Abstract
While it has been known for more than 75 years that physical activity is associated with increased mitochondrial content in muscle, the molecular mechanism for this adaptive process has only recently been elucidated. This brief review examines existing studies that have identified AMPK-activated protein kinase (AMPK) and several other key regulators of mitochondrial biogenesis, including peroxisome proliferator-activated receptor-γ coactivator-1α and -1β, calcium/calmodulin-dependent protein kinase IV, and nitric oxide. In addition, the potential role of mitochondrial dysfunction in the pathogenesis of insulin resistance associated with ageing and type 2 diabetes mellitus is also discussed.
Introduction
The association between physical activity and mitochondrial content has been known for more than 75 years (Needham, 1926). Some of the first observations to establish this association were done by comparing the mitochondrial content in the breast muscle of chickens, which fly infrequently, to the breast muscle of pigeons, which regularly fly for extended periods of time. These studies found that that pigeon breast muscle has more mitochondrial activity and content than the chicken breast muscle (Paul & Sperling, 1952). Other studies also demonstrated that continuously functioning muscles, like cardiac muscle, have more mitochondrial activity and content than sporadically functioning muscles, such as back muscle (Paul & Sperling, 1952). These early studies in animals suggested that muscles responsible for long-lasting and regular physical activity are capable of increasing their mitochondrial activity and content to fulfill their roles.
Endurance exercise and mitochondrial biogenesis
Endurance exercise studies during the late 1960s and early 1970s provided further evidence that repeated bouts of physical activity increase mitochondrial activity and content. Mitochondrial biogenesis was observed in 6-week-old rats subjected to treadmill exercise 5 days per week for 3 months. At the end of their training, the exercising rats' skeletal muscle contained a higher concentration of cytochrome c and increased activities of key mitochondrial enzymes (Holloszy, 1967). Further studies established the same trend in humans (Morgan et al. 1971; Gollnick et al. 1972; Hoppeler et al. 1973). Fink and colleagues compared a subset of exercise-trained individuals, elite distance runners, and sedentary individuals and found that the runners have a much greater percentage of oxidative, slow-twitch skeletal muscle fibres and more succinate dehydrogenase activity than sedentary controls (Fink et al. 1977). Yet despite the long-recognized link between mitochondrial biogenesis and stimuli such as endurance exercise, the critical factors regulating mitochondrial biogenesis have remained elusive until recently.
Newly discovered factors regulating mitochondrial biogenesis
The first major regulator of mitochondrial biogenesis discovered was peroxisome proliferator-activated receptor-γ coactivator-1α (PGC-1α) (Puigserver et al. 1998; Wu et al. 1999). Puigserver and colleagues induced PGC-1α mRNA expression in mice by exposing them to cold (4°C) for either 3 or 12 h at a time. Doing so also increased the mRNA expression levels of ATP synthetase (β subunit), cytochrome c-oxidase II, and cytochrome c-oxidase IV. Irrcher et al. (2003) used electrical stimulation in rats to provide further in vivo evidence of a link between PGC-1α and mitochondrial biogenesis. They detected a rise in PGC-1α protein expression as the electrical stimulation induced an increase in cytochrome c-oxidase activity. Lastly, Wu et al. (1999) further investigated the link between PGC-1α and mitochondrial biogenesis by examining mitochondrial DNA content and mitochondrial content in mouse myotube cells expressing PGC-1α. They found increases in both, thus clearly illustrating a strong connection between PGC-1α and mitochondrial biogenesis.
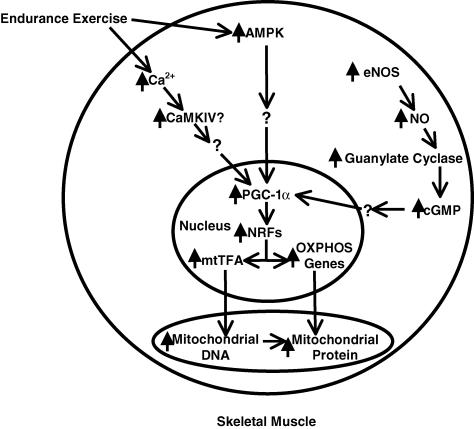
PGC-1α is a cotranscriptional regulation factor that induces mitochondrial biogenesis by activating a group of transcription factors, including nuclear respiratory factor 1 (NRF1) and nuclear respiratory factor 2 (NRF2), which activate mitochondrial transcription factor A (mtTFA). Previous studies demonstrated that NRF1 and NRF2 are important contributors to the sequence of events culminating in the increase in transcription of key mitochondrial enzymes, and they have been shown to interact with mtTFA, which initiates the replication and transcription of mitochondrial DNA (Clayton, 1992; Scarpulla, 1997; Virbasius & Scarpulla, 1994). Wu et al. (1999) further explored the relationship between PGC-1α and the NRFs in a series of experiments that provided evidence that PGC-1α modulates the NRFs via physical interaction and PGC-1α-induced mitochondrial biogenesis requires NRFs. Exercise studies further linked PGC-1α and the NRFs to mitochondrial biogenesis as an acute bout of swimming increased PGC-1α protein expression and NRF-1 binding to the δ-aminolevulinate synthase (δ-ALAS) promoter and NRF-2 binding to the cytochrome c-oxidase IV promoter (Baar et al. 2002). Taken together, these studies illustrate that PGC-1α plays an indispensable role in mediating a pathway that connects stimuli such a cold or exercise to an internal metabolic response like mitochondrial biogenesis via the NRF transcription factors (Fig. 1).
