Abstract
Skeletal muscle is composed of specialized fibre types that enable it to fulfil complex and variable functional needs. Muscle fibres of Xenopus laevis, a frog formerly classified as a toad, were the first to be typed based on a combination of physiological, morphological, histochemical and biochemical characteristics. Currently the most widely accepted criterion for muscle fibre typing is the myosin heavy chain (MHC) isoform composition because it is assumed that variations of this protein are the most important contributors to functional diversity. Yet this criterion has not been used for classification of Xenopus fibres due to the lack of an effective protocol for MHC isoform analysis. In the present study we aimed to resolve and visualize electrophoretically the MHC isoforms expressed in the iliofibularis muscle of Xenopus laevis, to define their functional identity and to classify the fibres based on their MHC isoform composition. Using a SDS-PAGE protocol that proved successful with mammalian muscle MHC isoforms, we were able to detect five MHC isoforms in Xenopus iliofibularis muscle. The kinetics of stretch-induced force transients (stretch activation) produced by a fibre was strongly correlated with its MHC isoform content indicating that the five MHC isoforms confer different kinetics characteristics. Hybrid fibre types containing two MHC isoforms exhibited stretch activation kinetics parameters that were intermediate between those of the corresponding pure fibre types. These results clearly show that the MHC isoforms expressed in Xenopus muscle are functionally different thereby validating the idea that MHC isoform composition is the most reliable criterion for vertebrate skeletal muscle fibre type classification. Thus, our results lay the foundation for the unequivocal classification of the muscle fibres in the Xenopus iliofibularis muscle and for gaining further insights into skeletal muscle fibre diversity.
Skeletal muscle is composed of specialized fibre types that enable it to fulfil complex and variable functional needs. One of the most important, and earliest used, preparations for studying skeletal muscle diversity is the iliofibularis muscle of Xenopus laevis, a frog formerly classified as toad. Investigations on this preparation, carried out in several laboratories, led to the classification of the anuran fibres into three groups: twitch, intermediate and tonic (Lännergren & Smith, 1966; Smith & Ovalle, 1973; Lännergren, 1975, 1978, 1979; Van der Laarse et al. 1986; Rowlerson & Spurway, 1988). Twitch fibres display action-potential-induced force responses that are relatively fast, tonic fibres respond slowly and in a graded manner to graded levels of membrane depolarization, and intermediate fibres display both twitch and tonic membrane and contractile characteristics.
Since the 1980s, the focus of muscle research has shifted from anurans to mammals, and there are many studies showing the great diversity of the mammalian skeletal muscle (for review see Pette & Staron, 1990; Schiaffino & Reggiani, 1996; Bottinelli, 2001). Compared with the current knowledge of mammalian muscle fibre diversity, our understanding of anuran skeletal muscle fibre diversity lags noticeably behind and this must be redressed considering that single fibres from frogs have been extensively used for studying many basic aspects of muscle contraction.
As reviewed by Lännergren (1975, 1992), histochemical staining techniques applied to cross-sections of Xenopus muscle revealed interfibre differences with respect to carbohydrate metabolism, rates of myofibrillar ATPase activity and sensitivity of myofibrillar ATPase to alkaline or acidic pH. Using native gel electrophoresis, some authors (Lännergren & Hoh, 1984; Lännergren, 1987) detected five different isomyosins (i.e. myosin molecules comprising combinations of various isoforms of its constituent hexameric subunits) in the iliofibularis muscle of Xenopus laevis. Based on a combination of morphological (such as diameter, degree of transparency, morphology of nerve terminals, location of fibres within the muscle), molecular (isomyosins) and mechanical properties (such as force responses to electrical, K+ or acetylcholine stimulation, force–velocity relationship) Xenopus muscle fibres have been allocated to one of five fibre types: three twitch fibre types (types 1, 2 and 3), one tonic fibre type (type 5) and one intermediate fibre type (type 4).
Since the head portion of the myosin heavy chains (MHC) contains the active site for ATP hydrolysis and plays the key role in the process of force generation (for review, see Huxley, 2000), MHC isoform composition, determined electrophoretically, has become the preferred criterion for muscle fibre classification into specific types. This method originates mainly from studies of mammalian skeletal muscle fibres that showed that MHCs exist as several isoforms that can be resolved and identified by SDS-PAGE (Pette & Staron, 1990; Schiaffino & Reggiani, 1996). The MHC isoform-based method of fibre type classification was validated by Galler and colleagues (Galler et al. 1994, 1996, 1997; Andruchov et al. 2004a) who showed that the mammalian MHC isoforms are functionally different. More specifically, this group demonstrated, using skinned single fibre preparations, that the MHC isoform composition of mammalian muscle fibres is strongly correlated with the kinetics of force transients produced by a rapid change in length of maximally Ca2+-activated fibres and that this correlation holds for both stretch and release experiments (Galler et al. 1996). In a stretch experiment, the force rises simultaneously with the length change, and decays when the new fibre length is reached. The force decay is followed by a delayed force increase (stretch activation; Heinl et al. 1974; Galler, 1994). The force decay after the stretch may reflect the detachment of myosin heads while the subsequent delayed force increase may reflect their simultaneous reattachment and force generation. The time-to-peak of the delayed force increase (t3 value) is strictly different in mammalian fibre types containing different MHC isoforms, and no overlaps of t3 values were observed between different fibre types expressing only one MHC isoform.
In this context it is worth noting that Lutz and colleagues (Lutz et al. 1998a, b) identified four MHC isoforms in the frog Rana pipiens using SDS-PAGE, monoclonal antibodies and mRNA analyses. Single fibres of this species were found to contain either one (pure fibres) or two (hybrid fibres) MHC isoforms. Four MHC isoforms were also identified by SDS-PAGE in skeletal muscles of another anuran, the cane toad Bufo marinus (Nguyen & Stephenson, 1999). Here, in addition to pure fibre types, hybrid fibres with seven different combinations of two or three MHC isoforms were also detected (Nguyen & Stephenson, 2002). So far there is no information on the MHC isoform composition of skeletal muscle from Xenopus laevis.
The aim of the present study was to resolve, visualize and define the functional identity of the MHC isoforms expressed in the iliofibularis muscle of Xenopus laevis. Using a SDS-PAGE protocol that proved successful with mammalian muscle MHC isoforms, we were able to detect five MHC isoforms in Xenopus iliofibularis muscle. The kinetics of stretch-induced force transients produced by a fibre was strongly correlated with its MHC isoform content showing that each of the five MHC isoforms displays different kinetics. Hybrid fibre types containing two MHC isoforms exhibited stretch activation kinetics parameters that were intermediate between those of the corresponding pure fibre types. Taken together results obtained in this study lay the foundation for the unequivocal classification of the muscle fibres in the Xenopus iliofibularis muscle based on their composition of functionally different MHC isoforms and for gaining further insights into the diversity of vertebrate skeletal muscle fibres.
