Abstract
5′AMP-activated protein kinase (AMPK) is an energy sensor activated by perturbed cellular energy status such as during muscle contraction. Activated AMPK is thought to regulate several key metabolic pathways. We used sex comparison to investigate whether AMPK signalling in skeletal muscle regulates fat oxidation during exercise. Moderately trained women and men completed 90 min bicycle exercise at 60% V̇O2peak. Both AMPK Thr172 phosphorylation and α2AMPK activity were increased by exercise in men (∼200%, P < 0.001) but not significantly in women. The sex difference in muscle AMPK activation with exercise was accompanied by an increase in muscle free AMP (∼164%, P < 0.01), free AMP/ATP ratio (159%, P < 0.05), and creatine (∼42%, P < 0.001) in men but not in women (NS), suggesting that lack of AMPK activation in women was due to better maintenance of muscle cellular energy balance compared with men. During exercise, fat oxidation per kg lean body mass was higher in women than in men (P < 0.05). Regression analysis revealed that a higher proportion of type 1 muscle fibres (∼23%, P < 0.01) and a higher capillarization (∼23%, P < 0.05) in women than in men could partly explain the sex difference in α2AMPK activity (r = −0.54, P < 0.05) and fat oxidation (r = 0.64, P < 0.05) during exercise. On the other hand, fat oxidation appeared not to be regulated via AMPK. In conclusion, during prolonged submaximal exercise at 60% V̇O2peak, higher fat oxidation in women cannot be explained by higher AMPK signalling but is accompanied by improved muscle cellular energy balance in women probably due to sex specific muscle morphology.
It has been shown in some (Tarnopolsky et al. 1990; Phillips et al. 1993; Horton et al. 1998; Friedlander et al. 1998; Friedlander et al. 1999; Carter et al. 2001a; Venables et al. 2005) but not all (Burguera et al. 2000; Steffensen et al. 2002; Roepstorff et al. 2002; Riddell et al. 2003; Perreault et al. 2004) previous studies that relative fat oxidation during exercise is higher in women than in men. In those studies where no significant sex difference has been observed in relative fat oxidation during exercise, the tendency has still been in favour of higher relative fat oxidation in women than in men (Burguera et al. 2000; Steffensen et al. 2002; Roepstorff et al. 2002; Riddell et al. 2003; Perreault et al. 2004). Therefore, it seems reasonable to conclude that there is a small sex difference in relative fat oxidation during exercise, but that this sex difference is not always possible to detect in studies with a limited number of subjects. The mechanism(s) behind the higher relative fat oxidation seen during exercise in women compared with men are at present unknown.
5′AMP-activated protein kinase (AMPK) acts as an energy sensor in skeletal muscle (Hardie & Carling, 1997; Winder, 2001). Activation of AMPK is thought to increase energy producing pathways while switching off energy consuming pathways (Hardie & Carling, 1997; Winder, 2001). Exercise is one situation in which AMPK is activated in skeletal muscle (Fujii et al. 2000; Wojtaszewski et al. 2000), thereby phosphorylating and inactivating acetyl-CoA carboxylase β (ACCβ), which should lead to a decreased production of malonyl-CoA (Rasmussen & Winder, 1997; Winder, 1998). Malonyl-CoA is a known inhibitor of carnitine palmitoyl-CoA transferase I (CPT I), the enzyme catalysing the entry of long-chain fatty acyl-CoA into mitochondria for oxidation. It follows that a decrease in malonyl-CoA could have the potential to relieve inhibition of CPT I and allow for elevated fat oxidation, although measured concentrations of malonyl-CoA in skeletal muscle greatly exceed the IC50 of CPT I for malonyl-CoA (McGarry et al. 1983). In line with AMPK being involved in regulating fat oxidation, several rodent studies have shown increased AMPK activity and decreased ACC activity and malonyl-CoA concentration when going from rest to exercise, a transition characterized by markedly elevated fat oxidation (Winder & Hardie, 1996; Rasmussen & Winder, 1997; Winder, 1998). Thus, it is generally believed that AMPK signalling through the ACC–malonyl CoA–CPT I pathway is a very important mechanism to regulate fat oxidation in skeletal muscle during exercise. This contention is widespread despite recent human studies showing that high fat oxidation during exercise with low muscle glycogen or after short-term exercise training cannot be explained by increased signalling from AMPK via ACC to CPT I (McConell et al. 2005; Roepstorff et al. 2005a). Moreover, although some human studies have found a decrease in muscle malonyl-CoA content from rest to exercise (Dean et al. 2000; Roepstorff et al. 2005a), this has not been a consistent finding (Odland et al. 1996; Odland et al. 1998). Since women, compared with men, are thought to oxidize more fat during exercise, sex comparison would be a suitable model to further investigate whether AMPK regulates fat oxidation during exercise. If so, one would expect a more marked skeletal muscle AMPK activation by exercise in women than in men.
In the present study, we investigated fat oxidation as well as skeletal muscle AMPK expression and activation during 90 min bicycle exercise at 60% V̇O2peak in women and men. It was found that fat oxidation during exercise was higher in women than in men and that AMPK Thr172 phosphorylation and α2AMPK activity during exercise were both higher in men than in women. These findings suggest that AMPK signalling is not a major regulator of fat oxidation during prolonged exercise in humans.
Methods
Subjects
Nine women and eight men, all moderately trained, were recruited to participate in the study (Table 1). All subjects were young, healthy, non-smokers, and they participated on a regular basis (3–4 h per week) in leisure time activities such as running, cycling, strength training, and different game sports. Body mass index was in the range of 20–25. The women had a peak oxygen uptake (V̇O2peak) of 40–55 ml O2 min−1 (kg body mass)−1 and the men had a V̇O2peak of 50–65 ml O2 min−1 (kg body mass)−1. Female and male groups had similar V̇O2peak (kg lean body mass)−1, habitual physical activity level, and exercise training history (Table 1). All women were eumenorrheic with a menstrual cycle length between 28 and 35 days and did not take any oral contraception. Pre-experimental testing and the main experiments in women were carried out during the midfollicular phase of their menstrual cycle (day 7–10 from first day of the menstrual cycle). Before volunteering, subjects were given full oral and written information about the course of the study and possible risks associated with participation. Written consent was obtained from each subject. The study was approved by The Copenhagen Ethics Comittee (KF-01-046/02) and conformed to the code of ethics of the World Medical Association (Declaration of Helsinki II).
Table 1. Subject characteristics.
| Women | Men | |
|---|---|---|
| (n = 9) | (n = 8) | |
| Age (years) | 24 ± 1 | 25 ± 1 |
| Height (m) | 1.71 ± 0.03* | 1.85 ± 0.02 |
| Body mass (BM) (kg) | 65.0 ± 2.3* | 79.5 ± 2.8 |
| Body fat (%) | 24.7 ± 1.5* | 12.1 ± 2.3 |
| Lean body mass (LBM) (kg) | 48.9 ± 1.9* | 69.9 ± 3.1 |
| V̇O2peak, | ||
| ″lmin−1 | 3.2 ± 0.1* | 4.4 ± 0.2 |
| ″ml (kg BM)−1 min−1 | 48.8 ± 1.3* | 55.6 ± 1.2 |
| ″ml (kg LBM)−1 min−1 | 65.0 ± 1.7 | 63.4 ± 0.8 |
| Maximal work load (watts) | 264 ± 13* | 364 ± 14 |
| Maximal citrate synthase activity (μmol (g d.w.)−1 min−1) | 31.7 ± 2.5(*) | 26.2 ± 1.1 |
| Training history | ||
| ″Frequency (workouts week−1) | 3.2 ± 0.6 | 3.0 ± 0.4 |
| ″Duration (h week−1) | 3.5 ± 0.6 | 3.2 ± 0.5 |
Data are mean ± s.e.m. *Sex difference, P < 0.001; *tendency towards sex difference, P = 0.06.
Pre-experimental protocol
To determine V̇O2peak all subjects performed an incremental exercise test on a Monark Ergomedic 839E bicycle ergometer (Monark, Varberg, Sweden). In addition, they filled out a questionnaire regarding habitual physical activity and exercise training. Body composition was determined by hydrostatic weighing (Siri, 1956) with a correction for residual lung volume measured by the oxygen dilution method (Lundsgaard & van Slyke, 1917). The determination of body composition was carried out after a 4 h fasting period where subjects refrained from all food and liquids.
Diet
For eight days preceding the main trial, all subjects consumed an isoenergetic diet containing 65 energy per cent (E%) carbohydrate, 20 E% fat, and 15 E% protein. The constituents of the diet were weighed out at the laboratory and delivered to the subjects. The amount of energy to be consumed was individually determined from body weight, sex, and habitual physical activity level based on guidelines from the World Health Organization (FAO/WHO/UNU, 1985). To ensure that subjects kept their body mass steady during the 8 day dietary regimen, energy consumption during the experimental diet was adjusted after 4 days based on daily monitoring of body mass.