Figure 1. Critical factors involved in mitochondrial biogenesis.
CaMKIV, AMPK, and NO cause an increase in PGC-1α gene transcription, which results in gene expression of the NRFs, to which PGC-1α also binds and activates, and finally, mtTFA expression and initiation of mitochondrial DNA replication. The NRFs also induce OXPHOS gene transcription, and the resulting nuclear-encoded proteins then travel to the mitochondria. Lastly, mtTFA is able to transcribe mitochondrial DNA, which leads to the production of mitochondrial-encoded proteins.
A homologue of PGC-1α, termed peroxisome proliferator-activated receptor-γ coactivator-1β (PGC-1β), has also been found recently, and studies have demonstrated that it also regulates mitochondrial biogenesis. In L6 myoblasts expressing PGC-1β, mitochondrial biogenesis was observed (Meirhaeghe et al. 2003). Lin et al. (2002) also found that PGC-1β interacts with NRF-1 to mediate its transcriptional activity (Lin et al. 2002). Stimuli like cold and exercise, however, did not increase PGC-1β mRNA expression, thus suggesting that PGC-1α and PGC-1β are stimulated independently. Once stimulated, though, both PGC-1α and PGC-1β clearly regulate mitochondrial biogenesis through NRF-1 to enable the mitochondria to meet the energetic requirements of the cell.
Calcium/calmodulin-dependent protein kinase IV (CaMKIV) has also been identified as a major regulator of mitochondrial biogenesis. Previous studies have shown that CaMKIV influences gene expression in oxidative fibres of myocytes (Wu et al. 2000). To examine the potential role of CaMKIV in mitochondrial biogenesis, Wu and colleagues created a transgenic mouse with a skeletal muscle-specific constitutively active form of CaMKIV. They showed that the skeletal muscle of mice with constitutively active CaMKIV contain more copies of mitochondrial DNA and possess more mitochondria as a percentage of the myocytes' volume. In addition, mRNA expression of cytochrome b and carnitine palmitoyltranferase-1 was increased in the transgenic line (Wu et al. 2002). More recent studies, however, have created doubt about the role of CaMKIV in mitochondrial biogenesis. Akimoto et al. (2004) generated CaMKIV null mice, which had similar protein expression levels of PGC-1α and cytochrome c-oxidase IV to wild-type mice. Moreover, wild-type and CaMKIV null mice both increased their PGC-1α and cytochrome c-oxidase IV protein expression in response to voluntary running, thus indicating that CaMKIV is not required for mitochondrial biogenesis. Finally, Akimoto and colleagues did not detect CaMKIV protein expression in murine skeletal muscle. Thus, it remains unclear what physiological role CaMKIV plays in the regulation of muscle mitochondrial biogenesis in response to physical training.
Recently, nitric oxide (NO) has also been shown to have an effect upon mitochondrial biogenesis. HeLa cells expressing endothelial nitric oxide synthase eNOS (eNOS) displayed increases in mitochondrial DNA content, cytochrome c and cytochrome c-oxidase IV protein expression levels, and PGC-1α, NRF-1, and mtTFA mRNA expression (Nisoli et al. 2003). Nisoli and colleagues also found that the NO produced by eNOS activates guanylate cyclase to increase the amount of cyclic GMP (cGMP) present, which, through an unknown mechanism, transmits a signal to the nucleus that causes the induction of PGC-1α gene transcription and, consequently, mitochondrial biogenesis.
