Abstract
Calcium influxes through ionotropic glutamate receptors (AMPA and NMDA receptors, AMPARs and NMDARs) are considered to be critical for the shaping and refinement of neural circuits during synaptogenesis. Using a combined morphological and electrophysiological approach, we evaluated this hypothesis at the level of the nucleus tractus solitarii (NTS), a brainstem structure that is a gateway for many visceral sensory afferent fibres. We confirmed that in the NTS, the first excitatory synapses appeared at embryonic day 18. We next characterized the biophysical properties of NTS AMPARs. Throughout perinatal development, both evoked and miniature EPSCs recorded in the presence of an NMDAR blocker were insensitive to polyamines and had linear current–voltage relationships. This demonstrated that AMPARs at NTS excitatory synapses were calcium-impermeable receptors composed of a majority of GluR2 subunits. We then investigated the influence of calcium influxes through NMDARs on the development of NTS synaptic transmission. We found that NMDAR expression at synaptic sites did not precede AMPAR expression. Moreover, NMDAR blockade in utero did not prevent the development of AMPAR synaptic currents and the synaptic clustering of GluR2 subunits. Thus, our data support an alternative model of synaptogenesis that does not depend on calcium influxes through either AMPARs or NMDARs. This model may be particularly relevant to the formation of neural networks devoted to basic behaviours required at birth for survival.
The construction of neural circuits involves a series of events that ultimately lead to the adult pattern. The initial formation of the connectivity diagram relies on different processes such as axonal pathfinding and target selection that may not require electrical activity of neurons (Katz & Shatz, 1996; Crair, 1999; Craig et al. 2006). Later on, neuronal activity seems to be important for synapse formation through calcium induction of biochemical cascades (Aamodt & Constantine-Paton, 1999; Bregestovski & Spitzer, 2005). In particular, calcium influxes through ionotropic glutamate receptors are thought to be required for excitatory synapse maturation and stabilization. Excitatory synaptic transmission is generally mediated by the action of glutamate on two different ionotropic receptors, the NMDA receptor (NMDAR) and the AMPA receptor (AMPAR). The NMDAR is endowed with both ligand- and voltage-dependent properties. This makes it act as a coincidence detector and, hence, provides a molecular basis for developmental Hebbian theories (Crair, 1999; Contestabile, 2000). NMDARs seem to be predominant in initial glutamatergic transmission. Furthermore, in various brain regions including the cortex and the hippocampus, functional maturation by AMPAR delivery to nascent synapses is thought to occur via an NMDA-dependant, ‘long-term potentiation (LTP)-like’ mechanism. (Wu et al. 1996; Isaac et al. 1997; Zhu et al. 2000). The AMPAR is a tetramer consisting of various combinations of four basic subunits referred to as GluR1 to GluR4. AMPAR lacking the GLuR2 subunit are both inwardly rectifying and highly permeable to calcium (Kohler et al. 1993; Kumar et al. 2002). The early synaptic expression of GluR2-lacking AMPARs followed by a switch in the subunit composition of AMPARs appears to be a common feature of neuronal development in most brain regions (Kumar et al. 2002; Rohrbough & Spitzer, 1999; Furuta & Martin, 1999). Thus, calcium influx through GluR2-lacking AMPARs has been deemed important in the formation and the refinement of the structural and functional properties of neural networks during early synaptogenesis (Rohrbough & Spitzer, 1999; Kumar et al. 2002).
The medulla contains neural circuits responsible for basic functions such as respiration and feeding, which are essential to life. Obviously, the survival of newborn mammals relies upon their ability to breathe and ingest nutrients. Contrary to sensory-motor and cognitive functions, basic autonomic regulations are therefore required to be operative at birth. Among different nuclei involved in the control of autonomic functions, the nucleus tractus solitarii (NTS) plays a central role. This nucleus, located in the caudal brainstem, is a gateway for many primary afferents from cardiovascular, respiratory, gastrointestinal, and other visceral sensory receptors. This visceral information runs into different cranial nerves (that join centrally to form a fibre tract, the tractus solitarius, TS; Andresen & Kunze, 1994) and triggers autonomic reflexes that are mostly stereotyped responses. They are therefore less likely to require instruction by neural activity to properly develop. This leads us to hypothesize that, contrary to what occurs in forebrain structures, early synaptogenesis in the NTS is an activity-independent process which does not require calcium-permeable glutamate receptors. We tested this hypothesis in the present study, using both electrophysiology and immunocytochemistry approaches. We found that in the NTS the first synapses appeared at embryonic day 18 (E18). We showed that these synapses directly formed with GluR2-rich, calcium-impermeable AMPAR. Finally, we provided evidence that NMDAR activation was not mandatory to trigger AMPAR clustering into NTS synapses.
Methods
Animals
All procedures were in agreement with the European Communities Council directive (86/609/EEC). The day of birth was considered as postnatal day zero (P0). Embryos were taken from pregnant females purchased from Janvier Laboratory (France). The day of finding a sperm-positive vaginal smear was considered as embryonic day zero (E0). On days E18 to E20, embryos were removed by hysterectomy under a mixture of xylasine/ketamine (15 mg kg−1/50 mg kg−1) and immediately killed by decapitation. Developing rat pups (P0 to P20) were also used in addition to embryos. Chronic NMDA receptor blockade was performed on pregnant females by i.p. injections of MK-801, a non competitive NMDA receptor antagonist (Sigma France) as previously described (Vincent et al. 2004; Lachamp et al. 2005). Starting from E17, each animal received two daily injections of MK-801 (0.5 mg kg−1) at 8.00 am and 6.00 pm Embryos were removed on day E20.
In vitro slice preparation and electrophysiology
Slice preparation
Preparation of medullary slice from embryos or developing Wistar rats was made as described before (Lachamp et al. 2003, 2005). Briefly, animals were decapitated and the brainstem was quickly removed, cooled to 4°C and cut on a vibratome (Leica VT1000 S, Leica Microsystemes SAS France), into 300 μm-thick medullary slices, in oxygenated (95% O2–5% CO2, pH 7.4) saline containing (mm) 130 NaCl, 3 KCl, 0.5 CaCl2, 4 MgCl2, 26 NaHCO3, 1.25 KH2PO4, 10 glucose, 0.5 ascorbate, 2 pyruvate, 3 myoinositol, 0.05 acid amino-phosphonovalerate (APV). Slices were then allowed to recover for 1 h in a similar saline containing 2 mm CaCl2, 3 mm MgCl2 and no APV at room temperature.
Electrophysiology
For recordings, slices were perfused in a chamber at 1–3 ml min−1 with a similar physiological solution containing 2 mm CaCl2, 3 mm MgCl2 and a mixture of GABAA receptors blockers (μm: 20 biccuculine, 50 picrotoxine) at 32°C. At depolarized potentials, AMPA EPSCs were isolated in the presence of 100 μm APV. Since the NTS is functionally divided into different subnuclei in the adult rat, we restricted our study to the medial NTS region on subpostremal slices. The dorsal motor nucleus of the vagus nerve and the tractus solitarius (TS) were taken as anatomical landmarks to reproducibly target the recording region. Therefore only one slice was selected per animal.
Patch electrodes (2.5–5 MΩ) contained (mm): 120 caesium methane sulphonate, 10 NaCl, 1 MgCl2, 1 CaCl2, 10 EGTA, 2 ATP, 0.3 GTP, 10 Glucose, 10 Hepes, 10 TEA, 4 4AP (pH 7.4) and 0.1 spermine (except for experiments in which polyamines were bath applied). The junction potential was about 8 mV and was not corrected for.
Bipolar stimulations of the TS were applied through a tungsten electrode (1–10 V; 50–200 μs) at low frequency (0.1–0.5 Hz). In embryonic slices, EPSCs were evoked using a patch electrode with a broken tip, filled with external solution. Stimulation strength and duration were kept constant throughout the experiment.
Recordings of miniatures EPSCs (mEPSCs) were performed in the presence of 1 μm tetrodotoxin (TTX) in the external solution. In experiments designed to test the sensitivity of AMPARs to extracellular polyamines, the following compounds were used (mm): 1 spermine or 9 × 10−3 1-naphtyl acetyl spermine (NASPM), a synthetic analogue of Joro spider toxin (Koike et al. 1997). After collecting control mEPSCs, spermine or NASPM were bath-applied for 5 min at least before collecting data. Two-hundred or more mEPSCs were collected before and after polyamine application over time epochs lasting 5–10 min. This procedure has already been described to probe for the subunit composition of AMPARs (Ju et al. 2004).
Drugs
TTX and NASPM were purchased from Alomon Laboratories (Jerusalem, Israel) and Tocris Cookson (Bristol, UK), respectively. Other chemicals were obtained from Sigma-Aldrich (France).
