Abstract
AMP-activated protein kinase (AMPK) has emerged as a key regulator of energy metabolism in the heart. The high energy demands of the heart are primarily met by the metabolism of both fatty acids and glucose, both processes being regulated by AMPK. During myocardial ischaemia a rapid activation of AMPK occurs, resulting in an activation of both glucose uptake and glycolysis, as well as an increase in fatty acid oxidation. This activation of AMPK has the potential to increase energy production and to inhibit apoptosis, thereby protecting the heart during the ischaemic stress. However, at clinically relevant high levels of fatty acids, ischaemic-induced activation of AMPK also stimulates fatty acid oxidation during and following ischaemia. This can contribute to ischaemic injury secondary to an inhibition of glucose oxidation, which results in a decrease in cardiac efficiency. In a number of other non-cardiac tissues, AMPK has been shown to have pro-apoptotic effects. As a result, the question of whether AMPK activation benefits or harms the ischaemic heart remains controversial. The role of AMPK in cardiac hypertrophy is also controversial. Activation of AMPK inhibits protein synthesis, and may be an adaptive response to pathological cardiac hypertrophy. However, none of mouse models of AMPK deficiency (excluding those that may involve the γ2 subunit mutations) demonstrate increased cardiac mass, suggesting that AMPK is not essential for restriction of cardiac growth. In addition to the potential effects of AMPK on myofibrillar hypertrophy associated with pressure overload, there is also controversy with respect to the cardiac hypertrophy associated with the γ2 subunit mutations. In the cardiac hypertrophy associated with glycogen overload, both activating and inactivating mutations of AMPK in mice are associated with a marked cardiac hypertrophy. This review will address the issue of whether AMPK activation acts as an enemy or ally to the ischaemic and hypertrophied heart. Resolving this issue has important implications as to whether therapeutic approaches to protect the ischaemic heart should be developed which either activate or inhibit AMPK.
Introduction
5′-AMP-activated protein kinase (AMPK) has emerged as a key kinase controlling many cellular processes, particularly pathways involved cellular energy status (Hardie & Carling, 1997). AMPK is activated during metabolic stress, and not only activates a number of energy producing metabolic pathways, but also inhibits energy consuming pathways (Hardie & Carling, 1997). This has resulted in AMPK being coined a ‘fuel gauge’ in the cell, a ‘low fuel warning system’ or a ‘master switch’ for cellular energy levels (Hardie & Carling, 1997). This role of AMPK as a fuel gauge is particularly relevant in the heart, which has a very high energy demand, and over a 24 h period produces and consumes more energy (in the form of ATP) than any other organ. There are very little energy reserves in the heart, and if ATP production ceased the heart's ATP supply would be completely exhausted within seconds. Therefore, it stands to reason that AMPK should have a very important role as a ‘fuel gauge’ in the heart. This is indeed the case, and as will be discussed, AMPK activation can increase both glucose and fatty acid metabolism in times of increased metabolic demand.
The role of AMPK as a fuel gauge is particularly important in the setting of cardiac ischaemia or hypoxia. Ischaemic heart disease is a major problem in western society, being the major cause of death and disability. We originally demonstrated that cardiac AMPK is rapidly activated during ischaemia (Kudo et al. 1995,1996), an observation now confirmed by numerous laboratories (Beauloye et al. 2001b,2002; Russell et al. 2004; Baron et al. 2005; Li et al. 2005; Shibata et al. 2005; Sakamoto et al. 2006). This activation of AMPK results in a stimulation of glucose uptake, glycolysis and fatty acid oxidation (Kudo et al. 1996; Russell et al. 1999, 2004; Beauloye et al. 2002; Hopkins et al. 2003). These metabolic effects can be both beneficial and harmful during ischaemia and during reperfusion following ischaemia. Ischaemia can also induce apoptosis in the heart. While pro-apoptotic effects of AMPK have been demonstrated (Capano & Crompton, 2006), cardiac studies overwhelming suggest an anti-apoptotic effect of AMPK (Hickson-Bick et al. 2000; Russell et al. 2004; Shibata et al. 2005). Therefore, the complex relationship between AMPK action, myocardial ischaemia and apoptosis remains to be completely elucidated.
In addition to its actions on cardiac energy metabolism, AMPK also has important actions on the cardiac hypertrophic process (MacRae et al. 1995; Gollob et al. 2001a; Tian et al. 2001; McLeod & Proud, 2002; Arad et al. 2003; Gollob, 2003; Horman et al. 2003; Patel et al. 2003; Browne et al. 2004; Browne & Proud, 2004; Chan et al. 2004; Shibata et al. 2004; Davies et al. 2005). AMPK activation inhibits cardiac protein synthesis and the cardiac hypertrophic process (MacRae et al. 1995; McLeod & Proud, 2002; Gollob, 2003; Horman et al. 2003; Browne et al. 2004; Browne & Proud, 2004; Chan et al. 2004; Shibata et al. 2004). On the other hand both activating and inactivating AMPK mutations have been shown to contribute to cardiac hypertrophy (Gollob et al. 2001a; Arad et al. 2003; Patel et al. 2003; Davies et al. 2005).
Despite the explosion of interest in AMPK's action on the heart, many questions remain to be answered. Controversy also exists as to the potential beneficial versus harmful effects of AMPK in the setting of ischaemia, apoptosis and cardiac hypertrophy. This review will address these complex issues.
The regulation of cardiac AMPK by AMP and AMPKKs
Cellular control of AMPK has been widely studied, and the role of 5′-AMP and upstream phosphorylation of the α subunit of AMPK by AMPK kinases (AMPKKs) has been widely reported (Hardie & Carling, 1997). Similar control mechanisms exist in the heart, and the rapid activation of AMPK in times of stress appears to involve both increases in [AMP] and activation of upstream AMPKKs. Elucidating the role of AMP in the control of AMPK has been hampered by the complexity of accurately measuring cytoplasmic [AMP], since total cellular AMP content does not reflect cytoplasmic [AMP]. However, studies by Frederich & Balschi (2002) have carefully examined the relationship between AMPK and AMP, using a 31P-NMR approach in which cytoplasmic [AMP] concentrations can be calculated. These authors showed that cardiac AMPK is activated by AMP concentrations in the low micromolar range. This correlation of low AMP concentrations with increased AMPK activity suggests that [AMP] could activate AMPK in the absence of ischaemia (such as in response to increased workload). However, [ATP] in the 4 mm range increases the concentrations of AMP necessary to activate AMPK by 10- to 20-fold. Since aerobic [ATP] is in the 7 mm range, the issue of whether [AMP] is an important regulator of AMPK activation in the absence of ischaemia or hypoxia remains unresolved.
Similar to other organ systems, AMPK phosphorylation is a key mechanism controlling AMPK activation in the heart. Phosphorylation of Thr172 in the activation loop of the α1 and α2 subunit by upstream AMPK kinases (AMPKKs) is a key mechanism by which cardiac AMPK is activated during times of metabolic stress (Hardie & Carling, 1997). To date, two AMPKKs have been identified in the heart, the tumour suppressor kinase LKB1 and a calmodulin-dependent protein kinase kinase (CamKK). While LKB1 is highly expressed in heart (Sakamoto et al. 2006), CamKK is expressed only at low levels (Anderson et al. 1998). To date the role of either of these AMPKKs in the control of AMPK phosphorylation and activation has not been clearly defined. However, progress is being made. For example, we (Altarejos et al. 2005) and others (Baron et al. 2005) have shown that an increase in AMPK phosphorylation during myocardial ischaemia is accompanied by a stimulation of AMPKK activity. However, we (Altarejos et al. 2005) and others (Sakamoto et al. 2004) found that this activation of AMPKK is not associated with an activation of LKB1. This suggests that either CamKK is involved in AMPK phosphorylation during ischaemia, there is the presence of additional, as yet unidentified AMPKKs in the heart, or that LKB1 is constitutively active and metabolic stress can make AMPK a better substrate for LKB1. Indeed, recent evidence in endothelial cells suggests that PKC-ζ phosphorylates LKB1 at Ser428, resulting in an increased association of LKB1 with AMPK and consequent AMPK Thr172 phosphorylation by LKB1 (Xie et al. 2006). Whether a similar pathway exists in the heart has yet to be determined.
Hormonal regulation of AMPK also involves the phosphorylation control of AMPK. Adiponectin has been shown to result in a phosphorylation and activation of cardiac AMPK (Shibata et al. 2004), although this is not a consistent finding (Onay-Besikci et al. 2004). In contrast, insulin inhibits cardiac AMPK activity (Gamble & Lopaschuk, 1997), as well as AMPK phosphorylation (Beauloye et al. 2001b; Kovacic et al. 2003). Exactly how insulin inhibits AMPK is not clear, although it appears to be directly mediated by Akt (Kovacic et al. 2003; Soltys et al. 2006) via a hierarchical mechanism whereby Ser485/Ser491 phosphorylation of AMPK α1/α2 by Akt reduces LKB1 phosphorylation of Thr172 of AMPK (Horman et al. 2005; Soltys et al. 2006). Confusing this issue is the observation that AMPK can inhibit cardiac insulin signalling as well, possibly via activation of an atypical protein kinase C (Longnus et al. 2005). Further characterization of the upstream AMPKKs in the heart should help clarify the relationship between hormonal control of AMPK and the phosphorylation and activation of AMPK.
Control of energy metabolism in the heart by AMPK
One of the key roles of AMPK in the heart is the regulation of energy metabolism. In the normal, healthy heart, the large amounts of ATP necessary to sustain contractile function and basal metabolism are generated primarily by mitochondrial oxidative metabolism, with a small amount derived from glycolysis. While the heart is an omnivore and can use many different energy substrates (fatty acids, glucose, lactate, ketones, amino acids), mitochondrial ATP is primarily produced by the oxidation of fatty acids and of pyruvate (derived from either glycolysis or lactate). In the normal heart approximately 10–40% of the ATP is produced via pyruvate oxidation, while the remaining 60–90% of the ATP is derived from the oxidation of fatty acids. The rate of flux through these different pathways is affected by arterial substrate concentration, hormones, coronary flow, inotropic state and nutritional status (Saddik et al. 1993). These effects are mediated by various enzymes and substrate/product relationships. In addition, AMPK also appears to play an important role in regulating both fatty acid and glucose metabolism.
The rate at which fatty acids are taken up and oxidized by the heart is dependent on their plasma concentration, their transport into the cell, as well as the control of their intracellular utilization. There are a number of membrane transporters and enzymes involved in the transfer of substrates from the cytosol into the mitochondrial matrix. At the sarcolemmal membrane of the working heart, fatty acid translocase (FAT/CD36) has been shown to account for as much as 50% of the fatty acid taken up into the cell and subsequently used for oxidation (Kuang et al. 2004). A key site of control for the transport of fatty acids into the mitochondria is the enzyme carnitine palmitoyl transferase 1 (CPT-1), which is located on the outer mitochondrial membrane. This enzyme is inhibited by malonyl CoA, the levels of which are indirectly regulated by AMPK. In the heart, levels of malonyl CoA are controlled by the dynamic rates of its synthesis and degradation. Malonyl CoA is synthesized from acetyl CoA in the heart by acetyl CoA carboxylase (Thampy, 1989), while degradation of malonyl CoA occurs by the reverse reaction via decarboxylation back to acetyl CoA by the enzyme malonyl CoA decarboxylase (Dyck et al. 1998). Interestingly, both enzymes involved in the control of cardiac malonyl CoA levels have been suggested to be under phosphorylation control by AMPK (Table 1). Phosphorylation of ACC by AMPK has been well documented (Hopkins et al. 2003) and although controversial, it has been suggested that MCD is also a phosphorylation target of AMPK (Saha et al. 2000). Therefore, AMPK plays a distinct and important role in regulating both malonyl CoA levels and fatty acid oxidation in the heart.
Table 1. Cardiac targets of AMPK.
| Target | Direct/indirect | Effect | Metabolic action | References |
|---|---|---|---|---|
| Glucose metabolism | ||||
| ″Glucose transporters | Indirect | Increased SL localization | Increased glucose uptake | Russell et al. (2004) |
| ″Phosphofructokinase-2 | Direct | Stimulation | Increased glycolysis | Marsin et al. (2000) |
| ″Glycogen synthase | ? | Possible inhibition | Decreased glycogen synthesis | Carling & Hardie (1989); Wojtaszewski et al. (2002) |
| ″Glycogen phosporylase | ? | Possible stimulation | Increased glycogenolysis | Carling & Hardie (1989) |
| ″p38 MAPK/TAB1 complex | Direct | Stimulation | Increased GLUT4 transport | Li et al. (2005) |
| Fatty acid metabolism | ||||
| ″Lipoprotein lipase | Direct | Increased translocation | Increased fatty acid supply | An et al. (2005) |
| ″CD36 | ? | Increased SL localization | Increased fatty acid uptake | Luiken et al. (2003) |
| ″Acetyl CoA carboxylase | Direct | Inhibition | Increased fatty acid oxidation | Kudo et al. (1995,1996) |
| ″Malonyl CoA decarboxylase | ? | Possible stimulation | Increased fatty acid oxidation | Saha et al. (2000); Habinowski et al. (2001) |
| Protein synthesis/hypertrophy | ||||
| ″eEF2 kinase | Direct | Stimulation | Decreased protein synthesis | Browne et al. (2004) |
| ″mTOR | ? | Possible inhibition | Decreased protein synthesis | Horman et al. (2003); Cheng et al. (2004) |
| ″TSC2 | ? | Possible stimulation | Decreased protein synthesis | Inoki et al. (2003) |
SL, sarcolemma; CPT 1, carnitine palmitoyltransferase 1; mTOR, mammalian target of rapamycin; GLUT4, glucose transporter; eEF2 kinase, eukaryotic elongation factor-2 kinase; TSC2, tuberous sclerosis complex 2.
