Abstract
In this study, we examined the contribution of the actin cytoskeleton to T-cell receptor (TCR)-initiated signalling in cytotoxic T lymphocytes (CTLs). We demonstrate that cytoskeletal remodelling is required for sustaining TCR-stimulated signals that lead to degranulation by CTLs. Disruption of the actin cytoskeleton in CTLs already undergoing signalling responses results in an almost immediate loss of essentially all protein tyrosine phosphorylation. This signal reversal is not restricted to tyrosine phosphorylation, as disruption of the actin cytoskeleton also reverses the phosphorylation of the more downstream serine/threonine kinase extracellular signal regulated kinase (Erk). An intact cytoskeleton and cell spreading are not sufficient for maintaining signals, as stabilization of actin filaments, at a point when peak tyrosine phosphorylation is occurring, also leads to the rapid loss of protein tyrosine phosphorylation. Disruption of tyrosine kinase activity after TCR signals are maximally induced causes the immediate reversal of tyrosine phosphorylation as well as cytoskeletal disruption, as indicated by loss of cell spreading, adhesion and CTL degranulation. Taken together, our results indicate that actin remodelling occurs co-temporally with ongoing tyrosine kinase activity, leading to CTL degranulation. We hypothesize that continuous actin remodelling is important for sustaining productive signals, even after downstream signalling molecules such as Erk have been activated, and that the actin cytoskeleton is not solely required for initiating and maintaining the T cell in contact with its stimulus.
Keywords: cellular activation, protein kinases/phosphatases, signal transduction
Introduction
A number of cytoskeletal rearrangements are associated with T-cell activation, including those required for conjugation, movement of receptors, formation of the immunological synapse and directional secretion of cytolytic granules and cytokines (reviewed in references 1 and 2). It has been established that the microtubule organizing centre of a T cell reorients towards the antigen-presenting cell (APC) or target cell and talin, a cytoskeleton protein, accumulates at the interface between a cytotoxic T lymphocyte (CTL) and its specific target cell.3 Plate-bound anti-CD3 has been shown to induce actin polymerization in Jurkat cells,4 and this actin polymerization is dynamically regulated by the tyrosine-phosphorylated adaptor protein linker for activation of T cells (LAT), downstream of the T-cell receptor (TCR).5 A number of elegant studies over the past decade have revealed many of the molecular mechanisms that govern actin dynamics in T cells (reviewed in references 1, 2, 6 and 7).
Cytoskeletal rearrangements are not only associated with T-cell activation but also essential for various aspects of this process. It was shown a number of years ago that disruption of the cytoskeleton by cytochalasins inhibits the formation of conjugates between CTLs and cognate target cells.8 Cytochalasins can block CTL activation stimulated with purified matrix-bound allogeneic class I major histocompatibility complex (MHC).9 There exists a clear requirement for the actin cytoskeleton in the sustained signals in CD4+ T cells, leading to increased intracellular Ca2+ and the production of interferon-γ.10 Cytochalasin D inhibits cell spreading stimulated with plate-bound anti-CD3.5 Interestingly, we established that, although cytochalasin D inhibits the induction of tyrosine phosphorylation signals triggered in CTLs with plate-bound anti-CD3, this drug has absolutely no inhibitory effect on tyrosine phosphorylation when those same CTLs are stimulated with soluble cross-linked antibodies, suggesting that the cytoskeleton is not required for TCR-generated signals per se, but only for those mounted with antibodies presented on a solid matrix.11 More recently, studies with knockout mice deficient in various signalling molecules, such as the tyrosine kinase Itk, the guanine nucleotide exchange factor Vav and the cytoskeleton regulator WASP, have shown defects in various aspects of T-cell function, including delayed kinetics of activation, decreased adhesion with APCs and decreased actin polymerization at the cell contact point.1,2,6,7 For example, it has been demonstrated that Vav is required for actin-dependent clustering of the TCR when antigen is presented on APCs.12 From these and numerous additional studies, it is clear that cytoskeletal rearrangements are important for T-cell activation.
Although the cytoskeleton has a defined role in the initial formation of stable conjugates and adhesion of cells to plate-bound anti-CD3 to allow optimal TCR ligation, it is not clear if the cytoskeleton is important for mediating or maintaining signal transduction events downstream of TCR ligation. There is some evidence to suggest that the cytoskeleton might be important for signalling downstream of the initiation of signals. Addition of cytochalasins after CTLs have adhered to purified class I MHC disrupts degranulation up to approximately 30 min after the initiation of the response.9 Furthermore, the addition of cytochalasins during an ongoing Ca2+ flux immediately disrupts the Ca2+ flux without affecting the stability of T-cell–APC conjugates.10 The role of the cytoskeleton in maintaining signalling responses is not known. It is possible that the cytoskeleton is important for bringing together various signalling molecules to the correct subcellular location for proper function and perhaps for providing a scaffold for these proteins to associate long enough to initiate downstream pathways. It is also not known if the cytoskeleton plays any role in signalling responses once the initial cytoskeletal rearrangements have occurred and signalling pathways have been initiated.
In the current study, we examined the contribution of the cytoskeleton to tyrosine phosphorylation and Erk activity triggered by the TCR complex in CTLs. Our data indicate that the requirement for the cytoskeleton in signals emanating from the TCR is continuous; even after tyrosine phosphorylation and Erk signals have peaked, there remains a requirement for actin remodelling. We demonstrate that the presence of actin filaments is not sufficient to initiate or sustain signals, but that continuous actin remodelling is important, arguing against the possibility that the actin cytoskeleton is serving solely as a scaffold for signalling proteins. Finally, our results indicate that signalling and cytoskeletal rearrangements must occur co-temporally and do not occur in a purely linear, sequential fashion.
Materials and methods
Cell lines, antibodies and reagents
The allo-specific mouse CTL clones Cl 11 and AB.1 (murine H-2d anti-Kb) have been described previously.13 These were grown in HEPES-buffered RPMI supplemented with heat-inactivated fetal bovine serum, sodium pyruvate, non-essential amino acids, l-glutamine, penicillin-streptomycin and β-mercaptoethanol. Cells were stimulated weekly with irradiated C57BL/6 J spleen cells in media supplemented with interleukin (IL)-2 and experiments were performed 4–6 days after stimulation. The growth of hybridomas producing monoclonal antibodies (mAbs) specific for CD3 (145-2C11), TCR (H57-597), CD45 (I3/2) and antiphosphotyrosine (PY-72) and the purification of the antibodies has been described previously.14 Anti-Erk (Erk1 + Erk2) mAb was purchased from Zymed (San Francisco, CA). Horseradish peroxidase (HRP)-coupled goat anti-mouse antibody and goat anti-hamster immunoglobulin G (IgG) were purchased from Jackson Immunologicals (West Grove, PA). Phorbol myristate acetate (PMA) and cytochalasin D and E were purchased from Sigma Chemicals (St. Louis, MO). Ionomycin, latrunculin A, jasplakinolide and PP1 were purchased from Calbiochem (San Diego, CA).
