Abstract
Loss of neural input to skeletal muscle fibres induces atrophy and degeneration with evidence of mitochondria-mediated cell death. However, the effect of denervation on the permeability transition pore (PTP), a mitochondrial protein complex implicated in cell death, is uncertain. In the present study, the impact of 21 days of denervation on the sensitivity of the PTP to Ca2+-induced opening was studied in isolated muscle mitochondria. Muscle denervation increased the sensitivity to Ca2+-induced opening of the PTP, as indicated by a significant decrease in calcium retention capacity (CRC: 111 ± 12 versus 475 ± 33 nmol (mg protein)−1 for denervated and sham, respectively). This phenomenon was partly attributable to in vivo mitochondrial and whole muscle Ca2+ overload. Cyclosporin A, which inhibits PTP opening by binding to cyclophilin D (CypD), was significantly more potent in mitochondria from denervated muscle and restored CRC to the level observed in mitochondria from sham-operated muscles. In contrast, the CypD independent inhibitor trifluoperazine was equally effective at inhibiting PTP opening in sham and denervated animals and did not correct the difference in CRC between groups. This phenomenon was associated with a significant increase in the content of the PTP regulating protein CypD relative to several mitochondrial marker proteins. Together, these results indicate that Ca2+ overload in vivo and an altered expression of CypD could predispose mitochondria to permeability transition in denervated muscles.
Several progressive neuromuscular diseases are associated with severe atrophy and loss of muscle fibres resulting in muscle wasting as the main clinical presentation. There is growing evidence that activation of cell death through apoptosis, and in a lesser manner necrosis, is involved in the loss of muscle fibres (Lu et al. 1997; Borisov & Carlson, 2000; Tews, 2002; Siu & Alway, 2005). In addition, apoptosis also occurs in localized segments of the remaining myofibres and may play a role in atrophic remodelling (Allen et al. 1997, 1999; Leeuwenburgh et al. 2005). These phenomena have been described in denervation disorders (Tews & Goebel, 1997b; Yoshimura & Harii, 1999; Borisov & Carlson, 2000; Siu & Alway, 2005), muscular dystrophy (Sandri, et al. 1995, 2001), inactivity (Leeuwenburgh et al. 2005; Siu et al. 2005a) and ageing-induced sarcopenia (Leeuwenburgh et al. 2005; Siu et al. 2005a,b).
Although the mechanisms responsible for the activation of cell death pathways in muscle are not well defined, there is evidence that at least in denervation atrophy, mitochondria may play a role. Indeed, several studies have reported an increase in the bax/bcl-2 ratio in various denervation disorders (Tews & Goebel, 1996, 1997a, b; Tews, 2002; Siu & Alway, 2005), which was suggested to induce permeation pathways through mitochondrial membranes. In addition, recent studies have shown that 2 weeks of denervation in rat muscle leads to the release of several mitochondrial pro-apoptotic proteins (e.g. cytochrome c, AIF, smac/Diablo) and to the activation of downstream events including the activation of caspase 9 and 3 and DNA fragmentation (Siu & Alway, 2005).
One of the well-described mechanisms of membrane permeation in mitochondria involves the opening of the permeability transition pore (PTP) (see Zoratti & Szabo, 1995 for extensive review). The PTP is a high conductance non-specific pore presumably formed by a supramolecular complex spanning the double membrane system of the mitochondria mainly at contact sites. Although it is increasingly recognized that the molecular composition of the PTP is probably variable (He & Lemasters, 2002; Kim et al. 2003; Zoratti et al. 2005), the prevailing hypothesis is that the adenylate translocator (ANT), the porin pore (VDAC) and the regulatory matrix protein cyclophilin-D (CypD) are the major proteins forming the complex (Crompton, 1999; Halestrap et al. 2000). Opening of the PTP results in matrix swelling, loss of membrane potential (ΔΨ), uncoupling of oxidative phosphorylation, and ATP hydrolysis and has also been shown to cause the release of several mitochondrial pro-apoptotic proteins including cytochrome c, AIF, Smac/Diablo, endonuclease G and Omi/HtrA2 (Di Lisa & Bernardi, 1998; Bernardi, 1999; Bernardi et al. 1999; Crompton, 1999; Suleiman et al. 2001). Although the regulation of PTP gating varies depending on the tissue studied, accumulation of Ca2+ in the matrix is the obligatory and most important trigger for its opening (Zoratti & Szabo, 1995; Bernardi, 1999). Several factors, including the type of substrate used, membrane potential, redox state of pyridine nucleotides, reactive oxygen species, adenylate concentration and pH can, however, act as coregulators by affecting the sensitivity of the pore to Ca2+ (Zoratti & Szabo, 1995; Bernardi, 1999).
There is currently little data available on the PTP in skeletal muscle. Opening of the PTP was shown to mediate the myotoxic effect of the anaesthetic bupivacaine, which could be prevented by inhibiting the pore with cyclosporin-A (CsA) (Irwin et al. 2002). More recently, mitochondrial dysfunction caused by an increased vulnerability to PTP opening was observed in mice harbouring a knockout of the collagen VI gene, which is involved in Bethlem myopathy and Ullrich congenital muscular dystrophy (Irwin et al. 2003). However, whether the vulnerability of mitochondria to PTP opening is altered in denervated muscle remains largely unknown. In the present study, surgical denervation was used to determine whether loss of neural input and/or its functional sequellae alters the sensitivity of isolated mitochondria to PTP opening and if this could be associated with changes in some factors favouring permeability transition.
