Abstract
AMP-activated protein kinase (AMPK) is involved in cellular energy homeostasis. Its functions have been extensively studied in muscles and liver. AMPK stimulates pathways which increase energy production (glucose transport, fatty acid oxidation) and switches off pathways which consume energy (lipogenesis, protein synthesis, gluconeogenesis). This has led to the concept that AMPK has an interesting pharmaceutical potential in situations of insulin resistance and it is indeed the target of existing drugs and hormones which improve insulin sensitivity. Adipose tissue is a key player in energy metabolism through the release of substrates and hormones involved in metabolism and insulin sensitivity. Activation of AMPK in adipose tissue can be achieved through situations such as fasting and exercise. Leptin and adiponectin as well as hypoglycaemic drugs are activators of adipose tissue AMPK. This activation probably involves changes in the AMP/ATP ratio and the upstream kinase LKB1. When activated, AMPK limits fatty acid efflux from adipocytes and favours local fatty acid oxidation. Since fatty acids have a key role in insulin resistance, especially in muscles, activating AMPK in adipose tissue might be found to be beneficial in insulin-resistant states, particularly as AMPK activation also reduces cytokine secretion in adipocytes.
Adipose tissue: energy and endocrinology
Storage of energy when food is available is a determinant of survival in periods of increased energy expenditure or decreased energy availability. Quantitatively, the main form of energy storage is represented by triglycerides in the adipocytes of adipose tissue. Adipose tissue is also composed of endothelial cells, fibroblasts and macrophages which are found in the stroma–vascular fraction. New adipocytes can be formed throughout life from this fraction depending upon the nutritional and hormonal conditions. The origin of the lipids stored can be either the diet or de novo synthesis from non-lipid substrates (lipogenic process). This process is active in rodent adipose tissue but relatively minor in human adipose tissue. In order to take up lipids from plasma, adipocytes synthesize a specific enzyme called lipoprotein lipase which is exported to the luminal side of vascular endothelium where it can hydrolyse triglyceride-rich lipoproteins such as chylomicrons and VLDLs (very low density lipoproteins) to yield fatty acids and free glycerol. Fatty acids enter into the adipocytes through transporters and are re-esterified with glycerol phosphate to form triglycerides stored in a single lipid droplet in white adipocytes. This droplet is surrounded by a membrane, itself covered with a protein called perilipin found exclusively in adipocytes. When needed, triglycerides are hydrolysed (lipolysis) into fatty acids and glycerol which are exported back into the blood. Lipolysis requires several enzymes acting successively and key enzymes are adipose tissue triglyceride lipase and hormone-sensitive lipase (HSL). HSL activity is regulated acutely through several mechanisms including reversible phosphorylation and translocation from the cytosol to the surface of the lipid droplet. Insulin favours lipid storage through the activation of lipogenesis, lipoprotein lipase synthesis and export to the vascular endothelium, and triglyceride esterification through the production of glycero-phosphate from glucose. In contrast adrenergic hormones produced either by the adrenal medulla or by the local sympathetic innervation activate lipolysis through binding to a β-agonist receptor and production of cAMP.
It is now well appreciated that, in addition to its functions related to energy storage and release, adipose tissue is also an endocrine organ, strongly involved in overall energy homeostasis and substrate partitioning. The most important hormones produced by adipose tissue are leptin and adiponectin. Leptin is a cytokine produced in proportion to the amount of adipose tissue and which acts in specific brain hypothalamic nuclei to reduce food intake and in rodents to activate thermogenesis (Friedman, 2000). Leptin also has actions outside of the brain, one of which is to stimulate fatty acid oxidation in muscles and liver, at least in part through AMP-activated protein kinase (AMPK) activation (Minokoshi et al. 2002).
Adiponectin belongs to the complement 1q family. It is one of the most abundant transcripts in adipocytes and its plasma concentration is high. It circulates and signals as a homomultimer. In contrast to leptin, its secretion and plasma concentration are inversely related to adiposity. Plasma adiponectin concentrations are decreased in obese and type 2 diabetic rodents, primates and humans (Tsao et al. 2002). Adiponectin is considered to be an insulin-sensitizing hormone since it activates muscle glucose utilization but also induces muscle and hepatic fatty acid oxidation (accumulation of fatty acids or fatty acyl-CoAs in insulin-sensitive cells is deleterious for insulin signalling) and decreases hepatic glucose production (Fruebis et al. 2001; Berg et al. 2002; Matsuzawa et al. 2004). It has been shown, at least in the liver, that adiponectin effects require AMPK activation (Yamauchi et al. 2002). Cytokines such as interleukin-6 (IL-6) and tumour necrosis factor (TNFα) are produced by adipose tissue although probably not specifically by adipocytes but also by cells from the stroma–vascular fraction and can favour insulin resistance in insulin-sensitive tissues.
AMPK is involved in other tissues in the maintenance of cellular as well as body energy homeostasis. When activated, it inhibits energy-consuming processes and activates energy-producing processes (see other contributions in this issue). Adipose tissue is a major component of energy homeostasis and a key player in the regulation of insulin sensitivity through fatty acid release and hormone secretion. Understanding the function of AMPK in adipocytes is thus crucial for assessing the importance of this enzyme in overall energy metabolism.
Structure of AMPK in adipose tissue
AMPK exists in the cell as a heterotrimeric complex with a catalytic (α) and two regulatory subunits (β and γ) (Woods et al. 1996a). Several isoforms have been identified for each subunit (α1, α2, β1, β2, γ1, γ2, γ3), that can lead to the formation of 12 different complexes. These combinations confer different properties to the AMPK complexes (Hardie & Carling, 1997) and show relative tissue specificity (Cheung et al. 2000). Muscle cells mainly express AMPK complexes containing the α2 catalytic subunit and liver expresses both α1 and α2 isoforms (Stapleton et al. 1996; Woods et al. 1996b). In adipose tissue, the α1 catalytic subunit is the predominant isoform expressed and accounts for the major part of AMPK activity (Lihn et al. 2004; Daval et al. 2005). Although the functional significance of these different complexes remains unclear, it can be emphasized that AMPK complexes containing the α1 isoform are less sensitive to AMP (Salt et al. 1998). At present there is no data concerning the respective expression of other AMPK subunits in adipocytes.
