Abstract
Cardiac troponin T (cTnT) is an essential component of the thin filament regulatory unit (RU) that regulates Ca2+ activation of tension in the heart muscle. Because there is coupling between the RU and myosin crossbridges, the functional outcome of cardiomyopathy-related mutations in cTnT may be modified by the type of myosin heavy chain (MHC) isoform. Ca2+ activation of tension and ATPase activity were measured in muscle fibres from normal rat hearts containing α-MHC isoform and propylthiouracil (PTU)-treated rat hearts containing β-MHC isoform. Muscle fibres from normal and PTU-treated rat hearts were reconstituted with two different mutations in rat cTnT; the deletion of Glu162 (cTnTE162DEL) and the deletion of Lys211 (cTnTK211DEL). α-MHC and β-MHC isoforms had contrasting impact on tension-dependent ATP consumption (tension cost) in cTnTE162DEL and cTnTK211DEL reconstituted muscle fibres. Significant increases in tension cost in α-MHC-containing muscle fibres corresponded to 17% (P < 0.01) and 23% (P < 0.001) when reconstituted with cTnTE162DEL and cTnTK211DEL, respectively. In contrast, tension cost decreased when these two cTnT mutants were reconstituted in muscle fibres containing β-MHC; by approximately 24% (P < 0.05) when reconstituted with cTnTE162DEL and by approximately 17% (P = 0.09) when reconstituted with cTnTK211DEL. Such differences in tension cost were substantiated by the mechano-dynamic analysis of cTnT mutant reconstituted muscle fibres from normal and PTU-treated rat hearts. Our observation demonstrates that qualitative changes in MHC isoform alters the nature of cardiac myofilament dysfunction induced by mutations in cTnT.
Cardiac troponin T (cTnT) is a subunit of the troponin (Tn) complex, which binds Ca2+ in the heart muscle. cTnT plays an important role in regulating Ca2+-activated tension by interacting with tropomyosin (Tm) and other thin filament regulatory proteins (Gordon et al. 2000). Several mutations in human cTnT (hcTnT) are known to be causal in familial hypertrophic cardiomyopathy (FHC) (Gomes & Potter, 2004; Tardiff, 2005). Two of these mutations in hcTnT are the deletion of Glu160 (hcTnTE160DEL) and the deletion of Lys210 (hcTnTK210DEL). hcTnTE160DEL mutation leads to ventricular hypertrophy and incidences of sudden death (Watkins et al. 1995) and the cTnTK210DEL mutation causes an early onset of ventricular dilatation and diminished contractile function, and frequently causes heart failure (Kamisago et al. 2001). The impact of mutations on the sequence of events that eventually lead to heart failure is not well understood. This issue takes on new significance in view of our recent findings that cTnT participates in regulating the dynamics of crossbridge (XB) cycling kinetics (Chandra et al. 2006), which suggests that mutations may interfere with important functions of cTnT.
A complication in the interpretation of some of the previous mutation studies is that such studies were undertaken with the use of the transgenic mouse (TG) that expressed a specific mutated sarcomeric gene in the heart. Although TG mouse models of FHC will continue to play an important role in the study of heart failure, some inherent limitations in the use of the mouse must always be noted (Kass et al. 1998). Many of the determinants of myocardial contractility in the rapidly contracting small ventricles of mouse hearts are significantly different from those of larger mammals (Li et al. 1997; Bers, 2000; Rice et al. 2000; Georgakopoulos & Kass, 2001; Stull et al. 2002). At the myofilament level, one of the major differences between the hearts of smaller and larger mammals is in the type of force generator, myosin heavy chain (MHC), present in the thick filament. Hearts of smaller animals contain predominantly the fast cycling α-MHC isoform, whereas the hearts of larger animals express the slow cycling β-MHC isoform (McNally et al. 1989). Given that the kinetic properties of MHC isoforms are the major determinants of the dynamic properties of left ventricular function, it is not surprising that the heart fails to adapt in certain forms of heart disease when the ratio of these two functionally diverse MHC isoforms is altered (Dillmann, 1980; Swynghhedauw, 1986; Miyata et al. 2000).
Coupling between the mechanical cycle (heart rate) and biochemical processes controlled by MHC and thin filament regulatory proteins (Rouslin & Broge, 1996; Campbell et al. 2004; Chandra et al. 2006) suggests an important link between myocardial contractility and heart muscle adaptation. This is consistent with the experimental observation that the spontaneous heart rate decreased significantly when the slower cycling β-MHC isoform was expressed in the mouse heart (Tardiff et al. 2000). A small increase in the level of β-MHC in the TG mouse hearts led to maladaptation of the heart as indicated by a significant systolic dysfunction (Tardiff et al. 2000), whereas a small increase in α-MHC in rat cardiac myocytes augmented power output (Herron & McDonald, 2002). Expression of nearly 40% of fast cycling α-MHC in rabbit hearts conferred protection against experimentally induced tachycardia (James et al. 2005).
Functional coupling between the thin filament regulatory unit (RU; Tm–Tn), and force-bearing crossbridges (XBs) suggest that the left ventricular function may be modulated by the RU through an impact on XB kinetics (Razumova et al. 2000). Thus, the manner in which the heart adapts to changes in contractility depends not only on MHC isoform, but also on changes in the composition of the RU. Whether or not the mutation-dependent triggers in the thin filament are affected by differences in MHC isoforms is the focus of this study. Our study demonstrates that the consequence of a mutation in cTnT is dependent on the type of MHC isoform.
Methods
PCR mutagenesis
Full-length cDNA clones for adult rat cardiac troponin I (cTnI) and rat cardiac troponin C (cTnC) were isolated, as previously described (Chandra et al. 2006). Adult rat cTnT DNA (Jin & Lin, 1989) was a gift from Dr J. J. Lin (University of Iowa, IA, USA). All rat cTnT DNA constructs used in this study were tagged with the human c-myc epitope (Tardiff et al. 1998; Chandra et al. 2001). Amino acids Glu162 (E162) and Lys211 (K211) in our rat cTnT sequence correspond to E160 and K210 in the human cTnT sequence, respectively. To delete the codons for E162 and K211 in the rat cTnT DNA clone, we used a standard PCR mutagenesis protocol (Hi-fidelity, Roche). Two separate PCR reactions were carried out. Reaction 1 in each case was designed to amplify the DNA fragment from the 5′-end of the cTnT sequence to the E162 or the K211 region of cTnT and reaction 2 in each case was designed to amplify the DNA fragment from the E162 or the K211 region of cTnT to the 3′-end of the cTnT sequence. The 3′-end of PCR product from reaction 1 had a sequence overlap of 30 nucleotides at the 5′-end of the PCR product from reaction 2. Oilgonucleotide primers for the first set of cTnTE162DEL mutagenesis are as follows. Primer 1, 5′-GCAGAATTCAGGCATATGGAGCAGAAGCTGATCTCCGAGGAGGACCTGTCTGACGCCGAGGAAGAGGTG-3′; primer 2, 5′-CCTGTTCTCCTCCTCACGCCGGGCCCTCTC-3′. Oilgonucleotide primers for the second set of cTnTE162DEL mutagenesis are as follows. Primer 1, 5′-GAGAGGGCCCGGCGTGAGGAGGAGAACAGG-3′; primer 2, 5′-TGCTGGAATTCAGGATCCCTATTTCCAACGCCCGGTGACTTT-3′. Oilgonucleotide primers for the first set of cTnTK211DEL mutagenesis are as follows. Primer 1, 5′-GCAGAATTCAGGCATATGGAGCAGAAGCTGATCTCCGAGGAGGACCTGTCTGACGCCGAGGAAGAGGTG-3′; primer 2, 5′-CCTCTCTGCCAGAATCTTCTTCTTCTCTCG-3′. Oilgonucleotide primers for the second set of cTnTK211DEL mutagenesis are as follows. Primer 1, 5′-CGAGAGAAGAAGAAGATTCTGGCAGAGAGG-3′; primer 2, 5′-TGCTGGAATTCAGGATCCCTATTTCCAACGCCCGGTGACTTT-3′. PCR-amplified DNA fragments from reactions 1 and 2 were gel-purified, mixed in equal proportions and the full-length cTnT products were amplified in the final PCR using appropriate 3′ and 5′ oligonucleotide primers. Final PCR products were digested with Nde I-BamH I restriction enzymes and subcloned into the pSBETa expression vector. Clones containing proper DNA inserts were sequenced.