Methods
Muscle denervation procedure
Female Sprague-Dawley rats (Charles River, Saint-Constant, Quebec, Canada), weighing 225–250 g at the beginning of the experiment, were housed in an environmentally controlled room (23°C, 12: 12 h light–dark cycle) and provided water and food ad libitum. Animals were randomly assigned to one of two groups: muscle denervation or sham-operated control where n = 24 animals per group. Following anaesthesia (ketamine–xylazine: 61.5/7.7 mg kg−1, i.p.) muscle denervation was performed bilaterally by extracting a 10 mm segment of the sciatic nerve through an incision in the mid-posterolateral area of the thigh. The incision was closed with silk sutures and the animals were administered buprenorphine (0.05 mg kg−1, i.p.) as postoperative analgesia. The incision site was washed with antibacterial solution to prevent infection. Daily inspection of the animals' hindlimbs was performed, verifying for absence of the toe-spread reflex. Body mass was evaluated every 48 h as an indication of tolerance to the denervation procedure. Any animal demonstrating signs of distress or automutilation of the distal ends of the feet and/or toes was immediately excluded from the experimental protocol. All procedures were approved by the animal ethics committee of the Université de Montréal and were in accordance with the guidelines of the Canadian Council of Animal Care.
Materials
All chemicals were purchased from Sigma (St Louis, MO, USA), with the exception of Cyclosporin A (Tocris, Ellisville, MO, USA), and Calcium Green-5N (Molecular Probes, Eugene, OR, USA).
Mitochondrial isolation procedure
Isolation of mitochondria was performed as previously described (Fontaine et al. 1998) with minor modifications. Twenty-one days following surgical denervation, animals were anaesthetized (ketamine–xylazine: 61.5/7.7 mg kg−1, i.p.) and the plantar group of muscles (soleus, plantaris, gastrocnemius) was dissected from the surrounding connective tissue, rapidly removed, trimmed clean of visible connective tissue, weighed and placed in 10 ml of ice-cold mitochondrial isolation buffer (mm: 150 sucrose, 75 KCl, 50 Tris base, 1 KH2PO4, 5 MgCl2, 1 EGTA, 0.2% BSA, pH 7.4). Animals were subsequently killed by lethal overdose of ketamine–xylazine. All steps were performed at 4°C. Muscles were minced with scissors, incubated for 1 min with Nagarse protease (0.2 mg ml−1) and homogenized using a motor-driven Teflon pestle homogenizer. The homogenate volume was completed to 40 ml with cold isolation buffer and centrifuged at 700 g for 10 min. The supernatant was decanted and centrifuged at 10 000 g for 10 min. The pellet was resuspended in 40 ml of suspension buffer (mm: 250 sucrose, 10 Tris base, 0.1 EGTA, pH 7.4) and centrifuged at 8000 g for 10 min. The final mitochondrial pellet was resuspended in 0.5 ml of suspension buffer and protein concentrations were determined by the bicinchoninic acid method.
Respirometry and enzyme activity
Mitochondrial oxygen consumption was measured polarographically at 25°C, using Clark-type electrodes (Oxygraph, Hansatech Instruments, Kings Lynn, UK). Experiments were started with the addition of 0.3 mg mitochondria in 1 ml of respiration buffer (mm: 125 KCl, 10 Pi-Tris, 0.05 EGTA, 10 Mops, 2.5 MgCl2) supplemented with glutamate–malate (5: 1 mm). The medium was then supplemented with 0.25 mm ADP to measure maximal rate of oxidative phosphorylation (VADP).
For the measurement of the activity of the respiratory chain complex IV cytochrome oxidase (COX), aliquots of the mitochondrial suspension were treated with 0.1% Triton X-100 for 60 min on ice. Following centrifugation for 5 min at 10 000 g, COX activity in the supernatant was determined spectrophotometrically as previously described (Burelle & Hochachka, 2002) and reported in milliunits per milligram protein.
Ca2+ challenge
Mitochondria (0.15 mg ml−1) were incubated at 25°C in 1.5 ml of buffer (mm: 250 sucrose, 10 Mops, 0.05 EGTA, 10 Pi-Tris, pH 7.3) containing glutamate–malate (5: 2.5 mm) or succinate–rotenone (5 mm−1 μm). All substrates were free acids buffered to pH 7.3 with Tris. Changes in extra-mitochondrial calcium concentration were monitored fluorimetrically (Hitachi, F4500 or Ocean Optics SD2000 spectrofluorometer) using Calcium Green-5N (1 μm, excitation–emission: 505–535 nm) as described by Ichas et al. (1997). Residual calcium concentration was adjusted to the same level at the beginning of every experiment by adding a small amount of EGTA. Unless stated otherwise, calcium pulses (83 nmol (mg prot.)−1) were then added at 2 min intervals until a Ca2+-induced mitochondrial Ca2+ release was observed. We have previously shown that this Ca2+ release is invariably accompanied by loss of ΔΨ and high amplitude swelling of the mitochondrial matrix (Marcil et al. 2005). Calcium retention capacity (CRC) was taken as the total amount of Ca2+ accumulated by mitochondria prior to the Ca2+ pulse triggering Ca2+ release. This value represents a reliable index of the threshold [Ca2+] required to open the PTP in the whole mitochondrial population studied.