Regulation of AMPK in adipose tissue
In adipose tissue, fasting and exercise activate AMPK (Park et al. 2002; Daval et al. 2005; Sponarova et al. 2005). Since both situations are concomitant with adrenergic stimulation, it could be anticipated that β-adrenergic agonists and their second messenger cAMP would stimulate AMPK activity. This is indeed the case (Haystead et al. 1990; Moule & Denton, 1998; Daval et al. 2005; Sponarova et al. 2005). It has been suggested that the effect of exercise on adipose tissue AMPK could also be secondary to the secretion of IL-6 by muscles (Kelly et al. 2004). Indeed, IL-6 is able to activate AMPK in F442A adipocytes and a decreased AMPK phosphorylation is found after exercise in adipose tissue of IL-6 knock-out mice.
Leptin (Orci et al. 2004) and adiponectin (Wu et al. 2003; Sell et al. 2006) are able to activate AMPK in adipose tissue. Hypoglycaemic drugs such as biguanides are also inducing an increase of AMPK activity in adipocytes (Daval et al. 2005; Huypens et al. 2005). More controversial results are found using thiazolidinediones, another class of hypoglycaemic agents which are ligands of the transcription factor PPARγ since Huypens et al. were unable to detect AMPK activation in 3T3-L1 adipocytes using 10 μm troglitazone (Huypens et al. 2005) whereas an increased AMPK activity was shown in vivo in adipose tissue of rats treated with pioglitazone (Saha et al. 2004) or rosiglitazone (Ye et al. 2004). In our own experiments, AMPK was activated by thiazolidinediones in isolated adipocytes but at concentrations higher than 200 μm (authors' unpublished results).
AMP and ATP concentrations in the cell are closely related due to the presence of the enzyme adenylate kinase. An increase in AMP is an exquisitely sensitive indicator of a decrease in the level of cellular energy charge. AMP activates AMPK by a complex mechanism involving allosteric effects and more importantly, the phosphorylation by upstream protein kinases of the threonine residue 172 within the activation loop of the α catalytic subunit (Hardie & Carling, 1997). Two upstream kinases have been characterized. LKB1 is a kinase which is constitutively active and phosphorylates AMPK when AMP concentration rises in the cell and binds to the γ subunit, thus transforming AMPK in a suitable substrate for LKB1 (Hawley et al. 2003; Woods et al. 2003; Shaw et al. 2004). The second kinase, calmodulin kinase kinase β, phosphorylates and activates AMPK in the presence of an increased calcium concentration, independently of an increase in AMP concentration (Hawley et al. 2005; Woods et al. 2005). In adipose tissue, several indirect arguments suggest that LKB1 is involved in AMPK activation. Treatment of adipocytes with AICAR, a drug which is transformed in the cell into ZMP, an analogue of AMP, activates AMPK in adipocytes (Sullivan et al. 1994; Corton et al. 1995; Salt et al. 2000; Lihn et al. 2004; Daval et al. 2005). In addition phenformin, a biguanide, induces AMPK activation and decreases ATP concentration (Daval et al. 2005). In transgenic mice expressing an uncoupling protein (UCP1) in white adipose tissue, the AMP/ATP ratio is increased and AMPK is activated (Matejkova et al. 2004). Finally, β-adrenergic lipolytic agents which induce AMPK stimulation are concomitant with a decrease in ATP concentration (Angel et al. 1971; Issad et al. 1995). To the best of our knowledge, a potential role of calmodulin kinase kinase β in AMPK activation has not been demonstrated in adipocytes.
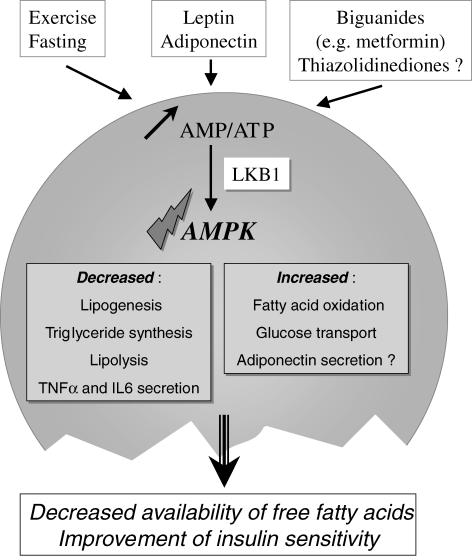
In conclusion, several situations which activate AMPK in adipose tissue are concomitant with an increased AMP/ATP ratio, strongly suggesting the involvement of the upstream kinase LKB1 (Fig. 1).
Figure 1.
AMPK activation in adipose tissue and its intracellular actions
AMPK and adipocyte differentiation
Indirect evidence suggests that AMPK activation can inhibit preadipocyte differentiation. AICAR treatment of 3T3-L1 or F442A preadipocytes inhibits adipocyte differentiation and blocks the expression of late adipogenic markers such as fatty acid synthase and the transcription factors PPARγ and C/EBPα and promotes apoptosis (Habinowski & Witters, 2001; Dagon et al. 2006). This is not totally unexpected if one considers that differentiation is an energy-consuming process involving new membrane (and thus lipid) and protein synthesis, two pathways which are strongly inhibited by AMPK activation (Bolster et al. 2002; Horman et al. 2002). However, in mice lacking the catalytic α1 subunit, the main catalytic isoform present in adipose tissue, the differentiation potential does not seem to be grossly affected since adipocytes are smaller but not more numerous (Daval et al. 2005). It is thus not clear whether AMPK has a physiological regulatory function in adipocyte differentiation.
AMPK and regulation of adipose tissue metabolism
Once activated, AMPK phosphorylates a number of proteins and modulates the transcription of genes implicated in the regulation of energy metabolism to switch on catabolic pathways that produce ATP and switch off anabolic pathways that consume ATP.