Antibody immobilization
Ninety-six-well flat-bottom plastic microtitre plates were incubated with 800 ng/well antibody (145-2C11 or H57-597) diluted in phosphate-buffered saline (PBS) at 37° for 90 min. Each well was washed twice with PBS (100 µl/well), blocked with 2% bovine serum albumin (BSA) in PBS (100 µl/well) at 37° for 30 min, washed twice, and used immediately for assay.
Degranulation assay
Degranulation, as measured by the release of serine esterases, was assayed as previously described.13 AB.1 or Clone 11 cells were washed three times by centrifugation in PBS. For immobilized antibody experiments, cells were added directly to the antibody-coated wells. For experiments with target cells, the plates were centrifuged for 4 min at 400 g to initiate conjugation. All degranulation assays were performed in 4% fetal calf serum (FCS) in RPMI. When used at the initiation of the assay, cytochalasin D or E was added at the indicated concentration to the clone cells, and then the cells were immediately added to the antibody-coated wells. Addition of cytochalasin D or E after the initiation of stimulation was accomplished by carefully adding a volume of 50 µl containing 30 µm cytochalasin D or E to 100 µl of cells. As a control, the same volume of vehicle was added to the cells. Cells were used at 1·5 × 105 cells/well in 150 µl and incubated at 37° for the indicated time, after which 25 µl of supernatant was assayed for N-benzyloxy-carbonyl-l-lysine thiobenzyl ester (BLT)-esterase activity.13 None of the inhibitors affected the viability of the cells during the period of assay and the effects of the cytochalasins were completely reversible. Each experiment was performed with triplicate samples and all experiments were repeated at least three times with identical results.
Sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS-PAGE) and antiphosphotyrosine immunoblotting
Protein tyrosine phosphorylation was assayed as previously described.15 Stimulation was performed as for the degranulation assay, except that 1·5 × 105 clone cells were triggered in 50 µl of serum-free media. After incubation at 37° for the indicated time, the cells were lysed by addition of 40 µl 2× Laemmli reducing sample buffer and the entire sample was subjected to electrophoresis on 7·5% SDS-PAGE gels. Anti-phosphotyrosine immunoblotting was performed with the phosphotyrosine-specific mAb PY-72, followed by rabbit anti-mouse antibody coupled to horseradish peroxidase. Erk phosphorylation was assessed as described previously.16 Blots were developed by chemiluminescence.
Results
Cytoskeletal changes are required for the stimulation of CTL degranulation, but not for the exocytosis of lytic granules
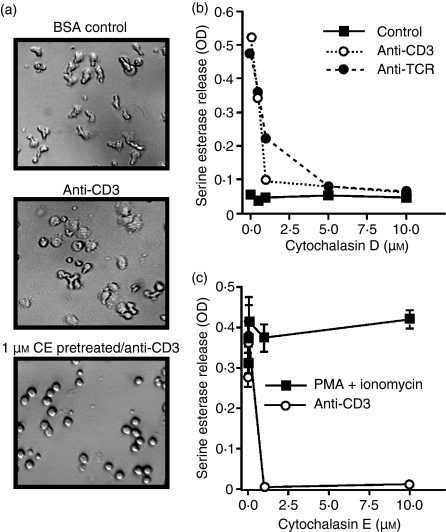
To determine if an intact cytoskeleton is required for TCR-triggered CTL degranulation, we stimulated Cl 11 cells with immobilized anti-CD3 or anti-TCR in the presence of cytochalasin D or cytochalasin E, which inhibits the addition of new actin monomers to actin filaments. We used concentrations of cytochalasins that have been previously shown to inhibit CTL conjugation8 and cell spreading on solid-phase anti-CD3.5 When cells were treated with either cytochalasin D or E, they appeared round and did not adhere to the plate or spread on the immobilized antibody (data not shown and Fig. 1a). Degranulation triggered with either anti-CD3 or anti-TCR was completely inhibited in the presence of cytochalasin D (Fig. 1b). Cytochalasin D had no impact on the minimal spontaneous degranulation from non-stimulated cells (Fig. 1b). Degranulation could be inhibited because actin polymerization is required for optimal TCR ligation and generation of signals or for the process of exocytosis of lytic granules, or both. To distinguish these possibilities, we triggered the CTL clone AB.1 to degranulate with the phorbol ester phorbol 12-myristate 13-acetate (PMA) and the calcium ionophore ionomycin in the presence of cytochalasin E. PMA- and ionomycin-induced degranulation was not inhibited by cytochalasin E (Fig. 1c), consistent with previously published results using TALL-104 leukaemia cells and latrunculin A, an actin polymerization inhibitor.17 These data indicate that the physical process of degranulation does not require an intact cytoskeleton, and suggest that there is a requirement for cytoskeletal function upstream of the pathways stimulated by PMA and ionomycin leading to degranulation.
Figure 1.
Cytochalasins inhibit signalling for degranulation, but not the exocytosis of lytic granules. (a) Cl 11 clone cells were left untreated and added to either control bovine serum albumin (BSA)-blocked wells (upper panel) or wells pre-coated with anti-CD3 (145-2C11) (middle panel). One group of cells was pretreated for 15 min with 1 µm cytochalasin E (CE) and then added to plate-bound anti-CD3 (lower panel). All photographs were taken 25 min after addition of cells to the wells. (b) Cl 11 cells were stimulated with immobilized anti-CD3 (2C11) or anti-T-cell receptor (TCR) (H57-597) in the presence of the indicated concentration of cytochalasin D. Serine esterase activity was measured in the supernatant at about 4 hr. (c) AB.1 clone cells were stimulated with plate-bound 145-2C11 (anti-CD3) or with 150 ng/ml phorbol 12-myristate 13-acetate (PMA) and 2 µm ionomycin in the presence of the indicated concentration of cytochalasin E for 4 hr before supernatants were assayed for serine esterase activity. The results are representative of results with both cytochalasin D and E on both clones AB.1 and Cl 11. The experiments were performed in triplicate and error bars indicate standard deviations. Note that for most samples the error bars are smaller than the symbols. OD, optical density.
