN. Hypocretin (orexin) activation and synaptic innervation of the locus coeruleus noradrenergic system. J Comp Neurol. 1999;415:145–159. [PubMed] [Google Scholar]
- Isomura Y, Sugimoto M, Fujiwara-Tsukamoto Y, Yamamoto-Muraki S, Yamada J, Fukuda A. Synaptically activated Cl− accumulation responsible for depolarizing GABAergic responses in mature hippocampal neurons. J Neurophysiol. 2003;90:2752–2756. doi: 10.1152/jn.00142.2003. [DOI] [PubMed] [Google Scholar]
- Jones BE. Arousal systems. Front Biosci. 2003;8:s438–s451. doi: 10.2741/1074. [DOI] [PubMed] [Google Scholar]
- Juhasz G, Emri Z, Kekesi KA, Salfay O, Crunelli V. Blockade of thalamic GABAB receptors decreases EEG synchronization. Neurosci Lett. 1994;172:155–158. doi: 10.1016/0304-3940(94)90685-8. [DOI] [PubMed] [Google Scholar]
- Kaupmann K, Cryan JF, Wellendorph P, Mombereau C, Sansig G, Klebs K, Schmutz M, Froestl W, Van Der Putten H, Mosbacher J, Brauner-Osborne H, Waldmeier P, Bettler B. Specific γ-hydroxybutyrate-binding sites but loss of pharmacological effects of γ-hydroxybutyrate in GABAB(1)-deficient mice. Eur J Neurosci. 2003;18:2722–2730. doi: 10.1111/j.1460-9568.2003.03013.x. [DOI] [PubMed] [Google Scholar]
- Kilduff TS, Peyron C. The hypocretin/orexin ligand-receptor system: Implications for sleep and sleep disorders. Trends Neurosci. 2000;23:359–365. doi: 10.1016/s0166-2236(00)01594-0. [DOI] [PubMed] [Google Scholar]
- Lambe EK, Aghajanian GK. Hypocretin (orexin) induces calcium transients in single spines postsynaptic to identified thalamocortical boutons in prefrontal slice. Neuron. 2003;40:139–150. doi: 10.1016/s0896-6273(03)00598-1. [DOI] [PubMed] [Google Scholar]
- Lee H, Chen CX, Liu YJ, Aizenman E, Kandler K. KCC2 expression in immature rat cortical neurons is sufficient to switch the polarity of GABA responses. Eur J Neurosci. 2005;21:2593–2599. doi: 10.1111/j.1460-9568.2005.04084.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Gao XB, Sakurai T, Van Den Pol AN. Hypocretin/orexin excites hypocretin neurons via a local glutamate neuron-A potential mechanism for orchestrating the hypothalamic arousal system. Neuron. 2002;36:1169–1181. doi: 10.1016/s0896-6273(02)01132-7. [DOI] [PubMed] [Google Scholar]
- Li Y, Van Den Pol AN. Direct and indirect inhibition by catecholamines of hypocretin/orexin neurons. J Neurosci. 2005;25:173–183. doi: 10.1523/JNEUROSCI.4015-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin L, Faraco J, Li R, Kadotani H, Rogers W, Lin X, Qiu X, De Jong PJ, Nishino S, Mignot E. The sleep disorder canine narcolepsy is caused by a mutation in the hypocretin (orexin) receptor 2 gene. Cell. 1999;98:365–376. doi: 10.1016/s0092-8674(00)81965-0. [DOI] [PubMed] [Google Scholar]
- Mamelak M, Escriu JM, Stokan O. The effects of gamma-hydroxybutyrate on sleep. Biol Psychiatry. 1977;12:273–288. [PubMed] [Google Scholar]
- Manfridi A, Brambilla D, Mancia M. Sleep is differently modulated by basal forebrain GABAA and GABAB receptors. Am J Physiol Regul Integr Comp Physiol. 2001;281:R170–R175. doi: 10.1152/ajpregu.2001.281.1.R170. [DOI] [PubMed] [Google Scholar]
- McGinty D, Szymusiak R. Hypothalamic regulation of sleep and arousal. Front Biosci. 2003;8:s1074–s1083. doi: 10.2741/1159. [DOI] [PubMed] [Google Scholar]
- Mercuri NB, Calabresi P, Stefani A, Stratta F, Bernardi G. GABA depolarizes neurons in the rat striatum: an in vivo study. Synapse. 1991;8:38–40. doi: 10.1002/syn.890080106. [DOI] [PubMed] [Google Scholar]
