Abstract
In the present study we used a combination of patch clamping and fast confocal Ca2+ imaging to examine the effects of activators of the nitric oxide (NO)/cGMP pathway on pacemaker activity in freshly dispersed ICC from the rabbit urethra, using the amphotericin B perforated patch configuration of the patch-clamp technique. The nitric oxide donor, DEA-NO, the soluble guanylyl cyclase activator YC-1 and the membrane-permeant analogue of cGMP, 8-Br-cGMP inhibited spontaneous transient depolarizations (STDs) and spontaneous transient inward currents (STICs) recorded under current-clamp and voltage-clamp conditions, respectively. Caffeine-evoked Cl− currents were unaltered in the presence of SP-8-Br-PET-cGMPs, suggesting that activation of the cGMP/PKG pathway does not block Cl− channels directly or interfere with Ca2+ release via ryanodine receptors (RyR). However, noradrenaline-evoked Cl− currents were attenuated by SP-8-Br-PET-cGMPs, suggesting that activation of cGMP-dependent protein kinase (PKG) may modulate release of Ca2+ via IP3 receptors (IP3R).When urethral interstitial cells (ICC) were loaded with Fluo4-AM (2 μm), and viewed with a confocal microscope, they fired regular propagating Ca2+ waves, which originated in one or more regions of the cell. Application of DEA-NO or other activators of the cGMP/PKG pathway did not significantly affect the oscillation frequency of these cells, but did significantly reduce their spatial spread. These effects were mimicked by the IP3R blocker, 2-APB (100 μm). These data suggest that NO donors and activators of the cGMP pathway inhibit electrical activity of urethral ICC by reducing the spatial spread of Ca2+ waves, rather than decreasing wave frequency.
Nitric oxide (NO) is the main inhibitory neurotransmitter in the urethra of a variety of species including rabbits, rats, sheep, pigs and humans (for review see Andersson & Wein, 2000). A number of studies have demonstrated that either electrical field stimulation of inhibitory nerves or exogenous application of NO can elevate cGMP levels in this tissue (Morita et al. 1992; Dokita et al. 1994; Persson & Andersson, 1994). Furthermore, mice lacking cGMP-dependent protein kinase G1 (PKG1, Persson et al. 2000) have significantly attenuated neurogenic relaxation of the urethra, suggesting that PKG1 is essential for nitrergic-mediated relaxation.
Although it is now well established that NO mediates it effects on the urethra via the cGMP/PKG1 pathway, little is known about how this inhibits urethral tone. One possibility is that NO reduces urethral tone by inhibiting the underlying spontaneous electrical activity. The effects of NO and NO donors on urethral electrical activity have been briefly examined in the rabbit urethra, but the results have been equivocal. Ito & Kimoto (1985) demonstrated that inhibitory junction potentials could be elicited by transmural nerve stimulation in the rabbit urethra and suggested that these were mediated via a non adrenergic non cholinergic (NANC) neurotransmitter. Although Hashitani et al. (1996) demonstrated that sodium nitroprusside application reduced the frequency of slow waves in the same tissue, they failed to demonstrate any hyperpolarization associated with this effect. Waldeck et al. (1998) were unable to demonstrate any effect of exogenous NO or inhibitory nerve stimulation on electrical activity in the same tissue. The results from the latter studies (Waldeck et al. 1998; Hashitani et al. 1996) combined with the insensitivity of the relaxant response to K+ channel blockers (Garcia-Pascual & Triguero, 1994; Costa, Labadia, Triguero, Jimenez & Garcia-Pascual, 2001) suggest that in contrast to other smooth muscles, K+ channels contribute little to NO-mediated relaxation in the urethra.
More recent studies have demonstrated that urethral tone may be generated (Sergeant et al. 2000) and modulated (Sergeant et al. 2002; Smet et al. 1996; Waldeck et al. 1998) by specialized pacemaker cells. These cells generate spontaneous transient inward Cl− currents in the rabbit urethra, which are thought to drive the surrounding smooth muscle in a manner similar to that demonstrated for interstitial cells of Cajal (ICC) in the gastrointestinal tract (Sanders, Koh & Ward, 2005). We have previously suggested that noradrenergic neurotransmission may, in part at least, be mediated via activation of urethral ICC. Immunohistochemical evidence presented by Smet et al. (1996) and Waldeck et al. (1998) would also support our contention that urethral ICC play a role in neurotransmission. Their studies demonstrated the presence of branched interstitial cells in the human, guinea-pig and rabbit urethras, respectively, that were immunopositive for cGMP, and support the idea that they may be important in mediating neurally released NO responses.
However, no study has examined in detail the effects of activating the cGMP/PKG pathway on electrical activity in isolated ICC from the rabbit urethra. The purpose of the present study was to examine the effects of activating the cGMP/PKG on electrical activity and Ca2+ waves, using a combination of electrophysiology and confocal imaging on freshly dispersed ICC from the rabbit urethra.
Methods
Cell dispersal
The bladder and urethra were removed from both male and female rabbits immediately after they had been killed by lethal injection of pentobarbitone. The most proximal 3 cm of the urethra was removed and placed in Krebs solution. This was then opened up longtitudinally and the urothelium removed by sharp dissection. Strips of tissue, 0·5 cm in width were cut into 1 mm3 pieces and stored in Hanks Ca2+ free solution for 30 min at 4°C prior to cell dispersal. Tissue pieces were incubated in dispersal medium containing (per 5 ml of Ca2+-free Hanks solution (see Solutions)): 15 mg collagenase (Sigma type 1A), 1 mg protease (Sigma type XXIV), 10 mg bovine serum albumin (Sigma) and 10 mg trypsin inhibitor (Sigma) for 10–15 min at 37°C. Tissue was then transferred to Ca2+-free Hanks solution, and stirred for a further 15–30 min to release single smooth muscle cells and ICC. These cells were plated in Petri dishes containing 100 μm Ca2+ Hanks solution and stored at 4°C for use within 8 h.
Using our dispersal procedure, both ICC and smooth muscle cells could be reliably isolated from the rabbit urethra as previously described (Sergeant et al. 2000). In the present study we focused on studying interstitial cells, which could be easily distinguished from smooth muscle cells using a number of criteria (Sergeant et al. 2000). Thus, ICC were highly branched, failed to contract in response to depolarizing current injection or application of noradrenaline, possessed abundant calcium-activated chloride current and normally fired spontaneous transient inward currents under voltage-clamp conditions.
Perforated-patch recordings from single cells
Currents were recorded using the perforated-patch configuration of the whole-cell patch-clamp technique (Rae et al. 1991). This circumvented the problem of current rundown encountered using the conventional whole-cell configuration. The cell membrane was perforated using the antibiotic amphotericin B (600 μg ml−1). Patch pipettes were initially front-filled by dipping into pipette solution, and then back-filled with the amphotericin B-containing solution. Pipettes were pulled from borosilicate glass capillary tubing (1.5 mm outer diameter, 1.17 mm inner diameter; Clark Medical Instruments) to a tip of diameter approximately 1–1.5 μm and resistance of 2–4 mΩ.
Voltage-clamp commands were delivered via an Axopatch 1D patch clamp amplifier (Axon Instruments) and membrane currents were recorded by a 12-bit AD/DA converter (Axodata 1200 or Labmaster, Scientific Solutions) interfaced to an Intel computer running pClamp software. During experiments, the dish containing the cells was continuously perfused with Hanks solution at 36 ± 1°C. Additionally the cell under study was continuously superfused by means of a custom-built close delivery system with a pipette of tip diameter 200 μm placed approximately 300 μm from the cell. The Hanks solution in the close delivery system could be switched to a drug-containing solution with a dead space time of less than 5 s. In all experiments, n refers to the number of cells studied, and each experimental set usually contained samples from a minimum of four animals. Summary data are presented as the mean ± standard error (s.e.m.), and statistical comparisons were made on raw data using Students' paired t test, taking P < 0.05 level as significant. In all figures *represents P < 0.05 and ** represents P < 0.01. The frequency of spontaneous transient inward currents (STICs) was obtained by measuring events that had amplitudes greater or equal to 100 pA and durations greater or equal to 500 ms. Similarly, any spontaneous transient depolarisation (STD) that had a duration of less than 500 ms was excluded from the analysis.
