Abstract
The LKB1→AMPK cascade is switched on by metabolic stresses that either inhibit ATP production (e.g. hypoxia, hypoglycaemia) or that accelerate ATP consumption (e.g. muscle contraction). Any decline in cellular energy status is accompanied by a rise in the cellular AMP: ATP ratio, and this activates AMPK by a complex and sensitive mechanism involving antagonistic binding of the nucleotides to two sites on the regulatory γ subunits of AMPK. Once activated by metabolic stress, AMPK activates catabolic pathways that generate ATP, while inhibiting cell growth and biosynthesis and other processes that consume ATP. While the AMPK system probably evolved in single-celled eukaryotes to maintain energy balance at the cellular level, in multicellular organisms its role has become adapted so that it is also involved in maintaining whole body energy balance. Thus, it is regulated by hormones and cytokines, especially the adipokines leptin and adiponectin, increasing whole body energy expenditure while regulating food intake. Some hormones may activate AMPK by an LKB1-independent mechanism involving Ca2+/calmodulin dependent protein kinase kinases. Low levels of activation of AMPK are likely to play a role in the current global rise in obesity and Type 2 diabetes, and AMPK is the target for the widely used antidiabetic drug metformin.
Introduction
A helpful analogy can be drawn between ATP and ADP and the chemicals in an electrical battery. Catabolism ‘charges up the battery’ by converting ADP to ATP, whereas most other tasks performed by the cell require energy and are driven, directly or indirectly, by hydrolysis of ATP to ADP or, less commonly, AMP. There is no a priori reason why these two opposing processes should always remain in balance, especially when the conditions experienced by the cell are fluctuating, but cells usually maintain their ATP: ADP ratio within rather narrow limits. How do they achieve this remarkable feat? While there are almost certainly multiple mechanisms, our central theme is that the AMP-activated protein kinase (AMPK) system is the key player.
With the benefit of hindsight, AMPK was discovered independently as soluble protein factors that, in the presence of ATP, caused time-dependent inactivation of the key regulatory enzymes of fatty acid and cholesterol synthesis, i.e. acetyl-CoA carboxylase (Carlson & Kim, 1973) and 3-hydroxy-3-methylglutaryl (HMG)-CoA reductase (HMGR) (Beg et al. 1973). Although these factors were correctly surmised to be protein kinases, it was to be 14 years before it was realized that they were in fact the same entity. The HMG-CoA reductase kinase activity was initially reported to require ADP, as well as ATP, for activity (Brown et al. 1975), but this was probably an artefact caused by contamination by adenylate kinase, and it was later shown that 5′-AMP was the true activator (Ferrer et al. 1985). Another key finding was that HMG-CoA reductase kinase was inactivated by protein phosphatases and reactivated by an upstream kinase (Ingebritsen et al. 1978), making this only the second protein kinase cascade to be discovered.
In 1987 our laboratory reported that the acetyl-CoA carboxylase kinase and HMG-CoA reductase kinase activities were both functions of a single protein kinase (Carling et al. 1987). Since it soon became clear that it was a true multisubstrate protein kinase, we followed the precedent set by cyclic AMP-dependent protein kinase (which had originally been termed phosphorylase kinase kinase) and named it after its allosteric activator, AMP (Munday et al. 1988). Unfortunately, some workers refer to it erroneously as ‘AMP-dependent protein kinase’ rather than the correct ‘AMP-activated protein kinase’. We were careful to avoid the former, partly because it does have a significant basal activity in the absence of AMP, and partly to avoid confusion with cyclic AMP-dependent protein kinase.
AMPK – structure and regulation
The modern era of research on AMPK arrived in 1994 with purification of the kinase to homogeneity, revealing that it contained three subunits (Davies et al. 1994; Mitchelhill et al. 1994), and the first cloning of DNA encoding a catalytic subunit (Woods et al. 1994). The kinase is now known to be a heterotrimeric complex comprising a catalytic α subunit and regulatory β and γ subunits. Each subunit is encoded by multiple genes (α1, α2; β1, β2; γ1, γ2, γ3) yielding at least 12 heterotrimeric combinations, with splice variants further adding to the diversity. Obvious orthologues of the α, β and γ subunits occur in all eukaryotic species for which genome sequences have been completed, including plants and fungi as well as animals, and even very primitive protozoa like Giardia lamblia (Hardie et al. 2003). This suggests that the AMPK system arose very early during eukaryotic evolution. In budding yeast (Saccharomyces cerevisiae), all three subunits are required for the response to starvation for nutrients, especially glucose (Schmidt & McCartney, 2000), while in the primitive green plant Physcomitrella patens the catalytic subunits are required for survival during periods of darkness (Thelander et al. 2004), which is the equivalent of starvation for a photosynthetic organism. Thus, the response to starvation may have been an ancient and critical function of the system.
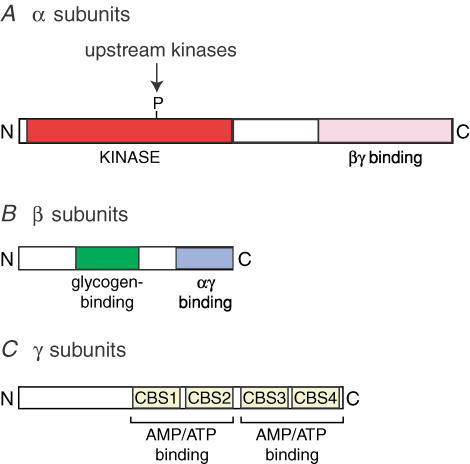
The three subunits of the kinase have a similar domain structure in all eukaryotes (Fig. 1). The α subunits contains a conventional serine/threonine kinase domain at the N-terminus, with the threonine residue whose phosphorylation is required for activity (Thr-172; Hawley et al. 1996) being located in the activation loop, the same region as in many other protein kinases that are activated by phosphorylation. The C-terminal region of the α subunit is required for formation of the complex with β and γ (Crute et al. 1998). The β subunits contain a C-terminal region required for complex formation, and a central ‘N-isoamylase domain’ that causes AMPK complexes to bind to glycogen (Hudson et al. 2003; Polekhina et al. 2003). Although this domain is the first region of an AMPK complex for which a crystal structure has been determined (Polekhina et al. 2005), its physiological role remains unclear. The γ subunits contain variable N-terminal regions followed by four tandem repeats of a sequence known as a CBS motif (Bateman, 1997). These are now known to act in pairs to form two modules called Bateman domains (Kemp, 2004), the function of which is discussed further below.
Figure 1. Typical domain structure of the α, β and γ subunits of AMPK and its homologues in lower eukaryotes.