Experimental protocol
The subjects arrived at the laboratory at 08.30 h after an overnight fast. Subjects had abstained from exercise training for 36 h before the trial. The subjects rested in the supine position for approx. 30 min. Then expired air was obtained in a Douglas bag for determination of resting pulmonary oxygen uptake and carbon dioxide excretion. A venous catheter was inserted into the antecubital arm vein for blood sampling and after an additional 15 min of rest blood was drawn. Then a muscle biopsy was obtained from the vastus lateralis muscle under local anaesthesia of the skin and fascia. Immediately afterwards, the subjects initiated a 90 min bicycle exercise bout at 60% V̇O2peak on a Monark Ergomedic 839E bicycle ergometer. Expired air was collected in Douglas bags after 10, 20, 30, 45, 60, 75 and 90 min. Blood samples were drawn after 30, 60 and 90 min. After 90 min of bicycling, the subjects terminated exercise and within 30 s another biopsy from the vastus lateralis muscle was obtained and frozen. The postexercise biopsy was obtained from the opposite leg compared with the pre-exercise biopsy. During the experimental trial, subjects were offered water ad libitum.
Breath samples
Expired volumes of air in the Douglas bags were measured with a chain-suspended Collins spirometer, and a small sample of mixed expiratory air was analysed for O2 (Servomex S-3A) and CO2 (Beckman LB2). The respiratory exchange ratio (RER) was calculated as the ratio between pulmonary CO2 excretion and O2 uptake. Whole-body fat oxidation rate was calculated by the following equation using the non-protein respiratory quotient and then expressed in kJ (kg LBM)−1 min−1 using a standard caloric equivalent for fat (Peronnet & Massicotte, 1991):
Blood samples
Blood glucose and lactate concentrations were measured automatically (ABL510, Radiometer Medical A/S, Copenhagen, Denmark). Concentrations of adrenaline and noradrenaline in plasma were determined by radioimmunoassay (KatCombi Radioimmunoassay, Immuno-Biological Laboratories GmbH, Hamburg, Germany).
Muscle biopsies
The biopsies were divided in two. One part was immediately frozen in liquid nitrogen and stored at −80°C for subsequent use. The other part was mounted in embedding medium, frozen in precooled isopentane, and stored at −80°C for subsequent histochemistry.
Later, 80 mg wet weight of muscle tissue from the first part was freeze-dried and dissected free of all visible adipose tissue, connective tissue, and blood under a microscope. The dissected muscle fibres were pooled and then divided into subpools for the respective analyses.
Maximal citrate synthase (CS) activity
Maximal activity of CS was measured fluorometrically (Lowry & Passonneau, 1972).
Muscle glycogen
The glycogen concentration was determined by a fluorometric method (Lowry & Passonneau, 1972).
Muscle lactate, creatine, phosphocreatine, and nucleotides
The freeze-dried and dissected muscle tissue was extracted with 3 n perchloric acid and neutralized with KHCO3. The muscle lactate, creatine and phosphocreatine content were determined fluorometrically according to Lowry & Passonneau (1972), while the muscle content of ATP, ADP, and AMP was measured by reverse-phase HPLC as previously described (Tullson et al. 1990). Concentrations of free ADP (ADPfree) and AMP (AMPfree) were estimated using the near-equilibrium nature of the creatine phosphokinase and the adenylate kinase reactions. First, the H+ concentration was estimated from the measured muscle lactate concentration (Mannion et al. 1993). Then, ADPfree was estimated from the H+ concentration and the measured Cr, PCr, and ATP concentrations using 1.66 × 109 m−1 as the creatine phosphokinase equilibrium constant (Lawson & Veech, 1979). Finally, AMPfree was estimated from the estimated ADPfree and the measured ATP concentration using 1.05 as the adenylate kinase equilibrium constant (Lawson & Veech, 1979).
Muscle lysates
Muscle lysates were prepared from freeze-dried and dissected muscle tissue as previously described (Roepstorff et al. 2004).
Western blotting
Expression of α1AMPK, α2AMPK, and ACCβ as well as phosphorylation of αAMPK Thr172 and ACCβ Ser221 were detected by Western blotting on the muscle lysates. The lysates were boiled in Laemmli buffer before being subjected to SDS-PAGE and immunoblotting. Primary antibodies were sheep anti-α1AMPK and sheep anti-α2AMPK (Woods et al. 1996) kindly provided by Professor D. G. Hardie, University of Dundee, UK. Primary phosphospecific antibodies were rabbit anti-αAMPK Thr172-phos (Cell Signalling Technology, Beverly, MA, USA) and rabbit anti-ACCα Ser79-phos (Upstate Biotechnology, Lake Placid, NY, USA). Secondary antibodies were horseradish peroxidase-conjugated anti-sheep and anti-rabbit (DAKO, Glostrup, Denmark). ACCβ contains a biotin moiety that is recognized by streptavidin, and therefore horseradish peroxidase-conjugated streptavidin (DAKO, Glostrup, Denmark) was used to detect ACCβ protein (Frosig et al. 2004). Antigen–antibody complexes were visualized using enhanced chemiluminescence (ECL+, Amersham Biosciences, UK) and quantified by a Kodak Image Station E440CF (Kodak, Glostrup, Denmark).
AMPK activity
α-Isoform-specific AMPK activity was determined in immunoprecipitates from muscle lysates as previously described (Wojtaszewski et al. 2003). Briefly, immunoprecipitates were prepared from 200 μg of muscle lysate protein using anti-α1AMPK or anti-α2AMPK antibodies (Woods et al. 1996). AMPK activity was measured in the immunoprecipitates using SAMS-peptide (HMRSAMSGLHLVKRR, 200 μm) as previously described (Wojtaszewski et al. 2000).
ATPase, periodic acid Schiff, and capillary stainings
Serial cross-sections (10 μm) were cut and stained for myofibrillar ATPase to identify type 1, 2A and 2X muscle fibres (Brooke & Kaiser, 1970). On two additional cross-sections, respectively, capillaries were stained using the method of Qu et al. (1997) and muscle glycogen was stained using periodic acid Schiff (PAS) staining (Pearse, 1968). The PAS staining was performed to obtain a measure of the fibre type specific glycogen utilization and thereby an indication of the fibre type recruitment pattern during the exercise bout. Stained sections were examined using a Zeiss Axiolab light microscope (Broch & Michelsen A/S, Birkerød, Denmark) coupled to a Sanyo VCC-2972 colour CCD camera. Muscle fibre type composition, glycogen content, and capillary density were analysed in all subjects by the same blinded observer using the TEMA image analysis software (CheckVision, Støvring, Denmark).
RNA purification and real-time RT-PCR
Total RNA was isolated from ∼25 mg muscle tissue as previously described (Roepstorff et al. 2005b). Expression of α1AMPK mRNA and α2AMPK mRNA was measured by real-time RT-PCR using previously described methodology (Nielsen et al. 2003). GAPDH was chosen as endogenous control, because in the present study GAPDH mRNA was not affected significantly by either sex or acute exercise (data not shown), which was in accordance with previous findings (Kiens et al. 2004).
Statistics
Data are presented as means ± s.e.m. For variables independent of time, Student's t test was performed to test for differences between women and men. For variables measured before and after exercise as well as variables measured before and during exercise, a two-way analysis of variance (ANOVA), with repeated measures for the time factor, was performed to test for sex differences or changes due to time. When a significant main effect of time was found, significant pairwise differences were detected using Tukey's post hoc test. Correlation analysis was performed by Pearson's product moment for two factors and linear multiple regression for three factors. In all cases, a probability of 0.05 was used as the level of significance.
Results
Workload
The average workload during the 90 min bicycle exercise trial was 132 ± 6 and 174 ± 7 W in women and men, respectively (P < 0.001). Pulmonary oxygen uptake (V̇o2) during exercise averaged 1.9 ± 0.1 and 2.6 ± 0.1 l O2 min−1 in women and men, respectively (P < 0.001). Expressed per kg lean body mass (LBM) the V̇02 averaged 38.7 ± 1.1 and 37.7 ± 0.6 ml O2 (kg LBM−1) min−1 during exercise in women and men, respectively (NS). Relative exercise intensity averaged 60 ± 1% V̇02 in both women and men. Heart rate was 54 ± 4 and 55 ± 2 beats min−1 at rest in women and men, respectively (NS). Heart rate increased from rest to exercise (P < 0.001), and during exercise averaged 150 ± 4 and 150 ± 6 beats min−1 in women and men, respectively (NS).