Data collection and analysis
Whole-cell patch-clamp recordings of NTS neurons were made with an Axopatch 200B (Axon instruments, Union City, CA, USA), filtered at 2 kHz and digitized at 20 kHz. Series resistance was monitored throughout the experiment and neurons in which this parameter was >25 MΩ or not stable were discarded.
mEPSC analysis was performed with the Mini Analysis software (Synaptosoft Inc., USA). The threshold for their detection was set at 3× root mean square noise level. Two minutes after break-in, 100–900 contiguous events per cell were analysed and averaged to yield mean mEPSC amplitude for each cell. The mean frequency of AMPA mEPSC was determined for each neuron as the number of events per second during a period from 2 to 10 min, as a function of the frequency. The decay time constant (τ) of synaptic currents was obtained by fitting the decay phase (t = 0 at the peak) with single or double exponential equations. At positive potentials, most EPSCs are composed of an initial fast AMPAR-mediated-current component, followed by a much slower NMDAR-mediated-current component of variable duration. Such dual-component EPSCs were best fitted to double exponential functions:
The weighted τ (tw) was calculated according to:
where Afast and Aslow are the relative amplitudes of the two exponential components.
Because the NMDAR mEPSC has decayed little from its peak when the AMPAR EPSC has fully decayed, the presence or absence of an AMPA component will have little consequence for the detectability of an NMDAR component. So, we used Aslow as an estimation of the amplitude of the NMDAR-mediated current component. Then the relative contribution (in percentage) of NMDAR mediated-component to the peak of the compound mEPSC was extrapolated as:
To check for the accuracy of the method, we subtracted the average mEPSC in normal saline to the average AMPAR-mediated mEPSC in the presence of APV, to derive the NMDAR component. Then the ratio of the NMDA EPSC peak to the EPSC peak was calculated. This method yielded similar results to the fitting procedure (n = 5, P = 0.6).
A peak-scaled non-stationary fluctuation analysis was made from an ensemble of mEPSCs (40–80) at a holding potential of −70 mV (Benke et al. 1998). mEPSCs were carefully selected for analysis by visual inspection based on the following criteria: fast rise time allowing for precise alignment; stable baseline holding current; absence of spurious fluctuations during the mEPSC decay. Estimation of the mean single-channel conductance of AMPARs was made with the Mini Analysis software (Synaptosoft Inc., USA; Traynelis et al. 1993; Benke et al. 1998). For this purpose the variance-amplitude relationship during the mEPSC decay was plotted. The shape of the relationship was fitted with the following equation:
where i was the mean single-channel AMPA current, I the mean current and N the number of channels activated at the peak, and σb2 the baseline variance. i was estimated as the slope of the linear fit of the first portion of the parabola, since the equation becomes linear when AMPAR open probability gets close to zero (Traynelis et al. 1993). The goodness of fit was assessed with a least-square algorithm. The number of open channels at the peak was calculated by dividing the average mEPSC amplitude by the unitary current (i). Unitary current was then converted to conductance according to average reversal potential measured from evoked EPSCs. There was no correlation between conductance and average EPSC amplitude, mean rise time, mean decay time, access resistance or background noise variance (data not shown, all P > 0.4). Thus, the range of values for conductance was mainly due to variations in AMPAR conductance between synapses (Benke et al. 1998).
Evoked EPSCs were offline analysed using p-clamp software. For the I–V analysis, average traces at each holding potential were obtained and aligned to the peak (typically 10–40 EPSCs were averaged). The mean amplitudes at negative potentials were fitted to a linear regression line. Reversal potential (Erev) values were estimated from the I–V relationship by linear interpolation. For calculating the rectification index (RI), we used the equation described in Kamboj et al. (1995):
Where I+40 and I−70 are, respectively, the EPSC current amplitude measured at +40 mV and at and −70 mV. The advantage of this method is that the RI is corrected for driving force and possible shifts in Erev. RI was also calculated by dividing average mEPSC conductance at +40 mV by average mEPSC conductance at −70 mV (100–200 events) according to reversal potentials derived from the I–V relationship of evoked EPSCs at similar ages. Paired-pulse ratio was measured at different developmental ages as described by Kim & Alger (2001). Briefly, a series (20–30 trials at a frequency of 0.05 Hz) of two electrical pulses separated by a 50 ms interval were applied to the TS, and the ratio of the mean amplitude of the second response to the mean amplitude of the first one was calculated.
Twenty to thirty voltage ramps were applied between +40 mV and −80 mV (120 mV s−1) in control conditions (TTX; 1 μm) and during the steady state of the inward current induced by bath application of kainate (100 μm in the presence of TTX, 1 min). I–V relationships for kainate-induced currents were obtained by subtracting kainate average current traces from control ones. RI for kainate-induced current was calculated as described above.
Statistical significance of the difference between means of two samples was computed with the non-parametric unpaired Mann–Whitney or Wilcoxon matched pairs test. When at least three samples were compared, the variance of the whole population was first analysed with the non-parametric Kruskhal–Wallis test. If the null hypothesis was rejected, the post hoc Dunn's test was then used to compare groups. The probability that data from two distributions belong to the same population was determined with the Kolmogorov–Smirnov test. Correlation was analysed with the Spearman rank-order test. Statistical data are given as mean ± standard deviation (s.d.) P < 0.05 was considered significant. Statistical tests were computed by using the Prism (Graphpad) or Statxact (Cytel studio) softwares.
Immunocytochemistry
We used antigen retrieval by microwave irradiation in order to increase detection of synaptically located antigens (Fritschy et al. 1998; see also Lachamp et al. 2005). Embryos (E18–E20) and developing rat pups (P0–P30) were killed by decapitation under halothane anaesthesia. Brainstems were removed and immediately frozen in cold isopentane (−50°C). Coronal medullary sections (14 μm thick) were obtained on a cryostat, thaw-mounted on gelatinized glass slides and fixed by immersion in phosphate buffer (0.1 m, pH 7.4) containing 0.5% paraformaldehyde under microwave irradiation (45 s, 800 W).
The following primary antibodies were used: mouse anti-GluR2 (Chemicon, Temecula CA; 3 μg ml−1), rabbit antisynaptophysin (Zymed, San Francisco, CA, USA; 1/50) and rabbit anti-VGLUT2 (Synaptic System, Goettingen Germany; 1/1000). Secondary antibodies were Alexa fluor 488-conjugated goat antimouse (Molecular Probe, Eugene, OR, USA; 1/200) and Alexa fluor 546-conjugated goat antirabbit (Molecular Probe, 1/200). All antibodies were diluted in saline phosphate buffer (PBS). Blocking steps were performed with 5% normal goat serum in PBS.
Confocal image acquisition was performed on a Leica TCS SP2 laser scanning microscope (Leica microsystems, Heidelberg, Germany) using the 488 nm band of an argon laser for excitation of Alexa Fluor 488 (spectral detection: 500–535 nm), and the 543 nm band of a helium–neon laser for excitation of Alexa Fluor 546 (spectral detection: 565–620 nm). High magnification images were acquired using a 63× oil immersion objective (NA: 1.32). Voxel size was adjusted to 58 nm in x and y and to 162 nm in z. Image editing was performed using Adobe Photoshop (Adobe Systems France, Paris, France).
Receptor clusters were counted on high magnification images (one channel single confocal sections, area sampled: 3500 μm2 in each animal). Immunoreactive puncta were extracted by threshold segmentation, and counted using the public domain NIH Image program (developed at the US National Institutes of Health). The effect of NMDA receptor blockade was evaluated by counting receptor clusters on NTS sections from MK-801-treated E20 embryos and age-matched control animals. Results were expressed as number of clusters per mm2 and analysed using the two-tailed Mann–Whitney test.
Results
Embryonic and postnatal development of miniature excitatory EPSCS (mEPSCs) in the NTS
Based on morphological criteria such as the presence of synaptic vesicles and dense membrane thickenings, the first identifiable synapses appear in the NTS around embryonic day 19 (Zhang & Ashwell, 2001). We therefore started our experiments as early as E18. Sixty cells were recorded at this stage. Among them 20% displayed no or a very small fast inward sodium current (<200 pA) when stepped from −70 mV to 0 mV. They were excluded from the study since they may have corresponded to very immature neurons or non-neural cells. The remaining cells were deemed as neurons since they displayed a fast inward current that amounted to at least 1 nA and was suppressed by TTX application (data not shown). Among them, 10 neurons were recorded at a holding potential of −70 mV for at least 4 min in the presence of TTX, bicuculine and picrotoxin to isolate AMPA miniature EPSCs (mEPSCs, Fig. 1A, see also Fig. 1G left). In this subset, 20% of neurons did not display any sign of synaptic activity and others showed a very low synaptic activity. Overall, mEPSC frequency was close to zero (m = 0.19 Hz ± 0.13, n = 10). At older ages studied, all cells recorded exhibited large fast inward sodium currents when subjected to a depolarizing step to 0 mV. As shown in Fig. 1A and B, a monotonic mEPSC frequency increase was observed with advancing neuron maturity from E18 to P19–20 (in Hz, E20: 0.47 ± 0.36, n = 10; P0–2: 0.69 ± 0.39, n = 10; P4–6: 1.63 ± 1.79, n = 8 and P19–20: 6.02 ± 3.9, n = 7).