Methods
Animals and muscle fibre dissection
Adult female Xenopus laevis (110–205 g) were kept in water at about 17°C. The animals were killed by decapitation following pithing, a strategy which complies with the ethical rules concerning animal experimentation at the University of Salzburg where the experiments were conducted. The iliofibularis muscles were quickly excised, blotted dry on filter paper, and placed in an ice-cooled Petri dish containing water-saturated viscous paraffin oil (07160 Merck) for up to 1 day. The dissection of fibres was performed on a base of transparent Sylgard gel (Dow Corning) that was placed on a black surface and the fibres were viewed under a stereo microscope at low intensity illumination from one side. This was useful for providing conditions similar to dark field microscopy. After one fibre was isolated, the largest and smallest diameters were measured, and then the fibre was mechanically skinned by rolling back the sarcolemma using fine forceps (Dumont no. 5), as previously described (Stephenson & Williams, 1981).
In order to increase the number of rare fibre types (especially tonic fibres) for functional analysis we intentionally selected these fibre types during dissection based on their location, diameter and microscopic appearance (Lännergren & Smith, 1966).
Experimental set-up and procedure for mechanical experiments
For mechanical experiments, segments of single skinned muscle fibres (typically 1.4–2.4 mm in length) were mounted horizontally between the two vertically orientated pins of an isometric apparatus (Galler & Hilber, 1994); one pin was attached to the force sensor (AE 801; SensoNor, Norway; 7.5 kHz), the other was attached to a stepping motor. The force transducer was fixed in a holder which was connected to a fine micrometer screw. The compliance of the fibre attachment system was 4 μm mN−1 (Galler & Hilber, 1994). This was measured by gluing the tips of the two attachment pins together and slightly displacing the force transducer with the micrometer screw. Rapid (about 1 ms) changes of fibre length were achieved by a feedback-controlled stepping motor based on a Ling vibrator. Stretches, 0.2–0.25% of fibre length (2.2–2.8 nm per half-sarcomere length) in amplitude, were applied to induce force transients. A cuvette transporting system enabled rapid changes of solutions. After attachment of the skinned fibres with the tissue glue Vetseal (Braun Melsungen, Germany), the fibre ends were fixed by superfusion with a fine rapidly downwards-directed stream of stained glutaraldehyde solutions (8% glutaraldehyde, 5% Toluidine blue, fixative) for 2–3 s. For this purpose the fibre was bathed in a rigor solution (10 mm imidazole, 2.5 mm EGTA, 1 mm MgCl2, 134 mm potassium propionate, pH 6.9) with a lower specific mass than the fixative. This procedure created a sharp boundary between the functional part of the fibre and the fixed ends, and improved considerably the maintenance of the sarcomere homogeneity and the stability of the mechanical properties during prolonged activation (Hilber & Galler, 1998). The composition of solutions used in mechanical experiments (relaxation, preactivation and maximum Ca2+-activation solutions) is given in Table 1. The pH of all solutions was 7.20 at the experimental temperature (15.0 ± 0.3°C). Free [Ca2+] and free [Mg2+] of the solutions were determined with ion-selective electrodes (Galler, 1999). Prior to the experiment, the preparations were adjusted to slack length in a relaxing solution (Table 1). Fibre dimensions were recorded and the sarcomere length was measured by He–Ne laser light diffractometry (632.8 nm). The average sarcomere length (SL) of all fibres measured at slack length was 2.20 ± 0.16 μm (n = 201), which falls in the range of SL values for maximal force production in anuran skeletal muscle fibres (2.0–2.2 μm in twitch fibres, Gordon et al. 1966; 2.0–2.4 μm in tonic fibres, Nasledov & Lebedinskaia, 1971).
Table 1. Composition of solutions (mm).
| Compound | Relaxation solution | Activation solution | Pre-activation solution |
|---|---|---|---|
| Hepes | 60 | 60 | 60 |
| Mg2+total | 10.3 | 8.12 | 8.5 |
| EGTA | 47.8 | 47.8 | 0.1 |
| Ca2+total | — | 48.1 | — |
| HDTA | — | — | 50 |
| ATPtotal | 8 | 8 | 8 |
| CP | 10 | 10 | 10 |
| Mg2+free | 1 | 1 | 1 |
| pCa | >9 | 4.0 | ∼7 |
The pH of all solutions was 7.20 at 15°C (adjusted with KOH). Hepes, N-(2-hydroxyethyl)-piperazine-N′-(2-ethanesulphonic acid) (H-4034, Sigma); Mg2+total, Mg(CO3 (OH)2), mag-nesium hydroxide carbonate (63081, Fluka); EGTA, ethylenglycol-bis-(β-aminoethylether)-N,N,N′,N′-tetraacetic acid (E-4378, Sigma), Ca2+total, CaCO3, calcium carbonate (2060, Merck); HDTA, 1,6-diaminohexane-N,N,N′,N′-tetraacetic acid (33020, Fluka); ATPtotal, Na2H2ATP, adenosine 5′-triphosphate disodium salt (A-2383, Sigma); CP, Na2HCP, phosphocreatine disodium salt (P-7936, Sigma); pCa = −log[Ca2+]. The protocol used for solution preparation was that described by Stephenson & Williams (1981)
For maximal activation, the fibre was transferred from the relaxing solution to the preactivating solution (Table 1, ∼1 min) and finally to the activating solution (Table 1). Maximal isometric tension was determined by dividing the force developed in the activating solution by the cross-sectional area of the fibre. The cross-sectional area was calculated assuming that it was ellipsoidal in shape with the two axes corresponding to the smallest and the largest value of the fibre diameter measured in the relaxing solution. All mechanical experiments were carried out at 15.0 ± 0.3°C.
Gel electrophoresis of MHC isoforms
The MHC isoform content of the single fibre segments was analysed after the mechanical experiments using the SDS-PAGE protocol of Andruchov et al. (2004a). The fibre segments were dissolved in the solubilizing buffer which contained 80 mm TRIS, pH 6.8, 2.3% (w/v) SDS, 710 mm 2-mercaptoethanol, 10 mm dithiotreitol, 12.5% (v/v) glycerol, 13.6% (w/v) sucrose, 0.025% (w/v) bromphenol blue, 0.1 mm phenylmethylsulphonyl fluoride (PMSF), 0.02 mm leupeptin and 0.001 mm pepstatin. The samples were boiled for 5 min. To each electrophoretic well, 8–10 μl of sample (containing approximately 0.6 nl of fibre and 0.1 μg of protein) was applied.
The MHC isoform composition was also determined in whole-muscle homogenates. For whole-muscle homogenates, the muscles were blotted dry, frozen quickly in liquid nitrogen and homogenized in 6 vols of the following solution: 0.3 m KCl, 0.1 m KH2PO4, 0.05 m K2HPO4, 1 mm ethylenediaminetetraacetic acid (EDTA), pH 6.5. After centrifugation (9000 g, 10 min), the supernatant was diluted with glycerol (50% v/v) and stored at −25°C until used. For electrophoretic analysis 1 vol. of this muscle extract was diluted with 9 vols of solubilizing buffer and boiled for 5 min.