Respiratory exchange ratio and fat oxidation
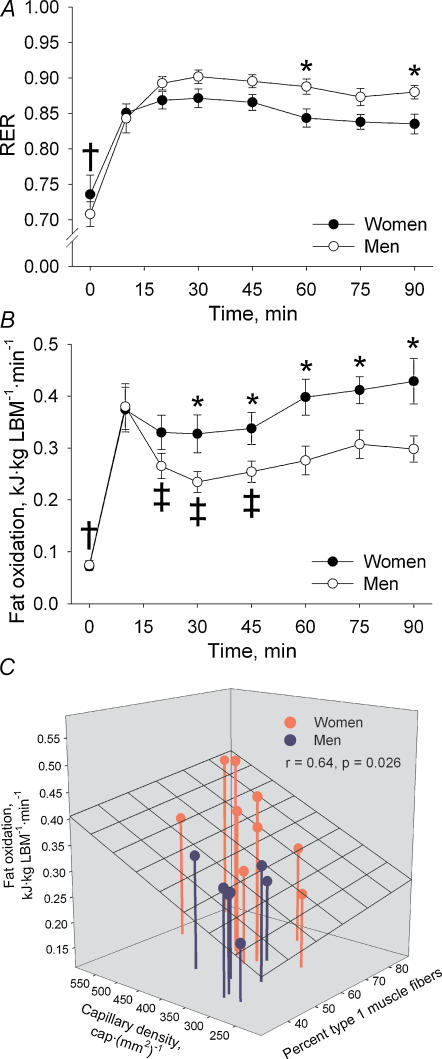
The respiratory exchange ratio (RER) at rest was 0.74 ± 0.03 and 0.71 ± 0.02 in women and men, respectively (NS) (Fig. 1A). During the initial 10 min of exercise RER increased (P < 0.001) in both women and men and thereafter RER did not change further throughout exercise (NS). RER was significantly lower in women than in men at 60 and 90 min of exercise (P < 0.05).
Figure 1. Respiratory exchange ratio (RER), fat oxidation and relationship between muscle morphology and fat oxidation in women and men during 90 min bicycling at 60% V̇O2peak.
A, RER. *Sex difference, P < 0.05; †different from exercise, P < 0.001. B, whole-body fat oxidation. *Sex difference, P < 0.05; †different from exercise in both sexes, P < 0.001; ‡different from 10 min in men, P < 0.05. C, relationship between capillary density and fibre type composition in the vastus lateralis muscle and whole-body fat oxidation during exercise analysed by multiple linear regression. LBM, lean body mass.
Whole-body fat oxidation per kg LBM was similar in women and men at rest and it increased (P < 0.001) from rest to 10 min of exercise in both sexes (Fig. 1B). In men a decrease was observed from 10 to 20 min of exercise after which fat oxidation did not change significantly throughout exercise. In women fat oxidation was constant during exercise. Fat oxidation was higher (P < 0.05) in women than in men during the last hour of exercise.
Blood glucose and lactate
At rest the blood concentrations of glucose and lactate did not differ significantly between women and men (Table 2). An increase (P < 0.05) was observed in blood glucose concentration from rest to 30 min of exercise after which a decrease (P < 0.05) was observed throughout exercise in both groups. The blood glucose concentration was higher in women than in men at 60 min of exercise (P < 0.05). The blood lactate concentration increased from rest to 30 min of exercise in both sexes (P < 0.05) and remained constant throughout exercise with no significant sex difference.
Table 2. Blood glucose and lactate as well as plasma adrenaline and noradrenaline concentrations in women and men at rest and during 90 min of bicycle exercise at 60% V̇O2peak.
| Rest | 30 min exercise | 60 min exercise | 90 min exercise | ||
|---|---|---|---|---|---|
| Blood glucose (mm) | Women | 4.6 ± 0.1 | 5.2 ± 0.2† | 5.1 ± 0.3† | 4.7 ± 0.1‡ |
| Men | 4.7 ± 0.1 | 5.0 ± 0.1† | 4.6 ± 0.1* | 4.4 ± 0.1‡ | |
| Blood lactate (mm) | Women | 0.9 ± 0.1 | 2.0 ± 0.4† | 1.7 ± 0.3† | 1.6 ± 0.2† |
| Men | 0.7 ± 0.1 | 2.0 ± 0.3† | 1.7 ± 0.1† | 1.8 ± 0.1† | |
| Plasma adrenaline (nm) | Women | 0.17 ± 0.10 | 0.53 ± 0.09 | 0.58 ± 0.10 | 1.02 ± 0.14† |
| Men | 0.09 ± 0.04 | 0.66 ± 0.16 | 1.14 ± 0.28† | 2.28 ± 0.50*†‡# | |
| Plasma noradrenaline (nm) | Women | 1.62 ± 0.49 | 6.40 ± 1.12† | 6.81 ± 1.16† | 7.07 ± 1.12†‡ |
| Men | 1.25 ± 0.33 | 5.74 ± 0.71† | 7.19 ± 1.19† | 8.43 ± 1.22†‡ |
Data are mean ± s.e.m.
Different from women, P < 0.05
different from rest, P < 0.05
different from 30 min, P < 0.05
different from 60 min, P < 0.05
Plasma adrenaline and noradrenaline
At rest, plasma adrenaline and noradrenaline concentrations did not differ significantly between women and men (Table 2). The plasma adrenaline concentration increased continuously (P < 0.05) from rest to 90 min of exercise and was higher in men than in women at the end of exercise (P < 0.05). The plasma noradrenaline concentration increased (P < 0.05) from rest to 30 min of exercise and remained elevated throughout exercise. No significant sex differences were observed in the plasma noradrenaline concentration.
Muscle glycogen
The glycogen concentration in the vastus lateralis muscle was similar in women and men at rest and decreased overall by 65% from rest to the end of exercise (P < 0.001) in women and men (Table 3).
Table 3. Glycogen, lactate, nucleotide, and creatine concentrations in the vastus lateralis muscle in women and men at rest and at 90 min of bicycle exercise at 60% V̇O2peak.
| Rest | 90 min exercise | |||
|---|---|---|---|---|
| Women | Men | Women | Men | |
| Glycogen (mmol (kg d.w.)−1) | 503 ± 36 | 481 ± 31 | 202 ± 25‡ | 141 ± 33‡ |
| Lactate (mmol (kg d.w.)−1) | 4.0 ± 0.9 | 6.3 ± 0.9 | 4.9 ± 1.0 | 7.5 ± 2.0 |
| ATP (mmol (kg d.w.)−1) | 22.3 ± 0.8 | 24.3 ± 0.8 | 23.3 ± 0.9 | 25.3 ± 1.0 |
| ADP (mmol (kg d.w.)−1) | 4.6 ± 0.5 | 4.9 ± 0.5 | 5.0 ± 0.5 | 5.0 ± 0.6 |
| AMP (mmol (kg d.w.)−1) | 0.35 ± 0.05 | 0.38 ± 0.05 | 0.38 ± 0.05 | 0.36 ± 0.06 |
| AMPfree (μmol (kg d.w.)−1) | 1.14 ± 0.31 | 1.36 ± 0.37 | 2.09 ± 0.58 | 3.59 ± 1.16† |
| AMPfree/ATP ratio (‰) | 0.052 ± 0.014 | 0.058 ± 0.017 | 0.095 ± 0.030 | 0.150 ± 0.052# |
| Creatine (Cr) (mmol (kg d.w.)−1) | 54 ± 4 | 58 ± 4 | 64 ± 4 | 82 ± 7*‡ |
| Phosphocreatine (PCr) (mmol (kg d.w.)−1) | 82 ± 5 | 84 ± 8 | 76 ± 8 | 73 ± 8 |
| PCr/Total Cr ratio (%) | 60 ± 3 | 58 ± 3 | 54 ± 4‡ | 47 ± 4‡ |
Data are mean ± s.e.m. d.w., dry weight; AMPfree, free AMP. AMPfree was calculated as described in Materials and Methods. Total creatine was calculated as the sum of Cr and PCr.
Different from women, P < 0.01; different from rest
P < 0.05
P < 0.01
P < 0.001
Muscle lactate, nucleotides and creatine
The muscle lactate concentration did not differ significantly between women and men and was not significantly higher after the 90 min exercise bout than at rest (Table 3).
All three measured nucleotides, ATP, ADP and AMP, remained unchanged from rest to 90 min of exercise (NS) and did not differ significantly between women and men (Table 3). The calculated concentration of AMPfree and the AMPfree/ATP ratio did not differ significantly between men and women at rest (Table 3). During exercise, an increase in the AMPfree concentration (164%, P < 0.01) and in the AMPfree/ATP ratio (158%, P < 0.05) was seen in men, while in women AMPfree and AMPfree/ATP did not change significantly.