Endogenous Ca2+ content
Muscles were sampled from one hindlimb and freeze-clamped in liquid N2 for determination of whole muscle Ca2+ content. Muscles from the contralateral limb were used for the isolation of mitochondria as described above except that all buffers were free of EGTA in order to avoid chelating Ca2+. Ground muscle samples and isolated mitochondrial pellets were diluted in 0.6 n HCl (1/10 w/v), homogenized with a polytron (2 × 10 s at a setting of 3) and sonicated (2 × 10 s at 40% of maximal power output). Following 30 min of incubation in boiling water, samples were centrifuged 5 min at 10 000 g and the supernatant was recovered. Ca2+ content in the supernatant was determined spectrophotometrically (VERSAmax, Molecular Devices) using an O-Cresolphthalein Complexone assay according the manufacturer's instructions (TECO Diagnostics, Anaheim, CA, USA). Results were expressed in nanomoles Ca2+ per milligram protein.
Immunoblot analysis
The protein expression of CypD, ANT and VDAC was determined in the isolated mitochondrial fraction. Samples were prepared for SDS-PAGE by dilutions with reducing sample buffer followed by a 10 min immersion in near-boiling water. Twenty micrograms of protein were loaded in each lane and resolved on 12% polyacrylamide mini-gels at room temperature. The gels were transferred to a PVDF membrane (Millipore). Equal sample loading was confirmed by Ponceau S stain (Sigma-Aldrich). The membrane was fixed for 10 min with 0.05% glutaraldehyde in phosphate-buffered saline with 0.1% Tween 20 (PBS-T) then blocked in 5% non-fat milk (CypD, VDAC) or 5% BSA (ANT) in PBS-T at room temperature for 90 min, and incubated overnight at 4°C with the following primary antibodies diluted in PBS-T with 5% BSA: anti-CypD (1: 2000 dilution, Affinity Bioreagents, Golden, CO, USA), anti-VDAC (1: 2000 dilution, Alexis Biochemicals, San Diego, CA, USA) and anti-ANT (1: 2000 dilution, Calbiochem San Diego, CA). Membranes were then incubated for 90 min at room temperature in secondary antibody solution (1: 50000 dilution; Jackson ImmunoResearch Laboratories, Inc., West Grove, PA, USA). Bands were visualized by enhanced chemiluminescence (Amersham) with film exposure times ranging from 5 to 45 min. Films were scanned and bands quantified by ImagePro software.
Statistical analysis
Data were analysed using ANOVA followed by Fisher's post hoc test. Where appropriate (i.e. in Ca2+ challenge experiments), Bonferonni's correction was applied to the P-value obtained to correct for multiple comparisons. A corrected P-value < 0.05 was considered significant.
Results
Morphometric parameters and mitochondrial protein isolation yield
Denervation for 21 days caused an atrophy of the hindlimb muscles as evidenced by a significant reduction in absolute muscle mass and muscle mass to body mass ratio (Table 1). The amount of protein recovered in the mitochondrial fraction was not significantly different between the two experimental groups. However, because of muscle atrophy, the total amount recovered per gram of muscle was significantly lower in denervated compared to sham-operated animals.
Table 1. Morphometric data and mitochondrial yield in muscle from sham and denervated animals.
| Sham | Denervated | |
|---|---|---|
| Body weight (g) | 262.3±4.3 | 262.0±3.7 |
| Hindlimb muscle weight (mg) | 1679±27 | 649±15* |
| Muscle mass to body mass ratio (mg g−1) | 6.42±0.009 | 2.49±0.05* |
| Mitochondrial isolation yield (mg prot.g−1) | 3.926±0.286 | 3.207±0.388 |
| Mitochondrial protein yield (mg) | 6.610±0.449 | 3.332±0.322* |
Significantly different from sham, P < 0.05
Respiratory parameters
Figure 1 shows the results of respirometry experiments aimed to determine oxidative capacity. In mitochondria from denervated muscles, basal ADP restricted (V0) and maximal rate of ADP-stimulated (VADP) respiration were significantly decreased relative to sham when expressed per milligram of protein. This reduction of respiratory activity was similar in magnitude to that of the respiratory chain marker enzyme COX, which was 1.8-fold lower in denervated compared to sham-operated animals. Consequently, when respiration rates were normalized per unit of COX, V0 and VADP were similar in the two experimental groups.
Figure 1. Respiratory function in mitochondria from sham and denervated animals.
A, basal ADP-restricted (Vo), and maximal rate of oxidative phosphorylation following addition of 1 mm ADP (VADP) expressed per mg of protein. B, the activity of the terminal respiratory chain enzyme COX in the mitochondrial fraction of sham-operated and denervated animals. C, V0 and VADP expressed per unit of COX present in the mitochondrial fraction of both experimental groups. For respirometry experiments mitochondria were energized with glutamate–malate (5, 2.5 mm). Data are presented as means ± s.e.m., for n = 6 and n = 5 in the denervated and sham groups, respectively. *Significantly different from sham (P < 0.05).