Expression and purification of recombinant rat cardiac troponin subunits
Recombinant rat cTnT, mutant rat cTnT, rat cTnC and rat cTnI (all in pSBETa plasmid DNA) were expressed in BL21 (DE3) cells (Novagen) and purified as previously described (Chandra et al. 2006). All pure protein fractions were extensively dialysed against deionized water containing 15 mm β-mercaptoethanol, lyophilized and stored at −80°C.
Reconstitution of recombinant rat cTnT into detergent-skinned rat cardiac muscle fibre bundles
All procedures were performed in accordance with the guidelines laid down by the Washington State University Institutional Animal Care and Use Committee. Sprague-Dawley rats were anaesthetized with sodium pentobarbital (50 mg kg−1 body weight), the hearts were rapidly excised and placed into ice-cold, high-relaxing solution containing (mm): MOPS 20 (pH 7.0), KCl 53, EGTA 10, MgCl2 6.81, Na2ATP 5.35 and DTT 1.0. The total ionic strength was 150 mm. A cocktail of protease inhibitors containing 4 μm benzamidine-HCl, 5 μm bestatin, 2 μm E-64, 10 μm leupeptin, 1 μm pepstatin and 200 μm phenylmethylsulfonyl fluoride were included in the buffer. Young adult Sprague-Dawley rats were treated with propylthiouracil (PTU) in the drinking water (0.6 g l−1) for 4–5 weeks. Shift from α- to β-MHC isoform expression in PTU-treated rat heart is well documented (Pope et al. 1980; Metzger et al. 1999; Herron et al. 2001; Rundell et al. 2004). Left ventricular papillary muscle fibre bundles from normal and PTU-treated rat hearts were isolated, dissected and detergent-skinned as previously described (Chandra et al. 2001). Exchange of rat muscle endogenous Tn complex with rat recombinant Tn complex containing mutant cTnT was based on the method previously described (Chandra et al. 2006). In brief, detergent-skinned muscle fibres were treated with the extraction solution containing cTnT and cTnI for approximately 3–4 h at room temperature (22°C) with stirring. The extraction buffer contained (mm): BES 50 (pH 7.0 at 20°C), KCl 180, 2,3-Butanedione monoxime (BDM) 10, EGTA 5, MgCl2 6.27, DTT 1.0 and MgATP2− 5, as well as 0.01% NaN3 and a cocktail of protease inhibitors. After washing muscle fibres with the extraction buffer, Ca2+-activated maximal tension was measured in solution at −log of free Ca2+ concentration (pCa) 4.3 to determine the residual tension. After reconstitution with cTnC (3 mg ml−1), Ca2+-activated tension and ATPase activity were measured at various pCa levels. All other details of reconstitution procedure are as previously described (Chandra et al. 2006). The composition of solutions of different pCa was calculated using the methods described by Fabiato & Fabiato (1979).
Measurement of tension-dependent ATP consumption in reconstituted rat cardiac muscle fibre bundles
Tension and ATPase activity were measured simultaneously at 20°C using the system previously described by Stienen et al. (1995) and de Tombe & Stienen (1995). Maximum activation buffer (pCa 4.3) contained (mm): potassium propionate 31, Na2ATP 5.95, MgCl2 6.61, EGTA 10, CaCl2 10.11, BES 50 (pH 7.0), NaN3 10, NADH 0.9 and phosphoenol pyruvate 10, with 4 mg ml−1 pyruvate kinase (500 U mg−1), 0.24 mg ml−1 lactate dehydrogenase (870 U mg−1) and 20 μm Diadenosine-5′ pentaphosphate (A2P5) as well as a cocktail of protease inhibitors. The ionic strength of the buffer was 180 mm. Detergent-skinned muscle fibre was attached to a motor and a force transducer using aluminium clips. Sarcomere length was measured, as previously described (de Tombe & Stienen, 1995). The resting sarcomere length was readjusted to 2.2 μm (after 2–3 cycles of full activation and relaxation) and the resting sarcomere length monitored using a He-Ne laser diffraction system. For ATPase measurements, near UV light (340 nm) was projected through the muscle chamber just below the muscle fibre, then split via a beam splitter (50/50) and detected at 340 nm (sensitive to change in [NADH]) and 400 nm (insensitive to [NADH]). The light intensity at 400 nm served as a reference signal. An analog divider and log amplifier produced a signal proportional to the amount of ATP consumed in the muscle chamber solution. ATP regeneration from ADP was coupled by enzymatic reactions as previously described (de Tombe & Stienen, 1995). These measurements allowed us to determine the tension cost in reconstituted muscle fibres (Campbell et al. 2004; Chandra et al. 2006).
Measurement of the rate constant of XB distortion in reconstituted rat cardiac muscle fibre bundles
XB distortion dynamics were determined in reconstituted muscle fibres as previously described (Campbell et al. 2004; Chandra et al. 2006). Previously, we have demonstrated a strong correlation between the rate constant of XB distortion (c) and the experimentally measured values of tension cost (Campbell et al. 2004; Chandra et al. 2006). The rate constant of XB distortion was determined (pCa 4.3) using buffer conditions as described for tension/ATPase measurements. In brief, muscle length-perturbation episodes of continuously varying sinusoidal frequencies (chirps) were delivered over time a period of 5 s to provide force and length information at frequencies between 1.0 and 40 Hz. Muscle fibre length (LM) was commanded to change at constant amplitude (0.5% of LM) during chirp perturbations and the resultant changes in force (ΔF) were measured. Experimentally measured change in force was fitted to a recruitment–distortion model to derive the rate constant of XB distortion. The differential equations and all other description of the model are as previously described (Campbell et al. 2004). In previous studies (Campbell et al. 2004; Chandra et al. 2006), the model was shown to fit the data well leaving very little residual error (R2 > 0.98) and the values of c were estimated with less than 1% error.
Polyacrylamide gel electrophoresis
Protein samples for gel electrophoresis and Western blot analysis were prepared and run on 12.5% SDS-polyacrylamide gels, as previously described (Chandra et al. 1999b). For Western blot analysis, proteins were transferred onto the Polyvinylidene fluoride (PVDF) membrane and probed using an anti-mouse primary antibody against the human c-myc epitope or an antibody against hcTnT as previously described (Tardiff et al. 1998; Montgomery et al. 2001). MHC from normal and PTU-treated rat heart muscle preparations were separated on 8% SDS-polyacrylamide gels, as previously described (Rundell et al. 2004).
