Abstract
The 5′-AMP-activated protein kinase (AMPK) is a potent regulator of skeletal muscle metabolism and gene expression. AMPK is activated both in response to in vivo exercise and ex vivo contraction. AMPK is therefore believed to be an important signalling molecule in regulating muscle metabolism during exercise as well as in adaptation of skeletal muscle to exercise training. The first part of this review is focused on different mechanisms regulating AMPK activity during muscle work such as alterations in nucleotide concentrations, availability of energy substrates and upstream AMPK kinases. We furthermore discuss the possible role of AMPK as a master switch in skeletal muscle metabolism with the main focus on AMPK in metabolic regulation during muscle work. Finally, AMPK has a well established role in regulating expression of genes encoding various enzymes in muscle, and this issue is discussed in relation to adaptation of skeletal muscle to exercise training.
Skeletal muscle is characterized by a dramatic increase in energy turnover in response to contraction and exercise. In order to maintain the muscle energy charge, both oxidative and non-oxidative ATP syntheses are increased. To feed the ATP production of the working muscle, carbohydrate originating from plasma glucose and muscle glycogen stores as well as lipid originating from plasma lipids and intramuscular triacylglycerol stores are oxidized (Holloszy et al. 1998; Richter et al. 2001; Kiens, 2006).
The ability of the skeletal muscle to produce ATP is dependent on content and activities of organelles and proteins responsible for substrate processing, oxidation and ATP production. Acutely, during exercise, the alterations in cytosolic messengers (e.g. Ca2+, free AMP, Pi, creatine, H+, lipid intermediates) are associated with activation of several signalling cascades and a rapid increase in uptake and intracellular mobilization of glucose and fatty acids. Simultaneously, signalling pathways eliciting transcription of genes encoding metabolic proteins are initiated. Such alterations may change muscle metabolic capacity in the period after exercise, and will in particular be manifested if these pathways are repeatedly activated as during regular physical activity. Even though the acute and chronic regulations may be different in nature and temporally separated, common initiating factors activated during exercise may exist. The 5′-AMP-activated protein kinase seems to be a candidate for this role because it can both regulate cell metabolism acutely and induce transcription of genes encoding various proteins.
The 5′-AMP-activated protein kinase (AMPK) is a multisubstrate serine/threonine protein kinase which is ubiquitously expressed and functions as an intracellular fuel sensor activated by depletion of high energy phospho compounds (Corton et al. 1994; Hardie et al. 1998). The overall function of AMPK is in response to cellular energy deprivation to increase the potential for ATP production while concurrently decreasing cellular energy-consuming anabolic processes (Corton et al. 1994; Kahn et al. 2005). The heterotrimeric AMPK holoenzyme consists of one catalytic subunit (α) and two functionally and structurally different regulatory subunits (β, γ). Two isoforms have been identified of the α- and β-subunit (α1 and -2, and β1 and -2) and three isoforms of the γ-subunit (γ1–3) (Kemp et al. 2003).
Activation of AMPK in exercising skeletal muscle
It is well established that muscle contraction is associated with a vast increase in energy turnover (>100-fold) and introduces a major energetic challenge to the muscle fibre (Sahlin et al. 1998). Under conditions of high energy turnover, AMP concentration increases with only a small decrease in ATP concentration. Even though free cytosolic AMP content is heavily buffered by binding to proteins and by deamination to inosine monophosphate (IMP), estimates of the pool of free cytosolic AMP suggested that it increases in response to contraction and exercise and that the increase is exercise intensity dependent (Tullson & Terjung, 1990; Rundell et al. 1993). AMP activates AMPK by binding to the two CBS domains on the γ-subunit, which activates AMPK directly by an allosteric mechanism and indirectly by rendering AMPK a better substrate for upstream kinase(s) and a worse substrate for phosphatases thus enhancing a potent activating phosphorylation on Thr172 on the α-subunit (Adams et al. 2004; Scott et al. 2004; Kahn et al. 2005). The binding of AMP to the γ-subunit is antagonized by ATP and the [ATP]/[AMPfree] ratio is therefore alleged to be the best predictor of AMPK activity (Corton et al. 1994), and reduction of this ratio is thought to be one of the major factors activating AMPK during muscle contraction and exercise (Park et al. 2002b; Chen et al. 2003).
A vast line of studies have shown that AMPK is activated in rodent muscle by electrical stimulation ex vivo and by motor nerve stimulation of both alive animals and perfused rat hindlimb in situ (Hutber et al. 1997; Vavvas et al. 1997; Hayashi et al. 1998; Derave et al. 2000). In vivo, exercise studies have furthermore shown that AMPK is activated in rat muscle during treadmill running and in human muscle during cycle exercise in a time and exercise-intensity-dependent manner (Winder & Hardie, 1996; Wojtaszewski et al. 2000; Fujii et al. 2000; Chen et al. 2003). In general, in rodent muscle both α1 and α2-associated AMPK complexes are activated during contraction/exercise whereas the majority of human studies display a more pronounced sensitivity and response of activation of α2-AMPK compared with α1-AMPK (Wojtaszewski et al. 2000; Musi et al. 2001; Park et al. 2002b; Chen et al. 2003). Although this may relate to both intensity and fibre type recruitment, it has become apparent that the expression pattern of AMPK isoforms varies between rodent and human muscle and between muscles types (Chen et al. 1999; Durante et al. 2002; Frosig et al. 2003). In mouse muscle for instance, α2-, γ1- and both β-isoforms are fairly evenly distributed between red and white muscle types whereas the α1-, γ2- and γ3-isoform content is higher in white muscle (Yu et al. 2004; Jorgensen et al. 2004b; Mahlapuu et al. 2004; authors' unpublished observations). Furthermore, a recent study investigated the isoform compositions of AMPK complexes in mixed human vastus lateralis muscle and found that only 3 of the 12 theoretically possible AMPK complexes were present in detectable amounts (α2β2γ1 ≫ α2β2γ3 = α1β2γ1) (Wojtaszewski et al. 2005). Even though AMPK in muscle has been shown to be sensitive to circulating factors such as leptin (Minokoshi et al. 2002) and adiponectin (Yamauchi et al. 2002), the activation of AMPK in exercising muscle seems to depend, at least in part, on local mechanisms. This is so, because AMPK activation is retained in contracting ex vivo incubated rodent muscle (Hayashi et al. 1998; Jorgensen et al. 2004b) and because AMPK activation during one-legged exercise in human is restricted to the exercising leg (authors' unpublished observations).
Interestingly, activation of α2-AMPK during ex vivo muscle contraction seems mainly to depend on covalent modification on α-Thr172 by the upstream LKB1 kinase, since knocking out LKB1 almost completely prevents both AICAR- and contraction-induced α2-AMPK signalling (Sakamoto et al. 2005). The increase in α-Thr172 AMPK-phosphorylation seems mainly to depend on conformational changes in AMPK which result in it becoming a better substrate for LKB1 as LKB1 activity is not increased during contraction of rodent muscle (Sakamoto et al. 2004; Hurst et al. 2005). However, LKB1 does not seem to be the only existing AMPK kinase as one study has recently shown increased activity of an unidentified AMPK kinase in exercising human muscle (Chen et al. 2003). Since some studies have shown that CaMK kinase phosphorylates AMPK in vitro (Hawley et al. 1995; Hurley et al. 2005), calcium signalling may also have a role in activating AMPK in muscle during exercise. Recent unpublished data from the authors' laboratory obtained in contracting mouse muscle supports this possibility.
Muscle glycogen seems to be an important factor in regulation of muscle AMPK activity. Several studies have shown that glycogen can be a powerful negative controller of AMPK because glycogen loading of muscle suppresses AMPK signalling in response to both exercise/contraction and AICAR (Derave et al. 2000; Wojtaszewski et al. 2002a, 2003). Interestingly, the AMPK β-subunit possesses a glycogen-binding domain binding AMPK to glycogen in vitro, but since incubation of AMPK with glycogen particles does not affect AMPK activity the mechanistic link does not seem to be via a direct interaction (Polekhina et al. 2003; Hudson et al. 2003). Furthermore, if the γ3-subunit is knocked out in mouse muscle then the inverse relationship between glycogen content and AMPK activity is no longer seen (Barnes et al. 2005) suggesting that only AMPK activity associated with γ3-complexes is affected by glycogen. Thus, the reduction in muscle glycogen during exercise could in part explain the steady increase in AMPK activity seen during prolonged bicycle exercise (Wojtaszewski et al. 2002b; Stephens et al. 2002; Rose et al. 2005) possibly due to a gradual relief from a glycogen related inhibition.
Interestingly, AMPK also seems to respond to availability of extra-cellular fuel sources by an as yet unknown mechanism(s). For instance, lowering of glucose in the medium of ex vivo incubated muscles and cultured β-cells increases AMPK activity (Salt et al. 1998; Itani et al. 2003), and also the AMPK homologue in yeast, SNF1, is activated by low availability of external glucose (Hardie et al. 1998). Two studies have investigated the influence of oral glucose ingestion on AMPK activation during exercise in human muscle (De Bock et al. 2005; Akerstrom et al. 2006). Both studies showed that AMPK activity expressed as phosphorylation of AMPK or its downstream substrate ACCβ was not affected significantly by glucose ingestion. However, further analysis in the study by Akerstrom et al. revealed that when AMPK signalling was measured as α-isoform specific kinase activity, α2- but not α1-AMPK activity was attenuated during exercise when glucose was ingested simultaneously. Moreover, in vitro studies have shown that long-chain fatty acid esters reduce AMPK activation by making AMPK a poorer substrate for LKB1 suggesting that muscle AMPK may be regulated by fatty acid (FA) availability (Taylor et al. 2005b). However, prolonged exposure to palmitate of resting ex vivo incubated muscle does not influence AMPK activity (Olsen & Hansen, 2002), and lowering plasma free fatty acid by prior ingestion of nicotinic acid (acipimox) also does not influence human muscle AMPK at rest (Watt et al. 2004a). On the other hand, lowering plasma free fatty acids (FFAs) during exercise in humans with nicotinic acid accelerates the exercise-induced AMPK activation (Watt et al. 2004a), but whether this is due to a reduction in cellular FA or the disturbance of muscle energy balance in not clear. Finally, an earlier study indicated that the energy-rich phosphocreatine inhibits AMPK activity allosterically, and the dramatic decrease in phosphocreatine during the onset of intensive exercise could be speculated to contribute to activation of AMPK by a ‘relief of inhibition’ mechanism (Ponticos et al. 1998). However, recent evidence has shown that phosphocreatine does not affect AMPK activity directly, although it may still be an important factor in regulating AMPK activation during exercise by buffering muscle ATP content and in turn diminishing AMP accumulation (Taylor et al. 2005a).
AMPK in acute regulation of muscle metabolism at rest and during exercise
In skeletal muscle, AMPK has been shown to have regulatory effects on fatty acid oxidation, glycogen metabolism and presumably also protein synthesis, but the most well-documented action of AMPK in skeletal muscle today is its ability to activate glucose uptake.