Lipogenesis and triglyceride synthesis
One of the first proteins identified as a target of AMPK was acetyl-CoA carboxylase (ACC) which synthetizes malonyl-CoA from acetyl-CoA and is a key enzyme of the lipogenic pathway (Sim & Hardie, 1988). The phosphorylation and thus inhibition of ACC by AMPK has been shown in several studies in vivo and in intact cells. In adipocytes, a direct effect of AMPK activation on ACC phosphorylation and activity was shown in rodent adipocytes using either AICAR (Sullivan et al. 1994) or expression of a constitutively active AMPK (Daval et al. 2005). This was concomitant with a decreased lipogenic rate (Sullivan et al. 1994). Conversely, overexpression of a dominant negative form of AMPK in adipocytes precludes the phosphorylation of ACC after AICAR or isoproterenol (isoprenaline) treatment (Daval et al. 2005). Exercise, which activates AMPK in adipose tissue is concomitant with a decreased ACC activity and malonyl-CoA concentration (Park et al. 2002). Exercise in rats also induces an increase in malonyl-CoA decarboxylase activity, thus further reducing malonyl-CoA concentrations, and a decrease in glycerol-acyl transferase activity, an enzyme involved in triglyceride synthesis. These effects are mimicked by AICAR treatment of the animals. As in the liver (Foretz et al. 1998; Leclerc et al. 1998), an activation of AMPK in adipocytes is concomitant with a decreased expression of lipogenic enzyme mRNA (Orci et al. 2004).
In conclusion, activation of AMPK in rodent adipocytes leads to a decreased lipogenic flux and a decreased triglyceride synthesis.
Lipolysis
The other major function of adipose tissue is the breakdown of triglycerides through the lipolytic pathway that occurs during fasting to provide fatty acids and glycerol as fuels for peripheral tissues. In adipocytes, AMPK activation using AICAR has been shown to inhibit β-adrenergic-induced lipolysis (Sullivan et al. 1994; Corton et al. 1995). Recent work (Daval et al. 2005) has confirmed these studies in a more direct way, showing that overexpression of a constitutively active AMPK in rat adipocytes was indeed inhibiting isoproterenol-induced lipolysis, whereas overexpression of a dominant negative form of AMPK had a converse effect. Other activators of AMPK such as biguanides also had an inhibitory action on lipolysis (Daval et al. 2005). These results are at variance with the study of Yin et al. in 3T3-L1 adipocytes (Yin et al. 2003) since these authors have shown that overexpression of a dominant negative form of AMPK inhibits isoproterenol-induced lipolysis suggesting, rather, a lipolytic action of AMPK activation. However, AMPK activity was not measured in these conditions and thus final conclusions from these experiments are difficult. Using the same cell line, we have demonstrated that AICAR and phenformin induce AMPK activity and strongly impair lipolysis (Daval et al. 2005). Interestingly, in mice lacking the predominant α1 AMPK isoform, the size of adipocytes is reduced and basal and isoproterenol-induced lipolysis is higher than that of control adipocytes (Daval et al. 2005). This argues in favour of an inhibitory role of AMPK activation on lipolysis.
Mice with a general knock-out of the AMPK α2 catalytic subunit have an increase in adipose mass due to adipocyte hypertrophy when fed a high fat diet and compared with high fat fed control mice (Villena et al. 2004). Since the AMPK α2 subunit represents only a very minor part of AMPK activity in adipose tissue, this adipocyte hypertrophy may be the consequence of the adaptation of adipose metabolism subsequent to the loss of AMPK α2 activity in other tissues such as muscle or liver.
What could be the mechanism accounting for lipolysis inhibition by AMPK? At present, two rate-limiting enzymes controlling the hydrolysis of triglycerides in adipocytes have been described. Hormone-sensitive lipase (HSL) was the first one to which a regulatory role was ascribed. HSL hydrolyses triglycerides, diglycerides and cholesteryl esters, although it has a much higher specific activity for diglycerides. Lipolytic agents such as β-adrenergic agonists acutely regulate HSL by increasing cAMP levels in the cell, thus activating cAMP-dependent protein kinase (protein kinase A; PKA) which in turn phosphorylates HSL and increases its intrinsic activity as well as promoting its translocation to the lipid droplet (Yeaman, 2004). HSL is a substrate for AMPK (Garton & Yeaman, 1990). AMPK phosphorylates Ser-565, precluding the further phosphorylation of the regulatory Ser-563 by PKA. Although it was later suggested that the true regulatory serines phosphorylated by PKA of HSL were Ser-659 and Ser-660 (Anthonsen et al. 1998), we have confirmed that activation of AMPK increases HSL phosphorylation on Ser-565 in adipocytes and more importantly that it precludes its isoproterenol-induced translocation to the lipid droplet, a major requirement for lipolysis activation (Daval et al. 2005).
The existence of a second regulatory lipase was discovered during the studies of HSL knock-out mice (Osuga et al. 2000; Wang et al. 2001; Haemmerle et al. 2002). In these mice, the amount of adipose tissue is decreased and its lipid composition is affected with a marked diacylglycerol accumulation. β-Agonist-induced lipolysis is also lower. However, basal lipolysis is normal and although a cholesteryl ester hydrolase activity is no longer detectable in the adipose tissue of HSL knock-out mice, a neutral triglyceride lipase activity is still present. This activity was identified as an adipose triglyceride lipase (ATGL) (Zimmermann et al. 2004). Recently, the genetic deletion of this ATGL has confirmed its importance in triglyceride hydrolysis as well as the likely role of HSL as a diglyceride (rather than a triglyceride) hydrolase (Haemmerle et al. 2006). Interestingly, in HSL knock-out mice, the residual triglyceride lipase activity (ATGL) is increased in the presence of β-agonists and part of this lipolytic response could be secondary to a translocation from the cytoplasm to the lipid droplet, as shown previously for HSL (Okazaki et al. 2002). Thus, it is obviously of interest to test whether ATGL is also a substrate for AMPK and whether its phosphorylation precludes its translocation to the lipid droplet.
