Abstract
The evolutionarily conserved serine/threonine kinase, AMP-activated protein kinase (AMPK), functions as a cellular fuel gauge that regulates metabolic pathways in glucose and fatty acid metabolism and protein synthesis. Recent data strongly implicate the AMPK–acetyl CoA carboxylase (ACC)–malonyl CoA pathway in the hypothalamus in the regulation of food intake, body weight and hepatic glucose production. Furthermore, data indicate that AMPK is a mediator of the effects of adipocyte-derived and gut-derived hormones and peptides on fatty acid oxidation and glucose uptake in peripheral tissues. Studies are now elucidating the potential role of kinases upstream of AMPK in these metabolic effects. In addition, recently, several novel downstream effectors of AMPK have been identified. The AMPK pathway in the hypothalamus and peripheral tissues coordinately integrates inputs from multiple hormones, peptides and nutrients to maintain energy homeostasis.
AMPK is a serine/threonine kinase that is evolutionarily conserved from yeast to mammals. It is a heterotrimeric protein consisting of an α catalytic subunit and two regulatory subunits, β and γ. AMPK is a fuel-sensing enzyme activated by physiological and pathological stresses that deplete cellular ATP, including hypoxia, ischaemia, glucose deprivation, uncouplers of oxidative phosphorylation, exercise and muscle contraction. Activation of AMPK represses ATP-consuming anabolic pathways and induces ATP-producing catabolic pathways. This is accomplished, at least in part, by regulating gene expression and the activities of key metabolic enzymes in fatty acid, cholesterol and glucose metabolism, and protein synthesis, as well as other metabolic pathways (Kahn et al. 2005). In addition to its role in the periphery, AMPK also regulates energy intake and body weight by mediating opposing effects of anorexigenic and orexigenic signals in the hypothalamus (Andersson et al. 2004; MS Kim et al. 2004; Minokoshi et al. 2004; Han et al. 2005; Kola et al. 2005). Thus, the coordinated regulation of the AMPK pathway in the hypothalamus and peripheral tissues plays a critical role in integrating hormonal and nutrient-derived signals that impact energy homeostasis. This review will focus on recent findings pertaining to the regulation of energy balance by AMPK in both the central nervous system (Fig. 1) and peripheral tissues (Fig. 2), and shed light on the upstream kinases and novel downstream targets for these actions of AMPK.
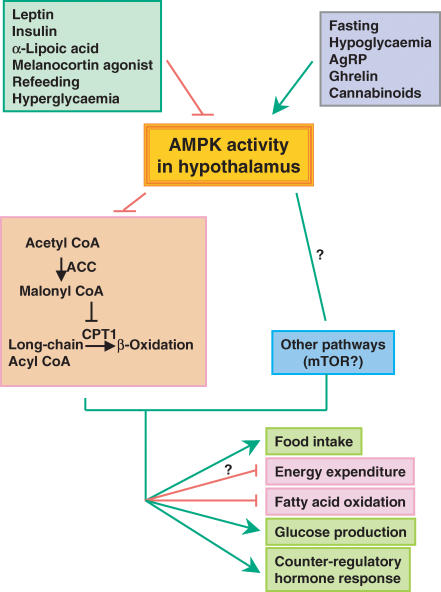
Figure 1. Multiple hormones, peptides, nutrients and altered metabolic states regulate AMPK activity in the hypothalamus.
Changes in AMPK activity may act through the acetyl CoA carboxylase (ACC)–malonyl CoA–CPT1 pathway to regulate food intake and other metabolic processes. Other pathways downstream of AMPK, including possibly mTOR (Cota et al. 2006), also mediate the effects of AMPK on energy balance and glucose homeostasis. An arrow represents stimulation, whereas a crossbar means inhibition.
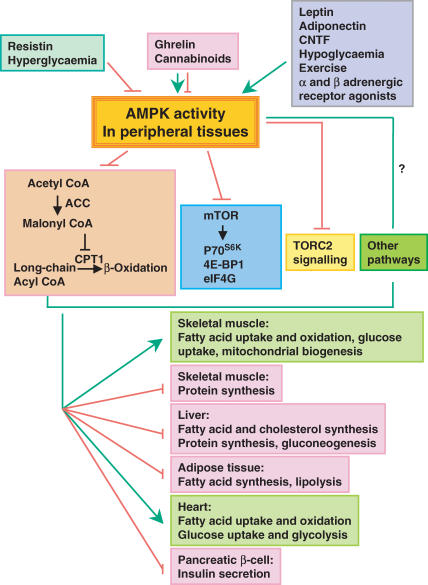
Figure 2. Hormones, nutrients and altered metabolic states regulate AMPK activity in peripheral tissues.
Changes in AMPK activity act through the acetyl CoA carboxylase (ACC)–malonyl CoA–CPT1 pathway to increase fatty acid oxidation (β-oxidation) and regulate other metabolic processes in multiple tissues. Other pathways downstream of AMPK also mediate its effects on glucose, fatty acid and protein metabolism. An arrow represents stimulation, whereas a crossbar means inhibition.
Hypothalamic AMPK activity regulates food intake and body weight (Fig. 1)
A number of recent studies demonstrate that hypothalamic AMPK activity regulates food intake. Hypothalamic AMPK is regulated under physiological conditions. Fasting results in increased AMPK activity in multiple hypothalamic regions, whereas refeeding inhibits it (Minokoshi et al. 2004). Activation of AMPK in the hypothalamus is sufficient to increase food intake, and body weight and suppression of hypothalamic AMPK activity is sufficient to decrease these parameters as demonstrated by experiments in which recombinant adenoviruses expressing constitutively active (CA) or dominant negative (DN) AMPK were injected into the basal medial hypothalamus in normal mice (Minokoshi et al. 2004). Changes in hypothalamic AMPK activity result in alterations of neuropeptide expression. Overexpressing DN-AMPK in medial basal hypothalamus suppresses mRNA expression of orexigenic neuropeptides, neuropeptide Y (NPY) and agouti-related peptide (AgRP) in arcuate hypothalamus (ARH), whereas overexpressing CA-AMPK enhances the fasting-induced increase in expression of NPY and AgRP in ARH and melanin-concentrating hormone in lateral hypothalamus (Minokoshi et al. 2004). These changes in neuropeptide expression are likely to mediate AMPK's effect on food intake, at least in part. Interestingly, these changes in neuropeptide expression are similar to those seen with administration of fatty acids intracerebroventricularly (i.c.v.) or manipulation of hypothalamic lipid metabolism (Obici et al. 2003).
Fasting and refeeding are accompanied by oscillations of hormones and nutrients in both the periphery and the central nervous system which may bring about the alterations in AMPK activity. Indeed, even relatively small increases in glucose levels which occur after refeeding may inhibit hypothalamic AMPK activity since i.c.v. injection of glucose suppresses AMPK activity in multiple hypothalamic areas (Minokoshi et al. 2004). The anorexigenic hormones leptin and insulin also inhibit hypothalamic AMPK activity. Leptin's effects on AMPK activity are localized to the arcuate and paraventricular (PVN) nuclei (Minokoshi et al. 2004) whereas high glucose or insulin inhibits AMPK in other hypothalamic regions as well. Melanocortin 4 (MC4) receptors appear to be involved in the effects of both leptin and refeeding on AMPK activity in the PVN. Administration i.c.v. of the melanocortin 4 receptor agonist, MT-II, inhibits AMPK activity in the PVN (Minokoshi et al. 2004). Furthermore, refeeding and leptin fail to inhibit hypothalamic AMPK activity in MC4 receptor knockout mice (Minokoshi et al. 2004). α-Lipoic acid, an antioxidant that reduces food intake when given by either intraperitoneal (i.p.) or i.c.v. injection in rats, also inhibits AMPK activity in the hypothalamus (MS Kim et al. 2004). The effect of α-lipoic acid on food intake is likely to be mediated by modulations of hypothalamic AMPK, as increasing AMPK activity by i.c.v. injection of the AMP activator 5′-aminoimidazole-4-carboxamide ribonucleoside (AICAR) or by adenovirus-mediated gene transfer of a constitutively active AMPK variant prevents the anorexigenic effect of α-lipoic acid (MS Kim et al. 2004).