Cardiac function is also dependent on the production of intracellular ATP derived from glucose metabolism. The initiation of glucose metabolism begins with the transport of glucose into the cell via the glucose transporters (GLUT) 1 and 4. While GLUT1 primarily maintains basal glucose uptake, GLUT4 can be mobilized from intracellular compartments to the sarcolemma in response to insulin and/or contractile stimulation, thus increasing glucose uptake (although GLUT1 has also been shown to translocate to the plasma membrane upon insulin stimulation; Fischer et al. 1997). Once glucose is within the cell, it has a number of fates, including being stored as glycogen or undergoing glycolytic metabolism. An important enzyme at the interface between carbohydrate oxidation and fatty acid metabolism is pyruvate dehydrogenase (PDH), which decarboxylates pyruvate to acetyl CoA. PDH activity is influenced not only by glycolysis, but also via an inhibitory effect exerted through fatty acid oxidation. In situations where the circulating free fatty acid levels are high, the oxidation of glucose and pyruvate, and the activity of PDH are decreased, due in part to an activation of pyruvate dehydrogenase kinase (PDK). Conversely, lowering plasma free fatty acid levels, or directly inhibiting fatty acid oxidation, decreases PDK, increases PDH, increases pyruvate oxidation and increases cardiac efficiency (cardiac work/O2 consumed) (Saddik et al. 1993; Hopkins et al. 2003).
When the energy demand of the heart is being met, the myocyte has the capacity to store glucose in the form of glycogen. This process is regulated by the enzyme glycogen synthase. However, when the demand for glucose increases, the myocyte begins to mobilize its glycogen stores by breaking down glycogen to glucose 1-phosphate (which is further metabolized to glucose 6-phosphate). This process is regulated by the enzyme glycogen phosphorylase. Both glycogen phosphorylase and glycogen synthase are regulated by complex mechanisms that involve allosteric regulation by metabolites, phosphorylation/dephosphorylation control, and/or feedback inhibition by either of the products (glycogen or glucose 1-phosphate).
Whether being mobilized from glycogen or being utilized directly from the uptake process, glucose (which eventually becomes glucose 6-phosphate) can be further metabolized within the cell to produce ATP. This catabolic process can be divided into two separate categories, namely glycolysis and glucose oxidation. Glycolysis is the initial sequence of reactions involved in the breakdown of glucose to pyruvate. This process can occur without the presence of oxygen while still producing ATP. The ATP produced via this process often contributes less than 10% of the overall ATP in the normally functioning myocardium. The pyruvate generated from glycolysis is further metabolized within the mitochondria to produce the majority of carbohydrate derived ATP (i.e. glucose oxidation).
A number of enzymes are involved in the regulation of glucose uptake, glycogen storage, glycogen mobilization and glucose metabolism. While many of these have multiple control mechanisms, a number of key enzymes in the glucose/glycogen metabolic pathways are also under phosphorylation/dephosphorylation control. AMPK has emerged as being centrally involved in the regulation of these enzymes and is thus a major regulator of cardiac energy metabolism (further discussions on glycolysis and glycogen metabolism are included below).
Role of AMPK in increased energy supply during increases in cardiac work
Acute increases in cardiac contractile function require an immediate increase in energy production, which results from an increase in both glucose metabolism and fatty acid oxidation. While AMPK is activated in response to exercise in skeletal muscle (Vavvas et al. 1997), it remains unclear whether cardiac AMPK is activated and contributes to the increase in energy production that occurs in response to physiological increases in cardiac work. Coven et al. (2003) showed an increase in both α1 and α2 AMPK activity in hearts of rats subjected to moderate- and high-intensity exercise. To further examine the functional relationship of AMPK in the heart during exercise, Musi et al. (2005) examined whether acute exercise increases AMPK in mouse hearts by using transgenic mice in which a cardiac-specific dominant-negative AMPKα2 subunit was expressed. Acute treadmill exercise resulted in an increase in AMPKα2 activity in wild-type mice, but not in the dominant-negative AMPKα2 mice. Regardless, of this lack of AMPK activation in the transgenic mice, no abnormalities in ATP levels, glycogen consumption or cardiac function were observed in these mice. However, the possibility of chronic adaptive changes in AMPK deficient mice to parallel exercise-signalling pathways cannot be ruled-out. In contrast to these exercise studies, Hall et al. (1996) observed that increasing cardiac work in swine hearts with dobutamine treatment is not associated with an increase in AMPK activity. In isolated rat hearts, stimulation of contractile function with adrenaline was also not associated with an increase in AMPK activity (Goodwin & Taegtmeyer, 1999). Furthermore, the increase in glycolysis observed in isolated rat hearts during increases in cardiac work is also not accompanied by an increase in AMPK activity (Beauloye et al. 2002). As a result, it remains unclear to what extent, if any, AMPK activation has in work-induced increases in energy production in the heart.
AMPK activation during ischaemia-reperfusion
Cardiac energy metabolism during ischaemia-reperfusion
Myocardial ischaemia is a major health problem in our society, and results in a decrease in mitochondrial oxidative metabolism, and a decrease in ATP production. During mild ischaemia (such as seen in angina pectoris), energy supply from mitochondrial glucose and fatty acid oxidation cannot keep up with energy demand, and glycolysis accelerates in an attempt to compensate for a decrease in mitochondrial ATP supply. During severe ischaemia (such as seen during a myocardial infarct or during cardiac surgery), oxidative metabolism dramatically decreases, and the major source of ATP production is via glycolysis. However, under these conditions glycolysis becomes uncoupled from glucose oxidation, resulting in the accumulation of deleterious by-products of glycolysis (lactate and protons) within the cardiac cells. This can lead to the re-direction of ATP away from myocardial contraction and towards the clearance of glycolytic by-products. This leads to a decrease in both cardiac function and efficiency (Dennis et al. 1991; Liu et al. 1996). Upon reperfusion of the reversibly injured ischaemic myocardium, contractile function will recover once energy production has been restored. The extent of this recovery is dependent on the type of substrate metabolized by the heart during reperfusion. However, fatty acid oxidation can be dramatically accelerated during reperfusion, with over 95% of acetyl CoA-derived ATP originating from fatty acids (see Kantor et al. 1999 for review). These high rates of fatty acid oxidation following ischaemia are due both to an increase in circulating fatty acid levels that occurs during ischaemia (see Kantor et al. 1999 for review), and to direct alterations in the subcellular control of fatty acid oxidation. High rates of fatty acid oxidation can dramatically inhibit glucose oxidation rates via the Randle cycle (Randle et al. 1965), since fatty acid-derived acetyl CoA decreases the production of glucose-derived acetyl CoA via inhibition of the pyruvate dehydrogenase complex. This results in a continued low rate of glucose oxidation, an uncoupling from glycolysis, and a continued decrease in cardiac efficiency during the critical period of reperfusion.
AMPK activation during myocardial ischaemia-reperfusion
While it is not clear to what extent AMPK becomes activated in the heart during physiological increases in cardiac work, a rapid and dramatic activation of AMPK during ischaemia has been well established. We first demonstrated that AMPK activation occurs during myocardial ischaemia (Kudo et al. 1995,1996), a finding now reproduced by numerous laboratories (Saddik et al. 1993; Kudo et al. 1995, 1996; Russell et al. 1999; Marsin et al. 2000). This activation of AMPK presumably occurs as an approach to restore myocyte ATP levels, primarily turning on ATP generating pathways. It is now clear that AMPK activation can increase both glucose and fatty acid metabolism in the ischaemic heart.
AMPK increases cardiac glucose utilization by a number of different mechanisms. AMPK activation promotes the translocation of GLUT4 to the sarcolemma of the myocyte, which promotes glucose uptake (Russell et al. 2004; Li et al. 2005). This may involve the interaction of AMPK with the scaffolding protein TAB1, inducing p38 MAPK autophosphorylation and activation (Li et al. 2005). AMPK also phosphorylates and activates phosphofructokinase 2 (Marsin et al. 2000), which produces fructose 2,6-bisphosphate, a potent stimulator of glycolysis. Since AMPK has an important role in promoting glucose uptake and glycolysis (Young et al. 1996; Merrill et al. 1997; Marsin et al. 2000), the activation of AMPK during ischaemia may benefit the heart by increasing glucose utilization (and subsequently anaerobic ATP synthesis).
A second major metabolic consequence of AMPK activation during and following ischaemia is a stimulation of fatty acid oxidation (Kudo et al. 1995, 1996; Makinde et al. 1997). While overall oxidative rates during ischaemia are limited by oxygen supply to the muscle, during and following ischaemia, AMPK activation can lower cardiac malonyl CoA levels, secondary to phosphorylation and inhibition of ACC (Kudo et al. 1995, 1996). Unfortunately, in the presence of an ischaemic-induced limitation of overall oxidative metabolism, the AMPK-dependent acceleration of fatty acid oxidation occurs at the expense of glucose oxidation, and has the potential to be detrimental in the setting of ischaemia–reperfusion (Kudo et al. 1996). During reperfusion, a continued activation of AMPK also contributes to fatty acid oxidation providing the major part of the ATP production during the critical period of reperfusion, again at the expense of glucose oxidation. As will be discussed, the effects of AMPK on cardiac fatty acid oxidation have the potential to be an ‘enemy’ to the ischaemic heart; at the same time it acts as an ‘ally’ by stimulating glucose uptake, glycogenolysis and glycolysis.
The role of AMP and upstream AMPKKs in ischaemic-induced activation of AMPK
During severe ischaemia, 5′-AMP levels can rise dramatically in the heart. A subsequent activation of AMPK by 5′-AMP probably contributes to the effects of AMPK on both glucose metabolism and fatty acid oxidation. However, during low flow ischaemia AMPK can be phosphorylated and activated independent of measurable changes in total cellular AMP content (Beauloye et al. 2001b; Altarejos et al. 2005). Frederich et al. (2005) also showed that hypoxia can increase cardiac AMPK phosphorylation and activity independent of cytosolic [AMP]. As a result, although increases in [AMP] may play a role in ischaemic and hypoxia-induced activation of cardiac AMPK, it is clear that other mechanisms of activation also occur.
The ischaemic- or hypoxic-induced activation of AMPK is accompanied by increase in a rapid phosphorylation of AMPK (Russell et al. 1999; Beauloye et al. 2001a,b; Altarejos et al. 2005; Baron et al. 2005). This implicates an ischaemic-induced activation of AMPK by an upstream AMPKK. In support of this, our group (Altarejos et al. 2005; Soltys et al. 2006) and Baron et al. (2005) showed that AMPKK activity is increased in the ischaemic heart. However, we (Altarejos et al. 2005) and others (Sakamoto et al. 2006) have also shown that this activation of AMPKK is not associated with an activation of LKB1. This suggests the presence of yet unidentified additional AMPKKs in the heart.
Despite the fact that LKB1 is not activated during ischaemia, a role for LKB1 in ischaemic-induced phosphorylation and activation of AMPK cannot be ruled out. Like other tissues, it is possible that an increase in [AMP] could make AMPK a better substrate for LKB1. In support of this, in mice hearts deficient in LKB1 there is a decreased phosphorylation and activation of the AMPKα2 during ischaemia, despite the absence of a change in LKB1 activity. Of interest is that the ischaemic-induced activation of AMPKα1 remains unchanged (Sakamoto et al. 2006). This suggests that LKB1 does contribute to the ischaemic-induced activation of AMPK, but that additional AMPKKs are also involved in this. Alternatively, the α1 isoform of AMPK may be expressed at higher levels than the α2 isoform in endothelial cells within the heart and it is the endothelial cell-specific α1 isoform that is not affected by cardiomyocyte-specific loss of LKB1.
Is AMPK activation beneficial or harmful during and following ischaemia?