- Moragues N, Ciofi P, Lafon P, Tramu G, Garret M. GABAA receptor epsilon subunit expression in identified peptidergic neurons of the rat hypothalamus. Brain Res. 2003;967:285–289. doi: 10.1016/s0006-8993(02)04270-1. [DOI] [PubMed] [Google Scholar]
- Muraki Y, Yamanaka A, Tsujino N, Kilduff TS, Goto K, Sakurai T. Serotonergic regulation of the orexin/hypocretin neurons through the 5-HT1A receptor. J Neurosci. 2004;24:7159–7166. doi: 10.1523/JNEUROSCI.1027-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narita M, Nagumo Y, Hashimoto S, Narita M, Khotib J, Miyatake M, Sakurai T, Yanagisawa M, Nakamachi T, Shioda S, Suzuki T. Direct involvement of orexinergic systems in the activation of the mesolimbic dopamine pathway and related behaviors induced by morphine. J Neurosci. 2006;26:398–405. doi: 10.1523/JNEUROSCI.2761-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishino S, Ripley B, Overeem S, Lammers GJ, Mignot E. Hypocretin (orexin) deficiency in human narcolepsy. Lancet. 2000;355:39–40. doi: 10.1016/S0140-6736(99)05582-8. [DOI] [PubMed] [Google Scholar]
- Obrietan K, Van Den Pol AN. GABA neurotransmission in the hypothalamus: developmental reversal from Ca2+ elevating to depressing. J Neurosci. 1995;15:5065–5077. doi: 10.1523/JNEUROSCI.15-07-05065.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyron C, Faraco J, Rogers W, Ripley B, Overeem S, Charnay Y, Nevsimalova S, Aldrich M, Reynolds D, Albin R, Li R, Hungs M, Pedrazzoli M, Padigaru M, Kucherlapati M, Fan J, Maki R, Lammers GJ, Bouras C, Kucherlapati R, Nishino S, Mignot E. A mutation in a case of early onset narcolepsy and a generalized absence of hypocretin peptides in human narcoleptic brains. Nat Med. 2000;6:991–997. doi: 10.1038/79690. [DOI] [PubMed] [Google Scholar]
- Peyron C, Tighe DK, Van Den Pol AN, De Lecea L, Heller HC, Sutcliffe JG, Kilduff TS. Neurons containing hypocretin (orexin) project to multiple neuronal systems. J Neurosci. 1998;18:9996–10015. doi: 10.1523/JNEUROSCI.18-23-09996.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter JT, Nieves D. Presynaptic GABAB receptors modulate thalamic excitation of inhibitory and excitatory neurons in the mouse barrel cortex. J Neurophysiol. 2004;92:2762–2770. doi: 10.1152/jn.00196.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puigcerver A, Van Luijtelaar EL, Drinkenburg WH, Coenen AL. Effects of the GABAB antagonist CGP 35348 on sleep-wake states, behaviour, and spike-wave discharges in old rats. Brain Res Bull. 1996;40:157–162. doi: 10.1016/0361-9230(96)00046-9. [DOI] [PubMed] [Google Scholar]
- Roberts DC. Preclinical evidence for GABAB agonists as a pharmacotherapy for cocaine addiction. Physiol Behav. 2005;86:18–20. doi: 10.1016/j.physbeh.2005.06.017. [DOI] [PubMed] [Google Scholar]
- Sakurai T. Roles of orexin/hypocretin in regulation of sleep/wakefulness and energy homeostasis. Sleep Med Rev. 2005;9:231–241. doi: 10.1016/j.smrv.2004.07.007. [DOI] [PubMed] [Google Scholar]
- Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, Williams SC, Richardson JA, Kozlowski GP, Wilson S, Arch JR, Buckingham RE, Haynes AC, Carr SA, Annan RS, McNulty DE, Liu WS, Terrett JA, Elshourbagy NA, Bergsma DJ, Yanagisawa M. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92:573–585. doi: 10.1016/s0092-8674(00)80949-6. [DOI] [PubMed] [Google Scholar]
