Abstract
It is now becoming evident that the liver has an important role in the control of whole body metabolism of energy nutrients. In this review, we focus on recent findings showing that AMP-activated protein kinase (AMPK) plays a major role in the control of hepatic metabolism. AMPK integrates nutritional and hormonal signals to promote energy balance by switching on catabolic pathways and switching off ATP-consuming pathways, both by short-term effects on phosphorylation of regulatory proteins and by long-term effects on gene expression. Activation of AMPK in the liver leads to the stimulation of fatty acid oxidation and inhibition of lipogenesis, glucose production and protein synthesis. Medical interest in the AMPK system has recently increased with the demonstration that AMPK could mediate some of the effects of the fat cell-derived adiponectin and the antidiabetic drugs metformin and thiazolidinediones. These findings reinforce the idea that pharmacological activation of AMPK may provide, through signalling and metabolic and gene expression effects, a new strategy for the management of metabolic hepatic disorders linked to type 2 diabetes and obesity.
Introduction
All living organisms possess mechanisms for repeatedly reassessing the status of long-term energy stores and, when the need arises, making compensatory adjustments to adapt their metabolism to the nutritional environment. Mammals have developed a complex series of nutrient cues, relieved by hormones or impulses from the central nervous system, to regulate whole-body metabolism in response to acute deficiencies or to prolonged excess or shortage of nutrient supply. Hepatic metabolism plays a key role in the regulation of whole-body energy status since the liver is the major site for storage and release of carbohydrates and for fatty acid synthesis. Indeed, during the postprandial period, net glucose uptake permits the repletion of hepatic glycogen stores, the excess dietary carbohydrates being then converted into triglycerides to promote long-term energy storage. By contrast, in the fasted state, a series of metabolic modifications induces net glucose output and lipid breakdown in the liver to maintain whole body homeostasis.
AMP-activated protein kinase (AMPK) is a phylogenetically conserved serine/threonine protein kinase which has been proposed to act as a ‘metabolic master switch’ mediating the cellular adaptation to environmental or nutritional stress factors. Once activated, AMPK leads to a concomitant inhibition of energy-consuming biosynthetic pathways, such as fatty acid and sterol synthesis, and activation of ATP-producing catabolic pathways, such as fatty acid oxidation. In the last few years, AMPK has come to be regarded as a point of conversion of regulatory signals monitoring systemic and cellular energy status. Among its identified roles, AMPK has been implicated in the control of hepatic glucose and lipid homeostasis by many additional effects both on genes and on short-term regulation of specific enzymes. In the light of recent observations suggesting that early changes in hepatic metabolism could initiate the subsequent development of insulin resistance, type 2 diabetes and obesity, it has been proposed that AMPK could provide a link in metabolic diseases underlying defects in energy metabolism. In the present review, we update those topics and discuss new findings that suggest that AMPK may be a promising pharmacological target for the treatment of type 2 diabetes and obesity.
Structure and regulation of AMPK by upstream kinases in liver
AMPK exists as a heterotrimeric complex consisting of a catalytic subunit α and two regulatory subunits β and γ (Hardie, 2004). Two to three isoforms of each subunit (α1, α2, β1, β2, γ1, γ2, and γ3) encoded by different genes are known, giving rise to a large variety of heterotrimeric combinations. It has been demonstrated that α1- and α2-containing complexes account each for about half of total AMPK activity in liver extracts, β1- and β2-containing complexes for 95% and 5%, respectively, and the γ1-containing complexes for 90%, the γ2-containing complexes for 10% and γ3-containing complexes for only minor activity (Cheung et al. 2000). There is no evidence for any selective association between α1 and α2 isoforms and the various β or γ isoforms in the liver. Nevertheless, the AMP dependence of the different isoform combinations is markedly affected by the identity of the α and γ isoforms present in the heterotrimeric complex suggesting that the allosteric AMP-binding site might involve the γ subunit, as well as the α subunit (Cheung et al. 2000).
In response to stresses that deplete ATP, the intracellular AMP: ATP ratio increases due to the action of adenylate kinase, resulting in the activation of AMPK (Hardie, 2004). AMPK is stimulated allosterically by AMP, which binds to the cystathionine β-synthase tandem repeats in the γ subunit, but is also activated by phosphorylation on residue Thr172, which lies within the activation loop of the kinase domain on the α-subunit (Hardie, 2004). The discovery of AMPK kinases came from the identification of upstream kinases for the sucrose non-fermenting 1 (SNF1) complex, the yeast orthologue of AMPK. The nearest relative kinases in mammals were LKB1 and calmodulin-dependent protein kinase kinase β (Hardie, 2004; Birnbaum, 2005). The major AMPK kinase activity in liver seems to correspond to LKB1 since it has been shown that deletion of LKB1 in the liver results in a proportional decrease of AMPK phosphorylation at Thr172 rendering AMPK insensitive to stimuli which normally activate it (Shaw et al. 2005).
Activators of hepatic AMPK
Numerous studies have reported AMPK activation in hepatic cells by intracellular changes in AMP: ATP ratio, resulting from any stress that depletes cellular ATP such as metabolic poisons targeting mitochondria (arsenite, antimycin A, azide, oligomycin and 2,4-dinitrophenol), heat shock and hypoxia (Hardie, 2004). Since the liver seldom suffers from hypoxia and the liver ATP concentration changes little under physiological conditions, hepatic AMPK activation should only occur as an ultimate cellular protection in these cases. However, evidence showing that hepatic AMPK could be activated in response to physiological stimuli such as exercise and nutrient deprivation (Hardie, 2004), and to physiopathological situations such as prolonged starvation (Assifi et al. 2005; Dentin et al. 2005), ischaemia–reperfusion injury (Peralta et al. 2001) and chronic alcohol consumption (You et al. 2004) suggests that AMPK might have a wider role in the liver. Moreover, the recent findings showing that hepatic AMPK is activated by adiponectin (Yamauchi et al. 2002) and by two different antidiabetic drugs, metformin (Zhou et al. 2001) and thiazolidinediones (TZDs) (Saha et al. 2004), further reinforced therapeutical interest, identifying AMPK as a potential target for the treatment of metabolic diseases such as obesity and type 2 diabetes. Most of these effects, including those of metformin, are believed to be independent of any apparent changes in the AMP: ATP ratio, but this assumption is mainly based on results obtained in other tissues than liver. Furthermore, the AMP: ATP ratio was also systematically calculated using total adenine nucleotide concentrations. This latter should be taken with caution, given that AMPK is only regulated by the cytosolic AMP: ATP ratio. Finally, since mitochondrial oxidative phosphorylation is crucial for liver energetics, subtle modifications of the cellular energetic state could be sufficient to activate AMPK.