The muscle creatine concentration did not differ between women and men at rest (NS) (Table 3). The muscle creatine concentration increased from rest to 90 min of exercise in men (P < 0.001) but not in women (NS). Thus, at 90 min of exercise it was 28% higher in men than in women (P < 0.01). The muscle phosphocreatine concentration did not differ significantly between rest and 90 min of exercise and was not significantly different between women and men (Table 3). The ratio between the concentrations of phosphocreatine and total creatine decreased (P < 0.001) from rest to 90 min of exercise in both women and men with no significant sex differences either at rest or at 90 min of exercise.
AMPK
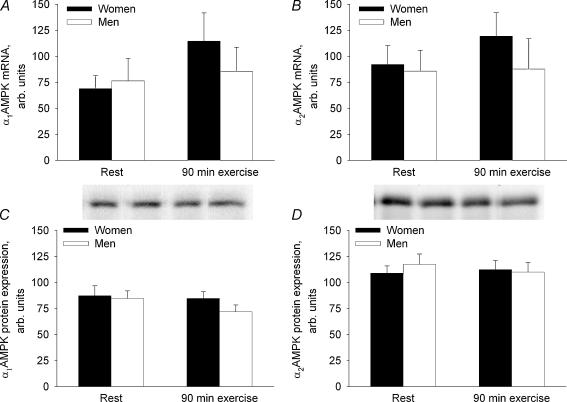
The α1AMPK and α2AMPK mRNA and protein expression in the vastus lateralis muscle did not differ significantly between women and men and did not change during the 90 min exercise bout (NS) (Fig. 2).
Figure 2. mRNA and protein expression of α1AMPK and α2AMPK in the vastus lateralis muscle in women and men before and at 90 min of bicycling at 60% V̇O2peak.
The target mRNA content was normalized to the GAPDH mRNA content. A, α1AMPK mRNA. B, α2AMPK mRNA. C, α1AMPK protein. D, α2AMPK protein.
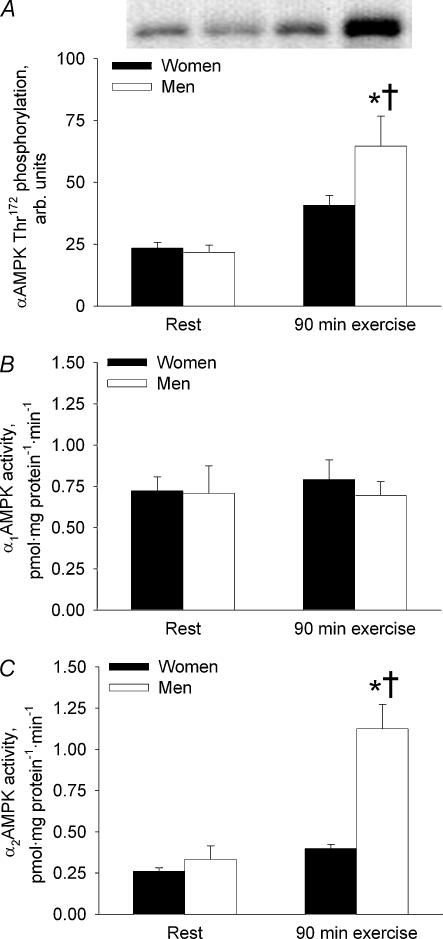
The αAMPK Thr172 phosphorylation did not differ significantly between women and men at rest (Fig. 3A). A 198% increase (P < 0.001) was observed from rest to 90 min of exercise in men, whereas in women the exercise-induced 74% increase in αAMPK Thr172 phosphorylation was only borderline-significant (P = 0.052). At 90 min of exercise, αAMPK Thr172 phosphorylation was 59% higher (P < 0.01) in men than in women.
Figure 3. AMPK phosphorylation and activity in the vastus lateralis muscle in women and men before and at 90 min of bicycling at 60% V̇O2peak.
A, αAMPK Thr172 phosphorylation. B, α1AMPK activity. C, α2AMPK activity. *Different from women, P < 0.01; †different from rest, P < 0.001.
α1AMPK activity was not significantly different between men and women and did not change from rest to 90 min of exercise (NS) (Fig. 3B).
α2AMPK activity did not differ significantly between men and women at rest (Fig. 3C). In men, a 210% increase (P < 0.001) was observed from rest to 90 min of exercise, whereas in women α2AMPK activity did not change significantly during exercise. At 90 min of exercise, α2AMPK activity was 137% higher in men than in women (P < 0.01).
ACC
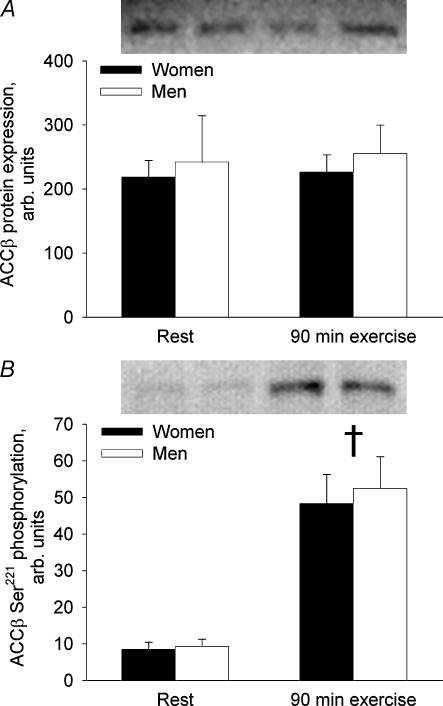
ACCβ protein expression was not significantly different between men and women and did not change from rest to 90 min of exercise (NS) (Fig. 4A). Phosphorylation of ACCβ on Ser221 increased 466% from rest to the end of exercise (P < 0.001) and did not differ significantly between women and men at rest or at 90 min of exercise (Fig. 4B).
Figure 4. ACCβ protein expression and phosphorylation in the vastus lateralis muscle in women and men before and at 90 min of bicycling at 60% V̇O2peak.
A, ACCβ protein. B, ACCβ Ser221 phosphorylation. †Different from rest, P < 0.001.
Histochemistry
Women had a higher relative number of type 1 muscle fibres compared with men (P < 0.01), while the higher relative number of type 2A and 2X fibres in men than in women did not reach statistical significance (Table 4). When expressed as area per cent, the proportion of type 1 fibres was higher in women than in men (P < 0.001), whereas the proportion of type 2A fibres was higher in men than in women (P < 0.05). The mean area of type 1 and 2X muscle fibres did not differ significantly between women and men, while type 2A fibres were larger in men than in women (P < 0.001).
Table 4. Fibre type composition and capillary density in the vastus lateralis muscle of women and men.
| Women | Men | |
|---|---|---|
| Fibre type composition (number percentage) | ||
| ″Type 1 | 67.9 ± 3.6** | 54.6 ± 2.8 |
| ″Type 2A | 23.3 ± 3.6 | 30.2 ± 2.0 |
| ″Type 2X | 8.7 ± 3.0 | 15.2 ± 3.0 |
| Fibre type composition (area percentage) | ||
| ″Type 1 | 68.2 ± 3.0*** | 49.2 ± 2.9 |
| ″Type 2A | 24.2 ± 3.8* | 35.6 ± 2.2 |
| ″Type 2X | 7.6 ± 2.6 | 15.3 ± 3.1 |
| Mean area per fibre (μm2) | ||
| ″Type 1 | 4267 ± 177 | 4490 ± 233 |
| ″Type 2A | 4407 ± 224*** | 5895 ± 189 |
| ″Type 2X | 4327 ± 473 | 5021 ± 339 |
| Capillary density | ||
| ″Capillaries per fibre | 2.0 ± 0.2 | 2.2 ± 0.1 |
| ″Capillaries per mm2 | 435 ± 32* | 355 ± 17 |
Data are mean ± s.e.m. Sex difference
P < 0.05
P < 0.01
P < 0.001
The mean number of capillaries surrounding each muscle fibre was similar in women and men (NS), but due to the higher mean muscle fibre area in men, the capillary density was lower in men than in women (P < 0.05) (Table 4).