- Sakurai T, Nagata R, Yamanaka A, Kawamura H, Tsujino N, Muraki Y, Kageyama H, Kunita S, Takahashi S, Goto K, Koyama Y, Shioda S, Yanagisawa M. Input of orexin/hypocretin neurons revealed by a genetically encoded tracer in mice. Neuron. 2005;46:297–308. doi: 10.1016/j.neuron.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Samson WK, Taylor MM, Ferguson AV. Non-sleep effects of hypocretin/orexin. Sleep Med Rev. 2005;9:243–252. doi: 10.1016/j.smrv.2004.07.006. [DOI] [PubMed] [Google Scholar]
- Saper CB, Chou TC, Scammell TE. The sleep switch: hypothalamic control of sleep and wakefulness. Trends Neurosci. 2001;24:726–731. doi: 10.1016/s0166-2236(00)02002-6. [DOI] [PubMed] [Google Scholar]
- Saper CB, Scammell TE, Lu J. Hypothalamic regulation of sleep and circadian rhythms. Nature. 2005;437:1257–1263. doi: 10.1038/nature04284. [DOI] [PubMed] [Google Scholar]
- Scharf MB, Brown D, Woods M, Brown L, Hirschowitz J. The effects and effectiveness of gamma-hydroxybutyrate in patients with narcolepsy. J Clin Psychiatry. 1985;46:222–225. [PubMed] [Google Scholar]
- Schuler V, Luscher C, Blanchet C, Klix N, Sansig G, Klebs K, Schmutz M, Heid J, Gentry C, Urban L, Fox A, Spooren W, Jaton AL, Vigouret J, Pozza M, Kelly PH, Mosbacher J, Froestl W, Kaslin E, Korn R, Bischoff S, Kaupmann K, Van Der Putten H, Bettler B. Epilepsy, hyperalgesia, impaired memory, and loss of pre- and postsynaptic GABAB responses in mice lacking GABAB(1) Neuron. 2001;31:47–58. doi: 10.1016/s0896-6273(01)00345-2. [DOI] [PubMed] [Google Scholar]
- Scrima L, Hartman PG, Johnson FH, Jr, Hiller FC. Efficacy of gamma-hydroxybutyrate versus placebo in treating narcolepsy-cataplexy: double-blind subjective measures. Biol Psychiatry. 1989;26:331–343. doi: 10.1016/0006-3223(89)90048-6. [DOI] [PubMed] [Google Scholar]
- Scrima L, Hartman PG, Johnson FH, Jr, Thomas EE, Hiller FC. The effects of gamma-hydroxybutyrate on the sleep of narcolepsy patients: a double-blind study. Sleep. 1990;13:479–490. doi: 10.1093/sleep/13.6.479. [DOI] [PubMed] [Google Scholar]
- Sergeeva OA, Andreeva N, Garret M, Scherer A, Haas HL. Pharmacological properties of GABAA receptors in rat hypothalamic neurons expressing the epsilon-subunit. J Neurosci. 2005;25:88–95. doi: 10.1523/JNEUROSCI.3209-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snead OC, 3rd, Gibson KM. Gamma-hydroxybutyric acid. N Engl J Med. 2005;352:2721–2732. doi: 10.1056/NEJMra044047. [DOI] [PubMed] [Google Scholar]
- Staley KJ, Proctor WR. Modulation of mammalian dendritic GABAA receptor function by the kinetics of Cl− and HCO3− transport. J Physiol. 1999;519:693–712. doi: 10.1111/j.1469-7793.1999.0693n.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein V, Nicoll RA. GABA generates excitement. Neuron. 2003;37:375–378. doi: 10.1016/s0896-6273(03)00056-4. [DOI] [PubMed] [Google Scholar]
- Stevens DR, Kuramasu A, Haas HL. GABAB-receptor-mediated control of GABAergic inhibition in rat histaminergic neurons in vitro. Eur J Neurosci. 1999;11:1148–1154. doi: 10.1046/j.1460-9568.1999.00519.x. [DOI] [PubMed] [Google Scholar]
- Thannickal T, Moore RY, Nienhuis R, Ramanathan L, Gulyani S, Aldrich M, Cornford M, Siegel JM. Reduced number of hypocretin neurons in human narcolepsy. Neuron. 2000;27:469–474. doi: 10.1016/s0896-6273(00)00058-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsujino N, Yamanaka A, Ichiki K, Muraki Y, Kilduff TS, Yagami K, Takahashi S, Goto K, Sakurai T. Cholecystokinin activates orexin/hypocretin neurons through the cholecystokinin A receptor. J Neurosci. 2005;25:7459–7469. doi: 10.1523/JNEUROSCI.1193-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulloor J, Mavanji V, Saha S, Siwek DF, Datta S. Spontaneous REM sleep is modulated by the activation of the pedunculopontine tegmental GABAB receptors in the freely moving rat. J Neurophysiol. 2004;91:1822–1831. doi: 10.1152/jn.01104.2003. [DOI] [PubMed] [Google Scholar]