Ca2+ imaging of single ICCs
After isolation of single cells, these were plated onto glass-bottomed dishes (WillCo) and left to stick down for 30 min on the microscope stage. They were then incubated in the dark for 15 min at room temperature with an acetoxymethylester form of the Ca2+-sensitive fluorescent dye Fluo4-AM (2 μm) and washed with warmed normal Hanks solution for 30 min prior to experimentation.
Cells were maintained at 37°C in normal Hanks solution and imaged using either an iXon 887 EMCCD camera (Andor Technology, Belfast; 512 × 512 pixels, pixel size 16 × 16 μm) coupled to a Nipkow spinning disk confocal head (CSU22, Yokogawa, Japan) or a MegaXR10 GenIII+ICCD (Stanford Photonics, USA; 1024 × 1000 pixels, pixel size 7 × 7 μm) attached to a Nipkow spinning disk confocal head (CSU10, Visitech UK). A krypton-argon laser (Melles Griot UK) at 488 nm was used to excite the Fluo-4, and the emitted light was detected at wavelengths >510 nm. Images were usually acquired at 5 or 15 frames s−1 and analysed using custom-written macros for Image J. These macros were kindly written by Dr Tony Collins, Wright Cell Imaging Facility, University Health Network, Toronto, Canada.
Image analysis
To analyse the data from confocal microscopy experiments, the mean camera background was first subtracted. Images were then normalized to obtain F/F0 by dividing the entire image by the mean intensity of the cell during a quiescent phase (i.e. the minimum fluorescence obtained between waves). Whole cell wave intensity was determined by drawing a region of interest (ROI) around the cell and plotting out the mean intensity.
To obtain post hoc linescan images, a 1 pixel-thick line was drawn centrally through the entire length of the cell and the ‘reslice’ command in Image J was invoked.
To measure wave propagation, the F/F0 images were thresholded to 75% of the peak wave intensity, and the distance at which the wave remained above threshold was taken as the propagation distance.
Solutions
The composition of the solutions used was as follows (mm): (1) Hanks solution: 129.8 Na+, 5.8 K+, 135 Cl−, 4.17 HCO3−, 0.34 HPO42−, 0.44 H2PO4−, 1.8 Ca2+, 0.9 Mg2+, 0.4 SO42−, 10 glucose, 2.9 sucrose and 10 Hepes, pH adjusted to 7.4 with NaOH. (2) Cs+ perforated patch pipette solution: 133 Cs+, 135 Cl−, 1.0 Mg2+, 0.5 EGTA, 10 Hepes, pH adjusted to 7.2 with CsOH. (3) Krebs solution: 146.2 Na+, 5.9 K+, 133.3 Cl−, 25 HCO3−, 1.2 H2PO4−, 2.5 Ca2+, 1.2 Mg2+ and 11 glucose, pH maintained at 7.4 by bubbling with 95% O2, 5% CO2. (4) Ca2+-free Hanks solution: 129.8 Na+, 5.8 K+, 135 Cl−, 4.17 HCO3−, 0.34 HPO42−, 0.44 H2PO4−, 2.7 Mg2+, 0.4 SO42−, 10 glucose, 2.9 sucrose, 5 EGTA and 10 Hepes, pH adjusted to 7.4 with NaOH.
Drugs used
The following drugs were used: amphotericin B (Sigma), 3-(5-hydroxymethyl-2-furyl)-1-benzyl indazole (YC-1, 100 mm stock in H2O) diethylamine nitric oxide (DEA-NO, Tocris; 100 mm stock in 10 mm NaOH), 8-Br-cGMP (Tocris, 100 mm stock in H2O), β-Phenyl-1, N2-etheno-8-bromoguanosine-3′, 5′-cyclic monophosphorothioate- (SP-8-Br-PET-cGMPs, Biolog, 10 mM stock in H2O), noradrenaline (Levophed, Zanofi Winthrop, added directly to Hanks), caffeine (Sigma, added directly to Hanks), 2-aminoethoxy diphenyl-borate (2-APB, Acros, 100 mm stock in 50% ethanol), 2-nitro-4-carboxyphenyl-N,N-diphenylcarbamate (NCDC, Sigma, 100 mm stock in DMSO). All drugs were diluted to their final concentrations in Hanks solution. Drug vehicles had no effect on the currents or Ca2+ oscillations studied.
Results
Effect of DEA -NO on spontaneous electrical activity of isolated ICC
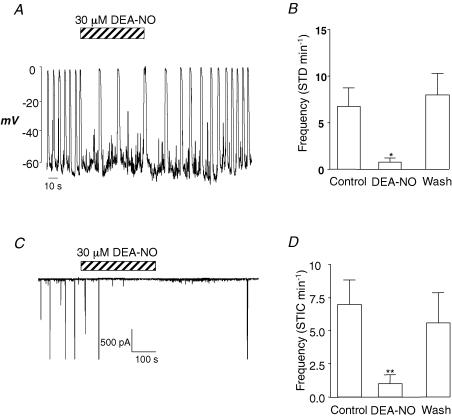
When isolated ICCs were held under current clamp with Cs+-rich pipettes, a small background hyperpolarizing current had to be injected to bring membrane potential to ∼ −60 mV. In the absence of any drugs, the ICC in Fig. 1A, fired STDs at a frequency of 12 min−1. Upon application of DEA-NO (30 μm), the frequency of STDs was reduced to ∼2 min−1, and returned towards control upon washout. Figure 1B shows a summary of four similar experiments in which the frequency of STDs was measured before, during and after washout of DEA-NO. Application of the NO donor significantly reduced STD frequency from 6.8 ± 1.9 min−1 to 0.8 ± 0.5 min−1 (P < 0.05).
Figure 1. DEA-NO reversibly inhibits slow waves and STICs in urethral ICC.
A, the effect of DEA-NO (30 μm) on a spontaneously active single ICC under current-clamp conditions. B, summary data of the effects of DEA-NO on STD frequency in four experiments recorded under the same conditions as A. C, a typical effect of DEA-NO on STICs recorded from an ICC held under voltage clamp at −60 mV. D, a summary of the effect of DEA-NO on STIC frequency, obtained from six experiments recorded under the same conditions as C.
To test the effects of NO on spontaneous transient inward currents (STICs), which, it has been previously shown, underlie STDs (Sergeant et al. 2000), we examined the effects of DEA-NO on STICs in ICC held at −60 mV under voltage-clamp conditions. As Fig. 1C suggests DEA-NO (30 μm) rapidly and reversibly abolished the STICs. In nine similar experiments (Fig. 3D), DEA-NO significantly reduced the frequency of STICs from 7 ± 1.8 to 1 ± 0.6 min−1 (P < 0.01). Upon washout, the frequency of STICs returned 5.6 ± 2.3 min−1, which was not significantly different from the control.
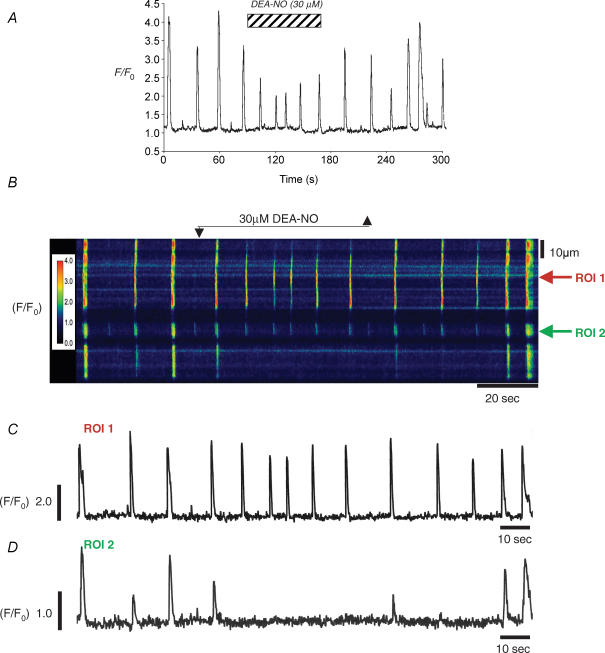
Figure 3. DEA-NO reduces whole-cell Ca2+ waves by reducing their spatial spread.