Cell spreading is not sufficient for ongoing signalling and degranulation
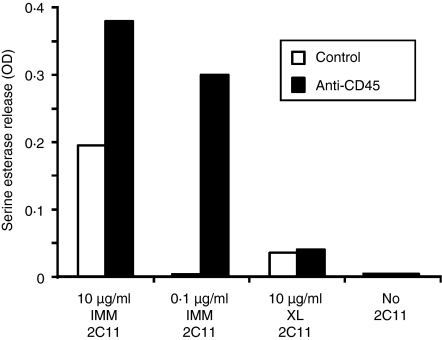
We have demonstrated that degranulation only occurs when CTLs are stimulated with plate- or bead-bound anti-CD3 or TCR, and not with cross-linked antibodies to CD3 or TCR.11 The obvious differences between these two stimulation procedures are the polarized signal and the tight adhesion and cell spreading that occur with solid-phase stimulation. If cell spreading and cytoskeletal rearrangements were indeed the only difference between the two stimulation conditions, one would predict that, if cell spreading could be induced in conjunction with cross-linked anti-CD3, degranulation should ensue. We have previously shown that immobilized anti-CD45 induces dramatic cell spreading of CTL clones.18 Anti-CD45 did not induce degranulation, but greatly enhanced degranulation to suboptimal anti-CD3 co-immobilized on the plate (Fig. 2). However, when cells stimulated with cross-linked anti-CD3 were plated on the immobilized anti-CD45, there was no increased degranulation over background (Fig. 2), although, upon visual inspection, these cells were spread on the plate and appeared very similar to cells stimulated with plate-bound anti-CD3 or anti-TCR (data not shown). These results suggest that induction of the cytoskeletal changes associated with cell spreading is not sufficient to stimulate degranulation in conjunction with non-polarized TCR-initiated signals. However, this does not imply that actin-dependent cell spreading or polarization is not required for CTL degranulation, as the data presented in Fig. 1 clearly indicate that the actin cytoskeleton is absolutely required for TCR-stimulated degranulation.
Figure 2.
Cell adherence and spreading are not sufficient to trigger degranulation in the presence of cross-linked anti-CD3. AB.1 clone cells were stimulated with suboptimal (0·1 µg/ml) or stimulatory (10 µg/ml) amounts of 145-2C11 immobilized on plastic (IMM) or 10 µg/ml of biotin-coupled 145-2C11 cross-linked with streptavidin (XL) in the presence or absence of anti-CD45 (I3/2) immobilized at 10 µg/ml. Serine esterase activity was assayed 4 hr after adding the cells to the plate. These data are representative of three different experiments.
Dynamic actin polymerization is required for ongoing tyrosine phosphorylation
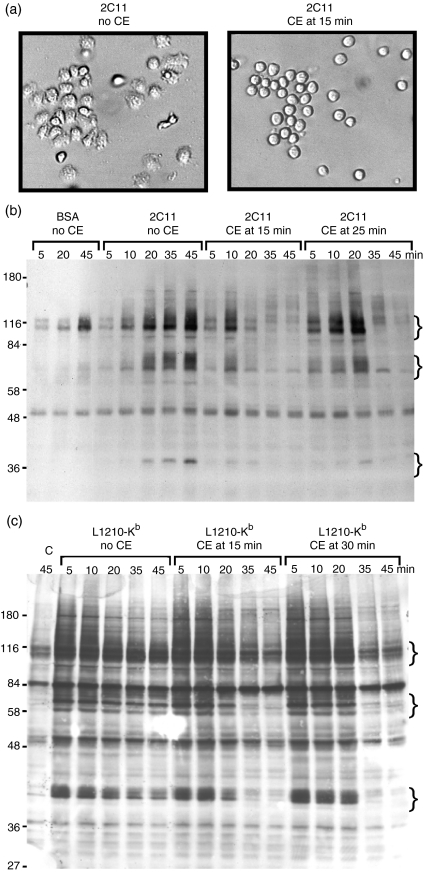
Our above-mentioned data suggest that cell spreading per se is not required for signalling, but it is possible that the tight adhesion and cell spreading are required for cells to make appropriate contact with the immobilized antibody on the plate to initiate signals and that, once the signals are underway, there is no longer a requirement for the cytoskeleton. To test this possibility, we determined the impact of addition of cytochalasin E to cells exhibiting peak tyrosine phosphorylation, in response to an immobilized TCR stimulus, on continued tyrosine phosphorylation. We used cytochalasin E for these studies as our preliminary data indicated that, at 10 µm, cytochalasin E could rapidly (within 5 min) and reproducibly disrupt cell spreading without any noticeable cytotoxicity or obvious membrane blebbing (Fig. 3a). Although this is a concentration that is about 10-fold higher than is required for the inhibition of degranulation, lower concentrations did not immediately reverse the adhesion of cells to the plate (data not shown). As we have reported previously,11 tyrosine phosphorylation was initiated at about 5 min after cells were added to the anti-CD3-bound plate and was sustained until about 45–60 min (Fig. 3b), during which time the cells remained bound and spread on the plate. That tyrosine phosphorylation could be observed at 5 min indicates that enough cells had already made sufficient contact with the plastic to initiate the tyrosine kinase cascade. When cytochalasin E was added at 15 min after the initiation of the response, the number of TCR-induced tyrosine phosphorylated proteins was significantly reduced by 20 min (Fig. 3b). The proteins that became phosphorylated and dephosphorylated included, but were not limited to, ZAP-70, Pyk2, FAK, Vav, Cbl and paxillin (data not shown). Interestingly, when cytochalasin E was added at 25 min, a time well beyond that at which all of the cells had spread on the immobilized antibody and tyrosine phosphorylation remained high, there was a rapid and dramatic reduction in the level of tyrosine phosphorylation (Fig. 3b). No additional tyrosine phosphorylation was observed at time points after addition of cytochalasin E. The cytochalasin E was not directly inhibiting tyrosine kinase activity, as induction of tyrosine kinase activity induced with soluble cross-linked anti-CD3 is not inhibited under these conditions (reference 11 and data not shown). Similar results have been observed with cytochalasin D and latrunculin A, additional inhibitors of actin polymerization.
Figure 3.