The lipid droplet membrane is covered with perilipin, a hydrophobic phosphoprotein. Phosphorylation of perilipin by PKA induces its relocation away from the lipid droplet membrane, allowing HSL (and probably ATGL) to reach its substrates (Tansey et al. 2004). Perilipin knock-out mice have indeed an enhanced basal lipolysis. Whether perilipin is a target of AMPK which when phosphorylated by this enzyme would be unable to relocate away from the droplet membrane is presently unknown.
To summarize, AMPK is activated in conditions of increased lipolysis such as exercise and fasting. This activation inhibits fatty acid and triglyceride synthesis and could limit lipolysis. This latter finding might seem counter-intuitive if one considers AMPK as an enzyme which in case of energy shortage should rather enhance energy availability (here fatty acids through lipolysis) for cells. However, a high rate of lipolysis could be very demanding for adipocyte energy homeostasis since part of the fatty acids can be reactivated into acyl-CoA, a reaction which consumes ATP and generates AMP. Alternatively, accumulation of free fatty acids into the adipocyte could be deleterious for energy-producing processes since they are well-known mitochondrial uncouplers (Kadenbach, 2003). Activation of AMPK would then be a feedback mechanism limiting the cellular energy drain associated with lipolysis in adipocytes.
Fatty acid oxidation
Two models of AMPK activation in adipose tissue are concomitant with an increased fatty acid oxidation. In the first one, the uncoupling mitochondrial protein UCP-1 is overexpressed in adipocytes leading to an increase in the AMP/ATP ratio, activation of AMPK, inactivation of ACC and a decreased lipogenesis (Matejkova et al. 2004). This induces an increased capacity for fatty acid oxidation which could be due to a decreased concentration of malonyl-CoA, alleviating the inhibition on carnitine palmitoyl-transferase I which catalyses the entry of fatty acids in mitochondria and constitutes the rate-limiting enzyme of fatty acid oxidation. UCP-1 overexpression is also concomitant with mitochondrial biogenesis in adipocytes (Rossmeisl et al. 2002). Interestingly, these mice are resistant to nutrient-induced obesity.
In a second model, Orci and coworkers (Orci et al. 2004) have induced hyperleptinaemia using an adenoviral-mediated overexpression of leptin in the liver. In adipose tissue of these hyper-leptinized rats, UCP-1 and UCP-2 expression is increased, AMPK activity is induced and leads to the phosphorylation and inactivation of ACC. There is also a strong mitochondrial biogenesis, features that could lead to the ‘rapid transformation of white adipocytes into fat-oxidizing machines’ (Orci et al. 2004). In these animals, hyperleptinaemia induces a depletion in body fat stores (Shimabukuro et al. 1997) and the authors suggest that this is due to oxidation of fatty acids within the adipocytes inasmuch as these adipocytes release glycerol but no fatty acids (Wang et al. 1999). Interestingly, leptin had no effect during diet-induced obesity implying a leptinergic blockade in adipocytes during overnutrition.
Although activation of AMPK is probably not responsible for all the metabolic characteristics of these models, the results nevertheless suggest that, similar to its effects in other tissues, AMPK activation in adipocytes induces increased fatty acid oxidation.
Glucose transport
AMPK activation stimulates glucose transport through increased GLUT4 translocation in muscles (Kahn et al. 2004). Only a few studies have addressed the potential role of AMPK in glucose uptake in adipose cells. Studies performed in 3T3-L1 adipocytes have reported that treatment of differentiated adipocytes with AICAR enhances basal glucose uptake by a mechanism independent of insulin signalling (Salt et al. 2000; Sakoda et al. 2002). However, overexpression of a dominant negative form of AMPK in 3T3-L1 adipocytes treated with AICAR abolishes AMPK activation without affecting the increase in glucose uptake (Sakoda et al. 2002), raising the question of a direct involvement of AMPK in AICAR-stimulated glucose transport in this model. A third study performed in primary rat adipocytes has shown that adiponectin activates AMPK and increases glucose uptake (Wu et al. 2003). In this study, the inhibition of AMPK by pharmacological compounds abolishes the adiponectin-stimulated glucose transport and it occurs without affecting insulin-stimulated glucose uptake. This suggests a role of AMPK in glucose transport in adipocytes which could involve a mechanism independent of the insulin signalling pathway. However, whether AMPK induces the translocation of GLUT4 to the membranes of adipocytes remains unclear.
AMPK and adipokine secretion
As stated above, adipose tissue is now considered as an endocrine organ involved in energy homeostasis, food intake and inflammation. In human adipose tissue, AICAR has been shown to increase the expression of the insulin-sensitizing hormone adiponectin (Lihn et al. 2004; Sell et al. 2006). A study performed in 3T3-L1 has shown the converse, reporting an inhibition of adiponectin expression and secretion in response to AMPK activation by the anti-diabetic drug metformin (Huypens et al. 2005). Metabolic and insulin-sensitizing effects of metformin have been shown to be in part mediated through the activation of AMPK (Zhou et al. 2001). However, type 2 diabetic patients treated with metformin display no change in serum adiponectin concentration or adipocyte adiponectin content (Phillips et al. 2003; Tiikkainen et al. 2004). The role of AMPK in the regulation of adiponectin expression and secretion thus remains unclear.
In human adipose tissue, inhibitory effects of AICAR on the expression and secretion of two pro-inflammatory cytokines, TNFα and interleukin-6 (IL-6) have been reported (Lihn et al. 2004; Sell et al. 2006). Since TNFα inhibits adiponectin expression (Kappes & Loffler, 2000), it has been suggested that the decrease in TNFα protein may be involved in an up-regulation of adiponectin expression and that the effects of AICAR on adiponectin may be indirect. The inhibition of TNFα and IL-6 secretion by AMPK could be beneficial, since inflammation is thought to contribute to the development of disorders associated with obesity such as insulin resistance. Activation of AMPK in adipose tissue by decreasing TNFα and IL-6 and indirectly increasing adiponectin secretion may thus contribute to the prevention or counteraction of insulin resistance in obese patients. However, the demonstration of a more direct effect of AMPK on cytokine secretion awaits additional experiments.