A gradient SDS-PAGE was carried out on 0.75 mm-thick, 16 cm long slab gels using a SE 600 system (Hoefer, San Francisco, USA). For the separating gel, a gel solution containing 7.428% (w/v) acrylamide and 0.072% (w/v) N,N′-methylene-bis-acrylamide (bottom gel, pore size parameters: T, 7.500%; C, 0.960%) and a gel solution containing 9.409% (w/v) acrylamide and 0.091% (w/v) N,N′-methylene-bis-acrylamide (top gel, pore size parameters: T, 9.500%; C, 0.958%) were mixed. In addition, both gel solutions contained 380 mm Tris/HCl (pH 8.8), 40% (v/v) glycerol, 0.07% (w/v) SDS, 0.032% (w/v) ammonium persulphate and 0.16% (v/v) N,N,N′,N′-tetramethylethylenediamine (TEMED). The separating gel was allowed to polymerize at room temperature for 1–1.5 h. The stacking gel contained 3.871% (w/v) acrylamide, 0.039% (w/v) N,N′-methylene-bis-acrylamide, 120 mm TRIS/HCl (pH 6.8), 3.2% (v/v) glycerol, 0.1% (w/v) SDS, 0.076% (w/v) ammonium persulphate and 0.14% (v/v) TEMED (T, 3.91%; C, 0.997%). The stacking gel was allowed to polymerize at room temperature for 40 min. The gels were run at constant voltage (140 V) for 34 h with a running-water cooling system. The running buffer contained 0.1% (w/v) SDS, 25 mm TRIS/HCl and 195 mm glycine. After electrophoresis, the gels were silver stained. For quantitative analysis of MHC isoforms the gels were scanned (HP ScanJet 6100C) and the densities of the protein bands were determined using ImageJ software (http://www.nih.gov).
Statistical analyses
Results, presented as means ± s.d., were tested for normality using the Kolmogorov-Smirnov normality test (Sigmastat, Jandel Scientific) and then were analysed for statistical significance using one-way ANOVA with Student-Neuman-Keuls post hoc test or an unpaired Student's t test (Sigmastat, Jandel Scientific; OriginLab Corporation), as appropriate, with P < 0.05 being regarded as statistically significant.
Results
MHC isoforms and fibre types in Xenopus iliofibularis
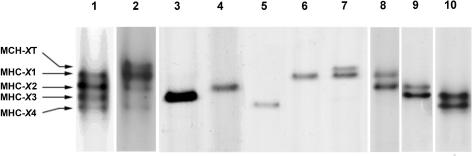
Electrophoretic analyses of MHC isoforms in Xenopus iliofibularis muscle homogenates have revealed five protein bands, which can be seen in the electrophoretograms of samples from two regions of the muscle (Fig. 1, lanes 1 and 2). Only four of these bands were found to be present as single entities in single fibres. Fibres containing only one MHC isoform are referred to as ‘pure fibres’ and those containing two or more MHC isoforms are referred to as ‘hybrid fibres’. Representatives of the four pure fibre types are shown in Fig. 1, lanes 3–6. The fifth MHC isoform band, which had the lowest electrophoretic mobility, was detected only together with one or two of the other four MHC isoform bands in hybrid fibres (see for example the electrophoretogram of the fibre in lane 7, Fig. 1). Representative electrophoretograms of three other types of hybrid fibres found in this study are shown in Fig. 1, lanes 8–10. It is worth noting that out of the 201 single fibres investigated for MHC isoform composition in this study, 105 fibres were pure fibres and 96 were hybrid fibres.
Figure 1. Silver-stained SDS-PAGE of myosin heavy chain (MHC) isoforms of iliofibularis muscle of Xenopus laevis.
Lanes 1 and 2, MHC isoforms of extracts of two different parts of iliofibularis muscles. The other lanes show MHC isoforms of single fibres for which mechanical properties were previously measured, lanes: 3, MHC-X3; 4, MHC-X2; 5, MHC-X4; 6, MHC-X1; 7, MHC-XT and MHC-X1; 8, MHC-X1 and MHC-X2; 9, MHC-X2 and MHC-X3; 10, MHC-X3 and MHC-X4.
All hybrid fibres expressing the MHC isoform of lowest electrophoretic mobility displayed the characteristics of tonic fibres with respect to microscopic appearance, diameter and location described by Lännergren & Smith (1966). Therefore, this MHC isoform band was named MHC-XT (where X stands for Xenopus and T for tonic). The remaining four MHC isoform bands were named, in order of increasing electrophoretic mobility, MHC-X1, MHC-X2, MHC-X3 and MHC-X4. In this study, the pure fibres expressing MHC-X1, MHC-X2, MHC-X3 or MHC-X4 are referred to as type X1, X2, X3 and X4 fibres, respectively. The nomenclature used for hybrid fibres describes the coexpressed MHC isoforms (e.g. fibre type X1 + 2 coexpresses MHC-X1 and MHC-X2; fibre type XT + 3 + 4 coexpresses MHC-XT, MHC-X3 and MHC-X4).
The population of single fibres isolated in this study from Xenopus iliofibularis muscle yielded nine different hybrid fibre types containing combinations of two or three MHC isoforms (Table 2). The hybrid fibres with two MHC isoforms contained the following MHC isoform combinations: MHC-X1 and MHC-X2 (type X1 + 2 fibres, n = 19), MHC-X2 and MHC-X3 (type X2 + 3 fibres, n = 17), MHC-X3 and MHC-X4 (type X3 + 4 fibres, n = 7), MHC-XT and MHC-X1 (type XT + 1 fibres, ‘tonic’ fibres, n = 27), MHC-X1 and MHC-X3 (type X1 + 3, n = 3) and MHC-X1 and MHC-X4 (type X1 + 4, n = 7). The hybrid fibres containing three MHC isoforms displayed the following combinations: MHC-X1, MHC-X2 and MHC-X3 (type X1 + 2 + 3, n = 3), MHC-X1, MHC-X3 and MHC-X4 (type X1 + 3 + 4, n = 10), and MHC-XT, MHC-X3 and MHC-X4 (type XT + 3 + 4, n = 3).
Table 2. MHC isoform content, resting sarcomere length (SL), and diameter of Xenopus laevis iliofibularis muscle fibre types.
| Fibre type | n | MHC-T (%) | MHC-X1 (%) | MHC-X2 (%) | MHC-X3 (%) | MHC-X4 (%) | SL (μm) | Diameter (μm) | Range of diameter (μm) |
|---|---|---|---|---|---|---|---|---|---|
| X1 | 56 | — | 100 | — | — | — | 2.21 ± 0.18 | 129 ± 16 | 90–145 |
| X2 | 16 | — | — | 100 | — | — | 2.21 ± 0.18 | 82 ± 11 | 65–105 |
| X3 | 16 | — | — | — | 100 | — | 2.18 ± 0.14 | 66 ± 7 | 45–75 |
| X4 | 17 | — | — | — | — | 100 | 2.22 ± 0.16 | 48 ± 8 | 35–55 |
| XT + 1 | 27 | 54 ± 12 | 46 ± 12 | — | — | — | 2.18 ± 0.18 | 39 ± 7 | 30–50 |
| X1 + 2 | 19 | — | 54 ± 11 | 46 ± 11 | — | — | 2.19 ± 0.16 | 97 ± 16 | 60–125 |
| X2 + 3 | 17 | — | — | 54 ± 12 | 46 ± 12 | — | 2.18 ± 0.13 | 75 ± 12 | 55–100 |
| X3 + 4 | 7 | — | — | — | 60 ± 16 | 40 ± 16 | 2.21 ± 0.09 | 58 ± 9 | 45–75 |
| X1 + 3 | 3 | — | 50 ± 4 | — | 50 ± 4 | — | 2.23 ± 0.12 | 55 ± 9 | 45–75 |
| X1 + 4 | 7 | — | 50 ± 12 | — | — | 50 ± 12 | 2.21 ± 0.16 | 46 ± 7 | 40–60 |
| X1 + 2 + 3 | 3 | — | 37 ± 7 | 34 ± 6 | 29 ± 8 | — | 2.2 ± 0.16 | 53 ± 10 | 40–65 |
| X1 + 3 + 4 | 10 | — | 21 ± 9 | — | 25 ± 5 | 54 ± 8 | 2.22 ± 0.13 | 43 ± 5 | 40–55 |
| XT + 3 + 4 | 3 | 13 ± 5 | — | — | 21 ± 3 | 66 ± 4 | 2.2 ± 0.08 | 45 ± 4 | 40–50 |
n, number of fibres investigated. All values represent means ± s.d. The content of MHC isoforms in single fibres was determined by densitometry of the protein bands in SDS-PAGE.
