Abstract
AMPK is a serine/threonine protein kinase, which serves as an energy sensor in all eukaryotic cell types. Published studies indicate that AMPK activation strongly suppresses cell proliferation in non-malignant cells as well as in tumour cells. These actions of AMPK appear to be mediated through multiple mechanisms including regulation of the cell cycle and inhibition of protein synthesis, de novo fatty acid synthesis, specifically the generation of mevalonate as well as other products downstream of mevalonate in the cholesterol synthesis pathway. Cell cycle regulation by AMPK is mediated by up-regulation of the p53–p21 axis as well as regulation of TSC2–mTOR (mammalian target of rapamycin) pathway. The AMPK signalling network contains a number of tumour suppressor genes including LKB1, p53, TSC1 and TSC2, and overcomes growth factor signalling from a variety of stimuli (via growth factors and by abnormal regulation of cellular proto-oncogenes including PI3K, Akt and ERK). These observations suggest that AMPK activation is a logical therapeutic target for diseases rooted in cellular proliferation, including atherosclerosis and cancer. In this review, we discuss about exciting recent advances indicating that AMPK functions as a suppressor of cell proliferation by controlling a variety of cellular events in normal cells as well as in tumour cells.
AMP-activated protein kinase (AMPK) is a member of a metabolite-sensing protein kinase family that is found in all eukaryotes (Hardie et al. 1998). AMPK has been proposed to act as a cellular fuel sensor, and is a potent mediator of exercise-induced glucose uptake in skeletal muscles (Hardie, 2003).
AMPK consists of a heterotrimeric complexes consisting of a catalytic α subunit and regulatory β and γ subunits, and functions as a protein serine/threonine kinase (Hardie & Carling, 1997). AMPK is activated under conditions that deplete cellular ATP and elevate AMP levels such as glucose deprivation, hypoxia, ischaemia and heat shock, which are associated with an increased AMP/ATP ratio (Kemp et al. 2003). In addition, it is also activated by several hormones and cytokines such as adiponectin (Yamauchi et al. 2002) and leptin (Minokoshi et al. 2002), and by the anti-diabetic drug metformin (Zhou et al. 2001; Shaw et al. 2005). Once activated, AMPK phosphorylates and inactivates a number of metabolic enzymes involved in ATP-consuming cellular events including fatty acid, cholesterol and protein synthesis, involving acetyl-Co enzyme A carboxylase (ACC) and HMG-CoA reductase, and also activates ATP-generating processes, including the uptake and oxidation of glucose and fatty acids (Hardie, 2005; Luo et al. 2005). A number of molecules and signalling pathways have been reported to be regulated by AMPK through direct phosphorylation as an AMPK substrate and also indirectly by gene expression regulation (Hardie, 2004).
Since its discovery, investigations into the regulation and the effects of AMPK have expanded very rapidly. Among these, research on the regulation of cellular proliferation by AMPK is becoming one of the important areas. In this review, we focus on the effect of AMPK activation on cellular proliferation and discuss the possibility that AMPK might be a therapeutic target for proliferative disorders such as atherosclerosis and cancers.
Cell proliferation is regulated by the cell cycle machinery
Mammalian cell proliferation is governed by the cell cycle machinery. Cell cycle progression is a tightly controlled series of events that are positively regulated by cyclin-dependent kinases (CDKs) and their cyclin-regulatory subunits (Sherr, 1996), and negatively regulated by CDK inhibitors (CDKIs) and tumour suppressor genes (Grana & Reddy, 1995). Mitogenic factors bind to their receptors and initiate a series of events resulting in the activation of CDKs that in turn regulate cell cycle progression, DNA synthesis and replication and mitosis. Although a number of growth factors reportedly utilize distinct signalling pathways to promote DNA synthesis, the distinct signalling pathways activated by individual growth factors must converge upon common downstream regulators of the cell cycle such as CDKs and CDKIs (Lukas et al. 1996). The final common pathway leading to G0/G1/S transition is the CDK-induced hyperphosphorylation of the retinoblastoma gene product (Rb), which functions as a molecular switch that commits the cell to DNA replication. Hyperphosphorylation of Rb results in the release of the transcription factor E2F, which induces the expression of genes required for the progression through S, G2 and M phases (Weinberg, 1995). In the absence of its phosphorylation by CDKs, Rb binds and sequesters E2F, thereby preventing transcriptional activation and sequent induction of E2F-dependent genes. CDKIs, such as p21CIP and p27KIP, negatively regulate this process by inhibiting cyclin/CDKs activity and phosphorylation of Rb, resulting in G1 arrest (Hunter & Pines, 1994). Progression of the cell cycle is regulated by the balance between the levels and activities of cyclin–CDK complexes, CDKIs and other growth suppressor proteins such as p53 (Grana & Reddy, 1995).
Tumor suppressor p53 expression and its function are tightly regulated by its phosphorylation state. Cellular stresses, such as γ-irradiation and glucose deprivation, induce the phosphorylation of p53 at Ser-15 (Shieh et al. 1997; Jones et al. 2005). The phosphorylated p53 induces cell-growth arrest and/or apoptosis through the transcriptional regulation of p53 response genes such as p21CIP and p53AIP1, which is carefully discussed in a recent review (Bode & Dong, 2004).
AMPK causes G1 cell cycle arrest via up-regulation of the p53–p21 axis
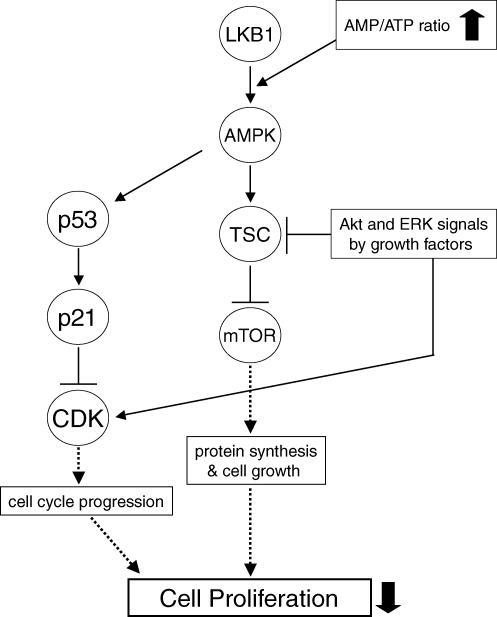
Recently, AMPK activation by the widely used AMP-mimetic 5-aminoimidazole-4-carboxamide ribonucleoside (AICAR) has been shown to cause cell cycle arrest in hepatoma HepG2 cells (Imamura et al. 2001), mouse embryonic fibroblasts (Jones et al. 2005), human aortic smooth muscle cells (SMCs) and rabbit aortic strips (Igata et al. 2005). These reports also supported the hypothesis that the mechanism of cell cycle arrest by AMPK activation involves accumulation of the tumour suppressor protein p53 by phosphorylation of its Ser-15 residue (Ser-18 in mice), and the accumulated p53 protein up-regulates one of the CDKIs, p21CIP protein by a transcriptional mechanism (Fig. 1). Whether phosphorylation of Ser-15 on p53 is mediated by AMPK itself or by another protein kinase, which is co-immunoprecipitated with AMPK, has not been elucidated. Importantly, the amino acid sequence around Ser-15 is not a good fit to the AMPK consensus recognition motif. Future studies will provide the complete understanding for the regulation of p53 protein by AMPK activation.