Glucose uptake
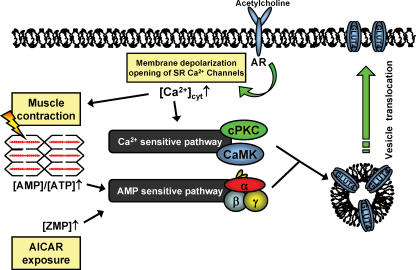
It was initially shown by Merrill et al. (1997) that the adenosine analogue 5-amino-4-imidazolecarboxamide riboside (AICAR) increased glucose uptake and AMPK activity in perfused rat muscle. This association between AMPK activity and glucose transport has been verified as causal by studies in ex vivo incubated muscles from transgenic mice where ablation or reduction of AMPK signalling by either over-expressing a kinase-dead α2-AMPK construct or knocking out the catalytic α2-isoform or the regulatory γ3-isoform completely abolished AICAR-stimulated glucose uptake (Mu et al. 2001; Jorgensen et al. 2004b; Barnes et al. 2004; Fujii et al. 2005). Activation of AMPK by AICAR or hypoxia increases the plasma membrane content of the transmembrane glucose transporter GLUT4, and in this way AMPK seems to control muscle glucose uptake, at least in part (Kurth-Kraczek et al. 1999; Mu et al. 2001; Koistinen et al. 2003) (Fig. 1).
Figure 1. Two pathways thought to regulate muscle glucose uptake during contraction.
The increase in cytosolic Ca2+ initiates contraction and is also suggested to be a feed-forward stimulus to glucose transport. Lowering of the muscle energy charge is in addition suggested to regulate muscle glucose in a feed-back manner. AR: acetylcholine receptor; SR: sarcoplasmatic reticulum.
Based on these observations, and because AMPK is activated during muscle contractions, AMPK has been proposed to be a key player in initiating signalling to contraction-stimulated glucose uptake (Merrill et al. 1997; Hayashi et al. 1998)(Fig. 1). Although obvious, such a connection has been surprisingly difficult to verify even with the use of genetic approaches. Several different genetic strategies have been used to evaluate the relationship between contraction-induced AMPK activation, GLUT4 translocation and glucose transport. Two transgenic mouse models with a muscle specific over-expression of a kinase-dead α2-AMPK construct displayed partially reduced contraction-stimulated glucose uptake in the EDL muscle (Mu et al. 2001; Fujii et al. 2005). Furthermore, in both mouse models force production was compromised during the ex vivo experimental conditions applied which could play some role in the observed reduction in contraction-stimulated glucose uptake (Mu et al. 2001; Fujii et al. 2005). In contrast, the soleus muscle from the AMPK kinase-dead mouse has normal force production ex vivo yet glucose transport is substantially reduced, although not totally eliminated (Mu et al. 2001) (T. E. Jensen, A. J. Rose & the authors, unpublished observations). However, the α1-isoform is only partially displaced by the kinase-dead α2 over-expression and the muscle still expresses some α1-AMPK activity which seems to be regulated during contractions (T. E. Jensen, A. J. Rose & the authors, unpublished observations). Similar to the data obtained in the α1- or α2-AMPK knockout models, in which ex vivo contraction-stimulated glucose uptake and force production is normal (Jorgensen et al. 2004b), the interpretation of the data from the two transgenic models may be biased by this residual AMPK activity. Taken together, these findings might be interpreted to mean that if both α1- and α2-AMPK activity are markedly decreased then contraction-induced glucose uptake is diminished, but as long as one of the two α-isoforms is expressed normally then contraction-induced glucose uptake is not decreased, at least during the ex vivo conditions studied. Recently, data obtained in mice with a muscle-specific knockout of LKB1 was reported. In this model, covalent activation of α2-AMPK is largely prevented during contraction and glucose uptake is severely blunted (Sakamoto et al. 2005). Although this model depicts LKB1 as a major upstream regulator of AMPK during contraction it does not necessarily link AMPK and glucose transport directly, as LKB1 regulates several other kinases the role of which is not fully elucidated (Sakamoto et al. 2004). Finally, it should be committed to memory when evaluating results obtained in transgenic animals that the risk of compensatory alterations of alternative signalling pathways always exists which may affect the interpretations of data.
The signalling pathway(s) by which AMPK regulates the sarcolemma GLUT4 content is far from clear but recent evidence suggests that the Rab binding protein AS160 may be involved in AMPK regulated glucose uptake in muscle (Bruss et al. 2005). Studies in 3T3-L1 adipocytes/fibroblasts have shown that AS160 is a negative regulator of Rab-dependent GLUT4 vesicle translocation and is phosphorylated and inactivated by Akt in addition to AMPK (Kane et al. 2002; Zeigerer et al. 2004). This view is reinforced by the observation that mutating several potential Akt sites on AS160 decreases insulin-induced membrane translocation of GLUT4 in adipocytes (Sano et al. 2003; Zeigerer et al. 2004). Interestingly, studies in rat muscle have shown that AS160 is phosphorylated both by AICAR and ex vivo contraction, suggesting that AS160 is involved in AMPK-dependent GLUT4 translocation (Bruss et al. 2005). This view is supported by recent observations showing that lowering AMPK signalling in white muscle by either α2- or a γ3-knockout (KO) or over-expressing the kinase-dead AMPK construct severely blunts AICAR-induced increases in AS160 phosphorylation indicating that AMPK complexes containing α2- and γ3-complexes are necessary for AICAR-induced AS160 phosphorylation in muscle (Treebak et al. 2006). During contraction, the increase in AS160 phosphorylation is still completely prevented in α2-AMPK KO and AMPK kinase-dead muscles but not in γ3-AMPK KO muscles indicating that contraction-induced AS160 phosphorylation is independent of AMPK γ3-complexes (Treebak et al. 2006). It has previously been shown that AICAR-induced glucose uptake is completely prevented in muscle from the α2- and γ3-AMPK KO mice and the AMPK kinase-dead mouse, while contraction-induced glucose uptake ex vivo is normal in α2- and γ3-AMPK KO muscles and only partially reduced in AMPK kinase dead muscles (Mu et al. 2001; Barnes et al. 2004; Jorgensen et al. 2004b; Fujii et al. 2005). Thus, the relationship between AMPK activity, AS160 phosphorylation and contraction-induced glucose uptake is not clear at this point.
Taken together, these investigations clearly show that AMPK can regulate glucose uptake in resting mouse muscle and that AMPK likely plays a partial role in contraction-stimulated glucose uptake in mouse muscle. However, all studies above employed high-intensity electrically induced isometric contraction which could have led to supra-physiological activation of AMPK, and extrapolation of these results to in vivo exercise is not straightforward. Even though several exercise studies in general have reported good agreements between the average increase in glucose uptake and AMPK activity in muscle biopsies from bicycling human subjects (Wojtaszewski et al. 2003; Nielsen et al. 2002), correlations do not necessarily imply causality. Hence, it seems evident that it is still too early to define the role of AMPK in regulating muscle glucose uptake during exercise, and that more in vivo approaches using transgenic mice or specific inhibitor strategies seem warranted to elucidate this aspect. Furthermore, alternative signalling pathways to AMPK such as Ca2+-dependent signalling molecules (e.g. CaMK and PKC) may be involved (Rose & Richter, 2005; Jessen & Goodyear, 2005) (Fig. 1).
Glycogen metabolism
Glycogen is an important energy source for the working muscle, especially at moderate to high intensities, and it is broken down by glycogen phosphorylase (GP) and glycogen disbranching enzyme to provide glycosyl units for glycolysis and oxidative phosphorylation. After a bout of exercise, or in response to insulin, glycogen is under the consumption of ATP (re)synthesized from cellular glucose by a chain of enzymes, and the incorporation of UDP-glucose to glycogen by glycogen synthase (GS) is believed to be the rate limiting step (Roach, 2002)(Fig. 2). Regulation of GP and GS is complex and depends on both allosteric and covalent mechanisms. Originally GP-kinase was found to be a target for AMPK, potentially enhancing GP activity and glycogenolysis (Carling & Hardie, 1989; Young et al. 1996). However, more recent in vitro evidence has shown that GP-kinase is unlikely to be an AMPK substrate suggesting that AMPK does not regulate glycogenolysis (Beyer et al. 2000). The finding that glycogen breakdown during treadmill running in muscle from either the AMPK kinase-dead mouse, or the α2- or γ3-AMPK KO mouse is not compromised (Mu et al. 2003; Barnes et al. 2004; Jorgensen et al. 2005) could support such a view.
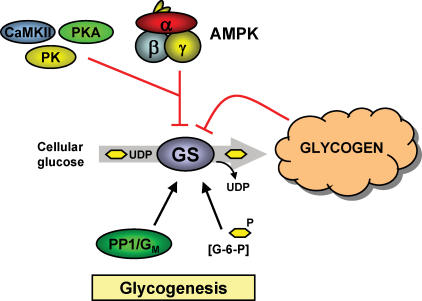
Figure 2. Glycogen synthesis.
Glycogen is synthesized by incorporation of cellular UDP-glucose into the glycogen particle. Glycogen synthase (GS) is believed to be the rate limiting step in this process. GS activity is increased by glucose-6-phophate (G-6-P) and phosphatase activity (PP1/G). Conversely, GS activity is reduced by increasing levels of glycogen and by phosphorylation on at least nine serine residues (e.g. Site2/Ser7) induced by several protein kinases where AMPK, calmodulin-activated protein kinase II (CaMKII), protein kinase A (PKA) and glycogen phosphorylase kinase (PK) are believed to be important Site2 kinases. Black arrow: activation; red arrow: inhibition/deactivation
In contrast to GP, several lines of in vivo and in vitro evidence have shown that AMPK phosphorylates GS on Site2 (Ser7) with a concomitant reduction in GS activity (Carling & Hardie, 1989; Jorgensen et al. 2004a; Halse et al. 2003) which is in agreement with the role of AMPK as a protector of cellular energy homeostasis (Fig. 2). In mouse muscle, the α2-isoform seems to be the most important α-AMPK isoform in phosphorylating GS because knocking out the α2-subunit completely prevented AICAR-induced deactivation of GS (Jorgensen et al. 2004a). A logical assumption from this finding could be that the α1-isoform is of minor importance in regulation of GS activity in response to AICAR. Even though it seems obvious that AMPK covalently modulates GS activity, it has been difficult to establish that activation of AMPK in fact lowers the rate of glycogenesis in resting muscle. A somewhat surprising finding was that chronic activation of AMPK with AICAR in rat muscle actually results in glycogen accumulation rather than lowering glycogen content (Winder et al. 2000). This is probably because activation of AMPK with AICAR in addition to directly inhibiting GS activity also increases glucose uptake and subsequently the cellular content of the allosteric GS activator, glucose-6-P. An AMPK-dependent increase in glucose-6-P would therefore be expected to induce an allosteric activation of GS potentially overruling the deactivating Site2 phosphorylation. This idea is indirectly supported by the finding that if basal AMPK signalling is increased by, for example, natural mutations or transgenic approaches, glycogen content is increased (Milan et al. 2000; Barnes et al. 2004) and if AMPK signalling is lowered then muscle glycogen is lowered (Mu et al. 2001; Jorgensen et al. 2004b) in spite of reduced kinase activity towards Site2 on GS (Jorgensen et al. 2004a). Despite these observations in resting muscle, it could be argued that one function of the activation of AMPK during exercise/contraction is to counteract activation of GS and thus the ATP and glucose consuming glycogenesis.