Given the critical role for the AMP-activated protein kinase (AMPK) in regulating intracellular energy metabolism in response to acute energy crises, it is perhaps not surprising that AMPK has also been identified as a major regulator of mitochondrial biogenesis in response to chronic energy depletion (Hardie, 2004; Hardie & Sakamoto, 2006; Kahn et al. 2005). In order to examine the link between chronic energy deprivation and mitochondrial biogenesis, our laboratory fed β-guanadinopropionic acid (β-GPA), a chronic pharmacological activator of AMPK that works by chronically depleting muscle phosphocreatine stores, to rats for 8 weeks. This resulted in chronic AMPK activation in skeletal muscle and increases in NRF-1 binding activity, δ-ALAS mRNA expression, cytochrome c protein expression, and mitochondrial content, thus clearly demonstrating that AMPK activation promotes mitochondrial biogenesis through PGC-1α and the NRFs (Bergeron et al. 2001). Other pharmacological studies have also established a link between chronic AMPK activation and the up-regulation of key mitochondrial enzymes in skeletal muscle. Winder et al. (2000) administered 5′-aminoimidazole-4-carboxamide-1-β-d-ribofuranoside (AICAR) to rats for 4 weeks and observed increased protein expression of cytochrome c and δ-ALAS and increased activities of citrate synthase, malate dehydrogenase and succinate dehydrogenase in skeletal muscle. AICAR-induced AMPK activation has also been shown to increase uncoupling protein-3 mRNA and protein expression (Zhou et al. 2000; Putnam et al. 2003).
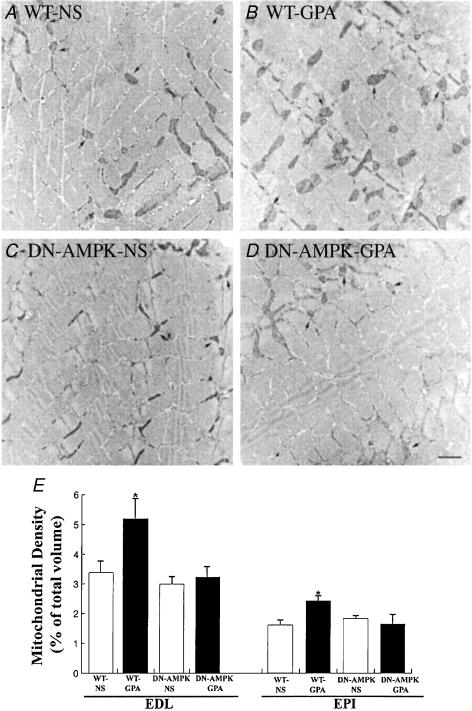
To determine if AMPK is required for mitochondrial biogenesis, transgenic mice expressing a dominant-negative mutant form of AMPK in skeletal muscle and wild-type mice were fed β-GPA for 8 weeks. While AMPK was activated in the skeletal muscle of the wild-type mice in response to β-GPA feeding, the transgenic mice fed β-GPA showed no similar increase in AMPK activation. Moreover, PGC-1α mRNA expression, cytochrome c protein expression levels, mitochondrial DNA content and mitochondrial density were all increased in the wild-type mice, and none of these parameters were increased in the β-GPA transgenic mice lacking a functional form of AMPK (Fig. 2) (Zong et al. 2002). Clearly, these data demonstrate that AMPK is necessary for mitochondrial biogenesis in response to chronic energy deprivation, and it appears likely that pharmacologically activated AMPK conveys its signal to induce mitochondrial biogenesis via the PGC-1α–NRF pathway.
Figure 2. Effect of β-GPA treatment on mitochondrial content as assessed by electron microscopy.
Transgenic mice expressing a dominant-negative mutant form of AMPK in skeletal muscle and wild-type mice were fed β-GPA for 8 weeks. Mitochondrial content was then assessed by electron microscopy of the epitrochlearis (EPI) muscle of wildtype (WT) mouse (saline treated) (A), WT mouse (GPA treated) (B), dominant-negative AMPK transgenic mouse (saline treated) (C), and dominant-negative AMPK transgenic mouse (GPA treated) (D). Arrows point to mitochondria. (Scale bar, 1 μm.) E, mitochondria density (% of total volume) in the extensor digitorum longus (EDL) (n = 10 in each group) and EPI muscles of each group of mice (n = 5 in each group). *P < 0.05 compared with other groups. From Zhong et al. (2002). ©2002 National Academy of Sciences, USA.
Exercise studies also suggest that AMPK works through PGC-1α to promote mitochondrial biogenesis. Six hours of low intensity swimming resulted in increases in AMPK activation and PGC-1α mRNA expression (Terada et al. 2002). Electrical stimulation designed to mimic endurance exercise also activated AMPK and increased PGC-1α protein expression (Atherton et al. 2005). These results demonstrate that the physiological activation of AMPK also leads to changes in PGC-1α mRNA and protein expression, although exercise has been shown to increase PGC-1α mRNA expression in the absence of a functional copy of AMPK-α2 (Jorgensen et al. 2005). Exploring further how exercise affects AMPK and PGC-1α and whether this leads to an increase in mitochondrial biogenesis are important issues that need to be addressed.
