Abstract
β-Adrenergic stimulation modulates ventricular currents and sinus cycle length (CL). We investigated how changes in CL affect the current induced by isoprenaline (Iso) during the action potential (AP) of guinea-pig ventricular myocytes. Action-potential clamp was applied at CLs of 250 and 1000 ms to measure: (1) the net current induced by 0.1 μm Iso (IIso); (2) the L-type Ca2+ current ICaL and slow delayed rectifier current IKs components of IIso (IIsoCa and IIsoK), identified as the Iso-induced current sensitive to nifedipine and HMR1556, respectively; and (3) IIso persisting after inhibition of both ICa and IKs (IisoR). The pause dependency of IKs and its modulation were evaluated in voltage-clamp experiments. The rate dependency of the duration of the action potential at 90% repolarization (APD90) and its modulation by isoprenaline were tested in current-clamp experiments. At a CL of 250 ms IIso was inward during initial repolarization and reversed at 59% of APD90. At a CL of 1000 ms IIso became mostly inward in all cells. Switching to shorter CL did not change IIsoCa and IIsoK amplitudes, but moved their peak amplitudes to earlier repolarization; IIsoR was independent of CL. Acceleration of IIsoK at shorter CL was based on faster pause dependency of IKs activation rate. The ‘restitution’ of activation rates was modulated by isoprenaline. The APD90–CL relation was rotated anticlockwise by isoprenaline and crossed the control curve at a CL of 150 ms (400 beats min−1). We conclude that: (1) isoprenaline induced markedly different current profiles according to pacing rate, involving CL-dependent ICa and IKs modulation; (2) the effect of isoprenaline on APD90 was CL dependent, and negligible during tachycardia; and (3) during sympathetic activation, repolarization stability may involve matched modulation of sinus rate and repolarizing currents.
Adrenergic stimulation of ventricular muscle directly modulates membrane currents and Ca2+ handling. At the same time adrenergic action on the sinus node increases heart rate, which on its own has profound effects on cell electrophysiology and excitation–contraction coupling. Thus, the physiological response of the ventricle to adrenergic stimulation reflects the interplay of direct and rate-induced effects. Whether such interplay may consist of a simple summation or have a more complex nature is largely unknown.
There are diverse conditions under which adrenergic activation is not accompanied by the expected (quantitatively appropriate) increase in ventricular activation rate; they are all characterized by an abnormal susceptibility to ventricular arrhythmias, mostly attributed to repolarization abnormalities (Zlotikamien et al. 1990; Swan et al. 1999; Priori et al. 1999). Whilst in some conditions the mismatch between neural activity and rate response stands out as the causative abnormality (Ueda et al. 2004), in others it can enhance the arrhythmogenic effect of primary (Gladman et al. 1996) and drug-induced repolarization defects (Pinski et al. 2002). This leads to the hypothesis that inadequate increase in heart rate may, per se, change the ventricular response to adrenergic activation from normal to arrhythmogenic.
In this study we describe a remarkable rate dependency of the net membrane current induced by β-adrenergic stimulation during the ventricular action potential in guinea-pig myocytes. We also address the mechanism of such a dependency, by studying separately the contributions of the currents mostly affected by β-adrenergic stimulation (slow delayed rectifier current IKs and L-type Ca2+ current ICaL). Focus on these currents is also justified by their time-dependent kinetics and by the importance of their balance in determining repolarization.
Methods
The present investigation conforms to the Guide to the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH publication no. 85-23, revised 1996) and to the guidelines for Animal Care endorsed by the University of Milan.
Myocyte isolation and recording apparatus
Hartley guinea-pigs (body weight ∼250 g) were killed by cervical dislocation under combined 20 mg ketamine–1.5 mg xylazine anaesthesia. Ventricular myocytes were isolated by using a retrograde coronary perfusion method previously published (Zaza et al. 1998), with minor modifications. Isolated myocytes were resuspended in 1 mm CaCl2 Tyrode solution containing gentamycin (10 μg ml−1) and stored at room temperature until use. Rod-shaped, Ca2+-tolerant myocytes were used within 12 h from dissociation. Measurements were performed only in quiescent myocytes with clear-cut striations. Myocytes in suspension were placed in a 30 mm Petri dish, with a plastic ring to reduce total volume to ∼1 ml. The dish was perfused at 2 ml min−1 with Tyrode solution containing (mm): 154 NaCl, 4 KCl, 2 CaCl2, 1 MgCl2, 5 Hepes-NaOH and 5.5 d-glucose, adjusted to pH 7.35 with NaOH. The cell under study was held within 300 μm from the tip (1 mm) of a thermostated manifold pipette allowing for rapid switch between solutions (measured equilibration time < 2 s). The solution temperature was monitored at the pipette tip with a fast-response digital thermometer (BAT-12, Physitemp, Clifton, NJ, USA) and kept at 36 ± 0.5°C.
Membrane potential and current measurements
Measurements were performed by the patch-clamp technique in the whole-cell configuration (Axon Multiclamp 700A, Axon Instruments). Electrodes with a tip resistance between 2.5 and 3 MΩ were pulled from borosilicate glass pipettes. The pipette solution contained (mm): 110 potassium aspartate, 23 KCl, 0.4 CaCl2 (calculated free Ca2+ of 10−7 m), 3 MgCl2, 5 Hepes-KOH, 1 EGTA-KOH, 0.4 GTP-Na salt, 5 ATP-Na salt, and 5 creatine phosphate Na salt, adjusted to pH 7.3 with KOH. In order to minimize impact on Ca2+-dependent conductances, pipette Ca2+ and EGTA concentrations were selected to achieve a small residual buffering capacity (Fabiato & Fabiato, 1979), as suggested by the persistence of mechanical activity in the patched myocytes. Membrane capacitance (85.4 ± 4 pF) and series resistance (8.8 ± 0.4 MΩ) were measured in every cell (n = 82) according to the required compensation current (Axon Multiclamp 700A), but left uncompensated. An average junction potential of +5.5 mV, measured upon moving the electrode tip from Tyrode solution to pipette (intracellular) solution was also left uncompensated. Current signals were filtered at 2 kHz, digitized through a 12-bit A–D converter (Axon Digidata 1200; sampling rate, 5 kHz). Trace acquisition and analysis was controlled by dedicated software (Axon pClamp 8.0).
Experimental protocols
Total isoprenaline-induced current (IIso) during ventricular electrical activity was evaluated at cycle lengths (CLs) of 250 and 1000 ms by the action-potential-clamp technique (AP clamp; Doerr et al. 1990; Rocchetti et al. 2001). Isoprenaline-induced current was obtained by subtracting the current recorded in control conditions from that recorded in the presence of 0.1 μm isoprenaline.