Conclusion
In the liver, AMPK is part of a mechanism which coordinates changes of lipid metabolism from anabolism to catabolism in case of energy shortage. It includes an inhibition of lipid synthesis and an increased lipid oxidation mediated by a decreased malonyl-CoA content due to an inhibition of ACC activity (Assifi et al. 2005). In adipocytes, a similar role for AMPK is conceivable in case of energy shortage or higher energy demand (exercise) since in these situations the observed activation of AMPK can lead to an inhibition of fatty acid synthesis and an activation of fatty acid oxidation. However, in adipocytes AMPK also inhibits lipolysis (Fig. 1). All these actions of AMPK will tend to decrease the availability of fatty acids in the plasma. Since fatty acids have a key role in the onset of insulin resistance, especially in muscles, activating AMPK in adipose tissue might be extremely beneficial in insulin-resistant states such as type 2 diabetes, particularly as AMPK activation also reduces inflammatory cytokine secretion in adipocytes. A number of questions remain nevertheless unsolved: Can AMPK be stimulated in adipose tissue by non-AMP-dependent mechanisms? Is there any difference in AMPK distribution and responsiveness in subcutaneous and deep visceral adipose tissues (an important question if one considers that visceral fat might be a preferred target)? Since many of the studies have been performed on rodent adipocytes or cell lines do they really apply to human adipocytes?
Acknowledgments
The personal work presented in this review was supported by contracts QLG1-CT-2001-01488 Ampdiamet and LSHM-CT- 2004-005272 Exgenesis from the European Union.
References
- Angel A, Desai K, Halperin ML. Free fatty acid and ATP levels in adipocytes during lipolysis. Metabolism. 1971;20:87–99. doi: 10.1016/0026-0495(71)90062-x. [DOI] [PubMed] [Google Scholar]
- Anthonsen MW, Ronnstrand L, Wernstedt C, Degerman E, Holm C. Identification of novel phosphorylation sites in hormone-sensitive lipase that are phosphorylated in response to isoproterenol and govern activation properties in vitro. J Biol Chem. 1998;273:215–221. doi: 10.1074/jbc.273.1.215. [DOI] [PubMed] [Google Scholar]
- Assifi MM, Suchankova G, Constant S, Prentki M, Saha AK, Ruderman NB. AMP-activated protein kinase and coordination of hepatic fatty acid metabolism of starved/carbohydrate-refed rats. Am J Physiol Endocrinol Metab. 2005;289:E794–E800. doi: 10.1152/ajpendo.00144.2005. [DOI] [PubMed] [Google Scholar]
- Berg AH, Combs TP, Scherer PE. ACRP30/adiponectin: an adipokine regulating glucose and lipid metabolism. Trends Endocrinol Metab. 2002;13:84–89. doi: 10.1016/s1043-2760(01)00524-0. [DOI] [PubMed] [Google Scholar]
- Bolster DR, Crozier SJ, Kimball SR, Jefferson LS. AMP-activated protein kinase suppresses protein synthesis in rat skeletal muscle through down-regulated mammalian target of rapamycin (mTOR) signaling. J Biol Chem. 2002;277:23977–23980. doi: 10.1074/jbc.C200171200. [DOI] [PubMed] [Google Scholar]
- Cheung PC, Salt IP, Davies SP, Hardie DG, Carling D. Characterization of AMP-activated protein kinase gamma-subunit isoforms and their role in AMP binding. Biochem J. 2000;346:659–669. [PMC free article] [PubMed] [Google Scholar]
- Corton JM, Gillespie JG, Hawley SA, Hardie DG. 5-Aminoimidazole-4-carboxamideribonucleoside. A specific method for activating AMP-activated protein kinase in intact cells? Eur J Biochem. 1995;229:558–565. doi: 10.1111/j.1432-1033.1995.tb20498.x. [DOI] [PubMed] [Google Scholar]
- Dagon Y, Avraham Y, Berry EM. AMPK activation regulates apoptosis, adipogenesis, and lipolysis by eIF2alpha in adipocytes. Biochem Biophys Res Commun. 2006;340:43–47. doi: 10.1016/j.bbrc.2005.11.159. [DOI] [PubMed] [Google Scholar]
- Daval M, Diot-Dupuy F, Bazin R, Hainault I, Viollet B, Vaulont S, Hajduch E, Ferre P, Foufelle F. Anti-lipolytic action of AMP-activated protein kinase in rodent adipocytes. J Biol Chem. 2005;280:25250–25257. doi: 10.1074/jbc.M414222200. [DOI] [PubMed] [Google Scholar]
- Foretz M, Carling D, Guichard G, Ferré P, Foufelle F. AMP-activated protein kinase inhibits the glucose-activated expression of fatty acid synthase gene in rat hepatocytes. J Biol Chem. 1998;273:14767–14771. doi: 10.1074/jbc.273.24.14767. [DOI] [PubMed] [Google Scholar]
- Friedman JM. Obesity in the new millennium. Nature. 2000;404:632–634. doi: 10.1038/35007504. [DOI] [PubMed] [Google Scholar]
- Fruebis J, Tsao TS, Javorschi S, Ebbets-Reed D, Erickson MR, Yen FT, Bihain BE, Lodish HF. Proteolytic cleavage product of 30-kDa adipocyte complement-related protein increases fatty acid oxidation in muscle and causes weight loss in mice. Proc Natl Acad Sci U S A. 2001;98:2005–2010. doi: 10.1073/pnas.041591798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garton AJ, Yeaman SJ. Identification and role of the basal phosphorylation site on hormone-sensitive lipase. Eur J Biochem. 1990;191:245–250. doi: 10.1111/j.1432-1033.1990.tb19116.x. [DOI] [PubMed] [Google Scholar]