Clinical relevance of reduced mitochondrial function and content in the pathogenesis of insulin resistance and Type 2 diabetes mellitus
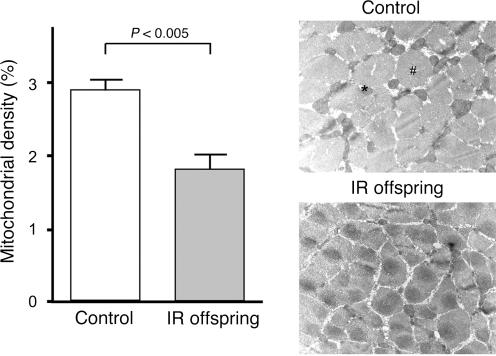
The link between mitochondria and diabetes was first observed in patients with a rare maternally transmitted form of diabetes-associated deafness, which was found to be due to a 10.4 kb mitochondrial DNA deletion encoding a mitochondrial tRNA (Ballinger et al. 1992). These patients have severe β-cell dysfunction, are insulin dependent, and resemble patients with type 1 diabetes. Recent studies by our group have found that a much more mild form of mitochondrial dysfunction may be responsible for causing insulin resistance associated with type 2 diabetes mellitus (T2DM). Using 31P magnetic resonance spectroscopy to non-invasively assess muscle mitochondrial rates of ATP synthesis, our group found a 30% reduction in rates of ATP synthesis in the muscle of young, lean and sedentary insulin-resistant offspring of parents with T2DM (Petersen et al. 2004). These young, lean and sedentary insulin-resistant individuals also had increased intramyocellular lipid content, which has previously been shown to cause insulin resistance in muscle and was hypothesized to be secondary to lower mitochondrial activity (Shulman, 2000). In order to understand whether the reduced mitochondrial activity could be attributed to reduced mitochondrial content and/or mitochondrial dysfunction, our group performed muscle biopsy studies to assess mitochondrial content by electron microscopy in a similar group of young, lean and sedentary insulin-resistant offspring (Morino et al. 2005). Using this approach, we found that these insulin-resistant subjects had a 38% reduction in mitochondrial content compared to the control subjects (Fig. 3). These data suggest that the reduced mitochondrial content may be the major factor responsible for the reduced mitochondrial activity and provide further evidence to support the hypothesis that hereditary mitochondrial dysfunction, due to reduced mitochondrial content, contributes to the development of insulin resistance and T2DM.
Figure 3. Mitochondrial density and gene expression data of young, lean and sedentary insulin-resistant offspring of parents with type 2 diabetes and control subjects.
Mitochondrial density in control subjects (n = 6) and insulin-resistant offspring (n = 8), assessed by electron microscopy. #, muscle fibre; *, mitochondrion.
Alterations in nuclear gene expression essential for mitochondrial biogenesis have also been found in type 2 diabetics. Specifically, type 2 diabetics and first-degree relatives of patients with T2DM displayed reduced NRF-1-dependent gene expression and reduced PGC-1α and PGC-1β gene expression in muscle compared with non-type 2 diabetic subjects (Patti et al. 2003). In addition, genes involved in mitochondrial oxidative phosphorylation that are regulated by PGC-1α were expressed less in type 2 diabetics than in non-diabetics (Mootha et al. 2003). However, in contrast to these results, recent studies by our group did not detect any changes in the mRNA or protein expression levels of PGC-1α, PGC-1β, NRF1, NRF2, or mtTFA of the young, lean, sedentary insulin-resistant offspring of the type 2 diabetic parents, suggesting that the observed reductions in PGC-1 responsive genes in the previous studies were secondary to T2DM, obesity, or some other acquired factors (Yechoor et al. 2002). Furthermore, these data suggest that other as-of-yet unidentified nuclear-encoded factors regulating mitochondrial biogenesis may be responsible for the observed reduction in mitochondrial content. A key question that remains to be answered is whether the reduced mitochondrial content observed in the young, lean and sedentary insulin-resistant offspring is responsible for the increased intramyocellular lipid content or whether it is secondary in nature.