The individual contributions of ICaL and IKs modulation to IIso were evaluated by recording the currents sensitive to the selective blockers nifedipine (5 μm) and HMR1556 (1 μm; Thomas et al. 2003), respectively (obtained as current in control conditions minus current in the presence of the blocker). For the purpose of this analysis, results are presented as the change in each current (ICaL or IKs) produced by isoprenaline, rather than the current itself. The isoprenaline-induced ICaL and IKs are referred to as IIsoCa and IIsoK, respectively. The current induced by isoprenaline in the presence of complete ICaL and IKs inhibition (IIsoR) was also evaluated. Where specified, individual current and action potential traces were plotted on time scales normalized to the duration of the action potential at 90% repolarization (APD90). This allowed averaging of traces obtained from different cells and favoured assessment of the contribution of each current to a specific phase of repolarization, independently of absolute APD90. Nonetheless, it should be kept in mind that differences in APD90 are obscured by this representation. Dynamic current–voltage (I–V) relations were obtained in AP-clamp experiments by plotting membrane current traces versus the respective action potential waveform. Reversibility of the effects of the test solution was checked in all AP-clamp experiments, as required to rule out artifactual current changes due to time-dependent run-down. Quantification of the reversibility of the isoprenaline effect (the most prone to run-down) is provided in the online supplement (Fig. 1S).
Pause dependency of IKs kinetics was analysed by a traditional voltage-clamp protocol. Two activating steps (S1 and S2 to +20 mV) were separated by a pause (at −80 mV) of variable duration (Fig. 5A). Time-dependent IKs activation during S2 (IKs reactivation) was fitted by a single exponential to estimate the reactivation time constant (τreact); the initial activation delay (about 60 ms) was not included in the fit (Tzounopoulos et al. 1998). Contaminating currents were minimized by adding E-4031 (5 μm, blocking the rapid delayed-rectifier current, IKr), 4-aminopyridine (3 mm, blocking the transient outward current, Ito), nifedipine (5 μm, blocking ICaL) and nickel (5 mm, blocking ICaL, the T-type Ca2+ current, ICaT and the Na+/Ca2+ exchanger current INCX) to extracellular solutions and increasing pipette EGTA to 5 mm. The chromanol derivative HMR1556 (1 μm), a selective IKs blocker, was used to confirm that the current recorded in the modified Tyrode solution was IKs (see online supplement).
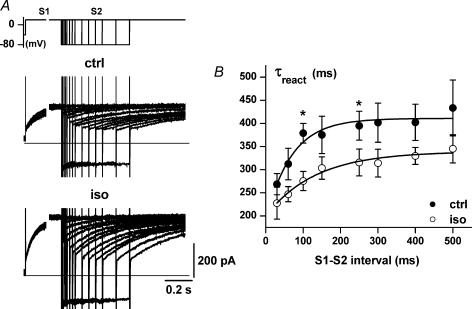
Figure 5. Pause dependency of IKs reactivation rate.
A, sample recordings of IKs (protocol shown in the inset) under control conditions (ctrl) and in the presence of isoprenaline (Iso). B, restitution of activation kinetics. The time constant of IKs reactivation (τreact), i.e. activation during S2, is plotted as a function of the S1–S2 interval. Control (•) and isoprenaline (○) data points were fitted (continuous lines) to estimate the time constant of the restitution process (τrest, see textl; n = 5). The overall difference between control and isoprenaline data is highly significant (P < 0.001 with ANOVA for repeated measurements); for individual points *P < 0.05 (Bonferroni's correction).
The rate dependency of APD90 was measured under current-clamp conditions during stimulation, delivered through the patch pipette (2 ms, amplitudes between 1.8 and 2.2 nA) at various cycle lengths (CLs, range 0.2–1.0 s). APD90 was measured from every beat by custom-made software. APD90 values were obtained from the average of at least five consecutive action potentials recorded after achievement of a steady state at each CL and after 1 min quiescence. The APD90–CL relation was well fitted by the logistic type function (Zaza et al. 1991; Malfatto et al. 2003) APD = APDmax × CLS/(CL50S + CLS) with APDmax constrained at the APD90 value measured after prolonged (1 min) quiescence. The CL at 50% APDmax (CL50) and slope factor (S) were estimated from the fitting.
Chemicals
HMR1556 and 9-anthracene-carboxylic acid (9-AC) were dissolved in DMSO, nifedipine in ethanol, and E-4031 in distilled water. The final DMSO or ethanol concentrations did not exceed 0.1%. HMR1556 and E-4031 were generous gifts of Aventis (Frankfurt, Germany) and Sanofi Recherche (Montpellier, France), respectively; all other chemicals were purchased from Sigma (St Louis, MO, USA).
Statistical analysis
Current magnitude was expressed as ‘peak’ and ‘mean’ values. The latter was calculated by integrating the current trace over repolarization and dividing the result by repolarization time. Current magnitudes were divided by cell capacitance to obtain current densities. Least-square fitting was used to estimate process kinetics. Student's t test or ANOVA for paired or unpaired measurements were applied as appropriate to test for significance bewteen means. Post hoc comparison between individual means was performed by Student's t test after Bonferroni's correction. Data are expressed and plotted as the mean ± s.e.m. value; ± s.e.m. intervals are also shown for average trace recordings. Statistical significance was defined as P < 0.05 (n.s., not significant). The sample size for each experiment is specified in the respective figure legend.
Results
Rate dependency of total isoprenaline-induced current (IIso)
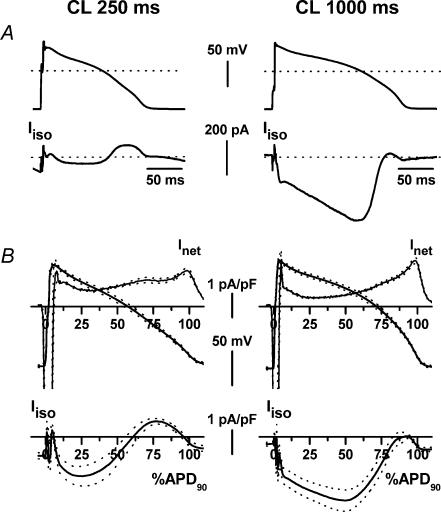
A representative example of IIso recorded at CLs of 250 and 1000 ms in the same myocyte is shown in Fig. 1A. Figure 1B shows average IIso profiles and the respective action potential waveforms obtained from all the cells tested after normalization of the time scale to APD90 (see Methods). During early repolarization IIso was inward, decreasing or reversing as repolarization progressed.