The relatively high proportion of the ‘tonic’ fibres (30 out of 201) is not representative of their natural abundance in the iliofibularis muscle because, as mentioned earlier, these fibres were intentionally selected for during dissection based on their location, diameter and microscopic appearance (Lännergren & Smith, 1966) to increase their number for functional analysis. Type XT + 1 fibres, containing 0.46 ± 0.12 MHC-XT (mean ± s.d.), made up the largest proportion (27 out of 30) of hybrid fibres expressing the MHC-XT isoform.
Properties of MHC isoform-based fibre types from Xenopus iliofibularis muscle
Functional analyses were performed on all pure fibres and on the relatively large group (n = 70) of hybrid fibres coexpressing two MHC isoforms (types XT + 1, X1 + 2, X2 + 3 and X3 + 4 fibres). The hybrid fibre types X1 + 3 and X1 + 4, and hybrid fibres containing three MHC isoforms were not included in functional analysis because of their low number. For reliable functional analysis, a much larger number of such fibres, with comparable MHC isoform ratios, would be required.
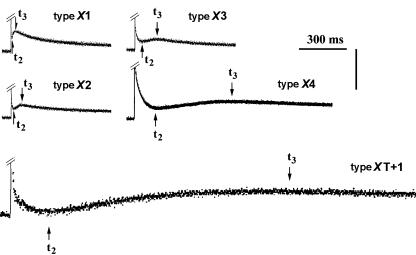
The force transients developed by maximally Ca2+-activated fibres following stepwise stretches (within about 1 ms) are shown in Fig. 2. The two parameters describing the time course of the stretch-induced force transients are: t2, the time from the beginning of the stretch to the onset of delayed force rise, and t3, the time from the beginning of the stretch to the peak of the delayed force rise. The two stretch activation parameters t2 and t3 values of individual fibres are strongly correlated (r2 = 0.98). In experiments with XT + 1 fibres, the force decay following the delayed force increase was not very pronounced, and thus, the peak of the force transient was not obvious. In this case, a straight line fitting the slow force decay was drawn and the point of deviation of the force trace from this line was taken as the t3 value.
Figure 2. Force responses following stepwise stretch (∼0.25% of fibre length) of maximally Ca2+-activated skeletal muscle fibres of Xenopus laevis at 15°C.
t2, time from the beginning of the stretch to the onset of the delayed force increase; t3, time-to-peak of the delayed force increase. Vertical bar: 0.1 mN for types X1, X2 and X3, 0.08 mN for type X4, and 0.06 mN for type XT + 1.
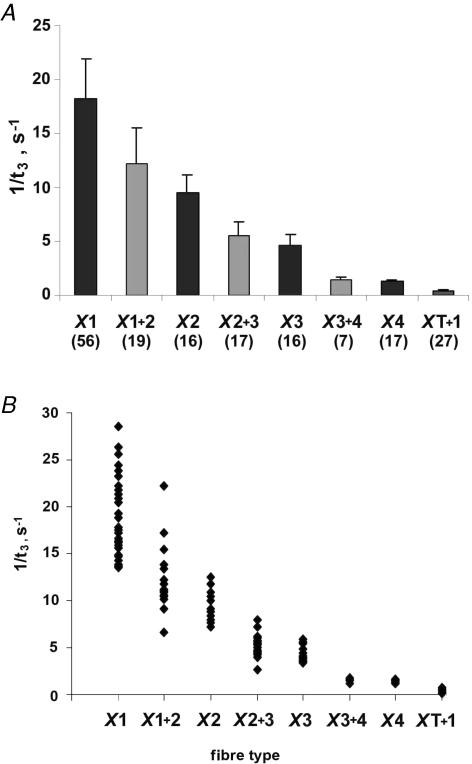
The kinetics of the force transients markedly differed (up to 50 times) between different fibre types. As shown in Table 3, the order from fastest to slowest kinetics was: type X1 > type X1 + 2 > type X2 > type X2 + 3 > type X3 > type X3 + 4 > type X4 > type XT + 1. Figure 3A shows means ± s.d. of 1/t3 for pure and hybrid fibre types. Hybrid fibre types exhibited 1/t3 values that fell between those of the corresponding pure fibre types. Figure 3B shows a scatterplot of 1/t3 values for pure and hybrid fibre types of Xenopus laevis iliofibularis muscle. It can be seen that no overlap of 1/t3 values exists between pure fibre types. Kolmogorov-Smirnov tests (P > 0.2) showed that the t3 values of all pure fibre types (X1, X2, X3 and X4) were normally distributed. This suggests that the fibres displaying only one MHC protein band are indeed pure fibres, because if any of the individual MHC bands MHC-X1, MHC-X2, MHC-X3 and MHC-X4 were made of more than one isoform, then one would not have expected a simple normal distribution for t3. For example, no normal distribution was found when the Kolmogorov-Smirnov test was applied to X1 + 2 and XT + 1 hybrid fibres (P < 0.05).
Table 3. Stretch activation kinetics and maximal isometric tension of MHC-based fibre types from Xenopus iliofibularis muscle.