Vulnerability to Ca2+-induced PTP opening
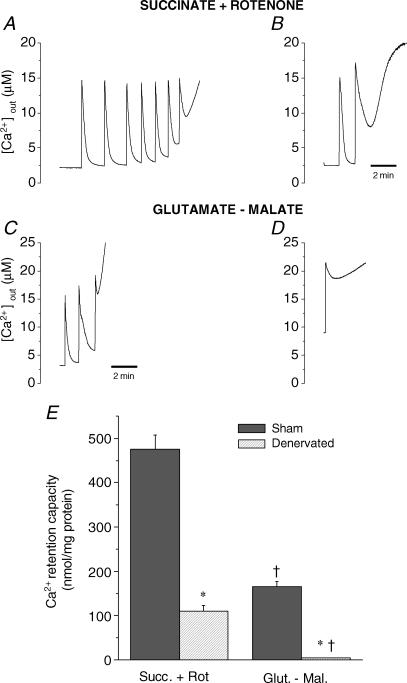
Figure 2 shows representative Ca2+ uptake traces in mitochondria from sham and denervated muscles submitted to progressive Ca2+ loading. When energized with the complex II substrate succinate, mitochondria from sham muscle were able to accumulate 475 ± 33 nmol Ca2+ (mg protein)−1 before Ca2+ was released as a result of PTP opening. In contrast, under the same conditions 4.3-fold less Ca2+ was required to trigger pore opening in mitochondria from denervated muscle (111 ± 12 nmol Ca2+ (mg protein)−1) and the Ca2+ uptake kinetics were substantially slower compared to sham (time to 50% uptake: 48 ± 13 versus 14 ± 1 s, P < 0,05).
Figure 2. Response to Ca2+ challenge in mitochondria from sham and denervated animals.
Typical Calcium Green 5N tracing of muscle mitochondria (0.15 mg ml−1) energized with succinate–rotenone (5 mm, 1 μm) and glutamate–malate (5, 2.5 mm) in sham operated (A and C) and denervated animals (B and D). Tracings show progressive Ca2+ accumulation followed by release of accumulated Ca2+ secondary to PTP opening. Each spike indicates the addition of a calcium pulse of 83 nmol mg−1. E, average Ca2+ retention capacity of mitochondria in the various experimental conditions. Means + s.e.m. are presented for n = 8 and 9 in the sham and denervated groups, respectively. *Significantly different from sham (P < 0.05), †Significantly different from Succinate + Rotenone within the same experimental group (P < 0.05).
In energized mitochondria, the regulation of PTP opening is known to vary according to the type of substrate oxidized, with substrates feeding complex I acting as sensitizers compared to substrates for complex II (Fontaine et al. 1998; Bernardi, 1999; Leverve & Fontaine, 2001; Marcil et al. 2005). Therefore, experiments were also performed in the presence of the complex I donors glutamate–malate (Fig. 2). In line with previous results, CRC in the presence of glutamate–malate was significantly lower than in the presence of the complex II substrate succinate in the two experimental groups. However, CRC was still reduced in denervated compared to sham-operated animals.
In order to determine whether changes in CRC could be due to changes in Ca2+ present in the mitochondrial matrix at baseline, endogenous Ca2+ levels were determined in the mitochondria and whole muscle. Figure 3 indicates that mitochondria from denervated muscles had a greater Ca2+ content compared to sham, which was accompanied by a substantial muscle Ca2+ overload.
Figure 3. Effect of denervation on endogenous mitochondrial and whole muscle Ca2+ content in sham and denervated animals.
Endogenous Ca2+ content measured in the whole muscle homogenate and the mitochondrial fraction of sham and denervated animals. Data are presented as means + s.e.m., for n = 8 and n = 9 in the denervated and sham groups, respectively. *Significantly different from sham (P < 0.05).
As indicated by the lower (∼2-fold) content of both inner (COX, ANT) and outer membrane (VDAC) markers (Figs 1 and 5), denervation appeared to reduce the number of mitochondria present in the mitochondrial fraction. Therefore, CRC was also determined with a fixed amount of COX (Fig. 4). Under these conditions, the difference in CRC observed between the two experimental groups was significantly less (2.4-fold) than the results obtained with a fixed amount of protein (4.3-fold). In addition, if endogenous Ca2+ present in mitochondria at baseline was taken into account, the difference in CRC between sham and denervated muscles decreased further to 1.5-fold, but remained significant.
Figure 5. Cyclophilin-D, VDAC and ANT content of mitochondrial fraction from sham and denervated animals.
Representative immunoblots of cyclophilin D (CypD: 21 kDa), voltage dependent anion channel (VDAC: 31 kDa) and adenine nucleotide translocase (ANT: 31 kDa) in muscle mitochondrial fraction isolated from sham and denervated animals. Each line represents a single mitochondrial preparation in each group. Densitometric analysis for each protein represents the mean + s.e.m. of 4 preparations in each experimental group. A and B show CypD content expressed relative to that of VDAC and ANT, respectively. C shows CypD content relative to the activity of COX measured in the same samples. *Significantly different from sham (P < 0.05).
Figure 4. Total Ca2+ retention capacity and effect of PTP inhibitors in mitochondria from sham and denervated animals.
Total Ca2+ retention capacity in absence and presence of the PTP inhibitors cyclosporin-A (1 μm) and trifluoperazine (10 μm). Values are expressed as the sum of endogenous Ca2+ (filled bars) and of Ca2+ added during the in vitro challenge and are expressed per unit of the marker enzyme cytochrome oxidase (COX). Means + s.e.m. are presented for n = 8 and 9 in the sham and denervated groups, respectively. *Significantly different from sham (P < 0.05), †Significantly different from baseline (P < 0.05).