Figure 1. Cycle-length dependency of total isoprenaline-induced current (IIso).
A, sample IIso recordings at the two CLs with the corresponding AP waveforms (absolute time scales). B, top panels show average AP waveform and net transmembrane current density (Inet = −dV/dt) required to generate it; bottom panels show IIso density. In B the time scale was normalized to APD90. Average recordings (n = 21) and ± s.e.m. interval are shown by continuous and dotted lines, respectively.
At a CL of 250 ms, the inward component of IIso was consistently followed by an outward one, of sizable magnitude and extending to the end of repolarization. On average, the inward component achieved a maximum in early phase 2 (time to peak, tpeak, at 28.5 ± 2.9% of APD90), current reversal occurred at 59.3 ± 4.1% of APD90, and the contribution of the outward component to total IIso was 43.5 ± 6.4%.
At a CL of 1000 ms inward IIso became strongly predominant; its amplitude increased and its peak moved to the transition between phase 2 and 3 (tpeak at 48.9 ± 4.8% of APD90; P < 0.05 versus CL 250 ms). At this CL, the current did not reverse in three of 21 cells and in the remaining ones it became outward only at the very end of repolarization (80.1 ± 1.9% of APD90); thus, on average, IIso was inward during the whole repolarization.
Diastolic IIso was inward at both CLs and larger at a CL of 250 ms (−0.77 ± 0.1 pA pF−1 versus −0.30 ± 0.07 pA pF−1; P < 0.05).
ICaL and IKs strongly contribute to repolarization and are well-known targets of β-adrenergic modulation (Reuter, 1983; Li et al. 1999; Volders et al. 2003). Thus, further experiments were performed to establish the individual contribution of these conductances to IIso.
Rate dependency of IIsoCa (isoprenaline-induced ICa)
To evaluate the contribution of calcium currents to the rate dependency of IIso, we studied modulation of the current sensitive to blockade by nifedipine. This current may include ICaL plus all the other current components sensitive to changes in subsarcolemmal Ca2+, including IKs (Nitta et al. 1994). The contribution of the latter current was evaluated in a set of preliminary experiments (not shown), in which nifedipine-sensitive current was measured with and without IKs blockade (HMR1556, 1 μm). At a CL of 250 ms HMR1556 resulted in a trend for an increase in IIsoCa, thus suggesting potential Ca2+-mediated enhancement of IKs. Thus, all subsequent experiments on IIsoCa were performed in the presence of HMR1556 (1 μm, added to all solutions). Under such conditions IIsoCa should include only the contributions of ICaL and the Na+–Ca2+ exchanger current (INCX).
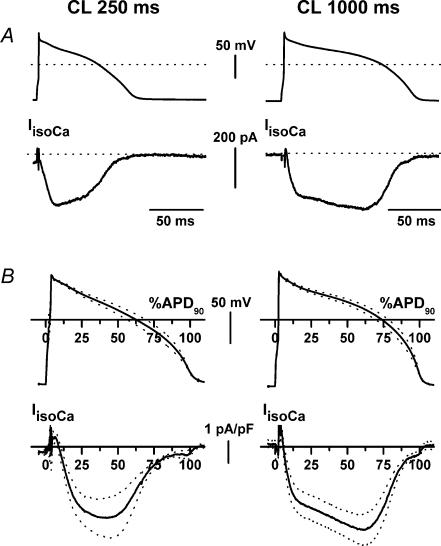
Isoprenaline-induced ICa was obtained as the difference between nifedipine-sensitive currents recorded in the presence and absence of isoprenaline. Figure 2A shows a representative example of IIsoCa recorded from the same myocyte at the two CLs. Average action potential waveforms and the respective IIsoCa profiles obtained in a sample are shown in Fig. 2B. At a CL of 250 ms IIsoCa consisted of a large inward current, monotonically increasing to a peak during the plateau phase of the action potential (tpeak at 35.6 ± 3.9% of APD90). Its maximum (Ipeak) and mean densities (Imean) were 3.5 ± 0.9 and 1.76 ± 0.40 pA pF−1, respectively. At a CL of 1000 ms, IIsoCa activation included a slower phase leading to a delayed peak, which occurred at the transition between plateau and phase 3 repolarization (tpeak at 54.8 ± 6.8% of APD90; P < 0.05 versus CL 250 ms). Ipeak (3.94 ± 0.6 pA pF−1) and Imean (2.46 ± 0.46 pA pF−1) of IIsoCa densities were unchanged as compared to CL 250 ms.
Figure 2. Cycle-length dependency of isoprenaline-induced calcium current (IIsoCa).
Description and symbols as in Fig. 1. For average recordings n = 6.
Rate dependency of IIsoK (isoprenaline-induced IKs)
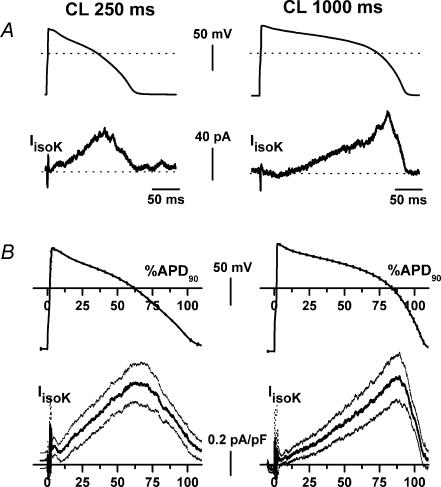
A representative example of IIsoK recorded at CLs of 250 and 1000 ms in the same myocyte is shown in Fig. 3A. Average action potential waveforms and the respective IIsoK profiles recorded in a sample are shown in Fig. 3B. Peak and mean IIsoK densities were not significantly different between the two CLs (peak, 0.93 ± 0.2 versus 0.99 ± 0.2 pA pF−1, n.s.; mean, 0.51 ± 0.13 versus 0.43 ± 0.13 pA pF−1, n.s.). As shown by the average recordings in Fig. 3B, the instantaneous (time-independent) component of IIsoK was almost null at a CL of 1000 ms (0.046 ± 0.050 pA pF−1) and tended to increase at the shorter CL (0.187 ± 0.09 pA pF−1, n.s., n = 6). Failure of this small change to achieve statistical significance was probably due to subtraction artifacts during the upstroke phase of individual recordings, which increased data dispersion.
Figure 3. Cycle-length dependency of isoprenaline-induced IKs (IIsoK).
Description and symbols as in Fig. 1. For average recordings n = 6.