In working muscle, GS activity is in general found to be increased except during very intense exercise where unchanged or even decreased GS activity has been reported (Nielsen & Wojtaszewski, 2004). Although likely to be complex in nature, one plausible explanation for the increase in GS activity is that the breakdown of muscle glycogen during exercise is associated with dephosphorylation of GS at Site3a and -3b which leads to an elevated GS activity (Nielsen & Wojtaszewski, 2004). However, concurrent phosphorylation of GS on Site2 would act to counter-regulate the effects mediated by dephosphorylation on Site3a and -3b. Studies in the α2-KO mouse suggest that α2-AMPK does not fulfil such a role as the increase in GS Site2 phosphorylation is normal both during treadmill exercised and in ex vivo contracted white muscle lacking the α2-isoform (authors' unpublished observations). However, it cannot entirely be ruled out that activation of the remaining α1-isoform in α2-KO muscle had normalized AMPK signalling towards GS during exercise/contraction. Also, it should be remembered that during exercise, several additional GS Site2 kinases (PKA, PKC, CaMK and GPK) are likely to be activated making it difficult to tease out the specific role of each single kinase (Fig. 2).
Fatty acid metabolism
Both resting and exercising muscle oxidize long-chain fatty acids (LCFAs) to generate ATP. These are either transported from the plasma into the muscle or are derived from hydrolysis of intracellular triacylglycerol (IMTG) stores. The transsarcolemmal uptake of LCFAs in muscle is believed to depend on both facilitated diffusion aided by fatty acid translocase (FAT/CD36) and the membrane associated fatty acid-binding protein (FABPpm) as well as simple diffusion (Kiens, 2006). Studies in giant sarcolemmal vesicles obtained from rat muscles have shown that with contractions, FAT/CD36 translocates to the plasma membrane with a parallel increase in fatty acid uptake and this mechanism has therefore been suggested as a regulatory step in contraction-induced LCFA uptake (Bonen et al. 2000). This view is in addition supported by the observation that activation of AMPK with AICAR increases FFA uptake in perfused rat muscle (Raney et al. 2005). It is also noteworthy that the lowering of AMPK activity by resistin in L6 skeletal muscle cells decreases cell surface FAT/CD36 content (Palanivel & Sweeney, 2005) and that activation of AMPK with AICAR in cardiac myocytes increases FAT/CD36 plasma membrane translocation (Luiken et al. 2003). A recent study has, however, shown that inhibition of ERK 1/2 without inhibition of AMPK completely prevented the contraction-induced increase in CD36 translocation to plasma membrane and LCFA uptake (Turcotte et al. 2005). Thus, the role of AMPK in contraction-induced uptake of LCFA of muscle is not clear.
Several studies have in addition shown that AMPK can enhance muscle LCFA oxidation. For instance activation of AMPK with AICAR, leptin or adiponectin increases palmitate oxidation in both perfused rat muscle and in vivo in mouse muscle and in cultured muscle cells (Merrill et al. 1998; Winder & Holmes, 2000; Minokoshi et al. 2002; Yamauchi et al. 2002). Studies in transgenic animals support these observations since expression of the activating γ3 R225Q mutation in muscle increased oleate oxidation and prevented accumulation of triglycerides in mice fed a high-fat diet (Barnes et al. 2004). Interestingly, recent data have shown that resistin lowers AMPK signalling in muscle-like cells and that this reduction is associated with a suppressed fatty acid oxidation (Palanivel & Sweeney, 2005).
The mechanism by which AMPK controls LCFA oxidation in muscle is believed to be at the level of mitochondrial entry of LCFA via phosphorylation of the muscle isoform of acetyl-CoA carboxylase (ACCβ) at Ser218. This interaction does not per se alter ACCβ carboxylase activity but desensitizes ACCβ to allosteric activation by cytosolic citrate (Vavvas et al. 1997) and also sensitizes ACCβ to inhibition by palmitoyl-CoA (Rubink & Winder, 2005). A decrease in activity of ACCβ will decrease formation of malonyl-CoA (Winder & Hardie, 1996) (Fig. 3). Some studies (Saha et al. 2000; Park et al. 2002a), but not all (Habinowski et al. 2001), suggests that AMPK also lowers cellular malonyl-CoA content by phosphorylating and activating malonyl-CoA decarboxylase (MCD), the enzyme responsible for decarboxylating malonyl-CoA to acetyl-CoA (Fig. 3).
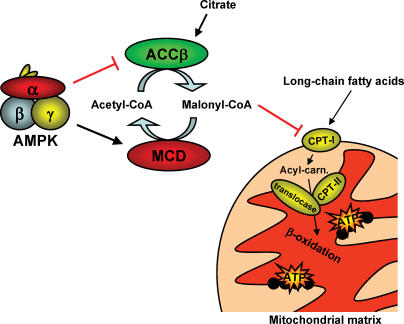
Figure 3. The mechanism by which AMPK is believed to stimulate fatty acid oxidation in the mitochondrial matrix.
Production of malonyl-CoA is reduced by inhibiting acetyl-CoA carboxylase-β activity (ACCβ) and possibly by activating malonyl-CoA decarboxylase (MCD) activity. Since malonyl-CoA inhibits carnitine palmitoyl transferase-I (CPT-I) activity, the reduction in malonyl-CoA content will relieve CPT-I from its inhibition leading to increased mitochondrial fatty acid uptake and oxidation. Red oval arrow: inhibition/deactivation. Acyl-carn: acyl-carnitine.
Because malonyl-CoA is an potent inhibitor of carnitine palmitoyl transferase-I (CPT-I), the rate limiting enzyme in mitochondrial FA uptake, a reduced level of malonyl-CoA relieves CPT-I from its inhibition in turn allowing carnitinization of LCFA-CoA and subsequent entry to the mitochondrial matrix for β-oxidation (McGarry & Brown, 1997) (Fig. 3). Since AMPK phosphorylates ACCβ (and MCD) and thereby lowers endogenous ACCβ activity during exercise/contraction, AMPK has been hypothesized to be a key player in enhancing muscle LCFA oxidation during exercise.
If the AMPK → ACCβ/(MCD) → malonyl-CoA axis is a central mechanism in up-regulating LCFA oxidation during exercise, then some degree of correlation between malonyl-CoA and lipid oxidation might be expected. While studies in treadmill exercised rats have shown a decrease in muscle malonyl-CoA at the same time as lipid oxidation presumably is increased (Winder & Hardie, 1996), studies of humans have shown conflicting results. This might to some extent be explained by the fact that the malonyl-CoA level is ∼100-fold lower in human than rat muscle (Winder et al. 1990; Dean et al. 2000; Roepstorff et al. 2005; Kraegen et al. 2006), perhaps suggesting that malonyl-CoA has a less important role in regulating FA oxidation in human muscle and/or that different mechanisms regulate the malonyl-CoA level in human muscle compared with rat muscle. Thus, in human muscle older studies failed to detect a decrease in malonyl-CoA during both prolonged submaximal and short-term bicycle exercise at various intensities (Odlland et al. 1996, 1998) while others have reported a decrease in muscle malonyl-CoA levels during submaximal bicycle exercise and one-legged exercise (Dean et al. 2000; Roepstorff et al. 2004b). However, in the study by Roepstorff et al. muscle lipid oxidation was manipulated by varying pre-exercise muscle glycogen levels but the decrease in malonyl-CoA concentration was identical in the two conditions despite differences in AMPK activity. In the study by Dean et al. it was found that malonyl-CoA concentrations only decreased at higher exercise intensities when lipid oxidation in fact decreased. Finally, a recent study showed that the higher lipid oxidation in females than males during exercise cannot be explained by a higher muscle AMPK activity in females. On the contrary, due to better maintenance of energy status in female muscle during exercise, AMPK activation was in fact lower in females than in males (Roepstorff et al. 2006). Taken together, these data may be interpreted to mean that while activation of AMPK and decrease in malonyl-CoA could be responsible for the increase in fat oxidation that occurs from rest to exercise, further fine tuning of fat oxidation during exercise is not carried out by the AMPK → ACCβ/(MCD) → malonyl-CoA axis. This fine tuning may be related to the muscle availability of free carnitine because carnitinization of LCFA-CoA by CPT-I is necessary for entry into the mitochondrial matrix (Roepstorff et al. 2005). However, a recent study has shown that electrically induced contraction of perfused rat muscle in situ by a low-intensity protocol increases FA uptake and oxidation without increasing AMPK activity or decreasing ACCβ activity (Raney et al. 2005). These findings imply that if AMPK is involved in regulating muscle FA metabolism during exercise this action seems to occur during more intensive exercise and that AMPK-independent mechanisms predominate during low-intensity muscle contraction (Raney et al. 2005).
Hormone sensitive lipase
AMPK has also been suggested to be involved in regulation of breakdown of IMTG during exercise via phosphorylation of the enzyme hormone sensitive lipase (HSL) thought to be important if not the rate-limiting enzyme in IMTG breakdown (Kiens, 2006). HSL activity can be modulated by phosphorylation of specific sites and five phosphorylation sites on HSL have so far been identified as regulatory sites. In vitro studies have demonstrated that Ser563, Ser659 and Ser660 are cAMP-dependent protein kinase A (PKA) targets on HSL (Holm, 2003) whereas Ser565 (Garton et al. 1989) and Ser600 (Shen et al. 2001) are targets for AMPK and MAPK, respectively. In adipocytes, activation of AMPK with AICAR has been found to inhibit subsequent HSL activation and lipolysis by β-adrenergic stimulation (Sullivan et al. 1994) suggesting an antilipolytic role of AMPK (Corton et al. 1995).
In skeletal muscle the role of AMPK in regulation of HSL activity and degradation of IMTG during exercise is controversial. No conclusive studies using genetic manipulation of AMPK expression in intact animals have been published. Available evidence stems mainly from human studies in which AMPK activity was manipulated by altering pre-exercise muscle glycogen levels. Thus, Roepstorff et al. (2004a) found that low pre-exercise muscle glycogen values as expected increased α2-AMPK activity in the vastus lateralis muscle and was associated with increased phosphorylation of HSL on Ser565 during exercise. However, there was no effect on HSL activity measured in vitro or on IMTG breakdown during exercise, leading the authors to conclude that AMPK activation and HSL phosphorylation on Ser565 do not play a major role in regulation of HSL activity during exercise. In another human study with a similar design, the different conclusion was that activation of AMPK can inhibit HSL activation by β-adrenergic stimulation (Watt et al. 2004b). This was further supported by studies in L6-myotubes in which constitutively active AMPK decreased HSL activity (Watt et al. 2006). However, in isolated contracting rat soleus muscle, HSL activity varied in the face of constant AMPK activity (Donsmark et al. 2004). Taken together, the available data do not allow any firm conclusions about the role of AMPK in regulation of HSL activity in skeletal muscle during exercise.
Protein synthesis
It is well established that global ribosomal synthesis of proteins is a potent consumer of cellular energy, a process among others requiring energy-rich aminoacyl-tRNA, mRNA and ATP/GTP. Protein synthesis can be regulated at several levels such as content of mRNA and ribosomes as well as the specific rate of translating mRNA to the nascent peptide where translation conventionally is subdivided into three phases, initiation, elongation and termination (Proud & Denton, 1997). Furthermore, short-term regulation of protein synthesis is believed to be predominately at the translation level (Bolster et al. 2004a). The acute regulation of protein synthesis is controlled by several classes of regulatory proteins modulated by the mTOR pathway or the eEF-2 kinase pathway. The mTOR pathway signals to initiation of mRNA translation by activating eukaryotic initiation factor 2 (eIF2) and S6 kinase (S6K1) and assembly of the eukaryotic initiator 4F complex where the latter is achieved by phosphorylating and inactivating eukaryotic initiation factor 4E binding proteins (4E-BP) (Proud, 2004). The calcium sensitive eukaryotic elongation factor 2 kinase (eEF2K or CaMKIII) phosphorylates and deactivates eukaryotic eEF2 thereby inhibiting its interacting with ribosomes and thus impairing the rate of peptide elongation (Carlberg et al. 1990).