Among chemical methods for activating AMPK in the liver, the use of 5-aminoimidazole-4-carboxamide-1-β-d-ribofuranoside (AICAR), a cell-permeant compound which is phosphorylated to the AMP analogue 5-aminoimidazole-4-carbonmide ribotide (ZMP), has been reported both in vitro (Corton et al. 1995) and in vivo (Pencek et al. 2005; Reiter et al. 2005). The accumulation of ZMP leads to AMPK activation by mimicking the effects of AMP on the kinase, i.e. direct allosteric activation and promotion of phosphorylation by upstream AMPK kinase (Corton et al. 1995). While AICAR is still the most widely used pharmacological activator of AMPK, it has been demonstrated that some of its actions could be independent of AMPK. All enzymes whose activities are influenced by AMP, such as fructose-1,6-bisphosphatase and 6-phosphofructo-2-kinase or glucokinase, are potential targets for ZMP. Furthermore, it has been reported that AICAR treatment decrease intracellular ATP concentration both in vitro (Corton et al. 1995) and in vivo (Pencek et al. 2005). It has been recently demonstrated using mice lacking both α1 and α2 catalytic subunits in the liver (AMPKα1α2LS−/−) that AICAR inhibits hepatic glucose phosphorylation by an AMPK-independent impairment of the glucose-induced translocation of GK following ATP depletion (Guigas et al. 2006). Lastly part of the detrimental effect of AICAR is likely to be due to an AMPK-independent inhibition of mitochondrial oxidative phosphorylation induced by a concomitant effect of ZMP on the mitochondrial respiratory-chain and a drop of adenine nucleotides and inorganic phosphate following its phosphorylation (B. Guigas, L. Hue & B. Viollet, unpublished results). Among the other AMPK activators previously described, metformin could also exert AMPK-independent effects in the liver, notably by its mild and specific inhibition of the mitochondrial respiratory-chain complex, which may be responsible for part of its therapeutic action.
Metabolic action of AMPK in liver
Lipid homeostasis
AMPK phosphorylates multiple targets in the liver in order to acutely switch on alternative catabolic pathways and switch off anabolic pathways (Table 1). Acetyl-CoA carboxylase (ACC) and 3-hydroxy-3-methylglutaryl-coenzyme A reductase (HMG-CoA reductase) were the first enzymes shown to be downstream targets for AMPK (Hardie, 2004). ACC and HMG-CoA reductase are, respectively, key enzymes in fatty acid and cholesterol synthesis, known as ATP-consuming biosynthetic pathways. ACC is an important rate-controlling enzyme for the synthesis of malonyl-CoA, which is both a critical precursor in the biosynthesis of fatty acids and a potent inhibitor of mitochondrial fatty acid oxidation. In the liver, two separate genes encode major isoforms of ACC, ACC1 and ACC2, which display distinct cellular distribution leading to the compartmentalization of cellular malonyl-CoA production. The malonyl-CoA synthesized by ACC1 is used in fatty acid synthesis, whereas the malonyl-CoA generated by ACC2 is involved in the control of fatty acid oxidation (Abu-Elheiga et al. 2001). Inhibition of ACC by AMPK leads to a fall in malonyl-CoA content and a subsequent decrease in fatty acid synthesis and increase in mitochondrial fatty acid oxidation via the allosteric regulation of carnitine palmitoyltransferase-1 (CPT-1), which catalyses the entry of long-chain fatty acyl-CoA into mitochondria. The transition from fasted to fed states is associated with nutritional and hormonal changes that lead to increased hepatic glycerolipid and fatty acid synthesis and decreased fatty acid oxidation and ketogenesis (Assifi et al. 2005). In re-fed rat, the activity of ACC increases dramatically in the first 1–3 h after re-feeding, which results in changes in hepatic malonyl-CoA concentration, and is associated with a coordinate reduction of AMPK activity, which remains low for at least 24 h (Assifi et al. 2005). Accordingly, insulin activates ACC to promote malonyl-CoA synthesis by inhibiting AMPK (Witters & Kemp, 1992). In addition, malonyl CoA decarboxylase (MCD), an enzyme involved in the turnover of malonyl-CoA, has been shown to be activated by AMPK in response to energy depletion, resulting in reduced malonyl-CoA levels and increased fatty acid oxidation (Assifi et al. 2005). Thus, in the liver, AMPK coordinates the changes in the activity of enzymes of lipid metabolism and regulates the partitioning of fatty acid between oxidative and biosynthetic pathways (Fig. 1). This has been illustrated by AMPKα2−/− mice and liver-specific AMPKα2−/− mouse models, which both exhibit hypertriglyceridaemia (Viollet et al. 2003; Andreelli et al. 2006). Furthermore, AICAR infusion resulted in a decrease in plasma triglyceride concentrations in both lean and obese Zucker rats, which can likely be attributed to an inhibition of hepatic lipogenesis (Bergeron et al. 2001). These results are consistent with in vitro findings demonstrating AICAR-induced inhibition of mitochondrial glycerol-3-phosphate acyltransferase (GPAT) activity and subsequent inhibition of triacylglycerol synthesis (Muoio et al. 1999). Since mitochondrial GPAT and CPT1 are both located on the outer mitochondrial membrane, mitochondrial GPAT competes directly with CPT1 for acyl-CoA substrates and AMPK would regulate acyl-CoA channelling towards β-oxidation and away from glycerolipid biosynthesis (Fig. 1).
Table 1. Hepatic targets of AMPK.
| Target | Enzymatic properties | Phosphorylation site(s) | Biological effect(s) | References |
|---|---|---|---|---|
| ACC1 | ↓ Activity | Ser79 | ↓ Lipogenesis | Munday et al. (1988) |
| ACC2 | ↓ Activity | Ser218 | ↑ Oxidation | Winder et al. (1997) |
| eEF2Kinase | ↑ Activity | Ser398 | ↓ Protein synthesis | Horman et al. (2002) |
| Browne et al. (2004) | ||||
| GPAT | ↓ Activity | ? | ↓ Triglyceride synthesis | Muoio et al. (1999) |
| HMG-CoA reductase | ↓ Activity | Ser872 | ↓ Cholesterol synthesis | Clarke & Hardie (1990) |
| MCD | ↑ Activity | ? | ↓ Malonyl CoA | Assifi et al. (2005) |
| PFK-2/FBPase-2 | Weak kinetic effects | Ser22, Ser32 | ? | Guigas et al. (2006) |
| mTOR | ↓ Activity | Thr2446 | ↓ Protein synthesis | Cheng et al. (2004) |
| TSC2 | ↑ Activity | Thr1227, Ser1345 | ↓ Protein synthesis | Inoki et al. (2003) |
Figure 1. Regulation of lipid metabolism by hepatic AMPK.
Activation of AMPK leads to the inhibition of cholesterol synthesis by phosphorylation of HMG-CoA reductase. By inhibiting ACC and activating MCD, AMPK increases fatty acid oxidation via the regulation of levels of malonyl-CoA, which is both a critical precursor for biosynthesis of fatty acids and a potent inhibitor of CPT-1, the enzyme that controls the transfer of long-chain fatty acyl-CoA into the mitochondria. AMPK inhibits also GPAT, the first committed enzyme in glycerolipid synthesis. The net resulting effect of AMPK activation is to inhibit energy-consuming lipogenic pathways (fatty acid, triglyceride and sterol synthesis) in favour of fatty acid oxidation. FA-CoA: fatty acyl-CoA.
Glucose homeostasis
Since AMPK is usually considered as part of a mechanism involved in energy sparing, a potential role for AMPK in the regulation of the energy-consuming process of hepatic gluconeogenesis (de novo synthesis of glucose from three-carbon precursors) has been considered. Results obtained with pharmacological compounds and adenovirus-mediated AMPK activation/inactivation strategies have demonstrated that AMPK plays a role in the control of glucose production by the liver. It has been first shown that systemic infusion of AICAR in normal and insulin-resistant obese rats leads to the inhibition of hepatic glucose production (Bergeron et al. 2001). In addition, it has been also reported that metformin, which activates AMPK, is able to suppress glucose production in primary cultured hepatocytes (Zhou et al. 2001). These findings were supported by the observation that treatment of hepatocytes in primary culture with adenovirus expressing a constitutively active form of AMPKα2 (AMPKα2-CA) reduced glucose output (Foretz et al. 2005). The potent effects of circulating adipocyte-derived hormones on whole-body glucose metabolism recently highlighted the involvement of AMPK in the control of glucose output by the liver. Indeed, a physiological link has been established between circulating resistin levels and hepatic AMPK activity in the maintenance of blood glucose levels (Satoh et al. 2004). Furthermore, it was recently demonstrated that the hypoglycaemic effects of adiponectin appear to be mediated by hepatic AMPK activation (Yamauchi et al. 2002). This was corroborated by the incapacity of adiponectin to regulate hepatic glucose production in the absence of the AMPKα2 subunit in the liver (Andreelli et al. 2006).