The orexigenic molecules, cannabinoids and ghrelin, stimulate hypothalamic AMPK activity (Andersson et al. 2004; Kola et al. 2005). Cannabinoids potently stimulate food intake acting via the presynaptic cannabinoid type 1 receptor (CB1) in the ventromedial hypothalamus (Jamshidi & Taylor, 2001). Ghrelin is predominantly expressed in the gastric mucosa and acts on receptors in the arcuate and ventromedial hypothalamus (Zigman et al. 2006). Circulating levels of ghrelin rise before meals and following food deprivation and i.c.v. administration of ghrelin increases food intake and body weight (Cummings et al. 2001; Nakazato et al. 2001; Cummings et al. 2002; Cota et al. 2003). These findings suggest that hypothalamic AMPK integrates nutrient-derived and hormonal or peptide-derived signals from both anorexigenic and orexigenic pathways to regulate food intake and body weight.
The downstream effectors which mediate the effects of hypothalamic AMPK on food intake are of key interest. Some studies show that inhibition of AMPK by leptin (Andersson et al. 2004) or α-lipoic acid (MS Kim et al. 2004) is associated with decreased phosphorylation of its downstream target, acetyl-CoA carboxylase (ACC), leading to stimulation of ACC activity and increases in cellular malonyl-CoA levels, which would inhibit mitochondrial carnitine palmitoyltransferase 1 (CPT1) and fatty acid oxidation. In support of this mechanism, inhibiting CPT1 in the hypothalamus suppresses food intake (Obici et al. 2003). In addition, adeno-associated virus-mediated gene transfer of malonyl-CoA decarboxylase, which is involved in the degradation of malonyl-CoA, into the medial basal hypothalamus of rats, results in increased food intake and progressive weight gain (He et al. 2006). Taken together, these data strongly support the importance of this pathway in the regulation of food intake.
Hypothalamic AMPK activity regulates hypoglycaemia-induced counter-regulatory hormone responses
Appropriate counter-regulatory hormone responses are critical for the recovery from hypoglycaemia, which is clinically important in the treatment of people with either type 1 or type 2 diabetes. Glucose is the primary energy source for neurons. Within the brain, glucose-sensing neurons located in the ventromedial hypothalamus have been thought to play an important role in the counter-regulatory hormone response to hypoglycaemia (Song et al. 2001). Inhibition of intracellular glucose utilization by 2-deoxyglucose activates hypothalamic AMPK activity (MS Kim et al. 2004). In addition, activating AMPK by AICAR in the ventromedial hypothalamus increases hepatic glucose production during a hypoglycaemic clamp (McCrimmon et al. 2004). These data suggest that neurons and/or glial cells in hypothalamus may sense the changes of glucose levels and participate in the counter-regulatory response by altering AMPK activity. Indeed, recent data demonstrate that hypoglycaemia induced by insulin activates AMPK in the arcuate nucleus/ventromedial hypothalamus and paraventricular hypothalamus (Han et al. 2005). Activation of hypothalamic AMPK by i.c.v. AICAR administration under euglycaemic conditions increases circulating levels of counter-regulatory hormones, including corticosterone and glucagon (Han et al. 2005). In addition, inhibiting hypothalamic AMPK activity by either i.c.v. injection of compound C or adenovirus-mediated gene transfer of DN-AMPK prevents the hypoglycaemia-induced rise of counter-regulatory hormones and the recovery from hypoglycaemia (Han et al. 2005). These data strongly suggest that hypothalamic AMPK plays an important role in the counter-regulatory hormone response and the recovery from hypoglycaemia.
AMPK regulates fatty acid oxidation and glucose metabolism in peripheral tissues (Fig. 2)
In the periphery, AMPK regulates a variety of metabolic pathways that result in suppression of ATP-consuming anabolic pathways and induction of ATP-producing catabolic pathways (Kahn et al. 2005). These include stimulating fatty acid uptake and oxidation and glucose uptake in multiple tissues; stimulating mitochondrial biogenesis in skeletal muscle; stimulating glycolysis in heart; inhibiting fatty acid synthesis in liver and adipocytes; inhibiting cholesterol synthesis and glucongenesis in liver; inhibiting protein synthesis in liver and muscle; inhibiting lipolysis in adipocytes; and inhibiting insulin secretion from pancreatic β-cells (Kahn et al. 2005). These effects of AMPK are achieved by regulating the activities of key metabolic enzymes in these metabolic pathways as well as by longer-term regulation of gene expression (Kahn et al. 2005).
Adipokines: leptin, adiponectin and resistin
A major advance in the understanding of AMPK's physiological function is the discovery that AMPK mediates leptin's effect on fatty acid oxidation in skeletal muscle (Minokoshi et al. 2002). Leptin administration in mice results in a dual effect on α2 AMPK in skeletal muscle: a rapid but transient activation and a more sustained activation lasting up to 6 h. The transient stimulation of muscle AMPK by leptin is exerted directly at the level of muscle, whereas the longer-lasting effect of leptin is mediated through the hypothalamic–sympathetic nervous system axis and involves α-adrenergic receptors in muscle. Activation of AMPK by leptin in muscle is accompanied by suppression of ACC activity, which, in turn, stimulates fatty acid oxidation. In fact, AMPK activation appears to be necessary for leptin's effect on ACC activity and thereby fatty acid oxidation in muscle (Minokoshi et al. 2002). Activation of AMPK is also important for adiponectin's effect of increasing fatty acid oxidation and glucose uptake in muscle and inhibiting gluconeogenesis in liver (Tomas et al. 2002; Yamauchi et al. 2002).
Obesity and diabetes are associated with excessive accumulation of ectopic lipids in non-adipose tissues. This is seen in leptin-resistant or leptin-deficient obese states (Unger, 2002). Adenovirus-mediated leptin gene transfer in normal leptin-sensitive rats, which leads to increased circulating leptin levels, results in rapid disappearance of not only triglycerides stored in white fat, but also of the triglyceride content in non-adipose tissues, such as skeletal muscle, liver and pancreas, independent of reduced food intake (Chen et al. 1996; Shimabukuro et al. 1997; Orci et al. 2004). This is mostly due to increased fatty acid oxidation and decreased fatty acid esterification (Shimabukuro et al. 1997). These metabolic changes most likely result from activation of AMPK, which inhibits ACC activity, and from suppression of lipogenic enzymes, such as fatty acid synthase (FAS) and stearoyl CoA desaturase 1. The expression of peroxisome proliferator-activated receptor γ coactivator-1α (PGC1α), which is a master regulator of mitochondrial biogenesis (Puigserver et al. 1998; Wu et al. 1999), the mitochondrial enzyme cytochrome c oxidase subunit IV and uncoupling proteins are also up-regulated in white adipose tissue (Orci et al. 2004). All of these changes would contribute to the reduction of tissue triglyceride content. In addition, leptin administration is therapeutic for cardiac lipotoxicity (Lee et al. 2004). In obese/diabetic fa/fa rats who do not respond to leptin treatment because of defects in the leptin receptor, administration of the AMPK activator AICAR results in leptinomimetic effects, leading to the prevention of ectopic lipid deposition and diabetes (Yu et al. 2004). Therefore, in leptin-sensitive states, AMPK may mediate leptin's effect on fatty acid metabolism, thus preventing ectopic lipid storage and improving insulin sensitivity. In leptin-resistant states which include diet-induced obesity, pharmacological activation of AMPK in peripheral tissues may be a strategy to bypass leptin resistance and help protect individuals from developing insulin resistance and type 2 diabetes.