Activation of the AMPK complex by AMP occurs by three independent mechanisms (Hardie et al. 1999): (i) allosteric activation of the phosphorylated enzyme; (ii) promotion of phosphorylation of Thr-172 by the upstream kinase; (iii) inhibition of dephosphorylation of Thr-172 by protein phosphatases. This triple mechanism means that the system is ultrasensitive, i.e. a small change in concentration of the input (AMP) can be converted into a much larger change in the final output (kinase activity) (Hardie et al. 1999). The Bateman domains on the γ subunits are now known to form the AMP binding sites: mutations in these domains in the γ2 isoform cause hereditary heart disease, and prevent both AMP binding and activation by AMP (Scott et al. 2004). Since mutations in either the N-terminal or C-terminal Bateman domain can prevent activation, it seems likely that AMP has to be bound at both sites for activation to occur. Binding to the two sites is also highly co-operative, with a Hill coefficient close to 2. This implies that one site has no affinity for AMP until the nucleotide has bound at the other, and represents a further mechanism to increase the sensitivity of the system. The Bateman domains also bind ATP in a manner that is mutually exclusive with AMP, although their affinity for ATP is lower than that for AMP and much lower than that of the catalytic domain (Scott et al. 2004). Since ATP binding does not cause any of the three activating effects of AMP, high concentrations of ATP inhibit activation of AMPK by antagonizing binding of AMP.
We started this review by arguing that the AMPK system was a sensor of cellular energy status, but why does it respond to AMP and ATP rather than ADP and ATP? Eukaryotic cells universally express the enzyme adenylate kinase, which catalyses the reaction 2ADP ⇌ ATP + AMP and maintains it close to equilibrium at all times. It is easy to show (Hardie et al. 2003) that this causes the cellular AMP: ATP ratio to vary approximately as the square of the ADP: ATP ratio. The former ratio is thus a much more sensitive indicator of compromised energy status than the latter. It is interesting that many of the features of regulation of the AMPK system seem to be geared to maximizing its sensitivity.
Identification of the upstream kinases
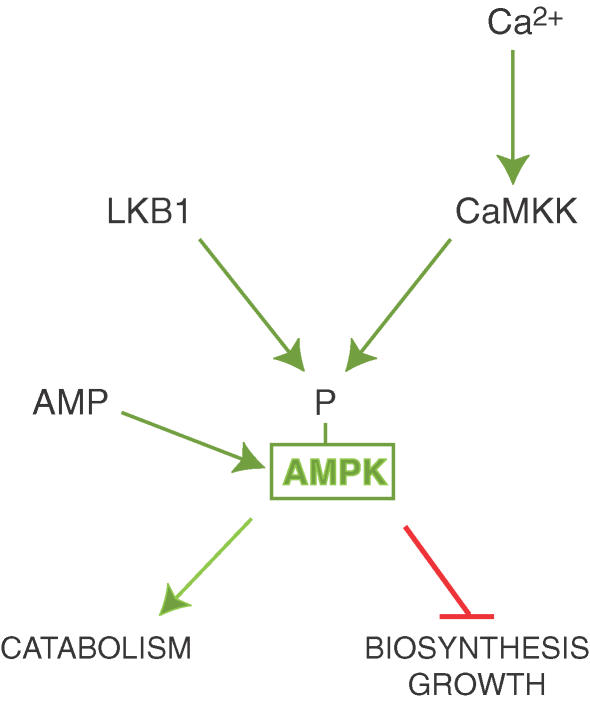
Although evidence was reported in 1978 that AMPK (then called HMG-CoA reductase kinase) was activated by an upstream kinase (Ingebritsen et al. 1978), it was to be 25 years before the latter was identified. An ‘AMP-activated protein kinase kinase’ (AMPKK) was partially purified in 1996 (Hawley et al. 1996), but attempts to purify it to homogeneity proved frustrating. However, after the completion of the budding yeast genome project, three protein kinases acting upstream of the yeast homologue of AMPK (the SNF1 complex) were identified by various genome-wide screening approaches (Hong et al. 2003; Sutherland et al. 2003). While none had a clear and obvious mammalian homologue, their kinase domains were most closely related to those of the protein kinase LKB1, and the calmodulin-dependent protein kinase kinases, CaMKKα and CaMKKβ. In a rapid flurry of activity, evidence was obtained that all three, but especially LKB1 and CaMKKβ, can phosphorylate Thr-172 and thus activate AMPK in intact cells and in vivo (Hawley et al. 2003, 2005; Woods et al. 2003, 2005; Shaw et al. 2004; Hurley et al. 2005; Sakamoto et al. 2005, 2006).
LKB1 exists as a complex with two accessory subunits, STRAD and MO25, and the AMPKK partially purified earlier (Hawley et al. 1996) was shown to be a complex between LKB1 and the α isoforms of STRAD and MO25 (Hawley et al. 2003). Intriguingly, LKB1 had been originally identified in humans as a tumour suppressor, indicating for the first time a link between AMPK and cancer. LKB1 is the gene mutated in the rare autosomal dominant human genetic disorder, Peutz–Jeghers syndrome (PJS) (Hemminki et al. 1998; Jenne et al. 1998). PJS subjects develop numerous benign tumours in the gastrointestinal tract, but also have a 20-fold increased risk of developing malignant tumours at other sites (Giardiello et al. 2000). Moreover, mutations in the LKB1 gene are also seen in some sporadic cancers, especially adenocarcinoma of the lung (Sanchez-Cespedes et al. 2002). LKB1 is required for activation of AMPK in response to treatments that elevate AMP or AMP mimetic agents, both in cultured cells (Hawley et al. 2003) and in skeletal muscle in vivo (Sakamoto et al. 2006). However, the LKB1 complex itself is not regulated by AMP and appears to be constitutively active (Lizcano et al. 2004; Sakamoto et al. 2004), with activation of the cascade being produced by the binding of AMP to AMPK causing it to become a better substrate for LKB1 (Hawley et al. 2003) (Fig. 2).
Figure 2. Upstream regulators of AMPK.
LKB1 and CaMKKs phosphorylate the same residue (Thr-172) on the α subunit of AMPK. Phosphorylation by LKB1, but not by CaMKKs, is stimulated by binding of AMP to AMPK.
Surprisingly, increases in AMP do not stimulate phosphorylation of Thr-172 by the CaMKKs, which is triggered instead by a rise in Ca2+ (Fig. 2) (Hawley et al. 2005; Hurley et al. 2005; Woods et al. 2005). While LKB1 is ubiquitously expressed, a limited survey of different tissues suggest that the CaMKKs are mainly expressed in neural tissues (Anderson et al. 1998). Experiments with muscle-specific knockouts of LKB1 in mice suggest that the Ca2+-mediated pathway cannot be a significant player in skeletal muscle (Sakamoto et al. 2005), but a more thorough investigation of the distribution of the Ca2+-mediated pathway needs to be performed.