Figure 1. Mechanisms of the inhibition of cell proliferation by AMPK activation.
Grow stimulation elicits activation of Akt and ERK, resulting in cell proliferation by activating both CDKs to progress cell cycle and mTOR pathway to promote protein synthesis essential for cell growth and proliferation (through pathways indicated by dashed arrows). AMPK activation overcomes the growth-stimulatory signalling via activation of p53–p21 axis as well as inhibition of mTOR signalling, resulting in suppression of cell proliferation.
The proliferative components of vascular diseases are varied and include restenosis after coronary artery intervention, a vasculopathy that accompanies cardiac transplantation and the processes in primary atherosclerosis. The entry of vascular cells, especially smooth muscle cells (SMCs), into the cell cycle plays an important role in the development and progression of these proliferative conditions. The majority of SMCs in intact blood vessels reside in the G0 phase of the cell cycle, and a growth-stimulating event(s) such as balloon injury or platelet-derived growth factor (PDGF) stimulation, must occur in order to trigger the SMC proliferation response. Our group reported that AMPK activation in SMCs stimulated with serum or PDGF-BB resulted in cell cycle arrest at the G1 phase and inhibited cell proliferation (Igata et al. 2005). Consistent with our report, AICAR inhibited angiotensin II-stimulated SMC thymidine incorporation and administration of AICAR prevented neointimal formation in the rat balloon injury model (Nagata et al. 2004). These observations indicate that AMPK activation can overcome growth-promoting signalling from a variety of stimuli and can maintain SMCs in a quiescent state similar to G0 phase. Thus, AMPK activation in SMCs might be a therapeutic target to prevent or treat vascular proliferative diseases.
AMPK activation by AICAR has also been recently reported to inhibit proliferation of various cancer cell lines in vitro and in vivo by increasing p21CIP, p27KIP and p53 (Rattan et al. 2005). Notably, AICAR inhibited proliferation in embryonic fibroblasts from both wild-type and LKB1 knock-out mice, suggesting that AICAR itself may also have a role in the inhibition of cancer cell growth. As discussed in the next section, AMPK is a target of the tumour suppressor gene lkb1 that encodes the protein kinase LKB1, which is a major regulator of AMPK activity.
Tumor suppressor LKB1 is upstream of AMPK and inhibits cell proliferation
LKB1 is a serine/threonine protein kinase and is the product of the gene lkb1, which is mutated in cells from patients with Peutz-Jeghers syndrome (PJS) (Boudeau et al. 2003). PJS is characterized by mucocutaneous melanin pigmentation, gastrointestinal polyposis and markedly increased risk of cancer (Giardiello et al. 2000). Mutations in PJS patients were shown to be loss-of-function mutations of LKB1 (Mehenni et al. 1998; Ylikorkala et al. 1999). Overexpression of wild type LKB1 in two cancer cell lines, HeLa and G361, which do not express endogenous LKB1, suppressed the proliferation of these cells by inducing a G1 cell cycle arrest (Tiainen et al. 1999). Catalytically inactive LKB1 mutants including some of those isolated from PJS patients failed to suppress cell growth, indicating the proliferation-inhibitory and anti-tumour effects of LKB1. LKB1 also associates with p53 (Karuman et al. 2001) and induces p21CIP up-regulation resulting in cell cycle arrest in a p53-dependent manner in G361 melanoma cells (Tiainen et al. 2002), providing further evidence that LKB1 can function as a tumour suppressor.
A microarray analysis reported that overexpression of LKB1 in A549 cells induced expression of several p53 responsive genes, suggesting that LKB1 regulates the p53 pathway (Jimenez et al. 2003). Although numerous studies of LKB1 had shown its involvement in the cancer-prone PJS, at that time the downstream molecular system had not been identified. Recently, however, LKB1 has been convincingly shown to phosphorylate AMPK and serves as an essential component of physiological AMPK activation (Hawley et al. 2003; Shaw et al. 2004b) (Fig. 1). LKB1 phosphorylates AMPK at Thr-172 in the activation loop of the kinase domain in the catalytic α-subunit. The identified human mutations of LKB1 typically occur in the catalytic domain and cause a loss of its kinase activity. This failure of LKB1 phosphorylation impairs its downstream signalling of AMPK (Forcet et al. 2005). Today connection between LKB1- and AMPK-induced cell cycle arrest seemed to be clear (Kyriakis, 2003; Hardie, 2004) although no published work has shown the direct evidence for LKB1–AMPK association in AMPK-dependent cell cycle arrest.
Interestingly, some different effects between LKB1- and AMPK-induced cell cycle arrest seem to be present. Jimenez et al. (2003) reported that introduction of wild-type LKB1 in A549 cells increased the mRNA expression of PTEN (phosphatase with sequence homology to tensin) and resulted in decreased Akt signalling. PTEN functions as a lipid phosphatase metabolizing the phosphatidylinositol 3,4,5-trisphosphate (PIP3) second messenger which stimulates proliferation and survival of cells (Cantley, 2002). On the other hand, Ouchi et al. (2004) reported that AMPK activation by adiponectin increased Akt signalling. We also observed that AMPK activation in both vascular endothelial cells and SMCs by AICAR treatment prolonged Akt phosphorylation compared with vehicle-treated controls (H. Motoshima et al. author observations). Further studies are required for the full understanding of the role of LKB1 and AMPK signalling in cell proliferation.