Data analysis
Data from the normalized pCa–tension measurements were fitted to the Hill equation using a non-linear least-square regression procedure to obtain the pCa50 (−log of free Ca2+ concentration required for half maximal activation) and the Hill coefficient (n). pCa50 and n were determined separately from each muscle fibre experiment and the values averaged. pCa50 values were converted to [Ca2+] to calculate the percentage increase or decrease in Ca2+ sensitivity of mutant cTnT reconstituted muscle fibres. Data were analysed using two-way ANOVA, with one factor being mutation (cTnTE162DEL, cTnTK211DEL or cTnT) and the other factor being PTU treatment (control or PTU-treated). The interaction effect was evaluated to test the hypothesis that PTU treatment (i.e. change in MHC composition) altered the effect of mutation in cTnT. Subsequent post hoc multiple comparisons using a Holm-Bonferroni corrected t test (Glantz, 2002) were then conducted as indicated, to compare each mutation with wild-type cTnT. Statistical significance was assumed for P < 0.05. Data are expressed as means ± s.e.m.
Results
Shift from α- to β-MHC isoform expression in PTU-treated rat heart muscle fibres
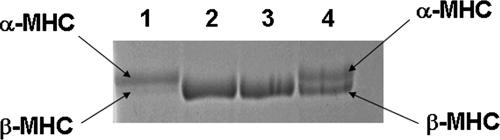
PTU treatment to induce changes in MHC isoform expression has been well studied in the past (Pope et al. 1980; Metzger et al. 1999; Herron et al. 2001; Rundell et al. 2004). Such previous studies have shown that the only demonstrable change in myofilament proteins is that of MHC isoform, which shifts from predominantly α- to β-MHC in rat hearts after 4–5 weeks of treatment with PTU. To ascertain the shift in MHC isoform expression, we used a recently described method for the separation of MHC isoforms on SDS-PAGE (Rundell et al. 2004). Figure 1 demonstrates that there is a complete shift from α- to β-MHC isoform in the PTU-treated rat heart muscle preparations. The predominant MHC isoform present in normal rat heart preparations is α-MHC (lane 1 in Fig. 1), which shifts to nearly 100% β-MHC in rat hearts treated with PTU (lanes 2 and 3 in Fig. 1).
Figure 1. SDS-PAGE (8%) analysis of rat cardiac muscle fibre proteins from normal and PTU-treated rat hearts.
Rats were made hypothyroid by feeding with tap water containing 0.6 g l−1 PTU for approximately 4–5 weeks. SDS-PAGE was performed as previously described (Rundell et al. 2004). Lane 1, muscle protein preparation from normal rat heart; lanes 2 and 3, muscle protein preparations from two different PTU-treated rat hearts; lane 4, mixture of normal and PTU-treated rat heart muscle preparations to highlight the size differences between α- and β-MHC isoforms. Lanes 2 and 3 demonstrates a complete shift to β-MHC isoform in PTU-treated rat heart muscle preparations. SDS gels were run at least once and stained with Coomassie blue as described before (Rundell et al. 2004).
Exchange of mutant rat cardiac Tn into detergent-skinned rat cardiac muscle fibre bundles with different MHC isoforms
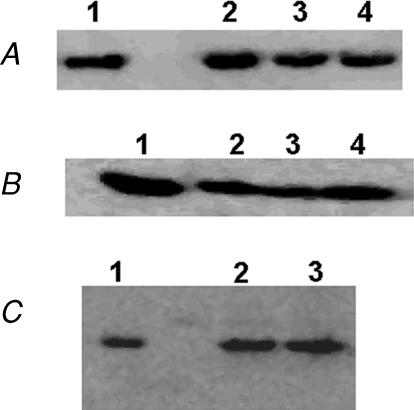
All cTnT constructs used in this study were tagged with the human c-myc epitope (Tardiff et al. 1998; Chandra et al. 2001). Tn exchange in rat cardiac muscle fibre bundles was performed as previously described (Chandra et al. 2006). We have previously demonstrated that the endogenous Tn complex is removed as a whole (Chandra et al. 1999a, 2006) by the exogenously added cTnT. To demonstrate the incorporation of wild-type cTnT (WT-cTnT) and mutant cTnT constructs in muscle fibres from normal and PTU-treated rat hearts, Western blot (Fig. 2A and B) was performed with the antibody against human c-myc epitope. In Fig. 2A and B, immunoreactivity is evident in lanes 2, 3 and 4, which correspond to muscle fibres reconstituted with WT-cTnT, cTnTE162DEL and cTnTK211DEL, respectively. As we have demonstrated previously, little or no immunoreactivity was evident (data not shown) when these reconstituted muscle fibres were probed with the antibody against cTnT, which demonstrated that the endogenous cTnT was replaced by c-myc-tagged cTnT. Furthermore, when these WT-cTnT/mutants + cTnI reconstituted muscle fibres were tested in pCa 4.3 solution, Ca2+-activated residual tension was minimal (∼1.0 mN mm−2). We have previously demonstrated that the Tn exchange protocol had no major impact on myofilament Ca2+ sensitivity, cooperativity, Ca2+-activated maximal tension and ATPase activity (Chandra et al. 2006). As previously observed by other investigators (Saggin et al. 1988; Fitzsimons et al. 1998; Metzger et al. 1999), Western blot analysis of heart muscle fibre preparations from normal and PTU-treated rat hearts (Fig. 2C) showed no shift in cTnT isoform expression.
Figure 2. Western blot analysis of rat cardiac muscle fibres reconstituted with recombinant c-myc-tagged cTnT constructs.
An antibody against either human c-myc epitope or human cTnT was used. Western blot analysis of reconstituted muscle fibres from normal rat hearts with antibody against human c-myc epitope (A), from PTU-treated rat hearts with antibody against human c-myc epitope (B) and from normal and PTU-treated rat hearts with antibody against hcTnT (C). A and B, lane identifications are as follows: lane 1, pure recombinant c-myc WT-cTnT; lane 2, muscle fibres reconstituted with c-myc WT-cTnT + cTnI + cTnC; lane 3, muscle fibres reconstituted with c-myc cTnTE162DEL + cTnI + cTnC; lane 4, muscle fibres reconstituted with c-myc cTnTK211DEL + cTnI + cTnC. No immunoreactivity was evident (data not shown) for the endogenous cTnT in reconstituted muscle fibres from both normal and PTU-treated rat cardiac muscle fibres. C, lane 1, pure recombinant cTnT; lane 2, muscle fibres from normal rat heart; lane 3, muscle fibres from PTU-treated rat heart. Lanes 2 and 3 demonstrate that there is only one adult cTnT isoform in these adult rat heart muscle fibres. Typically, Western blot was performed once or twice to detect different forms of cTnT.