Metabolic stresses that activate AMPK in intact cells and in vivo
Arguments discussed above suggest that the LKB1→AMPK cascade will be activated in a highly sensitive manner by even a very small decrease in cellular energy status. What conditions cause this to happen? Similar to its homologues in single celled eukaryotes like S. cerevisiae (Wilson et al. 1996), AMPK can be activated in cultured mammalian cells by glucose deprivation (Salt et al. 1998). However, regulation of AMPK by glucose in vivo may be normally restricted to specialized cells, including pancreatic β cells that secrete insulin in response to the level of glucose in the portal circulation, and cells in the hypothalamus that initiate feeding behaviour in response to hypoglycaemia. Like β cells, cells in the hypothalamus are unusual in that they express the high capacity glucose transporter, GLUT2, and the high Km isoform of hexokinase known as hexokinase IV or glucokinase (Kang et al. 2006). Thus, in these cell types the rate of glucose metabolism responds to physiological fluctuations in blood glucose, whereas in most other cells production of ATP from glucose would only decrease when blood glucose dropped to pathologically low levels. Both in β cells and in the hypothalamus there is evidence that AMPK activity correlates inversely with glucose over a physiologically relevant concentration range (Salt et al. 1998; da Silva Xavier et al. 2003; Minokoshi et al. 2004). Moreover, activation of AMPK by pharmacological means, or by over-expression of activated AMPK mutants, inhibits insulin release by pancreatic β cells, while in the hypothalamus it stimulates feeding behaviour (Salt et al. 1998; da Silva Xavier et al. 2003; Andersson et al. 2004; Minokoshi et al. 2004). These results suggest that the AMPK system is part of the mechanism by which these specialized cells sense fluctuations in glucose, although other mechanisms like the binding of ATP to KATP channels (Antcliff et al. 2005) are certainly also involved.
Other metabolic stresses that activate AMPK include ischaemia (Kudo et al. 1995) or hypoxia (Marsin et al. 2000), which have been shown to activate the kinase in heart muscle. Like regulation by glucose, activation of AMPK by hypoxia probably only occurs in many tissues under pathological conditions. However, just as there are specialized glucose-sensing cells in the pancreas and hypothalamus, there are specialized oxygen-sensing cells where regulation of AMPK by hypoxia may be a more normal physiological event. These include pulmonary artery smooth muscle cells, which contract in response to hypoxia and thus divert blood flow to oxygen-rich areas of the lung, and Type 1 cells in the carotid body, which stimulate afferent fibres leading to the brain in response to hypoxia, causing a compensatory increase in the rate of breathing (Gonzalez et al. 1994; Lopez-Barneo et al. 2001). In both cases, there is evidence that AMPK is involved in the oxygen sensing mechanism (Evans et al. 2005), a topic that is discussed in more detail elsewhere in this issue.
Another key metabolic stress that activates AMPK is exercise or contraction in skeletal muscle (Hardie & Sakamoto, 2006). The degree of activation of AMPK seems to be dependent on the level of metabolic stress caused by the contraction. For example, prior endurance training reduces the effect of the same intensity of exercise both in rodents (Durante et al. 2002) and humans (Nielsen et al. 2003). Interestingly, AMPK has also been found to be activated by strength exercise in endurance-trained humans, and by endurance exercise in strength-trained humans, but not vice versa (Coffey et al. 2006). This implies that the AMPK system may be particularly important during exercise of an intensity that is greater than that to which the individual is normally adapted. It seems likely, although not conclusively proven, that AMPK is activated by the increases in cellular AMP: ATP ratio that accompany such exercise. In mice, contraction of leg muscles produced by electrical stimulation of the sciatic nerve in situ causes increases in the ADP: ATP ratio and (as expected) even larger changes in the AMP: ATP ratio (Sakamoto et al. 2005). Intriguingly, the changes in nucleotide ratios are even greater in mice in which expression of the upstream kinase LKB1 in muscle is knocked out. In these mice the basal activity of the α2 isoform of AMPK is completely abolished, and the activities of the α1 and α2 isoforms do not increase in response to contraction (Sakamoto et al. 2005). These results confirm that the AMPK system helps to protect muscle against the metabolic stresses caused by contraction, in which the demand for ATP can be increased by more than 100-fold within seconds.
The response to metabolic stresses, such as starvation, is probably the key role for AMPK in single celled eukaryotes, and may have been its original raison d'être. However, it is now becoming clear that, during the course of evolution of multicellular organisms, hormones and cytokines have acquired the ability to regulate the AMPK system. Particularly interesting were findings that AMPK is activated by the adipokines, leptin in skeletal muscle (Minokoshi et al. 2002), and adiponectin in skeletal muscle and liver (Tomas et al. 2002; Yamauchi et al. 2002). This increases energy expenditure in muscle by increasing fatty acid oxidation and up-regulating mitochondrial biogenesis (see below). Conversely, leptin inhibits AMPK in the hypothalamus, consistent with the ability of the adipokine to inhibit food intake (Minokoshi et al. 2004). These results suggest that AMPK also has a key role in the regulation of body weight and whole body energy balance.
Downstream targets of AMPK
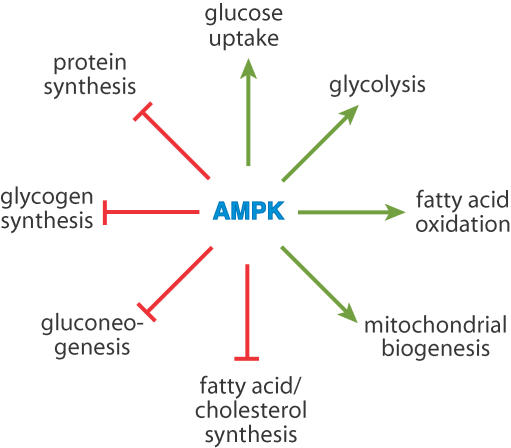
Some of the key effects of AMPK on energy metabolism are illustrated in Fig. 3. Many of the downstream effects were originally demonstrated using the compound 5-aminoimidazole-4-carboxamide (AICA) riboside, a nucleoside that is taken up into cells and converted to an AMP analogue, AICA riboside monophosphate (ZMP), which mimics all three effects of AMP on the AMPK system (Corton et al. 1995). As discussed above, the first targets of AMPK to be identified were acetyl-CoA carboxylase and HMG-CoA reductase, and using AICA riboside it was demonstrated that activation of AMPK caused consequent inhibition of fatty acid and cholesterol synthesis in heptocytes (Corton et al. 1995; Henin et al. 1995). AMPK activation also inhibits muscle glycogen synthesis via phosphorylation of glycogen synthase (Carling & Hardie, 1989; Jorgensen et al. 2004), protein synthesis in many cells via inhibition of the target-of-rapamycin (TOR) pathway (Inoki et al. 2003) and activation of elongation factor-2 kinase (Horman et al. 2002), and de novo glucose synthesis (gluconeogenesis) in the liver via phosphorylation of the transcriptional coactivator TORC2, which inhibits expression of genes involved in this pathway (Lochhead et al. 2000; Koo et al. 2005). This generalized inhibition of biosynthesis by AMPK (particularly its ability to inhibit the growth-promoting TOR pathway) may partly explain the tumour suppressor effects of the upstream kinase LKB1. In addition, AMPK activation appears to cause a G1 phase cell cycle arrest that is dependent on another tumour suppressor, p53 (Imamura et al. 2001; Jones et al. 2005). This arrest would prevent entry into S phase of the cell cycle when DNA replication takes place, the latter being another costly process in terms of ATP turnover.