At a Clof 250 ms the onset of time-dependent current was relatively fast, and IIsoK achieved a maximum at 86.1 ± 11 ms (tpeak at 59.6 ± 3.8% of APD90). At a CL of 1000 ms the onset of the time-dependent current was markedly slowed; as a consequence, peak IIsoK was achieved at 205.4 ± 34.1 ms (81.2 ± 2.8% of APD90; P < 0.05 versus CL 250 ms).
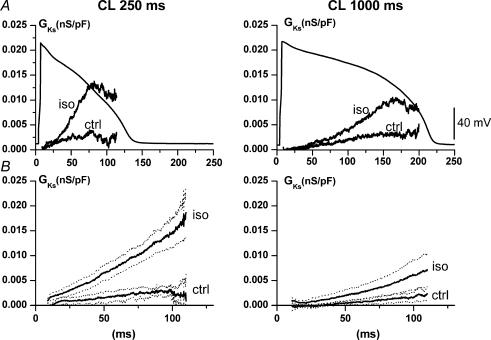
To analyse the difference in the rate of IIsoK onset between the two CLs independently of voltage profile, IKs conductance (GKs) profile was determined by dividing IKs by the corresponding K+ electromotive force (Em − EKs), at each instant during the action potential. EKs was −72.4 ± 1.5 mV as measured in preliminary experiments (see online supplement, Fig. 2S). GKs was determined at each CL in control conditions and during isoprenaline superfusion within the same myocyte. A representative example is shown in Fig. 4A, and the average of multiple recordings in Fig. 4B. The time-dependent GKs increased slightly as CL was shortened to 250 ms; this change was strongly amplified by isoprenaline, whose effect was significantly larger at a CL of 250 ms (onset rate, +356%) than at a CL of 1000 ms (onset rate, +149%; P < 0.05).
Figure 4. Cycle-length dependency of IKs conductance (GKs) and its modulation.
A, representative example of GKs profile at two CLs, in control (ctrl) and during isoprenaline (Iso). B, average GKs profiles (continuous lines) and their ± s.e.m. interval (dotted lines; n = 6).
The mechanism of CL-dependent enhancement of IKs activation rate, observed in AP-clamp experiments, was analysed with voltage clamp by a two-step (S1–S2) protocol (see Methods). The onset of IKs was clearly composed of a time-independent (instantaneous) component and a time-dependent one (Fig. 5). As the interval between pulses was shortened, the instantaneous current during S2 increased at the expense of time-dependent current, reflecting incomplete IKs deactivation (Fig. 5A). At the same time, the onset of time-dependent IKs became faster, reflecting increased reactivation rate (Fig. 5B), a facilitation phenomenon distinct from incomplete deactivation (see Discussion). The IKs reactivation time constant (τreact) increased with time from the previous pulse along an exponential ‘restitution’ process (Fig. 5B). The course of such restitution was quantified by its time constant (τrest), which was 68 ± 19 ms under control conditions. Isoprenaline slowed the restitution process (τrest, 132 ± 30 ms; P < 0.05 versus controls), thus extending facilitation of reactivation to longer interpulse intervals. Moreover, isoprenaline reduced the estimated steady-state value of τreact (339 ± 8 versus 410 ± 9 ms; P < 0.05).
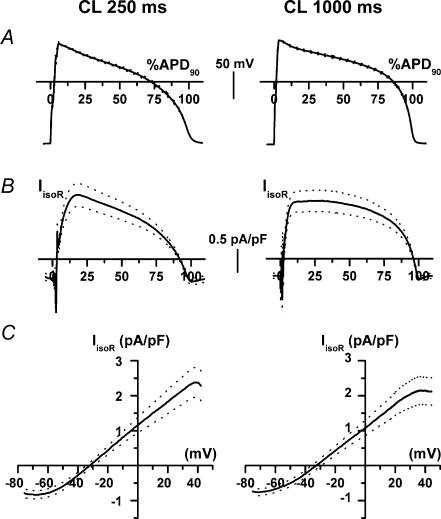
Residual IIso after ICaL and IKs blockade (IIsoR)
To test for the contribution of currents other than ICaL and IKs to IIso and its rate dependency, IIso was measured at the two CLs in the presence of 10 μm nifedipine and 1 μm HMR1556 (Fig. 6). Under such conditions isoprenaline induced a current change (residual IIso, IIsoR). The IIsoR profile mostly reflected the AP course, thus suggesting its origin from time-independent ‘background’ conductance(s) (Fig. 6B). The dynamic I–V relation (Fig. 6C) was linear between −60 and +40 mV. The current reversed at −31.93 ± 1.84 mV at a CL of 250 ms and at −34.9 ± 2.66 mV at a CL of 1000 ms (n.s.); the conductance (in the linear I–V region) was 36.1 ± 5.5 and 32.6 ± 4.5 pS pF−1 at CLs of 250 and 1000 ms, respectively (n.s.). Mean IIsoR density was also unchanged between the two CLs (1.62 ± 0.29 versus 1.77 ± 0.32 pA pF−1; n.s.). Minor differences in the current profiles at the two CLs reflected the different AP shapes; except for such differences, IIso measured in the absence of ICaL and IKs was rate independent. The IIsoR was completely and quickly reversible after isoprenaline wash out, which rules out the possibility of a leak current resulting from membrane damage. Exposure to 9-AC (100 μm), a blocker of cAMP-induced Cl− current (Levesque et al. 1993) was attempted, but IIsoR decrement was partial, required a long exposure time (minutes) and was irreversible. Thus, a 9-AC effect could not be safely discriminated by time-dependent current run-down. It should be stressed that 9-AC effect is generally slow in onset and decay; thus the present finding is not adequate to rule out the contribution of cAMP-induced ICl to IIsoR.
Figure 6. Cycle-length dependency of IIso after ICaL and IKs blockade (IIsoR).
A, average action potential waveforms. B, average IIsoR density recorded at the two CLs during the action potentials shown in A (time scales normalized to APD90). C, average dynamic I–V relations of IIsoR. Average recordings (n = 12) and their ± s.e.m. limits are shown by continuous and dotted lines, respectively.