Regulation of the mTOR pathway and cell proliferation by AMPK
The mammalian target of rapamycin (mTOR) is an evolutionarily conserved serine/threonine kinase and is a key regulator of protein translation/synthesis. This kinase is centrally involved in cell growth (i.e. an increase in cell size and cell mass) (Schmelzle & Hall, 2000; Fingar et al. 2002). Both cellular events, cell growth and cell division (proliferation), are distinct yet tightly coupled processes (Neufeld & Edgar, 1998; Conlon & Raff, 1999). During the G1/S/G2 phases of each cell division cycle, cell mass and DNA content double, and then daughter cells with appropriate cell mass and DNA content are produced after M phase, leading to cell division (Fingar et al. 2004).
The mTOR pathway integrates nutrient and mitogen signals to regulate cell growth and cell division. The mTOR pathway is activated by amino acids and by growth factors such as PDGF, epidermal growth factor (EGF) and insulin, and stimulates protein synthesis, cell growth and proliferation (Fig. 1). The growth factor-stimulated mTOR activation mediates the increased translation initiation of 5′-terminal oligopyrimidine tract-containing mRNAs, which encode components of the protein synthesis machinery. This event is one of the critical events involved in mammalian cell growth and proliferation. The most characterized downstream effectors of mTOR are the 70 kDa ribosomal protein S6 kinase 1 (p70S6K1 or S6K1) and the eukaryotic translation initiation factor 4E (eIF4E)-binding protein 1 (4E-BP1) (Fingar & Blenis, 2004; Hardie, 2005). Through these molecules, mTOR stimulates the initiation step of translation (Carrera, 2004).
Mitogens like PDGF and EGF stimulate the mTOR pathway by activating PI3-kinase and in turn Akt and the subsequent phosphorylation of TSC2 (a precursor of mTOR) by Akt at Ser-939 and Thr-1462, leading to the inhibition of TSC2 (Inoki et al. 2002; Manning et al. 2002). In the absence of growth-promoting stimuli, TSC2 (also called tuberin) binds to TSC1 (also called hamartin) to form a tumour suppressor complex, which has growth-inhibitory activity via suppression of mTOR (van Slegtenhorst et al. 1998). The TSC1–TSC2 complex has GTPase activity towards Rheb, a small GTP-binding protein that is required for mTOR activation (Garami et al. 2003). When phosphorylated by Akt, TSC2 loses its activity to inhibit Rheb, thus switching on the mTOR pathway. Regulation of TSC2 by the ERK pathway has also recently been reported by Ma et al. (2005). When ERK is activated by either growth factors, PMA, or introduction of constitutive active MEK (the kinase upstream of ERK), it phosphorylates TSC2 at Ser-540 and Ser-664, causing the dissociation of the TSC1–TSC2 complex and the attenuation of TSC2 activity, resulting in mTOR-dependent S6K1 phosphorylation and increased proliferation. For further information for the regulation of mTOR pathway, see a recent review article by Fingar & Blenis (2004).
Several groups have reported that activation of AMPK suppresses mTOR signalling by growth factors and amino acids (Bolster et al. 2002; Krause et al. 2002; Kimura et al. 2003) (Fig. 1). The molecular mechanism seems that activated AMPK by energy starvation phosphorylates TSC2 at Thr-1227 and Ser-1345, which sites are distinct from both the PKB sites and the ERK sites, and increases the activity of TSC1–TSC2 complex to inhibit mTOR (Inoki et al. 2003). Phosphorylation of TSC2 by AMPK is involved in the mechanism by which the TSC complex inhibits cell growth and protects cells from apoptosis induced by glucose starvation (Inoki et al. 2003; Shaw et al. 2004a). In addition, AMPK reportedly phosphorylates mTOR at Thr-2446 to reduce S6K1 phosphorylation by insulin, suggesting the inhibition of mTOR action (Cheng et al. 2004). Thus, AMPK directly and indirectly (via TSC2) suppresses mTOR activity to limit protein synthesis. Additionally, AMPK limits protein synthesis through the inhibition of translation elongation factor 2 (EF2) (Horman et al. 2002). Therefore, when AMPK is activated, cell growth and proliferation might be inhibited due to a limitation of protein synthesis. Thus, AMPK regulates cellular proliferation in response to energy status or nutrient availability.
Constitutive activation of PI3K-Akt signalling has been reported in many cancers including glioblastoma, melanoma, and advanced prostate cancer. AMPK activation is a feasible therapeutic strategy for these cancers since AMPK inhibits mTOR signalling downstream of Akt, and inhibition of mTOR pathway has been reported to inhibit tumour growth and metastasis in experimental animal models as well as in cultured cells.
Regulation of fatty acid synthesis and cell proliferation by AMPK
Many cancer cells exhibit a markedly increased rate of de novo fatty acid synthesis, which is typically quite low in non-malignant cells except for lipogenic cells such as adipocytes. Breast, prostate, colon and ovarian cancers express high levels of fatty acid synthase (FAS), a key enzyme for the de novo fatty acid biosynthesis (Kuhajda et al. 2000; Luo et al. 2005). Ongoing studies have shown that human cancers and their pre-neoplastic lesions constitutively overexpress FAS, and that the expression level of FAS in breast and ovarian cancers is associated with the malignant phenotype; inhibition of FAS suppresses cancer proliferation and induces apoptotic cell death, resulting in prolonged survival in hosts with cancer (Kuhajda et al. 2000; Menendez & Lupu, 2004). In prostate cancer cells, up-regulation of the lipogenic pathway by androgens is mediated by activation of the sterol regulatory element-binding protein (SREBP) transcription factors, which regulate the expression of many lipogenic enzymes, including FAS and ACC (Swinnen et al. 2004). Therefore, the inhibition of FAS activity or suppression of FAS expression has become a clear target for the pharmacological therapy of malignant tumours (Fig. 2).
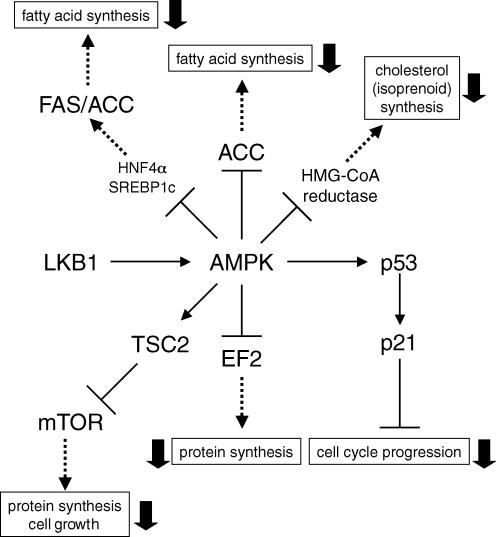
Figure 2. Various AMPK-regulated pathways which potentially modulate cell proliferation.