Recent results with animal models confirm the physiological importance of hepatic AMPK for whole-body glucose homeostasis. First, short-term activation of AMPK specifically in the liver by adenovirus-mediated expression of AMPKα2-CA is sufficient for controlling hyperglycaemia in murine models of diabetes (Foretz et al. 2005). Second, liver-specific AMPKα2−/− mice, which exhibited hyperglycaemia and glucose intolerance, presented increased fasted hepatic glucose production (Andreelli et al. 2006), demonstrating that the hepatic AMPKα2 isoform is essential to suppress hepatic glucose production and maintain fasting blood glucose levels in the physiological range. Furthermore, these results indicate that the remaining AMPKα1 in the liver is not sufficient to control hepatic glucose production. Third, AMPKα1α2LS−/− mice are resistant to the hypoglycaemic effect of AICAR indicating that hepatic AMPK has a crucial role in the control of blood glucose levels (B. Viollet & F. Andreelli, unpublished results). Lastly, in mice lacking LKB1 in the liver, AMPK was almost completely inactive and fasting blood glucose levels were highly increased (Shaw et al. 2005). In these mice, the antidiabetic drug metformin no longer normalized blood glucose levels providing the apparent possibility that LKB1-mediated activation of AMPK in the liver might be required to lower blood glucose levels in diabetic mice (Shaw et al. 2005). Nevertheless, recent studies have shown that LKB1 phosphorylates and activates at least 12 AMPK-related kinases (Lizcano et al. 2004) which could contribute to the action of metformin in the liver. In recent studies, overexpression of the salt-induced kinases (SIK) of the AMPK-related kinase family were found to inhibit hepatic gluconeogenesis (Screaton et al. 2004; Koo et al. 2005). These data raised the question whether the glucose-lowering function of LKB1 is mediated by AMPK-related kinases rather than AMPK itself.
Protein synthesis and cell proliferation
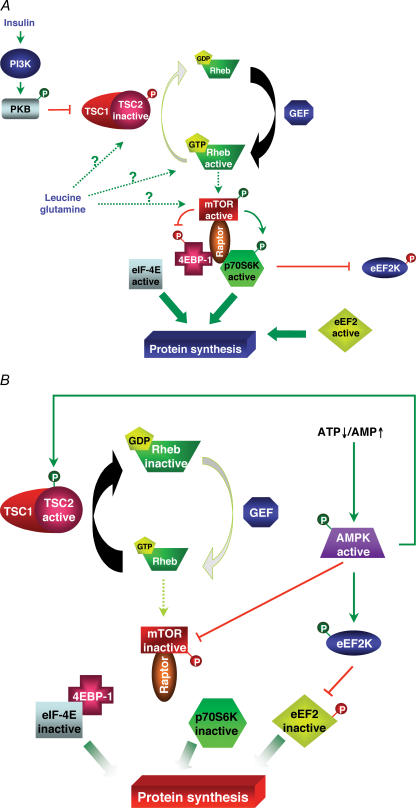
Protein synthesis is an anabolic pathway consuming a significant amount of ATP (4 ATP equivalents per peptide bond during the elongation step of protein synthesis). So it is logical that AMPK plays a fundamental role in the inhibition of protein synthesis (Fig. 2A). The control of protein synthesis is complex and involves (de)phosphorylation of several translation factors and ribosomal proteins (Proud, 2004) (Fig. 2B). One of the main targets of AMPK amongst these regulatory elements is the mammalian target of rapamycin (mTOR) (Sarbassov et al. 2005). mTOR is a protein kinase known to be activated in the liver by different anabolic agents, including insulin and certain amino acids like glutamine and leucine (Krause et al. 2002b). The mTOR pathway positively regulates cell growth by modulating a large number of processes including protein translation, autophagy, cell survival and proliferation (Sarbassov et al. 2005). Once activated, mTOR is able to phosphorylate two regulatory proteins, the p70 ribosomal S6 protein kinase (p70S6K) and the eukaryotic initiation factor 4E-binding protein-1 (4E-BP1). The phosphorylation of 4E-BP1 relieves its inhibitory action on eukaryotic initiation factor 4E (eIF-4E), which can then bind the mRNA cap and stimulate protein synthesis initiation. p70S6K phosphorylates several substrates including the S6 ribosomal protein and the translational regulator eukaryotic elongation factor-2 kinase (eEF2K), involved in the control of initiation and elongation steps, respectively (Proud, 2004). eEF2K is a dedicated calcium- and calmodulin-dependent kinase controlling the phosphorylation and inactivation of eukaryotic elongation factor-2 (eEF2). p70S6K phosphorylates directly and inactivates eEF2K. The resulting decrease in phosphorylation state of eEF2 observed in this condition leads to a stimulation of protein synthesis.
Figure 2. Diagrammatic representation of signalling involved in the regulation of the mTOR/p70S6K pathway by AMPK.
A, both insulin and amino acids stimulate the mTOR/p70S6K pathway to promote protein synthesis. Insulin, by activating the phosphatidylinositol-3-kinase/protein kinase B pathway, inhibits TSC2, and so, via the activation of Rheb, induces mTOR activation. On the other hand, the pathway used by amino acids like leucine or glutamine to activate the mTOR pathway is still not well defined. Once activated, mTOR, with the participation of Raptor, is able to phosphorylate 4EBP-1 and p70S6K. B, activated AMPK is able to phosphorylate and activate both TSC2 and eEF2K. Moreover, AMPK can inactivate directly mTOR. GEF: guanylate exchange factor.
AMPK activation can inhibit the mTOR pathway (Krause et al. 2002a; Reiter et al. 2005). Indeed, AMPK is able to directly phosphorylate mTOR on Thr2446 leading to its inactivation (Cheng et al. 2004). Moreover, upstream of mTOR, AMPK can also phosphorylate and activate the tuberous sclerosis complex 2 (TSC2) (Inoki et al. 2003). TSC2 is a GTPase activating protein that stimulates the intrinsic GTPase activity of the G protein Rheb, thereby promoting its conversion from the GTP-bound active state to the GDP-bound inactive state. Rheb, in its GTP-bound active state, activates mTOR by a not-well-defined mechanism. Therefore, AMPK-induced TSC2 activation promotes Rheb and mTOR inactivation, thereby inhibiting the initiation step of protein synthesis. Moreover, AMPK can directly phosphorylate and activate eEF2K, leading to eEF2 inhibition (Horman et al. 2002; Browne et al. 2004).