Cytochalasin E (CE) disrupts ongoing tyrosine phosphorylation stimulated with immobilized anti-CD3 or target cells. (a) Cl 11 cells were added to wells pre-coated with 145-2C11 (anti-CD3). After 15 min on the plates, carrier (left panel) or CE to a final concentration of 10 µm (right panel) was added to the cells. Photographs of the cells were taken within 5 min after addition of the inhibitor. Cl 11 cells were stimulated for the indicated time with (b) bovine serum albumin (BSA) or immobilized anti-CD3 or (c) L1210 target cells transfected with Kb. In both cases, CE was added to Cl 11 at a final concentration of 10 µm at the indicated time after addition of cells to immobilized 145-2C11 (b) or mixing with target cells (c). At the indicated time, cells were lysed with reducing sample buffer, and subjected to sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS-PAGE) and immunoblotting with antiphosphotyrosine antibodies. Lane ‘C’ in (c) refers to Cl 11 cells mixed with untransfected L1210 cells. The curly brackets indicate positions of groups of proteins that become dephosphorylated after CE addition. Molecular weight standards (kDa) are shown to the left of the gels in (b) and (c). The data shown are representative of four different experiments with Cl 11 or AB.1.
It could be argued that the requirement for ongoing cytoskeletal changes is a result of stimulating cells with immobile antibodies on a plastic surface and that, when cells are stimulated with antigen presented on a cell membrane, there will be no requirement for ongoing cytoskeletal rearrangements once the signal has been initiated. To directly test this possibility, we examined the induction of tyrosine phosphorylation using L1210 target cells. L1210 target cells are normally not recognized by AB.1 or Cl 11; however, if the allogeneic Kb class I MHC is expressed by transfection on these cells, they are lysed by these clones.19 As observed with stimulation using immobilized anti-CD3, addition of cytochalasin E to cells exhibiting peak tyrosine phosphorylation resulted in the rapid loss of tyrosine phosphorylated proteins (Fig. 3c). It should be noted that the kinetics of phosphorylation was much more rapid than that observed with immobilized antibodies, as peak levels of phosphorylation were observed already at 5 min and remained strong for the 45 min of the assay (Fig. 3c). In contrast with solid-phase anti-CD3 stimulation (Fig. 3b), there were significantly more proteins that became tyrosine phosphorylated after target cell stimulation, probably because many receptors besides the TCR were becoming engaged and triggered tyrosine phosphorylation, as well as receptors on the target cell that may initiate signals within the target cells (Fig. 3c). However, as with the stimulation on solid-phase anti-CD3, there was induction of phosphorylation of similar groups of proteins and, even after 30 min, continued tyrosine phosphorylation required an intact cytoskeleton. This is not a reflection of the time required for conjugate formation, as tight conjugates were already formed after the 4-min centrifugation. These results indicate that ongoing cytoskeletal rearrangements are required for target cell-induced tyrosine phosphorylation and that this is not an artefact of stimulating cells with an antibody bound to a solid matrix.
Maintenance of adherence to plate-bound anti-CD3 is not sufficient for maintaining tyrosine phosphorylation if actin turnover is inhibited
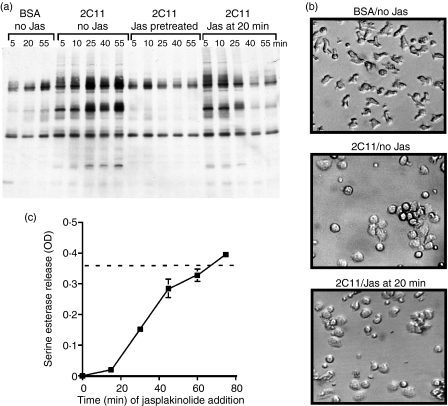
It is possible that the cytoskeleton is required to maintain tyrosine phosphorylation by providing a scaffold for signalling molecules. Treatment of cells with cytochalasin E causes the cells to almost immediately de-adhere from the plate and actin filaments are disrupted, resulting in the loss of potential molecular scaffolds. Jasplakinolide is a cell-permeable antifungal agent that binds to F-actin and inhibits actin filament disassembly, thereby stabilizing existing actin filaments.20,21 This agent would affect the dynamic turnover of actin filaments by depleting free globular actin and preventing subsequent actin rearrangements and polymerization. We employed this reagent to determine if adhesion to the plate-bound anti-CD3 was sufficient to allow ongoing signals in the absence of other cytoskeletal rearrangements. If cells were pretreated with jasplakinolide, we prevented all induction of tyrosine phosphorylation (Fig. 4a), and, although the cells clearly adhered to the antibody, cell spreading was not as extensive as that observed with the control cells (data not shown). When jasplakinolide was added to the cells 20 min after stimulation, tyrosine phosphorylation was reduced to almost background by 40 min (Fig. 4a), although the cells remained spread on the plate and should have been making sufficient contact with the antibody (Fig. 4b). Consistent with the tyrosine phosphorylation data, degranulation was essentially completely inhibited when jasplakinolide was added to the cells at 15–25 min after the cells were added to the immobilized anti-CD3 (Fig. 4c and data not shown). This does not eliminate the possibility that jasplakinolide has additional effects beyond induction of tyrosine phosphorylation; for example, inhibition of granule membrane fusion. Taken together, these results suggest that ongoing cytoskeletal changes, in addition to those necessary for cell spreading and adhesion, are required for continued tyrosine phosphorylation. If the cytoskeletal remodelling is terminated, continued tyrosine phosphorylation also ceases. These results suggest that the cytoskeleton is not merely acting as a scaffold for signalling molecules, as the scaffold would remain in place during this treatment, yet signalling was still blocked.
Figure 4.
Maintenance of cell spreading after immobilized anti-CD3 stimulation is not sufficient to continue tyrosine phosphorylation and degranulation. (a) AB.1 cells were incubated on immobilized bovine serum albumin (BSA) or 145-2C11 for the indicated time, after which cells were lysed in reducing sample buffer. Lysates were subjected to sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS-PAGE) and immunoblotting with antiphosphotyrosine. Cells were left untreated or pretreated for 15 min with 2 µm jasplakinolide (Jas), or jasplakinolide was added to the cells, to a final concentration of 2 µm, 20 min after cells were added to the immobilized 145-2C11. (b) Cl 11 cells were incubated on control BSA-blocked wells (top panel) or anti-CD3-coated wells for 20 min (lower two panels) at which time carrier (middle panel) or jasplakinolide (bottom panel) was added to a final concentration of 2 µm. Cells were incubated for an additional 30 min and photographed. (c) AB.1 cells were plated on immobilized 145-2C11 and jasplakinolide was added to a final concentration of 2 µm at the indicated time after initiation of the assay. The drug remained with the cells for the duration of the culture. Supernatants were assayed for serine esterase activity 3 hr after the initial addition of the cells to the plate. The assay was performed in triplicate and error bars indicate the standard deviations. The dotted line represents the average of the control stimulation with no drug in the assay. These data are representative of four individual experiments. OD, optical density.