Figure 1. Characterization of AMPAR-mediated transmission throughout the NTS development.
A, consecutive sample traces of miniature synaptic activity in an E18 (left) and a P6 (right) neuron. B–E, bar graphs showing average mEPSC frequency, amplitude, 20–80% rise time and τ as a function of age. Frequency but not amplitude increased with age. mEPSC rise times and decay times did not change from E18 to P4–6; in older neurons (P19–20) kinetics were significantly slower. F, mean cumulative distributions of mEPSC amplitudes at various ages (E18: n = 8, E20: n = 10, P0–2: n = 10, P4–6: n = 8, P19–20: n = 7, error bars indicate s.d., bin size = 5pA) G, left, 50 individual mEPSCs (grey traces) and mean mEPSC (black trace) superimposed, from an E18 (top) and a P2 (bottom) neuron. Right, corresponding current–variance relationships. Continuous lines represent the data fit from which γ was estimated. Broken lines represent the baseline variance. The weighted mean conductance (γ) was 11.1 pS at E18 and 11.4 pS at P2 for these neurons. H, bar graph showing the stability of the mean conductance at the three ages studied (E18–20: n = 7, P0–2: n = 8, P4–6: n = 7). For this figure and the following ones, asterisks indicate a group statistically different from the others (*P < 0.05; **P < 0.01; ***P < 0.001).
Surprisingly, in contrast to this developmental change in frequency, mEPSCs were indistinguishable with respect to their kinetics from E18 to P4–6 (E18: n = 8, E20: n = 10; P0–2: n = 10; P4–6: n = 8, Fig. 1D and E). Older neurons (P19–20, n = 7) had slower time course (20–80% rise time = 0.37 ± 0.08 ms; decay time τ = 2.79 ± 0.08 ms, Fig. 1D and E) than the younger ones (all data pooled together, mean rise 20–80% (RT): 0.22 ± 0.06, mean τ = 2.07 ± 0.59 (ms). Lastly, both mean amplitudes (in pA, E18: 25.2 ± 10.2, n = 8; E20: 22.9 ± 8.6, n = 10; P0–2: 25.3 ± 6.4, n = 10; P4–6: 21.6 ± 6.6, n = 8 and P19–20: 20.2 ± 7, n = 7, Fig. 1C) and cumulative amplitude distributions (Fig. 1F) were comparable throughout the developmental period studied.
Peak-scaled non-stationary fluctuation analysis was made on mEPSCs at different ages to estimate the mean single-channel conductance of synaptic AMPAR (Fig. 1G, see Methods). We found no significant difference between the three ages studied (E18–19: 11.4 ± 0.9 pS, n = 7; P0–2: 10.1 ± 2 pS, n = 8; P4–6: 11.1 ± 2.2, n = 7; Fig. 1H). The calculated number of open channels at the peak was not significantly different between groups, and averaged 28 ± 11 channels (n = 22).
Synapses between visceral afferent fibres and NTS neurons form with a unique AMPAR subunit composition
To assess AMPAR subunit composition, we first examined AMPAR-mediated EPSC rectification properties over a range of developmental stages (from E18 to P20). AMPAR-mediated EPSCs were elicited by stimulating the tractus solitarius (TS) in the presence of GABA and NMDAR antagonists. EPSCs were deemed monosynaptic by virtue of their short latency (3.14 ± 0.6 ms, n = 38; Fig. 2A, inset) and reduced jittering from stimulus onset (<200 μs, Doyle & Andresen, 2000; Lachamp et al. 2003). When using a paired-pulse protocol, all neurons exhibited a strong paired-pulse depression (data not shown). The amount of the depression as expressed by the paired-pulse ratio did not change during the perinatal period studied. It was 0.45 ± 0.29 at E18–19 (n = 11), 0.59 ± 0.24 at P0 (n = 13) and 0.50 ± 0.2 at P8–10 (n = 11).
Figure 2. Intracellular spermine (100 μm) does not alter the linearity of the I–V plots of evoked EPSCs.
A, inset, superimposed averaged recordings of evoked EPSCs (example from a P6 neuron) at various holding potentials (arrowhead: stimulation artefact). The plot shows normalized and superimposed I–V relationships of the pooled data for four age groups (E18–19: n = 7; P0–4: n = 11, P6–9: n = 12, P15–20: n = 8, for A and B) demonstrating the lack of rectification of EPSCs at all developmental stages. Each point of the plots represents an ensemble average of at leasteight experiments (error bars indicate s.d. when greater that the size of the symbol). Broken lines are extensions of linear regression fits of data points at negative potentials. B, rectification indices (see Methods) at different ages showing the absence of conductance reduction of evoked EPSCs at depolarized potentials. C, the plot represents two superimposed I–V relationships of the kainate-induced current at E20 and P1. Note the relative linearity of the curves. D, rectification indices for kainate-induced currents for two age groups (E18–20: n = 4; P0–4: n = 4).
I–V curves were obtained by averaging 10–40 EPSCs evoked at various holding potentials from −70 mV to +40 mV (Fig. 2A, inset). Since inward rectification depends on endogenous spermine, I–V relationship recordings were obtained with spermine (100 μm) in the pipette to prevent the loss of rectification induced by polyamine dialysis (Bowie & Mayer, 1995). Averaged I–V plots of AMPAR-mediated responses at various ages (E18–19, n = 7; P0–4, n = 11; P6–9, n = 12; P15–20, n = 8), normalized to the current amplitude recorded at −70 mV, are shown in Fig. 2A. Surprisingly we found that whatever the developmental stage, TS-evoked EPSCs were similar in magnitude at equipotential levels on either side of the reversal potential (the reversal potential was not significantly different among the studied groups and was 4.6 ± 4.8 mV, all data pooled together: n = 38). Rectification index (RI, see Methods) was stable throughout the various developmental stages and always close to 1 (E18–19: 1.05 ± 0.15; P0–4: 0.94 ± 0.16; P6–9: 0.95 ± 0.18; P15–20: 0.88 ± 0.11, Fig. 2B).
Despite the absence of synaptic rectifying AMPARs, NTS neurons may have expressed rectifying glutamate receptors at extrasynaptic sites. We therefore explored the apparent lack of rectification by studying the I–V relationship of kainite-induced currents in NTS neurons at two different ages (E18–20, n = 4 and P0–4, n = 4; see Methods). As illustrated in Fig. 2C, I–V curves reversed around 0 mV (all data pooled, mean value was 1.25 ± 3.5 mV; n = 8) and were linear at all ages studied as indicated by a RI close to one (E18–20: RI = 1.14 ± 0.07, n = 4; P0–4: RI = 1.07 ± 0.05, n = 4, Fig. 2D).
All NTS synapses form with similar AMPAr subunit composition throughout developmental stages
Other pharmacological approach was used to determine whether most synaptic AMPARs did contain a majority of GluR2 subunit. GluR2-containing AMPARs are unique not only in their lack of rectification but also in their lack of sensitivity to external polyamines (Washburn & Dingledine, 1996; Washburn et al. 1997). In addition, postnatal NTS neurons receive afferent inputs not only from visceral fibres conveyed by the TS but also from other sources (local networks and descending afferent inputs from higher nuclei; Fortin & Champagnat, 1993; Rinaman, 2003). Given that properties of AMPA-mediated EPSCs evoked by TS stimulation may differ in terms of their input specificity, we probed for external polyamine sensitivity of mEPSCs at two developmental postnatal ages (P0–2, n = 10 and P4–6, n = 8).
mEPSCs were isolated at holding potential of −70 mV, in the presence of a mixture of GABA and NMDA receptor antagonists and TTX. We used a small endogenous polyamine (PA), spermine (1 mm) and a synthetic acetylated polyamine, NASPM (9 μm). Its large phenylcyclohexyl head-group is known to prevent permeation of the blocker at very negative potentials when the voltage gradient is sufficiently high. Moreover an increase in the size of the terminal amino moiety, in the number of protonated amines and in the hydrophobicity of the spermine chain results in a markedly increased potency of the blocker (Washburn & Dingledine, 1996). Using one or the other of these two polyamines did not influence the results, so data were pooled.
The time course of pooled AMPAR-mediated EPSC amplitudes in response to extracellular polyamine is shown in Fig. 3A for P0–2 neurons and Fig. 3C for P4–6 neurons. Thus AMPAR-mediated EPSC mean amplitudes were unaffected in the two age groups by addition of PA (in pA, control: mean amplitude: 25.7 ± 6.6 and 21.9 ± 6.8; PA: 25.75 ± 6.2 and 23.2 ± 6.7 at P0–2 and P4–6, respectively). For each cell we compared the amplitude distributions before and during PA application, and they were always similar (see Methods, Fig. 3B and D). Lastly, at both ages AMPAR-mediated currents recorded with polyamine in the bath were indistinguishable from those recorded in control conditions with respect to their kinetics (in ms, P0–2: RT = 0.21 ± 0.04 and 0.22 ± 0.04; τ = 2.2 ± 0.4 and 2.3 ± 0.5; P4–6: RT = 0.26 ± 0.1 and 0.25 ± 0.07; τ = 2.1 ± 0.6 and 2.5 ± 0.6 for control and PA conditions, respectively). This lack of change in mEPSC time course is illustrated in the Fig. 3E and F, showing superimposed mean waveforms (in control and polyamine conditions) from representative experiments at P0 and P4.