The ability to increase the protein synthesis capacity of the cell is responsible for the ability of mTOR to drive cell growth and proliferation. Indeed, the signals that up-regulate mTOR activity are frequently activated in human cancers (Rao et al. 2004). AMPK is an anti-growth molecule because of its relationship with two tumour suppressor genes: LKB1 and TSC2 (Luo et al. 2005). LKB1 mutations result in Peutz–Jeghers syndrome and predisposition to cancers of the colon, pancreas, breast and other sites. Mutations of TSC1/TSC2 cause tuberous sclerosis which is associated with increased risk of cancers. Recently, the AMPK–TSC2–mTOR–eEF2 pathway has been linked to cell survival under hypoxic conditions (Liu et al. 2006). Moreover, AMPK activators, such as AICAR and metformin, inhibit the growth or the survival of some cancer cells, including hepatoma cells. The downstream targets of AMPK linked to these effects seem to include the mTOR pathway but also the tumour suppressor p53 and the cell-cycle inhibitor p21. These observations further suggest that AMPK provides a link between regulators of cellular metabolism and cell proliferation in cancer, which could be exploited for cancer treatment and/or prevention. Interestingly, a recent epidemiological study has revealed that diabetic patients treated with metformin are less prone to develop cancers (Evans et al. 2005). However, it remains to be demonstrated that this noticeable effect is mediated by AMPK.
Long-term effects of hepatic AMPK activation (regulation of gene transcription)
Although the action of AMPK in systemic energy balance is achieved by rapid and direct phosphorylation of metabolic enzymes, long-term effects have also been clearly demonstrated on gene expression. This has come from the finding that proteins related to all three AMPK subunits have been characterized in the yeast SNF1 complex, which is involved in the derepression of glucose-regulated genes through the modulation of the transcriptional activity of nuclear factors. Very interestingly, AMPKα2-containing complexes are found in both the nucleus and the cytoplasm (Salt et al. 1998) raising the possibility that AMPK might regulate gene expression, at least in part, by the phosphorylation of nuclear factors (Table 2).
Table 2. AMPK-targeted transcription factors.
| Target | Phosphorylation site | Phosphorylation effect | Target gene(s) | References |
|---|---|---|---|---|
| ChREBP | Ser568 | ↓ DNA binding | Glycolytic and lipogenic genes | Kawaguchi et al. (2002) |
| FoxO1 | ? | ↓ Protein stability | G6Pase | Barthel et al. (2002) |
| HNF4 | Ser304 | ↓ Protein stability | L-PK, ApoCIII, … | Leclerc et al. (2001); Hong et al. (2003) |
| SREBP1c | — | ↓ Gene expression | Glycolytic and lipogenic genes | Zhou et al. (2001); Foretz et al. (2005) |
| TORC2 | Ser171 | ↑ Cytoplasmic sequestration | Gluconeogenic genes | Koo et al. (2005) |
Gycolytic/lipogenic gene expression
It is now clearly established that AMPK plays an important role in the repression of glycolytic and lipogenic gene expression in the liver. AMPK activation by AICAR or by the use of AMPKα1-CA inhibits insulin/glucose-induced transcriptional stimulation of liver-type pyruvate kinase (L-PK), fatty acid synthase (FAS), Spot14 and ACC genes in primary cultured hepatocytes (Ferre et al. 2003). Expression of AMPKα2-CA in the liver by adenovirus-mediated gene transfer considerably decreased the refeeding-induced transcriptional activation of glycolytic and lipogenic genes and their upstream regulators, SREBP-1c (sterol regulatory element-binding protein-1c) and ChREBP (carbohydrate response element-binding protein) (Foretz et al. 2005). Similarly, activation of AMPK by metformin in the liver suppresses the expression of SREBP1c during the fasted–fed transition (Zhou et al. 2001). In addition, it has been demonstrated that activation of AMPK leads to the phosphorylation of ChREBP on Ser568, which caused a decrease in its DNA binding activity and subsequent transcriptional inhibition of glucose responsive genes (Kawaguchi et al. 2002). The nuclear hormone receptor HNF4α involved in L-PK gene transcription has been also described as a target of AMPK (Leclerc et al. 2001; Hong et al. 2003). AMPK can phosphorylate HNF4α on Ser304, a residue located in the dimerization domain of the protein, reducing the ability to form homodimers and to bind DNA. Phosphorylation of HNF4α leads to decreased protein stability and probably increases its degradation rate (Hong et al. 2003).
Polyunsaturated fatty acids (PUFAs) are known to play pivotal roles as ‘fuel partitioners’ in the liver via their unique ability to partition fatty acids away from lipid synthesis towards fatty acid oxidation. As AMPK exhibited the same properties, it has been hypothesized that PUFAs may directly regulate AMPK activity. PUFAs exert their effects by a concomitant up-regulation of gene expression involved in fatty acid oxidation and down-regulation of glycolytic and lipogenic gene expression by acting through the inhibition of SREBP1c gene transcription, mRNA stabilization and proteolytic cleavage and the suppression of ChREBP nuclear translocation. To address the role of AMPK in the inhibitory effect of PUFAs on glycolytic and lipogenic gene expression, a series of experiments have been performed in AMPKα1−/−, AMPKα2−/− (Dentin et al. 2005) and AMPKα1α2LS−/− mice (Fig. 3A). As shown in Fig. 3A, the inhibition of L-PK, FAS and ChREBP expression was sustained in livers of AMPKα1α2LS−/− mice fed with a PUFA-enriched diet. The lack of both AMPKα1 and -α2 isoforms did not affect ChREBP subcellular translocation into the nucleus under HCHO feeding and retention in the cytosol under PUFA conditions (Fig. 3B). Together, these data demonstrate that PUFAs inhibit ChREBP nuclear translocation and repress glycolytic/lipogenic gene expression by an AMPK-independent mechanism.
Figure 3. Effect of dietary PUFAs on L-PK and ChREBP gene expression and ChREBP localization in livers of AMPKα1α2LS−/− mice.
A, quantitative RT-PCR analysis of L-PK and ChREBP gene expression from livers of 24 h-fasted mice (F) and mice re-fed for 18 h with a high carbohydrate diet (HCHO) supplemented or not with PUFAs (PUFA) performed in control (filled bars) and AMPKα1α2LS−/− (open bars) mice. Results are means ± s.e.m.; n = 3/group. *Significantly different from mice re-fed with HCHO diet for 18 h (P < 0.005). B, cytosolic and nuclear ChREBP content from livers of 24 h-fasted control and AMPKα1α2LS−/− mice re-fed for 18 h upon HCHO diet supplemented or not with PUFAs. Expression of AMPKα catalytic subunits has been measured by using anti-pan-AMPKα antibodies. β-Actin protein levels are presented as loading control. A representative Western blot is shown; n = 3/group.