The question of whether AMPK activation is an ally or enemy to the ischaemic heart remains to be completely elucidated. Early studies in our laboratory implicated ischaemic-induced activation of AMPK as being detrimental to the ischaemic heart, primarily as the result of a stimulation of fatty acid oxidation and the subsequent inhibition of glucose oxidation (Fig. 1). These high fatty acid oxidation rates can contribute to a decrease in cardiac function and cardiac efficiency during the critical period of reperfusion. However, the majority of studies examining AMPK activation in the ischaemic heart suggest that AMPK activation is an adaptive response that allows the heart to generate ATP and thereby protect cardiac tissues in the presence of oxygen deprivation (Beauloye et al. 2001b; Baron et al. 2005; Sakamoto et al. 2006). Unfortunately, very few of these studies have actually examined what effect AMPK activation has directly on cardiac function.
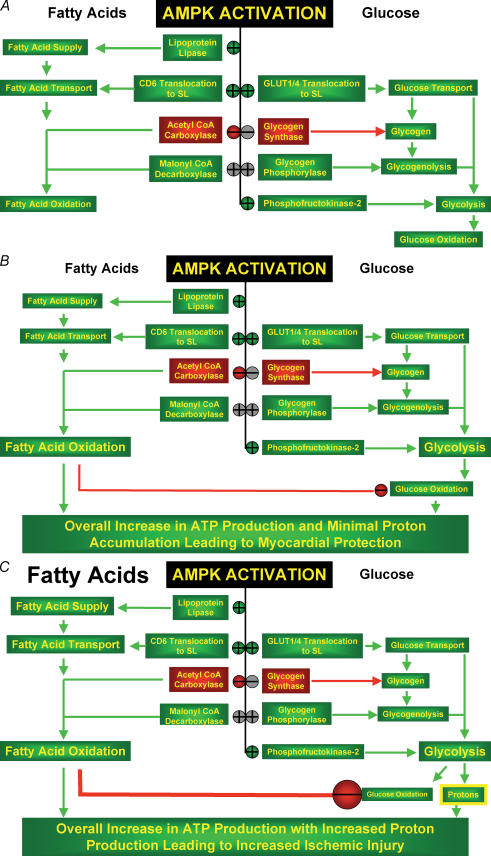
Figure 1. Proposed pathways by which activation of AMPK can benefit or harm the ischaemic-reperfused heart.
A, in the aerobic heart AMPK activation can activate or inhibit a number of processes that increase fatty acid oxidation and glycolysis. Ischaemia results in a rapid activation of AMPK in the heart. This results in a stimulation of glucose uptake, glycogenolysis and glycolysis. B, this can provide a potentially beneficial source of energy for the heart during an ischaemic-induced decrease in mitochondrial oxidative metabolism. AMPK activation also increases fatty acid oxidation, which unfortunately results in a parallel decrease in glucose oxidation rates. In the presence of low levels of fatty acids, this inhibition of glucose oxidation is minimal. C, in the presence of the high levels of fatty acids (which is seen in most clinical scenarios of ischaemia and reperfusion) a large decrease in glucose oxidation can increase proton production, due to an uncoupling of glycolysis from glucose oxidation. This can decrease cardiac efficiency and potentially contribute to ischaemic injury. Red symbols indicate inhibition, green symbols indicate activation, and grey symbols indicate possible direct or indirect targets of regulation.
A study by Russell et al. (2004) did examine what effect ischaemia had on cardiac function and apoptosis in an AMPKα2 kinase-dead (KD) transgenic mouse (K45Rα2). When isolated hearts perfused with low levels of fatty acids (0·4 mm oleate) were subjected to a low flow ischaemia, AMPKα1 and α2 activity, glucose uptake and lactate production were all increased in wild-type mice, but not in AMPKα2 KD mice. Hearts from AMPKα2 KD mice showed worse function during and following ischaemia, an increase in creatine kinase and lactate dehydrogenase release and an increase in caspase-3 and TUNEL positive cells, suggesting that AMPK activation was necessary to allow hearts to withstand an ischaemic insult. Of interest is that while fatty acid oxidation rapidly recovered during reperfusion of ischaemic hearts, both rates of fatty acid oxidation and glucose oxidation were not different between wild-type and AMPKα2 KD mice. Additional indirect support for a beneficial effect of AMPK activation during ischaemia was also observed by Shibata et al. (2005). Production of an in vivo infarct in adiponectin knock-out mice had increased infarcts, decreased phosphorylation of AMPK at Thr172 and increased TNFα and apoptosis. Administration of an adenovirus expressing adiponectin to mice resulted in decreased infarct size, increased phosphorylation of AMPK at Thr172, and decreased TNFα and apoptosis in the adiponectin deficient mice. This also suggests a beneficial effect of AMPK in the setting of ischaemia. However, these studies could not directly link alterations in AMPK to alterations in infarct size, and the protective effects of adiponectin were also proposed to act via other pathways, such as induction of cyclooxygenase-2-dependent synthesis of prostaglandin E2 (PGE2).
Brief periods of ischaemia prior to a prolonged period of ischaemia (called ‘ischaemic preconditioning’) can provide significant protection to the heart. Recently, activation of AMPK has been shown to occur in the setting of ischaemic preconditioning. Similar to what was previously observed in liver (Peralta et al. 2001), ischaemic preconditioning activates AMPK in the heart (Nishino et al. 2004; Depre et al. 2006). This activation of AMPK is associated with an up-regulation of GLUT4 expression, which occurs in a protein kinase C-dependent manner (Nishino et al. 2004). The activation of H11 kinase that occurs in preconditioned hearts is also associated with an activation of AMPK and promotes the translocation of AMPK to the nucleus (presumably resulting in an increase in transcription of metabolic enzymes such as GLUT4). This activation of AMPK and increase in glucose uptake in ischaemic preconditioning has the potential to benefit the heart. However, it has yet to be directly determined whether this activation of AMPK is associated with cardioprotection in the setting of ischaemic preconditioning.
Surprisingly, despite an extensive literature that has characterized the effects of ischaemia on AMPK activity and the effect AMPK activation has on energy metabolism in the ischaemic heart, almost none of these studies have directly linked alterations in AMPK activity with alterations in cardiac function. With the exception of the Russell et al. (2004), no study has linked a modification of AMPK activity with an alteration in cardiac function. As a result, it is difficult to state with clarity whether AMPK acts as an ally or an enemy in the ischaemic heart. AMPK activation during ischaemia has the potential to increase energy supply to the heart by increasing glucose uptake and glycolysis. However, it also has the potential to increase fatty acid oxidation, which by inhibiting glucose oxidation can increase proton production and decrease cardiac efficiency. It is interesting to note that the study of Russell et al. (2004) implying a beneficial effect of AMPK activation during ischaemia was performed in the presence of low concentrations of fatty acids, a condition in which glucose oxidation rates are very high. Indeed, in their study, measured glucose oxidation rates were abnormally high, which may have minimized any adverse effect of AMPK on fatty acid oxidation stimulation. It should be pointed out that in humans, during most clinically relevant situations of myocardial ischaemia, the heart is exposed to high levels of fatty acids, which can exceed 1 mm (Oliver et al. 1968; Mueller & Ayres, 1978; Lopaschuk et al. 1994). Determining the role of AMPK in the ischaemic heart is hampered by the lack of data using agonists or antagonists of AMPK in the ischaemic heart. The use of AICriboside in intact hearts as an approach to activate AMPK has proved problematic, while we have found that antagonists such as Compound C have been toxic to the heart even at doses that do not alter AMPK activity (unpublished observations). Even with the potential development of better AMPK agonists or antagonists, any short-term experiments in vitro need to be complemented by long-term experiments in intact animals to address whether AMPK deficiency enhances or inhibits myocardial ischaemic injury and necrosis. It is also important to recognize that none of the published in vitro heart studies can accurately recreate plasma concentrations of saturated and unsaturated FFAs and of triglycerides observed in vivo. More studies using in vivo ischaemia–reperfusion models would help to more clearly define whether AMPK deficiency is detrimental or protective to the heart. As a result, we believe the jury is still out as to whether AMPK is an ally or enemy to the ischaemic heart.
The role of AMPK in cardiac myocyte apoptosis
Apoptotic cell death is accelerated in many forms of heart disease, including ischaemic heart disease and heart failure. The role of AMPK in apoptosis is not clear, with both anti-apoptotic and pro-apoptotic actions being reported. In a number of cell types, including vascular smooth muscle cells, pancreatic cells and various cancerous cells, AMPK activation has been shown to be pro-apoptotic (Meisse et al. 2002; Kefas et al. 2003; Igata et al. 2005). This pro-apoptotic effect of AMPK has been attributed to inhibition of cell cycle progression, activation of the JNK pathway and caspase-3 activation, and up-regulation of the pro-apoptotic p53 protein (Igata et al. 2005). A recent study by Capano & Crompton (2006) in neonatal cardiomyocytes showed that AMPK can mediate the pro-apoptotic translocation of Bax to mitochondria, and that Bax translocation is an early step in ischaemia that occurs via AMPK activation of p38 MAPK. However, in the heart the majority of studies have suggested that AMPK activation is antiapoptotic. For, instance, AMPK activation is anti-apoptotic during palmitate-mediated apoptosis in neonatal cardiac myocytes (Hickson-Bick et al. 2000). Russell et al. (2004) investigated the role of AMPK during low flow ischaemia and postischaemic reperfusion. Using AMPKα2 KD transgenic mice, they found an increased left ventricular (LV) dysfunction, necrosis and apoptotic activity following ischaemia and reperfusion, concluding that the AMPK activation is cardioprotective and anti-apoptotic in the ischaemic heart (Russell et al. 2004). Adiponectin deficient mice subjected to ischaemia and reperfusion also exhibit higher levels of myocardial apoptosis (Shibata et al. 2005). In these mice, adiponectin supplementation inhibited apoptosis secondary to activation AMPK. These authors also demonstrated that neonatal rat ventricular myocytes treated with adiponectin were also resistant to hypoxia-induced apoptosis. This protection from apoptosis was inhibited when the cells were pretreated with a dominant negative mutant of AMPK, implicating AMPK as an important mediator of the anti-apoptotic effects of adiponectin. The reason for the conflicting results between intact heart studies and isolated myocytes is not clear. It is possible that the higher energy demand in the intact heart may explain these differences. Energy starvation (such as during ischaemia) is an important trigger of apoptosis. It is possible that the energy generating actions of AMPK in the intact heart may provide an anti-apoptotic action that can overcome the potential effects of AMPK activation on pro-apoptotic pathways such as p53 activation or mitochondrial Bax translocation. These possibilities remain to be investigated.
AMPK and cardiac hypertrophy
Cardiac (ventricular) hypertrophy is associated with increased cardiac myocyte cell volume, enhanced protein synthesis, changes in gene transcription and translation, and increased myofibrillar assembly (Sugden & Clerk, 1998). Cardiac hypertrophy often occurs in response to increased haemodynamic load arising from a variety of conditions including exercise, hypertension and valvular heart disease (for review see Lorell & Carabello, 2000). Initially, these morphological changes are adaptive responses that allow the heart to maintain cardiac output. A number of intracellular signalling pathways have been implicated in the complex regulation of hypertrophy and there appears to be a variety of molecular mechanisms that can cause hypertrophy (for review see Frey & Olson, 2003). Although the exact mechanism(s) have not yet been resolved, one signalling pathway that has emerged as being involved in the regulation of cardiac myocyte cell growth is the AMPK pathway. This section will address the potential effects of AMPK on myofibrillar hypertrophy associated with pressure overload, which is distinct from the cardiac hypertrophy associated with the γ2 subunit mutations (discussed later).
The earliest observations by Tian et al. (2001) have shown that the activity of both AMPKα1 and AMPKα2 are elevated in hypertrophic hearts subjected to chronic pressure overload. This increase in AMPK activity was associated with enhanced glucose uptake in the hypertrophic hearts. While fatty acid oxidation rates was not measured in these hearts, there were the characteristic decreases in the expression of CPT1 and MCAD, which is suggestive of a reduction in the fatty acid oxidative capacity of the hypertrophic heart. Although the authors do not suggest that AMPK activation is a cause of cardiac hypertrophy, the data from that study appear to implicate AMPK activation as a cause of cardiac hypertrophy (Tian et al. 2001). However, we have shown that during the development of Akt1-induced hypertrophy in neonatal rat cardiac myocytes AMPK is actually inactivated, which may be permissive for cardiac growth (Chan et al. 2004). In agreement with our study, adiponectin has been shown to attenuate hypertrophic growth via activation of AMPK and an AMPK-dependent signalling mechanism (Shibata et al. 2004). Further support of AMPK as a negative regulator of cardiac growth has been provided by Liao et al. (2005) who show that AMPK signalling is decreased in hearts from adiponectin deficient mice and that this decrease in AMPK activity exacerbates hypertrophic growth and heart failure following transverse aortic constriction. Although the reasons for these seemingly diametrically opposed roles for AMPK in the development of cardiac hypertrophy are not known, the increase in AMPK activity in pressure overload-induced hypertrophy was only shown to occur at a much later stage in the hypertrophic process (Tian et al. 2001), when energy status of the heart may necessitate AMPK activation. Indeed, the AMP/ATP ratio was increased more than 4-fold in the hypertrophic hearts as opposed to the control hearts, suggesting a metabolic stress (Tian et al. 2001). It is possible that AMPK activation may play dual roles in the development of cardiac hypertrophy. That is, pharmacological activation of AMPK in the early phase of cardiac hypertrophy may be able to prevent hypertrophic growth, while AMPK activation during pathological hypertrophy may be an adaptive response to the metabolic stress (Fig. 2).