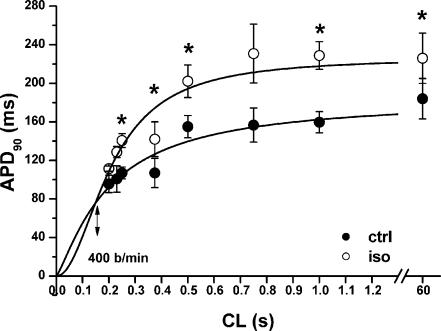
Rate dependency of isoprenaline effect on action potential duration
Under control conditions APD90 was a saturating function of CL (Fig. 7). Within the range of CLs tested, APD90 was increased by isoprenaline (0.1 μm). In the presence of isoprenaline, the APD90–CL relation was steeper (S coefficient, 2.05 ± 0.53 versus 1.24 ± 0.25; P < 0.05) and crossed the control curve at a CL of 150 ms, corresponding to a rate of 400 beats min−1. Isoprenaline increased APDmax (226 ± 26 versus 184 ± 21 ms; P < 0.05), but not CL50 (208 ± 21 versus 199 ± 25 ms; n.s.).
Figure 7. Cycle-length dependency of APD90 and its modulation.
APD90 measured during steady-state pacing at various CLs under control conditions (•) and in the presence of isoprenaline (○; n = 3 at all CLs). The continuous lines are logistic data fits, for which the asymptote was fixed at the 60 s APD90 value. The double-headed arrow indicates the heart rate at which the extrapolated APD90–CL relations cross (i.e. APD90 would be unaffected by isoprenaline). The overall difference between control and isoprenaline data is highly significant (P < 0.001 with ANOVA); for individual data points *P < 0.05 (Bonferroni's correction).
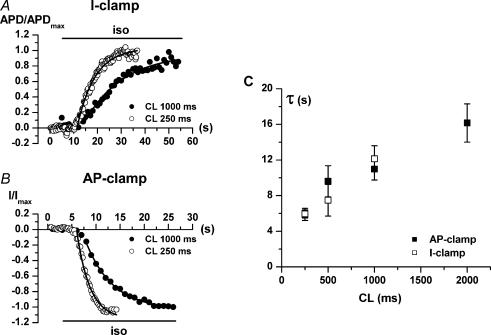
Kinetics of onset of isoprenaline effects
The kinetics by which isoprenaline-induced changes occur may be relevant to the extent to which they are expressed during the continuous fluctuations peculiar to physiological neural discharge. The analysis shown in Fig. 8 addresses the CL dependency of the kinetics of isoprenaline-induced changes in APD90 and transmembrane current. The onset of isoprenaline effects was characterized by an initial lag, followed by a time course well fitted by an exponential function. For both APD90 (current clamp, Fig. 8A) and transmembrane current (AP clamp, Fig. 8B), the rate of onset increased as CL was shortened. The exponential time constants (τ) for APD90 and membrane current were comparable and changed similarly as a function of CL (Fig. 8C). The CL dependency of τ was steeper at short CLs (τ doubled between 250 and 500 ms), showing saturation at longer CLs.
Figure 8. Rate of onset of isoprenaline effects on APD90 and membrane current.
The time of occurrence of peak inward IIso (at steady-state isoprenaline superfusion) was used in each cell as the reference point for current measurement. A and B show examples of the time course of the APD90 (current clamp) and membrane current (AP clamp) responses to isoprenaline (horizontal bar). To highlight CL-dependent changes in the kinetics of response, APD90 (APD) and current (I) were normalized to the steady-state value achieved at each CL (APDmax and Imax, respectively). Continuous lines represent fitting of the exponential phase of response. C, time constants (τ) of APD90 (□, n ≥ 8 at each CL) and membrane current (▪, n ≥ 7 at each CL) response as a function of CL.
Discussion
Interpretation of action-potential-clamp experiments
Any difference in net membrane current caused by receptor stimulation (primary effect) changes the action potential profile. Since most of cardiac channels are voltage gated, the change in voltage feeds back on currents, causing further changes (secondary effects). Such a loop eventually leads to a stable action potential profile, usually identified as the consequence of the intervention, which is possibly different from that resulting from the primary effect. The action-potential-clamp technique aims to identify the primary effect of receptor stimulation by interrupting the current–voltage interaction loop. At variance with traditional (square-wave) voltage-clamp experiments, the primary effect is analysed in the context of physiological voltage profiles and repetitive activity. While unsuited to the assessment of channel gating properties, this approach highlights features of the response to an intervention that may otherwise remain concealed.
Changes in diastolic interval as well as in action potential shape can contribute to the rate dependency of ionic conductances during phyisiological activity (Rocchetti et al. 2001). Action potential templates specific for each CL were used in the present experiments; thus, rate dependency included both the factors mentioned above, and no attempt was made to discriminate between them.
Rate dependency of IIso during ventricular action potential
Total isoprenaline-induced current (IIso) recapitulates all current changes directly or indirectly induced by β-receptor stimulation and represents the primary drive for the agonist-induced changes in the action potential contour. IIso includes channels of different selectivity, variably activated during the action potential course; this accounts for the biphasic profile of IIso. Such a profile was profoundly affected by stimulation rate, with changes in the inward:outward current ratio and in the timing of each component with respect to repolarization phase. Timing changes may be relevant because they strongly affect the voltage response to current flow. In particular, the transition between plateau and terminal repolarization is a critical phase because of its proximity to the threshold of autoregenerative phenomena leading either to full repolarization (IKr or IK1, the inward-rectifier current) (Rocchetti et al. 2001; Biliczki et al. 2002) or to abnormal depolarization (ICaL; January & Riddle, 1989; Zeng & Rudy, 1995), depending on net current balance. During this phase IIso was null or outward at short CL and strongly inward at long CL. This suggests that the safety margin for terminal repolarization (also named ‘repolarization reserve’; Biliczki et al. 2002) may be preserved during sympathetic activation only in the presence of an ‘appropriate’ increase in ventricular rate. In other words, robust repolarization may require a match between the responses of heart rate and ventricular currents to adrenergic activation. According to this interpretation, any mismatch condition (e.g. limiting the increase in ventricular rate) would alter repolarization reserve.
Rate dependency of IIso is reflected in rate dependency of APD90 modulation. Indeed, while APD90 was markedly prolonged by isoprenaline at long CLs, the fitted APD90–CL relations predicted that minimal APD90 change would occur at rates (400 beats min−1) compatible with adrenergically induced tachycardia in guinea-pigs (Hamlin et al. 2003). Although at long CL the IIso profile was suitable to facilitate early after-depolarizations, such phenomena seldom occurred under current-clamp conditions. This suggests that in the guinea-pig secondary current changes, occurring under current-clamp conditions, could still buffer the primary effect of β-receptor stimulation (seen under AP-clamp conditions), irrespective of stimulation rate. However, larger mammals are notoriously less resistant to repolarization abnormalities than guinea-pigs, possibly owing to differences in IKs expression (Biliczki et al. 2002); thus, their repolarization might be more sensitive to combined adrenergic activation and low rate.