In addition to inherited forms of mitochondrial dysfunction and reduced mitochondrial content, evidence is accumulating that acquired mitochondrial dysfunction also occurs with ageing. Using 31P and 13C magnetic resonance spectroscopy to non-invasively assess rates of mitochondrial oxidative-phosphorylation activity, our group found reductions in mitochondrial oxidative and phosphorylation activity in muscle of healthy, lean and sedentary elderly individuals, who also had increased intramyocellular and intrahepatocellular lipid content and insulin resistance (Petersen et al. 2003). Why mitochondrial dysfunction occurs with ageing remains unknown, but age-associated accumulation of mutations in mitochondrial DNA may play a role. Mice with a defective mitochondrial DNA polymerase have been shown to age prematurely (Trifunovic et al. 2004) and recently discovered point mutations in the displacement loop (D-loop), where mitochondrial DNA replication is regulated by mtTFA, were shown to accumulate with ageing (Michikawa et al. 1999; Del Bo et al. 2002).
Intriguingly, young insulin-resistant and obese Zucker diabetic fatty (ZDF) rats showed defects in AMPK phosphorylation and in PGC-1α protein expression levels, and endurance exercise on a treadmill partially restored these abnormalities (Sriwijitkamol et al. 2005). These experiments suggest that obesity may impair AMPK signalling and that physical activity reverses obesity's effect. Another recent study further supports this idea as rats fed a high-fat diet for 5 months had reduced AMPK activity and AMPK-α mRNA and protein expression levels, and pharmacological activation of AMPK using metformin increased AMPK activity and AMPK-α mRNA and protein expression levels (Liu et al. 2006). While rodent studies have demonstrated a potential link between excess lipid accumulation and impaired AMPK activity, human studies have not found any similar associations (Hojlund et al. 2004; Steinberg et al. 2004). Nevertheless, given its ability to activate glucose transport through a PI-3 kinase-independent pathway, promote fat oxidation via inhibition of acetyl-CoA carboxylase, and direct mitochondrial biogenesis via promoting increased expression of PGC-1α in skeletal muscle, AMPK is a potentially attractive target to treat and prevent insulin resistance in muscle of patients with T2DM.
Summary
While it has been known for decades that physical activity is associated with increased mitochondrial content, it has been only recently that some of the critical factors involved in the regulation of mitochondrial biogenesis have been identified. Given the potentially important role of mitochondrial dysfunction and reduced mitochondrial content in the pathogenesis of insulin resistance, understanding the molecular mechanisms regulating mitochondrial biogenesis and function may provide potentially important novel therapeutic targets to prevent and treat T2DM.
Acknowledgments
This work was supported by grants from the US Public Health Service (P01 DK-068229, P30 DK-45735, R01 DK-40936, U24 DK-59635, T32G-07499). G. I. Shulman is the recipient of a Distinguished Clinical Scientist Award from the American Diabetes Association.
References
- Akimoto T, Ribar TJ, Williams RS, Yan Z. Skeletal muscle adaptation in response to voluntary running in Ca2+/calmodulin-dependent protein kinase IV-deficient mice. Am J Physiol Cell Physiol. 2004;287:C1311–C1319. doi: 10.1152/ajpcell.00248.2004. [DOI] [PubMed] [Google Scholar]
- Atherton PJ, Babraj J, Smith K, Singh J, Rennie MJ, Wackerhage H. Selective adaptation of AMPK-PGC-1α or PKB-TSC2-mTOR signaling can explain specific adaptive responses to endurance or resistance training-like electrical muscle stimulation. FASEB J. 2005;19:786–788. doi: 10.1096/fj.04-2179fje. [DOI] [PubMed] [Google Scholar]
- Baar K, Wende AR, Jones TE, Marison M, Nolte LA, Chen M, Kelly DP, Holloszy JO. Adaptations of skeletal muscle to exercise: rapid increase in the transcriptional coactivator PGC-1. FASEB J. 2002;16:1879–1886. doi: 10.1096/fj.02-0367com. [DOI] [PubMed] [Google Scholar]
