Abstract
Interest in the interactions between nervous and immune systems involved in both pathological and homeostatic mechanisms of host defence has prompted studies of neuroendocrine immune modulation and cytokine involvement in neuropathologies. In this review we concentrate on a distinct area of homeostatic control of both normal and abnormal host defence activity involving the network of peripheral c-fibre nerve fibres. These nerve fibres have long been recognized by dermatologists and gastroenterologists as key players in abnormal inflammatory processes, such as dermatitis and eczema. However, the involvement of nerves can all too easily be regarded as that of isolated elements in a local phenomenon. On the contrary, it is becoming increasingly clear that neural monitoring of host defence activities takes place, and that involvement of central/spinal mechanisms are crucial in the co-ordination of the adaptive response to host challenge. We describe studies demonstrating neural control of host defence and use the specific examples of bone marrow haemopoiesis and contact sensitivity to highlight the role of direct nerve fibre connections in these activities. We propose a host monitoring system that requires interaction between specialized immune cells and nerve fibres distributed throughout the body and that gives rise to both neural and immune memories of prior challenge. While immunological mechanisms alone may be sufficient for local responsiveness to subsequent challenge, data are discussed that implicate the neural memory in co-ordination of host defence across the body, at distinct sites not served by the same nerve fibres, consistent with central nervous mediation.
Keywords: contact sensitivity, haemopoiesis, memory, nerve fibres, neuroimmunology
Neuroimmunology
It is first necessary to highlight increasingly troublesome issues of definition in this area of research. As a greater number of peptide mediators common to the immune, nervous and neuroendocrine systems are discovered, terms such as ‘cytokine’ and ‘neuropeptide’ become progressively more misleading. Cytokines and neuropeptides are potential mediators between the nervous and immune systems, as well as existing within each system. As evidence of this ‘neuro-immune dialogue’ accumulates, a point may be reached where a major reclassification of molecules becomes necessary. There are also problems regarding functions ascribed to specific mediators. Glucocorticoids are not just immunosuppressive (a phenomenon associated with chronic stress and high levels of these molecules), but are also involved in optimizing immune responses at low levels in acute stress (see below). Ablation of the sympathetic nervous system, or treatment with adrenergic agonists, can exacerbate or ameliorate symptoms in animal models of arthritis. In an adjuvant-induced model, β-agonists were found to be pro-inflammatory in the earlier, asymptomatic stage, but anti-inflammatory in the later, symptomatic phase.1 Chemical sympathectomy had a similar time-dependent effect in antigen-induced arthritis.2
Such issues can be confounding and add to the confusion concerning sources and targets of these mediators in vivo. These issues aside, there is a significant body of evidence supporting the integration of these systems in a co-ordinated host defence network. In this review we will concentrate on neural co-ordination of host defence and present evidence to show that this co-ordination is important in the orchestration of immunological activities.
Neuroimmunology encompasses both homeostasis and pathology
How do nervous, endocrine and immune systems interact? There have emerged several discrete categories of research into fields that each uses the term ‘neuroimmunology.’ Here we briefly review these groupings and identify some of the difficulties associated with their classification.
One area concerns ‘inappropriate’ immune responses – pathological processes involving injurious effects on the nervous system – including autoimmune reactions, such as multiple sclerosis,3 as well as cytokine mediation of inflammatory injury in stroke.4 Although the origin of cytokines involved in inflammatory brain injury may not necessarily be immunological, this highlights a semantic problem throughout all fields of neuroimmunology regarding peptide mediators. The pejorative terms ‘cytokines’ and ‘neuropeptides’ need careful use, as neither is limited to immune or nervous origin. Similarly, catecholamines, although predominantly sourced from sympathetic nerves and the adrenal medulla, can also be sourced from immunocytes (see below and Table 1). Such issues can be confounding and add to the confusion concerning the source and target of these mediators in vivo.
Table 1.
Mediators of neuroimmunomodulation
| Name | Source(s) | Targets, functions, effects | Refs |
|---|---|---|---|
| Substance P | Central nervous system, peptidergic nerve fibres (c-fibres), macrophages, granulocytes, lymphocytes | Released antidromically as part of nociception. Increases lymphocyte output from lymph nodes, stimulates lymphocyte proliferation, causes mast cell degranulation. Blocks T-cell adhesion to fibronectin and can augment cytokine secretion from antigen-exposed cells in vitro. Neurokinin receptors present on endothelia, smooth muscle, lymphocytes, Langerhans' cells, mast cells. Neurokinin receptor activation causes rises in intracellular Ca2+. Related peptides neurokinin A and B are also released by c-fibres. | 24,63,64,68, 69,164–168 |
| Calcitonin gene-related peptide (CGRP) | c-fibres, lymphocytes | Induces vasodilation, plasma extravasation, mast cell degranulation. Inhibits antigen presentation by Langerhans' cells. Alters T-cell cytokine secretion and adhesion to fibronectin. CGRP receptor present on T-cell surface. Stimulation of CGRP receptors on Langerhans' cells brings about signalling through adenylate cyclase and cAMP. | 16,100–102,169 |