Figure 4 also shows the results of experiments performed in the presence of inhibitors of the PTP. Incubation in the presence of 1 μm CsA, which inhibits pore opening by binding to the putative regulatory protein CypD (Davidson & Halestrap, 1990; Baines et al. 2005; Basso et al. 2005; Nakagawa et al. 2005; Schinzel et al. 2005), resulted in a 1.6 ± 0.1-fold increase in CRC in mitochondria from sham muscles. In contrast, in the denervated group the same treatment had a significantly more potent effect and increased CRC by 2.4 ± 0.2-fold. As a consequence, CsA was able to abolish the difference in CRC observed between the two experimental groups. In order to determine if this phenomenon was unique to CsA, experiments were also performed in the presence of trifluoperazine, which inhibits PTP opening through a CypD-independent mechanism (Bernardi et al. 1993). Incubation with trifluoperazine increased CRC in both experimental groups but did not correct for the difference in CRC observed between sham and denervated at baseline in the absence of inhibitors.
Immunoblot analysis of the mitochondrial fraction
Since the increased potency to pharmacological PTP inhibition in the denervated group appeared to be specific to CsA the mitochondrial content of CypD, the putative receptor for CsA (Davidson & Halestrap, 1990; Baines et al. 2005; Basso et al. 2005; Nakagawa et al. 2005; Schinzel et al. 2005) was determined (Fig. 5). No significant difference in the absolute content of CypD was observed between groups. However similar to what was observed with COX activity (Fig. 1) the content of VDAC and ANT, two abundant mitochondrial proteins was significantly lower in the mitochondrial fraction of denervated compared to sham-operated muscles. Therefore, following denervation, CypD appeared to be over expressed by 2- to 3-fold relative to each protein marker evaluated (Fig. 5).
Discussion
Recent studies have shown that mitochondria, through their capacity to signal cell death, may be involved in the atrophic remodelling of muscles following denervation (Tews & Goebel, 1996, 1997a, a, Tews, 2002; Siu & Alway, 2005) but the mechanisms remain largely undefined. In the present study we show that mitochondria isolated from denervated muscles display some respiratory dysfunction and a greater susceptibility to opening of the PTP, one of the mechanisms involved in mitochondria-mediated cell death. This phenomenon is due in part to the existence in vivo of a muscle and mitochondrial Ca2+ overload, which favours permeability transition. In addition, we provide evidence that mitochondria from denervated muscle have an increased CypD content relative to several marker proteins and display a selective increase in the sensitivity to CsA, suggesting that CypD further facilitates pore opening in the denervated state.
Effect of denervation on respiratory capacity
Similar to what has been previously reported (Joffe et al. 1981a), we observed that mitochondria isolated from denervated muscle display a reduction in V0 and VADP per milligram of protein, which would indicate that denervation caused a significant reduction in oxidative capacity. However, the amplitude of this reduction should be interpreted with caution. Indeed, while milligrams of protein is generally a good denominator to express respiratory parameters because the majority of mitochondrial proteins consist of respiratory chain enzymes, in some cases its use can introduce distortions in the results (Leary et al. 2003). For instance, in the present study denervation caused minimal changes in the protein concentration of the mitochondrial fraction (Table 1), whereas COX and other mitochondrial marker proteins were significantly reduced. Because the relationship between total protein and specific mitochondrial markers was altered by denervation, respiration was also expressed per unit of COX (Leary et al. 2003). When expressed this way, the effect of denervation on oxidative capacity was much less pronounced, which suggests that the negative impact of denervation on the metabolic potential of mitochondria could be less important than previously thought (Joffe et al. 1981a).
Effect of denervation on the susceptibility to Ca2+-induced PTP opening
Previous studies in rats denervated for 21 days have reported that respiratory uncoupling occurred prematurely in mitochondria when incubated in the presence of Ca2+, although the mechanisms were not investigated (Joffe et al. 1981b, c). The results we obtained in the present study clearly establish a role for the PTP in Ca2+-induced dysfunction. Indeed, CRC was substantially lower in denervated compared to sham-operated muscles when expressed per milligram of protein or per unit of COX and the CRC displayed sensitivity to classic PTP inhibitors, particularly CsA.
Ca2+-induced permeability transition is known to be modulated by a variety of physiological effectors, including the flow of electrons through complex I, which increases the sensitivity of the PTP to Ca2+-induced opening (Fontaine et al. 1998; Bernardi, 1999; Leverve & Fontaine, 2001; Marcil et al. 2005). However, in the present study, bypassing complex I using succinate did not abolish the difference in CRC between both experimental groups, suggesting that the greater sensitivity of mitochondria to pore opening in denervated muscle was not related to a mechanism involving electron flow through complex I.