A, a plot of whole-cell fluorescence before during and after application of DEA-NO. B, a psuedo-line scan of the same cell demonstrating that DEA-NO reversibly reduced wave spread. C, the oscillations at the site of origin were little affected by DEA-NO application, but they failed to spread to adjacent regions (D).
Effect of activating cGMP on spontaneous electrical activity in isolated ICC
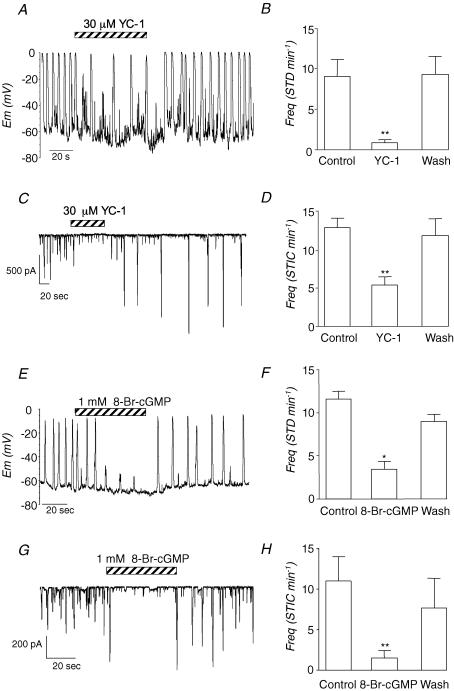
Since NO is thought to mediate its effects via the cGMP/PKG pathway, we next assessed if the effects of DEA-NO could be mimicked by the membrane-permeant activator of soluble guanylyl cyclase, YC-1 and the membrane permeant-analogue of cGMP, 8-Br-cGMP. As Fig. 2A–D suggests, activation of guanylyl cyclase with YC-1 (30 μm) decreased the frequency of STDs and STICs in ICCs in a manner similar to that observed with DEA-NO. Figure 2B and D shows summary data from a series of experiments in which the frequency of STDs and STICs, respectively, was measured before, during and after application of YC-1. The frequency of STDs was reduced from 9.0 ± 2.1 min−1 to 0.8 ± 0.5 min−1 (n = 5, P < 0.01). Similarly, STIC frequency was reversibly reduced in six experiments, from 12.8 ± 1.2 min−1 to 5.3 ± 1.4 min−1 in the presence of YC-1 (P < 0.01).
Figure 2. Activators of the cGMP/GK pathway inhibit electrical activity in ICC.
A and C, the effects of the GC activator YC-1 (30 μm) on STDs and STICS recorded under current clamp and voltage clamp, respectively. B and D, summary data of the effect of YC-1 on STD and STIC frequency obtained from five and six cells, respectively. E and G, the inhibitory effects of the membrane-permeant analogue of cGMP, 8-Br-cGMP (1 mm) on STDs and STICS recorded under current clamp and voltage clamp, respectively. F and H, summary data of the effect of 8-Br-cGMP on STDs and STICS obtained from four cells recorded under the same conditions as those in E and G, respectively.
When the effects of 8-Br-cGMP (1 mm) were examined (Fig. 2E–H), the frequency and amplitude of both STDs (Fig. 2E) and STICs (Fig. 2G) were reversibly reduced. Figure 2F and H shows summaries of four experiments in which 8-Br-cGMP decreased STD and STIC frequency from 11.6 ± 3.4 to 0.9 ± 1 (P < 0.01) and from 11 ± 3 min−1 to 1.5 ± 0.9 min−1, respectively (P < 0.05).
Effect of activating the GC/cGMP pathway on spontaneous Ca2+ events
In an attempt to understand how activating the cGMP/PKG-dependent pathway could alter the Ca2+ events underlying STICs in ICCs, we carried out a series of experiments on freshly dispersed ICCs loaded with the Ca2+ indicator Fluo4-AM. Under control conditions the Ca2+ events were quite variable from cell to cell as noted previously (Johnston et al. 2005). In approximately 50% of the cells studied, spontaneous Ca2+ waves were observed. These occurred at a mean frequency of 4.1 ± 0.5 min−1, had a mean amplitude of 4.4 ± 0.4 F/F0, full duration at half maximum amplitude (FDHM) of 2.9 ± 0.4 s, a mean spatial spread of 112 ± 12 μm, and a mean propagation speed of 27 ± 0.4 μm s−1 (n = 14). Since these events underlie the generation of STICs in urethral ICCs (Johnston et al. 2005), we focused on studying these waves in the present study.
Effect of DEA-NO on spontaneous Ca2+ events
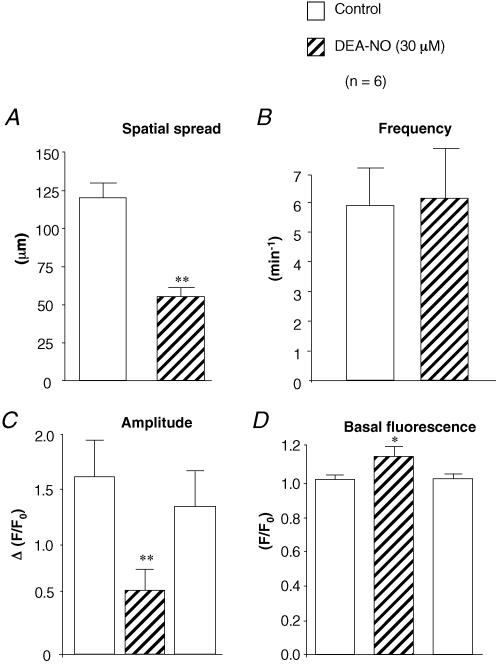
Figure 3 shows a typical example of an experiment in which the effect of DEA-NO was examined on a spontaneously active ICC recorded at 5 frames per second (fps). Figure 3A shows a plot of whole-cell fluorescence against time, and demonstrates that application of DEA-NO (30 μm) reduced the amplitude of the Ca2+ waves but had little effect on their frequency. In 17 similar experiments DEA-NO reduced the amplitude of the waves (ΔF/F0) from 1.62 ± 0.34 to 0.58 ± 0.13. The reason for the reduction in amplitude became apparent when the spatial spread of the Ca2+ waves was examined. Figure 3B shows a post hoc pseudo linescan (PLS) obtained by measuring the peak intensity of a 1 pixel-wide line drawn through the centre of the cell along its entire longitudinal axis and plotting data against time. As shown in Fig. 3B the results suggest that under control conditions the Ca2+ waves spread ∼80 μm. In the presence of 30 μm DEA-NO, spatial attenuation of the waves occurred. This effect was reversible upon washout. Figure 3C and D are profile plots through ROI 1, close to the site where the wave originated and ROI 2 which was 60 μm distal to the site of wave origin. Prior to drug application, two of the three control waves propagated without significant decrement from ROI 1 to ROI 2. However, in the presence of DEA-NO, although ROI 1 continued to fire (at a slightly increased frequency and reduced amplitude, in this example), none of these events were propagated to ROI 2. In six similar experiments summarized in Fig. 4, DEA-NO reversibly decreased the mean wave spread from 121 ± 10 μm to 55 ± 6 μm (P < 0.01), increased the basal fluorescence, and reduced the mean amplitude of the whole cell waves. However, the frequency of waves was unaffected by DEA-NO (5.9 ± 1.3 compared to 6.2 ± 1.2 min−1).
Figure 4. Summary data of the effect of DEA-NO.