Some evidence suggests that AMPK can reduce cellular energy expenditure by lowering protein synthesis. Interestingly, subcutaneous injection of AICAR in rat reduces incorporation of phenylalanine to proteins by ∼50% suggesting that the concomitant activation of α2-AMPK leads to an acute reduction of protein synthesis in resting rat muscle (Bolster et al. 2002). Since transfection of HEK293 kidney cells with a kinase-dead AMPK construct abolishes AICAR stimulated protein synthesis (Horman et al. 2002), the above effect of AICAR in vivo seems to depend on AMPK signalling rather than side-effects of AICAR induced by, for example, hypoglycaemia or other AMP-mediated effects in the cell. The mechanism by which AMPK inhibits protein synthesis potentially depends on down- and up-regulation of mTOR and eEF2 kinase signalling to 4E-BPs and eEF2, respectively (Horman et al. 2002; Inoki et al. 2003; Chan et al. 2004). In accordance, AICAR treatment of rat skeletal muscle and cardiomyocytes reduces both 4E-BP-dependent assembly of the eIF4 F complex and eEF2 activity (Bolster et al. 2002; Horman et al. 2003).
Studies in rat and untrained human have shown that global protein synthesis is reduced during acute dynamic exercise in a time- and intensity-dependent manner (Rennie & Tipton, 2000; Rennie, 2005). For instance, in electrically stimulated perfused rat muscle, protein synthesis is reduced by almost 90% in white muscle types (Bylund-Fellenius et al. 1984). Whether the exercise-induced activation of AMPK is directly involved in lowering global protein synthesis in muscle seems convoluted to address, because the mTOR pathway is activated by growth factors/hormones and amino acids and the eEF-2 kinase pathway is activated by Ca2+, and AMPK (Richter et al. 2004; Bolster et al. 2004b; Proud, 2004). However, studies in exercising humans have shown that the inhibitory Thr56 phosphorylation on eEF-2 is rapid and sustained during 90 min of exercise, contrasting with the more gradual increase in AMPK activity during the exercise bout (Rose et al. 2005). This could indicate a role of other regulatory pathways, e.g. Ca2+-dependent signalling towards mRNA translation during exercise. Due to the complex matrix of intracellular signalling potentially involved in regulating global muscle protein synthesis during exercise, it seems too early to conclude on the role of AMPK in the acute regulation of protein synthesis during muscle exercise. More causal approaches than correlative comparisons are clearly warranted.
AMPK in metabolic adaptation in muscle
In addition to the acute metabolic functions of AMPK, AMPK also regulates expression of specific genes. Repetitive pharmacological activation of AMPK in vivo results in protein expression of muscle mimicking some of the effects of exercise training. For instance, activation of AMPK by daily injections with AICAR or chronic intake of the creatine analogue β-guanadinopropionic acid (β-GPA) in rodent increases muscle expression of GLUT4 and hexokinase II (HKII) (Holmes et al. 1999; Winder et al. 2000; Zheng et al. 2001) (Fig. 4). These findings are supported by studies of the AMPK kinase-dead or the α2-KO mouse which showed that muscle mRNA content of GLUT4 and HKII fails to increase in response AICAR (Holmes et al. 2004; Jorgensen et al. 2005). Furthermore, chronic activation of AMPK with AICAR or β-GPA increases mitochondrial content and expression of mitochondrial proteins (Winder et al. 2000; Bergeron et al. 2001; Zong et al. 2002) (Fig. 4). Again, transgenic approaches to lower or abrogate muscle AMPK signalling strongly suggest a causal role of AMPK in this scheme. In the AMPK kinase-dead mouse, β-GPA-induced increase in mitochondrial content is totally abolished (Zong et al. 2002) and an α2-knockout hinders AICAR in increasing protein/mRNA of several mitochondrial markers (citrate synthaste, HAD, COX1, cytochrome c, CPT-I) (Jorgensen et al. 2005; authors' unpublished observations). In accordance with the decreased mitochondrial enzyme content, we have shown that the α2-KO mouse has a disturbed muscle energy balance during exercise as indicated by a reduced ATP content with a comparable increase in IMP content (Jorgensen et al. 2005). Thus, these data are in fact the first to demonstrate a crucial role for α2-AMPK in maintaining energy-status in muscle during in vivo exercise. Our findings are supported by recent observations showing that LKB1 knockout muscles have disturbed energy balance and α2-AMPK signalling during ex vivo contraction (Sakamoto et al. 2005).
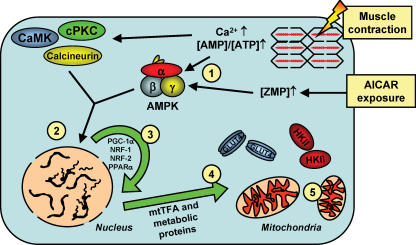
Figure 4. Cellular events proposed to be involved in inducing mitochondrial biogenesis and up-regulation of some metabolic proteins in muscle at rest and during exercise.
1, increases in the [AMP]/[ATP] ratio activates AMPK in exercising muscle and ZMP activates AMPK in resting muscle. 2, AMPK and several other signalling molecules increase transcription of nuclear genes encoding metabolic proteins and transcriptional modulators. 3, transcription modulators subsequently activate nuclear genes encoding various mitochondrial and metabolic enzymes as well as mitochondrial genes. 4, synthesis of mitochondrial and metabolic enzymes as well as transcription factor(s) important for activation of mitochondrial genes in the mitochondrial matrix. 5, formation of new mitochondria.
The mechanism(s) by which AMPK exerts its actions on GLUT4 protein expression and on mitochondrial biogenesis seems to depend on activation of several cellular transcription factors and coactivators. The coordinated induction of mitochondrial biogenesis in muscle is believed to involve the peroxisome proliferator-activated receptor-γ coactivator-1α (PGC-1α) (Scarpulla, 2002). This transcriptional coactivator increases expression of the transcription factors nuclear respiratory factor (NRF) 1 and 2, mitochondrial transcription factor A (mtTFA) and peroxisome proliferator-activated receptor-α (PPARα) (Wu et al. 1999; Scarpulla, 2002) (Fig. 4). Interestingly, PGC-1α mRNA and protein increases in response to activation of AMPK with AICAR, β-GPA or thyroid hormone in differentiated muscle and L6E9 muscle cells which could indicate that AMPK exerts its mitochondriogenic action, at least in part, by enhancing PGC-1α expression (Zong et al. 2002; Irrcher et al. 2003; Jorgensen et al. 2005). Furthermore, over-expression of both PGC-1α and NRF-1 in cultured muscle cell lines and mouse muscle increases GLUT4 protein and transcriptional activity of myocyte enhancer factor (MEF) isoforms (GLUT4 transcription factors) (Michael et al. 2001; Baar et al. 2003) which could indicate that AMPK increases GLUT4 expression by a PGC-1α-dependent pathway. Furthermore, activation of AMPK with AICAR increases GLUT4 promoter DNA binding activity of MEF isoforms as well as GLUT4 (Zheng et al. 2001; Ojuka et al. 2002). In contrast, signalling molecules downstream of AMPK connecting it to protein expression of HKII are far from understood and clearly requires more research.
Based on these findings, a key role of AMPK in inducing metabolic adaptations of skeletal muscle to exercise-training might be hypothesized but only a few studies have provided more conclusive results concerning this aspect. Holmes et al. (2004) investigated whether the acute exercise-induced increase in GLUT4 gene activation is compromised in muscle from the AMPK kinase-dead mice. They somewhat surprisingly reported that GLUT4 mRNA increased normally after treadmill running in muscle with impaired AMPK signalling. In concert with this observation, we have shown that PGC-1α, HKII and FOXO1 mRNA increase normally in muscle from the α2-KO mouse after treadmill running despite a ∼60% reduction in AMPK signalling during both rest and exercise (Jorgensen et al. 2005). A complicating aspect of the study in the α2-KO mice is that the remaining α1-isoform in α2-AMPK KO muscle is still activated and it could be argued that the remaining α1-isoform in α2-KO muscle was sufficient in initiating metabolic adaptations in muscle to exercise. On the other hand, if AMPK is mandatory in initiating metabolic adaptations of muscle to exercise, then the reduction in AMPK signalling should be expected to be manifested at some point. Disregarding these speculations, alternative signalling pathways to AMPK such as CaMK, PKC, MAPK or calcineurin may be involved (Fig. 4).
Concluding remarks
A large body of experimental evidence has clearly shown that pharmacological activation of AMPK in resting muscle has profound actions on metabolism of glucose, lipids and proteins as well as on the expression of a large number of genes. Because AMPK is activated in muscle by exercise/contraction it is tempting to assign an important role for AMPK in regulation of muscle metabolism and gene expression during exercise/contraction. However, it has been surprisingly difficult to establish such a role for AMPK unequivocally. One reason for this may be the many additional signalling pathways that are activated during exercise such as MAP kinases, PKCs, CaMKs and calcineurin (Figs 1, 2 and 4) which may have overlapping actions to AMPK. Another reason is the lack of potent and specific pharmacological tools to inhibit AMPK signalling in differentiated muscle. So far, the most conclusive evidence has come from studies using genetic manipulation of AMPK expression/function but even so the available mouse models have sometimes offered less than conclusive findings. While it is somewhat surprising that the role of AMPK in exercise-induced gene activation has not been demonstrated so far, the α2-AMPK KO mouse in fact has decreased expression of mitochondrial proteins and markedly disturbed balance of ATP and AMP in muscle during exercise. The reason for the disturbed energy balance could be related to decreased mitochondrial protein expression or disturbance in mobilization or uptake of substrates. In terms of substrate availability, a role for AMPK in contraction-induced glucose uptake is emerging whereas the jury is still out on the role of AMPK in lipid and protein metabolism during muscle contraction.
Acknowledgments
The authors were supported by the Danish Medical and Natural Science Research Councils, the Novo Nordisk Foundation, the Danish Diabetes Association, the Lundbeck Foundation and an Integrated Project (LSHM-CT-2004-005272) funded by the European Commission. J.F.P.W. is the recipient of a Hallas Møller fellowship from the Novo Nordisk Foundation and S.B.J. is supported by a postdoctoral research fellowship from the Danish Medical Research Council (271-05-0697).