In accordance with a previous study (Joffe et al. 1981c), we observed a significant elevation in the endogenous level of Ca2+ in mitochondria from denervated muscles. This phenomenon has been reported in other myopathies (Joffe et al. 1981c; Marchand et al. 2001) and is probably due to a progressive increase in intramuscular Ca2+ levels (Joffe et al. 1981c and Fig. 3) which appears to occur as a result of an increase in the leakiness of the sarcolemma and the abnormal morphology and functioning of the sarsoplasmic reticulum (Joffe et al. 1981c; Takekura et al. 2003). Since the accumulation of Ca2+ in the matrix is the main trigger for PTP opening (Zoratti & Szabo, 1995) this increase in endogenous Ca2+ clearly contributed to the increased mitochondrial vulnerability to pore opening in the denervated group. However, the fact that CRC still remained 1.5-fold lower in the denervated group after taking endogenous Ca2+ into account indicated that other factors further predisposed mitochondria to permeability transition.
In this regard CypD, a matrix peptidyl-prolyl cis–trans isomerase, was recently shown to play an important role in sensitizing the pore to Ca2+ (Baines et al. 2005; Basso et al. 2005; Nakagawa et al. 2005; Schinzel et al. 2005). Indeed, in mitochondria from Ppif−/− mice, which are devoid of CypD, the amount of Ca2+ required to trigger PTP opening is increased severalfold (Basso et al. 2005; Nakagawa et al. 2005; Schinzel et al. 2005). In addition, the inhibitory effect of CsA is completely abolished in these mice, which indicates that CypD expression is the key factor that confers sensitivity to CsA (Basso et al. 2005; Nakagawa et al. 2005; Schinzel et al. 2005). Consistent with these data, Baines et al. (2005) also reported that in mice over-expressing CypD in a cardiac-restricted manner, PTP opening occurred more readily and that CsA could prevent this effect. Our observations that CsA was selectively more potent in mitochondria from denervated muscle and was able to restore CRC to the level observed in sham-operated muscle therefore suggest a role for CypD in increasing the vulnerability to PTP opening following denervation. The fact that CypD was over expressed by 2- to 3-fold relative to all mitochondrial protein markers measured following denervation is consistent with this idea. To our knowledge, this is the first study to suggest a link between changes in CypD expression and PTP dysregulation in a non-transgenic model of disease.
Conclusion
In conclusion, the results reported in the present study indicate that conditions that substantially increase the vulnerability of mitochondria to undergo permeability transition are observed 21 days after denervation. This includes a significant increase in mitochondrial and whole muscle Ca2+ content and an increase in the expression of CypD, which further predispose the Ca2+ overloaded mitochondria to permeability transition. This observation is of significance as the occurrence of PTP opening in some mitochondria in vivo may partly account for the activation of mitochondria-mediated cell death that is known to occur in the process of denervation atrophy (Tews & Goebel, 1996, 1997a, b; Tews, 2002; Siu & Alway, 2005).
Acknowledgments
This work was supported by grants from the Natural Science and Engineering Council of Canada (NSERC: to Y.B) and by the Canadian Space Agency (to P.F.G) and by a Ph.D. Fellowship from NSERC to K.C. Y.B. is a Junior Investigator of the Fonds de Recherche en Sante du Quebec (FRSQ).
References
- Allen DL, Linderman JK, Roy RR, Bigbee AJ, Grindeland RE, Mukku V, Edgerton VR. Apoptosis: a mechanism contributing to remodeling of skeletal muscle in response to hindlimb unweighting. Am J Physiol. 1997;273:C579–C587. doi: 10.1152/ajpcell.1997.273.2.C579. [DOI] [PubMed] [Google Scholar]
- Allen DL, Roy RR, Edgerton VR. Myonuclear domains in muscle adaptation and disease. Muscle Nerve. 1999;22:1350–1360. doi: 10.1002/(sici)1097-4598(199910)22:10<1350::aid-mus3>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Baines CP, Kaiser RA, Purcell NH, Blair NS, Osinska H, Hambleton MA, Brunskill EW, Sayen MR, Gottlieb RA, Dorn GW, Robbins J, Molkentin JD. Loss of cyclophilin D reveals a critical role for mitochondrial permeability transition in cell death. Nature. 2005;434:658–662. doi: 10.1038/nature03434. [DOI] [PubMed] [Google Scholar]
- Basso E, Fante L, Fowlkes J, Petronilli V, Forte MA, Bernardi P. Properties of the permeability transition pore in mitochondria devoid of cyclophilin D. J Biol Chem. 2005;280:18558–18561. doi: 10.1074/jbc.C500089200. [DOI] [PubMed] [Google Scholar]
- Bernardi P. Mitochondrial transport of cations: channels, exchangers, and permeability transition. Physiol Rev. 1999;79:1127–1155. doi: 10.1152/physrev.1999.79.4.1127. [DOI] [PubMed] [Google Scholar]