Kinetic relationship between tyrosine phosphorylation and cytoskeletal rearrangements
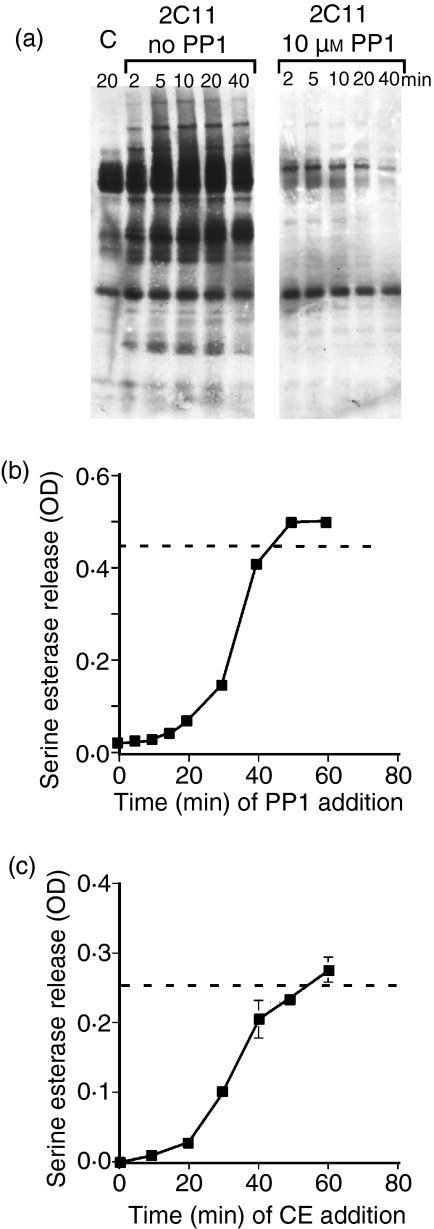
The above results suggest that cytoskeletal rearrangements are required to sustain tyrosine phosphorylation. We therefore determined the kinetic requirements for continuous tyrosine kinase activity leading to degranulation by making use of the src-family tyrosine kinase inhibitor PP1. We first showed that the concentration of PP1 used for these experiments was sufficient to inhibit tyrosine phosphorylation (Fig. 5a). None of the concentrations of inhibitor was toxic to the cells over the period of assay (data not shown). We then determined the length of time that tyrosine kinase activity is required for degranulation by adding PP1 at various times after addition of the cells to immobilized anti-CD3. Src-family tyrosine phosphorylation was required for at least 25–30 min for optimal degranulation (Fig. 5b). Interestingly, PP1 also very rapidly inhibited the adhesion of the cells to the immobilized anti-CD3 (data not shown), indicating that ongoing tyrosine phosphorylation is also required to maintain cytoskeletal integrity and adherence to the plate-bound antibody.
Figure 5.
Src-family kinase activity and cytoskeletal rearrangements are required for similar lengths of time for degranulation. (a) AB.1 cells were stimulated with immobilized 145-2C11 in the presence and absence of 10 µm PP1 for the indicated time. Cell lysates were then immunoblotted with antiphosphotyrosine. (b) AB.1 cells were plated on immobilized 145-2C11 and, at the indicated time, PP1 was added to a final concentration of 10 µm to the assay. Degranulation was assessed 3 hr later. (c) Cytochalasin E (CE) was added to AB.1 cells to a final concentration of 10 µm at the indicated time after addition to 145-2C11-coated wells. Degranulation was then measured 3 hr after initiation of the response. The dotted line indicates the average response of control cells in the same assay. These data are representative of four different experiments. OD, optical density.
To determine the length of time that actin polymerization is required for induction of degranulation by CTL clones, we added cytochalasin E to cells at various times after plating the cells on immobilized anti-CD3 and then measured degranulation at 3 hr after the initial addition of cells to the plate. These data indicate that ongoing cytoskeletal rearrangements must be occurring during the first 20–30 min of stimulation, depending on the clone being examined, to generate a functional response (Fig. 5c).
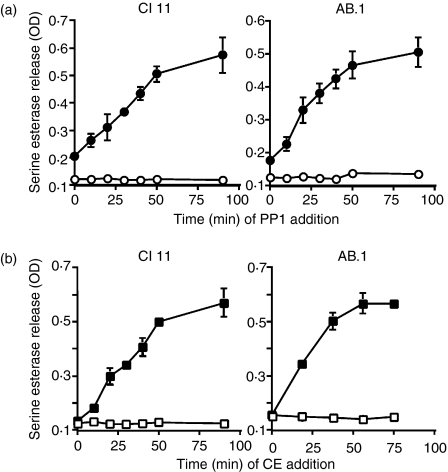
To confirm that these results are not unique to stimulation with anti-CD3, we also examined the length of time that tyrosine phosphorylation and cytoskeletal rearrangements are required for target cell-induced degranulation. Both CTL clones AB.1 and Cl 11 were stimulated with L1210 or L1210 transfected with Kb, and, at various times after mixing of the CTLs with the target cells, either PP1 (Fig. 6a) or cytochalasin E (Fig. 6b) was added to the culture. The cells were then cultured for 3 hr, after which the extent of degranulation was assessed. The time for which tyrosine phosphorylation and cytoskeletal rearrangements were required was significantly reduced with target cells compared with immobilized antibody, probably because the signalling occurs so rapidly and efficiently after the initiation of conjugation by centrifugation. In spite of this, the durations for which the actin remodelling and tyrosine phosphorylation were required for degranulation were remarkably similar for the two clones examined. These results suggest that tyrosine phosphorylation and cytoskeletal rearrangements are ongoing and dynamic and are required for similar times leading to signalling for degranulation.
Figure 6.
Tyrosine phosphorylation and cytoskeletal rearrangements are required for similar lengths of time to allow cytotoxic T lymphocyte (CTL) degranulation in response to target cells. AB.1 or Cl 11 cells were stimulated with L1210 target cells (open symbols) or L1210 target cells transfected with Kb (closed symbols). At various times after mixing of the CTLs and target cells, either PP1 (a) or cytochalasin E (CE) (b) was added to a final concentration of 10 µm to the assay. Degranulation was measured 3 hr after initiation of the culture. The assays were performed in triplicate and error bars represent standard deviations. Similar results were observed in two additional experiments with both cell lines. OD, optical density.