Summary barcharts showing the effect of DEA-NO (30 μm) on mean wave spread (μm) (A), mean frequency (min−1) (B), mean whole cell wave amplitude (ΔF/F0) (C) and mean basal Ca2+ fluorescence (F/F0) (D) in 6 cells. Experiments were performed under the same conditions as those shown in Fig. 3.
Effect of 8-Br-cGMP and YC-1 on spontaneous Ca2+ events
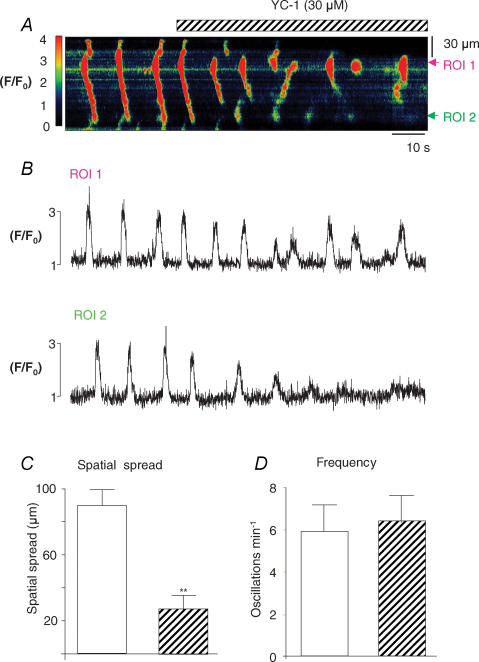
The reduction in spatial spread of the waves observed in the presence of DEA-NO was also apparent when either YC-1 or 8-Br-cGMP was applied. Figure 5A shows a typical example of the effects of activating a guanylyl cyclase (GC) with YC-1 (30 μm). Under control conditions the cell fired propagating waves, but upon application of YC-1 a reduction in the spatial spread of the Ca2+ wave was apparent without any significant reduction in wave frequency. Figure 5B shows profile plots of the fluorescence taken from two ROIs spaced 60 μm apart. In the absence of any drugs, the wave propagated without significant reduction in amplitude from ROI 1 to ROI 2. Although the waves still initiated close to ROI 1 in the presence of YC-1, they failed to propagate to ROI 2. Figure 5C and D shows summary data from seven experiments in which spatial spread was reduced from 89.7 ± 9.2 μm to 27.8 ± 5.5 μm (P < 0.01) by YC-1. However, frequency was little changed (5.9 ± 1.3 min−1 under control conditions compared to 6.1 ± 1.2 min−1 in the presence of YC-1).
Figure 5. The effect of activating GC on Ca2+ waves.
A, a line scan image of global Ca2+ waves in the absence and presence of YC-1 (30 μm). B, two profile plots taken from ROI 1 and ROI 2. C, summary graphs of the effects of YC-1 on mean Ca2+ spread (μm) and D, mean frequency (min−1).
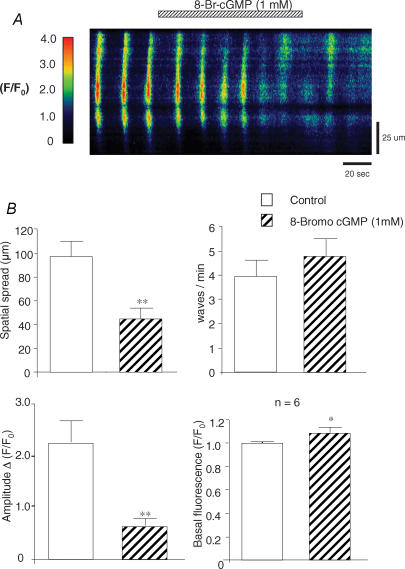
Figure 6A shows an example of spontaneous waves before and during application of 8-Br-cGMP (1 mm). Under control conditions, waves propagated throughout the cell (Fig. 6A). Application of 8-Br-cGMP increased basal fluorescence and reduced the spatial spread of the waves without affecting wave frequency. Summary data from six similar experiments is shown in Fig. 6B, where 8-Br-cGMP reduced the mean amplitude of the whole-cell waves, decreased the spatial spread of the waves from 98 ± 12 to 45 ± 9 μm, but again failed to significantly alter wave frequency (3.9 ± 0.7 min−1 before compared to 4.9 ± 0.7 min−1 during cGMP). In approximately 30% of experiments 8-Br-cGMP evoked a transient release of Ca2+ upon application, followed by a transient inhibition of spontaneous activity. These data were excluded from the above analysis.
Figure 6. The effect of 8-Br-cGMP on Ca2+ waves.
A line scan image showing global Ca2+ waves in the absence and presence of 8-Br-cGMP (1 mm). B, summary graphs showing the effects of cGMP on mean Ca2+ spread (μm), mean frequency (min−1), mean amplitude (ΔF/F0) and basal Ca2+ fluorescence (F/F0) n = 6.
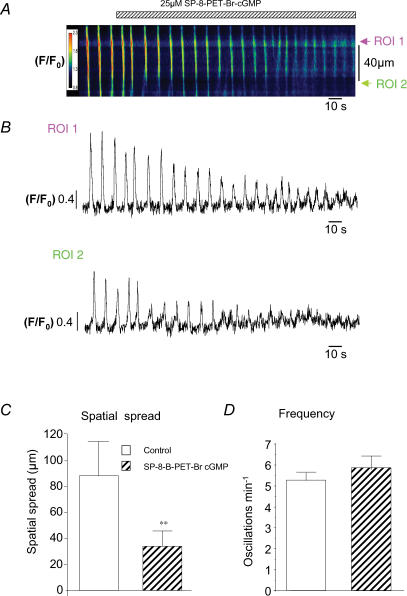
cGMP is thought to mediate its inhibitory effects via activation of PKG in the urethra. Therefore we tested if the PKG agonist, SP-8-Br-PET-cGMPs, produced similar effects to those obtained with DEA-NO, 8-Br-cGMP and YC-1. Figure 7A shows the effect of 25 μm SP-8-Br-PET-cGMPs on spontaneous Ca2+ waves. In its presence, both the amplitude and spatial spread of waves were decreased, but again, frequency was little affected. In Fig. 7B, the mean fluorescence was measured and plotted against time at two ROIs placed 40 μm apart. In this example, the amplitude of the wave at the initiation site was also reduced. Figure 7C and D shows summary data for five experiments in which application of SP-8-Br-PET-cGMPs (25 μm) decreased spatial spread from 89 ± 26 μm to 35 ± 12 μm (P < 0.01), but had little effect on Ca2+ wave frequency (5.3 ± 0.5 min−1 compared to 5.9 ± 0.5 min−1 P = 0.21).
Figure 7. The effect of the G-kinase activator SP-8-Br-PET-cGMPson Ca2+ waves.
A, a pseudo-line scan image of propagated Ca2+ waves in the absence and presence of the G-kinase activator SP-8-Br-PET-cGMPs (25 μm). B, profile plots taken from the two specific regions in the cell (labelled ROI 1 and ROI 2 in A placed 30 μm apart. C and D, summary data to demonstrate that G-kinase activation significantly reduced wave spatial spread but had no significant effect on wave frequency, respectively.