| Type X1 (n = 56) | Type X1 + 2 (n = 19) | Type X2 (n = 16) | Type X2 + 3 (n = 17) | Type X3 (n = 16) | Type X3 + 4 (n = 7) | Type X4 (n = 17) | Type XT + 1 (n = 27) | |
|---|---|---|---|---|---|---|---|---|
| t2 (ms) | 20.4 ± 5.4 | 33.8 ± 14.6 | 38.9 ± 9.6 | 65.8 ± 18.9 | 77.0 ± 15.3 | 275.0 ± 62.6 | 292.5 ± 106.5 | 871.2 ± 265.9 |
| (26.5) | (43.2) | (23.7) | (28.7) | (19.9) | (22.8) | (36.4) | (30.5) | |
| t3 (ms) | 57.8 ± 10.8 | 87.0 ± 21.3 | 108.8 ± 18.1 | 195.6 ± 58.1 | 224.2 ± 47.3 | 733.6 ± 115.1 | 797.1 ± 96.8 | 2868.5 ± 999.2 |
| (18.7) | (24.5) | (16.6) | (29.7) | (21.1) | (15.7) | (12.1) | (34.7) | |
| T0 | 411 ± 61 | 377 ± 62 | 358 ± 32 | 362 ± 44 | 360 ± 41 | 343 ± 36 | 325 ± 32 | 261 ± 23 |
| (mN mm−2) | (14.8) | (16.4) | (8.9) | (12.2) | (11.4) | (10.5) | (9.8) | (8.8) |
T0, maximal isometric tension. The results are given as means ± s.d.; the number in parentheses represents the coefficient of variation. Maximal isometric tension was calculated by relating maximal Ca2+-activated force to the cross-sectional area of the fibres determined in relaxation solution. t2, time from the beginning of the stretch to the onset of delayed force increase; t3, time from the beginning of the stretch to the peak of the delayed force increase. X1 versus all types, P < 0.01, and XT + 1 versus all types, P < 0.01 (ANOVA with Student-Neuman-Keuls post hoc test); for t2: within all types, P < 0.01, except X2 + 3 versus X3 and X3 + 4 versus X4, P > 0.1 (two-tailed Student's t test); for t3: within all types, P < 0.01, except X2 + 3 versus X3 and X3 + 4 versus X4, P > 0.2 (two-tailed Student's t test).
Figure 3. Rates of stretch-induced force transients in different fibre types of Xenopus laevis iliofibularis muscle expressed as 1/t3 in a bar graph (A) and a scatterplot (B).
A, the dark and light grey columns are means ± s.d. for fibre types containing one and two MHC isoforms, respectively, with the number of fibres for each fibre type given in parentheses. Significant differences were found between all fibres types (P < 0.01; two-tailed Student's t test) except for X2 + 3 versus X3, and X3 + 4 versus X4. Kolmogorov-Smirnov tests indicate normal distribution for t3 values of pure fibre types (P > 0.09 for type X1, P > 0.2 for types X2, X3 and X4) and lack of normal distribution for t3 values of tonic fibres (type XT + 1, P = 0.01).
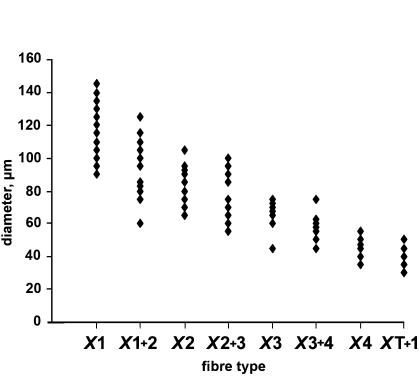
A scatterplot of fibre diameters (average of largest and smallest diameter values measured in oil before skinning) is shown in Fig. 4. Individual values fell within a broad range (30 and 145 μm) and the mean values for the fibre types decreased in the following order: type X1 > type X1 + 2 > type X2 > type X2 + 3 > type X3 > type X3 + 4 > type X4 > type XT + 1. A significant difference (P < 0.05) between diameters was found for all fibre types with the exception of the pairs X2 versus X2 + 3 (P = 0.18), X3 versus X3 + 4 (P = 0.13), and X4 versus X3 + 4 (P = 0.09) (one-way ANOVA with Student-Neuman-Keuls post hoc tests). The mean diameter value (and range) for each fibre type is given in Table 2.
Figure 4. Scatterplot of fibre diameter of Xenopus laevis muscle fibre types.
The diameter was calculated by taking the average of largest and smallest fibre diameter values measured in oil before skinning. A significant difference (P < 0.05, one-way ANOVA followed by Student-Neuman-Keuls post hoc test) was found between diameters of all fibre types with the exception of the pairs X2 versus X2 + 3 (P = 0.18), X3 versus X3 + 4 (P = 0.13) and X4 versus X3 + 4 (P = 0.09).
The average sarcomere length of fibres of different types, measured at slack length ranged between 2.18 and 2.22 μm. These values (see Table 2) were not significantly different between different fibre types (P > 0.2, ANOVA with Student-Neuman-Keuls post hoc test).
The maximal isometric tensions produced by Xenopus iliofibularis muscle fibres ranged typically between 250 and 380 mN mm−2. The mean values (±s.d.) for each fibre type are given in Table 3. Type XT + 1 fibres produced the smallest tensions (P < 0.01) and type X1 fibres produced the largest tensions (P < 0.02). The tension values for all other fibre types were not significantly different from each other (P > 0.1, Table 3).
Discussion
In the present study we resolved, visualized and defined the functional identity of the MHC isoforms expressed in the iliofibularis muscle of Xenopus laevis, which allowed us to classify for the first time the skeletal muscle fibres of this animal according to their MHC isoform composition. Our results provide compelling evidence that Xenopus muscle fibres containing different MHC isoforms have different functional characteristics with respect to stretch activation kinetics. The bi-univocal relationship between the MHC isoform composition of a Xenopus muscle fibre and its stretch activation kinetics (a functional parameter) validates the use of MHC polymorphism for fibre-type classification in frog skeletal muscle and confirms the view that MHC isoform composition is the most ‘reliable’ criterion for fibre-type classification not only for mammalian but generally for vertebrate skeletal muscle.
Fibre types based on functionally different MHC isoforms
Using a refined SDS-PAGE protocol, we identified five MHC isoforms in Xenopus iliofibularis muscle (MHC-XT, MHC-X1, MHC-X2, MHC-X3 and MHC-X4) and in analogy to the mammalian skeletal muscle fibre types (Pette & Staron, 1990; Schiaffino & Reggiani, 1996; Stephenson, 2001), we named the fibre types of Xenopus according to their MHC isoform content. Single fibres contained either one (pure fibre types) or more (two or three; hybrid fibre types) MHC isoforms in different combinations.
Mechanical investigations on maximally Ca2+-activated skinned fibre preparations revealed that the kinetics of stretch-induced force transients (time parameters t2 and t3) produced by single fibres containing different MHC isoforms were different and closely correlated with the MHC isoforms present in the fibres. Importantly, among pure fibres there was no overlap between the t3 (or 1/t3) values, indicating that there was a bi-univocal relationship between the MHC isoform present and t3 (or 1/t3). Also, all hybrid fibres containing approximately similar amounts of two MHC isoforms (Table 2), displayed kinetics of stretch-induced force transients that were intermediate between those displayed by the pure fibres containing only one of the two respective MHC isoforms. The slowest kinetics was displayed by the tonic fibres of type XT + 1.
The correlation between the kinetics of stretch-induced force transients and the MHC isoforms of Xenopus fibres is as tight as that reported earlier for mammalian skeletal muscle fibre types (mouse, Andruchov et al. 2004a; rat, Galler et al. 1994, 1997; rabbit, Andruchov et al. 2004b; human, Hilber et al. 1999). This indicates that the fibre type classification system based on functionally different MHC isoforms is valid for both mammalian and amphibian skeletal muscle fibres and therefore, by extrapolation, for vertebrates in general. The results also show clearly that the identity and number of the MHC isoforms expressed in a fibre confer the fibre-specific functional characteristics.