Impact of cTnTE162DEL and cTnTK211DEL mutants on muscle fibre bundles from normal and PTU-treated rat hearts
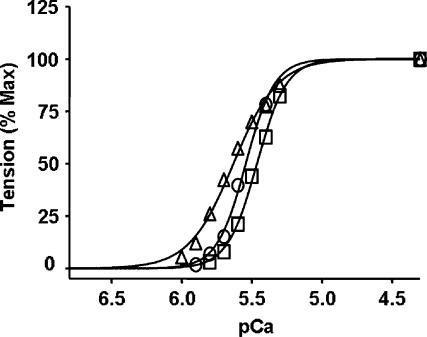
In reconstitution experiments, we used c-myc-tagged WT-cTnT as the control. In previous studies, we and others have demonstrated that the presence of c-myc epitope in cTnT has no significant effect on Ca2+ activation of tension and ATPase activity in detergent-skinned muscle preparations (Chandra et al. 2001; Montgomery et al. 2001) or intact heart function (Tardiff et al. 1998; Ertz-Berger et al. 2005). In the first line of experiments, the effects of WT-cTnT, cTnTE162DEL and cTnTK211DEL on Ca2+ activation of myofilaments were tested in cardiac muscle fibres from normal rat hearts, which contained predominantly the α-MHC isoform. When compared to WT-cTnT + cTnI + cTnC reconstituted muscle fibres, myofilament Ca2+ sensitivity, as measured by pCa50 values, increased significantly (23%) in cTnTE162DEL + cTnI + cTnC reconstituted muscle fibres and decreased significantly (23%) in cTnTK211DEL + cTnI + cTnC reconstituted muscle fibres (Fig. 3 and Table 1).
Figure 3. Normalized pCa–tension relations in reconstituted muscle fibres from normal rat hearts.
Ca2+-activated tension was measured at different pCa as described in the Methods for WT-cTnT + cTnI + cTnC (○), cTnTE162DEL + cTnI + cTnC (▵) and cTnTK211DEL + cTnI + cTnC (□). pCa50 and the Hill coefficient values (n) are listed in Table 1. Standard error bars are smaller than symbols. Number of determinations is at least eight for each.
Table 1. Normalized pCa–tension relationship in detergent-skinned rat heart muscle fibre bundles reconstituted with recombinant c-myc-tagged rat cTnT constructs.
| WT-cTnT | E162DEL | K211DEL | |
|---|---|---|---|
| Normal rat hearts | |||
| ″pCa50 | 5.55 ± 0.01 | 5.64 ± 0.02* | 5.46 ± 0.02* |
| ″n | 3.9 ± 0.2 | 2.8 ± 0.1* | 4.2 ± 0.2 |
| PTU-treated rat hearts | |||
| ″pCa50 | 5.54 ± 0.02 | 5.66 ± 0.03* | 5.47 ± 0.03* |
| ″n | 4.1 ± 0.2 | 3.1 ± 0.1* | 4.5 ± 0.3 |
Values are means ± s.e.m. Data from normalized pCa–tension measurements were fitted to the Hill equation by using a non-linear least square regression procedure to derive pCa50 and the Hill coefficient (n). pCa50 and n were determined separately from each muscle fibre experiment and the values averaged. WT-cTnT represents WT-cTnT + cTnI + cTnC reconstituted muscle fibres; E162DEL represents cTnTE162DEL + cTnI + cTnC reconstituted muscle fibres and K211DEL represents cTnTK211DEL + cTnI + cTnC reconstituted muscle fibres. Number of determinations is at least eight for each. Statistical differences were analysed using two-way ANOVA as described in the Methods.
P < 0.05.
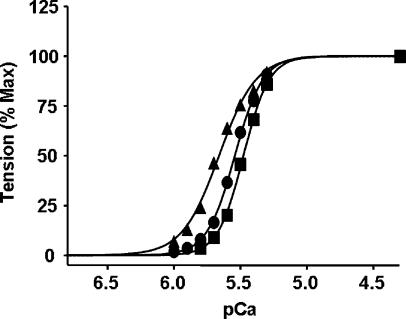
Next, we tested the effects of cTnTE162DEL and cTnTK211DEL on Ca2+ activation of cardiac muscle fibres from PTU-treated rat hearts, which contained nearly 100% β-MHC isoform. The impact of mutant cTnTs on Ca2+ sensitivity of muscle fibres containing β-MHC isoform (Fig. 4) was similar to that of muscle fibres from normal rat hearts which contained α-MHC isoform (Fig. 3). Ca2+ sensitivity of cTnTE162DEL + cTnI + cTnC reconstituted muscle fibres increased significantly (32%) and that of cTnTK211DEL + cTnI + cTnC reconstituted muscle fibres decreased significantly (17%). pCa50 values and the Hill coefficient values from the Hill fits are listed in Table 1. Data presented in Table 1 demonstrate that rat cardiac myofilament Ca2+ sensitivity is not MHC isoform dependent. cTnTE162DEL mutant decreased the Hill coefficient values in both α-MHC- and β-MHC-containing cardiac muscle fibres.
Figure 4. Normalized pCa–tension relations in reconstituted muscle fibres from PTU-treated rat hearts.
Ca2+-activated tension was measured at different pCa as described in the Methods for WT-cTnT + cTnI + cTnC (•), cTnTE162DEL + cTnI cTnC (▴) and cTnTK211DEL cTnI cTnC (▪). pCa50 and the Hill coefficient values (n) are listed in Table 1. Standard error bars are smaller than symbols. Number of determinations is at least eight for each.
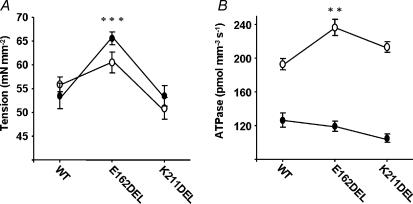
Compared to muscle fibres from normal rat hearts, Ca2+-activated maximal tension was not affected significantly in muscle fibres from PTU-treated rat hearts. In this study, both the interaction effect (P = 0.23) and the main effect for muscle fibres from normal versus PTU-treated groups (P = 0.33) were not significant (Fig. 5A). Our observation is substantiated by two independent previous studies which demonstrated that a shift from α- to β-MHC in rat cardiac muscle had no significant impact on Ca2+-activated maximal tension (Metzger et al. 1999; Rundell et al. 2004). However, the effect of cTnTE162DEL mutation on maximal tension was significant (P < 0.001). Ca2+-activated maximal tension increased significantly when muscle fibres containing either α-MHC or β-MHC were reconstituted with the cTnTE162DEL mutant (Fig. 5A). On the other hand, cTnTK211DEL mutant had no significant effect on Ca2+-activated maximal tension in muscle fibres containing either α-MHC or β-MHC (Fig. 5A).
Figure 5. Ca2+-activated maximal tension and ATPase activity in reconstituted muscle fibres from normal and PTU-treated rat hearts.
Ca2+-activated maximal tension (A) and ATPase activity (B) were measured in maximal activation buffer (pCa 4.3). For both A and B (○) represents experiments in muscle fibres from normal rat hearts and (•) represents experiments in muscle fibres from PTU-treated rat hearts. For both A and B, WT represents WT-cTnT + cTnI + cTnC reconstituted muscle fibres; E162DEL represents cTnTE162DEL + cTnI + cTnC reconstituted muscle fibres and K211DEL represents cTnTK211DEL + cTnI + cTnC reconstituted muscle fibres. Number of determinations is at least eight for each. Data were analysed using two-way ANOVA as described in the Methods. Subsequent post hoc multiple comparisons were made using a Holm-Bonferroni corrected t test (Glantz, 2002) to compare each mutation versus WT within PTU-treated and normal groups. **P < 0.01, ***P < 0.001.