AMPK activation may regulate cell proliferation not only by activation of p53–p21 axis and inhibition of mTOR signalling, but also by suppression of the mevalonate synthesis pathway and de novo fatty acid synthesis. When AMPK is activated, various pathways indicated by dashed arrows are suppressed.
The inhibition of FAS expression by AMPK activation was previously reported in primary cultured hepatocytes (Foretz et al. 1998; Leclerc et al. 1998; Woods et al. 2000). Furthermore, AMPK-induced suppression of FAS gene expression either by AICAR or an anti-diabetic drug metformin in liver cells was explained by the strong suppressive effect on SREBP1c expression in vivo and in vitro (Zhou et al. 2001). Indeed, activation of AMPK by either AICAR or rosiglitazone, reduces expression of FAS and ACC resulting in suppression of proliferation in prostate-cancer cells (Xiang et al. 2004). Thus, another potential mechanism by which AMPK might function in cancer therapy is via inhibition of FAS expression (Fig. 2).
Regulation of cholesterol synthesis pathway by AMPK
Statins are the most commonly used drugs to decrease cholesterol levels acting via inhibition of 3-hydroxy-3-methylglutaryl-Co enzyme A (HMG-CoA) reductase, the rate-limiting enzyme in cholesterol synthesis. These block the conversion of HMG-CoA to mevalonate in the cholesterol synthesis pathway (Fig. 3). Recently statins have been demonstrated to reduce mortality related to cardiovascular diseases in patients with hypercholesterolaemia and their clinical use is more spreading. Furthermore, it has been reported that statins have a number of vasoprotective effects to improve endothelial function, reduce free radical formation and the risk for plaque rapture, inhibit inflammatory reaction between monocytes and vascular tissues as well as reduce cholesterol levels. For additional information of statins, see a review by Elrod & Lefer (2005). Since AMPK activation has also been reported to phosphorylate and inhibit HMG-CoA reductase activity and reduce cholesterol levels (Corton et al. 1994; Henin et al. 1995), some beneficial vaso- and cardio-protective effects, but not all, of statins described above are expected by AMPK activation in vascular tissues although investigations targeting this issue are very limited.
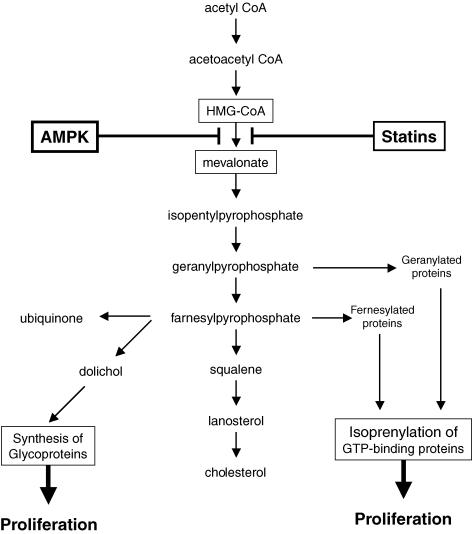
Figure 3. AMPK inhibits synthesis of mevalonate and its downstream products.
AMPK activation, like statins, inhibits HMG-CoA reductase and suppresses the production of downstream metabolites including dolichol and isoprenylated GTP-binding proteins, all of which positively regulate cell proliferation.
In recent years, statins have also been reported to exert anti-tumour and anti-proliferative activities in in vivo studies with experimental animals and in in vitro studies, and to reduce cancer incidence in patients treated with statins in several case-controlled studies. Compared with its vascular effects, available information is limited for the anti-cancer effects of statins. A putative mechanism was reported to be the decrease in mevalonate synthesis and other products (including dolichol, geranylpyrophosphate and farnesylpyrophosphate) downstream of mevalonate. Some of these are essential for cell proliferation and survival. AMPK activation is able to inhibit HMG-CoA reductase, decrease mevalonate synthesis and reduce the products downstream from mevalonate (Fig. 3). Therefore, the anti-cancer effects of AMPK activation might be mediated at least in part through the inhibition of this pathway.
Regulation of AMPK and anti-proliferative effect by adiponectin
AMPK was originally thought to be a regulator of cellular energy balance by sensing the intracellular AMP: ATP ratio. Recent studies have reported that extracellular hormonal stimulation by adiponectin and leptin, both of which are peptide hormones secreted by adipose tissue, also could activate AMPK (Yamauchi et al. 2002; Minokoshi et al. 2002). Adiponectin has a number of beneficial anti-diabetic and anti-atherosclerotic effects (Goldstein & Scalia, 2004). Adiponectin has been reported to inhibit vascular SMC proliferation (Arita et al. 2002) and myelomonocytic lineage cells (Yokota et al. 2000). We also reported that globular adiponectin activated AMPK in adipocytes (Wu et al. 2003) and suppressed endothelial cell proliferation induced by a low dose of oxidized low density lipoprotein (Motoshima et al. 2004). We revealed that an AMPK activator, AICAR, suppressed proliferation of human aortic SMCs stimulated by either 15% serum or PDGF-BB mediated through AMPK activation using a pharmacological or dominant-negative AMPK-mediated inhibition of AMPK (Igata et al. 2005). We also observed that another AMPK activator phenformin also inhibited the proliferation of both endothelial cells and vascular smooth muscle cells (VSMCs) (H. Motoshima et al. authors observations). These anti-proliferative effects on vascular cells by different reagents might be mediated through AMPK activation.
Plasma adiponectin has been shown to be decreased in patients with breast (Miyoshi et al. 2003; Mantzoros et al. 2004), endometrial (Dal Maso et al. 2004) and gastric cancer (Ishikawa et al. 2005). Interestingly, potential anti-cancer effects of adiponectin have been suggested, and it has been shown to directly inhibit the proliferation of cultured breast cancer cells (Kang et al. 2005). Brakenhielm et al. (2004) also reported that adiponectin inhibited tumour growth in a mouse model mediated by the potent inhibition of endothelial cell proliferation, consistent with our observations. Further investigations into the relation between adiponectin and cancer are appearing at a rapid pace. Future studies will provide important information as to whether the anti-tumour activity of adiponectin is mediated through activation of AMPK.
Very recently, two clinical studies showing that metformin also provides some protective effect against cancer in diabetic patients who are treated with it rather than other medications, were published (Evans et al. 2005; Bowker et al. 2006). In metformin-treated patients, several anti-proliferative and anti-tumour mechanisms via AMPK activation described here might be contributing to the reduced risk of cancer.