Gluconeogenic gene expression
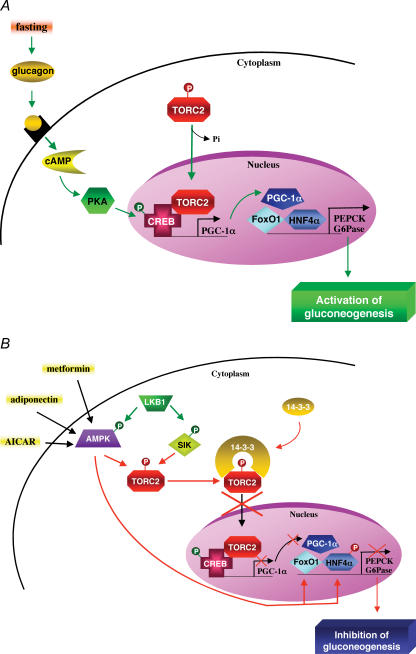
Activation of AMPK in primary culture of hepatocytes was shown to reduce gene expression of the key gluconeogenic enzymes, phosphoenolpyruvate carboxykinase (PEPCK) and glucose-6-phosphatase (G6Pase) (Foretz et al. 2005). Administration of full-length adiponectin is known to reduce both blood glucose level and the expression of gluconeogenic genes. The concomitant injection of adenovirus encoding a dominant negative form of AMPK (AMPK-DN) prevented the inhibition of PEPCK and G6Pase gene expression by adiponectin (Yamauchi et al. 2002) suggesting that AMPK may regulate directly transcriptional regulators of the gluconeogenic programme in hepatocytes. Recently, TORC2 (transducer of regulated CREB activity 2) has emerged as a critical regulator of gluconeogenesis in mice (Koo et al. 2005). Under fasting conditions, glucagon triggers the transcription of gluconeogenic genes via the cAMP-responsive factor CREB (CRE binding protein) and subsequent recruitment of the coactivator CBP and TORC2. This leads to the expression of the coactivator PGC-1α, which in turn drives the transcription of PEPCK and G6Pase genes in association with the transcription factor HNF4α and the forkhead family activator FoxO1 (Fig. 4A). TORC2 activity is tightly regulated by a series of phosphorylation events blocking its nuclear accumulation. Interestingly, AMPK and the AMPK-related kinases SIK1 and -2 were found to phosphorylate Ser171 of TORC2, promoting binding to the 14-3-3 proteins and its sequestration out of the nucleus (Screaton et al. 2004; Koo et al. 2005) (Fig. 4B). The S171A mutant partially rescued SIK1- and AICAR-mediated suppression of G6Pase, PEPCK and PGC-1α expression in primary hepatocytes, suggesting that TORC2 might be a critical downstream target of both AMPK and AMPK-related kinase SIK1 in the regulation of hepatic gluconeogenesis (Koo et al. 2005). Thus, the regulation of both nuclear import and nuclear export has emerged as one of the most efficient mechanisms to adapt gene expression to the cell environment by restricting the access of transcriptional regulators to their target genes. It is also noteworthy that glucose starvation and AICAR treatment strongly repress G6Pase expression due to the complete disappearance of the transcription factor FoxO1 (Barthel et al. 2002), indicating that AMPK may also control transcription by regulating protein stability or degradation (Table 2).
Figure 4. Transcriptional control of gluconeogenesis by TORC2 and AMPK.
A, in response to fasting, the cAMP-responsive CREB coactivator TORC2 controls the gluconeogenic programme in liver via its nuclear translocation and association with CREB transcription factor, driving the expression of the PGC1α coactivator. Expression of the coactivator PGC-1α in turn drives the transcription of key gluconeogenic enzymes such as PEPCK and G6Pase in association with the transcription factor HNF4α and the forkhead family activator FoxO1. B, activity of TORC2 is controlled by AMPK and AMPK-related kinase SIK phosphorylation, which determines whether TORC2 becomes localized in the nucleus. Phosphorylated TORC2 is sequestered in the cytoplasm via a phosphorylation-dependent interaction with 14-3-3 proteins. Moreover, AMPK can also control gluconeogenic gene transcription by regulating stability or degradation of HNF4α and FoxO1 transcription factors.
Cytochrome P450 gene expression
AMPK has been implicated in the regulation of the drug-metabolizing enzyme cytochrome P450 (CYP) gene family (Rencurel et al. 2005). The CYP gene plays a crucial role in the transformation of xenobiotics by the liver and their expression is strongly induced in response to phenobarbital (PB) administration. Incubation of hepatoma cells with PB caused a dose-dependent increase in AMPK activity that was accompanied by a decrease in intracellular ATP levels. Expression of AMPKα1-CA mimics the phenobarbital induction of CYP2B gene expression in HepG2 and primary human hepatocytes. Conversely, expression of AMPK-DN inhibits the induction of these genes in response to phenobarbital treatment. These data support a role for AMPK in the induction of CYP gene expression by barbiturate drugs. The action of AMPK could be mediated by changes in the nuclear localization or activity of the nuclear receptor constitutive androstane receptor (CAR) responsible for the PB induction of CYP2B genes (Kodama et al. 2004). Interestingly, CAR is also involved in the PB-induced transcriptional repression of the PEPCK gene (Kodama et al. 2004).
Hepatic AMPK as a therapeutic target for metabolic disorders
AMPK activation for hyperglycaemic states
Because of its favourable global metabolic effects when activated, it is tempting to consider AMPK as a possible therapeutic target in the prevention and treatment of type 2 diabetes and insulin resistance. An important hallmark of type 2 diabetes is an increase in hepatic glucose production. Suppression of gluconeogenesis, a key metabolic pathway for hepatic glucose output, has been shown to improve overall glycaemic control in both human patients and type 2 diabetic animal models. It has been demonstrated that hepatic AMPK activation abolishes hyperglycaemia in diabetic ob/ob and STZ-induced diabetic mice by suppression of gluconeogenesis (Foretz et al. 2005). These results demonstrate that AMPK activation may have an effect under conditions of severe insulinoresistance or insulinopenia and that insulin signalling is therefore not required for this effect. Metformin is now a mainstay of therapy in the treatment of type 2 diabetes and is also an effective agent to decrease the risk of development of the disease. The ability of metformin to suppress hepatic glucose production and to lower blood glucose levels requires LKB1/AMPK signals (Zhou et al. 2001; Shaw et al. 2005). TZDs are a class of antidiabetic drugs that increase systemic insulin sensitivity both in rodents and humans. TZDs are ligands for PPARγ, a member of the nuclear hormone receptor family of transcription factors, which is expressed in adipose tissue and to some extent in liver and other tissues. The molecular pathway of AMPK activation by TZDs is still unclear, but activation has been recently attributed to their ability to increase adiponectin plasma levels, since activation of AMPK by rosiglitazone treatment is diminished in adiponectin KO mice (Nawrocki et al. 2006). Alternatively, this effect could also be due to decreased intracellular ATP levels in liver in response to TZD treatment (Saha et al. 2004).
Circulating levels of adiponectin are decreased in individuals with obesity, atherosclerosis and insulin resistance, suggesting that its deficiency may have a causal role in the etiopathogenesis of these diseases. Therefore, adiponectin replacement in humans may represent a promising approach to prevent and/or treat obesity, insulin resistance and type 2 diabetes. It has been reported that an acute increase in circulating adiponectin levels triggers a transient decrease in basal glucose levels by inhibiting both the expression of hepatic gluconeogenic enzymes and the rate of endogenous glucose production in both wild-type mice and a type 2 diabetes mouse model (Berg et al. 2001). The action of full length adiponectin on hepatic glucose production is dependent on phosphorylation and activation of AMPK in the liver (Yamauchi et al. 2002). Lack of action of adiponectin on hepatic glucose production when AMPKα2 catalytic subunit is missing strongly supports the concept that adiponectin's effect is strictly dependent on AMPK (Andreelli et al. 2006).
AMPK for hepatic steatosis management
Non-alcoholic fatty liver disease is a clinicopathological term that encompasses a disease spectrum ranging from simple triglyceride accumulation in hepatocytes (hepatic steatosis) to hepatic steatosis with inflammation (steatohepatitis), fibrosis and cirrhosis. Metformin and TZDs markedly reduced hepatic steatosis both in rodents (Lin et al. 2000) and humans (Bajaj et al. 2003), presumably acting through hepatic AMPK activation.
Chronic ethanol administration in mice, a model mimicking the chronic alcohol consumption in humans, leads to hepatic steatosis and is associated with a significant increase in the abundance of the mature (active) form of SREBP1c in the liver, an increased expression of lipogenic genes and an inhibition of AMPK and peroxisome proliferator-activated receptor α (PPARα), two critical signalling molecules controlling the pathways of hepatic fatty acid oxidation (You et al. 2004). Transcriptional control by AMPK may be important for the treatment of liver steatosis because AMPK activation suppresses expression of SREBP1c (Zhou et al. 2001; Foretz et al. 2005). In addition, chronic ethanol consumption significantly decreased circulating concentrations of adiponectin in mice (Xu et al. 2003). Delivery of recombinant full-length adiponectin into these mice alleviated liver steatosis and injury through activation of fatty acid oxidation and by decrease of lipogenesis.