- Bernardi P, Scorrano L, Colonna R, Petronilli V, Di Lisa F. Mitochondria and cell death. Mechanistic aspects and methodological issues. Eur J Biochem. 1999;264:687–701. doi: 10.1046/j.1432-1327.1999.00725.x. [DOI] [PubMed] [Google Scholar]
- Bernardi P, Veronese P, Petronilli V. Modulation of the mitochondrial cyclosporin A-sensitive permeability transition pore. I. Evidence for two separate Me2+ binding sites with opposing effects on the pore open probability. J Biol Chem. 1993;268:1005–1010. [PubMed] [Google Scholar]
- Borisov AB, Carlson BM. Cell death in denervated skeletal muscle is distinct from classical apoptosis. Anat Rec. 2000;258:305–318. doi: 10.1002/(SICI)1097-0185(20000301)258:3<305::AID-AR10>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Burelle Y, Hochachka PW. Endurance training induces muscle-specific changes in mitochondrial function in skinned muscle fibers. J Appl Physiol. 2002;92:2429–2438. doi: 10.1152/japplphysiol.01024.2001. [DOI] [PubMed] [Google Scholar]
- Crompton M. The mitochondrial permeability transition pore and its role in cell death. Biochem J. 1999;341:233–249. [PMC free article] [PubMed] [Google Scholar]
- Davidson AM, Halestrap AP. Partial inhibition by cyclosporin A of the swelling of liver mitochondria in vivo and in vitro induced by sub-micromolar [Ca2+], but not by butyrate. Evidence for two distinct swelling mechanisms. Biochem J. 1990;268:147–152. doi: 10.1042/bj2680147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Lisa F, Bernardi P. Mitochondrial function as a determinant of recovery or death in cell response to injury. Mol Cell Biochem. 1998;184:379–391. [PubMed] [Google Scholar]
- Fontaine E, Eriksson O, Ichas F, Bernardi P. Regulation of the permeability transition pore in skeletal muscle mitochondria. Modulation by electron flow through the respiratory chain complex I. J Biol Chem. 1998;273:12662–12668. doi: 10.1074/jbc.273.20.12662. [DOI] [PubMed] [Google Scholar]
- Halestrap AP, Doran E, Gillespie JP, O'Toole A. Mitochondria and cell death. Biochem Soc Trans. 2000;28:170–177. doi: 10.1042/bst0280170. [DOI] [PubMed] [Google Scholar]
- He L, Lemasters JJ. Regulated and unregulated mitochondrial permeability transition pores: a new paradigm of pore structure and function? FEBS Lett. 2002;512:1–7. doi: 10.1016/s0014-5793(01)03314-2. [DOI] [PubMed] [Google Scholar]
- Ichas F, Jouaville LS, Mazat JP. Mitochondria are excitable organelles capable of generating and conveying electrical and calcium signals. Cell. 1997;89:1145–1153. doi: 10.1016/s0092-8674(00)80301-3. [DOI] [PubMed] [Google Scholar]
- Irwin WA, Bergamin N, Sabatelli P, Reggiani C, Megighian A, Merlini L, Braghetta P, Columbaro M, Volpin D, Bressan GM, Bernardi P, Bonaldo P. Mitochondrial dysfunction and apoptosis in myopathic mice with collagen VI deficiency. Nat Genet. 2003;35:367–371. doi: 10.1038/ng1270. [DOI] [PubMed] [Google Scholar]
- Irwin W, Fontaine E, Agnolucci L, Penzo D, Betto R, Bortolotto S, Reggiani C, Salviati G, Bernardi P. Bupivacaine myotoxicity is mediated by mitochondria. J Biol Chem. 2002;277:12221–12227. doi: 10.1074/jbc.M108938200. [DOI] [PubMed] [Google Scholar]
- Joffe M, Savage N, Isaacs H. Biochemical functioning of mitochondria in normal and denervated mammalian skeletal muscle. Muscle Nerve. 1981a;4:514–519. doi: 10.1002/mus.880040608. [DOI] [PubMed] [Google Scholar]
- Joffe M, Savage N, Isaacs H. Ca2+-uptake properties of two populations of mitochondria from normal and denervated rat soleus muscle. Biochem J. 1981b;200:671–677. doi: 10.1042/bj2000671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joffe M, Savage N, Isaacs H. Increased muscle calcium. A possible cause of mitochondrial dysfunction and cellular necrosis in denervated rat skeletal muscle. Biochem J. 1981c;196:663–667. doi: 10.1042/bj1960663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J-S, He L, Lemasters JJ. Mitochondrial permeability transition: a common pathway to necrosis and apoptosis. Biochem Biophys Res Commun. 2003;304:463. doi: 10.1016/s0006-291x(03)00618-1. [DOI] [PubMed] [Google Scholar]
- Leary SC, Lyons CN, Rosenberger AG, Ballantyne JS, Stillman J, Moyes CD. Fiber-type differences in muscle mitochondrial profiles. Am J Physiol Regul Integr Comp Physiol. 2003;285:R817–R826. doi: 10.1152/ajpregu.00058.2003. [DOI] [PubMed] [Google Scholar]
- Leeuwenburgh C, Gurley CM, Strotman BA, Dupont-Versteegden EE. Age-related differences in apoptosis with disuse atrophy in soleus muscle. Am J Physiol Regul Integr Comp Physiol. 2005;288:R1288–R1296. doi: 10.1152/ajpregu.00576.2004. [DOI] [PubMed] [Google Scholar]