- Habinowski SA, Witters LA. The effects of AICAR on adipocyte differentiation of 3T3-L1 cells. Biochem Biophys Res Commun. 2001;286:852–856. doi: 10.1006/bbrc.2001.5484. [DOI] [PubMed] [Google Scholar]
- Haemmerle G, Lass A, Zimmermann R, Gorkiewicz G, Meyer C, Rozman J, et al. Defective lipolysis and altered energy metabolism in mice lacking adipose triglyceride lipase. Science. 2006;312:734–737. doi: 10.1126/science.1123965. [DOI] [PubMed] [Google Scholar]
- Haemmerle G, Zimmermann R, Hayn M, Theussl C, Waeg G, Wagner E, et al. Hormone-sensitive lipase deficiency in mice causes diglyceride accumulation in adipose tissue, muscle, and testis. J Biol Chem. 2002;277:4806–4815. doi: 10.1074/jbc.M110355200. [DOI] [PubMed] [Google Scholar]
- Hardie DG, Carling D. The AMP-activated protein kinase – fuel gauge of the mammalian cell? Eur J Biochem. 1997;246:259–273. doi: 10.1111/j.1432-1033.1997.00259.x. [DOI] [PubMed] [Google Scholar]
- Hawley SA, Boudeau J, Reid JL, Mustard KJ, Udd L, Makela TP, Alessi DR, Hardie DG. Complexes between the LKB1 tumor suppressor, STRAD alpha/beta and MO25 alpha/beta are upstream kinases in the AMP-activated protein kinase cascade. J Biol. 2003;2:28. doi: 10.1186/1475-4924-2-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawley SA, Pan DA, Mustard KJ, Ross L, Bain J, Edelman AM, Frenguelli BG, Hardie DG. Calmodulin-dependent protein kinase kinase-beta is an alternative upstream kinase for AMP-activated protein kinase. Cell Metab. 2005;2:9–19. doi: 10.1016/j.cmet.2005.05.009. [DOI] [PubMed] [Google Scholar]
- Haystead TA, Moore F, Cohen P, Hardie DG. Roles of the AMP-activated and cyclic-AMP-dependent protein kinases in the adrenaline-induced inactivation of acetyl-CoA carboxylase in rat adipocytes. Eur J Biochem. 1990;187:199–205. doi: 10.1111/j.1432-1033.1990.tb15295.x. [DOI] [PubMed] [Google Scholar]
- Horman S, Browne G, Krause U, Patel J, Vertommen D, Bertrand L, et al. Activation of AMP-activated protein kinase leads to the phosphorylation of elongation factor 2 and an inhibition of protein synthesis. Curr Biol. 2002;12:1419–1423. doi: 10.1016/s0960-9822(02)01077-1. [DOI] [PubMed] [Google Scholar]
- Huypens P, Quartier E, Pipeleers D, Van de Casteele M. Metformin reduces adiponectin protein expression and release in 3T3-L1 adipocytes involving activation of AMP activated protein kinase. Eur J Pharmacol. 2005;518:90–95. doi: 10.1016/j.ejphar.2005.06.016. [DOI] [PubMed] [Google Scholar]
- Issad T, Combettes M, Ferre P. Isoproterenol inhibits insulin-stimulated tyrosine phosphorylation of the insulin receptor without increasing its serine/threonine phosphorylation. Eur J Biochem. 1995;234:108–115. doi: 10.1111/j.1432-1033.1995.108_c.x. [DOI] [PubMed] [Google Scholar]
- Kadenbach B. Intrinsic and extrinsic uncoupling of oxidative phosphorylation. Biochim Biophys Acta. 2003;1604:77–94. doi: 10.1016/s0005-2728(03)00027-6. [DOI] [PubMed] [Google Scholar]
- Kahn BB, Alquier T, Carling D, Hardie DG. AMP-activated protein kinase: Ancient energy gauge provides clues to modern understanding of metabolism. Cell Metab. 2004;1:15–25. doi: 10.1016/j.cmet.2004.12.003. [DOI] [PubMed] [Google Scholar]
- Kappes A, Loffler G. Influences of ionomycin, dibutyryl-cycloAMP and tumour necrosis factor-alpha on intracellular amount and secretion of apM1 in differentiating primary human preadipocytes. Horm Metab Res. 2000;32:548–554. doi: 10.1055/s-2007-978684. [DOI] [PubMed] [Google Scholar]
- Kelly M, Keller C, Avilucea PR, Keller P, Luo Z, Xiang X, et al. AMPK activity is diminished in tissues of IL-6 knockout mice: the effect of exercise. Biochem Biophys Res Commun. 2004;320:449–454. doi: 10.1016/j.bbrc.2004.05.188. [DOI] [PubMed] [Google Scholar]
- Leclerc I, Kahn A, Doiron B. The 5′-AMP-activated protein kinase inhibits the transcriptional stimulation by glucose in liver cells, acting through the glucose response complex. FEBS Lett. 1998;431:180–184. doi: 10.1016/s0014-5793(98)00745-5. [DOI] [PubMed] [Google Scholar]
- Lihn AS, Jessen N, Pedersen SB, Lund S, Richelsen B. AICAR stimulates adiponectin and inhibits cytokines in adipose tissue. Biochem Biophys Res Commun. 2004;316:853–858. doi: 10.1016/j.bbrc.2004.02.139. [DOI] [PubMed] [Google Scholar]
- Matejkova O, Mustard KJ, Sponarova J, Flachs P, Rossmeisl M, Miksik I, et al. Possible involvement of AMP-activated protein kinase in obesity resistance induced by respiratory uncoupling in white fat. FEBS Lett. 2004;569:245–248. doi: 10.1016/j.febslet.2004.06.002. [DOI] [PubMed] [Google Scholar]
- Matsuzawa Y, Funahashi T, Kihara S, Shimomura I. Adiponectin and metabolic syndrome. Arterioscler Thromb Vasc Biol. 2004;24:29–33. doi: 10.1161/01.ATV.0000099786.99623.EF. [DOI] [PubMed] [Google Scholar]