AMPK for protective effects of ischaemic preconditioning in liver transplantation
Hepatic ischaemia–reperfusion injury associated with liver transplantation and hepatic resections is a critical problem in clinical practice. Preconditioning (a short period of ischaemia and reperfusion) is associated with a beneficial effect during major hepatic surgery in patients subjected to complex hepatic resections in which long periods of ischaemia are necessary. Ischaemic preconditioning prevents ATP degradation and concomitant intracellular accumulation of AMPK induced by subsequent ischaemia (Peralta et al. 2000). Increases in AMP levels during ischaemia activate AMPK while inhibition of AMPK abolishes completely the effects of preconditioning, indicating that AMPK is essential to promote the preconditioning effect (Peralta et al. 2001). So, activation of AMPK in liver by preconditioning may represent a new strategy to reduce the hepatic injury associated with ischaemia–reperfusion in humans.
Conclusion
The liver is highly sensitive to changes in metabolic demands and variation in nutritional and hormonal signals will act on the hepatic AMPK system to regulate whole body energy metabolism. The AMPK system plays a major role in the regulation of glucose and lipid metabolism through its acute effects on energy metabolism pathways and long-term effects involving changes in gene expression. The relationship between AMPK activation and beneficial metabolic effects in diabetic rodent models has provided the rationale for the development of new therapeutic strategies based on pharmacological but also nutritional use of AMPK activators in order to prevent or reverse hepatic disorders linked to type 2 diabetes and obesity.
Perspectives
AMPK activation in the liver entails metabolic consequences that are beneficial for the diabetic patients. This holds true for the inhibition of gluconeogenesis, which helps to maintain glycaemia. Many other known or potential effects of AMPK on liver metabolism remain, however, to be investigated. This is the case for glycogen and cholesterol metabolism. A decrease in the latter would mimic the effects of statins and benefit those patients already prone to cardiovascular complications. By contrast, the expected stimulation of fatty acid oxidation and ketogenesis by AMPK might lead to ketoacidosis, a dreadful complication of diabetes. The inhibition of protein synthesis by AMPK could favour protein degradation and lead to a negative nitrogen balance together with enhanced urea synthesis, a pathway known to consume 4 mol of ATP per mol of urea. Clearly, these effects of AMPK on protein metabolism and on the detoxifying capacity of the liver deserve serious consideration. In conclusion, care should be taken that the well-documented beneficial effects of pharmacological activation of AMPK on glucose metabolism do not mask other metabolic effects that could be detrimental for diabetic patients.
Acknowledgments
Work cited from the authors' laboratories was supported by integrated projects from the European Commission (Grants QLG1-CT-2001-01488/ampdiamet and LSHM-CT-2004-005272/exgenesis), Association pour l'Etude des Diabètes et des Maladies Métaboliques (ALFEDIAM), Programme National de Recherche sur le Diabète (PNRD), Association de Recherche sur le Diabète (ARD), Institut Benjamin Delessert and the Fonds National de la Recherche Scientifique. M.F. is supported by a postdoctoral fellowship from the European Union (exgenesis), B.G. is recipient of the ICP-‘Michel de Visscher’ Fellowship, and L.B. is Research Associate of the Belgian Fund for Scientific Research (Belgium).
References
- Abu-Elheiga L, Matzuk MM, Abo-Hashema KA, Wakil SJ. Continuous fatty acid oxidation and reduced fat storage in mice lacking acetyl-CoA carboxylase 2. Science. 2001;291:2613–2616. doi: 10.1126/science.1056843. [DOI] [PubMed] [Google Scholar]
- Andreelli F, Foretz M, Knauf C, Cani PD, Perrin C, Iglesias MA, Pillot B, Bado A, Tronche F, Mithieux G, Vaulont S, Burcelin R, Viollet B. Liver adenosine monophosphate-activated kinase-α2 catalytic subunit is a key target for the control of hepatic glucose production by adiponectin and leptin but not by insulin. Endocrinology. 2006;147:2432–2441. doi: 10.1210/en.2005-0898. [DOI] [PubMed] [Google Scholar]
- Assifi MM, Suchankova G, Constant S, Prentki M, Saha AK, Ruderman NB. AMP-activated protein kinase and coordination of hepatic fatty acid metabolism of starved/carbohydrate-refed rats. Am J Physiol Endocrinol Metab. 2005;289:E794–E800. doi: 10.1152/ajpendo.00144.2005. [DOI] [PubMed] [Google Scholar]
- Bajaj M, Suraamornkul S, Pratipanawatr T, Hardies LJ, Pratipanawatr W, Glass L, Cersosimo E, Miyazaki Y, DeFronzo RA. Pioglitazone reduces hepatic fat content and augments splanchnic glucose uptake in patients with type 2 diabetes. Diabetes. 2003;52:1364–1370. doi: 10.2337/diabetes.52.6.1364. [DOI] [PubMed] [Google Scholar]
- Barthel A, Schmoll D, Kruger KD, Roth RA, Joost HG. Regulation of the forkhead transcription factor FKHR (FOXO1a) by glucose starvation and AICAR, an activator of AMP-activated protein kinase. Endocrinology. 2002;143:3183–3186. doi: 10.1210/endo.143.8.8792. [DOI] [PubMed] [Google Scholar]
- Berg AH, Du Combs TPX, Brownlee M, Scherer PE. The adipocyte-secreted protein Acrp30 enhances hepatic insulin action. Nat Med. 2001;7:947–953. doi: 10.1038/90992. [DOI] [PubMed] [Google Scholar]
- Bergeron R, Previs SF, Cline GW, Perret P, Russell RR, 3rd, Young LH, Shulman GI. Effect of 5-aminoimidazole-4-carboxamide-1-β-d-ribofuranoside infusion on in vivo glucose and lipid metabolism in lean and obese Zucker rats. Diabetes. 2001;50:1076–1082. doi: 10.2337/diabetes.50.5.1076. [DOI] [PubMed] [Google Scholar]
- Birnbaum MJ. Activating AMP-activated protein kinase without AMP. Mol Cell. 2005;19:289–290. doi: 10.1016/j.molcel.2005.07.012. [DOI] [PubMed] [Google Scholar]
- Browne GJ, Finn SG, Proud CG. Stimulation of the AMP-activated protein kinase leads to activation of eukaryotic elongation factor 2 kinase and to its phosphorylation at a novel site, serine 398. J Biol Chem. 2004;279:12220–12231. doi: 10.1074/jbc.M309773200. [DOI] [PubMed] [Google Scholar]
- Cheng SW, Fryer LG, Carling D, Shepherd PR. Thr2446 is a novel mammalian target of rapamycin (mTOR) phosphorylation site regulated by nutrient status. J Biol Chem. 2004;279:15719–15722. doi: 10.1074/jbc.C300534200. [DOI] [PubMed] [Google Scholar]