Figure 3. Key processes of energy metabolism that are regulated by AMPK.
A green arrow indicates activation, whereas a red line with a bar at the end indicates inhibition. Some of these processes are regulated by multiple effects of AMPK. For example, glucose uptake and fatty acid synthesis are regulated both acutely (with no change in gene expression) and chronically (via effects on gene expression).
As well as conserving ATP by inhibiting biosynthetic pathways, AMPK activation stimulates catabolic pathways that generate ATP. In skeletal muscle it stimulates glucose uptake (Merrill et al. 1997), both via translocation of the glucose transporter GLUT4 to the plasma membrane (Kurth-Kraczek et al. 1999) and, in the longer term, by increasing its expression (Holmes et al. 1999). Studies of mice in which AMPK activation has been knocked out by various means show that AMPK is at least partly responsible for the increased glucose uptake during muscle contraction (Mu et al. 2001; Sakamoto et al. 2005). In other cells, AMPK activation increases the intrinsic activity of the glucose transporter GLUT1 by an unknown mechanism (Barnes et al. 2002). In some cell types such as cardiac myocytes (Marsin et al. 2000) and monocytes and macrophages (Marsin et al. 2002), AMPK phosphorylates and activates 6-phosphofructo-2-kinase, thus increasing the glycolytic activator fructose-2,6-bisphosphate, stimulating ATP production by glycolysis in response to hypoxia. By phosphorylation of the ACC-2 (-β) isoform of acetyl-CoA carboxylase AMPK lowers malonyl-CoA, relieving inhibition of uptake of fatty acids into mitochondria via the carnitine carrier system, and thus stimulating fatty acid oxidation (Merrill et al. 1997). As well as these acute effects on glucose and fatty acid oxidation, AMPK also up-regulates mitochondrial biogenesis (Winder et al. 2000), thus increasing the capacity of tissues for aerobic production of ATP. This appears to involve increased expression of the transcriptional coactivator PGC-1α (Zong et al. 2002).
Although many of the targets initially identified for AMPK are involved in energy metabolism, examples of targets involved in other processes are increasingly being found. For example, ion channels have the potential to initiate very significant ATP turnover, due to their ability to rapidly dissipate concentration gradients across membranes when in the open state. Transepithelial NaCl transport, an energetically costly process for epithelial cells, is inhibited by effects of AMPK both on the cystic fibrosis transmembrane regulator Cl− channel (CFTR) (Hallows et al. 2000, 2003a, b) and the amiloride-sensitive Na+ channel (ENaC) (Carattino et al. 2005; Woollhead et al. 2005). In our view there are likely to be many other undiscovered targets for AMPK within ion channels and pumps, and this is an area that is ripe for further exploration.
Conclusions and medical perspectives
To summarize, AMPK is activated by metabolic stresses that either inhibit ATP production (e.g. hypoxia, hypoglycaemia) or accelerate ATP consumption (e.g. muscle contraction). Once activated by such metabolic stresses, it switches on catabolic pathways that generate ATP, while switching off biosynthetic pathways and other processes that consume ATP. Its key roles in maintaining energy balance, both at the single cell and the whole body levels, suggest that it will also be an important player in the derangements of energy metabolism that occur in conditions like obesity, Type 2 diabetes and the metabolic syndrome. There is no current evidence that mutations or altered expression of AMPK is a common cause of Type 2 diabetes in humans, but the latter is strongly correlated with obesity and a sedentary lifestyle, and a low activation state of AMPK in the periphery, due to over-nutrition and lack of exercise, may be a contributory factor in its onset. Consistent with this, two of the major classes of drug currently widely used to treat Type 2 diabetes, i.e. biguanides like metformin (Zhou et al. 2001) and the thiazolidinediones (Fryer et al. 2002), have been reported to activate AMPK. Both appear to do this indirectly by inhibiting complex I of the respiratory chain (El-Mir et al. 2000; Owen et al. 2000; Brunmair et al. 2004). The thiazolidinediones also have other effects, but there is now good evidence, from studies of mice that are deficient in the upstream kinase LKB1 in the liver, that the blood glucose lowering effects of metformin are mediated entirely by AMPK (Shaw et al. 2005). Given the increasing prevalence of Type 2 diabetes, with estimates of almost 200 million people (5% of the adult population) suffering from the condition worldwide in 2003 (www.idf.org), and given that metformin is used for treatment of over 120 million, the growing attention on the AMPK system is likely to continue for the foreseeable future.
Acknowledgments
The authors are supported by an Integrated Project (LSHM-CT-2004-005272) under Framework Programme 6 of the European Commission, and a Programme Grant from the Wellcome Trust.
References
- Anderson KA, Means RL, Huang QH, Kemp BE, Goldstein EG, Selbert MA, Edelman AM, Fremeau RT, Means AR. Components of a calmodulin-dependent protein kinase cascade. Molecular cloning, functional characterization and cellular localization of Ca2+/calmodulin-dependent protein kinase kinase β. J Biol Chem. 1998;273:31880–31889. doi: 10.1074/jbc.273.48.31880. [DOI] [PubMed] [Google Scholar]
- Andersson U, Filipsson K, Abbott CR, Woods A, Smith K, Bloom SR, Carling D, Small CJ. AMP-activated protein kinase plays a role in the control of food intake. J Biol Chem. 2004;279:12005–12008. doi: 10.1074/jbc.C300557200. [DOI] [PubMed] [Google Scholar]