Figure 3. Extracellular application of polyamine does not affect AMPAR-mediated transmission.
A,C, pooled data (P0–2: n = 10; P4–6: n = 8) showing the time course of mEPSC amplitude (holding potential = −70 mV), before and during polyamine application (black bar). (Note that polyamines were bath-applied for 5 min at least before collecting data, also see Methods) B,D, representative examples of cumulative distributions of mEPSC amplitudes in control and during polyamine application in a P0 and P6 neuron. Inset: normalized distributions of mean amplitudes in both conditions (same data as main graph, bin size = 4 pA). E,F, averaged and superimposed traces obtained in control and polyamine conditions, from a P0 (left) and a P6 (right) neuron, showing no change in mEPSC kinetics. Error bars indicate s.d.
In conclusion, TS-evoked or spontaneous excitatory activities recorded during the whole development stages were mediated by AMPARs mainly composed of the GluR2 subunit.
Developmental changes in GluR2 immunoreactivity in the NTS
To correlate electrophysiological results with the expression of AMPAR subunits, we next assayed GluR2 content via immunocytochemistry and tested whether NTS neurons might alter their GluR2 expression throughout development. Double immunofluorescence confocal microscopy was used to assess GluR2 levels in NTS neurons at various age groups, from the earlier synaptogenesis stage (E18) to adult neurons.
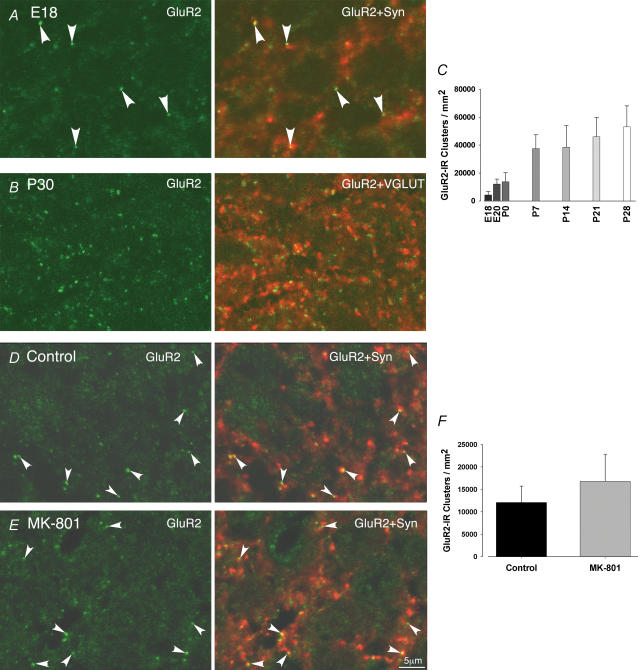
The pattern of GluR2 staining in the adult NTS was similar to that reported previously (Lachamp et al. 2003). When viewed at high magnification (Fig. 4B), GluR2 imunoreactivity appeared to be composed of numerous puncta which is consistent with labelling occurring at synaptic sites. This synaptic location of GluR2-immunoreactive puncta was further confirmed by the simultaneous detection of glutamatergic terminals using VGLUT immunoreactivity (Fig. 4B, see Lachamp et al. 2006). More interestingly, similar GluR2 staining was present as early as E18 (see Fig. 4A). Virtually all GluR2-immunoreactive patches were apposed to synaptophysin immunolabelling (Fig. 4A). They were therefore likely to correspond to accumulation of GluR2-containing AMPARs at differentiating synaptic sites. We then quantified the density of GluR2-immunoreactive puncta throughout development. The densities increased from E18 to P28: GluR2 immunoreactivity increased by approximately threefold between E18 and P0. Then it doubled over the first postnatal week before reaching a steady state around P21–28 (Fig. 4C).
Figure 4. Evolution of AMPAR density throughout the NTS development (upper part) and effect of NMDAR blockade on GluR2-clustering in utero (lower part).
A, left, distribution of GluR2 immunofluorescence (green) in the NTS at E18; right, most GluR2-containing AMPAR clusters are apposed to the presynaptic marker synaptophysin (red). B, left, distribution of GluR2 immunofluorescence (green) in the NTS at P30; right, virtually all GluR2 clusters are apposed to presynaptic marker VGLUT (red). C, evolution of AMPAR cluster densities in the NTS throughout development. D, left, GluR2 immunofluorescence at E20 from a control embryo (green); right, GluR2 clusters are apposed to synaptophysin immunolabelling (red) in the NTS as indicated by head stars. E, left, GluR2 immunofluorescence at E20 from a MK-801-treated rat (green); right, GluR2 clusters are apposed to synaptophysin immunolabelling in the NTS (red). F, densities of AMPAR clusters in NTS neuron from control (n = 4) and MK-801-treated (n = 4) E20 embryos. The increase after MK-801 is not significant. Error bars indicate s.d.
Development of synaptic NMDARs and their role in the development of the GluR2-mediated transmission in NTS neurons
The lack of developmental switch of AMPAR subtype and the presence of the GluR2 subunit as soon as the earlier synaptogenesis stage in NTS neurons fundamentally differs from previously described glutamatergic synaptogenesis models. Because of the literature implicating the NMDAR in the activity-dependent production of the AMPA EPSCs (Isaac et al. 1997; Liao et al. 1999; Wu et al. 1996), we firstly investigated the development of synaptic responses mediated by NMDAR. Secondly, we tested the influence of their chronic blockade on the development of AMPAR-mediated-transmission.
From E20, when held at a potential of +40 mV, all neurons exhibited mEPSCs with a fast and a slow component in their decaying phase that could be fitted to a double exponential function (Fig. 5A, see Methods). Application of APV suppressed the slowly decaying phase hence revealing the AMPA component of mEPSCs (Fig. 5A). However, at the very beginning of synaptogenesis (E18), neurons displayed AMPAR mEPSCs with no NMDA or a weak NMDA component, as exemplified in Fig. 5B. We therefore quantified the relative contribution of the NMDA component for average mEPSCs in each neuron (see Methods). The mean NMDAR-mediated component dramatically increased from E18 to E20 (Fig. 5C–D; E18: n = 7, E19: n = 6, E20: n = 5). The mean relative contribution of the NMDAR-mediated-component to the peak of the compound mEPSC was: 35 ± 25% at E18, 38.6 ± 25.8% at E19 and 65.8 ± 14.5% at E20. Furthermore, at E18, 30% of neurons did not display an NMDA component on mEPSCs. We also searched for an NMDA component in TS-evoked synaptic responses at E18–19 (n = 12). In 25% of neurons, the decaying phase was best fitted to a single exponential and unaffected after APV application (Fig. 5E). In the remaining cells, the decaying phase was well fitted to a double exponential which yielded to various amounts of relative NMDA proportion (Fig. 5F). When compared, distributions of NMDA components were not statistically different between E18 and E19 (data not shown). These results showed an upregulation of the synaptic NMDA component during embryonic life, and suggested that development of AMPAR- and NMDAR-mediated transmissions occurred independently.
Figure 5. Development of synaptic NMDAR-mediated responses in the NTS.
A, superimposed example of scaled, averaged mEPSCs (200 mEPSCs at least were averaged) obtained in control condition (TTX) and after blockade of NMDARs by APV at E20 (holding potential = +40 mV). At E20, the NMDAR-mediated component of the average mEPSC was obtained by subtracting the APV trace from the control one (grey trace). B, example of averaged, scaled mEPSCs (black trace) at E18. The NMDAR-mediated component was rather small. A,B, the grey lines are the fits of the decay times to a double exponential function in control conditions. C, bar graph showing the increase of the mean relative contribution (%) of NMDAR-mediated component to the peak of the compound mEPSC (see Methods). during prenatal development (E18: n = 7; E19: n = 6; E20: n = 5). Error bars indicate s.d. D, normalized distributions of the mean relative contribution (%) of the NMDA response to the peak of the mean mEPSC among the cell population, at the same prenatal ages (E18: n = 7; E19: n = 6; E20: n = 5, bin size = 5%). Note that at E18, 30% of the cells did not express a synaptic NMDA component, whereas at E20, the mean relative NMDAR-mediated response was at least of 45%. E, averaged evoked EPSCs (eEPSCs, mean of 20 consecutive responses, holding potential = +40 mV) from an E18 neuron in control (black trace) and after application of APV (grey trace). Note the absence of an NMDA component in the synaptic response. The grey line is the fit of the decay time to a single exponential function (see Methods). F, normalized distribution of the mean relative contribution (%) of the NMDAR component to the peak of the mean eEPSC among the cell population at E18–19 (n = 12, bin size = 5%). Note that 25% of cells were devoid of synaptically evoked NMDAR-mediated currents.