- Cheung PC, Salt IP, Davies SP, Hardie DG, Carling D. Characterization of AMP-activated protein kinase γ-subunit isoforms and their role in AMP binding. Biochem J. 2000;346:659–669. [PMC free article] [PubMed] [Google Scholar]
- Clarke PR, Hardie DG. Regulation of HMG-CoA reductase: identification of the site phosphorylated by the AMP-activated protein kinase in vitro and in intact rat liver. EMBO J. 1990;9:2439–2446. doi: 10.1002/j.1460-2075.1990.tb07420.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corton JM, Gillespie JG, Hawley SA, Hardie DG. 5-Aminoimidazole-4-carboxamide ribonucleoside. A specific method for activating AMP-activated protein kinase in intact cells? Eur J Biochem. 1995;229:558–565. doi: 10.1111/j.1432-1033.1995.tb20498.x. [DOI] [PubMed] [Google Scholar]
- Dentin R, Benhamed F, Pegorier JP, Foufelle F, Viollet B, Vaulont S, Girard J, Postic C. Polyunsaturated fatty acids suppress glycolytic and lipogenic genes through the inhibition of ChREBP nuclear protein translocation. J Clin Invest. 2005;115:2843–2854. doi: 10.1172/JCI25256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans JM, Donnelly LA, Emslie-Smith AM, Alessi DR, Morris AD. Metformin and reduced risk of cancer in diabetic patients. BMJ. 2005;330:1304–1305. doi: 10.1136/bmj.38415.708634.F7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferre P, Azzout-Marniche D, Foufelle F. AMP-activated protein kinase and hepatic genes involved in glucose metabolism. Biochem Soc Trans. 2003;31:220–223. doi: 10.1042/bst0310220. [DOI] [PubMed] [Google Scholar]
- Foretz M, Ancellin N, Andreelli F, Saintillan Y, Grondin P, Kahn A, Thorens B, Vaulont S, Viollet B. Short-term overexpression of a constitutively active form of AMP-activated protein kinase in the liver leads to mild hypoglycemia and fatty liver. Diabetes. 2005;54:1331–1339. doi: 10.2337/diabetes.54.5.1331. [DOI] [PubMed] [Google Scholar]
- Guigas B, Bertrand L, Taleux N, Foretz M, Wiernsperger N, Vertommen D, Andreelli F, Viollet B, Hue L. 5-Aminoimidazole-4-carboxamide-1-β-D-ribofuranoside and metformin inhibit hepatic glucose phosphorylation by an AMP-activated protein kinase-independent effect on glucokinase translocation. Diabetes. 2006;55:865–874. doi: 10.2337/diabetes.55.04.06.db05-1178. [DOI] [PubMed] [Google Scholar]
- Hardie DG. The AMP-activated protein kinase pathway – new players upstream and downstream. J Cell Sci. 2004;117:5479–5487. doi: 10.1242/jcs.01540. [DOI] [PubMed] [Google Scholar]
- Hong YH, Varanasi US, Yang W, Leff T. AMP-activated protein kinase regulates HNF4α transcriptional activity by inhibiting dimer formation and decreasing protein stability. J Biol Chem. 2003;278:27495–27501. doi: 10.1074/jbc.M304112200. [DOI] [PubMed] [Google Scholar]
- Horman S, Browne G, Krause U, Patel J, Vertommen D, Bertrand L, Lavoinne A, Hue L, Proud C, Rider M. Activation of AMP-activated protein kinase leads to the phosphorylation of elongation factor 2 and an inhibition of protein synthesis. Curr Biol. 2002;12:1419–1423. doi: 10.1016/s0960-9822(02)01077-1. [DOI] [PubMed] [Google Scholar]
- Inoki K, Zhu T, Guan KL. TSC2 mediates cellular energy response to control cell growth and survival. Cell. 2003;115:577–590. doi: 10.1016/s0092-8674(03)00929-2. [DOI] [PubMed] [Google Scholar]
- Kawaguchi T, Osatomi K, Yamashita H, Kabashima T, Uyeda K. Mechanism for fatty acid ‘sparing’ effect on glucose-induced transcription: regulation of carbohydrate-responsive element-binding protein by AMP-activated protein kinase. J Biol Chem. 2002;277:3829–3835. doi: 10.1074/jbc.M107895200. [DOI] [PubMed] [Google Scholar]
- Kodama S, Koike C, Negishi M, Yamamoto Y. Nuclear receptors CAR and PXR cross talk with FOXO1 to regulate genes that encode drug-metabolizing and gluconeogenic enzymes. Mol Cell Biol. 2004;24:7931–7940. doi: 10.1128/MCB.24.18.7931-7940.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo SH, Flechner L, Qi L, Zhang X, Screaton RA, Jeffries S, Hedrick S, Xu W, Boussouar F, Brindle P, Takemori H, Montminy M. The CREB coactivator TORC2 is a key regulator of fasting glucose metabolism. Nature. 2005;437:1109–1111. doi: 10.1038/nature03967. [DOI] [PubMed] [Google Scholar]
- Krause U, Bertrand L, Hue L. Control of p70 ribosomal protein S6 kinase and acetyl-CoA carboxylase by AMP-activated protein kinase and protein phosphatases in isolated hepatocytes. Eur J Biochem. 2002a;269:3751–3759. doi: 10.1046/j.1432-1033.2002.03074.x. [DOI] [PubMed] [Google Scholar]
- Krause U, Bertrand L, Maisin L, Rosa M, Hue L. Signalling pathways and combinatory effects of insulin and amino acids in isolated rat hepatocytes. Eur J Biochem. 2002b;269:3742–3750. doi: 10.1046/j.1432-1033.2002.03069.x. [DOI] [PubMed] [Google Scholar]
- Leclerc I, Lenzner C, Gourdon L, Vaulont S, Kahn A, Viollet B. Hepatocyte nuclear factor-4α involved in type 1 maturity-onset diabetes of the young is a novel target of AMP-activated protein kinase. Diabetes. 2001;50:1515–1521. doi: 10.2337/diabetes.50.7.1515. [DOI] [PubMed] [Google Scholar]
- Lin HZ, Yang SQ, Chuckaree C, Kuhajda F, Ronnet G, Diehl AM. Metformin reverses fatty liver disease in obese, leptin-deficient mice. Nat Med. 2000;6:998–1003. doi: 10.1038/79697. [DOI] [PubMed] [Google Scholar]
- Liu L, Cash TP, Jones RG, Keith B, Thompson CB, Simon MC. Hypoxia-induced energy stress regulates mRNA translation and cell growth. Mol Cell. 2006;21:521–531. doi: 10.1016/j.molcel.2006.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lizcano JM, Goransson O, Toth R, Deak M, Morrice NA, Boudeau J, Hawley SA, Udd L, Makela TP, Hardie DG, Alessi DR. LKB1 is a master kinase that activates 13 kinases of the AMPK subfamily, including MARK/PAR-1. EMBO J. 2004;23:833–843. doi: 10.1038/sj.emboj.7600110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Z, Saha AK, Xiang X, Ruderman NB. AMPK, the metabolic syndrome and cancer. Trends Pharmacol Sci. 2005;26:69–76. doi: 10.1016/j.tips.2004.12.011. [DOI] [PubMed] [Google Scholar]
- Munday MR, Campbell DG, Carling D, Hardie DG. Identification by amino acid sequencing of three major regulatory phosphorylation sites on rat acetyl-CoA carboxylase. Eur J Biochem. 1988;175:331–338. doi: 10.1111/j.1432-1033.1988.tb14201.x. [DOI] [PubMed] [Google Scholar]