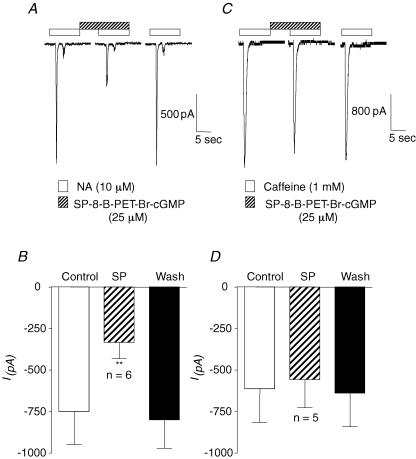
Since release of Ca2+ from RyRs and IP3Rs plays an important role in the initiation and propagation of Ca2+ waves in urethral ICC (Johnston et al. 2005), we next tested if Ca2+ release from these stores was affected by activation of the cGMP/PKG pathway. Noradrenaline (NA, 10 μm) and caffeine (1 mm) were used to induce Ca2+ release via IP3Rs and RyRs, respectively, in cells held under voltage clamp at −60 mV. Under control conditions, NA application for 10 s induced a transient Ca2+-activated Cl− current, due to release of Ca2+ via IP3Rs (Sergeant et al. 2002). As Fig. 8A suggests, activation of PKG with SP-8-Br-PET-cGMPs (25 μm) reversibly reduced the amplitude of these currents. Summary data from six similar experiments shown in Fig. 8B demonstrate that PKG activation decreased NA-evoked Cl− currents from −750 ± 198 pA to −333 ± 98 pA (P < 0.01). In contrast, as Fig. 8C demonstrates, SP-8-Br-PET-cGMPs (25 μm) had little effect on Ca2+ release via RyRs evoked by 1 mm caffeine (Sergeant, 2000). The bar chart in Fig. 8D shows summary data from five similar experiments. The mean amplitude of the caffeine-evoked currents was −614 ± 203 pA under control conditions compared to −557 ± 169 pA in SP-8-Br-PET-cGMPs (P = 0.2). Ca2+ release evoked by 1 mm caffeine was unaffected in the presence of 2-APB (100 μm, n = 4), suggesting that this concentration of caffeine did not cause release of Ca2+ from IP3Rs. In four separate experiments, we also examined the effects of SP-8-Br-PET-cGMPs (25 μm) on Cl− currents evoked by 10 mm caffeine in cells held at −60 mV. Under control conditions the peak current evoked by 10 mm caffeine was −1027 ± 256 pA, compared to −1049 ± 371 pA in the presence of SP-8-Br-PET-cGMPs. These data suggest that activation of PKG inhibits release of Ca2+ from IP3Rs but not RyRs. Furthermore, the inability of SP-8-Br-PET-cGMPs to inhibit Cl− currents activated by caffeine suggests that the PKG activator does not directly inhibit Cl− channels.
Figure 8. Activating PKG inhibits Ca2+ release from IP3Rs but not RyRs.
A shows NA-evoked currents recorded under voltage clamp at −60 mV, before, during and after activation of PKG with SP-8-Br-PET-cGMPs; 80 s intervals were left between successive intervals of NA. B, summary data for the effect of SP-8-Br-PET-cGMPs on NA-evoked currents. C, caffeine-evoked currents before, during and after activation of PKG with SP-8-Br-PET-cGMPs. Caffeine was applied at 80 s intervals. D, summary data for the effect of SP-8-Br-PET-cGMPs on caffeine-evoked currents.
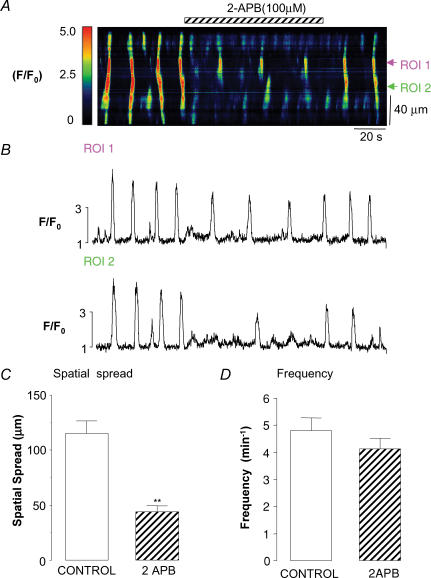
The data above support the idea that activators of PKG modulate Ca2+ release via IP3Rs. We therefore tested the effect of inhibiting IP3Rs with 2-APB on spontaneous Ca2+ waves, to examine if it mimicked the effect of activating the cGMP/PKG pathway. Figure 9A shows a typical example of one such experiment in which Ca2+ waves were recorded at 15 fps. After the cell had fired four waves, 2-APB (100 μm) was applied. Although the cell still produced oscillations, their spatial spread was markedly attenuated. The frequency of waves was reduced in this experiment, but in 11 similar experiments it was not significantly altered (from 4.8 ± 0.5 min−1 under control conditions compared to 4.1 ± 0.4 min−1 in 2-APB, P = 0.14, Fig. 9D). However, in the same 11 experiments, application of 2-APB significantly reduced the spatial spread of the Ca2+ waves from 115 ± 11 μm to 44 ± 5 μm (P < 0.01, Fig. 9C). In a separate series of experiments, we also examined the effects of inhibiting phospholipase C with 100 μm NCDC. In six cells, NCDC application significantly decreased the spatial spread of the Ca2+ waves from 121 ± 19 μm to 45 ± 9 μm (P < 0.01) without affecting wave frequency or basal Ca2+. The above data are consistent with the idea that activation of the cGMP/PKG pathway inhibits Ca2+ release via IP3Rs.
Figure 9. Inhibition of Ca2+ release via IP3Rs mimics the effect of NO on Ca2+ waves.
A, pseudo-line scan image of propagated Ca2+ waves in the absence and presence of the IP3R inhibitor 2-APB (100 μm). B, two profile plots taken from two ROIs placed 40 μm apart. C and D, summaries of the effect of 2-APB on spatial spread and wave frequency.
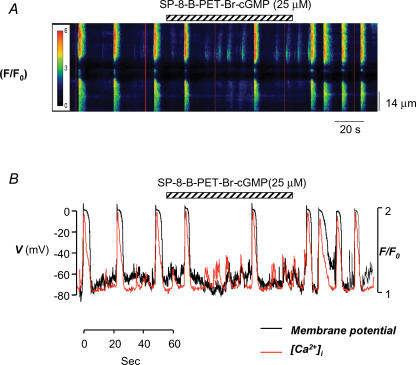
Figure 10 shows recordings from an ICC in which we simultaneously recorded membrane potential and Ca2+ waves before and during SP-8-Br-PET-cGMPs application. Hyperpolarizing background current was injected to bring membrane potential to ∼ −70 mV. The red vertical lines in Fig. 10A are artifacts caused by the regular emission of an LED used to synchronize the fluorescence capture and electrophysiological recordings. Figure 10B shows a plot of whole-cell fluorescence (in red) and membrane potential (in black) from the same experiment. Prior to PKG activation, the cell produced large phasic increases in Ca2+, which triggered full STDs. Occasionally smaller Ca2+ release events were detected. These produced small-amplitude transient fluctuations in membrane potential that depolarized the cell by <25 mV, but failed to reach threshold (−40 mV) to activate the L-type Ca2+ channels and elicit full STDs. In the presence of SP-8-Br-PET-cGMPs, the Ca2+ waves were clearly attenuated, and small-amplitude Ca2+ release events that spread < 20 μm were apparent. In this experiment, one Ca2+ event was sufficiently large to depolarize the cell to ∼ −40 mV, and elicited a large STD after approximately 60 s exposure to SP-8-Br-PET-cGMPs. Upon washout of SP-8-Br-PET-cGMPs, wave amplitude and STD frequency returned towards control values.
Figure 10. PKG activation reduces STD frequency by inhibiting wave spread.
A, pseudo-line scan image of a spontaneously active ICC loaded with Fluo4-AM and held under current-clamp conditions. Application of SP-8-Br-PET-cGMPs (25 μm) reduced the frequency of Ca2+ oscillations and unmasked smaller Ca2+ release events. B, a plot of whole-cell fluorescence (red) and membrane potential (black) before during and after application SP-8Br-PET-cGMPs.