The adipocyte/macrophage-secreted hormone resistin inhibits AMPK phosphorylation and activity in liver, muscle and adipose tissue in vivo (Banerjee et al. 2004; Muse et al. 2004; Satoh et al. 2004) and diminishes AMPK phosphorylation and fatty acid oxidation in cultured muscle cells (Palanivel & Sweeney, 2005). Plasma resistin levels increase with high fat feeding which is associated with diminished AMPK phosphorylation in liver (Muse et al. 2004). Resistin antisense oligodeoxynucleotide given i.p. enhances AMPK phosphorylation in liver above levels in control, chow-fed mice. Therefore, inhibition of AMPK may contribute to the detrimental metabolic effects of resistin.
Peripheral effects of ciliary neurotrophic factor
Ciliary neurotrophic factor (CNTF) decreases food intake and body weight by inducing hypothalamic neurogenesis (Kokoeva et al. 2005). CNTF also stimulates both α1 and α2 AMPK activity, increases ACC phosphorylation and activates fatty acid oxidation in muscle (Watt et al. 2006). The effect of CNTF is exerted through binding to the heterodimeric receptor complexes that include the leukaemia inhibitory factor receptor and gp130β which bind with either the CNTF receptor α (CNTFRα) or the interleukin-6 receptor. However, unlike leptin, which has a direct effect on muscle via leptin receptors in muscle as well as an indirect effect via the hypothalamic–sympathetic nervous system axis (Minokoshi et al. 2002), the effect of CNTF is exerted only directly at the level of muscle. Administration i.c.v. of CNTF has no effect on muscle AMPK activity and is not capable of inducing the expression of genes involved in mitochondrial biogenesis and fatty acid oxidation, such as PGC1α and CPT1 (Watt et al. 2006).
Peripheral effects of ghrelin and cannabinoids
Both ghrelin and cannabinoids activate AMPK in the hypothalamus, which may contribute to their orexigenic effect (Kola et al. 2005). However, in peripheral tissues, regulation of AMPK by ghrelin and cannabinoids is a more complex process. Both ghrelin and cannabinoids activate α1 and α2 AMPK in heart, whereas they inhibit α1 and α2 AMPK in liver and adipose tissue. However, ghrelin and cannabinoids have no effect on AMPK in skeletal muscle (Kola et al. 2005). These data indicate that ghrelin and cannabinoids regulate AMPK in a tissue-specific manner. Ghrelin and cannabinoids exert versatile biological effects in multiple tissues, which may be mediated by their differential regulation of AMPK activity in these tissues. It has been reported that cannabinoids are beneficial in limiting damage and reducing infarct size during myocardial infarction (Joyeux et al. 2002). The beneficial effect of cannabinoids on ischaemic heart may be mediated by AMPK, as AMPK is activated during low-flow ischaemia and is important for glucose uptake and glycolysis in ischaemic heart. Thus, AMPK in heart plays a protective role in limiting damage and apoptotic activity associated with ischaemia and reperfusion (Russell et al. 2004). Ghrelin stimulates gluconeogenesis in hepatocytes (Murata et al. 2002), which is consistent with its effect of inhibiting AMPK activity in liver. In addition, both ghrelin and cannabinoids are implicated in promoting lipid storage in adipose tissue (Tschop et al. 2000; Cota et al. 2003; Tsubone et al. 2005), consistent with their effects of inhibiting AMPK in adipose tissue.
Regulation of AMPK activity by α- and β-adrenergic receptor signalling
The α-adrenergic receptors, which couple to G proteins such as Gq and trigger inositol trisphosphate release, activate AMPK in cultured cells (Kishi et al. 2000) and skeletal muscle in vivo and ex vivo (Minokoshi et al. 2002). α-Adrenergic receptors in muscle mediate the effect of leptin on AMPK activity and fatty acid oxidation that occurs indirectly through the hypothalamus and sympathetic nervous system since the latter effect can be blocked by α-adrenergic receptor blockers (Minokoshi et al. 2002).
The β-adrenergic agonist, isoproterenol (isoprenaline), stimulates AMPK activity in isolated adipocytes (Moule & Denton, 1998). In addition, the β-adrenergic receptor agonist norepinephrine (noradrenaline) activates AMPK as well as 2-deoxyglucose uptake in brown adipocytes both in vivo and in vitro (Inokuma et al. 2005; Hutchinson et al. 2005), whereas the α-adrenergic receptor agonists are without effect (Hutchinson et al. 2005). These data support the possibility that brown fat AMPK activity is regulated through the β-adrenergic receptor signalling. Data also suggest that uncoupling protein 1 may play a role in the regulation of AMPK activity in both brown and white adipocytes (Matejkova et al. 2004; Hutchinson et al. 2005; Inokuma et al. 2005).
AMPK integrates signals from hormones and nutrients to coordinately regulate energy balance through peripheral and central nervous system mechanisms
An increasing body of data suggests that AMPK mediates the actions of diverse hormones and nutrients. Accordingly, AMPK is regulated by these factors in both the central nervous system and periphery. Leptin's coordinated regulation of AMPK in the hypothalamus and periphery is necessary for its regulation of energy homeostasis (Minokoshi et al. 2002, 2004). In addition, ghrelin and cannabinoids activate AMPK in the hypothalamus, whereas they exert diverse effects on AMPK in different peripheral tissues (Kola et al. 2005).
On the other hand, AMPK activity in the hypothalamus may also be critical in mediating hormonal and nutrient regulation of physiological processes in the periphery. Activation of AMPK in the hypothalamus is necessary to trigger secretion of counter-regulatory hormones from peripheral tissues in response to hypoglycaemia (Han et al. 2005). Inhibition of hypothalamic AMPK by leptin may also be necessary for leptin's effect in skeletal muscle on AMPK activity and fatty acid oxidation (Y. Minokoshi & B. B. Kahn, unpublished data). In addition, the FAS inhibitor C75, when administrated i.c.v., inhibits hypothalamic AMPK activity, which may mediate its anorexigenic effect (EK Kim et al. 2004). However, i.c.v. administration of C75 also results in stimulation of fatty acid oxidation in skeletal muscle and reduction in circulating free fatty acid and ketone levels (Cha et al. 2005). At another point in the pathway, inhibition of hypothalamic CPT1 results in reduced hepatic glucose production (Obici et al. 2003). These findings indicate an integrated role of AMPK in mediating diverse metabolic actions of hormones and nutrients in both the hypothalamus and peripheral tissues. Many of the peripheral effects of AMPK on energy balance appear to be mediated through the central nervous system.