- Muoio DM, Seefeld K, Witters LA, Coleman RA. AMP-activated kinase reciprocally regulates triacylglycerol synthesis and fatty acid oxidation in liver and muscle: evidence that sn-glycerol-3-phosphate acyltransferase is a novel target. Biochem J. 1999;338:783–791. [PMC free article] [PubMed] [Google Scholar]
- Nawrocki AR, Rajala MW, Tomas E, Pajvani UB, Saha AK, Trumbauer ME, Pang Z, Chen AS, Ruderman NB, Chen H, Rossetti L, Scherer PE. Mice lacking adiponectin show decreased hepatic insulin sensitivity and reduced responsiveness to peroxisome proliferator-activated receptor γ agonists. J Biol Chem. 2006;281:2654–2660. doi: 10.1074/jbc.M505311200. [DOI] [PubMed] [Google Scholar]
- Pencek RR, Shearer J, Camacho RC, James FD, Lacy DB, Fueger PT, Donahue EP, Snead W, Wasserman DH. 5-Aminoimidazole-4-carboxamide-1-β-d-ribofuranoside causes acute hepatic insulin resistance in vivo. Diabetes. 2005;54:355–360. doi: 10.2337/diabetes.54.2.355. [DOI] [PubMed] [Google Scholar]
- Peralta C, Bartrons R, Riera L, Manzano A, Xaus C, Gelpi E, Rosello-Catafau J. Hepatic preconditioning preserves energy metabolism during sustained ischemia. Am J Physiol Gastrointest Liver Physiol. 2000;279:G163–G171. doi: 10.1152/ajpgi.2000.279.1.G163. [DOI] [PubMed] [Google Scholar]
- Peralta C, Bartrons R, Serafin A, Blazquez C, Guzman M, Prats N, Xaus C, Cutillas B, Gelpi E, Rosello-Catafau J. Adenosine monophosphate-activated protein kinase mediates the protective effects of ischemic preconditioning on hepatic ischemia-reperfusion injury in the rat. Hepatology. 2001;34:1164–1173. doi: 10.1053/jhep.2001.29197. [DOI] [PubMed] [Google Scholar]
- Proud CG. Role of mTOR signalling in the control of translation initiation and elongation by nutrients. Curr Top Microbiol Immunol. 2004;279:215–244. doi: 10.1007/978-3-642-18930-2_13. [DOI] [PubMed] [Google Scholar]
- Rao RD, Buckner JC, Sarkaria JN. Mammalian target of rapamycin (mTOR) inhibitors as anti-cancer agents. Curr Cancer Drug Targets. 2004;4:621–635. doi: 10.2174/1568009043332718. [DOI] [PubMed] [Google Scholar]
- Reiter AK, Bolster DR, Crozier SJ, Kimball SR, Jefferson LS. Repression of protein synthesis and mTOR signaling in rat liver mediated by the AMPK activator aminoimidazole carboxamide ribonucleoside. Am J Physiol Endocrinol Metab. 2005;288:E980–E988. doi: 10.1152/ajpendo.00333.2004. [DOI] [PubMed] [Google Scholar]
- Rencurel F, Stenhouse A, Hawley SA, Friedberg T, Hardie DG, Sutherland C, Wolf CR. AMP-activated protein kinase mediates phenobarbital induction of CYP2B gene expression in hepatocytes and a newly derived human hepatoma cell line. J Biol Chem. 2005;280:4367–4373. doi: 10.1074/jbc.M412711200. [DOI] [PubMed] [Google Scholar]
- Saha AK, Avilucea PR, Ye JM, Assifi MM, Kraegen EW, Ruderman NB. Pioglitazone treatment activates AMP-activated protein kinase in rat liver and adipose tissue in vivo. Biochem Biophys Res Commun. 2004;314:580–585. doi: 10.1016/j.bbrc.2003.12.120. [DOI] [PubMed] [Google Scholar]
- Salt I, Celler JW, Hawley SA, Prescott A, Woods A, Carling D, Hardie DG. AMP-activated protein kinase: greater AMP dependence, and preferential nuclear localization, of complexes containing the α2 isoform. Biochem J. 1998;334:177–187. doi: 10.1042/bj3340177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarbassov DD, Ali SM, Sabatini DM. Growing roles for the mTOR pathway. Curr Opin Cell Biol. 2005;17:596–603. doi: 10.1016/j.ceb.2005.09.009. [DOI] [PubMed] [Google Scholar]
- Satoh H, Nguyen MT, Miles PD, Imamura T, Usui I, Olefsky JM. Adenovirus-mediated chronic ‘hyper-resistinemia’ leads to in vivo insulin resistance in normal rats. J Clin Invest. 2004;114:224–231. doi: 10.1172/JCI20785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Screaton RA, Conkright MD, Katoh Y, Best JL, Canettieri G, Jeffries S, Guzman E, Niessen S, Yates JR, 3rd, Takemori H, Okamoto M, Montminy M. The CREB coactivator TORC2 functions as a calcium- and cAMP-sensitive coincidence detector. Cell. 2004;119:61–74. doi: 10.1016/j.cell.2004.09.015. [DOI] [PubMed] [Google Scholar]
- Shaw RJ, Lamia KA, Vasquez D, Koo SH, Bardeesy N, Depinho RA, Montminy M, Cantley LC. The kinase LKB1 mediates glucose homeostasis in liver and therapeutic effects of metformin. Science. 2005;310:1642–1646. doi: 10.1126/science.1120781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viollet B, Andreelli F, Jorgensen SB, Perrin C, Geloen A, Flamez D, Mu J, Lenzner C, Baud O, Bennoun M, Gomas E, Nicolas G, Wojtaszewski JF, Kahn A, Carling D, Schuit FC, Birnbaum MJ, Richter EA, Burcelin R, Vaulont S. The AMP-activated protein kinase α2 catalytic subunit controls whole-body insulin sensitivity. J Clin Invest. 2003;111:91–98. doi: 10.1172/JCI16567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winder WW, Wilson HA, Hardie DG, Rasmussen BB, Hutber CA, Call GB, Clayton RD, Conley LM, Yoon S, Zhou B. Phosphorylation of rat muscle acetyl-CoA carboxylase by AMP-activated protein kinase and protein kinase A. J Appl Physiol. 1997;82:219–225. doi: 10.1152/jappl.1997.82.1.219. [DOI] [PubMed] [Google Scholar]
- Witters LA, Kemp BE. Insulin activation of acetyl-CoA carboxylase accompanied by inhibition of the 5′-AMP-activated protein kinase. J Biol Chem. 1992;267:2864–2867. [PubMed] [Google Scholar]
- Xu A, Wang Y, Keshaw H, Xu LY, Lam KS, Cooper GJ. The fat-derived hormone adiponectin alleviates alcoholic and nonalcoholic fatty liver diseases in mice. J Clin Invest. 2003;112:91–100. doi: 10.1172/JCI17797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamauchi T, Kamon J, Minokoshi Y, Ito Y, Waki H, Uchida S, Yamashita S, Noda M, Kita S, Ueki K, Eto K, Akanuma Y, Froguel P, Foufelle F, Ferre P, Carling D, Kimura S, Nagai R, Kahn BB, Kadowaki T. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat Med. 2002;8:1288–1295. doi: 10.1038/nm788. [DOI] [PubMed] [Google Scholar]
- You M, Matsumoto M, Pacold CM, Cho WK, Crabb DW. The role of AMP-activated protein kinase in the action of ethanol in the liver. Gastroenterology. 2004;127:1798–1808. doi: 10.1053/j.gastro.2004.09.049. [DOI] [PubMed] [Google Scholar]
- Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenyk-Melody J, Wu M, Ventre J, Doebber T, Fujii N, Musi N, Hirshman MF, Goodyear LJ, Moller DE. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest. 2001;108:1167–1174. doi: 10.1172/JCI13505. [DOI] [PMC free article] [PubMed] [Google Scholar]