- Van Cauter E, Plat L, Scharf MB, Leproult R, Cespedes S, L'Hermite-Baleriaux M, Copinschi G. Simultaneous stimulation of slow-wave sleep and growth hormone secretion by gamma-hydroxybutyrate in normal young Men. J Clin Invest. 1997;100:745–753. doi: 10.1172/JCI119587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Den Pol AN, Gao XB, Obrietan K, Kilduff TS, Belousov AB. Presynaptic and postsynaptic actions and modulation of neuroendocrine neurons by a new hypothalamic peptide, hypocretin/orexin. J Neurosci. 1998;18:7962–7971. doi: 10.1523/JNEUROSCI.18-19-07962.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt KE, Nicoll RA. Glutamate and gamma-aminobutyric acid mediate a heterosynaptic depression at mossy fiber synapses in the hippocampus. Proc Natl Acad Sci U S A. 1999;96:1118–1122. doi: 10.1073/pnas.96.3.1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YF, Gao XB, Van Den Pol AN. Membrane properties underlying patterns of GABA-dependent action potentials in developing mouse hypothalamic neurons. J Neurophysiol. 2001;86:1252–1265. doi: 10.1152/jn.2001.86.3.1252. [DOI] [PubMed] [Google Scholar]
- Wu M, Zaborszky L, Hajszan T, Van Den Pol AN, Alreja M. Hypocretin/orexin innervation and excitation of identified septohippocampal cholinergic neurons. J Neurosci. 2004;24:3527–3536. doi: 10.1523/JNEUROSCI.5364-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie X, Moriarty S, Yamanaka A, Sakurai T, Kilduff TS. Modulation of hypocretin/orexin neurons in mouse hypothalamic slices by GABAB receptor activation. Abstr Soc Neurosci. 2004;318, 18 [Google Scholar]
- Xie X, Smart TG. Gamma-hydroxybutyrate hyperpolarizes hippocampal neurones by activating GABAB receptors. Eur J Pharmacol. 1992;212:291–294. doi: 10.1016/0014-2999(92)90347-7. [DOI] [PubMed] [Google Scholar]
- Xie X, Smart TG. Properties of GABA-mediated synaptic potentials induced by zinc in adult rat hippocampal pyramidal neurones. J Physiol. 1993;460:503–523. doi: 10.1113/jphysiol.1993.sp019484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanaka A, Beuckmann CT, Willie JT, Hara J, Tsujino N, Mieda M, Tominaga M, Yagami K, Sugiyama F, Goto K, Yanagisawa M, Sakurai T. Hypothalamic orexin neurons regulate arousal according to energy balance in mice. Neuron. 2003b;38:701–713. doi: 10.1016/s0896-6273(03)00331-3. [DOI] [PubMed] [Google Scholar]
- Yamanaka A, Muraki Y, Tsujino N, Goto K, Sakurai T. Regulation of orexin neurons by the monoaminergic and cholinergic systems. Biochem Biophys Res Commun. 2003a;303:120–129. doi: 10.1016/s0006-291x(03)00299-7. [DOI] [PubMed] [Google Scholar]
- Yamanaka A, Tsujino N, Funahashi H, Honda K, Guan JL, Wang QP, Tominaga M, Goto K, Shioda S, Sakurai T. Orexins activate histaminergic neurons via the orexin 2 receptor. Biochem Biophys Res Commun. 2002;290:1237–1245. doi: 10.1006/bbrc.2001.6318. [DOI] [PubMed] [Google Scholar]
- Yoshida K, McCormack S, Espana RA, Crocker A, Scammell TE. Afferents to the orexin neurons of the rat brain. J Comp Neurol. 2005;494:845–861. doi: 10.1002/cne.20859. [DOI] [PMC free article] [PubMed] [Google Scholar]