Functional implication of force transient kinetics
The high degree of correlation between the stretch activation parameters t2 and t3, and the MHC isoform lends further support to the idea that the force transients induced by rapid length changes reflect elementary steps of the cross-bridge cycle (Huxley & Simmons, 1971; Heinl et al. 1974; Ford et al. 1977). Indeed, studies of sinusoidal analysis and stretch activation kinetics provided strong evidence that the force decay after stepwise stretch (t2) is associated with detachment of myosin heads following ATP binding and the delayed force increase (t3) is associated with their re-attachment and force generation prior to the release of phosphate (Kawai & Brandt, 1980; Kawai & Zhao, 1993; Galler et al. 2005). Therefore, the tight correlation between MHC isoform and the values of both t2 and t3 (Table 3) indicates that both the rates of cross-bridge detachment from and attachment to actin are different for the individual MHC isoforms. In contrast, physiological properties such as the unloaded shortening velocity or the time course of the twitch force response, which depend on the cross-bridge cycle as a whole (especially on the slowest step of the whole cycle) rather than on the individual steps of cross-bridge attachment and detachment, may not be as tightly correlated with the MHC isoform composition of the fibre as the stretch activation parameters t2 and t3. It should be noted that the correlation between the time course of the twitch force and the MHC isoform should be even weaker since the twitch kinetics depends not only on the cycling cross-bridges, but also on other additional factors such as the level of sarcoplasmic reticulum (SR) Ca2+ loading and the SR pump rate.
Comparison of MHC isoform-based fibre types with fibre types defined by other criteria
How do fibre types of Xenopus iliofibularis muscle based on MHC composition compare with the fibre types described in the literature prior to this study? At first glance one may argue that direct comparisons of this kind cannot be made, simply because there are many more fibre types based on various combinations of MHC isoforms expressed than the five fibre types that were initially described by Lännergren & Smith (1966) and studied more intensively during the subsequent decades (Smith & Lännergren, 1968; Smith & Ovalle, 1973; Lännergren, 1975, 1978, 1987; Lännergren & Hoh, 1984; Van der Laarse et al. 1986; Horiuti, 1986; Rowlerson & Spurway, 1988; Spurway & Rowlerson, 1989; Stienen et al. 1995). However, at closer examination, the four pure fibre types (X1, X2, X3 and X4) and the fifth hybrid type (XT + 1) expressing MHC-XT in combination with MHC-X1 display quite distinct properties with respect to stretch activation kinetics, size and location characteristics that are similar to those of the five fibre types (1, 2, 3, 4 and 5) previously described by Lännergren and colleagues. Importantly, in addition to these five MHC isoform-based fibre types (X1, X2, X3, X4 and XT + 1), we found a large group of hybrid fibres expressing various combinations of MHC isoforms that had intermediate properties between those of the X1, X2, X3, X4, XT + 1 fibres. Therefore, it is likely that each of the five fibre types 1–5 described in previous studies would include not only the respective fibre types X1, X2, X3, X4 and XT + 1, but also several groups of hybrid fibres that reduce the sharp distinction that exists between the five main MHC-based fibre types X1, X2, X3, X4 and XT + 1. Since the stretch activation kinetics of the MHC isoform-based fibre types X1, X2, X3, X4 and XT + 1 decrease in the same order (X1 > X2 > X3 > X4 > XT + 1) as the unloaded shortening velocity of the isomyosin-based fibre types (1 > 2 > 3 > 4 > 5, Lännergren & Hoh, 1984), it is likely that fibres X1, X2, X3, X4 and XT + 1 belong to fibre types 1, 2, 3, 4 and 5, respectively. This correspondence also suggests that the five isomyosins described by Lännergren and Hoh (Lännergren & Hoh, 1984; Lännergren, 1992) may correspond to the five MHCs isoforms: MHC-X1, MHC-X2, MHC-X3, MHC-X4 and MHC-XT.
The broad correspondence between the isomyosin-based fibre types and the MHC isoform-based fibre types described here is further supported by differences in diameter between different fibre types. Thus, Lännergren & Smith (1966) and Lännergren & Hoh (1984) mention the following ranges of diameters for the fibre types with different isomyosins (in μm): 100–150 for type 1, 55–100 for type 2, 40–70 for type 3, 35–70 for type 4, and 35–70 for type 5. A comparison of these values with the mean diameters of our MHC-based fibre types, given in Table 2, strongly suggests that our type X1 and X2 fibres belong to the type 1 and type 2 fibres of Lännergren & Hoh (1984), respectively. However, since isomyosin-based fibre types 3, 4 and 5 display overlapping diameter values (Lännergren & Hoh, 1984), this parameter cannot be used to assess the relationship between MHC isoform-based fibre types X3, X4 and XT + 1 and isomyosin-based fibre types 3, 4 and 5.
Also, Lännergren & Smith (1966) reported fibre-type-related differences with respect to the location of fibres within the Xenopus iliofibularis muscle and to their appearance in dark-field microscopy. According to these authors, type 5 fibres (tonic fibres) are preferentially found in the ‘centre’ of the muscle close to the proximal tendon. Type 4 fibres are also found in the ‘centre’, but many of them are also located in the immediate surrounding of the ‘centre’ where type 3 fibres are abundant, while type 2 and type 1 fibres are preferentially located at the muscle periphery, occupying about two-thirds of the whole muscle cross-section. Type 1 fibres were also reported to be ‘pale’, type 2 and type 3 fibres to be ‘dark’, and type 5 (tonic fibres) to be ‘clear’. We did not rigorously collect data on the intramuscle location and the microscopic appearance of the MHC isoform-based fibre types described here, but our observations made during fibre dissection are in general agreement with the proposition that the five MHC isoform-based fibre types described in this study for Xenopus iliofibularis muscle belong to the five fibre types distinguished earlier by other investigators (Lännergren & Smith, 1966; Smith & Ovalle, 1973; Lännergren, 1975, 1978, 1979; Rowlerson & Spurway, 1988).
Hybrid fibres
The fibre population dissected in this study from the Xenopus iliofibularis muscle contained diverse combinations of two or more MHC isoforms. The nine different hybrid fibre types to which these fibres belonged were named according to their MHC isoform combinations: X1 + 2 (9.5% of all fibres investigated), X2 + 3 (8.5%), X3 + 4 (3.5%), X1 + 3 (1.5%), X1 + 4 (3.5%), X1 + 2 + 3 (1.5%), X1 + 3 + 4 (5%), XT + 1(13.4%), and XT + 3 + 4 (1.5%). Fibres containing more than one MHC isoform were also reported for various skeletal muscles of Rana pipiens, which were found to contain ‘type 1/2’ and ‘type 2/3’ fibres (Lutz et al. 1998a, b, 2001) and for the rectus abdominis muscle of the cane toad (Nguyen & Stephenson, 2002). Earlier studies of the Xenopus iliofibularis muscle described only about two different hybrid fibre types with no clear indication of their relative proportion in the adult animals. Lännergren (1987) identified two types of hybrid fibres, ‘1 + 2 fibres’ and ‘2 + 3 fibres’ based on fibre-type-related differences in isomyosin composition, and Rowlerson & Spurway, 1988) identified hybrid fibres of the type ‘F1/2’ and ‘F2/3’ using ATPase histochemistry and immunohistochemistry. An important result is that all fibres containing the ‘tonic’ MHC isoform XT displayed at least one of the other four MHC isoforms. Similarly, all fibres dissected by Stephenson and colleagues from the rectus abdominis muscle of the toad Bufo marinus that contained the ‘tonic’ MHC isoform (Nguyen & Stephenson, 2002; O'Connell et al. 2006) also contained 1–3 ‘twitch’ MHC isoforms.