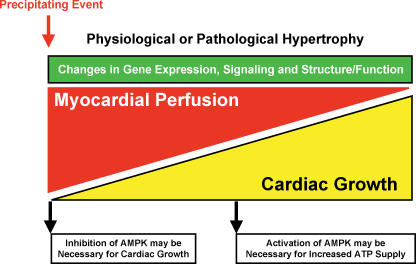
Figure 2. The involvement of AMPK in the development of cardiac hypertrophy.
Following the precipitating event, a number of mechanisms (including reduced myocardial perfusion) can promote cardiac myocyte growth. Hypertrophic growth can be adaptive (physiological hypertrophy) but can also become maladaptive (pathological hypertrophy). AMPK appears to have a dual role in the hypertrophic process, where in the early stages inhibition of AMPK may be necessary for cardiac growth (or if not directly involved in the pathway, pharmacological activation of AMPK in the early phase of cardiac hypertrophy may be able to prevent hypertrophic growth), while in the later stages AMPK activation may become essential for maintaining adequate ATP supply.
While the adaptive phase of AMPK activation during cardiac hypertrophy may be simply due to the decreased energy supply and/or energy reserve leading to increased AMP/ATP ratios, the putative preventative role of AMPK may be more complex. Due to the increase in cell size associated with cardiac hypertrophy, a necessary mediator of hypertrophy is protein synthesis (for review see Hannan et al. 2003). Recently, we have shown that pharmacological activation of AMPK inhibits protein synthesis associated with cardiac hypertrophy (Chan et al. 2004). In addition, activation of Akt results in the phosphorylation and inhibition of cardiac AMPK (Kovacic et al. 2003). As activation of Akt1 can also cause cardiac hypertrophy (Shioi et al. 2002; Shiojima et al. 2002; Latronico et al. 2004), we have suggested that a contributing factor to Akt1-induced hypertrophy is the inhibition of AMPK (Chan et al. 2004). Furthermore, we have shown that pharmacological activation of AMPK inhibits Akt1-induced hypertrophic growth via two independent signalling pathways that are known to mediate protein synthesis in the heart (the mTOR–p70S6 kinase–ribosomal protein S6 and the eEF2 kinase–eEF2 pathways) (Chan et al. 2004). These data are consistent with earlier reports demonstrating that these same signalling pathways are regulated by AMPK and may regulate protein synthesis in the cardiac myocyte (McLeod & Proud, 2002; Horman et al. 2003; Browne et al. 2004; Browne & Proud, 2004). While pharmacological activation of AMPK appears to be able to inhibit hypertrophic growth, there is no direct empirical evidence demonstrating that AMPK is an intrinsic component of hypertrophic signalling cascades. Indeed, none of the mouse models that involve decreased AMPK activity (excluding those that may involve the γ2 subunit mutations) demonstrate increased cardiac mass (Viollet et al. 2003; Xing et al. 2003; Jorgensen et al. 2004; Russell et al. 2004; Musi et al. 2005; Sakamoto et al. 2006), suggesting that AMPK is not essential for restriction of cardiac growth. However, one of the mutations in the γ2 subunit of AMPK has been suggested to render the enzyme inactive (possibly by excessive glycogen accumulation) and this decrease in AMPK activity may contribute to hypertrophic growth (Davies et al. 2005). This finding is consistent with AMPK activity being necessary for restricting cardiac growth. Although the precise involvement of AMPK in cardiac hypertrophy is currently unknown, many studies support the concept that during times of energy depletion, AMPK is activated and protein synthesis may be inhibited in order to conserve cellular energy status.
In addition to regulating protein synthesis, AMPK activation may inhibit cardiac hypertrophy via alternative signalling mechanisms. For example, adiponectin has been proposed to mediate both agonist-stimulated and aortic constriction-induced hypertrophy by activating AMPK and inhibiting ERK activation (Shibata et al. 2004). Since activation of ERK is only one component of the Gq-dependent mechanism involved in the control of hypertrophic growth (for review see Dorn & Force, 2005), it is likely that other signalling pathways may also be influenced by the adiponectin–AMPK signalling axis. Regardless of the precise regulatory signalling pathways controlled by AMPK during hypertrophy, the concept that AMPK may link adipokine signalling and the regulation of the hypertrophic cardiomyopathies associated with diabetes/obesity is an exciting avenue of research that is deserving of future investigation.
As mentioned, a number of changes in intracellular signalling pathways occur in response to increased haemodynamic load and/or agonist-stimulation causing cardiac hypertrophy. During the development of myocardial hypertrophy many of these changes are adaptive and beneficial to cardiac function. However, as hypertrophy progresses, many changes can become maladaptive and can contribute to the worsening of contractile performance leading to heart failure. How AMPK fits into this entire process is not clear. AMPK activation can inhibit protein synthesis associated with cardiac hypertrophy and may be able to prevent the development of pathological hypertrophy. However, this concept has not been proven in animal models of cardiac hypertrophy. Nevertheless, since AMPK is activated during metabolic stress it is logical to suggest that the inhibition of protein synthesis by AMPK occurs during times of depleted energy supply. This is particularly important given that protein synthesis consumes a high proportion of the cell's energy. Thus pharmacological activators of AMPK may prove to be beneficial in an effort to reduce and/or slow the progression of pathological cardiac hypertrophy (Fig. 2). The same pharmacological activators of AMPK may also be used in preventing or attenuating some hypertrophic cardiomyopathies associated with diabetes and obesity. Indeed, mimicking the AMPK-activating effects of adiponectin in patients with reduced adiponectin levels may prove to be one mechanism by which diabetic hypertrophic cardiomyopathies can be prevented in the human population.
In addition to controlling the growth process in hypertrophy, AMPK most likely plays a major role in the control of cardiac energy metabolism. During the development of pressure or volume overload-induced hypertrophy, significant alterations in cardiac energy metabolism have been reported (see Sambandam et al. 2002 for review). For example, there is a reduction in overall oxidative metabolism (glucose and fatty acid oxidation), and an increase in glycolysis (Allard et al. 1994b; Wambolt et al. 1999; Allard et al. 2000; Longnus et al. 2001). These changes presumably occur as a direct result of a mild ischaemia associated with reduced myocardial perfusion related to increased myocyte cell size (for review see Lehman & Kelly, 2002). In the early stages of pressure or volume overload-induced hypertrophy, the residual oxidative metabolism is still dominated by fatty acid oxidation (Allard et al. 1994b). However, as hypertrophic growth continues and myocardial perfusion worsens (Fig. 2), gene expression of enzymes involved in fatty acid utilization are down-regulated, while genes involved in glucose uptake and the glycolytic pathway are up-regulated (for reviews see Taegtmeyer, 2000; Lehman & Kelly, 2002). This switch to a more fetal metabolism is presumed to occur as an adaptive response to reduced oxygen availability (i.e. reducing the oxidation of fatty acids increases oxygen efficiency). Although these changes in gene expression are correlated with a worsening of myocardial perfusion, it is very likely that many other, as yet unidentified, factors also play major roles in this switch to a more fetal metabolism. Whatever the mechanism, prolonged inhibition of the heart's major source of energy (i.e. fatty acids) may eventually produce an energetically compromised heart that results in diminished functional capacity and the eventual progression to heart failure.
In chronic pressure overload-induced cardiac hypertrophy, it has been shown that AMPK activity increases (Tian et al. 2001), suggesting that AMPK activation is either (1) a cause of cardiac hypertrophy, (2) a necessary compensatory consequence of pressure-overload, or (3) a maladaptive alteration in the hypertrophic process. This increase in AMPK activity has been suggested to be a major contributor to enhanced glucose uptake in the hypertrophic heart (Tian et al. 2001), which may be a beneficial metabolic adaptation for an energy starved heart (Fig. 2). In this situation, AMPK activation would appear to be an ally to the hypertrophic heart. In contrast to this, it is possible that increased AMPK activity in the hypertrophic heart may actually become an ‘enemy’ to cardiac function by accelerating fatty acid oxidation rates such that they become the major oxidative substrate in the heart at the expense of glucose oxidation. This would uncouple glycolysis from glucose oxidation, resulting in increased proton production. This increase in proton production is detrimental to the ischaemia–reperfused myocardium (as discussed in the ischaemia–reperfusion section). This may explain why the hypertrophied hearts are more susceptible to ischaemia–reperfusion injury (Anderson et al. 1990; Gaasch et al. 1990; Allard et al. 1994a; Schonekess et al. 1996). With these possibilities in mind, it is currently unknown if AMPK activation is beneficial or detrimental to cardiac function in the already hypertrophic heart. In addition, the role of AMPK in the hypertrophic process may be variable depending on the severity of hypertrophy and/or where in the hypertrophic continuum (including heart failure) the heart resides (Fig. 2).
The role of abnormal AMPK activity, glycogen storage and hypertrophic cardiomyopathies associated with Wolff–Parkinson–White syndrome
AMPK consists of a catalytic subunit (α) and two regulatory subunits (β and γ) (Stapleton et al. 1994; Dyck et al. 1996; Gao et al. 1996; Woods et al. 1996). Each of these subunits has at least two highly homologous isoforms (α1, α2; β1, β2; γ1, γ2, γ3), suggesting that 12 various subunit combinations can exists. Recent molecular characterization of AMPK indicates that the β isoform is a scaffolding protein, which binds both the α and γ subunits (Thornton et al. 1998). The γ subunit of AMPK has been identified as the protein that binds AMP (Cheung et al. 2000) thus allowing the α subunit to undergo phosphorylation by upstream AMPK kinases. In many tissues and/or cells the most predominant holoenzyme contains the α1/β1/γ1 subunits, although heart also significantly expresses the α2/β2/γ2 subunits (Stapleton et al. 1996; Thornton et al. 1998; Cheung et al. 2000). Although no specific tissue expression of most of the isoforms exists, the γ3 isoform seems to be primarily expressed in skeletal muscle (Thornton et al. 1998). The roles of all these isoforms have not yet been fully elucidated, but it is becoming increasingly apparent that the γ subunits can be major regulators of the holoenzyme activity (Cheung et al. 2000; Lang et al. 2000; Milan et al. 2000; Blair et al. 2001; Franz et al. 2001; Gollob et al. 2001b; Hamilton et al. 2001; Daniel & Carling, 2002; Gollob, 2003; Hudson et al. 2003; Oliveira et al. 2003; Adams et al. 2004; Mahlapuu et al. 2004).
The putative AMP binding site(s) on AMPK have been proposed to be located within the four tandem cystathionine β-synthase (CBS) motifs found within the γ subunit (now termed the Bateman domains). Mutations within these regions have gained considerable attention of late because of their resulting phenotypes. For instance, a non-conserved mutation at amino acid 200 of the γ3 subunit of AMPK (resulting in an arginine to a glutamine mutation) in the Hampshire pig results in elevated skeletal muscle glycogen levels (the γ3 gene is named PRKAG3 and the mutation is designated PRKAG3 R200Q). This increase in skeletal muscle glycogen levels is highly suggestive that the PRKAG3 R200Q mutation is involved in alterations in AMPK activity and subsequent glucose metabolism. Although the exact mechanism by which this mutation causes excessive glycogen deposition is still not clear, it is likely that there is a mismatch between glucose uptake and glucose utilization. Similar mutations in the γ2 subunit have been found and the effect these mutations have on AMPK activity and glucose utilization in the heart is just now being defined (Fig. 3).
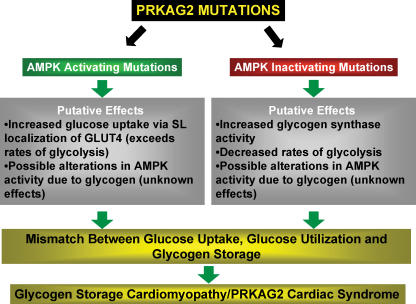
Figure 3. The PRKAG2 cardiac syndrome.
Mutations in the γ2 subunit of AMPK (the PRKAG2 gene) appear to cause both activating and inhibiting forms of cardiac AMPK. Various changes in the control of glucose utilization and storage ensue that result in a glycogen storage cardiomyopathy that is associated with the development of Wolff–Parkinson–White Syndrome and cardiac hypertrophy.