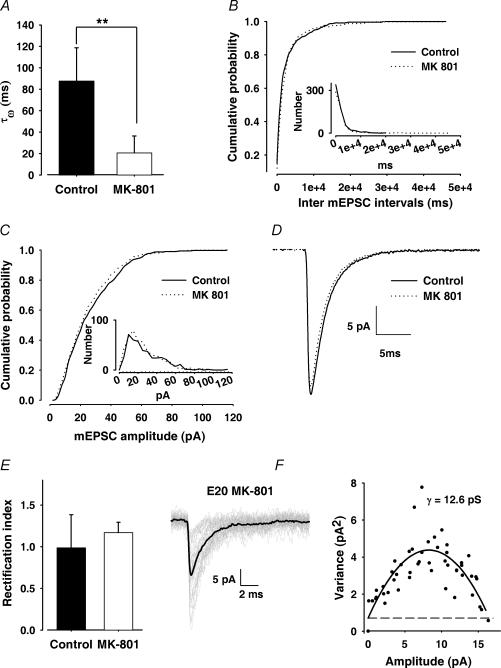
Chronic blockade of NMDARs was performed by chronic injection of MK-801 to pregnant female rats from E17 to E20 (see Methods). A positive control for NMDAR blockade in utero was obtained by analysing the residual NMDAR-mediated current component. The slow NMDAR-mediated current component was estimated on average mEPSC at a holding potential of +40 mV. Decay was fitted to a double exponential function and the weighted decay time constant was calculated. After chronic treatment with MK-801, the mean weighted decay time constant was drastically reduced in E20 neurons when compared to control animals (from 87.7 ± 30 ms for control rats to 18 ± 20 ms for treated animals, n = 5 in each condition, Fig. 6A). Addition of APV in the saline suppressed the slow component in control neurons (n = 5), but did not significantly alter the mean weighted decay time constant in most treated ones (two out of three, data not shown). The apparent persistent block of NMDARs and the remaining NMDA component observed in some neurons are likely to be related to the various durations of incubation time before recordings, since MK-801 dissociation may take hours (Huettner & Bean, 1988). Nevertheless, we cannot completely rule out a downregulation of NMDAR expression during fetal development due to the early NMDAR blockade. Whatever the explanation may be, MK-801 underwent placental transfer and was effective on embryo NMDARs (see also, Lachamp et al. 2005).
Figure 6. Effect of MK-801 chronic injection on the development of synaptic responses in the NTS.
A, bar graph illustrating the faster kinetics of mEPSC decay (recorded at depolarized potential) for MK-801-treated animals: The mean weighted τ (see Methods) in treated animals was drastically reduced, compared to control ones (n = 5 in both conditions). B,C, pooled cumulative distributions of inter-mEPSC intervals (C) and mean mEPSC amplitudes (n = 7 in both conditions; 100–300 events per cell). Insets: distribution of inter-mEPSC intervals and amplitudes (same data as main graph, bin size = 500 ms and 2 pA, respectively). D, averaged and superimposed traces from control and MK-801-treated animals showing no change in mEPSC kinetics. Traces were obtained by averaging events from all experiments in both conditions (n = 7 in both conditions). E, bar graph showing the lack of change of the rectification index in MK-801-treated animals. RI remained close to 1 (n = 7 in both conditions). F, left, 40 individual mEPSCs (grey traces) and mean mEPSCs (black trace) superimposed, recorded in an MK-801-treated animal; right, corresponding current–variance relationship. Continuous line represents the data fit from which γ was estimated; the broken line represents the baseline variance. The weighted mean conductance was 12.6 pS for this neuron. Error bars indicate s.d.
NMDAR blockade altered neither the frequency (in Hz, mean frequency = 0.36 ± 0.23 and 0.37 ± 0.37 in control and PA conditions, respectively, n = 7 for both groups) nor the amplitude (in pA, mean amplitude = 22.9 ± 8 and 23.7 ± 5 in control and PA conditions, respectively, n = 7 for both groups) of spontaneous miniature AMPAR-mediated events. Cumulative distributions of inter-mEPSC intervals (Fig. 6B, n = 7) and amplitudes (n = 7, Fig. 6C) were not significantly different between control and MK-801-treated neurons. Furthermore, biophysical properties of synaptic AMPARs were not modified by MK-801 treatment. AMPAR-mediated mEPSCs were indistinguishable from those recorded in control conditions with respect to their kinetics (Fig. 6D; mean RT = 0.24 ± 0.05 ms and 0.24 ± 0.03 ms, mean τ = 2.21 ± 0.48 ms and 2.36 ± 0.52 ms for control and MK-801-treated animals, respectively; n = 7 for both groups).The RI (calculated from average mEPSCs obtained at two holding potentials, see also Methods) was not significantly different between control and MK-801-treated animals (1.07 ± 0.17 and 1.16 ± 0.06, respectively; n = 5 for both groups, Fig. 6E). Lastly, the chronic NMDAR blockade did not change the mean AMPAR conductance estimated by non stationary fluctuation (NSF) analysis (mean conductance = 12.2 ± 1.8 pS). Figure 6F illustrates an example of 40 mEPSCs and mean waveform superimposed (left), and corresponding current variance relationship (right) in an MK-801-treated animal.
We also assessed synaptic GluR2 clustering after chronic application of MK-801. Consistent with electrophysiological data, NMDAR blockade failed to prevent synaptic AMPAR clustering. Numerous synaptic GluR2-immunoreactive puncta were apparent in the NTS neurons of both control and MK-801-treated embryos (Fig. 4D and E). As described above, all GluR2-immunoreactive patches were apposed to synaptophysin immunolabelling. The lack of effect of NMDAR blockade on AMPAR clustering was confirmed by quantitative analysis (Fig. 4F), which failed to demonstrate any significant difference between GluR2 puncta densities measured in control and MK-801-treated animals (12 ± 3.6 versus 17 ± 5.9 thousands of clusters per mm2; n = 4).
Discussion
Our findings support the view that the formation of excitatory synapses within the NTS involves a series of events that departs from that described in neocortical and hippocampal synapses. In the NTS, glutamatergic synaptogenesis occurs without expression of calcium-permeable AMPARs. In addition, synaptic clustering of GluR2-enriched receptors does not depend on NMDAR activation.
Characteristics of developing synaptic currents in the NTS
In the rat, NTS neurons are produced between E10 and E14 (Altman & Bayer, 1980) and vagal afferents start to emerge from the TS at E15 (Rinaman & Levitt, 1993). Synaptic vesicles are not visible before E19 (Zhang & Ashwell, 2001). Therefore recordings at E18 represented the very first functional synaptic activity in the NTS. The very low mEPSC frequency at E18 may reflect reduced release probability. However, paired-pulse depression was observed at all ages studied and did not change in magnitude over development, arguing for little change in release probability. In addition, the developmental mEPSC frequency increase correlated well to the dramatic increase in synaptic density reported to occur in the NTS before (Zhang & Ashwell, 2001) and after birth (Lachamp et al. 2002). Quantal currents remained relatively constant at developing NTS synapses, suggesting little change in postsynaptic AMPAR number and/or vesicular content. AMPAR-mediated mEPSC kinetics remained unaltered from E18 to the first postnatal week. Later on, a significant slowing down of both decay and rise times was observed. Holding currents, series resistances and reversal potentials were similar across different age groups arguing against compromise voltage-clamp conditions at P19–20. The dendritic arbor of NTS neurons becomes larger two weeks after birth (Vincent & Tell, 1999). Meanwhile, NTS synapses become preferentially located on dendrites rather than on the cell body (Miller et al. 1983). The apparent slow kinetics for mEPSCs at P19–20 were probably due to the remote electrotonic location of the synapses on distal dendritic shafts. As expected for electrotonic distortion, the faster phases of mEPSCs were two-fold more altered than the decay times. In addition, late expression of kainate receptors at synaptic sites may have contributed to the slowing down of EPSC kinetics.