In addition to the effect of cTnT mutants on myofilament Ca2+ sensitivity, another notable observation was the impact of cTnTE162DEL and cTnTK211DEL on Ca2+-activated maximal ATPase activity in muscle fibres containing α-MHC and β-MHC (Fig. 5B). The interaction effect for Ca2+-activated maximal ATPase activity was significant (P = 0.007), which indicated that β-MHC altered the effect of mutation in cTnT. ATPase activity increased when cTnTE162DEL and cTnTK211DEL were reconstituted into muscle fibres containing α-MHC (Fig. 5B). ATPase activity was approximately 23% higher (P < 0.001) in cTnTE162DEL reconstituted fibres and 11% higher (P = 0.2) in cTnTK211DEL reconstituted fibres. In contrast, there was a slight trend towards a decrease (non-significant) in Ca2+-activated maximal ATPase activity when these two cTnT mutants were reconstituted into muscle fibres containing β-MHC isoform (Fig. 5B). ATPase activity was approximately 7% lower (P = 0.2) for cTnTE162DEL reconstituted fibres and 18% lower (P = 0.5) for cTnTK211DEL reconstituted fibres.
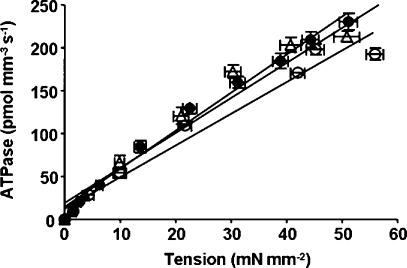
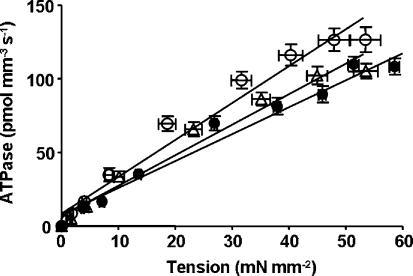
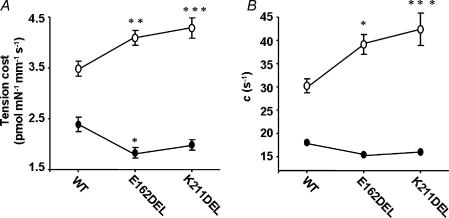
Because β-MHC altered the effect of mutation in cTnT on ATPase activity, we wanted to test whether α- and β-MHC isoforms have different effects on the mutation-induced impact on tension-dependent ATP consumption. Previous TG mouse studies have suggested a causative link between cTnT mutation-induced impact on altered cardiac muscle energetics and cardiac phenotypes (Montgomery et al. 2001; Javadpour et al. 2003; Chandra et al. 2005). We simultaneously measured steady-state isometric tension and ATPase activity at different pCa in reconstituted muscle fibres from normal and PTU-treated groups. Data were fitted using a linear regression analysis as shown in Figs 6 and 7. The slope of the tension–ATPase relationship is a measure of the amount of ATP hydrolysed for a given amount of tension produced (tension cost). Mean slope values from linear fits of several muscle fibre bundles reconstituted with WT-cTnT + cTnI + cTnC, cTnTE162DEL + cTnI + cTnC and cTnTK211DEL + cTnI + cTnC are summarized in Fig. 8A. The interaction effect for tension cost was significant (P = 0.0001), which demonstrated that β-MHC altered the effect of mutation in cTnT. Tension cost increased significantly by approximately 17% (P < 0.01) when α-MHC-containing muscle fibres were reconstituted with cTnTE162DEL and by approximately 23% (P < 0.001) when reconstituted with cTnTK211DEL. In contrast, tension cost decreased when these two cTnT mutants were reconstituted in muscle fibres containing β-MHC (Fig. 8A); by approximately 24% (P < 0.05) when reconstituted with cTnTE162DEL and by approximately 17% (P = 0.09, non-significant) when reconstituted with cTnTK211DEL.
Figure 6. Relationship between tension and ATPase activity in reconstituted rat cardiac muscle fibres from normal rat hearts.
Simultaneous measurement of tension and ATPase activity at different pCa are as described in the Methods. Averaged data from several experiments were fitted using a linear regression analysis. Departure from linearity was found to be non-significant in all cases. WT-cTnT + cTnI + cTnC reconstituted muscle fibres (○), R2 = 0.98; cTnTE162DEL + cTnI + cTnC reconstituted muscle fibres (•), R2 = 0.97; and cTnTK211DEL + cTnI + cTnC reconstituted muscle fibres (▵), R2 = 0.97. Comparison of mean slope values (tension cost) between reconstituted muscle fibres from normal and PTU-treated hearts are shown in Fig. 8A. Number of determinations is at least eight for each.
Figure 7. Relationship between tension and ATPase activity in reconstituted rat cardiac muscle fibres from PTU-treated rat hearts.
Simultaneous measurement of tension and ATPase activity at different pCa are as described in the Methods. Averaged data from several experiments were fitted using a linear regression analysis. Departure from linearity was found to be non-significant in all cases. WT-cTnT + cTnI + cTnC reconstituted muscle fibres (○), R2 = 0.95; cTnTE162DEL + cTnI + cTnC reconstituted muscle fibres (•), R2 = 0.96; and cTnTK211DEL + cTnI + cTnC reconstituted muscle fibres (▵), R2 = 0.97. Comparison of mean slope values (tension cost) between reconstituted muscle fibres from normal and PTU-treated hearts are shown in Fig. 8A. Number of determinations is at least eight for each.
Figure 8. α- and β-MHC-induced differences in tension cost and XB distortion rate constant (c).
Tension cost was determined (A) from the slopes of tension–ATPase relationships as shown in Figs 6 and 7. The rate constant of distortion (c) was determined (B) as described in the Methods. For A and B (○) represents muscle fibres from normal rat hearts and (•) represents muscle fibres from PTU-treated rat hearts. For both A and B, WT represents WT-cTnT + cTnI + cTnC reconstituted muscle fibres; E162DEL represents cTnTE162DEL + cTnI + cTnC reconstituted muscle fibres and K211DEL represents cTnTK211DEL + cTnI + cTnC reconstituted muscle fibres. Number of determinations is at least eight for each. Standard error bars are smaller than symbols in some cases. Data were analysed using two-way ANOVA as described in the Methods. Subsequent post hoc multiple comparisons were made using a Holm-Bonferroni corrected t test (Glantz, 2002) to compare each mutation versus WT within PTU-treated and normal groups. *P < 0.05, **P < 0.01, ***P < 0.001.
Impact of cTnTE162DEL and cTnTK211DEL mutants on the rate constant of XB distortion in muscle fibre bundles from normal and PTU-treated rat hearts
Results from the XB distortion dynamic measurements allowed us to validate the changes in tension cost as described above. XB distortion rate constant (c), estimated from model fitting, has a strong dependence on the kinetics of XB detachment. In previous studies (Campbell et al. 2004; Chandra et al. 2006), we have demonstrated that there is a strong correlation between c and the experimentally measured values of tension cost (ATPase/tension). Because the ratio of ATPase/tension is proportional to the rate constant of XB detachment (Brenner & Eisenberg, 1986), changes in the model-estimated c and tension cost can be correlated with changes in XB detachment kinetics.