A variety of mutations in the γ2 gene of AMPK (PRKAG2) have been shown to produce a glycogen storage cardiomyopathy characterized by ventricular pre-excitation (Wolff–Parkinson–White (WPW) syndrome), progressive conduction system disease (Blair et al. 2001; Gollob et al. 2001a,b; Arad et al. 2002; Vaughan et al. 2003), and in specialized cases, cardiac hypertrophy (MacRae et al. 1995; Gollob, 2003). The disease phenotype associated with patients with these mutations has been termed the PRKAG2 cardiac syndrome (Gollob, 2003). It is believed that conduction system abnormalities observed in the PRKAG2 cardiac syndrome originate from glycogen-filled myocytes causing pre-excitation muscular bypass tracts (Gollob et al. 2001b; Arad et al. 2002). These tracts independently connect the atrial and ventricular chambers and bypass the normal atrio-ventricular node. This bypass tract is used as a retrograde limb of the re-entrant circuit, thus resulting in a macro-re-entrant arrhythmia. However, this rapid anterograde bypass tract conduction that causes pre-excitation and ventricular fusion is not arrhythmogenic per se, nor is it associated with tachycardia. Although a number of mutations in the human PRKAG2 gene have been identified (Oliveira et al. 2003), two of the best-characterized mutations are the missense mutations that occur independently in the DNA coding for amino acid 488 and for amino acid 302 of the γ2 subunit (Gollob et al. 2001a; Arad et al. 2003; Patel et al. 2003). These mutations result in an asparagine and an arginine being converted to an isoleucine and a glutamine, respectively (termed PRKAG2 N488I and PRKAG2 R302Q mutations).
In transgenic mouse models, over-expression of the γ2 N488I mutation results in dramatic left ventricular hypertrophy (Arad et al. 2003). This hypertrophic growth is associated with significant accumulation (30-fold) of glycogen within the cardiac myocyte of the transgenic mice and has been attributed to an increase in AMPK activity (Arad et al. 2003). Using 31P-NMR spectroscopy of isolated mouse hearts and subsequent HPLC analysis it was reported that free and total AMP/ATP ratios were not different in wild-type and transgenic mice expressing the γ2 N488I mutation (Zou et al. 2005). Despite this, AMPKα2 activity was significantly increased in the transgenic mice expressing the γ2 N488I mutation at saturating AMP concentrations, but overall was less responsive to AMP (Zou et al. 2005). The importance of increased basal AMPKα2 activity in this pathway is consistent with another transgenic model where a dominant-negative mutant of AMPK can attenuate the disease phenotype associated with the γ2 N488I mutation (Ahmad et al. 2005). Overall, it appears that the γ2 N488I mutation causes a higher basal activity of cardiac AMPKα2, which is likely to be due to increased phosphorylation at Thr172 (Zou et al. 2005). In addition, this mutation in the γ2 subunit appears to render the holoenzyme insensitive to increased AMP concentrations (Zou et al. 2005).
Another transgenic mouse has been constructed that expresses the γ2 R302Q mutation (Sidhu et al. 2005). Similar to the PRKAG2 N488I mice, these mice also have increased cardiac glycogen levels, develop WPW syndrome, and display profound cardiac hypertrophy. In contrast to the γ2 N488I mutation, hearts from transgenic mice over-expressing the γ2 R302Q mutation have decreased AMPK activity (Sidhu et al. 2005). Since the γ2 N488I mutant and the γ2 R302Q mutant expressing mice have a very similar phenotype, it is difficult to reconcile how this remarkably similar phenotype occurs with opposite actions of AMPK. However, it is possible that dysregulation of AMPK activity (either activating or inhibiting) contributes to the development of WPW syndrome (Fig. 3). That is, perturbations in AMPK signalling must alter glucose uptake, glucose storage as glycogen and glucose utilization, and together these mismatches may contribute to the development of the conduction abnormalities associated with WPW syndrome, secondary to glycogen accumulation (Lang et al. 2000; Blair et al. 2001; Gollob et al. 2001a,b; Arad et al. 2002, 2003; Gollob, 2003; Light et al. 2003; Oliveira et al. 2003; Patel et al. 2003; Vaughan et al. 2003). In addition, it is possible that glycogen also feeds back and alters AMPK activity via a mechanism that is dependent on the γ2 mutations (Fig. 3). Indeed, a third transgenic mouse line harbouring the R531G mutation in the γ2 subunit of AMPK also develops a progressive cardiac phenotype consistent with that observed with the γ2 N488I and γ2 R302Q mutations (Davies et al. 2005). In this mouse, cardiac hypertrophy and glycogen accumulation developed by 4 weeks of age but was absent at 1 week of age (Davies et al. 2005). At 1 week of age, the transgenic mice expressing the γ2 R531G mutation had similar AMPK activities as wild-type mice but displayed reduced AMPK activity by 4 weeks of age, suggesting that AMPK activity is inhibited as a result of glycogen accumulation. Taken together, these transgenic mouse models suggest that the pathogenesis of WPW syndrome relating to mutations in the PRKAG2 gene is not a simple loss or gain of function mutation of AMPK and may be more complex than first proposed. Indeed, although profound glycogen accumulation may be one mechanism by which the conduction system abnormalities arise in patients with PRKAG2 mutations, there is also the possibility that various ion channels are regulated by AMPK, thus contributing to the observed arrhythmogenic activity in patients with some PRKAG2 mutations (Light et al. 2003). These possibilities also need to be explored.
Although there is still controversy as to whether some of the mutations in the γ2 subunit of AMPK are activating or inactivating mutations of AMPK, it is clear that two of the best-characterized mutations in the human PRKAG2 gene (γ2 R302Q and γ2 N488I) are associated with the PRKAG2 cardiac syndrome (Gollob, 2003). The PRKAG2 cardiac syndrome is defined by ventricular pre-excitation, progressive conduction system disease, and cardiac hypertrophy (Gollob, 2003), all of which may occur directly or indirectly as a result of altered glucose homeostasis within the cardiac myocyte. Evidence for the γ2 N488I mutation acting as an AMPK-activating mutation is growing, which suggests in this case that inappropriate activation of AMPK and/or diminished responsiveness to intracellular AMP concentrations can have profound morphological consequences in the heart. These data suggest that activation of AMPK may have dire consequences in the heart and may contribute to the development of the PRKAG2 cardiac syndrome. However, it must be considered that global activation of cardiac AMPK may not, in and of itself, be involved the PRKAG2 cardiac syndrome since the effect seems to be confined to only the AMPK complexes that contain the γ2 subunit. Since other γ isoforms also exist in the heart (Stapleton et al. 1996; Thornton et al. 1998; Cheung et al. 2000), it is likely that the AMPK holoenzyme containing the γ2 subunit does not account for 100% of the cardiac AMPK activity. This suggests the γ2 subunit-containing AMPK complex may have specialized roles and/or specific targets that are necessary mediators of glycogen accumulation and the resulting ventricular pre-excitation, progressive conduction system disease, and cardiac hypertrophy. Clarification of the targets of the γ2 subunit-containing AMPK complex are necessary before a clear understanding of how perturbations in AMPK signalling influence the development of this disease. Thus, it is too early to predict how pharmacological activation or inhibition of AMPK will influence the development of the PRKAG2 cardiac syndrome.
Summary
AMPK has a very important role in the heart in regulating energy metabolism and protein synthesis. Whether AMPK has a role in mediating the increased energy demand during increases in cardiac work has yet to be fully established. In contrast, activation of AMPK during myocardial ischaemia, apoptosis and cardiac hypertrophy has been clearly established. However, what is less clear is whether this activation of AMPK acts as an ally or enemy in response to these cardiac stresses. Better definition of the role of AMPK in cardiac physiology and pathology has the potential to develop new therapeutic strategies to treat both ischaemic heart disease and cardiac hypertrophy.
Acknowledgments
G.D.L. is an Alberta Heritage Foundation for Medical Research Medical Scientist. J.R.B.D. is an Alberta Heritage Foundation for Medical Research Senior Scholar and a Canada Research Chair in Molecular Biology of Heart Disease and Metabolism.
References
- Adams J, Chen ZP, Van Denderen BJ, Morton CJ, Parker MW, Witters LA, Stapleton D, Kemp BE. Intrasteric control of AMPK via the γ1 subunit AMP allosteric regulatory site. Protein Sci. 2004;13:155–165. doi: 10.1110/ps.03340004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad F, Arad M, Musi N, He H, Wolf C, Branco D, Perez-Atayde AR, Stapleton D, Bali D, Xing Y, Tian R, Goodyear LJ, Berul CI, Ingwall JS, Seidman CE, Seidman JG. Increased α2 subunit-associated AMPK activity and PRKAG2 cardiomyopathy. Circulation. 2005;112:3140–3148. doi: 10.1161/CIRCULATIONAHA.105.550806. [DOI] [PubMed] [Google Scholar]
- Allard MF, Flint JD, English JC, Henning SL, Salamanca MC, Kamimura CT, English DR. Calcium overload during reperfusion is accelerated in isolated hypertrophied rat hearts. J Mol Cell Cardiol. 1994a;26:1551–1563. doi: 10.1006/jmcc.1994.1175. [DOI] [PubMed] [Google Scholar]
- Allard MF, Schonekess BO, Henning SL, English DR, Lopaschuk GD. Contribution of oxidative metabolism and glycolysis to ATP production in hypertrophied hearts. Am J Physiol. 1994b;267:H742–H750. doi: 10.1152/ajpheart.1994.267.2.H742. [DOI] [PubMed] [Google Scholar]
- Allard MF, Wambolt RB, Longnus SL, Grist M, Lydell CP, Parsons HL, Rodrigues B, Hall JL, Stanley WC, Bondy GP. Hypertrophied rat hearts are less responsive to the metabolic and functional effects of insulin. Am J Physiol Endocrinol Metab. 2000;279:E487–E493. doi: 10.1152/ajpendo.2000.279.3.E487. [DOI] [PubMed] [Google Scholar]
- Altarejos JY, Taniguchi M, Clanachan AS, Lopaschuk GD. Myocardial ischemia differentially regulates LKB1 and an alternate 5′-AMP-activated protein kinase kinase. J Biol Chem. 2005;280:183–190. doi: 10.1074/jbc.M411810200. [DOI] [PubMed] [Google Scholar]
- An D, Pulinilkunnil T, Qi D, Ghosh S, Abrahani A, Rodrigues B. The metabolic ‘switch’ AMPK regulates cardiac heparin-releasable lipoprotein lipase. Am J Physiol Endocrinol Metab. 2005;288:E246–E253. doi: 10.1152/ajpendo.00211.2004. [DOI] [PubMed] [Google Scholar]
- Anderson PG, Allard MF, Thomas GD, Bishop SP, Digerness SB. Increased ischemic injury but decreased hypoxic injury in hypertrophied rat hearts. Circ Res. 1990;67:948–959. doi: 10.1161/01.res.67.4.948. [DOI] [PubMed] [Google Scholar]