- Antcliff JF, Haider S, Proks P, Sansom MS, Ashcroft FM. Functional analysis of a structural model of the ATP-binding site of the KATP channel Kir6.2 subunit. EMBO J. 2005;24:229–239. doi: 10.1038/sj.emboj.7600487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes K, Ingram JC, Porras OH, Barros LF, Hudson ER, Fryer LG, Foufelle F, Carling D, Hardie DG, Baldwin SA. Activation of GLUT1 by metabolic and osmotic stress: potential involvement of AMP-activated protein kinase (AMPK) J Cell Sci. 2002;115:2433–2442. doi: 10.1242/jcs.115.11.2433. [DOI] [PubMed] [Google Scholar]
- Bateman A. The structure of a domain common to archaebacteria and the homocystinuria disease protein. Trends Biochem Sci. 1997;22:12–13. doi: 10.1016/s0968-0004(96)30046-7. [DOI] [PubMed] [Google Scholar]
- Beg ZH, Allmann DW, Gibson DM. Modulation of 3-hydroxy-3-methylglutaryl coenzyme: a reductase activity with cAMP and with protein fractions of rat liver cytosol. Biochem Biophys Res Comm. 1973;54:1362–1369. doi: 10.1016/0006-291x(73)91137-6. [DOI] [PubMed] [Google Scholar]
- Brown MS, Brunschede GY, Goldstein JL. Inactivation of 3-hydroxy-3-methylglutaryl coenzyme A reductase in vitro. An adenine nucleotide-dependent reaction catalyzed by a factor in human fibroblasts. J Biol Chem. 1975;250:2502–2509. [PubMed] [Google Scholar]
- Brunmair B, Staniek K, Gras F, Scharf N, Althaym A, Clara R, Roden M, Gnaiger E, Nohl H, Waldhausl W, Furnsinn C. Thiazolidinediones, like metformin, inhibit respiratory complex I: a common mechanism contributing to their antidiabetic actions? Diabetes. 2004;53:1052–1059. doi: 10.2337/diabetes.53.4.1052. [DOI] [PubMed] [Google Scholar]
- Carattino MD, Edinger RS, Grieser HJ, Wise R, Neumann D, Schlattner U, Johnson JP, Kleyman TR, Hallows KR. Epithelial sodium channel inhibition by AMP-activated protein kinase in oocytes and polarized renal epithelial cells. J Biol Chem. 2005;280:17608–17616. doi: 10.1074/jbc.M501770200. [DOI] [PubMed] [Google Scholar]
- Carling D, Hardie DG. The substrate and sequence specificity of the AMP-activated protein kinase. Phosphorylation of glycogen synthase and phosphorylase kinase. Biochim Biophys Acta. 1989;1012:81–86. doi: 10.1016/0167-4889(89)90014-1. [DOI] [PubMed] [Google Scholar]
- Carling D, Zammit VA, Hardie DG. A common bicyclic protein kinase cascade inactivates the regulatory enzymes of fatty acid and cholesterol biosynthesis. FEBS Lett. 1987;223:217–222. doi: 10.1016/0014-5793(87)80292-2. [DOI] [PubMed] [Google Scholar]
- Carlson CA, Kim KH. Regulation of hepatic acetyl coenzyme A carboxylase by phosphorylation and dephosphorylation. J Biol Chem. 1973;248:378–380. [PubMed] [Google Scholar]
- Coffey VG, Zhong Z, Shield A, Canny BJ, Chibalin AV, Zierath JR, Hawley JA. Early signaling responses to divergent exercise stimuli in skeletal muscle from well-trained humans. FASEB J. 2006;20:190–192. doi: 10.1096/fj.05-4809fje. [DOI] [PubMed] [Google Scholar]
- Corton JM, Gillespie JG, Hawley SA, Hardie DG. 5-Aminoimidazole-4-carboxamide ribonucleoside: a specific method for activating AMP-activated protein kinase in intact cells? Eur J Biochem. 1995;229:558–565. doi: 10.1111/j.1432-1033.1995.tb20498.x. [DOI] [PubMed] [Google Scholar]
- Crute BE, Seefeld K, Gamble J, Kemp BE, Witters LA. Functional domains of the α1 catalytic subunit of the AMP-activated protein kinase. J Biol Chem. 1998;273:35347–35354. doi: 10.1074/jbc.273.52.35347. [DOI] [PubMed] [Google Scholar]
- Davies SP, Hawley SA, Woods A, Carling D, Haystead TAJ, Hardie DG. Purification of the AMP-activated protein kinase on ATP-γ-Sepharose and analysis of its subunit structure. Eur J Biochem. 1994;223:351–357. doi: 10.1111/j.1432-1033.1994.tb19001.x. [DOI] [PubMed] [Google Scholar]
- Durante PE, Mustard KJ, Park SH, Winder WW, Hardie DG. Effects of endurance training on activity and expression of AMP-activated protein kinase isoforms in rat muscles. Am J Physiol. 2002;283:E178–E186. doi: 10.1152/ajpendo.00404.2001. [DOI] [PubMed] [Google Scholar]
- El-Mir MY, Nogueira V, Fontaine E, Averet N, Rigoulet M, Leverve X. Dimethylbiguanide inhibits cell respiration via an indirect effect targeted on the respiratory chain complex I. J Biol Chem. 2000;275:223–228. doi: 10.1074/jbc.275.1.223. [DOI] [PubMed] [Google Scholar]
- Evans AM, Mustard KJ, Wyatt CN, Peers C, Dipp M, Kumar P, Kinnear NP, Hardie DG. Does AMP-activated protein kinase couple inhibition of mitochondrial oxidative phosphorylation by hypoxia to calcium signaling in O2-sensing cells? J Biol Chem. 2005;280:41504–41511. doi: 10.1074/jbc.M510040200. [DOI] [PubMed] [Google Scholar]
- Ferrer A, Caelles C, Massot N, Hegardt FG. Activation of rat liver cytosolic 3-hydroxy-3-methylglutaryl coenzyme A reductase kinase by adenosine 5′-monophosphate. Biochem Biophys Res Comm. 1985;132:497–504. doi: 10.1016/0006-291x(85)91161-1. [DOI] [PubMed] [Google Scholar]
- Fryer LG, Parbu-Patel A, Carling D. The anti-diabetic drugs rosiglitazone and metformin stimulate AMP-activated protein kinase through distinct pathways. J Biol Chem. 2002;277:25226–25232. doi: 10.1074/jbc.M202489200. [DOI] [PubMed] [Google Scholar]
- Giardiello FM, Brensinger JD, Tersmette AC, Goodman SN, Petersen GM, Booker SV, Cruz-Correa M, Offerhaus JA. Very high risk of cancer in familial Peutz–Jeghers syndrome. Gastroenterology. 2000;119:1447–1453. doi: 10.1053/gast.2000.20228. [DOI] [PubMed] [Google Scholar]
- Gonzalez C, Almaraz L, Obeso A, Rigual R. Carotid body chemoreceptors: from natural stimuli to sensory discharges. Physiol Rev. 1994;74:829–898. doi: 10.1152/physrev.1994.74.4.829. [DOI] [PubMed] [Google Scholar]
- Hallows KR, Kobinger GP, Wilson JM, Witters LA, Foskett JK. Physiological modulation of CFTR activity by AMP-activated protein kinase in polarized T84 cells. Am J Physiol Cell Physiol. 2003a;284:C1297–C1308. doi: 10.1152/ajpcell.00227.2002. [DOI] [PubMed] [Google Scholar]
- Hallows KR, McCane JE, Kemp BE, Witters LA, Foskett JK. Regulation of channel gating by AMP-activated protein kinase modulates cystic fibrosis transmembrane conductance regulator activity in lung submucosal cells. J Biol Chem. 2003b;278:998–1004. doi: 10.1074/jbc.M210621200. [DOI] [PubMed] [Google Scholar]