References
- Adams J, Chen ZP, Van Denderen BJ, Morton CJ, Parker MW, Witters LA, Stapleton D, Kemp BE. Intrasteric control of AMPK via the γ1 subunit AMP allosteric regulatory site. Protein Sci. 2004;13:155–165. doi: 10.1110/ps.03340004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akerstrom TCA, Birk JB, Klein DK, Erikstrup C, Plomgaard P, Pedersen BK, Wojtaszewki JFP. Oral glucose ingestion attenuates exercise-induced activation of 5′-AMP-activated protein kinase in human skeletal muscle. Biochem Biophys Res Commun. 2006;342:949–955. doi: 10.1016/j.bbrc.2006.02.057. [DOI] [PubMed] [Google Scholar]
- Baar K, Song Z, Semenkovich CF, Jones TE, Han DH, Nolte LA, Ojuka EO, Chen M, Holloszy JO. Skeletal muscle overexpression of nuclear respiratory factor 1 increases glucose transport capacity. FASEB J. 2003;17:1666–1673. doi: 10.1096/fj.03-0049com. [DOI] [PubMed] [Google Scholar]
- Barnes BR, Glund S, Long YC, Hjalm G, Andersson L, Zierath JR. 5′-AMP-activated protein kinase regulates skeletal muscle glycogen content and ergogenics. FASEB J. 2005;19:773–779. doi: 10.1096/fj.04-3221com. [DOI] [PubMed] [Google Scholar]
- Barnes BR, Marklund S, Steiler TL, Walter M, Hjalm G, Amarger V, Mahlapuu M, Leng Y, Johansson C, Galuska D, Lindgren K, Abrink M, Stapleton D, Zierath JR, Andersson L. The 5′-AMP-activated protein kinase γ3 isoform has a key role in carbohydrate and lipid metabolism in glycolytic skeletal muscle. J Biol Chem. 2004;279:38441–38447. doi: 10.1074/jbc.M405533200. [DOI] [PubMed] [Google Scholar]
- Bergeron R, Ren JM, Cadman KS, Moore IK, Perret P, Pypaert M, Young LH, Semenkovich CF, Shulman GI. Chronic activation of AMP kinase results in NRF-1 activation and mitochondrial biogenesis. Am J Physiol Endocrinol Metab. 2001;281:E1340–E1346. doi: 10.1152/ajpendo.2001.281.6.E1340. [DOI] [PubMed] [Google Scholar]
- Beyer A, Kitzerow A, Crute B, Kemp BE, Witters LA, Heilmeyer LM., Jr Muscle phosphorylase kinase is not a substrate of AMP-activated protein kinase. Biol Chem. 2000;381:457–461. doi: 10.1515/BC.2000.060. [DOI] [PubMed] [Google Scholar]
- Bolster DR, Crozier SJ, Kimball SR, Jefferson LS. AMP-activated protein kinase suppresses protein synthesis in rat skeletal muscle through down-regulated mammalian target of rapamycin (mTOR) signaling. J Biol Chem. 2002;277:23977–23980. doi: 10.1074/jbc.C200171200. [DOI] [PubMed] [Google Scholar]
- Bolster DR, Jefferson LS, Kimball SR. Regulation of protein synthesis associated with skeletal muscle hypertrophy by insulin-, amino acid- and exercise-induced signalling. Proc Nutr Soc. 2004a;63:351–356. doi: 10.1079/PNS2004355. [DOI] [PubMed] [Google Scholar]
- Bolster DR, Jefferson LS, Kimball SR. Regulation of protein synthesis associated with skeletal muscle hypertrophy by insulin-, amino acid- and exercise-induced signalling. Proc Nutr Soc. 2004b;63:351–356. doi: 10.1079/PNS2004355. [DOI] [PubMed] [Google Scholar]
- Bonen A, Luiken JJ, Arumugam Y, Glatz JF, Tandon NN. Acute regulation of fatty acid uptake involves the cellular redistribution of fatty acid translocase. J Biol Chem. 2000;275:14501–14508. doi: 10.1074/jbc.275.19.14501. [DOI] [PubMed] [Google Scholar]
- Bruss MD, Arias EB, Lienhard GE, Cartee GD. Increased phosphorylation of Akt substrate of 160 kDa (AS160) in rat skeletal muscle in response to insulin or contractile activity. Diabetes. 2005;54:41–50. doi: 10.2337/diabetes.54.1.41. [DOI] [PubMed] [Google Scholar]
- Bylund-Fellenius AC, Ojamaa KM, Flaim KE, Li JB, Wassner SJ, Jefferson LS. Protein synthesis versus energy state in contracting muscles of perfused rat hindlimb. Am J Physiol. 1984;246:E297–E305. doi: 10.1152/ajpendo.1984.246.4.E297. [DOI] [PubMed] [Google Scholar]
- Carlberg U, Nilsson A, Nygard O. Functional properties of phosphorylated elongation factor 2. Eur J Biochem. 1990;191:639–645. doi: 10.1111/j.1432-1033.1990.tb19169.x. [DOI] [PubMed] [Google Scholar]
- Carling D, Hardie DG. The substrate and sequence specificity of the AMP-activated protein kinase. Phosphorylation of glycogen synthase and phosphorylase kinase. Biochim Biophys Acta. 1989;1012:81–86. doi: 10.1016/0167-4889(89)90014-1. [DOI] [PubMed] [Google Scholar]
- Chan AY, Soltys CL, Young ME, Proud CG, Dyck JR. Activation of AMP-activated protein kinase inhibits protein synthesis associated with hypertrophy in the cardiac myocyte. J Biol Chem. 2004;279:32771–32779. doi: 10.1074/jbc.M403528200. [DOI] [PubMed] [Google Scholar]
- Chen Z, Heierhorst J, Mann RJ, Mitchelhill KI, Michell BJ, Witters LA, Lynch GS, Kemp BE, Stapleton D. Expression of the AMP-activated protein kinase β1 and β2 subunits in skeletal muscle. FEBS Lett. 1999;460:343–348. doi: 10.1016/s0014-5793(99)01371-x. [DOI] [PubMed] [Google Scholar]
- Chen ZP, Stephens TJ, Murthy S, Canny BJ, Hargreaves M, Witters LA, Kemp BE, McConell GK. Effect of exercise intensity on skeletal muscle AMPK signaling in humans. Diabetes. 2003;52:2205–2212. doi: 10.2337/diabetes.52.9.2205. [DOI] [PubMed] [Google Scholar]
- Corton JM, Gillespie JG, Hardie DG. Role of the AMP-activated protein kinase in the cellular stress response. Curr Biol. 1994;4:315–324. doi: 10.1016/s0960-9822(00)00070-1. [DOI] [PubMed] [Google Scholar]
- Corton JM, Gillespie JG, Hawley SA, Hardie DG. 5-Aminoimidazole-4-carboxamide ribonucleoside. A specific method for activating AMP-activated protein kinase in intact cells? Eur J Biochem. 1995;229:558–565. doi: 10.1111/j.1432-1033.1995.tb20498.x. [DOI] [PubMed] [Google Scholar]
- De Bock K, Richter EA, Russell AP, Eijnde BO, Derave W, Ramaekers M, Koninckx E, Leger B, Verhaeghe J, Hespel P. Exercise in the fasted state facilitates fibre type-specific intramyocellular lipid breakdown and stimulates glycogen resynthesis in humans. J Physiol. 2005;564:649–660. doi: 10.1113/jphysiol.2005.083170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean D, Daugaard JR, Young ME, Saha A, Vavvas D, Asp S, Kiens B, Kim KH, Witters L, Richter EA, Ruderman N. Exercise diminishes the activity of acetyl-CoA carboxylase in human muscle. Diabetes. 2000;49:1295–1300. doi: 10.2337/diabetes.49.8.1295. [DOI] [PubMed] [Google Scholar]
- Derave W, Ai H, Ihlemann J, Witters LA, Kristiansen S, Richter EA, Ploug T. Dissociation of AMP-activated protein kinase activation and glucose transport in contracting slow-twitch muscle. Diabetes. 2000;49:1281–1287. doi: 10.2337/diabetes.49.8.1281. [DOI] [PubMed] [Google Scholar]
- Donsmark M, Langfort J, Holm C, Ploug T, Galbo H. Contractions induce phosphorylation of the AMPK site Ser565 in hormone-sensitive lipase in muscle. Biochem Biophys Res Commun. 2004;316:867–871. doi: 10.1016/j.bbrc.2004.02.140. [DOI] [PubMed] [Google Scholar]
- Durante PE, Mustard KJ, Park SH, Winder WW, Hardie DG. Effects of endurance training on activity and expression of AMP-activated protein kinase isoforms in rat muscles. Am J Physiol Endocrinol Metab. 2002;283:E178–E186. doi: 10.1152/ajpendo.00404.2001. [DOI] [PubMed] [Google Scholar]
- Frosig C, Jorgensen SB, Hardie DG, Richter EA, Wojtaszewski JF. 5′AMP-activated protein kinase activity and protein expression are regulated by endurance training in human skeletal muscle. Am J Physiol Endocrinol Metab. 2003;286:E411–E417. doi: 10.1152/ajpendo.00317.2003. [DOI] [PubMed] [Google Scholar]
- Fujii N, Hayashi T, Hirshman MF, Smith JT, Habinowski SA, Kaijser L, Mu J, Ljungqvist O, Birnbaum MJ, Witters LA, Thorell A, Goodyear LJ. Exercise induces isoform-specific increase in 5′AMP-activated protein kinase activity in human skeletal muscle. Biochem Biophys Res Commun. 2000;273:1150–1155. doi: 10.1006/bbrc.2000.3073. [DOI] [PubMed] [Google Scholar]
- Fujii N, Hirshman MF, Kane EM, Ho RC, Peter LE, Seifert MM, Goodyear LJ. AMP-activated protein kinase α2 activity is not essential for contraction- and hyperosmolarity-induced glucose transport in skeletal muscle. J Biol Chem. 2005;280:39033–39041. doi: 10.1074/jbc.M504208200. [DOI] [PubMed] [Google Scholar]
- Garton AJ, Campbell DG, Carling D, Hardie DG, Colbran RJ, Yeaman SJ. Phosphorylation of bovine hormone-sensitive lipase by the AMP-activated protein kinase. A possible antilipolytic mechanism. Eur J Biochem. 1989;179:249–254. doi: 10.1111/j.1432-1033.1989.tb14548.x. [DOI] [PubMed] [Google Scholar]
- Habinowski SA, Hirshman M, Sakamoto K, Kemp BE, Gould SJ, Goodyear LJ, Witters LA. Malonyl-CoA decarboxylase is not a substrate of AMP-activated protein kinase in rat fast-twitch skeletal muscle or an islet cell line. Arch Biochem Biophys. 2001;396:71–79. doi: 10.1006/abbi.2001.2589. [DOI] [PubMed] [Google Scholar]
- Halse R, Fryer LG, McCormack JG, Carling D, Yeaman SJ. Regulation of glycogen synthase by glucose and glycogen: a possible role for AMP-activated protein kinase. Diabetes. 2003;52:9–15. doi: 10.2337/diabetes.52.1.9. [DOI] [PubMed] [Google Scholar]
- Hardie DG, Carling D, Carlson M. The AMP-activated/SNF1 protein kinase subfamily: metabolic sensors of the eukaryotic cell? Annu Rev Biochem. 1998;67:821–855. doi: 10.1146/annurev.biochem.67.1.821. [DOI] [PubMed] [Google Scholar]