Dysregulation of AMPK in diet-induced obesity and diabetes
Obesity is usually associated with leptin resistance (El-Haschimi et al. 2000). Given the importance of AMPK in mediating leptin's physiological effects, it is plausible to hypothesize that dysregulation of AMPK may contribute to leptin resistance in diet-induced obesity. Suppressing hypothalamic AMPK activity is necessary for leptin's effect on food intake and body weight, as overexpression of CA-AMPK in mediobasal hypothalamus abolishes leptin's anorexigenic effect (Minokoshi et al. 2004). In addition, transgenic mice overexpressing leptin in liver (LepTg) are lean and have enhanced glucose and lipid metabolism on a chow diet despite significantly elevated circulating leptin levels (Ogawa et al. 1999). However, LepTg mice become as obese and insulin resistant as their wild-type littermates on a high fat diet (Tanaka et al. 2005). These effects correlate inversely with alterations in muscle AMPK/ACC phosphorylation, i.e. the phosphorylation of AMPK and ACC in soleus muscle of LepTg on a chow diet is elevated compared to wild-type littermates, whereas this is abrogated in LepTg mice on a high fat diet for 15 weeks. These data suggest that altered AMPK activity may contribute to leptin resistance.
Feeding mice a high fat diet for 12 weeks results in leptin resistance as evidenced by the fact that exogenous leptin fails to suppress food intake (Martin et al. 2006). High fat feeding also causes dysregulation of AMPK in hypothalamus and muscle (Martin et al. 2006). In muscle of high-fat-fed mice, basal AMPK activity is elevated and leptin fails to further stimulate it. In the hypothalamus of these mice, basal AMPK activity is suppressed, and leptin fails to further suppress it. These data indicate that dysregulation of AMPK occurs during diet-induced obesity and leptin loses its ability to coordinately regulate AMPK in the hypothalamus and periphery. In a study in rats, high fat feeding for 5 months impairs both the phosphorylation and protein expression of the AMPK α subunit in gastrocnemius muscle (Liu et al. 2006) but the responses of AMPK activity to leptin were not assessed.
The tumour suppressor LKB1 and the Ca2+–calmodulin-dependent protein kinase kinases are upstream kinases for AMPK
Although it has long been known that phosphorylation of the AMPK α catalytic subunit at Thr172 is essential for the activation of AMPK (Hawley et al. 1996), the upstream AMPK kinase (AMPKK) remained elusive until recent identification of three closely related yeast protein kinases, Elm1, Pak1 and Tos3. These act upstream of the yeast homologue of AMPK (the SNF1 complex) (Hong et al. 2003; Sutherland et al. 2003). The most closely related mammalian homologues to these kinases are the tumour suppressor LKB1, and members of the Ca2+–calmodulin-dependent protein kinase kinase (CaMKK) family, CaMKKα and β. Further studies demonstrated that these protein kinases phosphorylate and activate AMPK (Hawley et al. 2003, 2005; Woods et al. 2003; Shaw et al. 2004, 2005; Hong et al. 2005; Hurley et al. 2005; Sakamoto et al. 2005, 2006).
LKB1 exists as a heterotrimeric complex with two other proteins, mouse protein 25 (MO25) and STE20-related adaptor protein (Hawley et al. 2003). AMPKK activities purified from rat liver can be immunoprecipitated using anti-LKB1 antibodies. In addition, blocking LKB1 activity in cells abolishes AMPK activation in response to different stimuli (Hawley et al. 2003; Woods et al. 2003). Deficiency of LKB1 in skeletal muscle and heart of transgenic mice prevents contraction-stimulated AMPK activation and reduces glucose uptake in skeletal muscle as well as ischaemia-mediated AMPK α2 but not α1 activation in heart (Sakamoto et al. 2005, 2006). In addition, deletion of LKB1 in liver results in a nearly complete loss of AMPK activity, leading to hyperglycaemia with elevated gluconeogenic and lipogenic gene expression (Shaw et al. 2005). These findings clearly demonstrate that LKB1 is a physiologically important upstream kinase for AMPK. The antidiabetic agent metformin which activates AMPK did not improve glucose control in liver-LKB1-deficient mice supporting the importance of the LKB1–AMPK pathway in the liver in the therapeutic effects of metformin.
Intriguingly, most attempts to identify physiological regulation of LKB1 have failed and LKB1 is not regulated by stimuli that activate AMPK (Lizcano et al. 2004; Sakamoto et al. 2004). Endurance training increases LKB1 and MO25 protein levels in skeletal muscle; however, the AMPKK activity is either unaltered or decreased (Taylor et al. 2004, 2005). In addition, perfusing rat liver with glucagon results in increased LKB1 phosphorylation at Ser248 (Kimball et al. 2004), a site that is phosphorylated by protein kinase A and p90RSK (p90 ribosomal s6 kinase). The phosphorylation of Ser248 is essential for the LKB1 effect to suppress cell growth although it does not affect LKB1 activity (Sapkota et al. 2001).
LKB1 was originally identified as a tumour suppressor gene mutated in the rare hereditary form of cancer termed Peutz-Jeghers syndrome (Jenne et al. 1998). This may indicate a connection between AMPK cascade and cancer. However, it should be noted that AMPK is only one of the 11 AMPK-related kinases that are phosphorylated by LKB1 (Lizcano et al. 2004). Therefore, it is possible that some of the functions of LKB1 may be mediated by the AMPK-related kinases, rather than AMPK itself.
Recent data demonstrate that CAMKKα and β are also upstream kinases for AMPK. AMPK is activated by Ca2+ ionophores in LKB1-deficient cells, and this activation is prevented by either CaMKK small interfering RNAs or the CaMKK inhibitor STO-609 (Hawley et al. 2005; Hurley et al. 2005; Woods et al. 2005). Unlike LKB1, whose expression is ubiquitous, the expression of CaMKKα and β is more restricted with high levels in the brain, including hypothalamus, and low expression in peripheral tissues (Anderson et al. 1998; Sakagami et al. 2000; Vinet et al. 2003). Given the importance of AMPK in the regulation of food intake and body weight in the hypothalamus, it is plausible that CaMKKs may be a part of the AMPK regulatory pathway in the brain. In addition, further studies are needed to clarify the potential role of CaMKKs in regulating AMPK activity in the periphery.
Identification of novel downstream targets for AMPK
TORC2
One of the most exciting findings in the identification of downstream target(s) for AMPK is the discovery that the transducer of regulated CREB (cyclic AMP response element binding protein) activity 2 (TORC2) signalling is regulated by AMPK in liver. Hepatic gluconeogenesis plays a key role in the maintenance of glucose homeostasis. AMPK suppresses hepatic gluconeogenesis by blocking the expression of gluconeogenic genes (Yamauchi et al. 2002) but the mechanism for this has not been known. The CREB coactivator TORC2 is essential in the regulation of hepatic gluconeogenesis by mediating CREB-dependent transactivation of PGC1α and its gluconeogenic targets, phosphoenolpyruvate carboxykinase (PEPCK) and glucose-6-phosphatase (G6Pase) (Koo et al. 2005). The activity and localization of TORC2 is regulated by phosphorylation events. Phosphorylation of TORC2 at Ser171 results in nuclear exclusion and sequestration in the cytoplasm (Koo et al. 2005). Recently, activation of AMPK by AICAR in primary rat hepatocytes was shown to phosphorylate TORC2, prevent its nuclear entry and block the expression of PEPCK and G6Pase (Koo et al. 2005). In addition, in liver of LKB1-deficient mice, in which AMPK activity is low (see above), expression of gluconeogenic genes is elevated causing hyperglycaemia. TORC2 remains unphosphorylated and is predominantly localized in the nucleus (active state) (Shaw et al. 2005). Knockdown of TORC2 with a small hairpin RNA results in decreased TORC2 and PGC1α expression and reduction of hyperglycaemia in these mice. These data demonstrate that TORC2 is an important downstream mediator of AMPK's effect of down-regulating gluconeogenic gene expression and thereby suppressing hepatic gluconeogenesis.