- Ballinger SW, Shoffner JM, Hedaya EV, Trounce I, Polak MA, Koontz DA, Wallace DC. Maternally transmitted diabetes and deafness associated with a 10.4 kb mitochondrial DNA deletion. Nat Genet. 1992;1:11–15. doi: 10.1038/ng0492-11. [DOI] [PubMed] [Google Scholar]
- Bergeron R, Ren JM, Cadman KS, Moore IK, Perret P, Pypaert M, Young LH, Semenkovich CF, Shulman GI. Chronic activation of AMP kinase results in NRF-1 activation and mitochondrial biogenesis. Am J Physiol Endocrinol Metab. 2001;281:E1340–E1346. doi: 10.1152/ajpendo.2001.281.6.E1340. [DOI] [PubMed] [Google Scholar]
- Clayton DA. Transcription and replication of animal mitochondrial DNAs. Int Rev Cytol. 1992;141:217–232. doi: 10.1016/s0074-7696(08)62067-7. [DOI] [PubMed] [Google Scholar]
- Del Bo R, Bordoni A, Martinelli Boneschi F, Crimi M, Sciacco M, Bresolin N, Scarlato G, Comi GP. Evidence and age-related distribution of mtDNA D-loop point mutations in skeletal muscle from health subjects and mitochondrial patients. J Neurol Sci. 2002;202:85–91. doi: 10.1016/s0022-510x(02)00247-2. [DOI] [PubMed] [Google Scholar]
- Fink WJ, Costill DL, Pollock ML. Submaximal and maximal working capacity of elite distance runners. Part II. Muscle fiber composition and enzyme activities. Ann N Y Acad Sci. 1977;301:323–327. doi: 10.1111/j.1749-6632.1977.tb38210.x. [DOI] [PubMed] [Google Scholar]
- Gollnick PD, Armstrong RB, Saubert CW, Piehl K, Saltin B. Enzyme activity and fiber composition in skeletal muscle of untrained and trained men. J Appl Physiol. 1972;33:312–319. doi: 10.1152/jappl.1972.33.3.312. [DOI] [PubMed] [Google Scholar]
- Hardie DG. AMP-activated protein kinase: a key system mediating metabolic responses to exercise. Med Sci Sports Exerc. 2004;36:28–34. doi: 10.1249/01.MSS.0000106171.38299.64. [DOI] [PubMed] [Google Scholar]
- Hardie DG, Sakamoto K. AMPK: a key sensor of fuel and energy status in skeletal muscle. Physiology. 2006;21:48–60. doi: 10.1152/physiol.00044.2005. [DOI] [PubMed] [Google Scholar]
- Hojlund K, Mustard KJ, Staehr P, Hardie DG, Beck-Nielsen H, Richter EA, Wojtaszewski JF. AMPK activity and isoform protein expression are similar in muscle of obese subjects with and without type 2 diabetes. Am J Phsyiol Endocrinol Metab. 2004;286:E239–E244. doi: 10.1152/ajpendo.00326.2003. [DOI] [PubMed] [Google Scholar]
- Holloszy JO. Biochemical adaptations in muscle. Effects of exercise on mitochondrial oxygen uptake and respiratory enzyme activity in skeletal muscle. J Biol Chem. 1967;242:2278–2282. [PubMed] [Google Scholar]
- Hoppeler H, Luthi P, Claassen H, Weibel ER, Howald H. The ultrastructure of normal human skeletal muscle. A morphometric analysis on untrained men, women and well-trained orienteers. Pflugers Arch. 1973;344:217–232. doi: 10.1007/BF00588462. [DOI] [PubMed] [Google Scholar]
- Irrcher I, Adhihetty PJ, Sheehan T, Joseph AM, Hood DA. PPARγ coactivator-1α expression during thyroid hormone- and contractile activity-induced mitochondrial adaptations. Am J Physiol Cell Physiol. 2003;284:C1669–C1677. doi: 10.1152/ajpcell.00409.2002. [DOI] [PubMed] [Google Scholar]
- Jorgensen SB, Wojtaszewski JF, Viollet B, Andreelli F, Birk JB, Hellsten Y, Schjerling P, Vaulont S, Neufer PD, Richter EA, Pilegaard H. Effects of α-AMPK knockout on exercise-induced gene activation in mouse skeletal muscle. FASEB J. 2005;19:1146–1148. doi: 10.1096/fj.04-3144fje. [DOI] [PubMed] [Google Scholar]
- Kahn BB, Alquier T, Carling D, Hardie DG. AMP-activated protein kinase: ancient energy gauge provides clues to modern understanding of metabolism. Cell Metab. 2005;1:15–25. doi: 10.1016/j.cmet.2004.12.003. [DOI] [PubMed] [Google Scholar]
- Lin J, Puigserver P, Donovan J, Tarr P, Spiegelman BM. Peroxisome proliferator-activated receptor γ coactivator 1β (PGC-1β), a novel PGC-1-related transcription coactivator associated with host cell factor. J Biol Chem. 2002;277:1645–1648. doi: 10.1074/jbc.C100631200. [DOI] [PubMed] [Google Scholar]