Maximal isometric tension in fibres of different types
Maximal isometric tension (force per cross-sectional area) was not significantly different between the fibre types X2, X3, X4 and related hybrid fibre types (X1 + 2, X2 + 3 and X3 + 4). Compared with X2, X3, X4, X1 + 2, X2 + 3 and X3 + 4 fibres, type X1 fibres produced tensions that were significantly higher by about 20%, and type XT + 1 fibres produced tensions that were significantly lower by about 30% (see Table 3). The finding that type X1 fibres produced higher tensions than the other fibre types is consistent with earlier reports. Thus, Lännergren (1987) found a larger tension in intact type 1 fibres of Xenopus iliofibularis muscle compared with other fibre types, and Lutz et al. (2001) found a larger tension in type 1 fibres compared with type 2 fibres. Please note that the fibre types of the latter study are thought to correspond to the fibre types defined by Lännergren & Smith (1966).
The ratio between the average maximal isometric tensions produced by type X1 fibres (highest values) and type XT + 1 fibres (lowest values) of Xenopus was around 1.6, which is similar to the ratio of about 1.5 found between the maximal isometric tensions produced by type IIB fibres (highest values) and type I fibres (lowest values) for a number of mammalian species (mouse, Andruchov et al. 2004a; rat, Andruchov et al. 2006; rabbit, Andruchov et al. 2004b; 22°C). By comparison, the ratio between the average values of t3 (and t2) for fibre types XT + 1 (highest values) and X1 (lowest values) in Xenopus and fibre types I (highest values) and IIB (lowest values) in the rabbit (Andruchov et al. 2006) was about 50 (43) (see Table 3) and about 35 (30) (Andruchov et al. 2004b), respectively. Based on these data, it appears that kinetics parameters differ much more between fibre types than parameters based on maximum isometric tension. This is true for both anuran and mammalian muscle, and thus it may represent a common characteristic of skeletal muscle fibre diversity.
In conclusion, this study shows that the iliofibularis muscle of Xenopus laevis contains five functionally distinct skeletal MHC isoforms, which display different stretch activation kinetics. These MHC isoforms are expressed in different combinations in single fibres thereby producing a large diversity of fibre types. The study strengthens the view that MHC isoform composition is the most reliable criterion for fibre type classification, and thus, a valuable tool for investigating the diversity and plasticity of vertebrate skeletal muscles.
Acknowledgments
This work was supported by a grant from the Austrian Fonds zur Förderung der Wissenschaftlichen Forschung (FWF-P16709-B09) to S.G., and by grants from the Australian Research Council and NHMRC to D.G.S. and G.M.S.
References
- Andruchov O, Andruchova O, Wang Y, Galler S. Kinetic properties of myosin heavy chain isoforms in mouse skeletal muscle: Comparison with rat, rabbit and human and correlation with amino acid sequence. Am J Physiol Cell Physiol. 2004a;287:C1725–C1732. doi: 10.1152/ajpcell.00255.2004. [DOI] [PubMed] [Google Scholar]
- Andruchov O, Andruchova O, Wang Y, Galler S. Dependence of cross-bridge kinetics on myosin light chain isoforms in rabbit and rat skeletal muscle fibres. J Physiol. 2006;571:231–242. doi: 10.1113/jphysiol.2005.099770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andruchov O, Wang Y, Andruchova O, Galler S. Functional properties of skinned rabbit skeletal and cardiac muscle preparations containing α-cardiac myosin heavy chain. Pflugers Arch. 2004b;448:44–53. doi: 10.1007/s00424-003-1229-2. [DOI] [PubMed] [Google Scholar]
- Bottinelli R. Functional heterogeneity of mammalian single muscle fibres: do myosin isoforms tell the whole story? Pflugers Arch. 2001;443:6–17. doi: 10.1007/s004240100700. [DOI] [PubMed] [Google Scholar]
- Ford LE, Huxley AF, Simmons RM. Tension responses to sudden length change in stimulated frog muscle fibres near slack length. J Physiol. 1977;269:441–515. doi: 10.1113/jphysiol.1977.sp011911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galler S. Stretch activation of skeletal muscle fibre types. Pflugers Arch. 1994;427:384–386. doi: 10.1007/BF00374550. [DOI] [PubMed] [Google Scholar]
- Galler S. Ca2+ Sr2+ force relationships and kinetic properties of fast-twitch rat leg muscle fibre subtypes. Acta Physiol Scand. 1999;167:131–141. doi: 10.1046/j.1365-201x.1999.0600x.x. [DOI] [PubMed] [Google Scholar]
- Galler S, Hilber K. Unloaded shortening of skinned mammalian skeletal muscle fibres. Effects of the experimental approach and passive force. J Muscle Res Cell Motil. 1994;15:400–412. doi: 10.1007/BF00122114. [DOI] [PubMed] [Google Scholar]
- Galler S, Hilber K, Pette D. Force responses following stepwise length changes of rat skeletal muscle fibre types. J Physiol. 1996;493:219–227. doi: 10.1113/jphysiol.1996.sp021377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galler S, Hilber K, Pette D. Stretch activation and myosin heavy chain isoforms of rat, rabbit and human skeletal muscle fibres. J Muscle Res Cell Motil. 1997;18:441–448. doi: 10.1023/a:1018646814843. [DOI] [PubMed] [Google Scholar]
- Galler S, Schmitt TL, Pette D. Stretch activation, unloaded shortening velocity, and myosin heavy chain isoforms of rat skeletal muscle fibres. J Physiol. 1994;478:513–521. doi: 10.1113/jphysiol.1994.sp020270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galler S, Wang G, Kawai M. Elementary steps of the cross-bridge cycle in fast-twitch fiber types from rabbit skeletal muscles. Biophys J. 2005;89:3248–3260. doi: 10.1529/biophysj.104.056614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon AM, Huxley AF, Julian FJ. The variation in isometric tension with sarcomere length in vertebrate muscle fibres. J Physiol. 1966;184:170–192. doi: 10.1113/jphysiol.1966.sp007909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinl P, Kuhn HJ, Rüegg JC. Tension responses to quick length changes of glycerinated skeletal muscle fibres from frog and tortoise. J Physiol. 1974;237:243–258. doi: 10.1113/jphysiol.1974.sp010480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilber K, Galler S. Improvement of the measurements on skinned muscle fibres by fixation of the fibre ends with glutaraldehyde. J Muscle Res Cell Motil. 1998;19:365–372. doi: 10.1023/a:1005393519811. [DOI] [PubMed] [Google Scholar]
- Hilber K, Galler S, Gohlsch B, Pette D. Kinetic properties of myosin heavy chain isoforms in single fibres from human skeletal muscle. FEBS Lett. 1999;455:267–270. doi: 10.1016/s0014-5793(99)00903-5. [DOI] [PubMed] [Google Scholar]