mTOR
The mammalian Target of Rapamycin (mTOR) is one of the downstream targets of AMPK (Bolster et al. 2002; Kimura et al. 2003; Cheng et al. 2004; Reiter et al. 2005). mTOR is a highly conserved serine/threonine kinase, which integrates nutrient and hormonal signals to control growth and development. mTOR stimulates protein synthesis and inhibits autophagy in the presence of mitogens and available nutrients, including amino acids. mTOR phosphorylates and regulates several of its downstream effectors involved in the control of protein translation, including p70 ribosomal S6 kinase (p70S6K), eukaryotic initiation factor 4E-binding protein 1 (4E-BP1) and eukaryotic initiation factor 4G (eIF4G) (Tokunaga et al. 2004; Deldicque et al. 2005). Upon depletion of ATP, activation of AMPK results in inhibition of mTOR signalling, thereby suppressing protein synthesis, which is an important pathway by which AMPK conserves cellular energy during low energy states (Tokunaga et al. 2004; Deldicque et al. 2005).
Recent data showed that the mTOR signalling pathway plays an important role in the regulation of food intake and body weight in the hypothalamus (Cota et al. 2006). mTOR is co-localized with AgRP/NPY and proopiomelanocortin neurons in arcuate hypothalamus. Leptin and refeeding activate mTOR signalling, whereas fasting down-regulates it. In addition, the amino acid l-leucine a potent stimulator of mTOR activity, inhibits food intake and reduces body weight, whereas the mTOR inhibitor, rapamycin, blocks the effect of both leucine and leptin on food intake and body weight (Cota et al. 2006).
These effects of the mTOR signalling pathway in the hypothalamus are consistent with the possibility that it may be a mediator of the hypothalamic effects of AMPK, since inhibition of AMPK would result in stimulation of mTOR signalling. It will be interesting to determine whether mTOR signalling mediates AMPK's effects on food intake and body weight in the hypothalamus.
Summary and future directions
Major advances have occurred in understanding the regulation and physiological functions of the evolutionarily conserved cellular energy gauge, AMPK. An increasing number of hormones and nutrients are being shown to regulate AMPK signalling and novel molecules and pathways are being identified as targets of AMPK. Hormones, peptides and nutrients may affect AMPK activity in both the hypothalamus and peripheral tissues. Furthermore, hypothalamic AMPK may regulate metabolic pathways in the periphery through neuronal circuits. Key questions include: What are the upstream events and molecules by which hormones and nutrients alter AMPK activity? How is the activity and/or localization of the recently identified upstream kinases in the AMPK cascade, LKB1 and CaMKKs, regulated in both the hypothalamus and peripheral tissues? Do changes in these upstream kinases contribute to the hormonal and metabolic regulation of AMPK? In addition, it will be important to further delineate the downstream targets involved in the effects of AMPK on regulation of whole body energy homeostasis.
Acknowledgments
This work was supported by NIH grants P01 DK56116 and R01 DK60839. The authors thank Y. Minokoshi and other current and former members of the Kahn laboratory for their invaluable contributions to the work described.
References
- Anderson KA, Means RL, Huang QH, Kemp BE, Goldstein EG, Selbert MA, Edelman AM, Fremeau RT, Means AR. Components of a calmodulin-dependent protein kinase cascade. J Biol Chem. 1998;273:31880–31889. doi: 10.1074/jbc.273.48.31880. [DOI] [PubMed] [Google Scholar]
- Andersson U, Filipsson K, Abbott CR, Woods A, Smith K, Bloom SR, Carling D, Small CJ. AMP-activated protein kinase plays a role in the control of food intake. J Biol Chem. 2004;279:12005–12008. doi: 10.1074/jbc.C300557200. [DOI] [PubMed] [Google Scholar]
- Banerjee RR, Rangwala SM, Shapiro JS, Rich AS, Rhoades B, Qi Y, et al. Regulation of fasted blood glucose by resistin. Science. 2004;303:1195–1198. doi: 10.1126/science.1092341. [DOI] [PubMed] [Google Scholar]
- Bolster DR, Crozier SJ, Kimball SR, Jefferson LS. AMPK-activated protein kinase suppresses protein synthesis in rat skeletal muscle through down-regulated mammalian target of rapamycin (mTOR) signaling. J Biol Chem. 2002;277:23977–23980. doi: 10.1074/jbc.C200171200. [DOI] [PubMed] [Google Scholar]
- Cha SH, Hu Z, Chohnan S, Lane MD. Inhibition of hypothalamic fatty acid synthase triggers rapid activation of fatty acid oxidation in skeletal muscle. Proc Natl Acad Sci U S A. 2005;102:14557–14562. doi: 10.1073/pnas.0507300102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G, Koyama K, Yuan X, Lee Y, Zhou YT, O'Doherty R, Newgard CB, Unger RH. Disappearance of body fat in normal rats induced by adenovirus-mediated leptin gene therapy. Proc Natl Acad Sci U S A. 1996;93:14795–14799. doi: 10.1073/pnas.93.25.14795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng SWY, Fryer LGD, Carling D, Shepherd PR. Thr2446 is a novel mammalian target of rapamycin (mTOR) phosphorylation site regulated by nutrient status. J Biol Chem. 2004;279:15719–15722. doi: 10.1074/jbc.C300534200. [DOI] [PubMed] [Google Scholar]
- Cota D, Marsicano G, Tschop M, Grubler Y, Flachskamm C, Schubert M, et al. The endogenous cannabinoid system affects energy balance via central orexigenic drive and peripheral lipogenesis. J Clin Invest. 2003;112:423–431. doi: 10.1172/JCI17725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cota D, Proulx K, Smith KA, Kozma SC, Thomas G, Woods SC, Seeley RJ. Hypothalamic mTOR signaling regulates food intake. Science. 2006;312:927–930. doi: 10.1126/science.1124147. [DOI] [PubMed] [Google Scholar]
- Cummings DE, Purnell JQ, Frayo RS, Schmidova K, Wisse BE, Weigle DS. A preprandial rise in plasma ghrelin levels suggests a role in meal initiation in humans. Diabetes. 2001;50:1714–1717. doi: 10.2337/diabetes.50.8.1714. [DOI] [PubMed] [Google Scholar]
- Cummings DE, Weigle DS, Frayo RS, Breen PA, Ma MA, Dellinger EP, Purnell JQ. Plasma ghrelin levels after diet-induced weight loss or gastric bypass surgery. N Engl J Med. 2002;346:1623–1630. doi: 10.1056/NEJMoa012908. [DOI] [PubMed] [Google Scholar]