Discussion
Although the cGMP/GK1 pathway contributes to NO-mediated relaxations in the urethra, little is known about the cellular targets for cGMP/GK1, or how NO affects electrical activity in the urethra. In vascular smooth muscle, NO is thought to activate K+ channels either directly (Bolotina et al. 1994) or indirectly via stimulation of the cGMP/PKG pathway (Carrier et al. 1997). Consequently, membrane hyperpolarization occurs, Ca2+ influx is reduced, and relaxation occurs (Carrier et al. 1997; Heppner et al. 2003). In the urethra however, the neurogenic relaxation appears to be resistant to K+ channel blockade (Costa et al. 2001), suggesting that these channels contribute little to NO-induced relaxation. Intracellular recordings by Waldeck et al. (1998) and Hashitani et al. (1996) have similarly failed to demonstrate any change in membrane potential induced by either NO donors or cGMP analogues in rabbit urethral circular muscle, suggesting that NO does not act by hyperpolarizing the tissue. However, the latter study demonstrated that sodium nitroprusside and 8-Br-cGMP reduced the frequency of STDs recorded from rabbit urethral circular smooth muscle.
Since this spontaneous activity is thought to arise from ICC in the urethra (Sergeant et al. 2000) it is conceivable that neurotransmitters may mediate their effects on tone by modulating activity in these cells. Burns et al. (1996) and Beckett et al. (2002) have provided compelling evidence to suggest that ICC play an important role in neurotransmission in the gastrointestinal tract, since knockout mice lacking ICC have severely attenuated neurogenic responses. A number of studies suggest that ICC may also contribute to neurotransmission in the urethra. Previous results from our laboratory demonstrate that urethral ICC respond to exogenous NA application, and when these ICC are pharmacologically ablated, the contractile responses to adrenergic neurotransmission are abolished (Sergeant et al. 2002). Smet et al. (1996) have provided immunohistochemical evidence in the guinea-pig and human urethra, that cells with a morphological appearance similar to ICC in the gut, may also play a role in inhibitory neurotransmission. Their demonstration that ICC were immunopositive for cGMP following incubation of the tissues with NO donors, suggests that these cells possess the biochemical pathways to respond to NO.
The results of the present study are consistent with the findings of Smet et al. (1996) and demonstrate that spontaneous electrical activity in ICC is inhibited by activators of the cGMP/PKG pathway. Thus, DEA-NO, YC-1 and 8-Br-cGMP reversibly decreased the frequency of STDs in cells studied under current clamp. Similarly, under voltage-clamp conditions, the amplitude and frequency of STICs were also reduced by activators of the cGMP/PKG pathway. A number of possibilities could account for these inhibitory effects on electrical activity: (1) Ca2+ influx was reduced; (2) Cl− channels were directly inhibited; or (3) Ca2+ mobilization from intracellular stores was altered. Although a number of studies have demonstrated that NO can inhibit Ca2+ influx through either voltage-gated Ca2+ channels (Blatter & Wier, 1994; Grassi et al. 2004) or capacitative Ca2+ entry (CCE) pathways (Trepakova et al. 1999), this is unlikely to account for our observations for a number of reasons. Firstly, inhibition of L-type Ca2+ channels in urethral ICC has no effect on the frequency of either STDs or STICs (Sergeant et al. 2000). Although complete inhibition of T-type Ca2+ channels with 100 μm Ni2+ has been demonstrated to decrease the frequency of STICs in urethral ICC (Bradley et al. 2005), the effects were rather modest (∼25% reduction). It is equally unlikely that inhibition of CCE is responsible, since blockade of CCE with either Gd3+ or La3+ failed to significantly alter STIC frequency in urethral ICC (Bradley et al. 2005).
The ability of caffeine to evoke robust Cl− currents in the presence of SP-8-Br-PET-cGMPs suggests that PKG activation did not directly block the Cl− currents in these cells. Furthermore, since caffeine is thought to enhance release of Ca2+ from the sarcoplasmic reticulum (SR) via RyR activation (Zucchi & Ronca-Testoni, 1997), it appears that Ca2+ release through these channels remained intact in the presence of the PKG agonist. In contrast, when Ca2+ release via IP3Rs was triggered with exogenous noradrenaline (Sergeant et al. 2002), the amplitude of the evoked current was significantly reduced by SP-8-Br-PET-cGMPs. These data suggest that activation of PKG may specifically interfere with release of Ca2+ from IP3R receptors, as has been suggested in rabbit corpus cavernosal and guinea-pig ileal myocytes (Craven et al. 2004; Chung et al. 2005).
To further examine how activation of the NO/cGMP pathway altered Ca2+ mobilization from intracellular stores, we carried out experiments on ICC loaded with Fluo4-AM. We found that the ICC displayed a variety of Ca2+ events, the most common consisting of spontaneous propagating Ca2+ waves as noted previously (Johnston et al. 2005). We previously demonstrated that these spontaneous waves were abolished by either ryanodine or tetracaine, suggesting that RyR activation was critical for the initiation of Ca2+ waves in ICC (Johnston et al. 2005). In contrast, the IP3R blocker 2-APB (Maruyama et al. 1997), reduced the amplitude of Ca2+ waves, indicating that the IP3Rs serve to amplify the initial Ca2+ signal and permit the propagation of waves (Johnston et al. 2005). 2-APB has been shown to be unselective in a number of studies, and has been demonstrated to block Ca2+ influx, and inhibit Ca2+ uptake in addition to its inhibitory effects on Ca2+ release on IP3R (Peppiat et al. 2003). However, we have found little evidence to suggest that it produces these effects in rabbit urethral ICC. Thus, it selectively blocks STICS but not STOCs in these cells, suggesting that it does not block Ca2+ release from RyRs but does inhibit release via IP3Rs (Sergeant et al. 2001). Similarly, it does not block caffeine-evoked Cl− currents, but does block IP3R-mediated Ca2+ release induced by noradrenaline (Sergeant et al. 2002) We have demonstrated that 2-APB can modestly block capacitative Ca2+ entry (20%) in urethral ICC (Bradley et al. 2005); other more potent blockers of CCE have little effect on spontaneous activity suggesting that CCE plays little role in modulating the spontaneous activity observed.
Although application of DEA-NO failed to reduce the frequency of the Ca2+ oscillations, it dramatically reduced whole-cell wave amplitude. These effects were mimicked by both 8-Br-cGMP and SP-8-Br-PET-cGMPs, supporting the idea that NO mediates its effects via cGMP and GK1 as suggested by Persson et al. (2000). The reduction in whole-cell wave amplitude was largely explained by a significant decrease in the spatial spread of waves. In some examples the wave amplitude at the initiation site was also reduced (see Fig. 7A). Although this latter effect could contribute to the reduction in the spatial spread of the waves, the fact that spatial attenuation was often observed without any significant reduction in the amplitude of the wave at the initiation site (see Fig. 3B & C), suggests that the cGMP/PKG pathway can inhibit wave propagation independently of an effect on the primary pacemaker Ca2+-release site. However, given that RyRs appear not to be modulated by cGMP/PKG in our cells, the above data also suggest that a subpopulation of primary pacemaker Ca2+ release sites consist of clusters of both RyRs and IP3Rs as has been suggested in hippocampal neurons (Koizumi et al. 1999), arterial smooth muscle (Boittin et al. 1998) and cardiac myocytes (Lipp et al. 2000).
Activation of the NO/cGMP pathway produced effects on Ca2+ waves that were similar to those of IP3R blockade with 2-APB or inhibition of phospholipase C (PLC) with NCDC. These data are consistent with the idea that NO may mediate its effects on urethral ICC by modulating Ca2+ release from IP3Rs or by inhibiting IP3 production. A number of studies have demonstrated that PKG can either reduce IP3 production (Murthy et al. 1993; Ruth et al. 1993; Ding & Abdel-Latif, 1997) or inhibit Ca2+ release from IP3Rs (Komalavilas & Lincoln, 1996; Tertyshnikova et al. 1998; Feil et al. 2002; Murthy & Zhou, 2003). The mechanism through which PKG inhibits Ca2+ release via IP3Rs is a matter of debate and varies between tissues. Murthy & Zhou (2003) suggested that PKG1α directly phosphorylated IP3Rs in gastric smooth muscle cells to inhibit Ca2+ release. However, Schlossmann et al. (2000) have argued that PKG1β first phosphorylates a novel protein – inositol 1,4,5-trisphophate receptor-associated cGMP kinase substrate (IRAG), which in turn modulates the IP3R to reduce Ca2+ release. In support of this, Fritsch et al. (2004) found that NO/PKG-dependent inhibition of Ca2+ signalling in human colonic myocytes was abolished in cells treated with antisense oligonucleotides raised against IRAG, suggesting that IRAG was essential for mediating the effects of NO in these myocytes. Whether a similar mechanism is responsible for the inhibitory effects of NO in the urethra remains to be determined.