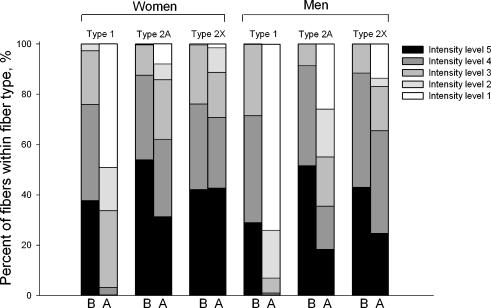
From the PAS staining it appeared that muscle glycogen content at rest was identical between women and men in all three muscle fibre types (Fig. 5). This finding supported the measurement of muscle glycogen content in crude muscle extracts (Table 3). During exercise, regardless of sex, muscle glycogen was markedly utilized in type 1 fibres with less utilization in type 2A fibres and almost no utilization in type 2X fibres. Furthermore, the slight trend towards a lower post-exercise muscle glycogen level in men than in women, seen with the glycogen analysis in crude muscle extracts (Table 3), was supported by the PAS staining. This trend was shown not to depend on fibre type (Fig. 5). Altogether, assuming that muscle fibre type specific glycogen utilization reflected fibre type recruitment during exercise, the PAS staining demonstrated that the fibre type recruitment pattern during the submaximal exercise bout was similar in women and men.
Figure 5. Fibre type specific glycogen utilization pattern in the vastus lateralis muscle of women and men during 90 min bicycling at 60% V̇O2peak.
B, before exercise. A, after exercise. Intensity levels 1–5 refer to the staining intensity of the PAS staining and are taken to semiquantitatively reflect the muscle glycogen content.
Correlation analyses
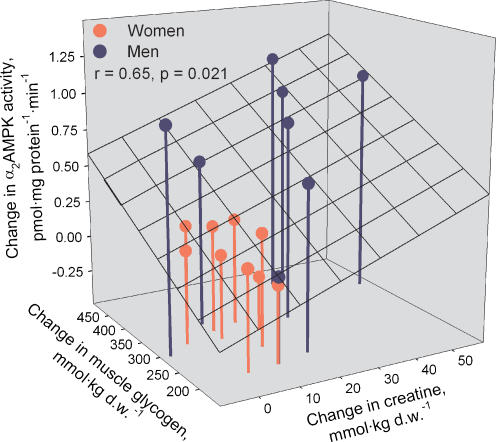
In an attempt to find potential factors to explain the change in α2AMPK activity during exercise, we tested whether the increase in α2AMPK activity during exercise correlated with muscle glycogen hydrolysis or any of the measures of cellular energy balance (changes in AMPfree, AMPfree/ATP, creatine or PCr/(Cr + PCr)). It was found that the change in α2AMPK activity during exercise correlated with changes in creatine (r = 0.56, P < 0.05) and the decline in muscle glycogen (r = 0.57, P < 0.05). Multiple linear regression revealed that the combined changes in creatine and muscle glycogen during exercise correlated significantly with the change in α2AMPK activity (r = 0.65, P < 0.05) (Fig. 6). To investigate whether the sex difference in cellular energy balance and AMPK activation during exercise was related to the sex specific muscle morphology, we tested any correlation between muscle morphology (fibre type 1 proportion and capillary density) and measures of cellular energy balance and AMPK activity. It was found that the proportion of type 1 fibres correlated negatively with the muscle creatine content during exercise (r = −0.62, P < 0.01) and with the increase in α2AMPK activity during exercise (r = −0.54, P < 0.05). Since AMPK activity and ACC phosphorylation could obviously not explain the sex difference seen in fat oxidation during exercise, we tested by linear regression whether sex specific muscle morphology was associated with the higher fat oxidation in women than in men during exercise. We found that fat oxidation during exercise correlated with the percentage of type 1 muscle fibres (r = 0.48, P = 0.05) and capillary density (r = 0.63, P < 0.01). Multiple linear regression revealed that the percentage of type 1 muscle fibres and capillary density in combination correlated significantly with fat oxidation during exercise (r = 0.64, P < 0.05) (Fig. 1C).
Figure 6. Relationship between changes in glycogen and creatine content and elevated activity of α2AMPK in the vastus lateralis muscle of women and men during 90 min bicycling at 60% V̇O2peak.
Discussion
The main findings of the present study were that α2AMPK activity, AMPK Thr172 phosphorylation, the free AMP and creatine concentrations, and free AMP/ATP ratio increased in skeletal muscle during 90 min submaximal bicycle exercise at 60% v02 in men but not significantly in women (Table 3 and Fig. 3A and C). These findings suggested that the lower exercise-induced muscle AMPK activation seen in women than in men was due to better maintenance of muscle cellular energy balance in women than in men during prolonged exercise. Since fat oxidation during exercise was higher in women than in men these findings also suggest that AMPK signalling is not a major regulator of fat oxidation during prolonged exercise in humans.
When considering the potential regulators of AMPK, it appears reasonable that skeletal muscle AMPK Thr172 phosphorylation and α2AMPK activity were higher in men than in women during exercise. In vitro AMPK activity is mainly regulated by phosphorylation on AMPK Thr172 by AMPK kinase(s) (Hawley et al. 1996) of which LKB1 is thought to be the most important (Hardie, 2005; Sakamoto et al. 2005). A high AMP/ATP ratio makes AMPK a better substrate for LKB1 (Hawley et al. 1996; Ponticos et al. 1998). Also, although the Cr/(PCr + Cr) ratio is probably not a direct regulator of AMPK activity (Taylor et al. 2006), increases in the Cr/(PCr + Cr) ratio can still inhibit AMPK activity through its effect on the AMP/ATP ratio via the creatine kinase equilibrium reaction. Moreover, low muscle glycogen content appears to increase the kinase activity acting on AMPK Thr172 (Derave et al. 2000; Wojtaszewski et al. 2003; Watt et al. 2004; Roepstorff et al. 2005a). In the present study, the ratio between AMPfree and ATP concentrations in muscle increased significantly during exercise in men but not in women, an increase in creatine concentration during exercise was also seen only in men (Table 3), and at the end of exercise the muscle glycogen level tended to be lower (P = 0.13) in men than in women (Table 3 and Fig. 5). Altogether, these factors may have caused the higher AMPK Thr172 phosphorylation and α2AMPK activity in men than in women during exercise. Indeed, multiple linear regression revealed that the increase in α2AMPK activity during exercise correlated with the increase in creatine and the decrease in muscle glycogen (Fig. 6) in accordance with previous findings in untrained and endurance trained men exercising at 80% v02 (Nielsen et al. 2003). Another factor that might have contributed to the sex difference in α2AMPK activity after 90 min exercise is the higher plasma adrenaline concentration in men than in women late during exercise (Table 2). Direct influence of adrenaline on α2AMPK activity is supported by a study showing that α-adrenergic stimulation, by phenylephrine, stimulated in vitro AMPK activity in rodent muscle (Minokoshi et al. 2002). Also, adrenaline stimulates glycogen breakdown in contracting muscle (Richter et al. 1982) and could thus indirectly influence AMPK activity.
Allosteric activation of AMPK, which is not detected in the in vitro assay, is regulated by the AMP/ATP ratio (Ponticos et al. 1998). In the present study, the AMPfree/ATP ratio increased during exercise in men but not in women (Table 3). Therefore, the data indicate that the in vivo sex difference in α2AMPK activation by exercise was at least as high as the sex difference in α2AMPK activity measured by the in vitro assay. Altogether, the data support the idea that the lower muscle AMPK activity in women than in men during exercise was due to better maintenance of cellular energy balance in women compared with men.
The notion that women are better able to maintain muscle cellular energy balance during exercise compared with men is supported by a previous study in which repeated bouts of high-intensity exercise induced a smaller muscle ATP reduction in women than in men (Esbjornsson-Liljedahl et al. 2002). In that study, the smaller ATP reduction by repeated bouts of exercise in women was ascribed to better IMP reamination during recovery periods, indicative of lower AMP/ATP and Cr/(Cr + PCr) ratios in women than in men (Esbjornsson-Liljedahl et al. 2002). Although intermittent high-intensity exercise is different from the prolonged moderate-intensity exercise bout undertaken in the present study, the latter is also characterized by intermittent contractile activity, in this case by each motor unit. A possible factor to explain the better maintenance of muscle cellular energy balance during exercise in women than in men could be that women have an improved ability to transport oxygen and substrates to the muscle cells and to oxidize energy substrates within the cells. The present (Table 4) and previous studies (Carter et al. 2001b; Steffensen et al. 2002) have all demonstrated that the proportion of the oxidative type 1 muscle fibres is higher in women than in men and that women have smaller muscle fibre cross-sectional areas, in particular of type 2 fibres. Also, the present study shows that women have higher muscle capillarization compared with men (Table 4). All these factors related to muscle morphology suggest that women have a shorter capillary-to-fibre diffusion distance and, due to higher proportion of oxidative fibres, should be better able to oxidize energy substrates compared with men. In the present study, arterio-venous oxygen and substrate kinetics across the exercising legs were not investigated. However, in a previous study from our laboratory it was observed that femoral venous blood flow per kg lean leg mass was higher in women than in men (∼13%, P < 0.05) during 90 min bicycle exercise at 60% v02 in both moderately trained and endurance trained subjects (Roepstorff et al. 2002; and unpublished observation). Also, in the same group of subjects leg oxygen uptake per kg lean leg mass tended to be higher in women than in men during exercise (∼7%, P = 0.14) (Roepstorff et al. 2002; and unpublished observation). Taken together, sex specific muscle morphology may explain the better maintenance of muscle cellular energy balance and, consequently, the smaller activation of muscle AMPK seen during prolonged submaximal exercise in women than in men. In support of this notion, in the present study there were significant correlations between the proportion of type 1 muscle fibres and the muscle creatine content during exercise (r = −0.62, P < 0.01) and between the proportion of type 1 muscle fibres and the increase in α2AMPK activity during exercise (r = −0.54, P < 0.05).