- Liu Y, Wan Q, Guan Q, Gao L, Zhao J. High-fat diet feeding impairs both the expression and activity of AMPKα in rats' skeletal muscle. Biochem Biophys Res Commun. 2006;339:701–707. doi: 10.1016/j.bbrc.2005.11.068. [DOI] [PubMed] [Google Scholar]
- Meirhaeghe A, Crowley V, Lenaghan C, Lelliott C, Green K, Stewart A, Hart K, Schinner S, Sethi JK, Yeo G, Brand MD, Cortright RN, O'Rahilly S, Montague C, Vidal-Puig AJ. Characterization of the human, mouse and rat PGC1β (peroxisome-proliferator-activated receptor-γ co-activator 1β) gene in vitro and in vivo. Biochem J. 2003;373:155–165. doi: 10.1042/BJ20030200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michikawa Y, Mazzucchelli F, Bresolin N, Scarlato G, Attardi G. Aging-dependent large accumulation of point mutations in the human mtDNA control region for replication. Science. 1999;286:774–779. doi: 10.1126/science.286.5440.774. [DOI] [PubMed] [Google Scholar]
- Mootha VK, Lindgren CM, Eriksson KF, Subramanian A, Sihag S, Lehar J, Puigserver P, Carlsson E, Ridderstrale M, Laurila E, Houstis N, Daly MJ, Patterson N, Mesirov JP, Golub TR, Tamayo P, Spiegelman B, Lander ES, Hirschhorn JN, Altshuler D, Groop LC. PGC-1α-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat Genet. 2003;34:267–273. doi: 10.1038/ng1180. [DOI] [PubMed] [Google Scholar]
- Morgan TE, Cobb LA, Short FA, Ross R, Gunn DR. Effect of long-term exercise on human muscle mitochondria. In: Pernow B, editor. Muscle Metabolism During Exercise. New York: Plenum; 1971. pp. 87–95. [Google Scholar]
- Morino K, Petersen KF, Dufour S, Befroy D, Frattini J, Shatzkes N, Neschen S, White MF, Bilz S, Sono S, Pypaert M, Shulman GI. Reduced mitochondrial density and increased IRS-1 serine phosphorylation in muscle of insulin-resistant offspring of type 2 diabetic parents. J Clin Invest. 2005;115:3587–3593. doi: 10.1172/JCI25151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Needham D. Red and white muscle. Phys Rev. 1926;6:1–28. [Google Scholar]
- Nisoli E, Clementi E, Paolucci C, Cozzi V, Tonello C, Sciorati C, Bracale R, Valerio A, Francolini M, Moncada S, Carruba MO. Mitochondrial biogenesis in mammals: the role of endogenous nitric oxide. Science. 2003;299:896–899. doi: 10.1126/science.1079368. [DOI] [PubMed] [Google Scholar]
- Patti ME, Butte AJ, Crunkhorn S, Cusi K, Berria R, Kashyap S, Miyazaki Y, Kohane I, Costello M, Saccone R, Landaker EJ, Goldfine AB, Mun E, DeFronzo R, Finlayson J, Kahn CR, Mandarino LJ. Coordinated reduction of genes of oxidative metabolism in humans with insulin resistance and diabetes: potential role of PGC1 and NRF1. Proc Natl Acad Sci U S A. 2003;100:8466–8471. doi: 10.1073/pnas.1032913100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul MH, Sperling E. Cyclophorase system XXIII. Correlation of cyclophorase activity and mitochondrial density in striated muscle. Proc Soc Exp Biol Med. 1952;79:352–354. doi: 10.3181/00379727-79-19375. [DOI] [PubMed] [Google Scholar]
- Petersen KF, Befroy D, Dufour S, Dziura J, Ariyan C, Rothman DL, DiPietro L, Cline GW, Shulman GI. Mitochondrial dysfunction in the elderly: possible role in insulin resistance. Science. 2003;300:1140–1142. doi: 10.1126/science.1082889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen KF, Dufour S, Befroy D, Garcia R, Shulman GI. Impaired mitochondrial activity in the insulin resistant offspring of patients with type 2 diabetes. N Engl J Med. 2004;350:664–671. doi: 10.1056/NEJMoa031314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puigserver P, Wu Z, Park CW, Graves R, Wright M, Spiegelman BM. A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell. 1998;92:829–839. doi: 10.1016/s0092-8674(00)81410-5. [DOI] [PubMed] [Google Scholar]