- Anderson KA, Means RL, Huang QH, Kemp BE, Goldstein EG, Selbert MA, Edelman AM, Fremeau RT, Means AR. Components of a calmodulin-dependent protein kinase cascade. Molecular cloning, functional characterization and cellular localization of Ca2+/calmodulin-dependent protein kinase kinase β. J Biol Chem. 1998;273:31880–31889. doi: 10.1074/jbc.273.48.31880. [DOI] [PubMed] [Google Scholar]
- Arad M, Benson DW, Perez-Atayde AR, McKenna WJ, Sparks EA, Kanter RJ, McGarry K, Seidman JG, Seidman CE. Constitutively active AMP kinase mutations cause glycogen storage disease mimicking hypertrophic cardiomyopathy. J Clin Invest. 2002;109:357–362. doi: 10.1172/JCI14571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arad M, Moskowitz IP, Patel VV, Ahmad F, Perez-Atayde AR, Sawyer DB, Walter M, Li GH, Burgon PG, Maguire CT, Stapleton D, Schmitt JP, Guo XX, Pizard A, Kupershmidt S, Roden DM, Berul CI, Seidman CE, Seidman JG. Transgenic mice overexpressing mutant PRKAG2 define the cause of Wolff-Parkinson-White syndrome in glycogen storage cardiomyopathy. Circulation. 2003;107:2850–2856. doi: 10.1161/01.CIR.0000075270.13497.2B. [DOI] [PubMed] [Google Scholar]
- Baron SJ, Li J, Russell RR, 3rd, Neumann D, Miller EJ, Tuerk R, Wallimann T, Hurley RL, Witters LA, Young LH. Dual mechanisms regulating AMPK kinase action in the ischemic heart. Circ Res. 2005;96:337–345. doi: 10.1161/01.RES.0000155723.53868.d2. [DOI] [PubMed] [Google Scholar]
- Beauloye C, Bertrand L, Krause U, Marsin AS, Dresselaers T, Vanstapel F, Vanoverschelde JL, Hue L. No-flow ischemia inhibits insulin signaling in heart by decreasing intracellular pH. Circ Res. 2001a;88:513–519. doi: 10.1161/01.res.88.5.513. [DOI] [PubMed] [Google Scholar]
- Beauloye C, Marsin AS, Bertrand L, Krause U, Hardie DG, Vanoverschelde JL, Hue L. Insulin antagonizes AMP-activated protein kinase activation by ischemia or anoxia in rat hearts, without affecting total adenine nucleotides. FEBS Lett. 2001b;505:348–352. doi: 10.1016/s0014-5793(01)02788-0. [DOI] [PubMed] [Google Scholar]
- Beauloye C, Marsin AS, Bertrand L, Vanoverschelde JL, Rider MH, Hue L. The stimulation of heart glycolysis by increased workload does not require AMP-activated protein kinase but a wortmannin-sensitive mechanism. FEBS Lett. 2002;531:324–328. doi: 10.1016/s0014-5793(02)03552-4. [DOI] [PubMed] [Google Scholar]
- Blair E, Redwood C, Ashrafian H, Oliveira M, Broxholme J, Kerr B, Salmon A, Ostman-Smith I, Watkins H. Mutations in the γ2 subunit of AMP-activated protein kinase cause familial hypertrophic cardiomyopathy: evidence for the central role of energy compromise in disease pathogenesis. Hum Mol Genet. 2001;10:1215–1220. doi: 10.1093/hmg/10.11.1215. [DOI] [PubMed] [Google Scholar]
- Browne GJ, Finn SG, Proud CG. Stimulation of the AMP-activated protein kinase leads to activation of eukaryotic elongation factor 2 kinase and to its phosphorylation at a novel site, serine 398. J Biol Chem. 2004;279:12220–12231. doi: 10.1074/jbc.M309773200. [DOI] [PubMed] [Google Scholar]
- Browne GJ, Proud CG. A novel mTOR-regulated phosphorylation site in elongation factor 2 kinase modulates the activity of the kinase and its binding to calmodulin. Mol Cell Biol. 2004;24:2986–2997. doi: 10.1128/MCB.24.7.2986-2997.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capano M, Crompton M. Bax translocates to mitochondria of heart cells during simulated ischaemia: involvement of AMP-activated and p38 mitogen-activated protein kinases. Biochem J. 2006;395:57–64. doi: 10.1042/BJ20051654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carling D, Hardie DG. The substrate and sequence specificity of the AMP-activated protein kinase. Phosphorylation of glycogen synthase and phosphorylase kinase. Biochim Biophys Acta. 1989;1012:81–86. doi: 10.1016/0167-4889(89)90014-1. [DOI] [PubMed] [Google Scholar]
- Chan AY, Soltys CL, Young ME, Proud CG, Dyck JR. Activation of AMP-activated protein kinase inhibits protein synthesis associated with hypertrophy in the cardiac myocyte. J Biol Chem. 2004;279:32771–32779. doi: 10.1074/jbc.M403528200. [DOI] [PubMed] [Google Scholar]
- Cheng SW, Fryer LG, Carling D, Shepherd PR. Thr2446 is a novel mammalian target of rapamycin (mTOR) phosphorylation site regulated by nutrient status. J Biol Chem. 2004;279:15719–15722. doi: 10.1074/jbc.C300534200. [DOI] [PubMed] [Google Scholar]
- Cheung PC, Salt IP, Davies SP, Hardie DG, Carling D. Characterization of AMP-activated protein kinase γ-subunit isoforms and their role in AMP binding. Biochem J. 2000;346:659–669. [PMC free article] [PubMed] [Google Scholar]
- Coven DL, Hu X, Cong L, Bergeron R, Shulman GI, Hardie DG, Young LH. Physiological role of AMP-activated protein kinase in the heart: graded activation during exercise. Am J Physiol Endocrinol Metab. 2003;285:E629–E636. doi: 10.1152/ajpendo.00171.2003. [DOI] [PubMed] [Google Scholar]
- Daniel T, Carling D. Functional analysis of mutations in the γ2 subunit of AMP-activated protein kinase associated with cardiac hypertrophy and Wolff-Parkinson-White syndrome. J Biol Chem. 2002;277:51017–51024. doi: 10.1074/jbc.M207093200. [DOI] [PubMed] [Google Scholar]
- Davies JK, Wells DJ, Liu K, Whitrow HR, Daniel TD, Grignani R, Lygate CA, Schneider JE, Noel G, Watkins H, Carling D. Characterisation of the role of the γ2 R531G mutation in AMP-activated protein kinase in cardiac hypertrophy and Wolff-Parkinson-White syndrome. Am J Physiol Heart Circ Physiol. 2005;290:H1942–H1951. doi: 10.1152/ajpheart.01020.2005. [DOI] [PubMed] [Google Scholar]
- Dennis SC, Gevers W, Opie LH. Protons in ischemia: where do they come from; where do they go to? J Mol Cell Cardiol. 1991;23:1077–1086. doi: 10.1016/0022-2828(91)91642-5. [DOI] [PubMed] [Google Scholar]
- Depre C, Wang L, Sui X, Qiu H, Hong C, Hedhli N, Ginion A, Shah A, Pelat M, Bertrand L, Wagner T, Gaussin V, Vatner SF. H11 kinase prevents myocardial infarction by preemptive preconditioning of the heart. Circ Res. 2006;98:280–288. doi: 10.1161/01.RES.0000201284.45482.e8. [DOI] [PubMed] [Google Scholar]
- Dorn GW, 2nd, Force T. Protein kinase cascades in the regulation of cardiac hypertrophy. J Clin Invest. 2005;115:527–537. doi: 10.1172/JCI24178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyck JR, Barr AJ, Barr RL, Kolattukudy PE, Lopaschuk GD. Characterization of cardiac malonyl-CoA decarboxylase and its putative role in regulating fatty acid oxidation. Am J Physiol. 1998;275:H2122–H2129. doi: 10.1152/ajpheart.1998.275.6.H2122. [DOI] [PubMed] [Google Scholar]
- Dyck JRB, Gao G, Widmer J, Stapleton D, Fernandez CS, Kemp BE, Witters LA. Regulation of 5′-AMP-activated protein kinase activity by the noncatalytic β and γ subunits. J Biol Chem. 1996;271:17798–17803. doi: 10.1074/jbc.271.30.17798. [DOI] [PubMed] [Google Scholar]
- Fischer Y, Thomas J, Sevilla L, Munoz P, Becker C, Holman G, Kozka IJ, Palacin M, Testar X, Kammermeier H, Zorzano A. Insulin-induced recruitment of glucose transporter 4 (GLUT4) and GLUT1 in isolated rat cardiac myocytes. Evidence of the existence of different intracellular GLUT4 vesicle populations. J Biol Chem. 1997;272:7085–7092. doi: 10.1074/jbc.272.11.7085. [DOI] [PubMed] [Google Scholar]
- Franz WM, Muller OJ, Katus HA. Cardiomyopathies: from genetics to the prospect of treatment. Lancet. 2001;358:1627–1637. doi: 10.1016/S0140-6736(01)06657-0. [DOI] [PubMed] [Google Scholar]
- Frederich M, Balschi JA. The relationship between AMP-activated protein kinase activity and AMP concentration in the isolated perfused rat heart. J Biol Chem. 2002;277:1928–1932. doi: 10.1074/jbc.M107128200. [DOI] [PubMed] [Google Scholar]
- Frederich M, Zhang L, Balschi JA. Hypoxia and AMP independently regulate AMP-activated protein kinase activity in heart. Am J Physiol Heart Circ Physiol. 2005;288:H2412–H2421. doi: 10.1152/ajpheart.00558.2004. [DOI] [PubMed] [Google Scholar]
- Frey N, Olson EN. Cardiac hypertrophy: the good, the bad, and the ugly. Annu Rev Physiol. 2003;65:45–79. doi: 10.1146/annurev.physiol.65.092101.142243. [DOI] [PubMed] [Google Scholar]
- Gaasch WH, Zile MR, Hoshino PK, Weinberg EO, Rhodes DR, Apstein CS. Tolerance of the hypertrophic heart to ischemia. Studies in compensated and failing dog hearts with pressure overload hypertrophy. Circulation. 1990;81:1644–1653. doi: 10.1161/01.cir.81.5.1644. [DOI] [PubMed] [Google Scholar]
- Gamble J, Lopaschuk GD. Insulin inhibition of 5′-adenosine monophosphate-activated protein kinase in the heart results in activation of acetyl coenzyme A carboxylase and inhibition of fatty acid oxidation. Metabolism. 1997;46:1270–1274. doi: 10.1016/s0026-0495(97)90229-8. [DOI] [PubMed] [Google Scholar]
- Gao G, Fernandez CS, Stapleton D, Auster AS, Widmer J, Dyck JR, Kemp BE, Witters LA. Non-catalytic β- and γ-subunit isoforms of the 5′-AMP-activated protein kinase. J Biol Chem. 1996;271:8675–8681. doi: 10.1074/jbc.271.15.8675. [DOI] [PubMed] [Google Scholar]
- Gollob MH. Glycogen storage disease as a unifying mechanism of disease in the PRKAG2 cardiac syndrome. Biochem Soc Trans. 2003;31:228–231. doi: 10.1042/bst0310228. [DOI] [PubMed] [Google Scholar]
- Gollob MH, Green MS, Tang AS, Gollob T, Karibe A, Ali Hassan AS, Ahmad F, Lozado R, Shah G, Fananapazir L, Bachinski LL, Roberts R, Hassan AS. Identification of a gene responsible for familial Wolff-Parkinson-White syndrome. N Engl J Med. 2001a;344:1823–1831. doi: 10.1056/NEJM200106143442403. [DOI] [PubMed] [Google Scholar]
- Gollob MH, Seger JJ, Gollob TN, Tapscott T, Gonzales O, Bachinski L, Roberts R. Novel PRKAG2 mutation responsible for the genetic syndrome of ventricular preexcitation and conduction system disease with childhood onset and absence of cardiac hypertrophy. Circulation. 2001b;104:3030–3033. doi: 10.1161/hc5001.102111. [DOI] [PubMed] [Google Scholar]
- Goodwin GW, Taegtmeyer H. Regulation of fatty acid oxidation of the heart by MCD and ACC during contractile stimulation. Am J Physiol. 1999;277:E772–E777. doi: 10.1152/ajpendo.1999.277.4.E772. [DOI] [PubMed] [Google Scholar]
- Habinowski SA, Hirshman M, Sakamoto K, Kemp BE, Gould SJ, Goodyear LJ, Witters LA. Malonyl-CoA decarboxylase is not a substrate of AMP-activated protein kinase in rat fast-twitch skeletal muscle or an islet cell line. Arch Biochem Biophys. 2001;396:71–79. doi: 10.1006/abbi.2001.2589. [DOI] [PubMed] [Google Scholar]
- Hall JL, Lopaschuk GD, Barr A, Bringas J, Pizzurro RD, Stanley WC. Increased cardiac fatty acid uptake with dobutamine infusion in swine is accompanied by a decrease in malonyl CoA levels. Cardiovasc Res. 1996;32:879–885. [PubMed] [Google Scholar]
- Hamilton SR, Stapleton D, O'Donnell JB, Jr, Kung JT, Dalal SR, Kemp BE, Witters LA. An activating mutation in the γ1 subunit of the AMP-activated protein kinase. FEBS Lett. 2001;500:163–168. doi: 10.1016/s0014-5793(01)02602-3. [DOI] [PubMed] [Google Scholar]