- Minokoshi Y, Kim Y, Peroni O, Fryer L, Muller C, Carling D, Kahn B. Leptin stimulates fatty-acid oxidation by activating AMP-activated protein kinase. Nature. 2002;415:268–269. doi: 10.1038/415339a. [DOI] [PubMed] [Google Scholar]
- Moule SK, Denton RM. The activation of p38 MAPK by the beta-adrenergic agonist isoproterenol in rat epididymal fat cells. FEBS Lett. 1998;439:287–290. doi: 10.1016/s0014-5793(98)01392-1. [DOI] [PubMed] [Google Scholar]
- Okazaki H, Osuga J, Tamura Y, Yahagi N, Tomita S, Shionoiri F, et al. Lipolysis in the absence of hormone-sensitive lipase: evidence for a common mechanism regulating distinct lipases. Diabetes. 2002;51:3368–3375. doi: 10.2337/diabetes.51.12.3368. [DOI] [PubMed] [Google Scholar]
- Orci L, Cook WS, Ravazzola M, Wang MY, Park BH, Montesano R, Unger RH. Rapid transformation of white adipocytes into fat-oxidizing machines. Proc Natl Acad Sci U S A. 2004;101:2058–2063. doi: 10.1073/pnas.0308258100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osuga J, Ishibashi S, Oka T, Yagyu H, Tozawa R, Fujimoto A, et al. Targeted disruption of hormone-sensitive lipase results in male sterility and adipocyte hypertrophy, but not in obesity. Proc Natl Acad Sci U S A. 2000;97:787–792. doi: 10.1073/pnas.97.2.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park H, Kaushik VK, Constant S, Prentki M, Przybytkowski E, Ruderman NB, Saha AK. Coordinate regulation of malonyl-CoA decarboxylase, sn-glycerol-3-phosphate acyltransferase, and acetyl-CoA carboxylase by AMP-activated protein kinase in rat tissues in response to exercise. J Biol Chem. 2002;277:32571–32577. doi: 10.1074/jbc.M201692200. [DOI] [PubMed] [Google Scholar]
- Phillips SA, Ciaraldi TP, Kong AP, Bandukwala R, Aroda V, Carter L, et al. Modulation of circulating and adipose tissue adiponectin levels by antidiabetic therapy. Diabetes. 2003;52:667–674. doi: 10.2337/diabetes.52.3.667. [DOI] [PubMed] [Google Scholar]
- Rossmeisl M, Barbatelli G, Flachs P, Brauner P, Zingaretti MC, Marelli M, et al. Expression of the uncoupling protein 1 from the aP2 gene promoter stimulates mitochondrial biogenesis in unilocular adipocytes in vivo. Eur J Biochem. 2002;269:19–28. doi: 10.1046/j.0014-2956.2002.02627.x. [DOI] [PubMed] [Google Scholar]
- Saha AK, Avilucea PR, Ye JM, Assifi MM, Kraegen EW, Ruderman NB. Pioglitazone treatment activates AMP-activated protein kinase in rat liver and adipose tissue in vivo. Biochem Biophys Res Commun. 2004;314:580–585. doi: 10.1016/j.bbrc.2003.12.120. [DOI] [PubMed] [Google Scholar]
- Sakoda H, Ogihara T, Anai M, Fujishiro M, Ono H, Onishi Y, et al. Activation of AMPK is essential for AICAR-induced glucose uptake by skeletal muscle but not adipocytes. Am J Physiol Endocrinol Metab. 2002;282:E1239–E1244. doi: 10.1152/ajpendo.00455.2001. [DOI] [PubMed] [Google Scholar]
- Salt I, Celler JW, Hawley SA, Prescott A, Woods A, Carling D, Hardie DG. AMP-activated protein kinase: greater AMP dependence, and preferential nuclear localization, of complexes containing the α2 isoform. Biochem J. 1998;334:177–187. doi: 10.1042/bj3340177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salt IP, Connell JM, Gould GW. 5-Aminoimidazole-4-carboxamide ribonucleoside (AICAR) inhibits insulin-stimulated glucose transport in 3T3-L1 adipocytes. Diabetes. 2000;49:1649–1656. doi: 10.2337/diabetes.49.10.1649. [DOI] [PubMed] [Google Scholar]
- Sell H, Dietze-Schroeder D, Eckardt K, Eckel J. Cytokine secretion by human adipocytes is differentially regulated by adiponectin, AICAR, and troglitazone. Biochem Biophys Res Commun. 2006;343:700–706. doi: 10.1016/j.bbrc.2006.03.010. [DOI] [PubMed] [Google Scholar]
- Shaw RJ, Kosmatka M, Bardeesy N, Hurley RL, Witters LA, DePinho RA, Cantley LC. The tumor suppressor LKB1 kinase directly activates AMP-activated kinase and regulates apoptosis in response to energy stress. Proc Natl Acad Sci U S A. 2004;101:3329–3335. doi: 10.1073/pnas.0308061100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimabukuro M, Koyama K, Chen G, Wang MY, Trieu F, Lee Y, Newgard CB, Unger RH. Direct antidiabetic effect of leptin through triglyceride depletion of tissues. Proc Natl Acad Sci U S A. 1997;94:4637–4641. doi: 10.1073/pnas.94.9.4637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim AT, Hardie DG. The low activity of acetyl-CoA carboxylase in basal and glucagon-stimulated hepatocytes is due to phosphorylation by the AMP-activated protein kinase and not cyclic AMP-dependent protein kinase. FEBS Lett. 1988;233:294–298. doi: 10.1016/0014-5793(88)80445-9. [DOI] [PubMed] [Google Scholar]
- Sponarova J, Mustard KJ, Horakova O, Flachs P, Rossmeisl M, Brauner P, et al. Involvement of AMP-activated protein kinase in fat depot-specific metabolic changes during starvation. FEBS Lett. 2005;579:6105–6110. doi: 10.1016/j.febslet.2005.09.078. [DOI] [PubMed] [Google Scholar]