An intact cytoskeleton is required to maintain Erk phosphorylation
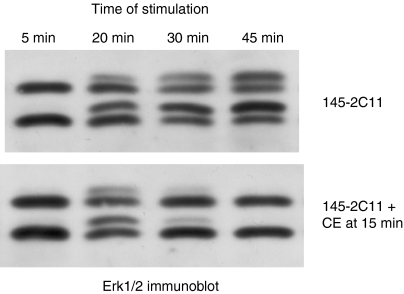
Activation of Erk is a downstream signalling event in T-cell activation. If the actin cytoskeleton were solely required for the recruitment of molecules to the TCR complex and associated cytoskeleton, then, once a downstream signalling molecule such as Erk was phosphorylated and activated, one would predict that its continued activation should not require actin remodelling. To determine if this is indeed the case, cells were allowed to interact with plate-bound anti-CD3 mAb for 15 min before the addition of cytochalasin E to disrupt actin polymerization. The activation of Erk1/Erk2 stimulated with immobilized anti-CD3, as measured by a mobility shift, was rapidly disrupted by cytochalasin E (Fig. 7), but cytochalasin E had no effect on Erk activation stimulated with cross-linked anti-CD3 or PMA (data not shown). Identical results were obtained with phosphorylation-specific Erk antibodies (data not shown). These data indicate that continued Erk activity is dependent on active cytoskeletal rearrangements.
Figure 7.
An intact cytoskeleton is required for sustained Erk activation. AB.1 T cells were added to wells that had been coated with anti-CD3 or bovine serum albumin (BSA). After 15 min, carrier (upper panel) or cytochalasin E (CE) (10 µm) (lower panel) was added to the wells and incubated until the indicated time from the initial addition of cells to the well. Whole cell lysates were harvested and immunoblots probed with anti-Erk. The degree of phosphorylation is indicated by the fraction of Erk1 and Erk2 with retarded mobility. These data are representative of three different experiments.
Discussion
The data presented herein suggest that ongoing actin remodelling is important for the maintenance of tyrosine kinase activity leading to T-cell activation. Our initial interpretation of the numerous studies in the literature showing that cytochalasins block signalling and T-cell responses, and our own data showing that cytochalasins inhibit signalling stimulated with plate-bound anti-CD3, but not soluble cross-linked anti-CD3,11 was that the cytochalasins blocked the initial and efficient interaction of T cells with the solid-phase stimulating antibody, thereby blocking the efficient generation of signals. In the current study, we set out to distinguish between the possible roles that the actin cytoskeleton could play in CTL activation. One possibility is that the actin cytoskeleton is solely a structural component required for tight conjugation between the CTL and its cognate target cell or solid-phase antibody stimulus and for the initial movement of molecules to the contact zone. Another possibility is that actin remodelling is important for regulating signalling events in cells, even after the initial contact with target cells.
We demonstrated that the cytoskeleton is not just stabilizing the adhesion of the cells to the plate to allow sustained contact with the plate-bound anti-CD3, as jasplakinolide allowed continued contact with the antibodies on the plate, and yet signals were almost immediately disrupted (Fig. 4). These results suggest that continued actin remodelling, not just cytoskeletal integrity, is important for sustaining signals that have already been initiated in the cell. The process of cell spreading and adhesion to the plates is also not sufficient to induce signals leading to degranulation of the CTL, as soluble cross-linked anti-CD3 does not co-operate with anti-CD45-induced (Fig. 2) or fibronectin-induced22 spreading and cytoskeletal remodelling to trigger degranulation. These data imply that cell spreading per se is not sufficient for T-cell activation, but instead that continuous actin remodelling plays an important role in activation.
The cytoskeleton is not required for all T-cell signalling; it depends on the stimulus used to activate the cell. As mentioned above, cytochalasins do not block soluble cross-linked anti-CD3-stimulated tyrosine phosphorylation11 or Erk activation (data not shown). Our results are in agreement with previous studies showing that cytochalasin D does not inhibit the Ca2+ flux triggered with soluble antibodies.10 We showed that CTL degranulation in response to PMA and ionomycin did not require cytoskeletal rearrangements (Fig. 1), which is consistent with our observation that PMA-stimulated Erk activation was not inhibited by cytochalasin (data not shown). Therefore, cytoskeletal remodelling seems to be crucial for regulating only those signals that are generated from polarized stimuli, such as plate- or bead-bound anti-CD3 or target cells, in the case of CTL.
We showed that the addition of cytochalasins after cells had adhered to plate-bound antibodies or target cells immediately blocked ongoing tyrosine phosphorylation and Erk phosphorylation. One possible interpretation of this result is that actin filaments serve as a scaffold for signalling molecules, perhaps to maintain the localization of these proteins. However, our results with jasplakinolide (Fig. 4) and anti-CD45-induced spreading (Fig. 2) indicate that stabilizing the actin filaments is not sufficient to maintain signals, arguing that the cytoskeleton is not merely providing a scaffold. Instead, our data support the notion that continuous actin remodelling is required for maintaining tyrosine phosphorylation, and that there is a feedback loop between tyrosine kinase activity and the actin cytoskeleton. This would imply that tyrosine kinase activity, or perhaps accessibility to tyrosine kinase substrates, is regulated by the actin cytoskeleton. There are at least two examples of the actin cytoskeleton regulating tyrosine kinase activity. The tyrosine kinase c-Abl binds directly to F-actin, which in turn inhibits its kinase activity.23 More recently, it was shown that actin depolymerization modestly induces the kinase activity of the Fer tyrosine kinase, which in turn phosphorylates the filamentous actin-binding protein cortactin, perhaps decreasing its cross-linking ability.24 Although these examples of F-actin regulation of tyrosine kinases have not been shown to be operational in T cells, there are probably additional protein tyrosine kinases that are, either directly or indirectly, regulated by the actin cytoskeleton in T cells. Paxillin is a potentially interesting link between the cytoskeleton and tyrosine kinases, as we have shown that this cytoskeletal adaptor protein associates with the tyrosine kinases Lck and Pyk2 in T cells.14
One could envision many mechanisms by which the actin cytoskeleton could regulate tyrosine phosphorylation within the cell. In addition to directly regulating protein tyrosine kinase activity, it is possible that the depolymerization of actin activates tyrosine phosphatases that rapidly dephosphorylate the substrate; however, we consider this unlikely as the jasplakinolide inhibition maintains actin filaments yet leads to rapid dephosphorylation. We therefore favour the model in which, during initiation of a T-cell response, there is an initial tyrosine kinase signal and phosphatases are not shut off or removed from the TCR complex, as many models of T-cell signalling suggest, but rather tyrosine phosphatases are at the complex continuously dephosphorylating tyrosine phosphorylated proteins, and tyrosine kinases must continuously re-phosphorylate these residues. This would imply that cells would favour these signalling pathways being in the ‘off’ position, and a continuous positive signal, regulated in part by the actin cytoskeleton, would be required to shift the equilibrium to the phosphorylation of proteins.