- Deldicque L, Theisen D, Francaux M. Regulation of mTOR by amino acids and resistance exercise in skeletal muscle. Eur J Appl Physiol. 2005;94:1–10. doi: 10.1007/s00421-004-1255-6. [DOI] [PubMed] [Google Scholar]
- El-Haschimi K, Pierroz DD, Hileman SM, Bjorbaek C, Flier JS. Two defects contribute to hypothalamic leptin resistance in mice with diet-induced obesity. J Clin Invest. 2000;105:1827–1832. doi: 10.1172/JCI9842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han S-M, Namkoong C, Jang PG, Park IS, Hong SW, Katakami H, et al. Hypothalamic AMP-activated protein kinase mediates counter-regulatory responses to hypoglycaemia in rats. Diabetologia. 2005;48:2170–2178. doi: 10.1007/s00125-005-1913-1. [DOI] [PubMed] [Google Scholar]
- Hawley SA, Boudeau J, Reid JL, Mustard KJ, Udd L, Makela TP, Alessi DR, Hardie DG. Complexes between the LKB1 tumor suppressor, STRADα/β and MO25α/β are upstream kinases in the AMP-activated protein kinase cascade. J Biol. 2003;2:28. doi: 10.1186/1475-4924-2-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawley SA, Davison M, Woods A, Davies SP, Beri RK, Carling D, Hardie DG. Characterization of the AMP-activated protein kinase kinase from rat liver and identification of threonine 172 as the major site at which it phosphorylates AMP-activated protein kinase. J Biol Chem. 1996;271:27879–27887. doi: 10.1074/jbc.271.44.27879. [DOI] [PubMed] [Google Scholar]
- Hawley SA, Pan DA, Mustard KJ, Ross L, Bain J, Edelman AM, Frenguelli GB, Hardie DG. Calmodulin-dependent protein kinase kinase β is an alternative upstream kinase for AMP-activated protein kinase. Cell Metab. 2005;2:9–19. doi: 10.1016/j.cmet.2005.05.009. [DOI] [PubMed] [Google Scholar]
- He W, Lam TK, Obici S, Rossetti L. Molecular disruption of hypothalamic nutrient sensing induces obesity. Nat Neurosci. 2006;9:227–233. doi: 10.1038/nn1626. [DOI] [PubMed] [Google Scholar]
- Hong SP, Leiper FC, Woods A, Carling D, Carlson M. Activation of yeast Snf1 and mammalian AMP-activated protein kinase by upstream kinases. Proc Natl Acad Sci U S A. 2003;100:8839–8843. doi: 10.1073/pnas.1533136100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong SP, Momcilovic M, Carlson M. Function of mammalian LKB1 and Ca2+/calmodulin-dependent protein kinase kinase α as Snf1-activating kinases in yeast. J Biol Chem. 2005;280:21804–21809. doi: 10.1074/jbc.M501887200. [DOI] [PubMed] [Google Scholar]
- Hurley RL, Anderson KA, Franzone JM, Kemp BE, Means AR, Witters LA. The Ca2+/calmodulin-dependent protein kinase kinases are AMP-activated protein kinase kinases. J Biol Chem. 2005;280:29060–29066. doi: 10.1074/jbc.M503824200. [DOI] [PubMed] [Google Scholar]
- Hutchinson DS, Chernogubova E, Dallner OS, Cannon B, Bengtsson T. β-Adrenoceptors, but not α-adrenoceptors, stimulate AMP-activated protein kinase in brown adipocytes independently of uncoupling protein-1. Diabetologia. 2005;48:2386–2395. doi: 10.1007/s00125-005-1936-7. [DOI] [PubMed] [Google Scholar]
- Inokuma K, Ogura-Okamatsu Y, Toda C, Kimura K, Yamashita H, Saito M. Uncoupling protein 1 is necessary for norepinephrine-induced glucose utilization in brown adipose tissue. Diabetes. 2005;54:1385–1391. doi: 10.2337/diabetes.54.5.1385. [DOI] [PubMed] [Google Scholar]
- Jamshidi N, Taylor DA. Anandamide administration into the ventromedial hypothalamus stimulates appetite in rats. Br J Pharmacol. 2001;134:1151–1154. doi: 10.1038/sj.bjp.0704379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenne DE, Reimann H, Nezu J, Friedel W, Loff S, Jeschke R, Muller O, Back W, Zimmer M. Peutz-Jeghers syndrome is caused by mutations in a novel serine threonine kinase. Nat Genet. 1998;18:38–43. doi: 10.1038/ng0198-38. [DOI] [PubMed] [Google Scholar]
- Joyeux M, Arnaud C, Godin-Ribuot D, Demenge P, Lamontagne D, Ribuot C. Endocannabinoids are implicated in the infarct size-reducing effect conferred by heat stress preconditioning in isolated rat hearts. Cardiovasc Res. 2002;55:619–625. doi: 10.1016/s0008-6363(02)00268-7. [DOI] [PubMed] [Google Scholar]
- Kahn BB, Alquier T, Carling D, Hardie DG. AMPK-activated protein kinase: ancient energy gauge provides clues to modern understanding of metabolism. Cell Metab. 2005;1:15–25. doi: 10.1016/j.cmet.2004.12.003. [DOI] [PubMed] [Google Scholar]
- Kim EK, Miller I, Aja S, Landree LE, Pinn M, McFadden J, Kuhajda FP, Moran TH, Ronnett GV. C75, a fatty acid synthase inhibitor, reduces food intake via hypothalamic AMP-activated protein kinase. J Biol Chem. 2004;279:19970–19976. doi: 10.1074/jbc.M402165200. [DOI] [PubMed] [Google Scholar]
- Kim MS, Park JY, Namkoong C, Jang PG, Ryu JW, Song HS, et al. Anti-obesity effects of α-lipoic acid mediated by suppression of hypothalamic AMP-activated protein kinase. Nat Med. 2004;10:727–733. doi: 10.1038/nm1061. [DOI] [PubMed] [Google Scholar]
- Kimball SR, Siegfried BA, Jefferson LS. Glucagon represses signaling through the mammalian target of rapamycin in rat liver by activating AMP-activated protein kinase. J Biol Chem. 2004;279:54103–54109. doi: 10.1074/jbc.M410755200. [DOI] [PubMed] [Google Scholar]
- Kimura N, Tokunaga C, Dalal S, Richardson C, Yoshino K-I, Hara K, et al. A possible linkage between AMPK-activated protein kinase (AMPK) and mammalian target of rapamycin (mTOR) signaling pathway. Genes Cells. 2003;9:65–79. doi: 10.1046/j.1365-2443.2003.00615.x. [DOI] [PubMed] [Google Scholar]
- Kishi K, Yuasa T, Minami A, Yamada M, Hagi A, Hayashi H, Kemp BE, Witters LA, Ebina Y. AMPK-activated protein kinase is activated by the stimulations of Gq-coupled receptors. Biochem Biophys Res Commun. 2000;276:16–22. doi: 10.1006/bbrc.2000.3417. [DOI] [PubMed] [Google Scholar]
- Kokoeva MW, Yin H, Flier JS. Neurogenesis in the hypothalamus of adult mice: potential role in energy balance. Science. 2005;310:679–683. doi: 10.1126/science.1115360. [DOI] [PubMed] [Google Scholar]
- Kola B, Hubina E, Tucci SA, Kirkham TC, Garcia EA, Mitchell SE, et al. Cannabinoids and ghrelin have both central and peripheral metabolic and cardiac effects via AMP-activated protein kinase. J Biol Chem. 2005;280:25196–25201. doi: 10.1074/jbc.C500175200. [DOI] [PubMed] [Google Scholar]
- Koo SH, Flechner L, Qi L, Zhang X, Screaton RA, Jeffries S, et al. The CREB coactivator TORC2 is a key regulator of fasting glucose metabolism. Nature. 2005;437:1109–1114. doi: 10.1038/nature03967. [DOI] [PubMed] [Google Scholar]