An important finding of the present study was that the higher relative fat oxidation in women than in men during exercise (Fig. 1) could not be ascribed to sex differences in muscle AMPK signalling through ACC. Thus, muscle ACCβ Ser221 phosphorylation did not differ between men and women and α2AMPK activation was in fact higher in men than in women during exercise (Figs 4B and 3C, respectively). Since ACCβ Ser221 phosphorylation has been used to reflect in vivo AMPK activity, the dissociation between AMPK activity and ACCβ Ser221 phosphorylation during exercise was somewhat surprising, although the same has been observed before in contracting skeletal muscle (Derave et al. 2000; Wojtaszewski et al. 2002; Nielsen et al. 2003). Possible explanations could be that allosteric regulators of AMPK can overrule the covalent regulation of AMPK on Thr172 or, alternatively, that ACCβ Ser221 phosphorylation is not always an optimal predictor of in vivo AMPK activity. In the present study, changes in potential allosteric regulators of AMPK (e.g. AMPfree/ATP and PCr/(PCr + Cr) ratios) supported the measured in vitro AMPK activity. Therefore, the latter of the two possibilities given above seems to be the most likely to explain the dissociation between AMPK activity and ACCβ Ser221 phosphorylation in the present study. No matter what, the similar ACCβ Ser221 phosphorylation seen in women and men at 90 min of exercise in the present study suggests that AMPK signalling through the ACC–malonyl CoA–CPT I pathway is not a major regulator of fat oxidation during prolonged exercise in humans. Still, it should be recognized that malonyl-CoA content and CPT I activity were not measured in the present study due to limited amounts of muscle tissue. Previously, it was shown that muscle CPT I activity did not differ between women and men (Berthon et al. 1998). It is possible that malonyl-CoA content may have been lower in women than in men due to regulation by factors other than ACC, although the higher proportion of type 1 muscle fibres in women is expected to induce higher malonyl-CoA content, since malonyl-CoA is higher in red than in white rat muscle (Saha et al. 1995). The higher AMPK activity in men, the similar ACC phosphorylation in women and men, and the higher fat oxidation in women during exercise in the present study confirm several previous exercise studies that have failed to demonstrate any correlation between muscle AMPK activity, ACC phosphorylation, and/or malonyl-CoA concentration on the one hand and fat oxidation rate on the other hand (Odland, et al. 1996, 1998; Dean et al. 2000; McConell et al. 2005; Roepstorff et al. 2005a; Wadley et al. 2006). What may then be the underlying cause of the higher fat oxidation seen in women compared with men? First, pH and/or free carnitine availability may be important in regulating CPT I activity and fat oxidation in skeletal muscle during exercise (Starritt et al. 2000; Bezaire et al. 2004; Roepstorff et al. 2005a). Second, the correlation between muscle morphology and fat oxidation seen in the present study (Fig. 1C) suggests that the higher fat oxidation in women than in men is, in part, due to factors associated with sex specific muscle morphology, i.e. due to factors different between type 1 and type 2 muscle fibres.
During exercise, muscle cellular energy balance and AMPK activation are known to be affected by the training status of the subjects (Nielsen et al. 2003) as well as the exercise intensity (Wojtaszewski et al. 2000; Wadley et al. 2006). Therefore, in the present study it was important to assure that the training status of the women and men was similar and that women and men performed comparable amounts of relative work during the exercise bout. Several observations suggest that both of these criteria were indeed fulfilled. First, women and men did not differ significantly in v02 per kg LBM, training history, and physical activity level (Table 1), suggesting that women and men were of similar training status. Although CS activity tended to differ significantly between women and men, the small sex difference was minor compared with the response usually induced by exercise training (Kiens et al. 2004). Second, the relative exercise intensity did not differ between women and men when expressed as v02 as a percentage of v02 (60 ± 1% in both women and men) or as v02 per kg LBM (38.7 ± 1.1 and 37.7 ± 0.6 ml O2 (kg LBM)−1 min−1 in women and men, respectively (NS)). Also, heart rate was identical in women (150 ± 4 beats min−1) and men (150 ± 6 beats min−1) during exercise. Although lactate threshold was not determined in the present study, the exercise induced rise in blood and muscle lactate concentrations were moderate and similar in women and men indicating that both women and men exercised at an intensity well below the lactate threshold. Finally, when assessed from the PAS staining against fibre type specific muscle glycogen, it appeared that the fibre type recruitment pattern during exercise was identical in women and men. Altogether, it seems that the difference between men and women in α2AMPK activation by exercise in the present study reflects a true sex difference.
In conclusion, the present findings suggest that the smaller AMPK activation by exercise in women than in men was due to better maintenance of muscle cellular energy balance during exercise in women compared with men. This might be explained by improved transport of oxygen and substrates to the muscle cells and generally higher capacity for oxidation of substrates within the muscle cells in women. In support of this contention, women had a higher proportion of oxidative type 1 muscle fibres, smaller muscle fibres, and higher capillary density compared with men and the former correlated negatively with exercise induced AMPK activation. The higher fat oxidation and lower AMPK activation during exercise in women than in men suggested that the sex difference in fat oxidation could not be explained by regulation of fat oxidation via AMPK. Rather, the higher fat oxidation in women than in men appears to be due to factors associated with sex specific muscle morphology, since fat oxidation correlated with the proportion of type 1 muscle fibres and capillary density.
Acknowledgments
We would like to thank Professor D. G. Hardie, University of Dundee, UK, for kindly providing the antiα1AMPK and antiα2AMPK antibodies. We acknowledge the skilled technical assistance of Irene Bech Nielsen, Betina Bolmgren, Jesper B. Birk, Winnie Taagerup, and Kristina Møller Kristensen. This study was supported by The Novo Nordisk Research Foundation, The Danish Diabetes Association, an Integrated Project (LSHM-CT-2004-005272) funded by the European Commission, The Danish Sports Research Council, Michaelsen Fonden, The Danish Medical and Natural Sciences Research Councils, and the Lundbeck Foundation. J.F.P.W. was supported by a Hallas Møller Fellowship from The Novo Nordisk Foundation.
References
- Berthon PM, Howlett RA, Heigenhauser GJ, Spriet LL. Human skeletal muscle carnitine palmitoyltransferase I activity determined in isolated intact mitochondria. J Appl Physiol. 1998;85:148–153. doi: 10.1152/jappl.1998.85.1.148. [DOI] [PubMed] [Google Scholar]
- Bezaire V, Heigenhauser GJ, Spriet LL. Regulation of CPT I activity in intermyofibrillar and subsarcolemmal mitochondria from human and rat skeletal muscle. Am J Physiol Endocrinol Metab. 2004;286:E85–E91. doi: 10.1152/ajpendo.00237.2003. [DOI] [PubMed] [Google Scholar]
- Brooke MH, Kaiser KK. Three ‘myosin adenosine triphosphatase’ systems: the nature of their pH lability and sulfhydryl dependence. J Histochem Cytochem. 1970;18:670–672. doi: 10.1177/18.9.670. [DOI] [PubMed] [Google Scholar]
- Burguera B, Proctor D, Dietz N, Guo Z, Joyner M, Jensen MD. Leg free fatty acid kinetics during exercise in men and women. Am J Physiol Endocrinol Metab. 2000;278:E113–E117. doi: 10.1152/ajpendo.2000.278.1.E113. [DOI] [PubMed] [Google Scholar]
- Carter SL, Rennie CD, Hamilton SJ, Tarnopolsky MA. Changes in skeletal muscle in males and females following endurance training. Can J Physiol Pharmacol. 2001b;79:386–392. [PubMed] [Google Scholar]
- Carter SL, Rennie C, Tarnopolsky MA. Substrate utilization during endurance exercise in men and women after endurance training. Am J Physiol Endocrinol Metab. 2001a;280:E898–E907. doi: 10.1152/ajpendo.2001.280.6.E898. [DOI] [PubMed] [Google Scholar]
- Dean D, Daugaard JR, Young ME, Saha A, Vavvas D, Asp S, Kiens B, Kim KH, Witters L, Richter EA, Ruderman N. Exercise diminishes the activity of acetyl-CoA carboxylase in human muscle. Diabetes. 2000;49:1295–1300. doi: 10.2337/diabetes.49.8.1295. [DOI] [PubMed] [Google Scholar]
- Derave W, Ai H, Ihlemann J, Witters LA, Kristiansen S, Richter EA, Ploug T. Dissociation of AMP-activated protein kinase activation and glucose transport in contracting slow-twitch muscle. Diabetes. 2000;49:1281–1287. doi: 10.2337/diabetes.49.8.1281. [DOI] [PubMed] [Google Scholar]
- Esbjornsson-Liljedahl M, Bodin K, Jansson E. Smaller muscle ATP reduction in women than in men by repeated bouts of sprint exercise. J Appl Physiol. 2002;93:1075–1083. doi: 10.1152/japplphysiol.00732.1999. [DOI] [PubMed] [Google Scholar]
- FAO/WHO/UNU. Energy and protein requirements. 1985 http://www.fao.org/DOCREP/003/AA040E/AA040E00.htm.