- Hallows KR, Raghuram V, Kemp BE, Witters LA, Foskett JK. Inhibition of cystic fibrosis transmembrane conductance regulator by novel interaction with the metabolic sensor AMP-activated protein kinase. J Clin Invest. 2000;105:1711–1721. doi: 10.1172/JCI9622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardie DG, Sakamoto K. AMPK: a key sensor of fuel and energy status in skeletal muscle. Physiology (Bethesda) 2006;21:48–60. doi: 10.1152/physiol.00044.2005. [DOI] [PubMed] [Google Scholar]
- Hardie DG, Salt IP, Hawley SA, Davies SP. AMP-activated protein kinase: an ultrasensitive system for monitoring cellular energy charge. Biochem J. 1999;338:717–722. [PMC free article] [PubMed] [Google Scholar]
- Hardie DG, Scott JW, Pan DA, Hudson ER. Management of cellular energy by the AMP-activated protein kinase system. FEBS Lett. 2003;546:113–120. doi: 10.1016/s0014-5793(03)00560-x. [DOI] [PubMed] [Google Scholar]
- Hawley SA, Boudeau J, Reid JL, Mustard KJ, Udd L, Makela TP, Alessi DR, Hardie DG. Complexes between the LKB1 tumor suppressor, STRADα/β and MO25α/β are upstream kinases in the AMP-activated protein kinase cascade. J Biol. 2003;2:28. doi: 10.1186/1475-4924-2-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawley SA, Davison M, Woods A, Davies SP, Beri RK, Carling D, Hardie DG. Characterization of the AMP-activated protein kinase kinase from rat liver, and identification of threonine-172 as the major site at which it phosphorylates and activates AMP-activated protein kinase. J Biol Chem. 1996;271:27879–27887. doi: 10.1074/jbc.271.44.27879. [DOI] [PubMed] [Google Scholar]
- Hawley SA, Pan DA, Mustard KJ, Ross L, Bain J, Edelman AM, Frenguelli BG, Hardie DG. Calmodulin-dependent protein kinase kinase-β is an alternative upstream kinase for AMP-activated protein kinase. Cell Metab. 2005;2:9–19. doi: 10.1016/j.cmet.2005.05.009. [DOI] [PubMed] [Google Scholar]
- Hemminki A, Markie D, Tomlinson I, Avizienyte E, Roth S, Loukola A, Bignell G, Warren W, Aminoff M, Hoglund P, Jarvinen H, Kristo P, Pelin K, Ridanpaa M, Salovaara R, Toro T, Bodmer W, Olschwang S, Olsen AS, Stratton MR, de la Chapelle A, Aaltonen LA. A serine/threonine kinase gene defective in Peutz–Jeghers syndrome. Nature. 1998;391:184–187. doi: 10.1038/34432. [DOI] [PubMed] [Google Scholar]
- Henin N, Vincent MF, Gruber HE, Van den Berghe G. Inhibition of fatty acid and cholesterol synthesis by stimulation of AMP-activated protein kinase. FASEB J. 1995;9:541–546. doi: 10.1096/fasebj.9.7.7737463. [DOI] [PubMed] [Google Scholar]
- Holmes BF, Kurth-Kraczek EJ, Winder WW. Chronic activation of 5′-AMP-activated protein kinase increases GLUT-4, hexokinase, and glycogen in muscle. J Appl Physiol. 1999;87:1990–1995. doi: 10.1152/jappl.1999.87.5.1990. [DOI] [PubMed] [Google Scholar]
- Hong SP, Leiper FC, Woods A, Carling D, Carlson M. Activation of yeast Snf1 and mammalian AMP-activated protein kinase by upstream kinases. Proc Natl Acad Sci U S A. 2003;100:8839–8843. doi: 10.1073/pnas.1533136100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horman S, Browne G, Krause U, Patel J, Vertommen D, Bertrand L, Lavoinne A, Hue L, Proud C, Rider M. Activation of AMP-activated protein kinase leads to the phosphorylation of elongation factor 2 and an inhibition of protein synthesis. Current Biol. 2002;12:1419–1423. doi: 10.1016/s0960-9822(02)01077-1. [DOI] [PubMed] [Google Scholar]
- Hudson ER, Pan DA, James J, Lucocq JM, Hawley SA, Green KA, Baba O, Terashima T, Hardie DG. A novel domain in AMP-activated protein kinase causes glycogen storage bodies similar to those seen in hereditary cardiac arrhythmias. Current Biol. 2003;13:861–866. doi: 10.1016/s0960-9822(03)00249-5. [DOI] [PubMed] [Google Scholar]
- Hurley RL, Anderson KA, Franzone JM, Kemp BE, Means AR, Witters LA. The Ca2+/calmoldulin-dependent protein kinase kinases are AMP-activated protein kinase kinases. J Biol Chem. 2005;280:29060–29066. doi: 10.1074/jbc.M503824200. [DOI] [PubMed] [Google Scholar]
- Imamura K, Ogura T, Kishimoto A, Kaminishi M, Esumi H. Cell cycle regulation via p53 phosphorylation by a 5′-AMP activated protein kinase activator, 5-aminoimidazole-4-carboxamide-1-β-d-ribofuranoside, in a human hepatocellular carcinoma cell line. Biochem Biophys Res Commun. 2001;287:562–567. doi: 10.1006/bbrc.2001.5627. [DOI] [PubMed] [Google Scholar]
- Ingebritsen TS, Lee H, Parker RA, Gibson DM. Reversible modulation of the activities of both liver microsomal hydroxymethylglutaryl coenzyme A reductase and its inactivating enzyme. Evidence for regulation by phosphorylation-dephosphorylation. Biochem Biophys Res Comm. 1978;81:1268–1277. doi: 10.1016/0006-291x(78)91273-1. [DOI] [PubMed] [Google Scholar]
- Inoki K, Zhu T, Guan KL. TSC2 mediates cellular energy response to control cell growth and survival. Cell. 2003;115:577–590. doi: 10.1016/s0092-8674(03)00929-2. [DOI] [PubMed] [Google Scholar]
- Jenne DE, Reimann H, Nezu J, Friedel W, Loff S, Jeschke R, Muller O, Back W, Zimmer M. Peutz–Jeghers syndrome is caused by mutations in a novel serine threonine kinase. Nat Genet. 1998;18:38–43. doi: 10.1038/ng0198-38. [DOI] [PubMed] [Google Scholar]
- Jones RG, Plas DR, Kubek S, Buzzai M, Mu J, Xu Y, Birnbaum MJ, Thompson CB. AMP-activated protein kinase induces a p53-dependent metabolic checkpoint. Mol Cell. 2005;18:283–293. doi: 10.1016/j.molcel.2005.03.027. [DOI] [PubMed] [Google Scholar]
- Jorgensen SB, Nielsen JN, Birk JB, Olsen GS, Viollet B, Andreelli F, Schjerling P, Vaulont S, Hardie DG, Hansen BF, Richter EA, Wojtaszewski JF. The α2–5′AMP-activated protein kinase is a site 2 glycogen synthase kinase in skeletal muscle and is responsive to glucose loading. Diabetes. 2004;53:3074–3081. doi: 10.2337/diabetes.53.12.3074. [DOI] [PubMed] [Google Scholar]