The GluR2 subunit and NTS synaptic development
According to the developmental model proposed by Malinow & Malenka, (2002), calcium influxes through NMDARs are mandatory to trigger synaptic insertion of GluR4 subunits, eventually replaced by GluR2-rich AMPAR (Zhu et al. 2000). This model conceptually resembles that proposed to explain LTP in which NMDAR activation triggers synaptic insertion of GluR2-lacking AMPARs (Malinow & Malenka, 2002; Plant et al. 2006). Both models provide a molecular basis for developmental and plasticity Hebbian theories (Crair, 1999; Contestabile, 2000). In the NTS, mEPSC recordings at depolarized potentials revealed an upregulation of the synaptic NMDA component during the embryonic life. Chronic blockade of NMDARs in pregnant rats alters neither the developmental increase in mEPSC frequency nor the biophysical characteristics of synaptic events (i.e. RI, kinetics and AMPAR unitary conductance). In addition, synaptic GluR2 clustering was not prevented either. Other studies have challenged the view of NMDA-driven synapse formation. Assembly of glutamatergic synapses in hippocampal cell culture occurs in the presence of glutamatergic receptor antagonists or TTX (Friedman et al. 2000; Gomperts et al. 2000; Luthi et al. 2001). Accumulation of GluR1 clusters at synapses in spinal neurons is independent of glutamatergic receptor activation (O'Brien et al. 1997). Moreover, NMDARs are not required to induce early synaptic GluR2 clustering in Purkinje neurons (Lachamp et al. 2005). Although chronic disabling of NMDAR function could trigger compensatory mechanisms for synapse formation (Zhu & Malinow, 2002), AMPAR delivery to synapses may involve other mechanisms. As described here and in other models (Rohrbough & Spitzer, 1999; O'Brien et al. 1997), insertion of AMPARs in NTS synapses may occur slightly before NMDAR delivery or concomitantly, but without the need for NMDAR activation.
Alteration in AMPAR properties appears to be a common feature of synaptic development. In spinal interneurons, Ca2+-permeable AMPAR are transiently expressed early in development (Rohrbough & Spitzer, 1999). In neocortex or hippocampus neurons, AMPAR-mediated EPSCs are characterized by an inwardly rectifying current–voltage (I–V) relationship and spermine sensitivity early in development that switch to a linear I–V relationship and a loss of spermine sensitivity (Kumar et al. 2002; Zhu et al. 2000). These changes are related to the replacement of AMPARs lacking the GluR2 subunit by AMPARs containing the edited form of GluR2 (Kumar et al. 2002; Zhu et al. 2000). A single GluR2 subunit is sufficient to confer calcium impermeability to heteromeric receptors (Burnashev et al. 1992; Hollmann et al. 1991; Geiger et al. 1995; Washburn et al. 1997). By contrast, the degree of inward rectification and polyamine sensitivity are regulated by the copy number of the GluR2 subunit within a receptor (Washburn et al. 1997). During development, GluR2 immunoreactivity sharply increases in several models (Pickard et al. 2000; Kumar et al. 2002). Synaptic insertion of GluR2 subunits has been thought to be independent of synaptic activity in developing animals, but numerous studies favour a role for calcium influx through Glur2-lacking AMPARs to trigger the switch of AMPAR subunits (Liu & Cull-Candy, 2000; Zhu et al. 2000; Kumar et al. 2002; Gardner et al. 2005; Plant et al. 2006).
To our surprise, synaptic AMPARs in NTS neurons already exhibited linear I–V relationship at the very beginning of synaptic formation. This strongly argues for the presence of AMPARs mainly formed with the association of GluR2 subunits at synaptic sites between vagal afferents and NTS neurons. Likewise, linear I–V curves for kainate-induced currents further suggest a reduced expression of rectifying glutamate receptors. After birth, the lack of external polyamine sensitivity of mEPSCs suggests that NTS developing synapses are homogeneous in terms of AMPAR composition whatever the afferent input at play. Accordingly, synaptic channel conductance remained constant during the period studied, suggesting that the stoichiometry of subunit combination did not change during the period studied. Our experiments do not allow us to determine the precise AMPAR subunit composition at E18. However homomeric assembly of Glur2 subunits is unlikely since it yields to very low unitary conductance (300 fS, Swanson et al. 1997). Instead, the conductance values estimated in our study were in the same range as those obtained for recombinant heteromeric GluR2/GluR4 receptor assemblies (Swanson et al. 1997). However, EPSC decay times of the latter combination are two-fold faster than those reported here. GluR1 and GluR4 subunits are weakly expressed in the adult NTS (Kessler & Baude, 1999). Therefore AMPAR may rather form with the assembly of GluR2–GLUR3 subunits. Finally, GluR2 subunits are present at NTS synaptic sites from the beginning of synaptogenesis, and develop in parallel with synapse formation. In conclusion, unlike the situation widely described in other systems, NTS synapse formation occurs with a primary insertion of calcium-impermeable AMPARs.
How do NTS synapses acquire AMPAR?
Since NTS AMPARs are never permeable to calcium, this raises the question of alternative mechanisms involved in AMPAR clustering at synaptic sites. The neuronal activity-regulated pentraxin (NARP) has been implicated in the aggregation of AMPARs at excitatory synapses in the spinal cord. This protein is mainly associated with synaptic vesicles and is released extracellularly during synaptic transmission (O'Brien et al.; 1999, 2002). The role of NARP would be limited to excitatory synapses occurring on the dendritic shaft (O'Brien et al. 1999, 2002; Mi et al. 2002). Interestingly, NTS neurons are relatively aspiny, and excitatory synapses mainly occur on dendritic shafts (Miller et al. 1983; Vincent et al. 2004). However, apparently normal synaptogenesis can be obtained in culture in the absence of vesicular release obtained by the deletion of genes encoding the presynaptic protein Munc-13 (Varoqueaux et al. 2002). In this mutant, AMPAR clustering appears normal when vesicular release is induced by application of α-latrotoxin (Varoqueaux et al. 2002). These observations suggest additional mechanisms for AMPAR clustering. Stargazin would be an interesting candidate since it controls synaptic AMPAR number via a direct interaction with PSD-95 (Chen et al. 2000; Schnell et al. 2002). This model may be particularly relevant for the NTS since this interaction is responsible for targeting the GluR2 subunit at synaptic sites. In addition, stargazin links the expression of synaptic NMDARs to AMPARs. This may explain the delayed expression of NMDAR observed in NTS neurons during the embryonic life.
Functional significance
Calcium influx through NMDARs and GluR2-lacking AMPARs has been described as an important step in synaptic development (Rohrbough & Spitzer, 1999; Kumar et al. 2002; Malinow & Malenka, 2002) and activity-dependent formation of plasticity (Liu & Cull-Candy, 2000; Mahanty & Sah, 1998). LTP requires the presence of GluR1 subunits and calcium influx through the AMPAR (Jia et al. 1996; Zamanillo et al. 1999; Meng et al. 2003; Plant et al. 2006). Altogether, these results emphasize the role of calcium-driven processes in inducing synaptic remodelling (Bregestovski & Spitzer, 2005). In contrast, the absence of calcium-permeable AMPARs in NTS developing synapses and the lack of effect of NMDAR blockade on NTS synapse formation raise the question of an activity-independent synaptogenesis (Crair, 1999; Craig et al. 2005). Whether calcium influx may be triggered by synaptic activation of voltage-gated calcium channels or depolarizing GABA inputs as described in other systems remains an open question for the NTS (Hartmann & Konnerth, 2005). Nevertheless, NTS synapses would form in a non-Hebbian manner. Unlike other systems, neural circuits responsible for autonomic functions must be operative at birth. Therefore formation of synaptic contacts should be less sensitive to alterations of fetal environment that may occur in utero or during delivery. After birth, visceral reflexes undergo maturational changes (Kasparov & Paton, 1997; Moss, 2002; Vincent & Tell, 1999, Vincent et al. 2004). In addition, environmental events during early postnatal life can influence the formation of autonomic neural circuits (Card et al. 2005).
In conclusion, our data support an alternative model of synaptogenesis to those described for other brain structures. Because calcium influxes through ionotropic glutamate receptors appear to be unnecessary for synapse formation, this model implies that instructive neural activity may be not mandatory for the primary construction of neural circuits (Crair, 1999; Craig et al. 2005). This model may be particularly relevant to the formation of neural networks devoted to basic behaviours required at birth for survival.
Acknowledgments
The study was supported by the Centre National de la Recherche Scientifique. The authors wish to thank Axel Fernandez and Jean-Claude Reynaud for expert technical assistance, Laurence Cathala for her help in analysing quantal transmission, and Agnes Baude, Marcel Crest and Christian Lüscher for critical reading of the manuscript.