- Friedlander AL, Casazza GA, Horning MA, Huie MJ, Piacentini MF, Trimmer JK, Brooks GA. Training-induced alterations of carbohydrate metabolism in women: women respond differently from men. J Appl Physiol. 1998;85:1175–1186. doi: 10.1152/jappl.1998.85.3.1175. [DOI] [PubMed] [Google Scholar]
- Friedlander AL, Casazza GA, Horning MA, Usaj A, Brooks GA. Endurance training increases fatty acid turnover, but not fat oxidation, in young men. J Appl Physiol. 1999;86:2097–2105. doi: 10.1152/jappl.1999.86.6.2097. [DOI] [PubMed] [Google Scholar]
- Frosig C, Jorgensen SB, Hardie DG, Richter EA, Wojtaszewski JF. 5′-AMP-activated protein kinase activity and protein expression are regulated by endurance training in human skeletal muscle. Am J Physiol Endocrinol Metab. 2004;286:E411–E417. doi: 10.1152/ajpendo.00317.2003. [DOI] [PubMed] [Google Scholar]
- Fujii N, Hayashi T, Hirshman MF, Smith JT, Habinowski SA, Kaijser L, Mu J, Ljungqvist O, Birnbaum MJ, Witters LA, Thorell A, Goodyear LJ. Exercise induces isoform-specific increase in 5′AMP-activated protein kinase activity in human skeletal muscle. Biochem Biophys Res Commun. 2000;273:1150–1155. doi: 10.1006/bbrc.2000.3073. [DOI] [PubMed] [Google Scholar]
- Hardie DG. New roles for the LKB1→AMPK pathway. Curr Opin Cell Biol. 2005;17:167–173. doi: 10.1016/j.ceb.2005.01.006. [DOI] [PubMed] [Google Scholar]
- Hardie DG, Carling D. The AMP-activated protein kinase – fuel gauge of the mammalian cell. Eur J Biochem. 1997;246:259–273. doi: 10.1111/j.1432-1033.1997.00259.x. [DOI] [PubMed] [Google Scholar]
- Hawley SA, Davison M, Woods A, Davies SP, Beri RK, Carling D, Hardie DG. Characterization of the AMP-activated protein kinase kinase from rat liver and identification of threonine 172 as the major site at which it phosphorylates AMP-activated protein kinase. J Biol Chem. 1996;271:27879–27887. doi: 10.1074/jbc.271.44.27879. [DOI] [PubMed] [Google Scholar]
- Horton TJ, Pagliassotti MJ, Hobbs K, Hill JO. Fuel metabolism in men and women during and after long-duration exercise. J Appl Physiol. 1998;85:1823–1832. doi: 10.1152/jappl.1998.85.5.1823. [DOI] [PubMed] [Google Scholar]
- Kiens B, Roepstorff C, Glatz JF, Bonen A, Schjerling P, Knudsen J, Nielsen JN. Lipid-binding proteins and lipoprotein lipase activity in human skeletal muscle: influence of physical activity and gender. J Appl Physiol. 2004;97:1209–1218. doi: 10.1152/japplphysiol.01278.2003. [DOI] [PubMed] [Google Scholar]
- Lawson JW, Veech RL. Effects of pH and free Mg2+ on the Keq of the creatine kinase reaction and other phosphate hydrolyses and phosphate transfer reactions. J Biol Chem. 1979;254:6528–6537. [PubMed] [Google Scholar]
- Lowry OH, Passonneau JV. A Flexible System of Enzymatic Analysis. New York: Academic Press; 1972. [Google Scholar]
- Lundsgaard C, van Slyke DD. Studies of Lung, I: Relation between thorax size and lung volume in normal adults. J Exp Medicine. 1917;27:65–85. doi: 10.1084/jem.27.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannion AF, Jakeman PM, Willan PL. Determination of human skeletal muscle buffer value by homogenate technique: methods of measurement. J Appl Physiol. 1993;75:1412–1418. doi: 10.1152/jappl.1993.75.3.1412. [DOI] [PubMed] [Google Scholar]
- McConell GK, Lee-Young RS, Chen ZP, Stepto NK, Huynh NN, Stephens TJ, Canny BJ, Kemp BE. Short-term exercise training in humans reduces AMPK signalling during prolonged exercise independent of muscle glycogen. J Physiol. 2005;568:665–676. doi: 10.1113/jphysiol.2005.089839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGarry JD, Mills SE, Long CS, Foster DW. Observations on the affinity for carnitine, and malonyl-CoA sensitivity, of carnitine palmitoyltransferase I in animal and human tissues. Demonstration of the presence of malonyl-CoA in non-hepatic tissues of the rat. Biochem J. 1983;214:21–28. doi: 10.1042/bj2140021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minokoshi Y, Kim YB, Peroni OD, Fryer LG, Muller C, Carling D, Kahn BB. Leptin stimulates fatty-acid oxidation by activating AMP-activated protein kinase. Nature. 2002;415:339–343. doi: 10.1038/415339a. [DOI] [PubMed] [Google Scholar]
- Nielsen JN, Mustard KJ, Graham DA, Yu H, MacDonald CS, Pilegaard H, Goodyear LJ, Hardie DG, Richter EA, Wojtaszewski JF. 5′-AMP-activated protein kinase activity and subunit expression in exercise-trained human skeletal muscle. J Appl Physiol. 2003;94:631–641. doi: 10.1152/japplphysiol.00642.2002. [DOI] [PubMed] [Google Scholar]
- Odland LM, Heigenhauser GJ, Lopaschuk GD, Spriet LL. Human skeletal muscle malonyl-CoA at rest and during prolonged submaximal exercise. Am J Physiol. 1996;270:E541–E544. doi: 10.1152/ajpendo.1996.270.3.E541. [DOI] [PubMed] [Google Scholar]
- Odland LM, Howlett RA, Heigenhauser GJ, Hultman E, Spriet LL. Skeletal muscle malonyl-CoA content at the onset of exercise at varying power outputs in humans. Am J Physiol. 1998;274:E1080–E1085. doi: 10.1152/ajpendo.1998.274.6.E1080. [DOI] [PubMed] [Google Scholar]
- Pearse AGE. Histochemistry. Theoretical and Applied. 3. London: J & A Churchill Ltd; 1968. [Google Scholar]
- Peronnet F, Massicotte D. Table of nonprotein respiratory quotient – An update. Can J Sport Sci. 1991;16:23–29. [PubMed] [Google Scholar]
- Perreault L, Lavely JM, Bergman BC, Horton TJ. Gender differences in insulin action after a single bout of exercise. J Appl Physiol. 2004;97:1013–1021. doi: 10.1152/japplphysiol.00186.2004. [DOI] [PubMed] [Google Scholar]
- Phillips SM, Atkinson SA, Tarnopolsky MA, MacDougall JD. Gender differences in leucine kinetics and nitrogen balance in endurance athletes. J Appl Physiol. 1993;75:2134–2141. doi: 10.1152/jappl.1993.75.5.2134. [DOI] [PubMed] [Google Scholar]
- Ponticos M, Lu QL, Morgan JE, Hardie DG, Partridge TA, Carling D. Dual regulation of the AMP-activated protein kinase provides a novel mechanism for the control of creatine kinase in skeletal muscle. EMBO J. 1998;17:1688–1699. doi: 10.1093/emboj/17.6.1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu Z, Andersen JL, Zhou S. Visualisation of capillaries in human skeletal muscle. Histochem Cell Biol. 1997;107:169–174. doi: 10.1007/s004180050101. [DOI] [PubMed] [Google Scholar]
- Rasmussen BB, Winder WW. Effect of exercise intensity on skeletal muscle malonyl-CoA and acetyl-CoA carboxylase. J Appl Physiol. 1997;83:1104–1109. doi: 10.1152/jappl.1997.83.4.1104. [DOI] [PubMed] [Google Scholar]