Conclusions
While the AMPK system is traditionally thought of as a sensor of cellular energy status and a regulator of metabolism, recent discoveries that a tumour-suppressor LKB1 is present upstream and two distinct tumour-suppressors p53 and TSC2 lie downstream, have now provided novel evidence that it may function as a suppressor of cell proliferation. AMPK promotes the maintenance of a resting cell phenotype in mature tissues which do not require proliferation to keep its function (like VSMCs in a large vessel) and also helps protect cells from transformation by oncogenic stimulation. Indeed, AMPK activation has been reported to suppress cell proliferation in a variety of cell types. These findings indicate that the AMPK system is playing a significant role in the regulation of cell proliferation.
Since AMPK can regulate a variety of signalling pathways described above, it will be interesting to determine which pathway(s) regulated by AMPK are most involved in the inhibition of cell proliferation. AMPK has pleiotropic effects that may affect cellular proliferation including: (1) inhibition of ACC resulting in suppression of fatty acid synthesis; (2) it inhibits HMG-CoA reductase resulting in suppression of synthesis of mevalonate and other products in the cholesterol synthesis pathway as well as cholesterol itself; (3) inhibition of the mTOR pathway resulting in inhibition of protein synthesis; and (4) inhibition of cell cycle progression by activating the p53–p21 axis. None of the available data has provided enough information to determine how much and how long activation of AMPK can serve to suppress protein synthesis, fatty acid synthesis as well as DNA synthesis, respectively. Further studies are required for a full understanding of this issue. Nevertheless, activation of AMPK has emerged as an important target for the prevention and treatment of atherosclerosis as well as potentially for cancer therapy.
Acknowledgments
This work was supported by Grants-in-Aid for Scientific Research from the Japan Society for the Promotion of Science, Japan (no. 16046219 and no. 16390266 to E.A) and by grant from the Japan Diabetes Foundation (to H.M.). Work in Dr Goldstein's laboratory is supported by NIH grants DK63018 and DK71360.
References
- Arita Y, Kihara S, Ouchi N, Maeda K, Kuriyama H, Okamoto Y, et al. Adipocyte-derived plasma protein adiponectin acts as a platelet-derived growth factor-BB-binding protein and regulates growth factor-induced common postreceptor signal in vascular smooth muscle cell. Circulation. 2002;105:2893–2898. doi: 10.1161/01.cir.0000018622.84402.ff. [DOI] [PubMed] [Google Scholar]
- Bode AM, Dong Z. Post-translational modification of p53 in tumorigenesis. Nat Rev Cancer. 2004;4:793–805. doi: 10.1038/nrc1455. [DOI] [PubMed] [Google Scholar]
- Bolster DR, Crozier SJ, Kimball SR, Jefferson LS. AMP-activated protein kinase suppresses protein synthesis in rat skeletal muscle through downregulated mTOR signaling. J Biol Chem. 2002;277:23977–23980. doi: 10.1074/jbc.C200171200. [DOI] [PubMed] [Google Scholar]
- Boudeau J, Sapkota G, Alessi DR. LKB1, a protein kinase regulating cell proliferation and polarity. FEBS Lett. 2003;546:159–165. doi: 10.1016/s0014-5793(03)00642-2. [DOI] [PubMed] [Google Scholar]
- Bowker SL, Majumdar SR, Veugelers P, Johnson JA. Increased cancer-related mortality for patients with type 2 diabetes who use sulfonylureas or insulin. Diabetes Care. 2006;29:254–258. doi: 10.2337/diacare.29.02.06.dc05-1558. [DOI] [PubMed] [Google Scholar]
- Brakenhielm E, Veitonmaki N, Cao R, Kihara S, Matsuzawa Y, Zhivotovsky B, Funahashi T, Cao Y. Adiponectin-induced antiangiogenesis and antitumor activity involve caspase-mediated endothelial cell apoptosis. Proc Natl Acad Sci U S A. 2004;101:2476–2481. doi: 10.1073/pnas.0308671100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantley LC. The phosphoinositide 3-kinase pathway. Science. 2002;296:1655–1657. doi: 10.1126/science.296.5573.1655. [DOI] [PubMed] [Google Scholar]
- Carrera AC. TOR signaling in mammals. J Cell Sci. 2004;117:4615–4616. doi: 10.1242/jcs.01311. [DOI] [PubMed] [Google Scholar]
- Cheng SW, Fryer LG, Carling D, Shepherd PR. Thr2446 is a novel mammalian target of rapamycin (mTOR) phosphorylation site regulated by nutrient status. J Biol Chem. 2004;279:15719–15722. doi: 10.1074/jbc.C300534200. [DOI] [PubMed] [Google Scholar]
- Conlon I, Raff M. Size control in animal development. Cell. 1999;96:235–244. doi: 10.1016/s0092-8674(00)80563-2. [DOI] [PubMed] [Google Scholar]
- Corton JM, Gillespie JG, Hardie DG. Role of the AMP-activated protein kinase in the cellular stress response. Curr Biol. 1994;4:315–324. doi: 10.1016/s0960-9822(00)00070-1. [DOI] [PubMed] [Google Scholar]
- Dal Maso L, Augustin LS, Karalis A, Talamini R, Franceschi S, Trichopoulos D, Mantzoros CS, La Vecchia C. Circulating adiponectin and endometrial cancer risk. J Clin Endocrinol Metab. 2004;89:1160–1163. doi: 10.1210/jc.2003-031716. [DOI] [PubMed] [Google Scholar]
- Elrod JW, Lefer DJ. The effects of statins on endothelium, inflammation and cardioprotection. Drug News Perspect. 2005;18:229–236. doi: 10.1358/dnp.2005.18.4.908656. [DOI] [PubMed] [Google Scholar]