- Kang L, Dunn-Meynell AA, Routh VH, Gaspers LD, Nagata Y, Nishimura T, Eiki J, Zhang BB, Levin BE. Glucokinase is a critical regulator of ventromedial hypothalamic neuronal glucosensing. Diabetes. 2006;55:412–420. doi: 10.2337/diabetes.55.02.06.db05-1229. [DOI] [PubMed] [Google Scholar]
- Kemp BE. Bateman domains and adenosine derivatives form a binding contract. J Clin Invest. 2004;113:182–184. doi: 10.1172/JCI20846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo SH, Flechner L, Qi L, Zhang X, Screaton RA, Jeffries S, Hedrick S, Xu W, Boussouar F, Brindle P, Takemori H, Montminy M. The CREB coactivator TORC2 is a key regulator of fasting glucose metabolism. Nature. 2005;437:1109–1011. doi: 10.1038/nature03967. [DOI] [PubMed] [Google Scholar]
- Kudo N, Barr AJ, Barr RL, Desai S, Lopaschuk GD. High rates of fatty acid oxidation during reperfusion of ischemic hearts are associated with a decrease in malonyl-CoA levels due to an increase in 5′-AMP-activated protein kinase inhibition of acetyl-CoA carboxylase. J Biol Chem. 1995;270:17513–17520. doi: 10.1074/jbc.270.29.17513. [DOI] [PubMed] [Google Scholar]
- Kurth-Kraczek EJ, Hirshman MF, Goodyear LJ, Winder WW. 5′-AMP-activated protein kinase activation causes GLUT4 translocation in skeletal muscle. Diabetes. 1999;48:1667–1671. doi: 10.2337/diabetes.48.8.1667. [DOI] [PubMed] [Google Scholar]
- Lizcano JM, Göransson O, Toth R, Deak M, Morrice NA, Boudeau J, Hawley SA, Udd L, Mäkelä TP, Hardie DG, Alessi DR. LKB1 is a master kinase that activates 13 protein kinases of the AMPK subfamily, including the MARK/PAR-1 kinases. EMBO J. 2004;23:833–843. doi: 10.1038/sj.emboj.7600110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lochhead PA, Salt IP, Walker KS, Hardie DG, Sutherland C. 5-Aminoimidazole-4-carboxamide riboside mimics the effects of insulin on the expression of the 2 key gluconeogenic genes PEPCK and glucose-6-phosphatase. Diabetes. 2000;49:896–903. doi: 10.2337/diabetes.49.6.896. [DOI] [PubMed] [Google Scholar]
- Lopez-Barneo J, Pardal R, Ortega-Saenz P. Cellular mechanism of oxygen sensing. Annu Rev Physiol. 2001;63:259–287. doi: 10.1146/annurev.physiol.63.1.259. [DOI] [PubMed] [Google Scholar]
- Marsin AS, Bertrand L, Rider MH, Deprez J, Beauloye C, Vincent MF, Van den Berghe G, Carling D, Hue L. Phosphorylation and activation of heart PFK-2 by AMPK has a role in the stimulation of glycolysis during ischaemia. Current Biol. 2000;10:1247–1255. doi: 10.1016/s0960-9822(00)00742-9. [DOI] [PubMed] [Google Scholar]
- Marsin AS, Bouzin C, Bertrand L, Hue L. The stimulation of glycolysis by hypoxia in activated monocytes is mediated by AMP-activated protein kinase and inducible 6-phosphofructo-2-kinase. J Biol Chem. 2002;277:30778–30783. doi: 10.1074/jbc.M205213200. [DOI] [PubMed] [Google Scholar]
- Merrill GM, Kurth E, Hardie DG, Winder WW. AICAR decreases malonyl-CoA and increases fatty acid oxidation in skeletal muscle of the rat. Am J Physiol. 1997;273:E1107–E1112. doi: 10.1152/ajpendo.1997.273.6.E1107. [DOI] [PubMed] [Google Scholar]
- Minokoshi Y, Alquier T, Furukawa N, Kim YB, Lee A, Xue B, Mu J, Foufelle F, Ferre P, Birnbaum MJ, Stuck BJ, Kahn BB. AMP-kinase regulates food intake by responding to hormonal and nutrient signals in the hypothalamus. Nature. 2004;428:569–574. doi: 10.1038/nature02440. [DOI] [PubMed] [Google Scholar]
- Minokoshi Y, Kim YB, Peroni OD, Fryer LG, Muller C, Carling D, Kahn BB. Leptin stimulates fatty-acid oxidation by activating AMP-activated protein kinase. Nature. 2002;415:339–343. doi: 10.1038/415339a. [DOI] [PubMed] [Google Scholar]
- Mitchelhill KI, Stapleton D, Gao G, House C, Michell B, Katsis F, Witters LA, Kemp BE. Mammalian AMP-activated protein kinase shares structural and functional homology with the catalytic domain of yeast Snf1 protein kinase. J Biol Chem. 1994;269:2361–2364. [PubMed] [Google Scholar]
- Mu J, Brozinick JT, Valladares O, Bucan M, Birnbaum MJ. A role for AMP-activated protein kinase in contraction- and hypoxia-regulated glucose ransport in skeletal muscle. Mol Cell. 2001;7:1085–1094. doi: 10.1016/s1097-2765(01)00251-9. [DOI] [PubMed] [Google Scholar]
- Munday MR, Campbell DG, Carling D, Hardie DG. Identification by amino acid sequencing of three major regulatory phosphorylation sites on rat acetyl-CoA carboxylase. Eur J Biochem. 1988;175:331–338. doi: 10.1111/j.1432-1033.1988.tb14201.x. [DOI] [PubMed] [Google Scholar]
- Nielsen JN, Mustard KJ, Graham DAYuH, MacDonald CS, Pilegaard H, Goodyear LJ, Hardie DG, Richter EA, Wojtaszewski JF. 5′-AMP-activated protein kinase activity and subunit expression in exercise-trained human skeletal muscle. J Appl Physiol. 2003;94:631–641. doi: 10.1152/japplphysiol.00642.2002. [DOI] [PubMed] [Google Scholar]
- Owen MR, Doran E, Halestrap AP. Evidence that metformin exerts its anti-diabetic effects through inhibition of complex 1 of the mitochondrial respiratory chain. Biochem J. 2000;348:607–614. [PMC free article] [PubMed] [Google Scholar]
- Polekhina G, Gupta A, Michell BJ, van Denderen B, Murthy S, Feil SC, Jennings IG, Campbell DJ, Witters LA, Parker MW, Kemp BE, Stapleton D. AMPK β-subunit targets metabolic stress-sensing to glycogen. Current Biol. 2003;13:867–871. doi: 10.1016/s0960-9822(03)00292-6. [DOI] [PubMed] [Google Scholar]
- Polekhina G, Gupta A, van Denderen BJ, Feil SC, Kemp BE, Stapleton D, Parker MW. Structural basis for glycogen recognition by AMP-activated protein kinase. Structure (Camb) 2005;13:1453–1462. doi: 10.1016/j.str.2005.07.008. [DOI] [PubMed] [Google Scholar]
- Sakamoto K, Goransson O, Hardie DG, Alessi DR. Activity of LKB1 and AMPK-related kinases in skeletal muscle: effects of contraction, phenformin, and AICAR. Am J Physiol Endocrinol Metab. 2004;287:E310–E317. doi: 10.1152/ajpendo.00074.2004. [DOI] [PubMed] [Google Scholar]