- Hannan RD, Jenkins A, Jenkins AK, Brandenburger Y. Cardiac hypertrophy: a matter of translation. Clin Exp Pharmacol Physiol. 2003;30:517–527. doi: 10.1046/j.1440-1681.2003.03873.x. [DOI] [PubMed] [Google Scholar]
- Hardie DG, Carling D. The AMP-activated protein kinase – fuel gauge of the mammalian cell? Eur J Biochem. 1997;246:259–273. doi: 10.1111/j.1432-1033.1997.00259.x. [DOI] [PubMed] [Google Scholar]
- Hickson-Bick DL, Buja ML, McMillin JB. Palmitate-mediated alterations in the fatty acid metabolism of rat neonatal cardiac myocytes. J Mol Cell Cardiol. 2000;32:511–519. doi: 10.1006/jmcc.1999.1098. [DOI] [PubMed] [Google Scholar]
- Hopkins TA, Dyck JR, Lopaschuk GD. AMP-activated protein kinase regulation of fatty acid oxidation in the ischaemic heart. Biochem Soc Trans. 2003;31:207–212. doi: 10.1042/bst0310207. [DOI] [PubMed] [Google Scholar]
- Horman S, Beauloye C, Vertommen D, Vanoverschelde JL, Hue L, Rider MH. Myocardial ischemia and increased heart work modulate the phosphorylation state of eukaryotic elongation factor-2. J Biol Chem. 2003;278:41970–41976. doi: 10.1074/jbc.M302403200. [DOI] [PubMed] [Google Scholar]
- Horman S, Vertommen D, Heath R, Neumann D, Mouton V, Woods A, Schlattner U, Wallimann T, Carling D, Hue L, Rider MH. Insulin antagonizes ischemia-induced Thr172 phosphorylation of AMP-activated protein kinase α-subunits in heart via hierarchical phosphorylation of Ser485/491. J Biol Chem. 2005;281:5335–5340. doi: 10.1074/jbc.M506850200. [DOI] [PubMed] [Google Scholar]
- Hudson ER, Pan DA, James J, Lucocq JM, Hawley SA, Green KA, Baba O, Terashima T, Hardie DG. A novel domain in AMP-activated protein kinase causes glycogen storage bodies similar to those seen in hereditary cardiac arrhythmias. Curr Biol. 2003;13:861–866. doi: 10.1016/s0960-9822(03)00249-5. [DOI] [PubMed] [Google Scholar]
- Igata M, Motoshima H, Tsuruzoe K, Kojima K, Matsumura T, Kondo T, Taguchi T, Nakamaru K, Yano M, Kukidome D, Matsumoto K, Toyonaga T, Asano T, Nishikawa T, Araki E. Adenosine monophosphate-activated protein kinase suppresses vascular smooth muscle cell proliferation through the inhibition of cell cycle progression. Circ Res. 2005;97:837–844. doi: 10.1161/01.RES.0000185823.73556.06. [DOI] [PubMed] [Google Scholar]
- Inoki K, Zhu T, Guan KL. TSC2 mediates cellular energy response to control cell growth and survival. Cell. 2003;115:577–590. doi: 10.1016/s0092-8674(03)00929-2. [DOI] [PubMed] [Google Scholar]
- Jorgensen SB, Viollet B, Andreelli F, Frosig C, Birk JB, Schjerling P, Vaulont S, Richter EA, Wojtaszewski JF. Knockout of the α2 but not α1 5′-AMP-activated protein kinase isoform abolishes 5-aminoimidazole-4-carboxamide-1-β-4-ribofuranosidebut not contraction-induced glucose uptake in skeletal muscle. J Biol Chem. 2004;279:1070–1079. doi: 10.1074/jbc.M306205200. [DOI] [PubMed] [Google Scholar]
- Kantor PF, Dyck JR, Lopaschuk GD. Fatty acid oxidation in the reperfused ischemic heart. Am J Med Sci. 1999;318:3–14. doi: 10.1097/00000441-199907000-00002. [DOI] [PubMed] [Google Scholar]
- Kefas BA, Cai Y, Ling Z, Heimberg H, Hue L, Pipeleers D, Van de Casteele M. AMP-activated protein kinase can induce apoptosis of insulin-producing MIN6 cells through stimulation of c-Jun-N-terminal kinase. J Mol Endocrinol. 2003;30:151–161. doi: 10.1677/jme.0.0300151. [DOI] [PubMed] [Google Scholar]
- Kovacic S, Soltys CL, Barr AJ, Shiojima I, Walsh K, Dyck JR. Akt activity negatively regulates phosphorylation of AMP-activated protein kinase in the heart. J Biol Chem. 2003;278:39422–39427. doi: 10.1074/jbc.M305371200. [DOI] [PubMed] [Google Scholar]
- Kuang M, Febbraio M, Wagg C, Lopaschuk GD, Dyck JR. Fatty acid translocase/CD36 deficiency does not energetically or functionally compromise hearts before or after ischemia. Circulation. 2004;109:1550–1557. doi: 10.1161/01.CIR.0000121730.41801.12. [DOI] [PubMed] [Google Scholar]
- Kudo N, Barr AJ, Barr RL, Desai S, Lopaschuk GD. High rates of fatty acid oxidation during reperfusion of ischemic hearts are associated with a decrease in malonyl CoA levels due to an increase in 5′-AMP-activated protein kinase inhibition of acetyl-CoA carboxylase. J Biol Chem. 1995;270:17513–17520. doi: 10.1074/jbc.270.29.17513. [DOI] [PubMed] [Google Scholar]
- Kudo N, Gillespie JG, Kung L, Witters LA, Schulz R, Clanachan AS, Lopaschuk GD. Characterization of 5′AMP-activated protein kinase activity in the heart and its role in inhibiting acetyl-CoA carboxylase during reperfusion following ischemia. Biochim Biophys Acta. 1996;1301:67–75. doi: 10.1016/0005-2760(96)00013-6. [DOI] [PubMed] [Google Scholar]
- Lang TYuL, Tu Q, Jiang J, Chen Z, Xin Y, Liu G, Zhao S. Molecular cloning, genomic organization, and mapping of PRKAG2, a heart abundant γ2 subunit of 5′-AMP-activated protein kinase, to human chromosome 7q36. Genomics. 2000;70:258–263. doi: 10.1006/geno.2000.6376. [DOI] [PubMed] [Google Scholar]
- Latronico MV, Costinean S, Lavitrano ML, Peschle C, Condorelli G. Regulation of cell size and contractile function by AKT in cardiomyocytes. Ann N Y Acad Sci. 2004;1015:250–260. doi: 10.1196/annals.1302.021. [DOI] [PubMed] [Google Scholar]
- Lehman JJ, Kelly DP. Gene regulatory mechanisms governing energy metabolism during cardiac hypertrophic growth. Heart Fail Rev. 2002;7:175–185. doi: 10.1023/a:1015332726303. [DOI] [PubMed] [Google Scholar]
- Li J, Miller EJ, Ninomiya-Tsuji J, Russell RR, 3rd, Young LH. AMP-activated protein kinase activates p38 mitogen-activated protein kinase by increasing recruitment of p38 MAPK to TAB1 in the ischemic heart. Circ Res. 2005;97:872–879. doi: 10.1161/01.RES.0000187458.77026.10. [DOI] [PubMed] [Google Scholar]
- Liao Y, Takashima S, Maeda N, Ouchi N, Komamura K, Shimomura I, Hori M, Matsuzawa Y, Funahashi T, Kitakaze M. Exacerbation of heart failure in adiponectin-deficient mice due to impaired regulation of AMPK and glucose metabolism. Cardiovasc Res. 2005;67:705–713. doi: 10.1016/j.cardiores.2005.04.018. [DOI] [PubMed] [Google Scholar]
- Light PE, Wallace CH, Dyck JR. Constitutively active adenosine monophosphate-activated protein kinase regulates voltage-gated sodium channels in ventricular myocytes. Circulation. 2003;107:1962–1965. doi: 10.1161/01.CIR.0000069269.60167.02. [DOI] [PubMed] [Google Scholar]
- Liu B, Clanachan AS, Schulz R, Lopaschuk GD. Cardiac efficiency is improved after ischemia by altering both the source and fate of protons. Circ Res. 1996;79:940–948. doi: 10.1161/01.res.79.5.940. [DOI] [PubMed] [Google Scholar]
- Longnus SL, Segalen C, Giudicelli J, Sajan MP, Farese RV, Van Obberghen E. Insulin signalling downstream of protein kinase B is potentiated by 5′AMP-activated protein kinase in rat hearts in vivo. Diabetologia. 2005;48:2591–2601. doi: 10.1007/s00125-005-0016-3. [DOI] [PubMed] [Google Scholar]
- Longnus SL, Wambolt RB, Barr RL, Lopaschuk GD, Allard MF. Regulation of myocardial fatty acid oxidation by substrate supply. Am J Physiol Heart Circ Physiol. 2001;281:H1561–H1567. doi: 10.1152/ajpheart.2001.281.4.H1561. [DOI] [PubMed] [Google Scholar]
- Lopaschuk GD, Collins-Nakai R, Olley PM, Montague TJ, McNeil G, Gayle M, Penkoske P, Finegan BA. Plasma fatty acid levels in infants and adults after myocardial ischemia. Am Heart J. 1994;128:61–67. doi: 10.1016/0002-8703(94)90010-8. [DOI] [PubMed] [Google Scholar]
- Lorell BH, Carabello BA. Left ventricular hypertrophy: pathogenesis, detection, and prognosis. Circulation. 2000;102:470–479. doi: 10.1161/01.cir.102.4.470. [DOI] [PubMed] [Google Scholar]
- Luiken JJ, Coort SL, Willems J, Coumans WA, Bonen A, Van Der Vusse GJ, Glatz JF. Contraction-induced fatty acid translocase/CD36 translocation in rat cardiac myocytes is mediated through AMP-activated protein kinase signaling. Diabetes. 2003;52:1627–1634. doi: 10.2337/diabetes.52.7.1627. [DOI] [PubMed] [Google Scholar]
- McLeod LE, Proud CG. ATP depletion increases phosphorylation of elongation factor eEF2 in adult cardiomyocytes independently of inhibition of mTOR signalling. FEBS Lett. 2002;531:448–452. doi: 10.1016/s0014-5793(02)03582-2. [DOI] [PubMed] [Google Scholar]
- MacRae CA, Ghaisas N, Kass S, Donnelly S, Basson CT, Watkins HC, Anan R, Thierfelder LH, McGarry K, Rowland E, et al. Familial hypertrophic cardiomyopathy with Wolff-Parkinson-White syndrome maps to a locus on chromosome 7q3. J Clin Invest. 1995;96:1216–1220. doi: 10.1172/JCI118154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahlapuu M, Johansson C, Lindgren K, Hjalm G, Barnes BR, Krook A, Zierath JR, Andersson L, Marklund S. Expression profiling of the γ-subunit isoforms of AMP-activated protein kinase suggests a major role for γ3 in white skeletal muscle. Am J Physiol Endocrinol Metab. 2004;286:E194–E200. doi: 10.1152/ajpendo.00147.2003. [DOI] [PubMed] [Google Scholar]
- Makinde AO, Gamble J, Lopaschuk GD. Upregulation of 5′-AMP-activated protein kinase is responsible for the increase in myocardial fatty acid oxidation rates following birth in the newborn rabbit. Circulation Res. 1997;80:482–489. doi: 10.1161/01.res.80.4.482. [DOI] [PubMed] [Google Scholar]
- Marsin AS, Bertrand L, Rider MH, Deprez J, Beauloye C, Vincent MF, Van den Berghe G, Carling D, Hue L. Phosphorylation and activation of heart PFK-2 by AMPK has a role in the stimulation of glycolysis during ischaemia. Curr Biol. 2000;10:1247–1255. doi: 10.1016/s0960-9822(00)00742-9. [DOI] [PubMed] [Google Scholar]
- Meisse D, Van de Casteele M, Beauloye C, Hainault I, Kefas BA, Rider MH, Foufelle F, Hue L. Sustained activation of AMP-activated protein kinase induces c-Jun N-terminal kinase activation and apoptosis in liver cells. FEBS Lett. 2002;526:38–42. doi: 10.1016/s0014-5793(02)03110-1. [DOI] [PubMed] [Google Scholar]
- Merrill GF, Kurth EJ, Hardie DG, Winder WW. AICA riboside increases AMP-activated protein kinase, fatty acid oxidation, and glucose uptake in rat muscle. Am J Physiol. 1997;273:E1107–E1112. doi: 10.1152/ajpendo.1997.273.6.E1107. [DOI] [PubMed] [Google Scholar]
- Milan D, Jeon JT, Looft C, Amarger V, Robic A, Thelander M, Rogel-Gaillard C, Paul S, Iannuccelli N, Rask L, Ronne H, Lundstrom K, Reinsch N, Gellin J, Kalm E, Roy PL, Chardon P, Andersson L. A mutation in PRKAG3 associated with excess glycogen content in pig skeletal muscle. Science. 2000;288:1248–1251. doi: 10.1126/science.288.5469.1248. [DOI] [PubMed] [Google Scholar]
- Mueller HS, Ayres SM. Metabolic response of the heart in acute myocardial infarction in man. Am J Cardiol. 1978;42:363–371. doi: 10.1016/0002-9149(78)90929-3. [DOI] [PubMed] [Google Scholar]
- Musi N, Hirshman MF, Arad M, Xing Y, Fujii N, Pomerleau J, Ahmad F, Berul CI, Seidman JG, Tian R, Goodyear LJ. Functional role of AMP-activated protein kinase in the heart during exercise. FEBS Lett. 2005;579:2045–2050. doi: 10.1016/j.febslet.2005.02.052. [DOI] [PubMed] [Google Scholar]