- Evans JM, Donnelly LA, Emslie-Smith AM, Alessi DR, Morris AD. Metformin and reduced risk of cancer in diabetic patients. BMJ. 2005;330:1304–1305. doi: 10.1136/bmj.38415.708634.F7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fingar DC, Blenis J. Target of rapamycin (TOR): an integrator of nutrient and growth factor signals and coordinator of cell growth and cell cycle progression. Oncogene. 2004;23:3151–3171. doi: 10.1038/sj.onc.1207542. [DOI] [PubMed] [Google Scholar]
- Fingar DC, Richardson CJ, Tee AR, Cheatham L, Tsou C, Blenis J. mTOR controls cell cycle progression through its cell growth effectors S6K1 and 4E-BP1/eukaryotic translation initiation factor 4E. Mol Cell Biol. 2004;24:200–216. doi: 10.1128/MCB.24.1.200-216.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fingar DC, Salama S, Tsou C, Harlow E, Blenis J. Mammalian cell size is controlled by mTOR and its downstream targets S6K1 and 4EBP1/eIF4E. Genes Dev. 2002;16:1472–1487. doi: 10.1101/gad.995802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forcet C, Etienne-Manneville S, Gaude H, Fournier L, Debilly S, Salmi M, Baas A, Olschwang S, Clevers H, Billaud M. Functional analysis of Peutz-Jeghers mutations reveals that the LKB1 C-terminal region exerts a crucial role in regulating both the AMPK pathway and the cell polarity. Hum Mol Genet. 2005;14:1283–1292. doi: 10.1093/hmg/ddi139. [DOI] [PubMed] [Google Scholar]
- Foretz M, Carling D, Guichard C, Ferre P, Foufelle F. AMP-activated protein kinase inhibits the glucose-activated expression of fatty acid synthase gene in rat hepatocytes. J Biol Chem. 1998;273:14767–14771. doi: 10.1074/jbc.273.24.14767. [DOI] [PubMed] [Google Scholar]
- Garami A, Zwartkruis FJ, Nobukuni T, Joaquin M, Roccio M, Stocker H, et al. Insulin activation of Rheb, a mediator of mTOR/S6K/4E-BP signaling, is inhibited by TSC1 and 2. Mol Cell. 2003;11:1457–1466. doi: 10.1016/s1097-2765(03)00220-x. [DOI] [PubMed] [Google Scholar]
- Giardiello FM, Brensinger JD, Tersmette AC, Goodman SN, Petersen GM, Booker SV, Cruz-Correa M, Offerhaus JA. Very high risk of cancer in familial Peutz–Jeghers syndrome. Gastroenterology. 2000;119:1447–1453. doi: 10.1053/gast.2000.20228. [DOI] [PubMed] [Google Scholar]
- Goldstein BJ, Scalia R. Adiponectin: a novel adipokine linking adipocytes and vascular function. J Clin Endocrinol Metab. 2004;89:2563–2568. doi: 10.1210/jc.2004-0518. [DOI] [PubMed] [Google Scholar]
- Grana X, Reddy EP. Cell cycle control in mammalian cells: role of cyclins, cyclin dependent kinases (CDKs), growth suppressor genes and cyclin-dependent kinase inhibitors (CKIs) Oncogene. 1995;11:211–219. [PubMed] [Google Scholar]
- Hardie DG. Minireview: the AMP-activated protein kinase cascade: the key sensor of cellular energy status. Endocrinology. 2003;144:5179–5183. doi: 10.1210/en.2003-0982. [DOI] [PubMed] [Google Scholar]
- Hardie DG. The AMP-activated protein kinase pathway – new players upstream and downstream. J Cell Sci. 2004;117:5479–5487. doi: 10.1242/jcs.01540. [DOI] [PubMed] [Google Scholar]
- Hardie DG. New roles for the LKB1 →AMPK pathway. Curr Opin Cell Biol. 2005;17:167–173. doi: 10.1016/j.ceb.2005.01.006. [DOI] [PubMed] [Google Scholar]
- Hardie DG, Carling D. The AMP-activated protein kinase – fuel gauge of the mammalian cell? Eur J Biochem. 1997;246:259–273. doi: 10.1111/j.1432-1033.1997.00259.x. [DOI] [PubMed] [Google Scholar]
- Hardie DG, Carling D, Carlson M. The AMP-activated/SNF1 protein kinase subfamily: metabolic sensors of the eukaryotic cell? Annu Rev Biochem. 1998;67:821–855. doi: 10.1146/annurev.biochem.67.1.821. [DOI] [PubMed] [Google Scholar]
- Hawley SA, Boudeau J, Reid JL, Mustard KJ, Udd L, Makela TP, Alessi DR, Hardie DG. Complexes between the LKB1 tumor suppressor, STRAD alpha/beta and MO25 alpha/beta are upstream kinases in the AMP-activated protein kinase cascade. J Biol. 2003;2:28. doi: 10.1186/1475-4924-2-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henin N, Vincent MF, Gruber HE, Van den Berghe G. Inhibition of fatty acid and cholesterol synthesis by stimulation of AMP-activated protein kinase. FASEB J. 1995;9:541–546. doi: 10.1096/fasebj.9.7.7737463. [DOI] [PubMed] [Google Scholar]
- Horman S, Browne G, Krause U, Patel J, Vertommen D, Bertrand L, et al. Activation of AMP-activated protein kinase leads to the phosphorylation of elongation factor 2 and an inhibition of protein synthesis. Curr Biol. 2002;12:1419–1423. doi: 10.1016/s0960-9822(02)01077-1. [DOI] [PubMed] [Google Scholar]
- Hunter T, Pines J. Cyclins and cancer. II: Cyclin D and CDK inhibitors come of age. Cell. 1994;79:573–582. doi: 10.1016/0092-8674(94)90543-6. [DOI] [PubMed] [Google Scholar]
- Igata M, Motoshima H, Tsuruzoe K, Kojima K, Matsumura T, Kondo T, et al. Adenosine monophosphate-activated protein kinase suppresses vascular smooth muscle cell proliferation through the inhibition of cell cycle progression. Circ Res. 2005;97:837–844. doi: 10.1161/01.RES.0000185823.73556.06. [DOI] [PubMed] [Google Scholar]
- Imamura K, Ogura T, Kishimoto A, Kaminishi M, Esumi H. Cell cycle regulation via p53 phosphorylation by a 5′-AMP activated protein kinase activator, 5-aminoimidazole-4-carboxamide-1-beta-D-ribofuranoside, in a human hepatocellular carcinoma cell line. Biochem Biophys Res Commun. 2001;287:562–567. doi: 10.1006/bbrc.2001.5627. [DOI] [PubMed] [Google Scholar]
- Inoki K, Li Y, Zhu T, Wu J, Guan KL. TSC2 is phosphorylated and inhibited by Akt and suppresses mTOR signalling. Nat Cell Biol. 2002;4:648–657. doi: 10.1038/ncb839. [DOI] [PubMed] [Google Scholar]
