In a recent issue of The Journal of Physiology, Telley et al. (2006) observed the dynamics of half sarcomere lengths during stretch of activated rabbit psoas myofibrils (see also Allen, 2006). They saw non-uniform lengthening of half-sarcomeres, but not the rapid, uncontrolled lengthening to beyond myofilament overlap predicted by Morgan (1990) in the ‘popping sarcomere hypothesis’. They claimed that they had provided the conditions for this to occur, that is, sarcomere lengthening that was rapid enough to reach the yield point of the force–velocity curve for lengthening (Katz, 1939), which took place on the descending limb of the curve of total (active plus passive) sarcomere tension as a function of length. Unfortunately, they did not provide evidence that either of these requirements had been met. In fact it remains uncertain what the force–velocity curve for lengthening and the isometric length–tension relation are for isolated myofibrils. The authors equated lengths beyond optimal filament overlap (half-sarcomere length of 1.2 μm in mammalian muscle) with the descending limb of the length–tension curve, and a large, 2.5-fold force enhancement during stretch with yielding. We would submit that sarcomere popping was not observed because the conditions for it to occur had not been met.
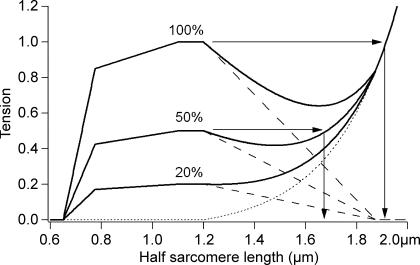
Lengths beyond optimum filament overlap have been equated with the descending limb of the length–tension relation in isometric contractions of maximally activated muscle fibres of the frog (Gordon et al. 1966). However, for isolated rabbit myofibrils, activated with pCa = 4.5 at 10°C, it is not at all clear that activation was maximal. If activation was submaximal, this could change the descending limb of the length–tension curve in two ways. First, a fall in active tension with no change in passive properties may mean that the passive tension curve rises more steeply than active tension falls, so that there is no descending limb (inflection) in the total tension curve, as shown schematically in Fig. 1 for 20% activation. Here it is assumed that the activation fraction does not change with length. Partial activation would be the result of low temperature reducing the tension generated per cross-bridge (Karatzaferi et al. 2004).
Figure 1. Length-independent partial activation.
Schematic active and passive length–tension curves for half-sarcomeres with maximal (100%) and partial (50% and 20%) activation. The continuous lines are the total tension, the dashed lines, active tension and dotted line passive tension (offset exponential). It is assumed that submaximal activation is a scaled version of full activation. At 100%, the half-sarcomeres are expected to ‘pop’ to 1.9 μm (beyond overlap, see arrows), at 50% they ‘pop’ to less than 1.7 μm, and at 20%, there is no descending limb at all, and no ‘popping’ is predicted. Active curve is Gordon et al. (1966) with a thin filament length of 1.1 μm for mammalian muscle, shown as half-sarcomere lengths, as in Telley et al. (2006).
Even if the level of submaximal activation is larger, so that there is a descending limb, ‘popped’ half-sarcomeres will not be as long as in maximally activated fibres. The popping sarcomere hypothesis predicts that sarcomeres will be extended to the length at which the sum of active plus passive tension at the stretched length is equal to their sum near the length where the stretch began (Morgan et al. 2000). In Fig. 1, for maximally activated frog tibialis anterior fibres, represented by the 100% curve, the popped length is beyond filament overlap. Partial activation, as shown by the 50% activation curve in Fig. 1, will reduce the popped length, which in this case would no longer be beyond overlap.
The second mechanism comes from the observations of Endo (1973) that for submaximal activation of skinned toad iliofibularis fibres, active tension can continue to rise with length well beyond optimum overlap. These observations support the interpretation that the percentage of activation depends on length, probably because Ca2+ concentrations are limiting and the myofilaments show a sarcomere length dependence in their sensitivity to Ca2+. Similar behaviour has been shown for submaximal activation of whole cat muscle, where the tension at a given stimulation rate, expressed as a fraction of maximally activated tension, depends on length (Joyce et al. 1969; Morgan et al. 2000). The rise in force from increasing activation with length would counter any fall from decreasing myofilament overlap, removing the descending limb, or moving it to longer lengths. Note that frog muscle is most easily activated maximally at low temperatures (0–4°C). Rabbit body temperature is normally close to 38°C, and activation is likely to be well below maximum at 10°C (Karatzaferi et al. 2004). Comparison of Telley et al.'s quoted specific tension with published values from the literature suggests that activation was only about 50%.
Any increase in passive stiffness would equally make it less likely that these myofibrils show a descending limb in their length tension curve at just beyond maximal overlap. There are several reasons to expect that this may be the case. Mammalian muscles typically have more passive tension than frog muscles. Skinned muscle fibres, and presumably myofibrils, often deteriorate by failing to fully relax. The choice by Telley et al. of half-sarcomere lengths only just beyond optimum overlap (1.2 μm) suggests that these isolated myofibrils have a high passive tension.
In the study by Telley et al. only a minority of half-sarcomeres had lengths beyond optimum filament overlap. None were beyond overlap before the stretch and < 20% were beyond overlap after the stretch (their Fig. 4), so most were definitely on the plateau or the ascending limb. It needs only a small shift in optimum length for none of the half-sarcomeres to have been on the descending limb.