- Lee Y, Naseem RH, Duplomb L, Park BH, Garry DJ, Richardson JA, Schaffer JE, Unger RH. Hyperleptinemia prevents lipotoxic cardiomyopathy in acyl CoA synthase transgenic mice. Proc Natl Acad Sci U S A. 2004;101:13624–13629. doi: 10.1073/pnas.0405499101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Wan Q, Guan Q, Gao L, Zhao J. High-fat diet feeding impairs both the expression and activity of AMPKα in rats' skeletal muscle. Biochem Biophys Res Commun. 2006;339:701–707. doi: 10.1016/j.bbrc.2005.11.068. [DOI] [PubMed] [Google Scholar]
- Lizcano JM, Goransson O, Toth R, Deak M, Morrice NA, Boudeau J, et al. LKB1 is a master kinase that activates 13 protein kinases of the AMPK subfamily, including the MARK/PAR-1 kinases. EMBO J. 2004;23:833–843. doi: 10.1038/sj.emboj.7600110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrimmon RJ, Fan X, Ding Y, Zhu W, Jacob RJ, Sherwin RS. Potential role for AMP-activated protein kinase in hypoglycemia sensing in the ventromedial hypothalamus. Diabetes. 2004;53:1953–1958. doi: 10.2337/diabetes.53.8.1953. [DOI] [PubMed] [Google Scholar]
- Martin TL, Alquier T, Asakura K, Furukawa N, Preitner F, Kahn BB. Diet-induced obesity alters AMP-kinase activity in hypothalamus and skeletal muscle. J Biol Chem. 2006;281 doi: 10.1074/jbc.M512831200. DOI: 10.1074/jbc.M512831200. [DOI] [PubMed] [Google Scholar]
- Matejkova O, Mustard KJ, Sponarova J, Flachs P, Rossmeisl M, Miksik I, Thomason-Hughes M, Hardie DG, Kopecky J. Possible involvement of AMP-activated protein kinase in obesity resistance induced by respiratory uncoupling in white fat. FEBS Lett. 2004;569:245–248. doi: 10.1016/j.febslet.2004.06.002. [DOI] [PubMed] [Google Scholar]
- Minokoshi Y, Alquier T, Furukawa N, Kim Y-B, Lee A, Xue B, et al. AMP-kinase regulates food intake by responding to hormonal and nutrient signals in the hypothalamus. Nature. 2004;428:569–574. doi: 10.1038/nature02440. [DOI] [PubMed] [Google Scholar]
- Minokoshi Y, Kim YB, Peroni OD, Fryer LGD, Muller C, Carling D, Kahn BB. Leptin stimulates fatty-acid oxidation by activating AMP-activated protein kinase. Nature. 2002;415:339–343. doi: 10.1038/415339a. [DOI] [PubMed] [Google Scholar]
- Moule SK, Denton RM. The activation of p38 MAPK by the β-adrenergic agonist isoproterenol in rat epididymal fat cells. FEBS Lett. 1998;439:287–290. doi: 10.1016/s0014-5793(98)01392-1. [DOI] [PubMed] [Google Scholar]
- Murata M, Okimura Y, Iida K, Matsumoto M, Sowa H, Kaji H, Kojima M, Kangawa K, Chihara K. Ghrelin modulates the downstream molecules of insulin signaling in hepatoma cells. J Biol Chem. 2002;277:5667–5674. doi: 10.1074/jbc.M103898200. [DOI] [PubMed] [Google Scholar]
- Muse ED, Obici S, Bhanot S, Monia BP, McKay RA, Rajala MW, Scherer PE, Rossetti L. Role of resistin in diet-induced hepatic insulin resistance. J Clin Invest. 2004;114:232–239. doi: 10.1172/JCI21270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakazato M, Murakami N, Date Y, Kojima M, Matsuo H, Kangawa K, Matsukura S. A role for ghrelin in the central regulation of feeding. Nature. 2001;409:194–198. doi: 10.1038/35051587. [DOI] [PubMed] [Google Scholar]
- Obici S, Feng Z, Arduini A, Conti R, Rossetti L. Inhibition of hypothalamic carnitine palmitoyltransferase-1 decreases food intake and glucose production. Nat Med. 2003;9:756–761. doi: 10.1038/nm873. [DOI] [PubMed] [Google Scholar]
- Ogawa Y, Masuzaki H, Hosoda K, Aizawa-Abe M, Suga J, Suda M, et al. Increased glucose metabolism and insulin sensitivity in transgenic skinny mice overexpressing leptin. Diabetes. 1999;48:1822–1829. doi: 10.2337/diabetes.48.9.1822. [DOI] [PubMed] [Google Scholar]
- Orci L, Cook WS, Ravazzola M, Wang MY, Park BH, Montesano R, Unger RH. Rapid transformation of white adipocytes into fat-oxidizing machines. Proc Natl Acad Sci U S A. 2004;101:2058–2063. doi: 10.1073/pnas.0308258100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palanivel R, Sweeney G. Regulation of fatty acid uptake and metabolism in L6 skeletal muscle cells by resistin. FEBS Lett. 2005;579:5049–5054. doi: 10.1016/j.febslet.2005.08.011. [DOI] [PubMed] [Google Scholar]
- Puigserver P, Wu Z, Park CW, Graves R, Wright M, Spiegelman BM. A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell. 1998;92:829–839. doi: 10.1016/s0092-8674(00)81410-5. [DOI] [PubMed] [Google Scholar]
- Reiter AK, Bolster DR, Crozier SJ, Kimball SR, Jefferson LS. Repression of protein synthesis and mTOR signaling in rat liver mediated by the AMPK activator aminoimidazole carboxamide ribonucleoside. Am J Physiol Endocrinol Metab. 2005;288:E980–E988. doi: 10.1152/ajpendo.00333.2004. [DOI] [PubMed] [Google Scholar]
- Russell RR, III, Li J, Coven DL, Pypaert M, Zechner C, Palmeri M, Giordano FJ, Mu J, Birnbaum MJ, Young LH. AMP-activated protein kinase mediates ischemic glucose uptake and prevents postischemic cardiac dysfunction, apoptosis, and injury. J Clin Invest. 2004;114:495–503. doi: 10.1172/JCI19297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakagami H, Umemiya M, Saito S, Kondo H. Distinct immunohistochemical localization of two isoforms of Ca2+/calmodulin-dependent protein kinase kinases in the adult rat brain. Eur J Neurosci. 2000;12:89–99. doi: 10.1046/j.1460-9568.2000.00883.x. [DOI] [PubMed] [Google Scholar]
- Sakamoto K, Goransson O, Hardie DG, Alessi DR. Activity of LKB1 and AMPK-related kinases in skeletal muscle: effects of contraction, phenformin, and AICAR. Am J Physiol Endocrinol Metab. 2004;287:E310–R317. doi: 10.1152/ajpendo.00074.2004. [DOI] [PubMed] [Google Scholar]
- Sakamoto K, McCarthy A, Smith D, Green KA, Hardie DG, Ashworth A, Alessi DR. Deficiency of LKB1 in skeletal muscle prevents AMPK activation and glucose uptake during contraction. EMBO J. 2005;24:1810–1820. doi: 10.1038/sj.emboj.7600667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto K, Zarrinpashneh E, Budas GR, Pouleur AC, Dutta A, Prescott AR, et al. Deficiency of LKB1 in heart prevents ischemia-mediated activation of AMPKα2 but not AMPKα1. Am J Physiol Endocrinol Metab. 2006;290:E780–E788. doi: 10.1152/ajpendo.00443.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapkota GP, Kieloch A, Lizcano JM, Lain S, Arthur JSC, Williams MR, Morrice N, Deak M, Alessi DR. Phosphorylation of the protein kinase mutated in Peutz-Jeghers cancer syndrome, LKB1/STK11, at Ser431 by p90RSK and cAMP-dependent protein kinase, but not its farnesylation at Cys433, is essential for LKB1 to suppress cell growth. J Biol Chem. 2001;276:19469–19482. doi: 10.1074/jbc.M009953200. [DOI] [PubMed] [Google Scholar]