- Sakamoto K, McCarthy A, Smith D, Green KA, Hardie DG, Ashworth A, Alessi DR. Deficiency of LKB1 in skeletal muscle prevents AMPK activation and glucose uptake during contraction. EMBO J. 2005;24:1810–1820. doi: 10.1038/sj.emboj.7600667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto K, Zarrinpashneh E, Budas GR, Pouleur AC, Dutta A, Prescott AR, Ashworth A, Jovanovic A, Alessi DR, Bertrand L. Deficiency of LKB1 in heart prevents ischemia-mediated activation of AMPKα2 but not AMPKα1. Am J Physiol. 2006;290:E780–E788. doi: 10.1152/ajpendo.00443.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salt IP, Johnson G, Ashcroft SJH, Hardie DG. AMP-activated protein kinase is activated by low glucose in cell lines derived from pancreatic β cells, and may regulate insulin release. Biochem J. 1998;335:533–539. doi: 10.1042/bj3350533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Cespedes M, Parrella P, Esteller M, Nomoto S, Trink B, Engles JM, Westra WH, Herman JG, Sidransky D. Inactivation of LKB1/STK11 is a common event in adenocarcinomas of the lung. Cancer Res. 2002;62:3659–3662. [PubMed] [Google Scholar]
- Schmidt MC, McCartney RR. β-subunits of Snf1 kinase are required for kinase function and substrate definition. EMBO J. 2000;19:4936–4943. doi: 10.1093/emboj/19.18.4936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott JW, Hawley SA, Green KA, Anis M, Stewart G, Scullion GA, Norman DG, Hardie DG. CBS domains form energy-sensing modules whose binding of adenosine ligands is disrupted by disease mutations. J Clin Invest. 2004;113:274–284. doi: 10.1172/JCI19874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw RJ, Kosmatka M, Bardeesy N, Hurley RL, Witters LA, DePinho RA, Cantley LC. The tumor suppressor LKB1 kinase directly activates AMP-activated kinase and regulates apoptosis in response to energy stress. Proc Natl Acad Sci U S A. 2004;101:3329–3335. doi: 10.1073/pnas.0308061100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw RJ, Lamia KA, Vasquez D, Koo SH, Bardeesy N, Depinho RA, Montminy M, Cantley LC. The kinase LKB1 mediates glucose homeostasis in liver and therapeutic effects of metformin. Science. 2005;310:1642–1646. doi: 10.1126/science.1120781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Silva Xavier G, Leclerc I, Varadi A, Tsuboi T, Moule SK, Rutter GA. Role for AMP-activated protein kinase in glucose-stimulated insulin secretion and preproinsulin gene expression. Biochem J. 2003;371:761–774. doi: 10.1042/BJ20021812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland CM, Hawley SA, McCartney RR, Leech A, Stark MJ, Schmidt MC, Hardie DG. Elm1p is one of three upstream kinases for the Saccharomyces cerevisiae SNF1 complex. Curr Biol. 2003;13:1299–1305. doi: 10.1016/s0960-9822(03)00459-7. [DOI] [PubMed] [Google Scholar]
- Thelander M, Olsson T, Ronne H. Snf1-related protein kinase 1 is needed for growth in a normal day-night light cycle. EMBO J. 2004;23:1900–1910. doi: 10.1038/sj.emboj.7600182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomas E, Tsao TS, Saha AK, Murrey HE, Zhang CcC, Itani SI, Lodish HF, Ruderman NB. Enhanced muscle fat oxidation and glucose transport by ACRP30 globular domain: acetyl-CoA carboxylase inhibition and AMP-activated protein kinase activation. Proc Natl Acad Sci U S A. 2002;99:16309–16313. doi: 10.1073/pnas.222657499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson WA, Hawley SA, Hardie DG. The mechanism of glucose repression/derepression in yeast: SNF1 protein kinase is activated by phosphorylation under derepressing conditions, and this correlates with a high AMP:ATP ratio. Current Biol. 1996;6:1426–1434. doi: 10.1016/s0960-9822(96)00747-6. [DOI] [PubMed] [Google Scholar]
- Winder WW, Holmes BF, Rubink DS, Jensen EB, Chen M, Holloszy JO. Activation of AMP-activated protein kinase increases mitochondrial enzymes in skeletal muscle. J Appl Physiol. 2000;88:2219–2226. doi: 10.1152/jappl.2000.88.6.2219. [DOI] [PubMed] [Google Scholar]
- Woods A, Dickerson K, Heath R, Hong SP, Momcilovic M, Johnstone SR, Carlson M, Carling D. Ca2+/calmodulin-dependent protein kinase kinase-β acts upstream of AMP-activated protein kinase in mammalian cells. Cell Metab. 2005;2:21–33. doi: 10.1016/j.cmet.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Woods A, Johnstone SR, Dickerson K, Leiper FC, Fryer LG, Neumann D, Schlattner U, Wallimann T, Carlson M, Carling D. LKB1 is the upstream kinase in the AMP-activated protein kinase cascade. Curr Biol. 2003;13:2004–2008. doi: 10.1016/j.cub.2003.10.031. [DOI] [PubMed] [Google Scholar]
- Woods A, Munday MR, Scott J, Yang X, Carlson M, Carling D. Yeast SNF1 is functionally related to mammalian AMP-activated protein kinase and regulates acetyl-CoA carboxylase in vivo. J Biol Chem. 1994;269:19509–19515. [PubMed] [Google Scholar]
- Woollhead AM, Scott JW, Hardie DG, Baines DL. Phenformin and 5-aminoimidazole-4-carboxamide-1-β-D-ribofuranoside (AICAR) activation of AMP-activated protein kinase inhibits transepithelial Na+ transport across H441 lung cells. J Physiol. 2005;566:781–792. doi: 10.1113/jphysiol.2005.088674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamauchi T, Kamon J, Minokoshi Y, Ito Y, Waki H, Uchida S, Yamashita S, Noda M, Kita S, Ueki K, Eto K, Akanuma Y, Froguel P, Foufelle F, Ferre P, Carling D, Kimura S, Nagai R, Kahn BB, Kadowaki T. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat Med. 2002;6:1288–1295. doi: 10.1038/nm788. [DOI] [PubMed] [Google Scholar]
- Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenyk-Melody J, Wu M, Ventre J, Doebber T, Fujii N, Musi N, Hirshman MF, Goodyear LJ, Moller DE. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest. 2001;108:1167–1174. doi: 10.1172/JCI13505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zong H, Ren JM, Young LH, Pypaert M, Mu J, Birnbaum MJ, Shulman GI. AMP kinase is required for mitochondrial biogenesis in skeletal muscle in response to chronic energy deprivation. Proc Natl Acad Sci U S A. 2002;99:15983–15987. doi: 10.1073/pnas.252625599. [DOI] [PMC free article] [PubMed] [Google Scholar]