The time course of IIso and APD90 changes during isoprenaline challenge displayed remarkable CL dependency. Since isoprenaline effects were slower than solution equilibration time (see Methods), their course is conceivably inherent to the biological response, whose mechanism may deserve further investigation. Faster onset implies that a larger proportion of steady-state effect will be effectively expressed during a rise in sympathetic activity, thus resulting in more efficient adaptation of repolarization at shorter CL. This emphasizes again the interplay between the response of ventricular currents and pacemaker activity in adrenergic modulation of repolarization. APD90 and membrane current were measured in separate groups of cells by different experimental approaches (current clamp versus AP clamp). The close similarity in the time course of their response suggests that IIso adequately describes primary modulation of repolarization by β-receptors.
Rate dependency of IIso components
Rate-dependent changes in IIsoCa and IIsoK mostly consisted of a shift in current distribution during repolarization. Indeed, neither mean nor peak current values varied significantly between the two CLs (but see section on Limitations of the study).
At short CL IIsoCa onset was monotonic and fast; a peak was achieved in the early plateau phase (36% of APD90). An increase in inward current with this timing may not jeopardize repolarization because the resulting positive shift in membrane potential would accelerate IKs activation; this, in turn, would balance the excess inward current. Moreover, at this time, ICaL may still be largely inactivated and unavailable for reactivation. At the longer CL IIsoCa continued to rise during the plateau phase, to peak in the proximity of the transition to final repolarization. As previously mentioned, this is a critical phase because ICaL may have partly recovered from inactivation and may enter an autoregenerative reactivation loop with membrane potential (January et al. 1988; January & Riddle, 1989). If this occurs, repolarization may be interrupted by early afterdepolarizations.
At shorter CL the instantaneous component of IIsoK showed a tendency to increase, but the most dramatic change was acceleration of the onset of the time-dependent component. This caused a shift in the distribution of IIsoK to an earlier phase of the action potential. Two mechanisms may contribute to rate-dependent changes in membrane current: (1) changes in the driving force, due to differences in the action potential contour and/or in the equilibrium potential of the permeant ion; and (2) changes in channel conductance, mainly due to the complex interplay between time-dependent gating and membrane potential time course. In the case of IIsoCa, discrimination between these mechanisms is difficult because the profile of intracellular Ca2+ transients is affected by both rate and β-receptor activation, thus making any estimate of Ca2+ driving force impossible. In contrast, in isolated myocytes K+ equilibrium potential can be assumed to be constant throughout the cycle; thus, the mechanism of the rate dependency of IKs and its adrenergic modulation could be investigated in more detail. The data presented in Fig. 4 clearly show that IIsoK acceleration at shorter CLs is caused by a conductance change, rather than simply reflecting a different voltage profile.
A potential contribution of cytosolic Ca2+ to IKs rate dependency was suggested by the observed trend in IIsoCa enhancement by HMR1556. However, CL dependency of IKs was mainly determined by the kinetics of channel gating. This is currently attributed to slow diastolic deactivation of IKs, which would lead to ‘accumulation’ of channels in the open state preferentially at short CLs (Zeng & Rudy, 1995; Stengl et al. 2003; Terrenoire et al. 2005). However, such a mechanism could account for the CL-dependent increase in the instantaneous component of GKs, but not for acceleration of the time-dependent one. Indeed, the latter component implies a faster transition from close to open states, i.e. a change in channel activation kinetics (Silva & Rudy, 2005). To address this issue, isoprenaline modulation of IKs gating was analysed by a square-wave voltage-clamp protocol, under conditions ruling out the contribution of changes in cytosolic Ca2+ (both ICaL and INCX blocked). Such experiments showed that IKs activation rate depends on the pause between two activations. Activation rate undergoes a restitution process with smaller time constants occurring at shorter pauses. The restitution process was slowed by isoprenaline, thus enhancing the range of diastolic intervals over which activation rate is CL dependent. A similar restitution of activation kinetics was reported for channels resulting from KCNE1 protein transfection in oocytes endogenously expressing KVLQT1 protein (Tzounopoulos et al. 1998). This observation was interpreted as a voltage-dependent transition between two sets of channel states, with different kinetics of the opening reaction. The present data extend this observation to native IKs and show that this process is modulated by β-adrenergic stimulation. The rate-dependent GKs increase observed in the present study under control conditions was smaller than that previously reported (Rocchetti et al. 2001). This quantitative discrepancy may reflect small differences in diastolic potential on which deactivation rate steeply depends.
After ICaL and IKs inhibition, isoprenaline activated a background current compatible with cAMP-induced ICl, though not obviously sensitive to 9-AC. It should be stressed that IIsoR might be a composite current and that the conditions under which IIsoR was measured are likely to affect the intracellular milieu. Thus, there is no guarantee that IIsoR is carried by an individual channel, or that it contributes to total IIso in its full extent; nonetheless, the sum IIsoCa + IIsoK + IIsoR roughly matched total IIso in terms of magnitude and dependency on CL (see online supplement, Fig. 5S). The IIsoR conductance was CL independent. At a CL of 250 ms, IIsoR driving force during late repolarization was reduced due to steeper action potential phase 2; thus, IIsoR cannot account for the increase in outward IIso observed at shorter CL.
Implications
The rate dependency of IIso distribution suggests that ventricular repolarization reserve might be reduced in all conditions characterized by a mismatch between increased sympathetic drive and heart rate response. Among the many lines of evidence in support of this view, two are particularly suggestive. The first one is the experimental observation that, after selective ablation of sympathetic nerves directed to the sinus node (right stellectomy), cats exposed to psychological stress developed repolarization abnormalities and arrhythmias (Schwartz et al. 1991). The second is a recent report of QT prolongation, ‘torsade de pointes’ ventricular tachycardia (TdP) and syncope upon sudden auditory stimuli in a patient with severe sinus node dysfunction caused by deficiency of the pacemaker current If (HCN4 mutation; Ueda et al. 2004). Notably, neither direct causes of repolarization abnormality nor signs of cardiomyopathy were found in this patient (Prof. A. Kimura, personal communication). These are extreme examples, in which arrhythmias were associated with severe bradycardia; nonetheless, milder mismatch between sympathetic drive and heart rate might reduce repolarization reserve and facilitate induction of TdP by other causes. This leads to the view that the extent of sinus rate response to sympathetic activation might be a factor in determining the widely variable penetrance and prognosis of congenital and drug-induced repolarization abnormalities (Priori, 2004; Shimizu et al. 2004). The practical implications of this view include reinforced indications to rate responsive pacing and restrictions in the use of ‘pure bradycardic agents’ (If blockers) in individuals exposed to risk of repolarization abnormality.