- Stapleton D, Mitchelhill KI, Gao G, Widmer J, Michell BJ, Teh T, et al. Mammalian AMP-activated protein kinase subfamily. J Biol Chem. 1996;271:611–614. doi: 10.1074/jbc.271.2.611. [DOI] [PubMed] [Google Scholar]
- Sullivan JE, Brocklehurst KJ, Marley AE, Carey F, Carling D, Beri RK. Inhibition of lipolysis and lipogenesis in isolated rat adipocytes with AICAR, a cell-permeable activator of AMP-activated protein kinase. FEBS Lett. 1994;353:33–36. doi: 10.1016/0014-5793(94)01006-4. [DOI] [PubMed] [Google Scholar]
- Tansey JT, Sztalryd C, Hlavin EM, Kimmel AR, Londos C. The central role of perilipin A in lipid metabolism and adipocyte lipolysis. IUBMB Life. 2004;56:379–385. doi: 10.1080/15216540400009968. [DOI] [PubMed] [Google Scholar]
- Tiikkainen M, Hakkinen AM, Korsheninnikova E, Nyman T, Makimattila S, Yki-Jarvinen H. Effects of rosiglitazone and metformin on liver fat content, hepatic insulin resistance, insulin clearance, and gene expression in adipose tissue in patients with type 2 diabetes. Diabetes. 2004;53:2169–2176. doi: 10.2337/diabetes.53.8.2169. [DOI] [PubMed] [Google Scholar]
- Tsao TS, Lodish HF, Fruebis J. ACRP30, a new hormone controlling fat and glucose metabolism. Eur J Pharmacol. 2002;440:213–221. doi: 10.1016/s0014-2999(02)01430-9. [DOI] [PubMed] [Google Scholar]
- Villena JA, Viollet B, Andreelli F, Kahn A, Vaulont S, Sul HS. Induced adiposity and adipocyte hypertrophy in mice lacking the AMP-activated protein kinase-α2 subunit. Diabetes. 2004;53:2242–2249. doi: 10.2337/diabetes.53.9.2242. [DOI] [PubMed] [Google Scholar]
- Wang SP, Laurin N, Himms-Hagen J, Rudnicki MA, Levy E, Robert MF, Pan L, Oligny L, Mitchell GA. The adipose tissue phenotype of hormone-sensitive lipase deficiency in mice. Obes Res. 2001;9:119–128. doi: 10.1038/oby.2001.15. [DOI] [PubMed] [Google Scholar]
- Wang MY, Lee Y, Unger RH. Novel form of lipolysis induced by leptin. J Biol Chem. 1999;274:17541–17544. doi: 10.1074/jbc.274.25.17541. [DOI] [PubMed] [Google Scholar]
- Woods A, Cheung PCF, Smith FC, Davison MD, Scott J, Beri RK, Carling D. Characterization of AMP-activated protein kinase β and γ subunits. J Biol Chem. 1996a;271:10282–10290. doi: 10.1074/jbc.271.17.10282. [DOI] [PubMed] [Google Scholar]
- Woods A, Dickerson K, Heath R, Hong SP, Momcilovic M, Johnstone SR, Carlson M, Carling D. Ca2+/calmodulin-dependent protein kinase kinase-beta acts upstream of AMP-activated protein kinase in mammalian cells. Cell Metab. 2005;2:21–33. doi: 10.1016/j.cmet.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Woods A, Johnstone SR, Dickerson K, Leiper FC, Fryer LG, Neumann D, et al. LKB1 is the upstream kinase in the AMP-activated protein kinase cascade. Curr Biol. 2003;13:2004–2008. doi: 10.1016/j.cub.2003.10.031. [DOI] [PubMed] [Google Scholar]
- Woods A, Salt I, Scott J, Hardie DG, Carling D. The α1 and α2 isoforms of the AMP-activated protein kinase have similar activities in rat liver but exhibit differences in substrate specificity in vitro. FEBS Lett. 1996b;397:347–351. doi: 10.1016/s0014-5793(96)01209-4. [DOI] [PubMed] [Google Scholar]
- Wu X, Motoshima H, Mahadev K, Stalker TJ, Scalia R, Goldstein BJ. Involvement of AMP-activated protein kinase in glucose uptake stimulated by the globular domain of adiponectin in primary rat adipocytes. Diabetes. 2003;52:1355–1363. doi: 10.2337/diabetes.52.6.1355. [DOI] [PubMed] [Google Scholar]
- Yamauchi T, Kamon J, Minokoshi Y, Ito Y, Waki H, Uchida S, et al. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat Med. 2002;8:1288–1295. doi: 10.1038/nm788. [DOI] [PubMed] [Google Scholar]
- Yeaman SJ. Hormone-sensitive lipase – new roles for an old enzyme. Biochem J. 2004;379:11–22. doi: 10.1042/BJ20031811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye JM, Dzamko N, Cleasby ME, Hegarty BD, Furler SM, Cooney GJ, Kraegen EW. Direct demonstration of lipid sequestration as a mechanism by which rosiglitazone prevents fatty-acid-induced insulin resistance in the rat: comparison with metformin. Diabetologia. 2004;47:1306–1313. doi: 10.1007/s00125-004-1436-1. [DOI] [PubMed] [Google Scholar]
- Yin W, Mu J, Birnbaum MJ. Role of AMP-activated protein kinase in cyclic AMP-dependent lipolysis in 3T3-L1 adipocytes. J Biol Chem. 2003;278:43074–43080. doi: 10.1074/jbc.M308484200. [DOI] [PubMed] [Google Scholar]
- Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenyk-Melody J, et al. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest. 2001;108:1167–1174. doi: 10.1172/JCI13505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann R, Strauss JG, Haemmerle G, Schoiswohl G, Birner-Gruenberger R, Riederer M, et al. Fat mobilization in adipose tissue is promoted by adipose triglyceride lipase. Science. 2004;306:1383–1386. doi: 10.1126/science.1100747. [DOI] [PubMed] [Google Scholar]