- Hawley SA, Selbert MA, Goldstein EG, Edelman AM, Carling D, Hardie DG. 5′-AMP activates the AMP-activated protein kinase cascade, and Ca2+/calmodulin activates the calmodulin-dependent protein kinase I cascade, via three independent mechanisms. J Biol Chem. 1995;270:27186–27191. doi: 10.1074/jbc.270.45.27186. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Hirshman MF, Kurth EJ, Winder WW, Goodyear LJ. Evidence for 5′-AMP-activated protein kinase mediation of the effect of muscle contraction on glucose transport. Diabetes. 1998;47:1369–1373. doi: 10.2337/diab.47.8.1369. [DOI] [PubMed] [Google Scholar]
- Holloszy JO, Kohrt WM, Hansen PA. The regulation of carbohydrate and fat metabolism during and after exercise. Front Biosci. 1998;3:D1011–D1027. doi: 10.2741/a342. [DOI] [PubMed] [Google Scholar]
- Holm C. Molecular mechanisms regulating hormone-sensitive lipase and lipolysis. Biochem Soc Trans. 2003;31:1120–1124. doi: 10.1042/bst0311120. [DOI] [PubMed] [Google Scholar]
- Holmes BF, Kurth-Kraczek EJ, Winder WW. Chronic activation of 5′-AMP-activated protein kinase increases GLUT-4, hexokinase, and glycogen in muscle. J Appl Physiol. 1999;87:1990–1995. doi: 10.1152/jappl.1999.87.5.1990. [DOI] [PubMed] [Google Scholar]
- Holmes BF, Lang DB, Birnbaum MJ, Mu J, Dohm GL. AMP kinase is not required for the GLUT4 response to exercise and denervation in skeletal muscle. Am J Physiol Endocrinol Metab. 2004;287:E739–E743. doi: 10.1152/ajpendo.00080.2004. [DOI] [PubMed] [Google Scholar]
- Horman S, Beauloye C, Vertommen D, Vanoverschelde JL, Hue L, Rider MH. Myocardial ischemia and increased heart work modulate the phosphorylation state of eukaryotic elongation factor-2. J Biol Chem. 2003;278:41970–41976. doi: 10.1074/jbc.M302403200. [DOI] [PubMed] [Google Scholar]
- Horman S, Browne G, Krause U, Patel J, Vertommen D, Bertrand L, Lavoinne A, Hue L, Proud C, Rider M. Activation of AMP-activated protein kinase leads to the phosphorylation of elongation factor 2 and an inhibition of protein synthesis. Curr Biol. 2002;12:1419–1423. doi: 10.1016/s0960-9822(02)01077-1. [DOI] [PubMed] [Google Scholar]
- Hudson ER, Pan DA, James J, Lucocq JM, Hawley SA, Green KA, Baba O, Terashima T, Hardie DG. A novel domain in AMP-activated protein kinase causes glycogen storage bodies similar to those seen in hereditary cardiac arrhythmias. Curr Biol. 2003;13:861–866. doi: 10.1016/s0960-9822(03)00249-5. [DOI] [PubMed] [Google Scholar]
- Hurley RL, Anderson KA, Franzone JM, Kemp BE, Means AR, Witters LA. The Ca2+/calmoldulin-dependent protein kinase kinases are AMP-activated protein kinase kinases. J Biol Chem. 2005;280:29060–29066. doi: 10.1074/jbc.M503824200. [DOI] [PubMed] [Google Scholar]
- Hurst D, Taylor EB, Cline TD, Greenwood LJ, Compton CL, Lamb JD, Winder WW. AMP-activated protein kinase kinase activity and phosphorylation of AMP-activated protein kinase in contracting muscle of sedentary and endurance-trained rats. Am J Physiol Endocrinol Metab. 2005;289:E710–E715. doi: 10.1152/ajpendo.00155.2005. [DOI] [PubMed] [Google Scholar]
- Hutber CA, Hardie DG, Winder WW. Electrical stimulation inactivates muscle acetyl-CoA carboxylase and increases AMP-activated protein kinase. Am J Physiol. 1997;272:E262–E266. doi: 10.1152/ajpendo.1997.272.2.E262. [DOI] [PubMed] [Google Scholar]
- Inoki K, Zhu T, Guan KL. TSC2 mediates cellular energy response to control cell growth and survival. Cell. 2003;115:577–590. doi: 10.1016/s0092-8674(03)00929-2. [DOI] [PubMed] [Google Scholar]
- Irrcher I, Adhihetty PJ, Sheehan T, Joseph AM, Hood DA. PPARγ coactivator-1α expression during thyroid hormone- and contractile activity-induced mitochondrial adaptations. Am J Physiol Cell Physiol. 2003;284:C1669–C1677. doi: 10.1152/ajpcell.00409.2002. [DOI] [PubMed] [Google Scholar]
- Itani SI, Saha AK, Kurowski TG, Coffin HR, Tornheim K, Ruderman NB. Glucose autoregulates its uptake in skeletal muscle: involvement of AMP-activated protein kinase. Diabetes. 2003;52:1635–1640. doi: 10.2337/diabetes.52.7.1635. [DOI] [PubMed] [Google Scholar]
- Jessen N, Goodyear LJ. Contraction signaling to glucose transport in skeletal muscle. J Appl Physiol. 2005;99:330–337. doi: 10.1152/japplphysiol.00175.2005. [DOI] [PubMed] [Google Scholar]
- Jorgensen SB, Nielsen JN, Birk JB, Olsen GS, Viollet B, Andreelli F, Schjerling P, Vaulont S, Hardie DG, Hansen BF, Richter EA, Wojtaszewski JF. The α2-5′AMP-activated protein kinase is a site 2 glycogen synthase kinase in skeletal muscle and is responsive to glucose loading. Diabetes. 2004a;53:3074–3081. doi: 10.2337/diabetes.53.12.3074. [DOI] [PubMed] [Google Scholar]
- Jorgensen SB, Viollet B, Andreelli F, Frosig C, Birk JB, Schjerling P, Vaulont S, Richter EA, Wojtaszewski JF. Knockout of the α2 but not alpha1 5′-AMP-activated protein kinase isoform abolishes 5-aminoimidazole-4-carboxamide-1-β-4-ribofuranoside but not contraction-induced glucose uptake in skeletal muscle. J Biol Chem. 2004b;279:1070–1079. doi: 10.1074/jbc.M306205200. [DOI] [PubMed] [Google Scholar]
- Jorgensen SB, Wojtaszewski JF, Viollet B, Andreelli F, Birk JB, Hellsten Y, Schjerling P, Vaulont S, Neufer PD, Richter EA, Pilegaard H. Effects of α-AMPK knockout on exercise-induced gene activation in mouse skeletal muscle. FASEB J. 2005;19:1146–1148. doi: 10.1096/fj.04-3144fje. [DOI] [PubMed] [Google Scholar]
- Kahn BB, Alquier T, Carling D, Hardie DG. AMP-activated protein kinase: ancient energy gauge provides clues to modern understanding of metabolism. Cell Metab. 2005;1:15–25. doi: 10.1016/j.cmet.2004.12.003. [DOI] [PubMed] [Google Scholar]
- Kane S, Sano H, Liu SC, Asara JM, Lane WS, Garner CC, Lienhard GE. A method to identify serine kinase substrates. Akt phosphorylates a novel adipocyte protein with a Rab GTPase-activating protein (GAP) domain. J Biol Chem. 2002;277:22115–22118. doi: 10.1074/jbc.C200198200. [DOI] [PubMed] [Google Scholar]
- Kemp BE, Stapleton D, Campbell DJ, Chen ZP, Murthy S, Walter M, Gupta A, Adams JJ, Katsis F, van Denderen B, Jennings IG, Iseli T, Michell BJ, Witters LA. AMP-activated protein kinase, super metabolic regulator. Biochem Soc Trans. 2003;31:162–168. doi: 10.1042/bst0310162. [DOI] [PubMed] [Google Scholar]
- Kiens B. Skeletal muscle lipid metabolism in exercise and insulin resistance. Physiol Rev. 2006;86:205–243. doi: 10.1152/physrev.00023.2004. [DOI] [PubMed] [Google Scholar]
- Koistinen HA, Galuska D, Chibalin AV, Yang J, Zierath JR, Holman GD, Wallberg-Henriksson H. 5-Amino-imidazole carboxamide riboside increases glucose transport and cell-surface GLUT4 content in skeletal muscle from subjects with type 2 diabetes. Diabetes. 2003;52:1066–1072. doi: 10.2337/diabetes.52.5.1066. [DOI] [PubMed] [Google Scholar]
- Kraegen EW, Saha AK, Preston E, Wilks D, Hoy AJ, Cooney GJ, Ruderman NB. Increased malonyl-CoA and diacylglycerol content and reduced AMPK activity accompany insulin resistance induced by glucose infusion in muscle and liver of rats. Am J Physiol Endocrinol Metab. 2006;290:E471–E479. doi: 10.1152/ajpendo.00316.2005. [DOI] [PubMed] [Google Scholar]
- Kurth-Kraczek EJ, Hirshman MF, Goodyear LJ, Winder WW. 5′-AMP-activated protein kinase activation causes GLUT4 translocation in skeletal muscle. Diabetes. 1999;48:1667–1671. doi: 10.2337/diabetes.48.8.1667. [DOI] [PubMed] [Google Scholar]
- Luiken JJ, Coort SL, Willems J, Coumans WA, Bonen A, van der Vusse GJ, Glatz JF. Contraction-induced fatty acid translocase/CD36 translocation in rat cardiac myocytes is mediated through AMP-activated protein kinase signaling. Diabetes. 2003;52:1627–1634. doi: 10.2337/diabetes.52.7.1627. [DOI] [PubMed] [Google Scholar]
- Mahlapuu M, Johansson C, Lindgren K, Hjalm G, Barnes BR, Krook A, Zierath JR, Andersson L, Marklund S. Expression profiling of the γ-subunit isoforms of AMP-activated protein kinase suggests a major role for γ3 in white skeletal muscle. Am J Physiol Endocrinol Metab. 2004;286:E194–E200. doi: 10.1152/ajpendo.00147.2003. [DOI] [PubMed] [Google Scholar]
- McGarry JD, Brown NF. The mitochondrial carnitine palmitoyltransferase system. From concept to molecular analysis. Eur J Biochem. 1997;244:1–14. doi: 10.1111/j.1432-1033.1997.00001.x. [DOI] [PubMed] [Google Scholar]
- Merrill GF, Kurth EJ, Hardie DG, Winder WW. AICA riboside increases AMP-activated protein kinase, fatty acid oxidation, and glucose uptake in rat muscle. Am J Physiol. 1997;273:E1107–E1112. doi: 10.1152/ajpendo.1997.273.6.E1107. [DOI] [PubMed] [Google Scholar]
- Merrill GF, Kurth EJ, Rasmussen BB, Winder WW. Influence of malonyl-CoA and palmitate concentration on rate of palmitate oxidation in rat muscle. J Appl Physiol. 1998;85:1909–1914. doi: 10.1152/jappl.1998.85.5.1909. [DOI] [PubMed] [Google Scholar]
- Michael LF, Wu Z, Cheatham RB, Puigserver P, Adelmant G, Lehman JJ, Kelly DP, Spiegelman BM. Restoration of insulin-sensitive glucose transporter (GLUT4) gene expression in muscle cells by the transcriptional coactivator PGC-1. Proc Natl Acad Sci U S A. 2001;98:3820–3825. doi: 10.1073/pnas.061035098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milan D, Jeon JT, Looft C, Amarger V, Robic A, Thelander M, Rogel-Gaillard C, Paul S, Iannuccelli N, Rask L, Ronne H, Lundstrom K, Reinsch N, Gellin J, Kalm E, Roy PL, Chardon P, Andersson L. A mutation in PRKAG3 associated with excess glycogen content in pig skeletal muscle. Science. 2000;288:1248–1251. doi: 10.1126/science.288.5469.1248. [DOI] [PubMed] [Google Scholar]