- Satoh H, Nguyen MTA, Miles PDG, Imamura T, Usui I, Olefsky JM. Adenovirus-mediated chronic ‘hyper-resistinemia’ leads to in vivo insulin resistance in normal rats. J Clin Invest. 2004;114:224–231. doi: 10.1172/JCI20785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw RJ, Kosmatka M, Bardeesy N, Hurley RL, Witters LA, DePinho RA, Cantley LC. The tumor suppressor LKB1 kinase directly activates AMP-activated kinase and regulates apoptosis in response to energy stress. Proc Natl Acad Sci U S A. 2004;101:3329–3335. doi: 10.1073/pnas.0308061100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw RJ, Lamia KA, Vasquez D, Koo SH, Bardeesy N, DePinho RA, Montminy M, Cantley LC. The kinase LKB1 mediates glucose homeostasis in liver and therapeutic effects of metformin. Science. 2005;310:1642–1646. doi: 10.1126/science.1120781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimabukuro M, Koyama K, Chen G, Wang MY, Trieu F, Lee Y, Newgard CB, Unger RH. Direct antidiabetic effect of leptin through triglyceride depletion of tissues. Proc Natl Acad Sci U S A. 1997;94:4637–4641. doi: 10.1073/pnas.94.9.4637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Z, Levin B, McArdle J, Bakhos N, Routh V. Convergence of pre- and post-synaptic influences on glucosensing neurons in the ventromedial hypothalamic nucleus. Diabetes. 2001;50:2673–2681. doi: 10.2337/diabetes.50.12.2673. [DOI] [PubMed] [Google Scholar]
- Sutherland CM, Hawley SA, McCartney RR, Leech A, Stark MJ, Schmidt MC, Hardie DG. Elm1p is one of three upstream kinases for the Saccharomyces serevisiae SNF1 complex. Curr Biol. 2003;13:1299–1305. doi: 10.1016/s0960-9822(03)00459-7. [DOI] [PubMed] [Google Scholar]
- Tanaka T, Hidaka S, Masuzaki H, Yasue S, Minokoshi Y, Ebihara K, et al. Skeletal muscle AMP-activated protein kinase phosphorylation parallels metabolic phenotype in leptin transgenic mice under dietary modification. Diabetes. 2005;54:2365–2374. doi: 10.2337/diabetes.54.8.2365. [DOI] [PubMed] [Google Scholar]
- Taylor EB, Hurst D, Greenwood LJ, Lamb JD, Cline TD, Sudweeks SN, Winder WW. Endurance training increases LKB1 and MO25 protein but not AMP-activated protein kinase kinase activity in skeletal muscle. Am J Physiol Endocrinol Metab. 2004;287:E1082–E1089. doi: 10.1152/ajpendo.00179.2004. [DOI] [PubMed] [Google Scholar]
- Taylor EB, Lamb JD, Hurst RW, Chesser DG, Ellingson WJ, Greenwood LJ, Porter BB, Herway ST, Winder WW. Endurance training increases skeletal muscle LKB1 and PGC-1α protein abundance: effects of time and intensity. Am J Physiol Endocrinol Metab. 2005;289:E960–E968. doi: 10.1152/ajpendo.00237.2005. [DOI] [PubMed] [Google Scholar]
- Tokunaga C, Yoshino YI, Yonezawa K. mTOR integrates amino acid- and energy-sensing pathways. Biochem Biophys Res Commun. 2004;313:443–448. doi: 10.1016/j.bbrc.2003.07.019. [DOI] [PubMed] [Google Scholar]
- Tomas E, Tsao TS, Saha AK, Murrey HE, Zhang Cc C, Itani SI, Lodish HF, Ruderman NB. Enhanced muscle fat oxidation and glucose transport by ACRP30 globular domain: acetyl-CoA carboxylase inhibition and AMP-activated protein kinase activation. Proc Natl Acad Sci U S A. 2002;99:16309–16313. doi: 10.1073/pnas.222657499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tschop M, Smiley DL, Heiman ML. Ghrelin induces adiposity in rodents. Nature. 2000;407:908–913. doi: 10.1038/35038090. [DOI] [PubMed] [Google Scholar]
- Tsubone T, Masaki T, Katsuragi I, Tanaka K, Kakuma T, Yoshimatsu H. Ghrelin regulates adiposity in white adipose tissue and UCP1 mRNA expression in brown adipose tissue in mice. Regul Pept. 2005;130:97–103. doi: 10.1016/j.regpep.2005.04.004. [DOI] [PubMed] [Google Scholar]
- Unger RH. Lipotoxicity disease. Annu Rev Med. 2002;53:319–336. doi: 10.1146/annurev.med.53.082901.104057. [DOI] [PubMed] [Google Scholar]
- Vinet J, Carra S, Blom JMC, Harey M, Brunello N, Barden N, Tascedda F. Cloning of mouse Ca2+/calmodulin-dependent protein kinase kinase β (CaMKKβ) and characterization of CaMKKβ and CaMKKα distribution in the adult mouse brain. Mol Brain Res. 2003;111:216–221. doi: 10.1016/s0169-328x(02)00698-8. [DOI] [PubMed] [Google Scholar]
- Watt MJ, Dzamko N, Thomas WG, Rose-John S, Ernst M, Carling D, Kemp BE, Febbraio MA, Steinberg GR. CNTF reverses obesity-induced insulin resistance by activating skeletal muscle AMPK. Nat Med. 2006;12:541–548. doi: 10.1038/nm1383. [DOI] [PubMed] [Google Scholar]
- Woods A, Dickerson K, Heath R, Hong SP, Momcilovic M, Hohnstone SR, Carlson M, Carling D. Ca2+/calmodulin-dependent protein kinase kinase-β acts upstream of AMP-activated protein kinase in mammalian cells. Cell Metab. 2005;2:21–33. doi: 10.1016/j.cmet.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Woods A, Johnstone SR, Dickerson K, Leiper FC, Fryer LGD, Neumann D, et al. LKB1 is the upstream kinase in the AMP-activated protein kinase cascade. Curr Biol. 2003;13:2004–2008. doi: 10.1016/j.cub.2003.10.031. [DOI] [PubMed] [Google Scholar]
- Wu Z, Puigserver P, Andersson U, Zhang C, Adelmant G, Mootha V, et al. Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell. 1999;98:115–124. doi: 10.1016/S0092-8674(00)80611-X. [DOI] [PubMed] [Google Scholar]
- Yamauchi T, Kamon J, Minokoshi Y, Ito Y, Waki H, Uchida S, et al. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat Med. 2002;8:1288–1295. doi: 10.1038/nm788. [DOI] [PubMed] [Google Scholar]
- Yu X, McCorkle S, Wang M, Lee Y, Li J, Saha AK, Unger RH, Ruderman NB. Leptinomimetic effects of the AMP kinase activator AICAR in leptin-resistant rats: prevention of diabetes and ectopic lipid deposition. Diabetologia. 2004;47:2012–2021. doi: 10.1007/s00125-004-1570-9. [DOI] [PubMed] [Google Scholar]
- Zigman JM, Jones JE, Lee CE, Saper CB, Elmquist JK. Expression of ghrelin receptor mRNA in the rat and the mouse brain. J Comp Neurol. 2006;494:528–548. doi: 10.1002/cne.20823. [DOI] [PMC free article] [PubMed] [Google Scholar]