Previous studies have suggested that continuous signals are required for T-cell activation and that disruption of receptor engagement results in the rapid diminution of downstream signals. Early studies with concanavalin A (ConA) stimulation of Jurkat cells demonstrated that signals were required for 2–4 hr for IL-2 production.25 Furthermore, increases in intracellular Ca2+ concentration, inositol tris-phosphate production and protein kinase C activity were all rapidly reversed after the addition of α-methyl mannoside to inhibit ConA binding to cells, suggesting that the cells must continually receive new signals to maintain the response and that mechanisms are in place to rapidly reverse these signals in the absence of input of new signals.25 More recently, examination of signalling at different temperatures suggested that tyrosine phosphorylation and dephosphorylation are occurring simultaneously and that, to keep the signal propagating, new signals must be generated continuously.26 An elegant study by Valitutti et al. showed that sustained signals are required for cytokine production by T cells, and that disruption of the actin cytoskeleton of pre-formed conjugates with actin-disrupting agents almost immediately reduces the ongoing Ca2+ flux and blocks cytokine production, without disrupting the conjugates.10 Based on previous studies and the data presented herein, we posit that continuous actin remodelling is important in maintaining the balance of signals to the ‘on’ position when polarized signals are provided to the cell.
Herein we determined the duration of signals required for degranulation. It would be of interest to know how this relates to the length of time that it takes for degranulation to occur. The length of time required for degranulation to occur has been difficult to measure accurately, because enzymatic assessment of released granzyme activity is generally employed. With the CTL clones used in this study, it was previously shown that degranulation was complete in about 1 hr.13 More recently, we have been able to measure degranulation by these CTLs using a fluorescence-activated cell sorter (FACS)-based assay for surface expression of CD107, a membrane marker found in the lysosomally derived granules from CTLs. We have found that increased CD107 can be detected starting at about 15 min and maximal levels are achieved at about 30–45 min after stimulation with target cells (J. He and H. L. Ostergaard, manuscript in preparation). Given the results presented here, that both cytoskeletal rearrangements and tyrosine phosphorylation are required for about 30–45 min for optimal degranulation, it appears that signalling and ongoing cytoskeletal rearrangements are required until membrane fusion actually occurs. A requirement for continuous signalling may be to maintain the CTL in a tight conjugate with the target cell until the lethal hit is delivered in a directional manner to minimize bystander damage of cells and indiscriminate killing. This type of degranulation is very different from degranulation of neutrophils triggered by various soluble mediators, where cytochalasins actually enhance degranulation.27 Furthermore, the requirement for ongoing signalling and cytoskeletal rearrangements is probably not for the actual process of exocytosis, as PMA- and ionomycin-stimulated degranulation is resistant to cytochalasins in these CTLs (Fig. 1). We therefore conclude that the ongoing signalling and cytoskeletal rearrangements are required for either initiating or maintaining the polarized signals required for directional degranulation by CTLs.
In summary, our data suggest that actin remodelling is required on an ongoing basis during the entire period required for tyrosine kinase activity and other signals leading to CTL degranulation. Cytoskeletal rearrangements do not appear to be required solely for cell spreading on the plate to allow optimal contact with antibodies, but are additionally required to maintain signalling cascades. These data suggest that, in addition to the actin cytoskeleton serving as a recruitment mechanism or scaffold for important signalling molecules, it is plausible that continuous remodelling is required to allow continued signalling, which in turn leads to additional actin remodelling. Although much has been learned over the past decade in dissecting how signals lead to cytoskeletal rearrangements, additional studies are required to understand how cytoskeletal rearrangements regulate signalling processes in T cells.
Acknowledgments
This work was supported by the National Cancer Institute of Canada with funds from the Canadian Cancer Society. A.S. was supported by summer studentships from the Alberta Heritage Foundation for Medical Research (AHFMR) and L.G.P was supported by a graduate studentship from AHFMR. H.L.O. is an AHFMR scientist. The authors would like to acknowledge Dr Kevin Kane for his helpful discussions, for critical reading of this manuscript and for the L1210 cells transfected with class I Kb.