Regardless of how activation of the cGMP/PKG pathway inhibits Ca2+ release via IP3Rs, it is tempting to speculate on how decreased Ca2+ wave amplitude leads to a decrease in STD frequency. We hypothesize that a reduction in the spatial spread of the Ca2+ signal in urethral ICCs would effectively limit the coupling between [Ca2+]i and the Cl− channels. As a consequence of this functional uncoupling, each Ca2+ oscillation fails to activate sufficient Cl− channels to depolarize the ICC and bring it into the range to fire STDs. This prevents activation of voltage-dependent Ca2+ channels, and limits the propagation of the pacemaking signal to the smooth muscle. In the absence of such a pacemaking ‘drive’, the ICC fail to rhythmically depolarize the smooth muscle and thus, the smooth muscle fails to fire action potentials. This decrease in action potential firing frequency in the smooth muscle leads to a reduction in smooth muscle cell [Ca2+]I, and ultimately reduces urethral tone.
Acknowledgments
The authors acknowledge the financial support of NIDDK (Grant R01-DK68565) and the Wellcome Trust (Grant: 064212). Louise Johnston was in receipt of a DEL (NI) Post Graduate Studentship and Gerard Sergeant is in receipt of a Health Research Board Post-Doctoral Fellowship. The authors would like to thank Dr Tony Collins, Wright Cell Imaging Facility, Toronto, Canada, for writing the Image J macros used in the data analysis.
References
- Andersson KE, Wein AJ. Pharmacology of the lower urinary tract: basis for current and future treatments of urinary incontinence. Pharmacol Rev. 2004;56:581–631. doi: 10.1124/pr.56.4.4. [DOI] [PubMed] [Google Scholar]
- Beckett EA, Horiguchi K, Khoyi M, Sanders KM, Ward SM. Loss of enteric motor neurotransmission in the gastric fundus of Sl/Sl(d) mice. J Physiol. 2002;543:871–887. doi: 10.1113/jphysiol.2002.021915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blatter LA, Wier WG. Nitric oxide decreases [Ca2+]i in vascular smooth muscle by inhibition of the calcium current. Cell Calcium. 1994;15:122–131. doi: 10.1016/0143-4160(94)90051-5. [DOI] [PubMed] [Google Scholar]
- Boittin FX, Coussin F, Macrez N, Mironneau C, Mironneau J. Inositol 1,4,5-trisphosphate- and ryanodine-sensitive Ca2+ release channel-dependent Ca2+ signalling in rat portal vein myocytes. Cell Calcium. 1998;23:303–311. doi: 10.1016/s0143-4160(98)90026-4. [DOI] [PubMed] [Google Scholar]
- Bolotina VM, Najibi S, Palacino JJ, Pagano PJ, Cohen RA. Nitric oxide directly activates calcium-dependent potassium channels in vascular smooth muscle. Nature. 1994;368:850–853. doi: 10.1038/368850a0. [DOI] [PubMed] [Google Scholar]
- Bradley E, Hollywood MA, McHale NG, Thornbury KD, Sergeant GP. Pacemaker activity in urethral interstitial cells is not dependent on capacitative calcium entry. Am J Physiol Cell Physiol. 2005;289:C625–C632. doi: 10.1152/ajpcell.00090.2005. [DOI] [PubMed] [Google Scholar]
- Burns AJ, Lomax AE, Torihashi S, Sanders KM, Ward SM. Interstitial cells of Cajal mediate inhibitory neurotransmission in the stomach. Proc Natl Acad Sci U S A. 1996;93:12008–12013. doi: 10.1073/pnas.93.21.12008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrier GO, Fuchs LC, Winecoff AP, Giulumian AD, White RE. Nitrovasodilators relax mesenteric microvessels by cGMP-induced stimulation of Ca-activated K channels. Am J Physiol. 1997;273:H76–H84. doi: 10.1152/ajpheart.1997.273.1.H76. [DOI] [PubMed] [Google Scholar]
- Chung SS, Ahn DS, Lee HG, Lee YH, Nam TS. Inhibition of carbachol-evoked oscillatory currents by the NO donor sodium nitroprusside in guinea-pig ileal myocytes. Exp Physiol. 2005;90:577–586. doi: 10.1113/expphysiol.2004.029611. [DOI] [PubMed] [Google Scholar]
- Costa G, Labadia A, Triguero D, Jimenez E, Garcia-Pascual A. Nitrergic relaxation in urethral smooth muscle: involvement of potassium channels and alternative redox forms of no. Naunyn Schmiedebergs Arch Pharmacol. 2001;364:516–523. doi: 10.1007/s002100100480. [DOI] [PubMed] [Google Scholar]
- Craven M, Sergeant GP, Hollywood MA, McHale NG, Thornbury KD. Modulation of spontaneous Ca2+-activated Cl− currents in the rabbit corpus cavernosum by the nitric oxide-cGMP pathway. J Physiol. 2004;556:495–506. doi: 10.1113/jphysiol.2003.058628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding KH, Abdel-Latif AA. Actions of C-type natriuretic peptide and sodium nitroprusside on carbachol-stimulated inositol phosphate formation and contraction in ciliary and iris sphincter smooth muscles. Invest Ophthalmol Vis Sci. 1997;38:2629–2638. [PubMed] [Google Scholar]
- Dokita S, Smith SD, Nishimoto T, Wheeler MA, Weiss RM. Involvement of nitric oxide and cyclic GMP in rabbit urethral relaxation. Eur J Pharmacol. 1994;266:269–275. doi: 10.1016/0922-4106(94)90136-8. [DOI] [PubMed] [Google Scholar]
- Feil R, Gappa N, Rutz M, Schlossmann J, Rose CR, Konnerth A, Brummer S, Kuhbandner S, Hofmann F. Functional reconstitution of vascular smooth muscle cells with cGMP-dependent protein kinase I isoforms. Circ Res. 2002;90:1080–1086. doi: 10.1161/01.res.0000019586.95768.40. [DOI] [PubMed] [Google Scholar]
- Fritsch RM, Saur D, Kurjak M, Oesterle D, Schlossmann J, Geiselhoringer A, Hofmann F, Allescher HD. InsP3R-associated cGMP kinase substrate (IRAG) is essential for nitric oxide-induced inhibition of calcium signalling in human colonic smooth muscle. J Biol Chem. 2004;279:12551–12559. doi: 10.1074/jbc.M313365200. [DOI] [PubMed] [Google Scholar]
- Garcia-Pascual A, Triguero D. Relaxation mechanisms induced by stimulation of nerves and by nitric oxide in sheep urethral muscle. J Physiol. 1994;476:333–347. doi: 10.1113/jphysiol.1994.sp020135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grassi C, D'Ascenzo M, Azzena GB. Modulation of Ca (v), 1 and Ca (v), 2.2 channels induced by nitric oxide via cGMP–dependent protein kinase. Neurochem Int. 2004;45:885–893. doi: 10.1016/j.neuint.2004.03.019. [DOI] [PubMed] [Google Scholar]
- Hashitani H, Van Helden DF, Suzuki H. Properties of spontaneous depolarizations in circular smooth muscle cells of rabbit urethra. Br J Pharmacol. 1996;118:1627–1632. doi: 10.1111/j.1476-5381.1996.tb15584.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heppner TJ, Herrera GM, Bonev AD, Hill-Eubanks D, Nelson MT. Ca2+ sparks and K(Ca) channels: novel mechanisms to relax urinary bladder smooth muscle. Adv Exp Med Biol. 2003;539:347–357. doi: 10.1007/978-1-4419-8889-8_26. [DOI] [PubMed] [Google Scholar]