- Nishino Y, Miura T, Miki T, Sakamoto J, Nakamura Y, Ikeda Y, Kobayashi H, Shimamoto K. Ischemic preconditioning activates AMPK in a PKC-dependent manner and induces GLUT4 up-regulation in the late phase of cardioprotection. Cardiovasc Res. 2004;61:610–619. doi: 10.1016/j.cardiores.2003.10.022. [DOI] [PubMed] [Google Scholar]
- Oliveira SM, Ehtisham J, Redwood CS, Ostman-Smith I, Blair EM, Watkins H. Mutation analysis of AMP-activated protein kinase subunits in inherited cardiomyopathies: implications for kinase function and disease pathogenesis. J Mol Cell Cardiol. 2003;35:1251–1255. doi: 10.1016/s0022-2828(03)00237-2. [DOI] [PubMed] [Google Scholar]
- Oliver MF, Kurien VA, Greenwood TW. Relation between serum-free-fatty acids and arrhythmias and death after acute myocardial infarction. Lancet. 1968;1:710–714. doi: 10.1016/s0140-6736(68)92163-6. [DOI] [PubMed] [Google Scholar]
- Onay-Besikci A, Altarejos JY, Lopaschuk GD. gAd-globular head domain of adiponectin increases fatty acid oxidation in newborn rabbit hearts. J Biol Chem. 2004;279:44320–44326. doi: 10.1074/jbc.M400347200. [DOI] [PubMed] [Google Scholar]
- Patel VV, Arad M, Moskowitz IP, Maguire CT, Branco D, Seidman JG, Seidman CE, Berul CI. Electrophysiologic characterization and postnatal development of ventricular pre-excitation in a mouse model of cardiac hypertrophy and Wolff-Parkinson-White syndrome. J Am Coll Cardiol. 2003;42:942–951. doi: 10.1016/s0735-1097(03)00850-7. [DOI] [PubMed] [Google Scholar]
- Peralta C, Bartrons R, Serafin A, Blazquez C, Guzman M, Prats N, Xaus C, Cutillas B, Gelpi E, Rosello-Catafau J. Adenosine monophosphate-activated protein kinase mediates the protective effects of ischemic preconditioning on hepatic ischemia-reperfusion injury in the rat. Hepatology. 2001;34:1164–1173. doi: 10.1053/jhep.2001.29197. [DOI] [PubMed] [Google Scholar]
- Randle PJ, Garland PB, Newsholme EA, Hales CN. The glucose fatty acid cycle in obesity and maturity onset diabetes mellitus. Ann N Y Acad Sci. 1965;131:324–333. doi: 10.1111/j.1749-6632.1965.tb34800.x. [DOI] [PubMed] [Google Scholar]
- Russell RR, 3rd, Bergeron R, Shulman GI, Young LH. Translocation of myocardial GLUT-4 and increased glucose uptake through activation of AMPK by AICAR. Am J Physiol. 1999;277:H643–H649. doi: 10.1152/ajpheart.1999.277.2.H643. [DOI] [PubMed] [Google Scholar]
- Russell RR, 3rd, Li J, Coven DL, Pypaert M, Zechner C, Palmeri M, Giordano FJ, Mu J, Birnbaum MJ, Young LH. AMP-activated protein kinase mediates ischemic glucose uptake and prevents postischemic cardiac dysfunction, apoptosis, and injury. J Clin Invest. 2004;114:495–503. doi: 10.1172/JCI19297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saddik M, Gamble J, Witters LA, Lopaschuk GD. Acetyl-CoA carboxylase regulation of fatty acid oxidation in the heart. J Biol Chem. 1993;268:25836–25845. [PubMed] [Google Scholar]
- Saha AK, Schwarsin AJ, Roduit R, Masse F, Kaushik V, Tornheim K, Prentki M, Ruderman NB. Activation of malonyl-CoA decarboxylase in rat skeletal muscle by contraction and the AMP-activated protein kinase activator 5-aminoimidazole-4-carboxamide-1-β-D-ribofuranoside. J Biol Chem. 2000a;275:24279–24283. doi: 10.1074/jbc.C000291200. [DOI] [PubMed] [Google Scholar]
- Sakamoto K, Goransson O, Hardie DG, Alessi DR. Activity of LKB1 and AMPK-related kinases in skeletal muscle; effects of contraction, phenformin and AICAR. Am J Physiol Endocrinol Metab. 2004;287:E310–E317. doi: 10.1152/ajpendo.00074.2004. [DOI] [PubMed] [Google Scholar]
- Sakamoto K, Zarrinpashneh E, Budas GR, Pouleur AC, Dutta A, Prescott AR, Vanoverschelde JL, Ashworth A, Jovanovic A, Alessi DR, Bertrand L. Deficiency of LKB1 in heart prevents ischemia-mediated activation of AMPKα2 but not AMPKα1. Am J Physiol Endocrinol Metab. 2006;290:E780–E788. doi: 10.1152/ajpendo.00443.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambandam N, Lopaschuk GD, Brownsey RW, Allard MF. Energy metabolism in the hypertrophied heart. Heart Fail Rev. 2002;7:161–173. doi: 10.1023/a:1015380609464. [DOI] [PubMed] [Google Scholar]
- Schonekess BO, Allard MF, Lopaschuk GD. Recovery of glycolysis and oxidative metabolism during postischemic reperfusion of hypertrophied rat hearts. Am J Physiol. 1996;271:H798–H805. doi: 10.1152/ajpheart.1996.271.2.H798. [DOI] [PubMed] [Google Scholar]
- Shibata R, Ouchi N, Ito M, Kihara S, Shiojima I, Pimentel DR, Kumada M, Sato K, Schiekofer S, Ohashi K, Funahashi T, Colucci WS, Walsh K. Adiponectin-mediated modulation of hypertrophic signals in the heart. Nat Med. 2004;10:1384–1389. doi: 10.1038/nm1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata R, Sato K, Pimentel DR, Takemura Y, Kihara S, Ohashi K, Funahashi T, Ouchi N, Walsh K. Adiponectin protects against myocardial ischemia-reperfusion injury through AMPK- and COX-2-dependent mechanisms. Nat Med. 2005;11:1096–1103. doi: 10.1038/nm1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shioi T, McMullen JR, Kang PM, Douglas PS, Obata T, Franke TF, Cantley LC, Izumo S. Akt/protein kinase B promotes organ growth in transgenic mice. Mol Cell Biol. 2002;22:2799–2809. doi: 10.1128/MCB.22.8.2799-2809.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiojima I, Yefremashvili M, Luo Z, Kureishi Y, Takahashi A, Tao J, Rosenzweig A, Kahn CR, Abel ED, Walsh K. Akt signaling mediates postnatal heart growth in response to insulin and nutritional status. J Biol Chem. 2002;277:37670–37677. doi: 10.1074/jbc.M204572200. [DOI] [PubMed] [Google Scholar]
- Sidhu JS, Rajawat YS, Rami TG, Gollob MH, Wang Z, Yuan R, Marian AJ, Demayo FJ, Weilbacher D, Taffet GE, Davies JK, Carling D, Khoury DS, Roberts R. Transgenic mouse model of ventricular preexcitation and atrioventricular reentrant tachycardia induced by an AMP-activated protein kinase loss-of-function mutation responsible for Wolff-Parkinson-White Syndrome. Circulation. 2005;111:21–29. doi: 10.1161/01.CIR.0000151291.32974.D5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soltys CL, Kovacic S, Dyck JR. Activation of cardiac AMP-activated protein kinase (AMPK) by LKB1 expression or chemical hypoxia is blunted by increased Akt activity. Am J Physiol Heart Circ Physiol. 2006;290:H2472–H2479. doi: 10.1152/ajpheart.01206.2005. [DOI] [PubMed] [Google Scholar]
- Stapleton D, Gao G, Michell BJ, Widmer J, Mitchelhill K, Teh T, House CM, Witters LA, Kemp BE. Mammalian 5′-AMP-activated protein kinase non-catalytic subunits are homologs of proteins that interact with yeast Snf1 protein kinase. J Biol Chem. 1994;269:29343–29346. [PubMed] [Google Scholar]
- Stapleton D, Mitchelhill KI, Gao G, Widmer J, Michell BJ, Teh T, House CM, Fernandez CS, Cox T, Witters LA, Kemp BE. Mammalian AMP-activated protein kinase subfamily. J Biol Chem. 1996;271:611–614. doi: 10.1074/jbc.271.2.611. [DOI] [PubMed] [Google Scholar]
- Sugden PH, Clerk A. Cellular mechanisms of cardiac hypertrophy. J Mol Med. 1998;76:725–746. doi: 10.1007/s001090050275. [DOI] [PubMed] [Google Scholar]
- Taegtmeyer H. Genetics of energetics: transcriptional responses in cardiac metabolism. Ann Biomed Eng. 2000;28:871–876. doi: 10.1114/1.1312187. [DOI] [PubMed] [Google Scholar]
- Thampy KG. Formation of malonyl coenzyme A in rat heart. Identification and purification of an isozyme of A carboxylase from rat heart. J Biol Chem. 1989;264:17631–17634. [PubMed] [Google Scholar]
- Thornton C, Snowden MA, Carling D. Identification of a novel AMP-activated protein kinase β subunit isoform that is highly expressed in skeletal muscle. J Biol Chem. 1998;273:12443–12450. doi: 10.1074/jbc.273.20.12443. [DOI] [PubMed] [Google Scholar]
- Tian R, Musi N, D'Agostino J, Hirshman MF, Goodyear LJ. Increased adenosine monophosphate-activated protein kinase activity in rat hearts with pressure-overload hypertrophy. Circulation. 2001;104:1664–1669. doi: 10.1161/hc4001.097183. [DOI] [PubMed] [Google Scholar]
- Vaughan CJ, Hom Y, Okin DA, McDermott DA, Lerman BB, Basson CT. Molecular genetic analysis of PRKAG2 in sporadic Wolff-Parkinson-White syndrome. J Cardiovasc Electrophysiol. 2003;14:263–268. doi: 10.1046/j.1540-8167.2003.02394.x. [DOI] [PubMed] [Google Scholar]
- Vavvas D, Apazidis A, Saha AK, Gamble J, Patel A, Kemp BE, Witters LA, Ruderman NB. Contraction-induced changes in acetyl-CoA carboxylase and 5′-AMP-activated kinase in skeletal muscle. J Biol Chem. 1997;272:13255–13261. doi: 10.1074/jbc.272.20.13255. [DOI] [PubMed] [Google Scholar]
- Viollet B, Andreelli F, Jorgensen SB, Perrin C, Geloen A, Flamez D, Mu J, Lenzner C, Baud O, Bennoun M, Gomas E, Nicolas G, Wojtaszewski JF, Kahn A, Carling D, Schuit FC, Birnbaum MJ, Richter EA, Burcelin R, Vaulont S. The AMP-activated protein kinase α2 catalytic subunit controls whole-body insulin sensitivity. J Clin Invest. 2003;111:91–98. doi: 10.1172/JCI16567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wambolt RB, Henning SL, English DR, Dyachkova Y, Lopaschuk GD, Allard MF. Glucose utilization and glycogen turnover are accelerated in hypertrophied rat hearts during severe low-flow ischemia. J Mol Cellular Cardiol. 1999;31:493–502. doi: 10.1006/jmcc.1998.0804. [DOI] [PubMed] [Google Scholar]
- Wojtaszewski JF, Jorgensen SB, Hellsten Y, Hardie DG, Richter EA. Glycogen-dependent effects of 5-aminoimidazole-4-carboxamide (AICA)-riboside on AMP-activated protein kinase and glycogen synthase activities in rat skeletal muscle. Diabetes. 2002;51:284–292. doi: 10.2337/diabetes.51.2.284. [DOI] [PubMed] [Google Scholar]
- Woods A, Cheung PC, Smith FC, Davison MD, Scott J, Beri RK, Carling D. Characterization of AMP-activated protein kinase β and γ subunits. Assembly of the heterotrimeric complex in vitro. J Biol Chem. 1996;271:10282–10290. doi: 10.1074/jbc.271.17.10282. [DOI] [PubMed] [Google Scholar]
- Xie Z, Dong Y, Zhang M, Cui MZ, Cohen RA, Riek U, Neumann D, Schlattner U, Zou MH. Activation of protein kinase C-ζ by peroxynitrite regulates LKB1-dependent AMP-activated protein kinase in cultured endothelial cells. J Biol Chem. 2006;281:6366–6375. doi: 10.1074/jbc.M511178200. [DOI] [PubMed] [Google Scholar]
- Xing Y, Musi N, Fujii N, Zou L, Luptak I, Hirshman MF, Goodyear LJ, Tian R. Glucose metabolism and energy homeostasis in mouse hearts overexpressing dominant negative α2 subunit of AMP-activated protein kinase. J Biol Chem. 2003;278:28372–28377. doi: 10.1074/jbc.M303521200. [DOI] [PubMed] [Google Scholar]
- Young ME, Radda GK, Leighton B. Activation of glycogen phosphorylase and glycogenolysis in rat skeletal muscle by AICAR – an activator of AMP-activated protein kinase. FEBS Lett. 1996;382:43–47. doi: 10.1016/0014-5793(96)00129-9. [DOI] [PubMed] [Google Scholar]
- Zou L, Shen M, Arad M, He H, Lofgren B, Ingwall JS, Seidman CE, Seidman JG, Tian R. N488I mutation of the γ2-subunit results in bidirectional changes in AMP-activated protein kinase activity. Circ Res. 2005;97:323–328. doi: 10.1161/01.RES.0000179035.20319.c2. [DOI] [PubMed] [Google Scholar]