- Inoki K, Zhu T, Guan KL. TSC2 mediates cellular energy response to control cell growth and survival. Cell. 2003;115:577–590. doi: 10.1016/s0092-8674(03)00929-2. [DOI] [PubMed] [Google Scholar]
- Ishikawa M, Kitayama J, Kazama S, Hiramatsu T, Hatano K, Nagawa H. Plasma adiponectin and gastric cancer. Clin Cancer Res. 2005;11:466–472. [PubMed] [Google Scholar]
- Jimenez AI, Fernandez P, Dominguez O, Dopazo A, Sanchez-Cespedes M. Growth and molecular profile of lung cancer cells expressing ectopic LKB1: down-regulation of the phosphatidylinositol 3′-phosphate kinase/PTEN pathway. Cancer Res. 2003;63:1382–1388. [PubMed] [Google Scholar]
- Jones RG, Plas DR, Kubek S, Buzzal M, Mu J, Xu Y, Birnbaum MJ, Thompson CB. AMP-activated protein kinase induces a p53-dependent metabolic checkpoint. Mol Cell. 2005;18:283–293. doi: 10.1016/j.molcel.2005.03.027. [DOI] [PubMed] [Google Scholar]
- Kang JH, Lee YY, Yu BY, Yang BS, Cho KH, Yoon do K, Roh YK. Adiponectin induces growth arrest and apoptosis of MDA-MB-231 breast cancer cell. Arch Pharm Res. 2005;28:1263–1269. doi: 10.1007/BF02978210. [DOI] [PubMed] [Google Scholar]
- Karuman P, Gozani O, Odze RD, Zhou XC, Zhu H, Shaw R, et al. The Peutz-Jegher gene product LKB1 is a mediator of p53-dependent cell death. Mol Cell. 2001;7:1307–1319. doi: 10.1016/s1097-2765(01)00258-1. [DOI] [PubMed] [Google Scholar]
- Kemp BE, Stapleton D, Campbell DJ, Chen ZP, Murthy S, Walter M, et al. AMP-activated protein kinase, super metabolic regulator. Biochem Soc Trans. 2003;31:162–168. doi: 10.1042/bst0310162. [DOI] [PubMed] [Google Scholar]
- Kimura N, Tokunaga C, Dalal S, Richardson C, Yoshino K, Hara K, et al. A possible linkage between AMP-activated protein kinase (AMPK) and mammalian target of rapamycin (mTOR) signalling pathway. Genes Cells. 2003;8:65–79. doi: 10.1046/j.1365-2443.2003.00615.x. [DOI] [PubMed] [Google Scholar]
- Krause U, Bertrand L, Hue L. Control of p70 ribosomal protein S6 kinase and acetyl-CoA carboxylase by AMP-activated protein kinase and protein phosphatases in isolated hepatocytes. Eur J Biochem. 2002;269:3751–3759. doi: 10.1046/j.1432-1033.2002.03074.x. [DOI] [PubMed] [Google Scholar]
- Kuhajda FP, Pizer ES, Li JN, Mani NS, Frehywot GL, Townsend CA. Synthesis and antitumor activity of an inhibitor of fatty acid synthase. Proc Natl Acad Sci U S A. 2000;97:3450–3454. doi: 10.1073/pnas.050582897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyriakis JM. At the crossroads: AMP-activated kinase and the LKB1 tumor suppressor link cell proliferation to metabolic regulation. J Biol. 2003;2:26. doi: 10.1186/1475-4924-2-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leclerc I, Kahn A, Doiron B. The 5′-AMP-activated protein kinase inhibits the transcriptional stimulation by glucose in liver cells, acting through the glucose response complex. FEBS Lett. 1998;431:180–184. doi: 10.1016/s0014-5793(98)00745-5. [DOI] [PubMed] [Google Scholar]
- Lukas J, Bartkova J, Bartek J. Convergence of mitogenic signalling cascades from diverse classes of receptors at the cyclin D-cyclin-dependent kinase-pRb-controlled G1 checkpoint. Mol Cell Biol. 1996;16:6917–6925. doi: 10.1128/mcb.16.12.6917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Z, Saha AK, Xiang X, Ruderman NB. AMPK, the metabolic syndrome and cancer. Trends Pharmacol Sci. 2005;26:69–76. doi: 10.1016/j.tips.2004.12.011. [DOI] [PubMed] [Google Scholar]
- Ma L, Chen Z, Erdjument-Bromage H, Tempst P, Pandolfi PP. Phosphorylation and functional inactivation of TSC2 by Erk implications for tuberous sclerosis and cancer pathogenesis. Cell. 2005;121:179–193. doi: 10.1016/j.cell.2005.02.031. [DOI] [PubMed] [Google Scholar]
- Manning BD, Tee AR, Logsdon MN, Blenis J, Cantley LC. Identification of the tuberous sclerosis complex-2 tumor suppressor gene product tuberin as a target of the phosphoinositide 3-kinase/akt pathway. Mol Cell. 2002;10:151–162. doi: 10.1016/s1097-2765(02)00568-3. [DOI] [PubMed] [Google Scholar]
- Mantzoros C, Petridou E, Dessypris N, Chavelas C, Dalamaga M, Alexe DM, et al. Adiponectin and breast cancer risk. J Clin Endocrinol Metab. 2004;89:1102–1107. doi: 10.1210/jc.2003-031804. [DOI] [PubMed] [Google Scholar]
- Mehenni H, Gehrig C, Nezu J, Oku A, Shimane M, Rossier C, et al. Loss of LKB1 kinase activity in Peutz–Jeghers syndrome, and evidence for allelic and locus heterogeneity. Am J Hum Genet. 1998;63:1641–1650. doi: 10.1086/302159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menendez JA, Lupu R. Fatty acid synthase-catalyzed de novo fatty acid biosynthesis: from anabolic-energy-storage pathway in normal tissues to jack-of-all-trades in cancer cells. Arch Immunol Ther Exp. 2004;52:414–426. [PubMed] [Google Scholar]
- Minokoshi Y, Kim YB, Peroni OD, Fryer LG, Muller C, Carling D, Kahn BB. Leptin stimulates fatty-acid oxidation by activating AMP-activated protein kinase. Nature. 2002;415:339–343. doi: 10.1038/415339a. [DOI] [PubMed] [Google Scholar]
- Miyoshi Y, Funahashi T, Kihara S, Taguchi T, Tamaki Y, Matsuzawa Y, Noguchi S. Association of serum adiponectin levels with breast cancer risk. Clin Cancer Res. 2003;9:5699–5704. [PubMed] [Google Scholar]
- Motoshima H, Wu X, Mahadev K, Goldstein BJ. Adiponectin suppresses proliferation and superoxide generation and enhances eNOS activity in endothelial cells treated with oxidized LDL. Biochem Biophys Res Commun. 2004;315:264–271. doi: 10.1016/j.bbrc.2004.01.049. [DOI] [PubMed] [Google Scholar]