It is interesting to note that model-estimated values of c varied in a manner similar to the experimentally derived values of tension cost (Fig. 8). The interaction effect for c was significant (P = 0.017), which demonstrated that β-MHC altered the effect of mutation in cTnT. For example, c increased significantly in α-MHC-containing normal muscle fibres by approximately 29% (P < 0.02) when reconstituted with cTnTE162DEL and by approximately 43% (P < 0.001) when reconstituted with cTnTK211DEL (Fig. 8A). In contrast, there was a small but non-significant decrease in c when these two cTnT mutants were reconstituted into muscle fibres containing β-MHC. For example, c in β-MHC-containing muscle fibres decreased by approximately 15% (P = 0.9) when reconstituted with cTnTE162DEL and by approximately 11% (P = 0.5) when reconstituted with cTnTK211DEL (Fig. 8B).
Discussion
The existence of multiple cTnT mutations and various cardiac phenotypes in FHC suggests that the primary molecular trigger in the sequence of events, which eventually leads to heart complications, varies with the nature of the mutation. Because there is functional coupling between the RU and XBs, it is expected that qualitative changes in MHC isoform will alter the nature of primary myofilament dysfunction induced by the mutation in cTnT. In view of our recent observation that cTnT participates in regulating the dynamics of XB cycling kinetics (Chandra et al. 2006), we wanted to test whether the effects of cTnT mutants on myofilament activation are modulated by changes in MHC isoform. To test our hypothesis, Ca2+ activation of tension, ATPase activity and myofilament dynamics were measured in mutant cTnT reconstituted muscle fibres from normal rat hearts (containing α-MHC) and PTU-treated rat hearts (containing β-MHC).
Effect of mutations in cTnT and MHC isoforms on rat cardiac myofilament Ca2+ sensitivity
The directionality of changes in myofilament Ca2+ sensitivity induced by the mutations in cTnT was not significantly affected by the type of MHC isoform. For example, cTnTE162DEL induced a significant increase in myofilament Ca2+ sensitivity and cTnTK211DEL induced a significant decrease in Ca2+ sensitivity, irrespective of the type of MHC isoform present in the myofilament. These observations demonstrate that the myofilament Ca2+ sensitivity is not MHC isoform-dependent and that the Ca2+-sensitive step that regulates the transition of the RU from the non-permissive to permissive states (McKillop & Geeves, 1993) is not affected by the differences between α- and β-MHC isoforms. Because the extent of subsequent non-permissive to permissive RU transition depends on strong XB binding, a step in this process is differentially affected by α- and β-MHC isoforms to bring about differences in tension cost. Previous transgenic and reconstitution studies have also shown that cTnTE162DEL increases myofilament Ca2+ sensitivity (Chandra et al. 2005) and cTnTK211DEL decreases myofilament Ca2+ sensitivity (Morimoto et al. 2002). However, in contrast to our previous TG mouse studies (Chandra et al. 2005), we observed a significant decrease in the Hill coefficient values when cTnTE162DEL was reconstituted into either α-MHC- or β-MHC-containing rat cardiac muscle fibres. A possible explanation is that our previous study used a TG mouse model that expressed approximately 50% of the mutant cTnT in the myofilament, whereas in this study we used a reconstitution protocol to incorporate mutant cTnT protein in the thin filament. The presence of both WT-cTnT and mutant cTnT in the thin filament of heterozygous mouse may be responsible for differences in myofilament cooperativity that we observed in our earlier study (Chandra et al. 2005). Although cTnTE162DEL reconstituted muscle fibres showed decreased myofilament cooperativity as assessed by the changes in Hill coefficient values, myofilament Ca2+ sensitivity increased significantly. The Hill coefficient is a composite index of myofilament cooperativity that includes cooperativity in Ca2+ binding to the RU and near-neighbour interactions, including RU–RU, XB–RU and XB–XB interactions (Razumova et al. 2000). Such near neighbour interactions may also influence Ca2+ binding to the RU in ways that are not well understood.
Effect of MHC isoform on cTnT mutation-induced changes in tension cost
cTnTE162DEL and cTnTK211DEL significantly increased tension cost in α-MHC-containing muscle fibres (Fig. 8A). In our previous study, TG mouse cardiac muscle fibres expressing cTnTE162DEL showed no significant increase in Ca2+-activated maximal tension, but demonstrated a significant increase in Ca2+-activated maximal ATPase activity. Because the slope of tension–ATPase relationship (tension cost) has been proposed as a measure of the rate of XB detachment (Brenner & Eisenberg, 1986), we concluded in our previous TG mouse study that cTnTE162DEL-induced increase in tension cost was due to an increase the rate of XB detachment (g). One way to validate this observation is to compare changes in tension cost with changes in rate constant of XB distortion (c), which is independently estimated by fitting the force response of small amplitude muscle length changes to the recruitment–distortion model (Campbell et al. 2004; Chandra et al. 2006). As demonstrated previously (Campbell et al. 2004; Chandra et al. 2006), model-estimated c has a strong dependence on g (tension cost), because XB distortion dynamics are principally determined by XB detachment kinetics (Campbell et al. 2004). The directionality of changes we observed in tension cost coincided with changes in c (Fig. 8). Therefore, our data suggest that the functional outcome of mutations in TnT varies depending on the type of MHC isoform present. Moreover, our observations also suggest that XB detachment kinetics are modulated by changes in cTnT structure (Chandra et al. 2005, 2006).
Structural changes in the RU have an impact on XB cycling kinetics
Differences in the composition of the RU have been linked to more prominent XB-dependent activation of force development in cardiac myofilaments (Fitzsimons et al. 2001; Chandra et al. 2006). Mutation in cTnT has been shown to increase XB detachment rate in mouse cardiac muscle fibres (Chandra et al. 2005). The pivotal location of cTnT in the thin filament (Gordon et al. 2000) may provide some clues as to how cTnT may modulate XB cycling kinetics. cTnT may modify the state of actin monomers in such a way that the kinetics of XB binding to actin is altered. cTnT may alter the strength of XB binding to actin in an indirect manner via its effect on TnI and Tm (Dahiya et al. 1994) or in a direct manner by its interaction with actin (Heeley & Smillie, 1988; Dahiya et al. 1994).
Because the strengths of cooperative interactions between the RU and XBs are influenced by multiple interactions within the myofilament (Greene & Eisenberg, 1980; Razumova et al. 2000), the dynamics of XB kinetic processes are likely to be affected by changes occurring within MHC as well as the RU. Just as Tn influences Tm binding to actin filaments, strongly bound XBs also modulate actin–RU interactions (Tobacman & Butters, 2000; Smith et al. 2003). Therefore, multiple amino acid sequence differences that exist in the head region of α- and β-MHC isoforms (McNally et al. 1989) are likely to have different effects on how XBs interact with actin and, importantly, how such interactions impact the feedback effect of XBs on the RU. In conclusion, our data demonstrate that the functional consequence of a mutation in cTnT is affected differently by the type of MHC isoform present in the thin filament. Therefore, our study suggests that some caution may be necessary when interpreting data from TG mouse studies that use mutant myofilament proteins. A limitation of this study is that we used a reconstitution method to incorporate mutant cTnT into cardiac myofilaments. The level of mutant protein expressed in human cardiomyopathy is one of the major ‘unknowns’ in the field. It is generally believed that the relative amount of mutant sarcomeric protein in human hearts may vary from very low to high levels. Therefore, conclusions drawn from our observations may not be applicable in some cases.