Limitations of the study
In action-potential-clamp experiments, individual currents were identified as being blocker-sensitive; thus, their magnitude and profile depend on the selectivity and potency of the blocker used. Experimental results and a detailed discussion on the methods used for current dissection in AP-clamp experiments are provided in the online supplement (Figs 3S and 4S).
Some intracellular Ca2+ buffering was required in all experiments; thus, the influence of cytosolic Ca2+ on membrane conductances might be underestimated. To minimize this potential artifact, buffering was limited to a minimum (see Methods) and was still compatible with cell contraction.
Recent reports suggest that the role of IKs in rate adaptation of APD90 may be larger in guinea-pigs than in larger mammals (Volders et al. 2003; Zicha et al. 2003), including man (Virag et al. 2001). This conclusion was based on the smaller magnitude and faster IKs deactivation found in larger mammals, which minimizes rate-dependent accumulation of the instantaneous IKs component (Stengl et al. 2003). According to this view, the value of the present findings would be limited by its species specificity. In contrast, also in the present experiments, changes in the instantaneous component contributed minimally to the rate dependency of IKs conductance (see Figs 3 and 4). Thus, also in the guinea-pig, incomplete diastolic deactivation may play a minor role. The major contribution to rate dependency of IKs was indeed based on acceleration of the time-dependent component, whose role can be readily appreciated only when the action potential waveform is used to drive membrane potential, as in the present experiments. Repolarization abnormalities resulting from IKs deficiency (Roden et al. 1996) suggest that, even if scantily expressed in isolated human myocytes, this current plays a functional role also in humans.
This study addresses the effect of β-adrenergic stimulation, which prevails in the modulation of repolarizing currents. Nonetheless, these currents may also be affected by α-receptors, which would also contribute under physiological conditions.
Acknowledgments
We are grateful to Professors A. Kimura (Medical and Dental University, Tokyo, Japan) and P. J. Schwartz (Department of Cardiology, University of Pavia, Italy) for providing information and insightful criticism to the manuscript and to Dr N. Szentandrassy (University of Debrecen, Hungary) for participating in some of the experiments. This work was partly funded by the Italian–Hungarian Cooperation program of the Italian Ministry of Foreign Affairs (to A. Zaza).
Supplemental material
The online version of this paper can be accessed at: DOI: 10.1113/jphysiol.2006.105015 http://jp.physoc.org/cgi/content/full/jphysiol.2006.105015/DC1 and contains supplemental material concerning 1) reversibility and specifity of current block used in AP-clamp experiments, 2) comparison between IIso and the sum of its individual components (IIsoCa + IIsoK + IIsoR), 3) experimental determination of the reversal potential of IKa.
This material can also be found as part of the full-text HTML version available from http://www.blackwell-synergy.com
References
- Biliczki P, Virag L, Iost N, Papp JG, Varro A. Interaction of different potassium channels in cardiac repolarization in dog ventricular preparations: role of repolarization reserve. Br J Pharmacol. 2002;137:361–368. doi: 10.1038/sj.bjp.0704881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doerr T, Denger R, Doerr A, Trautwein W. Ionic currents contributing to the action potential in single ventricular myocytes of the guinea pig studied with action potential clamp. Pflugers Arch. 1990;416:230–237. doi: 10.1007/BF00392058. [DOI] [PubMed] [Google Scholar]
- Fabiato A, Fabiato F. Calculator programs for computing the composition of the solutions containing multiple metals and ligands used for experiments in skinned muscle cells. J Physiol (Paris) 1979;75:463–505. [PubMed] [Google Scholar]
- Gladman G, Davis AM, Fogelman R, Hamilton RM, Gow RM. Torsade de pointes, acquired complete heart block and inappropriately long QT in childhood. Can J Cardiol. 1996;12:683–685. [PubMed] [Google Scholar]
- Hamlin RL, Kijtawornrat A, Keene BW, Hamlin DM. QT and RR intervals in conscious and anesthetized guinea pigs with highly varying RR intervals and given QTc-lengthening test articles. Toxicol Sci. 2003;76:437–442. doi: 10.1093/toxsci/kfg254. [DOI] [PubMed] [Google Scholar]
- January CT, Riddle JM. Early afterdepolarizations: mechanism of induction and block. A role for L-type Ca2+ current. Circ Res. 1989;64:977–990. doi: 10.1161/01.res.64.5.977. [DOI] [PubMed] [Google Scholar]
- January CT, Riddle JM, Salata JJ. A model for early afterdepolarizations: induction with the Ca++ channel agonist Bay K 8644. Circ Res. 1988;62:563–571. doi: 10.1161/01.res.62.3.563. [DOI] [PubMed] [Google Scholar]
- Levesque PC, Clark CD, Zakarov SI, Rosenshtraukh LV, Hume JR. Anion and cation modulation of the guinea-pig ventricular action potential during beta-adrenoceptor stimulation. Pflugers Arch. 1993;424:54–62. doi: 10.1007/BF00375102. [DOI] [PubMed] [Google Scholar]
- Li GR, Yang B, Feng J, Bosch RF, Carrier M, Nattel S. Transmembrane ICa contributes to rate-dependent changes of action potentials in human ventricular myocytes. Am J Physiol. 1999;276:H98–H106. doi: 10.1152/ajpheart.1999.276.1.H98. [DOI] [PubMed] [Google Scholar]
- Malfatto G, Facchini M, Zaza A. Characterization of the non-linear rate-dependency of QT interval in humans. Europace. 2003;5:163–170. doi: 10.1053/eupc.2002.0297. [DOI] [PubMed] [Google Scholar]
- Nitta J-I, Furukawa T, Marumo F, Sawanobori T, Hiraoka M. Subcellular mechanism for Ca2+-dependent enhancement of delayed rectifier K+ current in isolated membrane patches of guinea pig ventricular myocytes. Circ Res. 1994;74:96–104. doi: 10.1161/01.res.74.1.96. [DOI] [PubMed] [Google Scholar]