- Minokoshi Y, Kim YB, Peroni OD, Fryer LG, Muller C, Carling D, Kahn BB. Leptin stimulates fatty-acid oxidation by activating AMP-activated protein kinase. Nature. 2002;415:339–343. doi: 10.1038/415339a. [DOI] [PubMed] [Google Scholar]
- Mu J, Barton ER, Birnbaum MJ. Selective suppression of AMP-activated protein kinase in skeletal muscle: update on ‘lazy mice’. Biochem Soc Trans. 2003;31:236–241. doi: 10.1042/bst0310236. [DOI] [PubMed] [Google Scholar]
- Mu J, Brozinick JT, Jr, Valladares O, Bucan M, Birnbaum MJ. A role for AMP-activated protein kinase in contraction- and hypoxia-regulated glucose transport in skeletal muscle. Mol Cell. 2001;7:1085–1094. doi: 10.1016/s1097-2765(01)00251-9. [DOI] [PubMed] [Google Scholar]
- Musi N, Hayashi T, Fujii N, Hirshman MF, Witters LA, Goodyear LJ. AMP-activated protein kinase activity and glucose uptake in rat skeletal muscle. Am J Physiol Endocrinol Metab. 2001;280:E677–E684. doi: 10.1152/ajpendo.2001.280.5.E677. [DOI] [PubMed] [Google Scholar]
- Nielsen JN, Wojtaszewski JF. Regulation of glycogen synthase activity and phosphorylation by exercise. Proc Nutr Soc. 2004;63:233–237. doi: 10.1079/PNS2004348. [DOI] [PubMed] [Google Scholar]
- Nielsen JN, Wojtaszewski JF, Haller RG, Hardie DG, Kemp BE, Richter EA, Vissing J. Role of 5′AMP-activated protein kinase in glycogen synthase activity and glucose utilization: insights from patients with McArdle's disease. J Physiol. 2002;541:979–989. doi: 10.1113/jphysiol.2002.018044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odland LM, Heigenhauser GJ, Lopaschuk GD, Spriet LL. Human skeletal muscle malonyl-CoA at rest and during prolonged submaximal exercise. Am J Physiol. 1996;270:E541–E544. doi: 10.1152/ajpendo.1996.270.3.E541. [DOI] [PubMed] [Google Scholar]
- Odland LM, Howlett RA, Heigenhauser GJ, Hultman E, Spriet LL. Skeletal muscle malonyl-CoA content at the onset of exercise at varying power outputs in humans. Am J Physiol. 1998;274:E1080–E1085. doi: 10.1152/ajpendo.1998.274.6.E1080. [DOI] [PubMed] [Google Scholar]
- Ojuka EO, Jones TE, Nolte LA, Chen M, Wamhoff BR, Sturek M, Holloszy JO. Regulation of GLUT4 biogenesis in muscle: evidence for involvement of AMPK and Ca2+ Am J Physiol Endocrinol Metab. 2002;282:E1008–E1013. doi: 10.1152/ajpendo.00512.2001. [DOI] [PubMed] [Google Scholar]
- Olsen GS, Hansen BF. AMP kinase activation ameliorates insulin resistance induced by free fatty acids in rat skeletal muscle. Am J Physiol Endocrinol Metab. 2002;283:E965–E970. doi: 10.1152/ajpendo.00118.2002. [DOI] [PubMed] [Google Scholar]
- Palanivel R, Sweeney G. Regulation of fatty acid uptake and metabolism in L6 skeletal muscle cells by resistin. FEBS Lett. 2005;579:5049–5054. doi: 10.1016/j.febslet.2005.08.011. [DOI] [PubMed] [Google Scholar]
- Park SH, Gammon SR, Knippers JD, Paulsen SR, Rubink DS, Winder WW. Phosphorylation-activity relationships of AMPK and acetyl-CoA carboxylase in muscle. J Appl Physiol. 2002b;92:2475–2482. doi: 10.1152/japplphysiol.00071.2002. [DOI] [PubMed] [Google Scholar]
- Park H, Kaushik VK, Constant S, Prentki M, Przybytkowski E, Ruderman NB, Saha AK. Coordinate regulation of malonyl-CoA decarboxylase, sn-glycerol-3-phosphate acyltransferase, and acetyl-CoA carboxylase by AMP-activated protein kinase in rat tissues in response to exercise. J Biol Chem. 2002a;277:32571–32577. doi: 10.1074/jbc.M201692200. [DOI] [PubMed] [Google Scholar]
- Polekhina G, Gupta A, Michell BJ, van Denderen B, Murthy S, Feil SC, Jennings IG, Campbell DJ, Witters LA, Parker MW, Kemp BE, Stapleton D. AMPK β subunit targets metabolic stress sensing to glycogen. Curr Biol. 2003;13:867–871. doi: 10.1016/s0960-9822(03)00292-6. [DOI] [PubMed] [Google Scholar]
- Ponticos M, Lu QL, Morgan JE, Hardie DG, Partridge TA, Carling D. Dual regulation of the AMP-activated protein kinase provides a novel mechanism for the control of creatine kinase in skeletal muscle. EMBO J. 1998;17:1688–1699. doi: 10.1093/emboj/17.6.1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proud CG. The multifaceted role of mTOR in cellular stress responses. DNA Repair (Amst) 2004;3:927–934. doi: 10.1016/j.dnarep.2004.03.012. [DOI] [PubMed] [Google Scholar]
- Proud CG, Denton RM. Molecular mechanisms for the control of translation by insulin. Biochem J. 1997;328:329–341. doi: 10.1042/bj3280329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raney MA, Yee AJ, Todd MK, Turcotte LP. AMPK activation is not critical in the regulation of muscle FA uptake and oxidation during low-intensity muscle contraction. Am J Physiol Endocrinol Metab. 2005;288:E592–E598. doi: 10.1152/ajpendo.00301.2004. [DOI] [PubMed] [Google Scholar]
- Rennie MJ. Why muscle stops building when it's working. J Physiol. 2005;569:3. doi: 10.1113/jphysiol.2005.099424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rennie MJ, Tipton KD. Protein and amino acid metabolism during and after exercise and the effects of nutrition. Annu Rev Nutr. 2000;20:457–483. doi: 10.1146/annurev.nutr.20.1.457. [DOI] [PubMed] [Google Scholar]
- Richter EA, Derave W, Wojtaszewski JF. Glucose, exercise and insulin: emerging concepts. J Physiol. 2001;535:313–322. doi: 10.1111/j.1469-7793.2001.t01-2-00313.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter EA, Nielsen JN, Jorgensen SB, Frosig C, Birk JB, Wojtaszewski JF. Exercise signalling to glucose transport in skeletal muscle. Proc Nutr Soc. 2004;63:211–216. doi: 10.1079/PNS2004343. [DOI] [PubMed] [Google Scholar]
- Roach PJ. Glycogen and its metabolism. Curr Mol Med. 2002;2:101–120. doi: 10.2174/1566524024605761. [DOI] [PubMed] [Google Scholar]
- Roepstorff C, Halberg N, Hillig T, Saha AK, Ruderman NB, Wojtaszewski JF, Richter EA, Kiens B. Malonyl-CoA and carnitine in regulation of fat oxidation in human skeletal muscle during exercise. Am J Physiol Endocrinol Metab. 2005;288:E133–E142. doi: 10.1152/ajpendo.00379.2004. [DOI] [PubMed] [Google Scholar]
- Roepstorff C, Thiele M, Hillig T, Pilegaard H, Richter EA, Wojtaszewski JF, Kiens B. Higher skeletal muscle α2AMPK activation and lower energy charge and fat oxidation in men than in women during submaximal exercise. J Physiol. 2006;574:125–138. doi: 10.1113/jphysiol.2006.108720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roepstorff C, Vistisen B, Donsmark M, Nielsen JN, Galbo H, Green KA, Hardie DG, Wojtaszewski JF, Richter EA, Kiens B. Regulation of hormone-sensitive lipase activity and Ser563 and Ser565 phosphorylation in human skeletal muscle during exercise. J Physiol. 2004a;560:551–562. doi: 10.1113/jphysiol.2004.066480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roepstorff C, Vistisen B, Roepstorff K, Kiens B. Regulation of plasma long-chain fatty acid oxidation in relation to uptake in human skeletal muscle during exercise. Am J Physiol Endocrinol Metab. 2004b;287:E696–E705. doi: 10.1152/ajpendo.00001.2004. [DOI] [PubMed] [Google Scholar]
- Rose AJ, Broholm C, Kiillerich K, Finn SG, Proud CG, Rider MH, Richter EA, Kiens B. Exercise rapidly increases eukaryotic elongation factor 2 phosphorylation in skeletal muscle of men. J Physiol. 2005;569:223–228. doi: 10.1113/jphysiol.2005.097154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose AJ, Richter EA. Skeletal muscle glucose uptake during exercise: how is it regulated? Physiology (Bethesda) 2005;20:260–270. doi: 10.1152/physiol.00012.2005. [DOI] [PubMed] [Google Scholar]
- Rubink DS, Winder WW. Effect of phosphorylation by AMP-activated protein kinase on palmitoyl-CoA inhibition of skeletal muscle acetyl-CoA carboxylase. J Appl Physiol. 2005;98:1221–1227. doi: 10.1152/japplphysiol.00621.2004. [DOI] [PubMed] [Google Scholar]
- Rundell KW, Tullson PC, Terjung RL. AMP deaminase binding in rat skeletal muscle after high-intensity running. J Appl Physiol. 1993;74:2004–2006. doi: 10.1152/jappl.1993.74.4.2004. [DOI] [PubMed] [Google Scholar]
- Saha AK, Schwarsin AJ, Roduit R, Masse F, Kaushik V, Tornheim K, Prentki M, Ruderman NB. Activation of malonyl-CoA decarboxylase in rat skeletal muscle by contraction and the AMP-activated protein kinase activator 5-aminoimidazole-4-carboxamide-1-β-D-ribofuranoside. J Biol Chem. 2000;275:24279–24283. doi: 10.1074/jbc.C000291200. [DOI] [PubMed] [Google Scholar]
- Sahlin K, Tonkonogi M, Soderlund K. Energy supply and muscle fatigue in humans. Acta Physiol Scand. 1998;162:261–266. doi: 10.1046/j.1365-201X.1998.0298f.x. [DOI] [PubMed] [Google Scholar]
- Sakamoto K, Goransson O, Hardie DG, Alessi DR. Activity of LKB1 and AMPK-related kinases in skeletal muscle: effects of contraction, phenformin, and AICAR. Am J Physiol Endocrinol Metab. 2004;287:E310–E317. doi: 10.1152/ajpendo.00074.2004. [DOI] [PubMed] [Google Scholar]