References
- Aamodt SM, Constantine-Paton M. The role of neural activity in synaptic development and its implications for adult brain function. Adv Neurol. 1999;79:133–144. [PubMed] [Google Scholar]
- Altman S, Bayer SA. Development of the brain stem in the rat. I. Thymidine-autoradiographic study of the origin of neurons of the lower medulla. J Comp Neurol. 1980;194:1–25. doi: 10.1002/cne.901940102. [DOI] [PubMed] [Google Scholar]
- Andresen MC, Kunze DL. Nucleus tractus solitarius-gateway to neural circulatory control. Annu Rev Physiol. 1994;56:93–116. doi: 10.1146/annurev.ph.56.030194.000521. [DOI] [PubMed] [Google Scholar]
- Benke TA, Luthi A, Isaac JT, Collingridge GL. Modulation of AMPA receptor unitary conductance by synaptic activity. Nature. 1998;393:793–797. doi: 10.1038/31709. [DOI] [PubMed] [Google Scholar]
- Bowie D, Mayer ML. Inward rectification of both AMPA and kainate subtype glutamate receptors generated by polyamine-mediated ion channel block. Neuron. 1995;15:453–462. doi: 10.1016/0896-6273(95)90049-7. [DOI] [PubMed] [Google Scholar]
- Bregestovski P, Spitzer N. Calcium in the function of the nervous system: new implications. Cell Calcium. 2005;37:371–374. doi: 10.1016/j.ceca.2005.01.004. [DOI] [PubMed] [Google Scholar]
- Burnashev N, Khodorova A, Jonas P, Helm PJ, Wisden W, Monyer H, Seeburg PH, Sakmann B. Calcium-permeable AMPA-kainate receptors in fusiform cerebellar glial cells. Science. 1992;256:1566–1570. doi: 10.1126/science.1317970. [DOI] [PubMed] [Google Scholar]
- Card JP, Levitt P, Gluhovsky M, Rinaman L. Early experience modifies the postnatal assembly of autonomic emotional motor circuits in rats. J Neurosci. 2005;25:9102–9111. doi: 10.1523/JNEUROSCI.2345-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Chetkovich DM, Petralia RS, Sweeney NT, Kawasaki Y, Wenthold RJ, Bredt DS, Nicoll RA. Stargazin regulates synaptic targeting of AMPA receptors by two distinct mechanisms. Nature. 2000;408:936–943. doi: 10.1038/35050030. [DOI] [PubMed] [Google Scholar]
- Contestabile A. Roles of NMDA receptor activity and nitric oxide production in brain development. Brain Res Brain Res Rev. 2000;32:476–509. doi: 10.1016/s0165-0173(00)00018-7. [DOI] [PubMed] [Google Scholar]
- Craig AM, Graf ER, Linhoff MW. How to build a central synapse: clues from cell culture. Trends Neurosci. 2006;29:8–20. doi: 10.1016/j.tins.2005.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crair MC. Neuronal activity during development: permissive or instructive? Curr Opin Neurobiol. 1999;9:88–93. doi: 10.1016/s0959-4388(99)80011-7. [DOI] [PubMed] [Google Scholar]
- Doyle MW, Andresen MC. Reliability of monosynaptic sensory transmission in brain stem neurons in vitro. J Neurophysiol. 2000;85:2213–2223. doi: 10.1152/jn.2001.85.5.2213. [DOI] [PubMed] [Google Scholar]
- Fortin G, Champagnat J. Spontaneous synaptic activities in rat nucleus tractus solitarius neurons in vitro: evidence for re-excitatory processing. Brain Res. 1993;630:125–135. doi: 10.1016/0006-8993(93)90650-c. [DOI] [PubMed] [Google Scholar]
- Friedman HV, Bresler T, Garner CC, Ziv NE. Assembly of new individual excitatory synapses: time course and temporal order of synaptic molecule recruitment. Neuron. 2000;27:57–69. doi: 10.1016/s0896-6273(00)00009-x. [DOI] [PubMed] [Google Scholar]
- Fritschy JM, Weinmann O, Wenzel A, Benke D. Synapse-specific localization of NMDA and GABA(A) receptor subunits revealed by antigen-retrieval immunohistochemistry. J Comp Neurol. 1998;390:194–210. [PubMed] [Google Scholar]
- Furuta A, Martin LJ. Laminar segregation of the cortical plate during corticogenesis is accompanied by changes in glutamate receptor expression. J Neurobiol. 1999;39:67–80. [PubMed] [Google Scholar]
- Gardner SM, Takamiya K, Xia J, Suh JG, Johnson R, Yu S, Huganir RL. Calcium-permeable AMPA receptor plasticity is mediated by subunit–specific interactions with PICK1 and NSF. Neuron. 2005;45:903–915. doi: 10.1016/j.neuron.2005.02.026. [DOI] [PubMed] [Google Scholar]
- Geiger JR, Melcher T, Koh DS, Sakmann B, Seeburg PH, Jonas P, Monyer H. Relative abundance of subunit mRNAs determines gating and Ca2+ permeability of AMPA receptors in principal neurons and interneurons in rat CNS. Neuron. 1995;15:193–204. doi: 10.1016/0896-6273(95)90076-4. [DOI] [PubMed] [Google Scholar]
- Gomperts SN, Carroll R, Malenka RC, Nicoll RA. Distinct roles for ionotropic and metabotropic glutamate receptors in the maturation of excitatory synapses. J Neurosci. 2000;20:2229–2237. doi: 10.1523/JNEUROSCI.20-06-02229.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann J, Konnerth H. Determinants of postsynaptic Ca2+ signaling in Purkinje neurons. Cell Calcium. 2005;37:496–466. doi: 10.1016/j.ceca.2005.01.014. [DOI] [PubMed] [Google Scholar]
- Hollmann M, Hartley M, Heinemann S. Ca2+ permeability of KA-AMPA-gated glutamate receptor channels depends on subunit composition. Science. 1991;252:851–853. doi: 10.1126/science.1709304. [DOI] [PubMed] [Google Scholar]
- Huettner JE, Bean BP. Block of N-methyl-d-aspartate-activated current by the anticonvulsant MK-801: selective binding to open channels. Proc Natl Acad Sci USA. 1988;85:1307–1311. doi: 10.1073/pnas.85.4.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaac JT, Crair MC, Nicoll RA, Malenka RC. Silent synapses during development of thalamocortical inputs. Neuron. 1997;18:269–280. doi: 10.1016/s0896-6273(00)80267-6. [DOI] [PubMed] [Google Scholar]
- Jia Z, Agopyan N, Miu P, Xiong Z, Henderson J, Gerlai R, Taverna FA, Velumian A, MacDonald J, Carlen P, Abramow-Newerly W, Roder J. Enhanced LTP in mice deficient in the AMPA receptor GluR2. Neuron. 1996;17:945–956. doi: 10.1016/s0896-6273(00)80225-1. [DOI] [PubMed] [Google Scholar]
- Ju W, Morishita W, Tsui J, Gaietta G, Deerinck TJ, Adams SR, Garner CC, Tsien RY, Ellisman MH, Malenka RC. Activity-dependent regulation of dendritic synthesis and trafficking of AMPA receptors. Nat Neurosci. 2004;7:244–253. doi: 10.1038/nn1189. [DOI] [PubMed] [Google Scholar]
- Kamboj SK, Swanson GT, Cull-Candy SG. Intracellular spermine confers rectification on rat calcium-permeable AMPA and kainate receptors. J Physiol. 1995;486:297–303. doi: 10.1113/jphysiol.1995.sp020812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasparov S, Paton JF. Changes in baroreceptor vagal reflex performance in the developing rat. Pflugers Arch. 1997;434:438–444. doi: 10.1007/s004240050418. [DOI] [PubMed] [Google Scholar]
- Katz LC, Shatz C. Synaptic activity and the construction of cortical circuits. Science. 1996;274:1133–1138. doi: 10.1126/science.274.5290.1133. [DOI] [PubMed] [Google Scholar]
- Kessler JP, Baude A. Distribution of AMPA receptor subunits GluR1–4 in the dorsal vagal complex of the rat: a light and electron microscope immunocytochemical study. Synapse. 1999;34:55–67. doi: 10.1002/(SICI)1098-2396(199910)34:1<55::AID-SYN7>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Kim J, Alger BE. Random response fluctuations lead to spurious paired-pulse facilitation. J Neurosci. 2001;21:9608–9618. doi: 10.1523/JNEUROSCI.21-24-09608.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohler M, Burnashev N, Sakmann B, Seeburg PH. Determinants of Ca2+ permeability in both TM1 and TM2 of high affinity kainate receptor channels: Diversity by RNA editing. Neuron. 1993;10:491–500. doi: 10.1016/0896-6273(93)90336-p. [DOI] [PubMed] [Google Scholar]
- Koike M, Iino M, Osawa S. Blocking effect of 1-naphthyl acetyl spermine on Ca2+-permeable AMPA receptors in cultured rat hippocampal neurons. Neuroscience Res. 1997;29:27–36. doi: 10.1016/s0168-0102(97)00067-9. [DOI] [PubMed] [Google Scholar]
- Kumar SS, Bacci A, Kharazia V, Huguenard JR. A developmental switch of AMPA receptor subunits in neocortical pyramidal neurons. J Neurosci. 2002;22:3005–3015. doi: 10.1523/JNEUROSCI.22-08-03005.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachamp P, Balland B, Tell F, Baude A, Strube C, Crest M, Kessler JP. Early expression of AMPA receptors and lack of NMDA receptors in developing rat climbing fibre synapses. J Physiol. 2005;564:751–763. doi: 10.1113/jphysiol.2005.084517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachamp P, Balland B, Tell F, Crest M, Kessler JP. Synaptic localization of the glutamate receptor subunit GluR2 in the rat nucleus tractus solitarii. Eur J Neurosci. 2003;17:892–896. doi: 10.1046/j.1460-9568.2003.02494.x. [DOI] [PubMed] [Google Scholar]