- Pinski SL, Eguia LE, Trohman RG. What is the minimal pacing rate that prevents torsades de pointes? Insights from patients with permanent pacemakers. Pacing Clin Electrophysiol. 2002;25:1612–1615. doi: 10.1046/j.1460-9592.2002.01612.x. [DOI] [PubMed] [Google Scholar]
- Priori SG. Inherited arrhythmogenic diseases: the complexity beyond monogenic disorders. Circ Res. 2004;94:140–145. doi: 10.1161/01.RES.0000115750.12807.7E. [DOI] [PubMed] [Google Scholar]
- Priori SG, Barhanin J, Hauer RW, Haverkamp W, Jongsma HJ, Kleber AG, et al. Genetic and molecular basis of cardiac arrhythmias – Impact on clinical management. Eur Heart J. 1999;20:174–195. doi: 10.1053/euhj.1998.1220. [DOI] [PubMed] [Google Scholar]
- Reuter H. Calcium channel modulation by neurotransmitters, enzymes and drugs. Nature. 1983;301:569–574. doi: 10.1038/301569a0. [DOI] [PubMed] [Google Scholar]
- Rocchetti M, Besana A, Gurrola GB, Possani LD, Zaza A. Rate dependency of delayed rectifier currents during the guinea-pig ventricular action potential. J Physiol. 2001;534:721–732. doi: 10.1111/j.1469-7793.2001.00721.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roden DM, Lazzara R, Rosen M, Schwartz PJ, Towbin J, Vincent GM. Multiple mechanisms in the long-QT syndrome. Current knowledge, gaps, and future directions. The SADS Foundation Task Force on LQTS. Circulation. 1996;94:1996–2012. doi: 10.1161/01.cir.94.8.1996. [DOI] [PubMed] [Google Scholar]
- Schwartz PJ, Zaza A, Locati E, Moss AJ. Stress and sudden death. The case of the long QT syndrome. Circulation. 1991;83(Suppl. II):71–80. [PubMed] [Google Scholar]
- Shimizu W, Horie M, Ohno S, Takenaka K, Yamaguchi M, Shimizu M, et al. Mutation site-specific differences in arrhythmic risk and sensitivity to sympathetic stimulation in the LQT1 form of congenital long QT syndrome: multicenter study in Japan. J Am Coll Cardiol. 2004;44:117–125. doi: 10.1016/j.jacc.2004.03.043. [DOI] [PubMed] [Google Scholar]
- Silva J, Rudy Y. Subunit interaction determines IKs participation in cardiac repolarization and repolarization reserve. Circulation. 2005;112:1384–1391. doi: 10.1161/CIRCULATIONAHA.105.543306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stengl M, Volders PG, Thomsen MB, Spatjens RL, Sipido KR, Vos MA. Accumulation of slowly activating delayed rectifier potassium current (IKs) in canine ventricular myocytes. J Physiol. 2003;551:777–786. doi: 10.1113/jphysiol.2003.044040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swan H, Viitasalo M, Piippo K, Laitinen P, Kontula K, Toivonen L. Sinus node function and ventricular repolarization during exercise stress test in long QT syndrome patients with KvLQT1 and HERG potassium channel defects. J Am Coll Cardiol. 1999;34:823–829. doi: 10.1016/s0735-1097(99)00255-7. [DOI] [PubMed] [Google Scholar]
- Terrenoire C, Clancy CE, Cormier JW, Sampson KJ, Kass RS. Autonomic control of cardiac action potentials: role of potassium channel kinetics in response to sympathetic stimulation. Circ Res. 2005;96:e25–e34. doi: 10.1161/01.RES.0000160555.58046.9a. [DOI] [PubMed] [Google Scholar]
- Thomas GP, Gerlach U, Antzelevitch C. HMR 1556, a potent and selective blocker of slowly activating delayed rectifier potassium current. J Cardiovasc Pharmacol. 2003;41:140–147. doi: 10.1097/00005344-200301000-00018. [DOI] [PubMed] [Google Scholar]
- Tzounopoulos T, Maylie J, Adelman JP. Gating of IsK channels expressed in Xenopus oocytes. Biophys J. 1998;74:2299–2305. doi: 10.1016/S0006-3495(98)77939-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda K, Nakamura K, Hayashi T, Inagaki N, Takahashi M, Arimura T, et al. Functional characterization of a trafficking-defective HCN4 mutation, D553N, associated with cardiac arrhythmia. J Biol Chem. 2004;279:27194–27198. doi: 10.1074/jbc.M311953200. [DOI] [PubMed] [Google Scholar]
- Virag L, Iost N, Opincariu M, Szolnoky J, Szecsi J, Bogats G, et al. The slow component of the delayed rectifier potassium current in undiseased human ventricular myocytes. Cardiovasc Res. 2001;49:790–797. doi: 10.1016/s0008-6363(00)00306-0. [DOI] [PubMed] [Google Scholar]
- Volders PG, Stengl M, van Opstal JM, Gerlach U, Spatjens RL, Beekman JD, et al. Probing the contribution of IKs to canine ventricular repolarization: key role for β-adrenergic receptor stimulation. Circulation. 2003;107:2753–2760. doi: 10.1161/01.CIR.0000068344.54010.B3. [DOI] [PubMed] [Google Scholar]
- Zaza A, Malfatto G, Schwartz PJ. Sympathetic modulation of the relation between ventricular repolarization and cycle length. Circ Res. 1991;68:1191–1203. doi: 10.1161/01.res.68.5.1191. [DOI] [PubMed] [Google Scholar]
- Zaza A, Rocchetti M, Brioschi A, Cantadori A, Ferroni A. Dynamic Ca2+-induced inward rectification of K+ current during the ventricular action potential. Circ Res. 1998;82:947–956. doi: 10.1161/01.res.82.9.947. [DOI] [PubMed] [Google Scholar]
- Zeng J, Rudy Y. Early afterdepolarizations in cardiac myocytes: mechanism and rate dependence. Biophys J. 1995;68:949–964. doi: 10.1016/S0006-3495(95)80271-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zicha S, Moss I, Allen B, Varro A, Papp J, Dumaine R, et al. Molecular basis of species-specific expression of repolarizing K+ currents in the heart. Am J Physiol Heart Circ Physiol. 2003;285:H1641–H1649. doi: 10.1152/ajpheart.00346.2003. [DOI] [PubMed] [Google Scholar]
- Zlotikamien B, Kusniec J, Strasberg B, Erdman S, Sclarovsky S, Agmon J. Torsades de Pointes as a complication of bradyarrhythmias. Isr J Med Sci. 1990;26:102–105. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The online version of this paper can be accessed at: DOI: 10.1113/jphysiol.2006.105015 http://jp.physoc.org/cgi/content/full/jphysiol.2006.105015/DC1 and contains supplemental material concerning 1) reversibility and specifity of current block used in AP-clamp experiments, 2) comparison between IIso and the sum of its individual components (IIsoCa + IIsoK + IIsoR), 3) experimental determination of the reversal potential of IKa.
This material can also be found as part of the full-text HTML version available from http://www.blackwell-synergy.com