Acknowledgments
This work was supported by the grant R01 HL075643-02 from the National Institutes of Health (M.C.). We would like to thank Dr Kenneth B. Campbell for critical reading of the manuscript and Dr Bryan K. Slinker for help in statistical analysis. We would also like to thank Robert Kirkpatrick for his support with the instrumentation and software programming.
References
- Bers DM. Calcium fluxes involved in control of cardiac myocyte contraction. Circ Res. 2000;87:275–281. doi: 10.1161/01.res.87.4.275. [DOI] [PubMed] [Google Scholar]
- Brenner B, Eisenberg E. Rate of force generation in muscle: correlation with actomyosin ATPase activity in solution. Proc Natl Acad Sci U S A. 1986;83:3542–3546. doi: 10.1073/pnas.83.10.3542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell KB, Chandra M, Kirkpatrick RD, Slinker BK, Hunter WC. Interpreting cardiac muscle force-length dynamics using a novel functional model. Am J Physiol Heart Circ Physiol. 2004;286:H1535–H1545. doi: 10.1152/ajpheart.01029.2003. [DOI] [PubMed] [Google Scholar]
- Chandra M, Kim JJ, Solaro RJ. An improved method for troponin exchange in detergent skinned rat cardiac fibre bundles. Biochem Biophys Res Commun. 1999a;263:219–223. doi: 10.1006/bbrc.1999.1341. [DOI] [PubMed] [Google Scholar]
- Chandra M, Montgomery DE, Kim JJ, Solaro RJ. The N-terminal region of troponin T is essential for maximal activation of cardiac myofilaments. J Mol Cell Cardiol. 1999b;31:867–880. doi: 10.1006/jmcc.1999.0928. [DOI] [PubMed] [Google Scholar]
- Chandra M, Rundell VL, Tardiff JC, Leinwand LA, de Tombe PP, Solaro RJ. Ca2+ activation of myofilaments from transgenic mouse hearts expressing R92Q mutant cardiac troponin T. Am J Physiol Heart Circ Physiol. 2001;280:H705–H713. doi: 10.1152/ajpheart.2001.280.2.H705. [DOI] [PubMed] [Google Scholar]
- Chandra M, Tschirgi ML, Rajapakse I, Campbell KB. Troponin T modulates sarcomere length dependent recruitment of crossbridges in cardiac muscle. Biophys J. 2006;90:2867–2876. doi: 10.1529/biophysj.105.076950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandra M, Tschirgi ML, Tardiff JC. Increase in tension-dependent ATP consumption induced by cardiac troponin T mutation. Am J Physiol Heart Circ Physiol. 2005;289:H2112–H2119. doi: 10.1152/ajpheart.00571.2005. [DOI] [PubMed] [Google Scholar]
- Dahiya RC, Butters A, Tobacman LS. Equilibrium linkage analysis of cardiac thin filament assembly. Implications for the regulation of muscle contraction. J Biol Chem. 1994;269:29457–29461. [PubMed] [Google Scholar]
- de Tombe PP, Stienen GJM. Protein kinase A does not alter economy of force maintenance in skinned rat cardiac trabeculae. Circ Res. 1995;76:734–741. doi: 10.1161/01.res.76.5.734. [DOI] [PubMed] [Google Scholar]
- Dillmann WH. Diabetes mellitus induces changes in cardiac myosin of the rat. Diabetes. 1980;29:579–582. doi: 10.2337/diab.29.7.579. [DOI] [PubMed] [Google Scholar]
- Ertz-Berger BR, He H, Dowell C, Factor SM, Haim TE, Nunez S, Schwartz SD, Ingwall JS, Tardiff JC. Changes in the chemical and dynamic properties of cardiac troponin T cause discrete cardiomyopathies in transgenic mice. Proc Natl Acad Sci U S A. 2005;102:18219–18224. doi: 10.1073/pnas.0509181102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabiato A, Fabiato F. Calculator programs for computing the composition of the solutions containing multiple metals and ligands used for experiments in skinned muscle cells. J Physiol (Paris) 1979;75:463–505. [PubMed] [Google Scholar]
- Fitzsimons DP, Patel JR, Moss RL. Role of myosin heavy chain composition in kinetics of force development and relaxation in rat myocardium. J Physiol. 1998;513:171–183. doi: 10.1111/j.1469-7793.1998.171by.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzsimons DP, Patel JR, Moss RL. Cross-bridge interaction kinetics in rat myocardium are accelerated by strong binding of myosin to the thin filament. J Physiol. 2001;530:263–272. doi: 10.1111/j.1469-7793.2001.0263l.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgakopoulos D, Kass DA. Minimal force–frequency modulation of inotropy and relaxation of in situ murine heart. J Physiol. 2001;534:535–545. doi: 10.1111/j.1469-7793.2001.00535.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glantz SA. Primer of Biostatistics. 5. New York: McGraw-Hill; 2002. pp. 92–95. [Google Scholar]
- Gomes AV, Potter JD. Molecular and cellular aspects of troponin cardiomyopathies. Ann N Y Acad Sci. 2004;1015:214–224. doi: 10.1196/annals.1302.018. [DOI] [PubMed] [Google Scholar]
- Gordon AM, Homsher E, Regnier AM. Regulation of contraction in striated muscle. Physiol Rev. 2000;80:853–924. doi: 10.1152/physrev.2000.80.2.853. [DOI] [PubMed] [Google Scholar]
- Greene LE, Eisenberg E. Cooperative binding of myosin subfragment-1 to the actin-troponin-tropomyosin complex. Proc Natl Acad Sci U S A. 1980;77:2616–2620. doi: 10.1073/pnas.77.5.2616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heeley DH, Smillie LB. Interaction of rabbit skeletal muscle troponin T and F-actin at physiological ionic strength. Biochemistry. 1988;27:8227–8232. doi: 10.1021/bi00421a036. [DOI] [PubMed] [Google Scholar]
- Herron TJ, Korte SF, McDonald KS. Loaded shortening and power output in cardiac myocytes are dependent on myosin heavy chain isoform expression. Am J Physiol Heart Circ Physiol. 2001;281:H1217–H1222. doi: 10.1152/ajpheart.2001.281.3.H1217. [DOI] [PubMed] [Google Scholar]
- Herron TJ, McDonald KS. Small amounts of alpha-myosin heavy chain isoform expression significantly increase power output of rat cardiac myocyte fragments. Circ Res. 2002;90:1150–1152. doi: 10.1161/01.res.0000022879.57270.11. [DOI] [PubMed] [Google Scholar]
- James J, Martin L, Maike Krenz AS, Quatman C, Jones F, Klevitsky R, Gulick J, Robbins J. Forced expression of alpha-myosin heavy chain in the rabbit ventricle results in cardioprotection under cardiomyopathic conditions. Circulation. 2005;111:2339–2346. doi: 10.1161/01.CIR.0000164233.09448.B1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javadpour MM, Tardiff JC, Pinz I, Ingwall JS. Decreased energetics in murine hearts bearing the R92Q mutation in cardiac troponin T. J Clin Invest. 2003;112:768–775. doi: 10.1172/JCI15967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin JP, Lin JJ. Isolation and characterization of cDNA clones encoding embryonic and adult isoforms of rat cardiac troponin T. J Biol Chem. 1989;264:14471–14477. [PubMed] [Google Scholar]