- Nagata D, Takeda R, Sata M, Satonaka H, Suzuki E, Nagano T, Hirata Y. AMP-activated protein kinase inhibits angiotensin II-stimulated vascular smooth muscle cell proliferation. Circulation. 2004;110:444–451. doi: 10.1161/01.CIR.0000136025.96811.76. [DOI] [PubMed] [Google Scholar]
- Neufeld TP, Edgar BA. Connections between growth and the cell cycle. Curr Opin Cell Biol. 1998;10:784–790. doi: 10.1016/s0955-0674(98)80122-1. [DOI] [PubMed] [Google Scholar]
- Ouchi N, Kobayashi H, Kihara S, Kumada M, Sato K, Inoue T, Funahashi T, Walsh K. Adiponectin stimulates angiogenesis by promoting cross-talk between AMP-activated protein kinase and Akt signaling in endothelial cells. J Biol Chem. 2004;279:1304–1309. doi: 10.1074/jbc.M310389200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rattan R, Giri S, Singh AK, Singh I. 5-Aminoimidazole-4-carboxamide-1-beta-D-ribofuranoside inhibits cancer cell proliferation in vitro and in vivo via AMP-activated protein kinase. J Biol Chem. 2005;280:39582–39593. doi: 10.1074/jbc.M507443200. [DOI] [PubMed] [Google Scholar]
- Schmelzle T, Hall MN. TOR, a central controller of cell growth. Cell. 2000;103:253–262. doi: 10.1016/s0092-8674(00)00117-3. [DOI] [PubMed] [Google Scholar]
- Shaw RJ, Bardeesy N, Manning BD, Lopez L, Kosmatka M, DePinho RA, Cantley LC. The LKB1 tumor suppressor negatively regulates mTOR signaling. Cancer Cell. 2004a;6:91–99. doi: 10.1016/j.ccr.2004.06.007. [DOI] [PubMed] [Google Scholar]
- Shaw RJ, Kosmatka M, Bardeesy N, Hurley RL, Witters LA, DePinho RA, Cantley LC. The tumor suppressor LKB1 kinase directly activates AMP-activated kinase and regulates apoptosis in response to energy stress. Proc Natl Acad Sci U S A. 2004b;101:3329–3335. doi: 10.1073/pnas.0308061100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw RJ, Lamia KA, Vasquez D, Koo SH, Bardeesy N, Depinho RA, Montminy M, Cantley LC. The kinase LKB1 mediates glucose homeostasis in liver and therapeutic effects of metformin. Science. 2005;310:1642–1646. doi: 10.1126/science.1120781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherr CJ. Cancer cell cycles. Science. 1996;274:1672–1677. doi: 10.1126/science.274.5293.1672. [DOI] [PubMed] [Google Scholar]
- Shieh SY, Ikeda M, Taya Y, Prives C. DNA damage-induced phosphorylation of p53 alleviates inhibition by MDM2. Cell. 1997;91:325–334. doi: 10.1016/s0092-8674(00)80416-x. [DOI] [PubMed] [Google Scholar]
- Swinnen JV, Heemers H, van de Sande T, de Schrijver E, Brusselmans K, Heyns W, Verhoeven G. Androgens, lipogenesis and prostate cancer. J Steroid Biochem Mol Biol. 2004;92:273–279. doi: 10.1016/j.jsbmb.2004.10.013. [DOI] [PubMed] [Google Scholar]
- Tiainen M, Vaahtomeri K, Ylikorkala A, Makela TP. Growth arrest by the LKB1 tumor suppressor: induction of p21 (WAF1/CIP1) Hum Mol Genet. 2002;11:1497–1504. doi: 10.1093/hmg/11.13.1497. [DOI] [PubMed] [Google Scholar]
- Tiainen M, Ylikorkala A, Makela TP. Growth suppression by Lkb1 is mediated by a G1 cell cycle arrest. Proc Natl Acad Sci U S A. 1999;96:9248–9251. doi: 10.1073/pnas.96.16.9248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Slegtenhorst M, Nellist M, Nagelkerken B, Cheadle J, Snell R, van den Ouweland A, et al. Interaction between hamartin and tuberin, the TSC1 and TSC2 gene products. Hum Mol Genet. 1998;7:1053–1057. doi: 10.1093/hmg/7.6.1053. [DOI] [PubMed] [Google Scholar]
- Weinberg RA. The retinoblastoma protein and cell cycle control. Cell. 1995;81:323–330. doi: 10.1016/0092-8674(95)90385-2. [DOI] [PubMed] [Google Scholar]
- Woods A, Azzout-Marniche D, Foretz M, Stein SC, Lemarchand P, Ferre P, Foufelle F, Carling D. Characterization of the role of AMP-activated protein kinase in the regulation of glucose-activated gene expression using constitutively active and dominant negative forms of the kinase. Mol Cell Biol. 2000;20:6704–6711. doi: 10.1128/mcb.20.18.6704-6711.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Motoshima H, Mahadev K, Stalker TJ, Scalia R, Goldstein BJ. Involvement of AMP-activated protein kinase in glucose uptake stimulated by the globular domain of adiponectin in primary rat adipocytes. Diabetes. 2003;52:1355–1363. doi: 10.2337/diabetes.52.6.1355. [DOI] [PubMed] [Google Scholar]
- Xiang X, Saha AK, Wen R, Ruderman NB, Luo Z. AMP-activated protein kinase activators can inhibit the growth of prostate cancer cells by multiple mechanisms. Biochem Biophys Res Commun. 2004;321:161–167. doi: 10.1016/j.bbrc.2004.06.133. [DOI] [PubMed] [Google Scholar]
- Yamauchi T, Kamon J, Minokoshi Y, Ito Y, Waki H, Uchida S, et al. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat Med. 2002;8:1288–1295. doi: 10.1038/nm788. [DOI] [PubMed] [Google Scholar]
- Ylikorkala A, Avizienyte E, Tomlinson IP, Tiainen M, Roth S, Loukola A, et al. Mutations and impaired function of LKB1 in familial and non-familial Peutz–Jeghers syndrome and a sporadic testicular cancer. Hum Mol Genet. 1999;8:45–51. doi: 10.1093/hmg/8.1.45. [DOI] [PubMed] [Google Scholar]
- Yokota T, Oritani K, Takahashi I, Ishikawa J, Matsuyama A, Ouchi N, et al. Adiponectin, a new member of the family of soluble defense collagens, negatively regulates the growth of myelomonocytic progenitors and the functions of macrophages. Blood. 2000;96:1723–1732. [PubMed] [Google Scholar]
- Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenyk-Melody J, et al. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest. 2001;108:1167–1174. doi: 10.1172/JCI13505. [DOI] [PMC free article] [PubMed] [Google Scholar]