- Leverve XM, Fontaine E. Role of substrates in the regulation of mitochondrial function in situ. IUBMB Life. 2001;52:221–229. doi: 10.1080/15216540152846037. [DOI] [PubMed] [Google Scholar]
- Lu DX, Huang SK, Carlson BM. Electron microscopic study of long-term denervated rat skeletal muscle. Anat Rec. 1997;248:355–365. doi: 10.1002/(SICI)1097-0185(199707)248:3<355::AID-AR8>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Marchand E, Constantin B, Vandebrouck C, Raymond G, Cognard C. Calcium homeostasis and cell death in Sol8 dystrophin-deficient cell line in culture. Cell Calcium. 2001;29:85–96. doi: 10.1054/ceca.2000.0159. [DOI] [PubMed] [Google Scholar]
- Marcil M, Bourduas K, Ascah A, Burelle Y. Exercise training induces a respiratory substrate specific increase in Ca2+-induced permeability transition pore opening in heart mitochondria. Am J Physiol Heart Circ Physiol. 2005;290:H1549–1557. doi: 10.1152/ajpheart.00913.2005. [DOI] [PubMed] [Google Scholar]
- Nakagawa T, Shimizu S, Watanabe T, Yamaguchi O, Otsu K, Yamagata H, Inohara H, Kubo T, Tsujimoto Y. Cyclophilin d-dependent mitochondrial permeability transition regulates some necrotic but not apoptotic cell death. Nature. 2005;434:652–658. doi: 10.1038/nature03317. [DOI] [PubMed] [Google Scholar]
- Sandri M, Carraro U, Podhorska-Okolov M, Rizzi C, Arslan P, Monti D, Franceschi C. Apoptosis, DNA damage and ubiquitin expression in normal and mdx muscle fibers after exercise. FEBS Lett. 1995;373:291–295. doi: 10.1016/0014-5793(95)00908-r. [DOI] [PubMed] [Google Scholar]
- Sandri M, El Meslemani AH, Sandri C, Schjerling P, Vissing K, Andersen JL, Rossini K, Carraro U, Angelini C. Caspase 3 expression correlates with skeletal muscle apoptosis in Duchenne and facioscapulo human muscular dystrophy. A potential target for pharmacological treatment? J Neuropathol Exp Neurol. 2001;60:302–312. doi: 10.1093/jnen/60.3.302. [DOI] [PubMed] [Google Scholar]
- Schinzel AC, Takeuchi O, Huang Z, Fisher JK, Zhou Z, Rubens J, Hetz C, Danial NN, Moskowitz MA, Korsmeyer SJ. Cyclophilin D is a component of mitochondrial permeability transition and mediates neuronal cell death after focal cerebral ischemia. Proc Natl Acad Sci U S A. 2005;102:12005–12010. doi: 10.1073/pnas.0505294102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siu PM, Alway SE. Mitochondria-associated apoptotic signalling in denervated rat skeletal muscle. J Physiol. 2005;565:309–323. doi: 10.1113/jphysiol.2004.081083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siu PM, Pistilli EE, Alway SE. Apoptotic responses to hindlimb suspension in gastrocnemius muscles from young adult and aged rats. Am J Physiol Regul Integr Comp Physiol. 2005a;289:R1015–R1026. doi: 10.1152/ajpregu.00198.2005. [DOI] [PubMed] [Google Scholar]
- Siu PM, Pistilli EE, Butler DC, Alway SE. Aging influences cellular and molecular responses of apoptosis to skeletal muscle unloading. Am J Physiol Cell Physiol. 2005b;288:C338–C349. doi: 10.1152/ajpcell.00239.2004. [DOI] [PubMed] [Google Scholar]
- Suleiman MS, Halestrap AP, Griffiths EJ. Mitochondria: a target for myocardial protection. Pharmacol Ther. 2001;89:29–46. doi: 10.1016/s0163-7258(00)00102-9. [DOI] [PubMed] [Google Scholar]
- Takekura H, Tamaki H, Nishizawa T, Kasuga N. Plasticity of the transverse tubules following denervation and subsequent reinnervation in rat slow and fast muscle fibres. J Muscle Res Cell Motil. 2003;24:439–451. doi: 10.1023/a:1027356912404. [DOI] [PubMed] [Google Scholar]
- Tews DS. Apoptosis and muscle fibre loss in neuromuscular disorders. Neuromuscul Disord. 2002;12:613–622. doi: 10.1016/s0960-8966(02)00030-5. [DOI] [PubMed] [Google Scholar]
- Tews DS, Goebel HH. DNA fragmentation and BCL-2 expression in infantile spinal muscular atrophy. Neuromuscul Disord. 1996;6:265–273. doi: 10.1016/0960-8966(96)00018-1. [DOI] [PubMed] [Google Scholar]
- Tews DS, Goebel HH. Apoptosis-related proteins in skeletal muscle fibers of spinal muscular atrophy. J Neuropathol Exp Neurol. 1997a;56:150–156. doi: 10.1097/00005072-199702000-00005. [DOI] [PubMed] [Google Scholar]
- Tews DS, Goebel HH. DNA-fragmentation and expression of apoptosis-related proteins in muscular dystrophies. Neuropathol Appl Neurobiol. 1997b;23:331–338. [PubMed] [Google Scholar]
- Yoshimura K, Harii K. A regenerative change during muscle adaptation to denervation in rats. J Surg Res. 1999;81:139–146. doi: 10.1006/jsre.1998.5504. [DOI] [PubMed] [Google Scholar]
- Zoratti M, Szabo I. The mitochondrial permeability transition. Biochim Biophys Acta. 1995;1241:139–176. doi: 10.1016/0304-4157(95)00003-a. [DOI] [PubMed] [Google Scholar]
- Zoratti M, Szabo I, De Marchi U. Mitochondrial permeability transitions: how many doors to the house? Biochim Biophys Acta. 2005;1706:40–52. doi: 10.1016/j.bbabio.2004.10.006. [DOI] [PubMed] [Google Scholar]