- Richter EA, Ruderman NB, Gavras H, Belur ER, Galbo H. Muscle glycogenolysis during exercise: dual control by epinephrine and contractions. Am J Physiol Endocrinol Metab. 1982;242:E25–E32. doi: 10.1152/ajpendo.1982.242.1.E25. [DOI] [PubMed] [Google Scholar]
- Riddell MC, Partington SL, Stupka N, Armstrong D, Rennie C, Tarnopolsky MA. Substrate utilization during exercise performed with and without glucose ingestion in female and male endurance trained athletes. Int J Sport Nutr Exerc Metab. 2003;13:407–421. doi: 10.1123/ijsnem.13.4.407. [DOI] [PubMed] [Google Scholar]
- Roepstorff C, Halberg N, Hillig T, Saha AK, Ruderman NB, Wojtaszewski JF, Richter EA, Kiens B. Malonyl-CoA and carnitine in regulation of fat oxidation in human skeletal muscle during exercise. Am J Physiol Endocrinol Metab. 2005a;288:E133–E142. doi: 10.1152/ajpendo.00379.2004. [DOI] [PubMed] [Google Scholar]
- Roepstorff C, Schjerling P, Vistisen B, Madsen M, Steffensen CH, Rider MH, Kiens B. Regulation of oxidative enzyme activity and eukaryotic elongation factor 2 in human skeletal muscle: influence of gender and exercise. Acta Physiol Scand. 2005b;184:215–224. doi: 10.1111/j.1365-201X.2005.01442.x. [DOI] [PubMed] [Google Scholar]
- Roepstorff C, Steffensen CH, Madsen M, Stallknecht B, Kanstrup IL, Richter EA, Kiens B. Gender differences in substrate utilization during submaximal exercise in endurance-trained subjects. Am J Physiol Endocrinol Metab. 2002;282:E435–E447. doi: 10.1152/ajpendo.00266.2001. [DOI] [PubMed] [Google Scholar]
- Roepstorff C, Vistisen B, Donsmark M, Nielsen JN, Galbo H, Green KA, Hardie DG, Wojtaszewski JFP, Richter EA, Kiens B. Regulation of hormone-sensitive lipase activity and Ser563 and Ser565 phosphorylation in human skeletal muscle during exercise. J Physiol. 2004;560:551–562. doi: 10.1113/jphysiol.2004.066480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha AK, Kurowski TG, Ruderman NB. A malonyl-CoA fuel-sensing mechanism in muscle: effects of insulin, glucose, and denervation. Am J Physiol. 1995;269:E283–E289. doi: 10.1152/ajpendo.1995.269.2.E283. [DOI] [PubMed] [Google Scholar]
- Sakamoto K, McCarthy A, Smith D, Green KA, Grahame HD, Ashworth A, Alessi DR. Deficiency of LKB1 in skeletal muscle prevents AMPK activation and glucose uptake during contraction. EMBO J. 2005;24:1810–1820. doi: 10.1038/sj.emboj.7600667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siri WE. The gross composition of the body. Adv Biol Med Phys. 1956;4:239–279. doi: 10.1016/b978-1-4832-3110-5.50011-x. [DOI] [PubMed] [Google Scholar]
- Starritt EC, Howlett RA, Heigenhauser GJ, Spriet LL. Sensitivity of CPT I to malonyl-CoA in trained and untrained human skeletal muscle. Am J Physiol Endocrinol Metab. 2000;278:E462–E468. doi: 10.1152/ajpendo.2000.278.3.E462. [DOI] [PubMed] [Google Scholar]
- Steffensen CH, Roepstorff C, Madsen M, Kiens B. Myocellular triacylglycerol breakdown in females but not in males during exercise. Am J Physiol Endocrinol Metab. 2002;282:E634–E642. doi: 10.1152/ajpendo.00078.2001. [DOI] [PubMed] [Google Scholar]
- Tarnopolsky LJ, MacDougall JD, Atkinson SA, Tarnopolsky MA, Sutton JR. Gender differences in substrate for endurance exercise. J Appl Physiol. 1990;68:302–308. doi: 10.1152/jappl.1990.68.1.302. [DOI] [PubMed] [Google Scholar]
- Taylor EB, Ellingson WJ, Lamb JD, Chesser DG, Compton CL, Winder WW. Evidence against regulation of AMP-activated protein kinase and LKB1-STRAD-MO25 activity by creatine phosphate. Am J Physiol Endocrinol Metab. 2006;290:E661–669. doi: 10.1152/ajpendo.00313.2005. [DOI] [PubMed] [Google Scholar]
- Tullson PC, Whitlock DM, Terjung RL. Adenine-nucleotide degradation in slow-twitch red muscle. Am J Physiol. 1990;258:C258–C265. doi: 10.1152/ajpcell.1990.258.2.C258. [DOI] [PubMed] [Google Scholar]
- Venables MC, Achten J, Jeukendrup AE. Determinants of fat oxidation during exercise in healthy men and women: a cross-sectional study. J Appl Physiol. 2005;98:160–167. doi: 10.1152/japplphysiol.00662.2003. [DOI] [PubMed] [Google Scholar]
- Wadley GD, Lee-Young RS, Canny BJ, Wasuntarawat C, Chen ZP, Hargreaves M, Kemp BE, McConell GK. Effect of exercise intensity and hypoxia on skeletal muscle AMPK signaling and substrate metabolism in humans. Am J Physiol Endocrinol Metab. 2006;290:E694–702. doi: 10.1152/ajpendo.00464.2005. [DOI] [PubMed] [Google Scholar]
- Watt MJ, Steinberg GR, Chan S, Garnham A, Kemp BE, Febbraio MA. Beta-adrenergic stimulation of skeletal muscle HSL can be overridden by AMPK signaling. FASEB J. 2004;18:1445–1446. doi: 10.1096/fj.03-1067fje. [DOI] [PubMed] [Google Scholar]
- Winder WW. Intramuscular mechanisms regulating fatty acid oxidation during exercise. Adv Exp Med Biol. 1998;441:239–248. doi: 10.1007/978-1-4899-1928-1_22. [DOI] [PubMed] [Google Scholar]
- Winder WW. Energy-sensing and signaling by AMP-activated protein kinase in skeletal muscle. J Appl Physiol. 2001;91:1017–1028. doi: 10.1152/jappl.2001.91.3.1017. [DOI] [PubMed] [Google Scholar]
- Winder WW, Hardie DG. Inactivation of acetyl-CoA carboxylase and activation of AMP-activated protein kinase in muscle during exercise. Am J Physiol. 1996;270:E299–E304. doi: 10.1152/ajpendo.1996.270.2.E299. [DOI] [PubMed] [Google Scholar]
- Wojtaszewski JF, MacDonald C, Nielsen JN, Hellsten Y, Hardie DG, Kemp BE, Kiens B, Richter EA. Regulation of 5′AMP-activated protein kinase activity and substrate utilization in exercising human skeletal muscle. Am J Physiol Endocrinol Metab. 2003;284:E813–E822. doi: 10.1152/ajpendo.00436.2002. [DOI] [PubMed] [Google Scholar]
- Wojtaszewski JF, Mourtzakis M, Hillig T, Saltin B, Pilegaard H. Dissociation of AMPK activity and ACCβ phosphorylation in human muscle during prolonged exercise. Biochem Biophys Res Commun. 2002;298:309–316. doi: 10.1016/s0006-291x(02)02465-8. [DOI] [PubMed] [Google Scholar]
- Wojtaszewski JF, Nielsen P, Hansen BF, Richter EA, Kiens B. Isoform-specific and exercise intensity-dependent activation of 5′-AMP-activated protein kinase in human skeletal muscle. J Physiol. 2000;528:221–226. doi: 10.1111/j.1469-7793.2000.t01-1-00221.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods A, Salt I, Scott J, Hardie DG, Carling D. The α1 and α2 isoforms of the AMP-activated protein kinase have similar activities in rat liver but exhibit differences in substrate specificity in vitro. FEBS Lett. 1996;397:347–351. doi: 10.1016/s0014-5793(96)01209-4. [DOI] [PubMed] [Google Scholar]