- Putnam CT, Kiricsi M, Pearcey J, MacLean IM, Bamford JA, Murdoch GK, Dixon WT, Pette D. AMPK activation increases uncoupling protein-3 expression and mitochondrial enzyme activities in rat muscle without fibre type transitions. J Physiol. 2003;551:169–178. doi: 10.1113/jphysiol.2003.040691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarpulla RC. Nuclear control of respiratory chain expression in mammalian cells. J Bioenerg Biomembr. 1997;29:109–119. doi: 10.1023/a:1022681828846. [DOI] [PubMed] [Google Scholar]
- Shulman GI. Cellular mechanisms of insulin resistance. J Clin Invest. 2000;106:171–176. doi: 10.1172/JCI10583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sriwijitkamol A, Ivy JL, Christ-Roberts C, Defronzo RA, Mandarino LJ, Musi N. LKB1-AMPK signaling in muscle from obese insulin resistant zucker rats and effects of training. Am J Physiol Endocrinol Metab. 2005;290:E925–E932. doi: 10.1152/ajpendo.00429.2005. [DOI] [PubMed] [Google Scholar]
- Steinberg GR, Smith AC, Van Denderen BJ, Chen Z, Murthy S, Campbell DJ, Heigenhauser GJ, Dyck DJ, Kemp BE. AMP-activated protein kinase is not down-regulated in human skeletal muscle of obese females. J Clin Endocrinol Metab. 2004;89:4575–4580. doi: 10.1210/jc.2004-0308. [DOI] [PubMed] [Google Scholar]
- Terada S, Goto M, Kato M, Kawanaka K, Shimokawa T, Tabata I. Effects of low-intensity prolonged exercise on PGC-1 mRNA expression in rat epitrochlearis muscle. Biochem Biophys Res Commun. 2002;296:350–354. doi: 10.1016/s0006-291x(02)00881-1. [DOI] [PubMed] [Google Scholar]
- Trifunovic A, Wredenberg A, Falkenberg M, Spelbrink JN, Rovio AT, Bruder CE, Bohlooly YM, Gidlof S, Oldfors A, Wibom R, Tornell J, Jacobs HT, Larsson NG. Premature aging in mice expressing defective mitochondrial DNA polymerase. Nature. 2004;429:417–423. doi: 10.1038/nature02517. [DOI] [PubMed] [Google Scholar]
- Virbasius JV, Scarpulla RC. Activation of the human mitochondrial transcription factor A gene by nuclear respiratory factors: a potential regulatory link between nuclear and mitochondrial gene expression in organelle biogenesis. Proc Natl Acad Sci U S A. 1994;91:1309–1313. doi: 10.1073/pnas.91.4.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winder WW, Holmes BF, Rubink DS, Jensen EB, Chen M, Holloszy JO. Activation of AMP-activated protein kinase increases mitochondrial enzymes in skeletal muscle. J Appl Physiol. 2000;88:2219–2226. doi: 10.1152/jappl.2000.88.6.2219. [DOI] [PubMed] [Google Scholar]
- Wu H, Kanatous SB, Thurmond FA, Gallardo T, Isotani E, Bassel-Duby R, Williams RS. Regulation of mitochondrial biogenesis in skeletal muscle by CaMK. Science. 2002;296:349–352. doi: 10.1126/science.1071163. [DOI] [PubMed] [Google Scholar]
- Wu H, Naya FJ, McKinsey TA, Mercer B, Shelton JM, Chin ER, Simard AR, Michel RN, Bassel-Duby R, Olson EN, Williams RS. MEF2 responds to multiple calcium-regulated signals in the control of skeletal muscle fiber type. EMBO J. 2000;19:1963–1973. doi: 10.1093/emboj/19.9.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z, Puigserver P, Andersson U, Zhang C, Adelmant G, Mootha V, Troy A, Cinti S, Lowell B, Scarpulla RC, Spiegelman BM. Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell. 1999;98:115–124. doi: 10.1016/S0092-8674(00)80611-X. [DOI] [PubMed] [Google Scholar]
- Yechoor VK, Patti ME, Saccone R, Kahn CR. Coordinated patterns of gene expression for substrate and energy metabolism in skeletal muscle of diabetic mice. Proc Natl Acad Sci U S A. 2002;99:10587–10592. doi: 10.1073/pnas.142301999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou M, Lin BZ, Coughlin S, Vallega G, Pilch PF. UCP-3 expression in skeletal muscle: effects of exercise, hypoxia, and AMP-activated protein kinase. Am J Physiol Endocrinol Metab. 2000;279:E622–E629. doi: 10.1152/ajpendo.2000.279.3.E622. [DOI] [PubMed] [Google Scholar]
- Zong H, Ren JM, Young LH, Pypaert M, Mu J, Birnbaum MJ, Shulman GI. AMP kinase is required for mitochondrial biogenesis in skeletal muscle in response to chronic energy deprivation. Proc Natl Acad Sci U S A. 2002;25:15983–15987. doi: 10.1073/pnas.252625599. [DOI] [PMC free article] [PubMed] [Google Scholar]