- Horiuti K. Some properties of the contractile system and sarcoplasmatic reticulum of skinned slow fibres from Xenopus muscle. J Physiol. 1986;373:1–23. doi: 10.1113/jphysiol.1986.sp016032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huxley AF. Mechanics and models of the myosin motor. Philos Trans R Soc Lond B Biol Sci. 2000;355:433–440. doi: 10.1098/rstb.2000.0584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huxley AF, Simmons RM. Proposed mechanism of force generation in striated muscle. Nature. 1971;233:533–538. doi: 10.1038/233533a0. [DOI] [PubMed] [Google Scholar]
- Kawai M, Brandt PW. Sinusoidal analysis: a high resolution method for correlating biochemical reactions with physiological processes in activated skeletal muscles of rabbit, frog and crayfish. J Muscle Res Cell Motil. 1980;1:279–303. doi: 10.1007/BF00711932. [DOI] [PubMed] [Google Scholar]
- Kawai M, Zhao Y. Cross-bridge scheme and force per cross-bridge state in skinned rabbit psoas muscle fibres. Biophys J. 1993;65:638–651. doi: 10.1016/S0006-3495(93)81109-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lännergren J. Structure and function of twitch and slow fibres in amphibian skeletal muscle. In: Lennerstrand G, Bach-y-rita P, editors. Basic Mechanism of Ocular Motility and their Clinical Implications. Oxford and New York: Pergamon Press; 1975. pp. 63–84. [Google Scholar]
- Lännergren J. The force–velocity relation of isolated twitch and slow muscle fibres of Xenopus laevis. J Physiol. 1978;283:501–521. doi: 10.1113/jphysiol.1978.sp012516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lännergren J. An intermediate type of muscle fibre in Xenopus laevis. Nature. 1979;279:254–256. doi: 10.1038/279254a0. [DOI] [PubMed] [Google Scholar]
- Lännergren J. Contractile properties and myosin isoenzymes of various kinds of Xenopus twitch muscle fibres. J Muscle Res Cell Motil. 1987;8:260–273. doi: 10.1007/BF01574594. [DOI] [PubMed] [Google Scholar]
- Lännergren J. Fibre types in Xenopus muscle and their functional properties. In: Simmons RM, editor. Muscular Contraction. Cambridge: Cambridge University Press; 1992. pp. 181–188. [Google Scholar]
- Lännergren J, Hoh JF. Myosin isoenzymes in single muscle fibres of Xenopus laevis: analysis of five different functional types. Proc R Soc Lond B Biol Sci. 1984;222:401–408. doi: 10.1098/rspb.1984.0072. [DOI] [PubMed] [Google Scholar]
- Lännergren J, Smith RS. Types of muscle fibres in toad skeletal muscle. Acta Physiol Scand. 1966;68:263–274. [Google Scholar]
- Lutz GJ, Bremner SN, Bade MJ, Lieber RL. Identification of myosin light chains in Rana pipiens skeletal muscle and their expression patterns along single fibres. J Exp Biol. 2001;204:4237–4248. doi: 10.1242/jeb.204.24.4237. [DOI] [PubMed] [Google Scholar]
- Lutz GJ, Bremner S, Lajevardi N, Lieber RL, Rome LC. Quantitative analysis of muscle fibre type and myosin heavy chain distribution in the frog hindlimb: implication for locomotory design. J Muscle Res Cell Motil. 1998b;19:717–731. doi: 10.1023/a:1005466432372. [DOI] [PubMed] [Google Scholar]
- Lutz GJ, Cuizon DB, Ryan AF, Liber RL. Four novel myosin heavy chain transcripts define a molecular basis for muscle fibre types in Rana pipiens. J Physiol. 1998a;508:667–680. doi: 10.1111/j.1469-7793.1998.667bp.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasledov GA, Lebedinskaia II. Study of the contractile mechanism of frog tonic muscle fibres. Fiziol Zh USSR Im I M Sechenova. 1971;57:1307–1313. [PubMed] [Google Scholar]
- Nguyen LT, Stephenson GM. An electrophoretic study of myosin heavy chain expression in skeletal muscles of the toad Bufo marinus. J Muscle Res Cell Motil. 1999;20:687–695. doi: 10.1023/a:1005560431865. [DOI] [PubMed] [Google Scholar]
- Nguyen LT, Stephenson GM. Myosin heavy chain isoform expression and Ca2+-stimulated ATPase activity in single fibres of toad rectus abdominis muscle. J Muscle Res Cell Motil. 2002;23:147–156. doi: 10.1023/a:1020266422201. [DOI] [PubMed] [Google Scholar]
- O'Connell B, Blazev R, Stephenson GM. Electrophoretic and functional identification of two troponin C isoforms in toad skeletal muscle fibres. Am J Physiol Cell Physiol. 2006;290:C515–C523. doi: 10.1152/ajpcell.00307.2005. [DOI] [PubMed] [Google Scholar]
- Pette D, Staron RS. Cellular and molecular diversities of mammalian skeletal muscle fibers. Rev Physiol Biochem Pharmacol. 1990;116:1–76. doi: 10.1007/3540528806_3. [DOI] [PubMed] [Google Scholar]
- Rowlerson AM, Spurway NC. Histochemical and immunohistochemical properties of skeletal muscle fibres from Rana and Xenopus. Histochem J. 1988;20:657–673. doi: 10.1007/BF01845796. [DOI] [PubMed] [Google Scholar]
- Schiaffino S, Reggiani C. Molecular diversity of myofibrillar proteins: gene regulation and functional significance. Physiol Rev. 1996;76:371–423. doi: 10.1152/physrev.1996.76.2.371. [DOI] [PubMed] [Google Scholar]
- Smith RS, Lannergen J. Types of motor units in the skeletal muscle of Xenopus laevis. Nature. 1968;217:281–283. doi: 10.1038/217281a0. [DOI] [PubMed] [Google Scholar]
- Smith RS, Ovalle WKJ. Varieties of fast and slow extrafusal muscle fibres in amphibian hind limb muscles. J Anat. 1973;116:1–24. [PMC free article] [PubMed] [Google Scholar]
- Spurway NC, Rowlerson AM. Quantitative analysis of histochemical and immunohistochemical reactions in skeletal muscle fibres of Rana and Xenopus. Histochem J. 1989;21:461–474. doi: 10.1007/BF01845796. [DOI] [PubMed] [Google Scholar]
- Stephenson GM. Hybrid skeletal muscle fibres: a rare or common phenomenon? Clin Exp Pharmacol Physiol. 2001;28:692–702. doi: 10.1046/j.1440-1681.2001.03505.x. [DOI] [PubMed] [Google Scholar]
- Stephenson DG, Williams DA. Calcium activated force responses in fast- and slow-twitch skinned muscle fibres of the rat at different temperatures. J Physiol. 1981;317:281–302. doi: 10.1113/jphysiol.1981.sp013825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stienen GJM, Zaremba R, Elzinga G. ATP utilisation for calcium uptake and force production in skinned muscle fibres of Xenopus laevis. J Physiol. 1995;482:109–122. doi: 10.1113/jphysiol.1995.sp020503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Laarse WJ, Diegenbach PC, Hemminga MA. Calcium-stimulated myofibrillar ATPase activity correlates with shortening velocity of muscle fibres in Xenopus laevis. Histochem J. 1986;18:487–496. doi: 10.1007/BF01675616. [DOI] [PubMed] [Google Scholar]