- Sakamoto K, McCarthy A, Smith D, Green KA, Hardie DG, Ashworth A, Alessi DR. Deficiency of LKB1 in skeletal muscle prevents AMPK activation and glucose uptake during contraction. EMBO J. 2005;24:1810–1820. doi: 10.1038/sj.emboj.7600667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salt IP, Johnson G, Ashcroft SJ, Hardie DG. AMP-activated protein kinase is activated by low glucose in cell lines derived from pancreatic β cells, and may regulate insulin release. Biochem J. 1998;335:533–539. doi: 10.1042/bj3350533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sano H, Kane S, Sano E, Miinea CP, Asara JM, Lane WS, Garner CW, Lienhard GE. Insulin-stimulated phosphorylation of a Rab GTPase-activating protein regulates GLUT4 translocation. J Biol Chem. 2003;278:14599–14602. doi: 10.1074/jbc.C300063200. [DOI] [PubMed] [Google Scholar]
- Scarpulla RC. Nuclear activators and coactivators in mammalian mitochondrial biogenesis. Biochim Biophys Acta. 2002;1576:1–14. doi: 10.1016/s0167-4781(02)00343-3. [DOI] [PubMed] [Google Scholar]
- Scott JW, Hawley SA, Green KA, Anis M, Stewart G, Scullion GA, Norman DG, Hardie DG. CBS domains form energy-sensing modules whose binding of adenosine ligands is disrupted by disease mutations. J Clin Invest. 2004;113:274–284. doi: 10.1172/JCI19874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen WJ, Liang Y, Hong R, Patel S, Natu V, Sridhar K, Jenkins A, Bernlohr DA, Kraemer FB. Characterization of the functional interaction of adipocyte lipid-binding protein with hormone-sensitive lipase. J Biol Chem. 2001;276:49443–49448. doi: 10.1074/jbc.M104095200. [DOI] [PubMed] [Google Scholar]
- Stephens TJ, Chen ZP, Canny BJ, Michell BJ, Kemp BE, McConell GK. Progressive increase in human skeletal muscle AMPKα2 activity and ACC phosphorylation during exercise. Am J Physiol Endocrinol Metab. 2002;282:E688–E694. doi: 10.1152/ajpendo.00101.2001. [DOI] [PubMed] [Google Scholar]
- Sullivan JE, Brocklehurst KJ, Marley AE, Carey F, Carling D, Beri RK. Inhibition of lipolysis and lipogenesis in isolated rat adipocytes with AICAR, a cell-permeable activator of AMP-activated protein kinase. FEBS Lett. 1994;353:33–36. doi: 10.1016/0014-5793(94)01006-4. [DOI] [PubMed] [Google Scholar]
- Taylor EB, Ellingson WJ, Lamb JD, Chesser DG, Compton CL, Winder WW. Evidence against regulation of AMP-activated protein kinase and LKB1-STRAD-MO25 activity by creatine phosphate. Am J Physiol Endocrinol Metab. 2005a;290:E661–E669. doi: 10.1152/ajpendo.00313.2005. [DOI] [PubMed] [Google Scholar]
- Taylor EB, Ellingson WJ, Lamb JD, Chesser DG, Winder WW. Long-chain Acy-CoA esters inhibit phosphorylation of AMP-activated protein kinase at threonine 172 by LKB1/STRAD/MO25. Am J Physiol Endocrinol Metab. 2005b;288:E1055–E1061. doi: 10.1152/ajpendo.00516.2004. [DOI] [PubMed] [Google Scholar]
- Treebak JT, Glund S, Deshmukh A, Klein DK, Long YC, Jensen TE, Jorgensen SB, Viollet B, Anderson L, Neumann D, Wallimann T, Richter EA, Chibalin AV, Zierath JR, Wojtaszewki JFP. AMPK mediates AS160 phosphorylation in skeletal muscle: Dependency on AMPK catalytic and regulatory subunits. Diabetes. 2006 doi: 10.2337/db06-0175. in press. [DOI] [PubMed] [Google Scholar]
- Tullson PC, Terjung RL. Adenine nucleotide degradation in striated muscle. Int J Sports Med. 1990;11(Suppl. 2):S47–S55. doi: 10.1055/s-2007-1024854. [DOI] [PubMed] [Google Scholar]
- Turcotte LP, Raney MA, Todd MK. ERK1/2 inhibition prevents contraction-induced increase in plasma membrane FAT/CD36 content and FA uptake in rodent muscle. Acta Physiol Scand. 2005;184:131–139. doi: 10.1111/j.1365-201X.2005.01445.x. [DOI] [PubMed] [Google Scholar]
- Vavvas D, Apazidis A, Saha AK, Gamble J, Patel A, Kemp BE, Witters LA, Ruderman NB. Contraction-induced changes in acetyl-CoA carboxylase and 5′-AMP-activated kinase in skeletal muscle. J Biol Chem. 1997;272:13255–13261. doi: 10.1074/jbc.272.20.13255. [DOI] [PubMed] [Google Scholar]
- Watt MJ, Holmes AG, Pinnamaneni SK, Garnham AP, Steinberg GR, Kemp BE, Febbraio MA. Regulation of HSL serine phosphorylation in skeletal muscle and adipose tissue. Am J Physiol Endocrinol Metab. 2006;290:E500–E508. doi: 10.1152/ajpendo.00361.2005. [DOI] [PubMed] [Google Scholar]
- Watt MJ, Holmes AG, Steinberg GR, Mesa JL, Kemp BE, Febbraio MA. Reduced plasma FFA availability increases net triacylglycerol degradation, but not GPAT or HSL activity, in human skeletal muscle. Am J Physiol Endocrinol Metab. 2004a;287:E120–E127. doi: 10.1152/ajpendo.00542.2003. [DOI] [PubMed] [Google Scholar]
- Watt MJ, Steinberg GR, Chan S, Garnham A, Kemp BE, Febbraio MA. β-adrenergic stimulation of skeletal muscle HSL can be overridden by AMPK signaling. FASEB J. 2004b;18:1445–1446. doi: 10.1096/fj.03-1067fje. [DOI] [PubMed] [Google Scholar]
- Winder WW, Arogyasami J, Elayan IM, Cartmill D. Time course of exercise-induced decline in malonyl-CoA in different muscle types. Am J Physiol. 1990;259:E266–E271. doi: 10.1152/ajpendo.1990.259.2.E266. [DOI] [PubMed] [Google Scholar]
- Winder WW, Hardie DG. Inactivation of acetyl-CoA carboxylase and activation of AMP-activated protein kinase in muscle during exercise. Am J Physiol. 1996;270:E299–E304. doi: 10.1152/ajpendo.1996.270.2.E299. [DOI] [PubMed] [Google Scholar]
- Winder WW, Holmes BF. Insulin stimulation of glucose uptake fails to decrease palmitate oxidation in muscle if AMPK is activated. J Appl Physiol. 2000;89:2430–2437. doi: 10.1152/jappl.2000.89.6.2430. [DOI] [PubMed] [Google Scholar]
- Winder WW, Holmes BF, Rubink DS, Jensen EB, Chen M, Holloszy JO. Activation of AMP-activated protein kinase increases mitochondrial enzymes in skeletal muscle. J Appl Physiol. 2000;88:2219–2226. doi: 10.1152/jappl.2000.88.6.2219. [DOI] [PubMed] [Google Scholar]
- Wojtaszewski JF, Birk JB, Frosig C, Holten M, Pilegaard H, Dela F. Effects of type 2 diabetes and strength training on AMPK subunit isoform protein and mRNA expression in human skeletal muscle. J Physiol. 2005;564:563–573. doi: 10.1113/jphysiol.2005.082669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojtaszewski JF, Jorgensen SB, Hellsten Y, Hardie DG, Richter EA. Glycogen-dependent effects of 5-aminoimidazole-4-carboxamide (AICA)-riboside on AMP-activated protein kinase and glycogen synthase activities in rat skeletal muscle. Diabetes. 2002a;51:284–292. doi: 10.2337/diabetes.51.2.284. [DOI] [PubMed] [Google Scholar]
- Wojtaszewski JF, MacDonald C, Nielsen JN, Hellsten Y, Hardie GD, Kemp BE, Kiens B, Richter EA. Regulation of 5′AMP-activated protein kinase activity and substrate utilization in exercising human skeletal muscle. Am J Physiol Endocrinol Metab. 2003;284:E813–E822. doi: 10.1152/ajpendo.00436.2002. [DOI] [PubMed] [Google Scholar]
- Wojtaszewski JF, Mourtzakis M, Hillig T, Saltin B, Pilegaard H. Dissociation of AMPK activity and ACCβ phosphorylation in human muscle during prolonged exercise. Biochem Biophys Res Commun. 2002b;298:309–316. doi: 10.1016/s0006-291x(02)02465-8. [DOI] [PubMed] [Google Scholar]
- Wojtaszewski JF, Nielsen P, Hansen BF, Richter EA, Kiens B. Isoform-specific and exercise intensity-dependent activation of 5′-AMP-activated protein kinase in human skeletal muscle. J Physiol. 2000;528:221–226. doi: 10.1111/j.1469-7793.2000.t01-1-00221.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z, Puigserver P, Andersson U, Zhang C, Adelmant G, Mootha V, Troy A, Cinti S, Lowell B, Scarpulla RC, Spiegelman BM. Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell. 1999;98:115–124. doi: 10.1016/S0092-8674(00)80611-X. [DOI] [PubMed] [Google Scholar]
- Yamauchi T, Kamon J, Minokoshi Y, Ito Y, Waki H, Uchida S, Yamashita S, Noda M, Kita S, Ueki K, Eto K, Akanuma Y, Froguel P, Foufelle F, Ferre P, Carling D, Kimura S, Nagai R, Kahn BB, Kadowaki T. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat Med. 2002;8:1288–1295. doi: 10.1038/nm788. [DOI] [PubMed] [Google Scholar]
- Young ME, Radda GK, Leighton B. Activation of glycogen phosphorylase and glycogenolysis in rat skeletal muscle by AICAR – an activator of AMP-activated protein kinase. FEBS Lett. 1996;382:43–47. doi: 10.1016/0014-5793(96)00129-9. [DOI] [PubMed] [Google Scholar]
- Yu H, Fujii N, Hirshman MF, Pomerleau JM, Goodyear LJ. Cloning and characterization of mouse 5′-AMP-activated protein kinase γ3 subunit. Am J Physiol Cell Physiol. 2004;286:C283–C292. doi: 10.1152/ajpcell.00319.2003. [DOI] [PubMed] [Google Scholar]
- Zeigerer A, McBrayer MK, McGraw TE. Insulin stimulation of GLUT4 exocytosis, but not its inhibition of endocytosis, is dependent on RabGAP AS160. Mol Biol Cell. 2004;15:4406–4415. doi: 10.1091/mbc.E04-04-0333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng D, MacLean PS, Pohnert SC, Knight JB, Olson AL, Winder WW, Dohm GL. Regulation of muscle GLUT-4 transcription by AMP-activated protein kinase. J Appl Physiol. 2001;91:1073–1083. doi: 10.1152/jappl.2001.91.3.1073. [DOI] [PubMed] [Google Scholar]
- Zong H, Ren JM, Young LH, Pypaert M, Mu J, Birnbaum MJ, Shulman GI. AMP kinase is required for mitochondrial biogenesis in skeletal muscle in response to chronic energy deprivation. Proc Natl Acad Sci U S A. 2002;99:15983–15987. doi: 10.1073/pnas.252625599. [DOI] [PMC free article] [PubMed] [Google Scholar]