Abbreviations
- APC
antigen-presenting cell
- BSA
bovine serum albumin
- CTL
cytotoxic T lymphocyte
- Erk
extracellular signal regulated kinase
- HRP
horseradish peroxidase
- MHC
major histocompatibility complex
- mAb
monoclonal antibody
- PBS
phosphate-buffered saline
- PMA
phorbol myristate acetate
- TCR
T-cell receptor
References
- 1.Miletic AV, Swat M, Fujikawa K, Swat W. Cytoskeletal remodeling in lymphocyte activation. Curr Opin Immunol. 2003;15:261–8. doi: 10.1016/s0952-7915(03)00054-2. [DOI] [PubMed] [Google Scholar]
- 2.Fuller CL, Braciale VL, Samelson LE. All roads lead to actin: the intimate relationship between TCR signaling and the cytoskeleton. Immunol Rev. 2003;191:220–36. doi: 10.1034/j.1600-065x.2003.00004.x. [DOI] [PubMed] [Google Scholar]
- 3.Kupfer A, Singer SJ. Cell biology of cytotoxic and helper T cell functions: immunofluorescence microscopic studies of single cells and cell couples. Ann Rev Immunol. 1989;7:309–37. doi: 10.1146/annurev.iy.07.040189.001521. [DOI] [PubMed] [Google Scholar]
- 4.Parsey MV, Lewis GK. Actin polymerization and pseudopod reorganization accompany anti-CD3-induced growth arrest in Jurkat T cells. J Immunol. 1993;151:1881–93. [PubMed] [Google Scholar]
- 5.Bunnell SC, Kapoor V, Trible RP, Zhang W, Samelson LE. Dynamic actin polymerization drives T cell receptor-induced spreading: a role for the signal transduction adaptor LAT. Immunity. 2001;14:315–29. doi: 10.1016/s1074-7613(01)00112-1. [DOI] [PubMed] [Google Scholar]
- 6.Badour K, Zhang J, Siminovitch KA. The Wiskott–Aldrich syndrome protein: forging the link between actin and cell activation. Immunol Rev. 2003;192:98–112. doi: 10.1034/j.1600-065x.2003.00031.x. [DOI] [PubMed] [Google Scholar]
- 7.Sechi AS, Wehland J. Interplay between TCR signalling and actin cytoskeleton dynamics. Trends Immunol. 2004;25:257–65. doi: 10.1016/j.it.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 8.Golstein P, Foa C, MacLennan IC. Mechanism of T cell-mediated cytolysis: the differential impact of cytochalasins at the recognition and lethal hit stages. Eur J Immunol. 1978;8:302–9. doi: 10.1002/eji.1830080504. [DOI] [PubMed] [Google Scholar]
- 9.O'Rourke AM, Apgar JR, Kane KP, Martz E, Mescher MF. Cytoskeletal function in CD8- and T cell receptor-mediated interaction of cytotoxic T lymphocytes with class I protein. J Exp Med. 1991;173:241–9. doi: 10.1084/jem.173.1.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Valitutti S, Dessing M, Aktories K, Gallati H, Lanzavecchia A. Sustained signaling leading to T cell activation results from prolonged T cell receptor occupancy. Role of T cell actin cytoskeleton. J Exp Med. 1995;181:577–84. doi: 10.1084/jem.181.2.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Berg NN, Puente LG, Dawicki W, Ostergaard HL. Sustained TCR signaling is required for mitogen-activated protein kinase activation and degranulation by cytotoxic T lymphocytes. J Immunol. 1998;161:2919–24. [PubMed] [Google Scholar]
- 12.Wulfing C, Bauch A, Crabtree GR, Davis MM. The vav exchange factor is an essential regulator in actin-dependent receptor translocation to the lymphocyte-antigen-presenting cell interface. Proc Natl Acad Sci USA. 2000;97:10150–5. doi: 10.1073/pnas.97.18.10150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kane KP, Sherman LA, Mescher MF. Molecular interactions required for triggering alloantigen-specific cytolytic T lymphocytes. J Immunol. 1989;142:4153–60. [PubMed] [Google Scholar]
- 14.Ostergaard HL, Lou O, Arendt CW, Berg NN. Paxillin phosphorylation and association with Lck and Pyk2 in anti-CD3 or anti-CD45 stimulated T cells. J Biol Chem. 1998;273:5692–6. doi: 10.1074/jbc.273.10.5692. [DOI] [PubMed] [Google Scholar]
- 15.Ostergaard HL, Ma EA. Fibronectin induces phosphorylation of a 120-kDa protein and synergizes with the T cell receptor to activate cytotoxic T cell clones. Eur J Immunol. 1995;25:252–6. doi: 10.1002/eji.1830250141. [DOI] [PubMed] [Google Scholar]
- 16.Puente LG, Stone JC, Ostergaard HL. Evidence for PKC-dependent and independent activation of MAP kinase in T cells: Potential role of additional diacylglycerol binding proteins. J Immunol. 2000;165:6865–71. doi: 10.4049/jimmunol.165.12.6865. [DOI] [PubMed] [Google Scholar]
- 17.Lyubchenko TA, Wurth GA, Zweifach A. The actin cytoskeleton and cytotoxic T lymphocytes: evidence for multiple roles that could affect granule exocytosis-dependent target cell killing. J Physiol. 2003;547:835–47. doi: 10.1113/jphysiol.2002.033522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arendt CW, Hsi G, Ostergaard HL. Immobilized antibodies to CD45 induce rapid morphologic changes and increased tyrosine phosphorylation of p56lck-associated proteins in T cells. J Immunol. 1995;155:5095–103. [PubMed] [Google Scholar]
- 19.Durairaj M, Sharma R, Varghese JC, Kane KP. Requirement for Q226, but not multiple charged residues, in the class I MHC CD loop/D strand for TCR-activated CD8 accessory function. Eur J Immunol. 2003;33:676–84. doi: 10.1002/eji.200323499. [DOI] [PubMed] [Google Scholar]
- 20.Scott VR, Boehme R, Matthews TR. New class of antifungal agents: jasplakinolide, a cyclodepsipeptide from the marine sponge, Jaspis species. Antimicrob Agents Chemother. 1988;32:1154–7. doi: 10.1128/aac.32.8.1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bubb MR, Senderowicz AM, Sausville EA, Duncan KL, Korn ED. Jasplakinolide, a cytotoxic natural product, induces actin polymerization and competitively inhibits the binding of phalloidin to F-actin. J Biol Chem. 1994;269:14869–71. [PubMed] [Google Scholar]
- 22.Puente LG, Ostergaard HL. Beta 1/beta 3 integrin ligation is uncoupled from ERK1/ERK2 activation in cytotoxic T lymphocytes. J Leukoc Biol. 2003;73:391–8. doi: 10.1189/jlb.0402199. [DOI] [PubMed] [Google Scholar]
- 23.Woodring PJ, Hunter T, Wang JY. Inhibition of c-Abl tyrosine kinase activity by filamentous actin. J Biol Chem. 2001;276:27104–10. doi: 10.1074/jbc.M100559200. [DOI] [PubMed] [Google Scholar]
- 24.Fan L, Di Ciano-Oliveira C, Weed SA, Craig AW, Greer PA, Rotstein OD, Kapus A. Actin depolymerization-induced tyrosine phosphorylation of cortactin. the role of Fer kinase. Biochem J. 2004;380:581–91. doi: 10.1042/BJ20040178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weiss A, Shields R, Newton M, Manger B, Imboden J. Ligand–receptor interactions required for commitment to the activation of the interleukin 2 gene. J Immunol. 1987;138:2169–76. [PubMed] [Google Scholar]
- 26.Schade AE, Levine AD. Signal transduction through the T cell receptor is dynamically regulated by balancing kinase and phosphatase activities. Biochem Biophys Res Commun. 2002;296:637–43. doi: 10.1016/s0006-291x(02)00925-7. [DOI] [PubMed] [Google Scholar]
- 27.Bentwood BJ, Henson PM. The sequential release of granule constitutents from human neutrophils. J Immunol. 1980;124:855–62. [PubMed] [Google Scholar]