- Lachamp P, Crest M, Kessler JP. Vesicular glutamate transporters type 1 and 2 expression in axon terminals of the rat nucleus of the solitary tract. Neuroscience. 2006;137:73–81. doi: 10.1016/j.neuroscience.2005.08.048. [DOI] [PubMed] [Google Scholar]
- Lachamp P, Tell F, Kessler JP. Successive episodes of synapses production in the developing rat nucleus tractus solitarii. J Neurobiol. 2002;52:336–342. doi: 10.1002/neu.10091. [DOI] [PubMed] [Google Scholar]
- Liao D, Zhang X, O'Brien R, Ehlers MD, Huganir RL. Regulation of morphological postsynaptic silent synapses in developing hippocampal neurons. Nat Neurosci. 1999;2:37–43. doi: 10.1038/4540. [DOI] [PubMed] [Google Scholar]
- Liu SJ, Cull-Candy SG. Synaptic activity at calcium-permeable AMPA receptors induces a switch in receptor subtype. Nature. 2000;405:454–458. doi: 10.1038/35013064. [DOI] [PubMed] [Google Scholar]
- Luthi A, Schwyzer L, Mateos JM, Gahwiler BH, McKinney RA. NMDA receptor activation limits the number of synaptic connections during hippocampal development. Nat Neurosci. 2001;4:1102–1117. doi: 10.1038/nn744. [DOI] [PubMed] [Google Scholar]
- Mahanty NK, Sah P. Calcium-permeable AMPA receptors mediate long-term potentiation in interneurons in the amygdala. Nature. 1998;394:683–687. doi: 10.1038/29312. [DOI] [PubMed] [Google Scholar]
- Malinow R, Malenka RC. AMPA receptor trafficking and synaptic plasticity. Ann Rev Neurosci. 2002;25:103–126. doi: 10.1146/annurev.neuro.25.112701.142758. [DOI] [PubMed] [Google Scholar]
- Meng Y, Zhang Y, Jia Z. Synaptic transmission and plasticity in the absence of AMPA glutamate receptor GluR2 and GluR3. Neuron. 2003;39:163–176. doi: 10.1016/s0896-6273(03)00368-4. [DOI] [PubMed] [Google Scholar]
- Mi R, Tang X, Sutter R, Xu D, Worley P, O'Brien RJ. Differing mechanisms for glutamate receptor aggregation on dendritic spines and shafts in cultured hippocampal neurons. J Neurosci. 2002;22:7606–7616. doi: 10.1523/JNEUROSCI.22-17-07606.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AJ, McKoon M, Pinneau M, Silverstein R. Postnatal synaptic development of the nucleus tractus solitarius (NTS) of the rat. Dev Brain Res. 1983;8:205–213. doi: 10.1016/0165-3806(83)90005-6. [DOI] [PubMed] [Google Scholar]
- Moss IR. Maturation of respiratory control in the behaving mammal. Respir Physiol Neurobiol. 2002;132:131–144. doi: 10.1016/s1569-9048(02)00070-8. [DOI] [PubMed] [Google Scholar]
- O'Brien RJ, Mammen AL, Blackshaw S, Ehlers MD, Rothstein JD, Huganir RL. The development of excitatory synapses in cultured spinal neurons. J Neurosci. 1997;17:7339–7350. doi: 10.1523/JNEUROSCI.17-19-07339.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien RJ, Xu D, Mi R, Tang X, Hopf C, Worley P. Synaptically targeted Narp plays an essential role in the aggregation of AMPA receptors at excitatory synapses in cultured spinal neurons. J Neurosci. 2002;22:4487–4498. doi: 10.1523/JNEUROSCI.22-11-04487.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien RJ, Xu D, Petralia RS, Steward O, Huganir RL, Worley P. Synaptic clustering of AMPA receptors by the extracellular immediate-early gene product Narp. Neuron. 1999;23:309–323. doi: 10.1016/s0896-6273(00)80782-5. [DOI] [PubMed] [Google Scholar]
- Pickard L, Noel J, Henley JM, Collingridge GL, Molnar E. Developmental changes in synaptic AMPA and NMDA receptor distribution and AMPA receptor subunit composition in living hippocampal neurons. J Neurosci. 2000;20:7922–7931. doi: 10.1523/JNEUROSCI.20-21-07922.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plant K, Pelkey KA, Bortolotto ZA, Morita D, Terashima A, McBain CJ, Collingridge GL, Isaac JTR. Transient incorporation of native Glur2-lacking AMPA receptors during hippocampal long-term potentiation. Nature Neuroscience. 2006;9:602–604. doi: 10.1038/nn1678. [DOI] [PubMed] [Google Scholar]
- Rinaman L. Postnatal development of hypothalamic inputs to the dorsal vagal complex in rats. Physiol Behav. 2003;79:65–70. doi: 10.1016/s0031-9384(03)00105-7. [DOI] [PubMed] [Google Scholar]
- Rinaman L, Levitt P. Establishment of vagal sensorimotor circuits during fetal development in rats. J Neurobiol. 1993;24:641–659. doi: 10.1002/neu.480240509. [DOI] [PubMed] [Google Scholar]
- Rohrbough J, Spitzer NC. Ca2+-permeable AMPA receptors and spontaneous presynaptic transmitter release at developing excitatory spinal synapses. J Neurosci. 1999;19:8528–8541. doi: 10.1523/JNEUROSCI.19-19-08528.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnell E, Sizemore M, Karimzadegan S, Chen L, Bredt DS, Nicoll RA. Direct interactions between PSD-95 and stargazin control synaptic AMPA receptor number. Proc Natl Acad Sci USA. 2002;99:13902–13907. doi: 10.1073/pnas.172511199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson GT, Kamboj SK, Cull-Candy SG. Single-channel properties of recombinant AMPA receptors depend on RNA editing, splice variation, and subunit composition. J Neurosci. 1997;17:58–69. doi: 10.1523/JNEUROSCI.17-01-00058.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traynelis SF, Silver RA, Cull-Candy SG. Estimated conductance of glutamate receptor channels activated during EPSCs at the cerebellar mossy fiber–granule cell synapse. Neuron. 1993;11:279–289. doi: 10.1016/0896-6273(93)90184-s. [DOI] [PubMed] [Google Scholar]
- Varoqueaux F, Sigler A, Rhee J, Brose N, Enk C, Reim K, Rosenmund C. Total arrest of spontaneous and evoked synaptic transmission but normal synaptogenesis in the absence of Munc13-mediated vesicle priming. Proc Natl Acad Sci USA. 2002;99:9037–9042. doi: 10.1073/pnas.122623799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent A, Kessler JP, Baude A, Dipasquale E, Tell F. N-methyl-d-aspartate receptor activation exerts a dual control on postnatal development of nucleus tractus solitarii neurons in vivo. Neuroscience. 2004;126:185–194. doi: 10.1016/j.neuroscience.2004.03.012. [DOI] [PubMed] [Google Scholar]
- Vincent A, Tell F. Postnatal development of rat nucleus tractus solitarius neurons: morphological and electrophysiological evidence. Neuroscience. 1999;93:293–305. doi: 10.1016/s0306-4522(99)00109-8. [DOI] [PubMed] [Google Scholar]
- Washburn MS, Dingledine R. Block of alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors by polyamines and polyamine toxins. J Pharmacol Exp Ther. 1996;278:669–678. [PubMed] [Google Scholar]
- Washburn MS, Numberger M, Zhang S, Dingledine R. Differential dependence on GluR2 expression of three characteristic features of AMPA receptors. J Neurosci. 1997;17:9393–9406. doi: 10.1523/JNEUROSCI.17-24-09393.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G, Malinow R, Cline HT. Maturation of a central glutamatergic synapse. Science. 1996;274:972–976. doi: 10.1126/science.274.5289.972. [DOI] [PubMed] [Google Scholar]
- Zamanillo D, Sprengel R, Hvalby O, Jensen V, Burnashev N, Rozov A, Kaiser KM, Koster HJ, Borchardt T, Worley P, Lubke J, Frotscher M, Kelly PH, Sommer B, Andersen P, Seeburg PH, Sakmann B. Importance of AMPA receptors for hippocampal synaptic plasticity but not for spatial learning. Science. 1999;284:1805–1811. doi: 10.1126/science.284.5421.1805. [DOI] [PubMed] [Google Scholar]
- Zhang LL, Ashwell KW. The development of cranial nerve and visceral afferents to the nucleus of the solitary tract in the rat. Anat Embryol. 2001;204:135–151. doi: 10.1007/s004290100185. [DOI] [PubMed] [Google Scholar]
- Zhu JJ, Esteban JA, Hayashi Y, Malinow R. Postnatal synaptic potentiation: delivery of GluR4-containing AMPA receptors by spontaneous activity. Nat Neurosci. 2000;3:1098–1106. doi: 10.1038/80614. [DOI] [PubMed] [Google Scholar]
- Zhu JJ, Malinow R. Acute versus chronic NMDA receptor blockade and synaptic AMPA receptor delivery. Nat Neurosci. 2002;5:513–514. doi: 10.1038/nn0602-850. [DOI] [PubMed] [Google Scholar]