On the question of yield, the stretches applied were quite slow, and the tension records (Telley et al. Fig. 7) did not show the distinct yield point typically seen at optimum length with intact muscles or fibres. At 2–3 times isometric tension, the tension reached during stretch was much higher than is seen in whole mammalian muscles at body temperature. But without an isometric tension record at the final length for comparison (Morgan et al. 2000), or even a record of passive tension during a stretch, it is impossible to tell whether this is due to the low temperatures raising the yield point, a high passive stiffness, or the fact that the myofibril is being stretched over a range representing the ascending limb of its length–tension relation (cf. Joyce et al. 1969, Fig. 3).
It is notable that the observations of Telley et al. showed reducing sarcomere disorder during the stretch. This is the behaviour expected on the ascending limb of the length–tension curve, the region of sarcomere stability, but is very difficult to explain on the descending limb, the region of instability. Even the authors' own model, modified from Morgan (1990) by adding viscosity to the parallel elastic element of the Hill model for each half-sarcomere, led to an increase is disorder, though more slowly than without the viscosity. It is not clear whether the added viscosity is compatible with passive measurements on either intact fibres or isolated myofibrils. For a discussion of this, see Huxley (1980, p. 48).
The important contribution made by the work of Telley et al. is visualization of half-sarcomeres by means of fluorescent antibodies. This is particularly important for the study of active lengthenings since frequently the behaviour of the two halves of a sarcomere is very different. A popped half-sarcomere is often seen paired with the other half contracted down to a short length (Brown & Hill, 1991). We have argued that such behaviour supports the existence of elastic filaments which span the full length of the sarcomere (Proske & Morgan, 2001, Fig. 2). There are other observations made by Telley et al. with which we agree. The importance of sarcomere dynamics, the slow redistribution of sarcomere lengths after a stretch, and the ability of sarcomere nonuniformities to explain permanent extra tension after a stretch, are all in accord with our own findings over the years (Morgan, 1994).
In summary, it is not clear that the stretches applied to the myofibrils in the study of Telley et al. were on the descending limb of the curve of total tension against length, or indeed, whether these myofibrils had a descending limb to their length–tension curve at all. While isolated myofibrils may be an ‘ideal preparation’ for measuring half-sarcomere lengths, they are less than ideal when trying to determine whether or not a stretch is on the descending limb of their length–tension relation, a key requirement for observing popping sarcomeres.
References
- Allen DG. Why stretched muscles hurt – is there a role for half-sarcomere dynamics? J Physiol. 2006;573:4. doi: 10.1113/jphysiol.2006.109918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown LM, Hill L. Some observations on variations in filament overlap in tetanized muscle fibres and fibres stretched during a tetanus, detected in the electron microscope after rapid fixation. J Muscle Res Cell Motil. 1991;12:171–182. doi: 10.1007/BF01774036. [DOI] [PubMed] [Google Scholar]
- Endo M. Length dependence of activation of skinned muscle fibres by calcium. Cold Spring Harb Symp Quant Biol. 1973;37:168–171. [Google Scholar]
- Gordon AM, Huxley AF, Julian FJ. The variation in isometric tension with sarcomere length in vertebrate muscle fibres. J Physiol. 1966;184:170–192. doi: 10.1113/jphysiol.1966.sp007909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huxley AF. Reflections on Muscle. Liverpool: Liverpool University Press; 1980. [Google Scholar]
- Joyce GC, Rack PM, Westbury DR. The mechanical properties of cat soleus muscle during controlled lengthening and shortening movements. J Physiol. 1969;204:461–474. doi: 10.1113/jphysiol.1969.sp008924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karatzaferi C, Chinn MK, Cooke R. The force exerted by a muscle cross-bridge depends directly on the strength of the actomyosin bond. Biophys J. 2004;87:2532–2544. doi: 10.1529/biophysj.104.039909. erratum appears in Biophys J88, 757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B. The relation between force and speed in muscular contraction. J Physiol. 1939;96:45–64. doi: 10.1113/jphysiol.1939.sp003756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan DL. New insights into the behavior of muscle during active lengthening. Biophys J. 1990;57:209–221. doi: 10.1016/S0006-3495(90)82524-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan DL. An explanation for residual increased tension in striated muscle after stretch during contraction. Exp Physiol. 1994;79:831–838. doi: 10.1113/expphysiol.1994.sp003811. [DOI] [PubMed] [Google Scholar]
- Morgan DL, Whitehead NP, Wise AK, Gregory JE, Proske U. Tension changes in the cat soleus muscle following slow stretch or shortening of the contracting muscle. J Physiol. 2000;522:503–513. doi: 10.1111/j.1469-7793.2000.t01-2-00503.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proske U, Morgan DL. Muscle damage from eccentric exercise: mechanism, mechanical signs, adaptation and clinical applications. J Physiol. 2001;537:333–345. doi: 10.1111/j.1469-7793.2001.00333.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telley IA, Stehle R, Ranatunga KW, Pfitzer G, Stussi E, Denoth J. Dynamic behaviour of half-sarcomeres during and after stretch in activated psoas myofibrils: sarcomere asymmetry but no ‘sarcomere popping’. J Physiol. 2006;573:173–185. doi: 10.1113/jphysiol.2006.105809. [DOI] [PMC free article] [PubMed] [Google Scholar]