- Kamisago M, Sharma SD, DePalma SR, Solomon S, Sharma P, McDonough B, Smoot L, Mullen MP, Woolf PK, Wigle ED, Seidman JG, Seidman CE. Mutations in sarcomere protein genes as a cause of dilated cardiomyopathy. N Engl J Med. 2001;343:1688–1696. doi: 10.1056/NEJM200012073432304. [DOI] [PubMed] [Google Scholar]
- Kass DA, Hare JM, Georgakopoulos D. Murine cardiac function: a cautionary tail. Circ Res. 1998;82:519–522. doi: 10.1161/01.res.82.4.519. [DOI] [PubMed] [Google Scholar]
- Li L, Satoh H, Ginsburg KS, Bers DM. The effect of Ca2+–calmodulin-dependent protein kinase II on cardiac excitation–contraction coupling in ferret ventricular myocytes. J Physiol. 1997;501:17–31. doi: 10.1111/j.1469-7793.1997.017bo.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKillop DF, Geeves MA. Regulation of the interaction between actin and myosin subfragment 1: evidence for three states of the thin filament. Biophys J. 1993;65:693–701. doi: 10.1016/S0006-3495(93)81110-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNally EM, Kraft R, Bravo-Zehnder M, Taylor DA, Leinwand LA. Full-length rat alpha and beta cardiac myosin heavy chain sequences. Comparisons suggest a molecular basis for functional differences. J Mol Biol. 1989;210:665–671. doi: 10.1016/0022-2836(89)90141-1. [DOI] [PubMed] [Google Scholar]
- Metzger JM, Wahr PA, Michele DE, Albayya F, Westfall MV. Effects of myosin heavy chain isoform switching on Ca2+-activated tension development in single adult cardiac myocytes. Circ Res. 1999;84:1310–1317. doi: 10.1161/01.res.84.11.1310. [DOI] [PubMed] [Google Scholar]
- Miyata S, Minobe W, Bristow MR, Leinwand LA. Myosin heavy chain isoform expression in the failing and nonfailing human heart. Circ Res. 2000;86:386–390. doi: 10.1161/01.res.86.4.386. [DOI] [PubMed] [Google Scholar]
- Montgomery DE, Tardiff JC, Chandra M. Cardiac troponin T mutations: correlation between the type of mutation and the nature of myofilament dysfunction in transgenic mice. J Physiol. 2001;536:583–592. doi: 10.1111/j.1469-7793.2001.0583c.xd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morimoto S, Lu Q-W, Harada K, Takahashi-Yanaga F, Minakami R, Ohta M, Sasaguri T, Ohtsuki I. Ca2+-desensitizing effect of a deletion mutation delta K211 in cardiac troponin T that causes familial dilated cardiomyopathy. Proc Natl Acad Sci U S A. 2002;99:913–918. doi: 10.1073/pnas.022628899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope B, Hoh JF, Weeds A. The ATPase activities of rat cardiac myosin isoenzymes. FEBS Lett. 1980;118:205–208. doi: 10.1016/0014-5793(80)80219-5. [DOI] [PubMed] [Google Scholar]
- Razumova MV, Bukatina AE, Campbell KB. Different myofilament nearest-neighbor interactions have distinctive effects on contractile behavior. Biophys J. 2000;78:3120–3137. doi: 10.1016/S0006-3495(00)76849-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice JJ, Jafri MS, Winslow RL. Modeling short-term interval-force relations in cardiac muscle. Am J Physiol Heart Circ Physiol. 2000;278:H913–H931. doi: 10.1152/ajpheart.2000.278.3.H913. [DOI] [PubMed] [Google Scholar]
- Rouslin W, Broge CW. Isoform-independent heart rate-related variation in cardiac myofibrillar Ca2+-activated Mg2+-ATPase activity. Am J Physiol. 1996;270:C1271–C1276. doi: 10.1152/ajpcell.1996.270.5.C1271. [DOI] [PubMed] [Google Scholar]
- Rundell VLM, Manaves V, Martin AF, de Tombe PP. Impact of beta-myosin heavy chain isoform expression on cross-bridge cycling kinetics. Am J Physiol Heart Circ Physiol. 2004;288:H896–H903. doi: 10.1152/ajpheart.00407.2004. [DOI] [PubMed] [Google Scholar]
- Saggin L, Ausoni S, Gorza L, Sartore S, Schiaffino S. Troponin T switching in the developing rat heart. J Biol Chem. 1988;263:18488–18492. [PubMed] [Google Scholar]
- Smith DA, Maytum R, Geeves MA. Cooperative regulation of myosin–actin interactions by a continuous flexible chain I: actin-tropomyosin systems. Biophys J. 2003;84:3155–3167. doi: 10.1016/S0006-3495(03)70040-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stienen GJM, Zaremba R, Elzinga G. ATP utilization for calcium uptake and force production in skinned muscle fibres of Xenopus laevis. J Physiol. 1995;482:109–122. doi: 10.1113/jphysiol.1995.sp020503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stull LB, Leppo MK, Marbán E, Janssen PML. Physiological determinants of contractile force generation and calcium handling in mouse myocardium. J Mol Cell Cardiol. 2002;10:1367–1376. doi: 10.1006/jmcc.2002.2065. [DOI] [PubMed] [Google Scholar]
- Swynghhedauw B. Developmental and functional adaptation of contractile proteins in cardiac and skeletal muscles. Physiol Rev. 1986;66:710–771. doi: 10.1152/physrev.1986.66.3.710. [DOI] [PubMed] [Google Scholar]
- Tardiff JC. Sarcomeric proteins and familial hypertrophic cardiomyopathy: linking mutations in structural proteins to complex cardiovascular phenotypes. Heart Fail Rev. 2005;3:237–248. doi: 10.1007/s10741-005-5253-5. [DOI] [PubMed] [Google Scholar]
- Tardiff JC, Factor SM, Tompkins BD, Hewett TE, Palmer BM, Moore RL, Schwartz S, Robbins J, Leinwand LA. A truncated cardiac troponin T molecule in transgenic mice suggests multiple cellular mechanisms for familial hypertrophic cardiomyopathy. J Clin Invest. 1998;101:2800–2811. doi: 10.1172/JCI2389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tardiff JC, Hewett TE, Factor SM, Vikstrom KL, Robbins J, Leinwand LA. Expression of the beta (slow)-isoform of MHC in the adult mouse heart causes dominant-negative functional effects. Am J Physiol Heart Circ Physiol. 2000;278:H412–H419. doi: 10.1152/ajpheart.2000.278.2.H412. [DOI] [PubMed] [Google Scholar]
- Tobacman LS, Butters CA. A new model of cooperative myosin-thin filament binding. J Biol Chem. 2000;275:27587–27593. doi: 10.1074/jbc.M003648200. [DOI] [PubMed] [Google Scholar]
- Watkins H, McKenna WJ, Theirfelder L, Suk HJ, Anan R, Odonoghue A, Spirito P, Matsumori A, Moravec CS, Seidman JG, Seidman CE. Mutations in the gene for cardiac troponin T and α-Tm in hypertrophic cardiomyopathy. N Engl J Med. 1995;332:1058–1064. doi: 10.1056/NEJM199504203321603. [DOI] [PubMed] [Google Scholar]