- Ito Y, Kimoto Y. The neural and non-neural mechanisms involved in urethral activity in rabbits. J Physiol. 1985;367:57–72. doi: 10.1113/jphysiol.1985.sp015814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston L, Sergeant GP, Hollywood MA, Thornbury KD, McHale NG. Calcium oscillations in interstitial cells of the rabbit urethra. J Physiol. 2005;565:449–461. doi: 10.1113/jphysiol.2004.078097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koizumi S, Bootman MD, Bobanovic LK, Schell MJ, Berridge MJ, Lipp P. Characterization of elementary Ca2+ release signals in NGF-differentiated PC12 cells and hippocampal neurons. Neuron. 1999;22:125–137. doi: 10.1016/s0896-6273(00)80684-4. [DOI] [PubMed] [Google Scholar]
- Komalavilas P, Lincoln TM. Phosphorylation of the inositol 1,4,5-trisphosphate receptor. Cyclic GMP-dependent protein kinase mediates cAMP and cGMP dependent phosphorylation in the intact rat aorta. J Biol Chem. 1996;271:21933–21938. doi: 10.1074/jbc.271.36.21933. [DOI] [PubMed] [Google Scholar]
- Lipp P, Laine M, Tovey SC, Burrell KM, Berridge MJ, Li W, Bootman MD. Functional InsP3 receptors that may modulate excitation-contraction coupling in the heart. Curr Biol. 2000;10:939–942. doi: 10.1016/s0960-9822(00)00624-2. [DOI] [PubMed] [Google Scholar]
- Maruyama T, Kanaji T, Nakade S, Kanno T, Mikoshiba K. 2APB, 2-Aminoethoxydiphenyl borate, a membrane-penetrable modulator of Ins(1,4,5)P3 -induced Ca2+ release. J Biochem. 1997;122:498–505. doi: 10.1093/oxfordjournals.jbchem.a021780. [DOI] [PubMed] [Google Scholar]
- Morita T, Tsujii T, Dokita S. Regional difference in functional roles of cAMP and cGMP in lower urinary tract smooth muscle contractility. Urol Int. 1992;49:191–195. doi: 10.1159/000282424. [DOI] [PubMed] [Google Scholar]
- Murthy KS, Severi C, Grider JR, Makhlouf GM. Inhibition of IP3 and IP3-dependent Ca2+ mobilization by cyclic nucleotides in isolated gastric muscle cells. Am J Physiol. 1993;264:G967–G974. doi: 10.1152/ajpgi.1993.264.5.G967. [DOI] [PubMed] [Google Scholar]
- Murthy KS, Zhou H. Selective phosphorylation of the IP3R-I in vivo by cGMP-dependent protein kinase in smooth muscle. Am J Physiol Gastrointest Liver Physiol. 2003;284:G221–G230. doi: 10.1152/ajpgi.00401.2002. [DOI] [PubMed] [Google Scholar]
- Peppiatt CM, Collins TJ, Mackenzie L, Conway SJ, Holmes AB, Bootman MD, Berridge MJ, Seo JT, Roderick HL. 2-Aminoethoxydiphenyl borate (2-APB) antagonises inositol 1,4,5-trisphosphate-induced calcium release, inhibits calcium pumps and has a use-dependent and slowly reversible action on store-operated calcium entry channels. Cell Calcium. 2003;34:97–108. doi: 10.1016/s0143-4160(03)00026-5. [DOI] [PubMed] [Google Scholar]
- Persson K, Andersson KE. Non-adrenergic, non-cholinergic relaxation and levels of cyclic nucleotides in rabbit lower urinary tract. Eur J Pharmacol. 1994;268:159–167. doi: 10.1016/0922-4106(94)90185-6. [DOI] [PubMed] [Google Scholar]
- Persson K, Pandita RK, Aszodi A, Ahmad M, Pfeifer A, Fassler R, Andersson KE. Functional characteristics of urinary tract smooth muscles in mice lacking cGMP protein kinase type I. Am J Physiol Regul Integr Comp Physiol. 2000;279:R1112–R1120. doi: 10.1152/ajpregu.2000.279.3.R1112. [DOI] [PubMed] [Google Scholar]
- Rae J, Cooper K, Gates P, Watsky M. Low access resistance perforated patch recordings using amphotericin B. J Neurosci Meth. 1991;37:15–26. doi: 10.1016/0165-0270(91)90017-t. [DOI] [PubMed] [Google Scholar]
- Ruth P, Wang GX, Boekhoff I, May B, Pfeifer A, Penner R, Korth M, Breer H, Hofmann F. Transfected cGMP-dependent protein kinase suppresses calcium transients by inhibition of inositol 1,4,5-trisphosphate production. Proc Natl Acad Sci U S A. 1993;90:2623–2627. doi: 10.1073/pnas.90.7.2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders KM, Koh SD, Ward SM. Interstitial Cells of Cajal as Pacemakers in the Gastrointestinal Tract. Annu Rev Physiol. 2005;68:307–343. doi: 10.1146/annurev.physiol.68.040504.094718. October 24. [DOI] [PubMed] [Google Scholar]
- Schlossmann J, Ammendola A, Ashman K, Zong X, Huber A, Neubauer G, Wang GX, Allescher HD, Korth M, Wilm M, Hofmann F, Ruth P. Regulation of intracellular calcium by a signalling complex of IRAG, IP3 receptor and cGMP kinase Ibeta. Nature. 2000;404:197–201. doi: 10.1038/35004606. [DOI] [PubMed] [Google Scholar]
- Sergeant GP. PhD Thesis. The Queens University of Belfast; 2000. Specialised Pacemaking Cells in the Rabbit Urethra. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sergeant GP, Hollywood MA, McCloskey KD, Thornbury KD, McHale NG. Specialised pacemaking cells in the rabbit urethra. J Physiol. 2000;526:359–366. doi: 10.1111/j.1469-7793.2000.t01-2-00359.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sergeant GP, Thornbury KD, McHale NG, Hollywood MA. Characterization of norepinephrine-evoked inward currents in interstitial cells isolated from the rabbit urethra. Am J Physiol Cell Physiol. 2002;283:C885–C894. doi: 10.1152/ajpcell.00085.2002. [DOI] [PubMed] [Google Scholar]
- Smet PJ, Jonavicius J, Marshall VR, De Vente J. Distribution of nitric oxide synthase-immunoreactive nerves and identification of the cellular targets of nitric oxide in guinea-pig and human urinary bladder by cGMP immunohistochemistry. Neuroscience. 1996;71:337–348. doi: 10.1016/0306-4522(95)00453-x. [DOI] [PubMed] [Google Scholar]
- Tertyshnikova S, Yan X, Fein A. cGMP inhibits IP3-induced Ca2+ release in intact rat megakaryocytes via cGMP- and cAMP-dependent protein kinases. J Physiol. 1998;512:89–96. doi: 10.1111/j.1469-7793.1998.089bf.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trepakova ES, Cohen RA, Bolotina VM. Nitric oxide inhibits capacitative cation influx in human platelets by promoting sarcoplasmic/endoplasmic reticulum Ca2+-ATPase-dependent refilling of Ca2+ stores. Circ Res. 1999;84:201–209. doi: 10.1161/01.res.84.2.201. [DOI] [PubMed] [Google Scholar]
- Waldeck K, Ny L, Persson K, Andersson KE. Mediators and mechanisms of relaxation in rabbit urethral smooth muscle. Br J Pharmacol. 1998;123:617–624. doi: 10.1038/sj.bjp.0701645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucchi R, Ronca-Testoni S. The sarcoplasmic reticulum Ca2+ channel/ryanodine receptor: modulation by endogenous effectors, drugs and disease states. Pharmacol Rev. 1997;49:1–51. [PubMed] [Google Scholar]