| Vasoactive intestinal peptide (VIP) and pituitary adenylate cyclase-activating peptide (PACAP) | Produced at nerve terminals in the CNS and PNS, as well as in lymphoid organs | Suppresses the induction of contact hypersensitivity. Inhibits Langerhans' cells antigen presentation in vitro. Down-regulates expression of macrophage costimulatory molecules. Released by sensory nerves upon damage/inflammation. Receptors present on cutaneous nerves, macrophages, Langerhans' cells and T cells. Receptor activation induces cAMP formation in Langerhans' cells. | 77,170,171 |
| Neuropeptide Y | Nerve terminals in the CNS and PNS, lymphoid organs, lymphocytes and monocytes | Suppresses natural killer cell activity. Co-released with norepinephrine from sympathetic terminals following depolarization, participating in neurogenic inflammation. NPY receptors expressed by dorsal root ganglion neurones. | 172–175 |
| Somatostatin | Nerve terminals in the CNS and PNS, lymphoid organs | Specific somatostatin receptors present on lymphocyte surface, Langerhans' cells. Along with CGRP and NPY, directly induces T-cell adhesion to fibronectin. Also directly stimulates T-cell cytokine secretion. Can enhance or reduce lymphocyte proliferation. | 112,124,176–178 |
| GnRH-I, -II (gonadotrophin- releasing hormone) | Neuronal cells, T lymphocytes | Increases surface expression and transcription of a laminin receptor in T cells. Laminin receptor thought to be important in facilitating T-cell extravasation and holding of memory T lymphocytes in areas of challenge. Also induces chemotaxis towards SDF-1/CXCL12. | 179 |
| α-melanocyte stimulating hormone (α-MSH) | Keratinocytes, Langerhans' cells, melanocytes | Synthesis by keratinocytes up-regulated by immunological challenge. May induce hapten-specific tolerance by modulating levels of interleukin-10. Decreases endothelial cell adherence, dendritic costimulatory molecule expression, antagonizes interleukin-1 activity. Melanocortin receptors expressed throughout the skin. POMC peptides stimulate melanogenesis. | 126,180–187 |
| Corticotropin-releasing hormone (CRH) and urocortin | Throughout the skin, may be transported to the skin via sensory nerves in rodents. Lymphocytes | Keratinocytes, mast cells, endothelia all carry CRH receptors. Can prompt mast cell degranulation, despite antinflammatory and antiproliferative properties. Stimulates POMC activity in cutaneous fibroblasts. | 125–128,130,131 |
| Adrenocorticotrophic hormone (ACTH) | Anterior pituitary, skin cells, leucocytes | Derived from POMC. Cells throughout the skin express melanocortin receptors responsive to ACTH and MSH. Induces expression of preprotachykinin and neurokinin receptor. POMC peptides stimulate melanogenesis. | 126,187–189 |
| Nerve growth factor (NGF) | Mast cells, fibroblasts and other cell types at sites of injury/inflammation | Binds TrkA receptors on sensory afferents. Essential for nerve fibre growth/development/ maintenance. Can influence mast cell activity. | 126 |
| Serotonin (5-HT) | Afferent nociceptors, mast cells (rodents only). Stored by lymphocytes and platelets | Enhances plasma extravasation induced by capsaicin. Proinflammatory, vasodilator. Noradrenergic termini in lymphoid organs may take up 5-HT for later release. Immune cells, keratinocytes, unmyelinated axons all thought to express HT receptors. | 86,190 |
| Endorphins and enkephalins | Langerhans' cells, mononuclear leucocytes, keratinocytes | Antinociceptive properties. Production stimulated by proinflammatory stimuli (e.g. TNF-α). Enhances mononuclear cell chemotaxis and interleukin-2 production. Opioid receptors present on keratinocytes. | 191–195 |
| Epinephrine and norepinephrine | Lymphocytes, macrophages, sympathetic neurones, keratinocytes, adrenal medulla (under acute stress) | Norepinephrine co-localizes with neuropeptide Y in lymphoid organs. Intradermal injection can suppress the sensitization and elicitation phases of contact sensitivity. Decreases proinflammatory cytokine expression, promote anti-inflammatory cytokine production. Can be an activator of the immune response; depends on the type of recipient cell and its activational and maturational state. Stimulation with physiological concentrations of catecholamines stimulates adenylate cyclase activity and cyclic AMP production. Keratinocytes, almost all leucocytes, including Langerhans' cells and mast cells, express adrenoreceptors. Α2-agonists inhibit interleukin-12 secretion by dendritic cells and therefore T helper 1 cell development. This inhibition is mediated through increases in intracellular cyclic AMP concentration. | 196–198 |
| Histamine | Mast cell granules, basophils. Platelets, dendritic cells and T cells produce histamine de novo | Potent vasodilator. Activates submucosal neurones. Increasesinterleukin-10 secretion, inhibits interleukin-12 secretion by dendritic cells and T helper 1 cell development as a result. This inhibition is mediated through increases in intracellular cAMP. Increased levels of CD80, CD86 and MHC class II on dendritic cells. Histamine receptors: expressors include neurones, endothelia, dendritic cells, T and B cells. T helper 1 cells predominantly express H1R. H2R is primarily coupled to adenylate cyclase – also capable of signalling through PLC. T helper 2 cells predominantly express H2R. | 199–202 |
| Acetylcholine | Cholinergic innervation, keratinocytes | Cholinergic receptors present on keratinocytes and lymphocytes. Induces norepinephrine secretion from lymphocytes. Depresses TNF-α secretion without affecting interleukin-10 levels. | 80,81,203,204 |
| Melatonin | Nervous system, immune system | Mammalian skin produces melatonin from serotonin. Melatonin receptors expressed in mammalian skin. Modulates lymphocyte proliferation and the T helper 1/T helper 2 cytokine balance. | 190,205,206 |
| ATP and ADP | Keratinocytes, nociceptors (in response to noxious stimuli) | Nucleotide (P2X/Y) receptors present on sensory nerve fibres – ATP responsive? ATP may exert an inhibitory effect on dendritic cell migration, prolonging exposure to antigen. | 207 |
| Potassium | Nervous system and other tissues (in response to noxious stimuli) | Activates T-cell adhesion to fibronectin, facilitating migration. Brings about β1-inegrin adhesion in the same way as ‘neuropeptides’; T-cell membrane depolarization and opening of the T-cell Kv1.3 voltage-gated K+ channel. | 208,209 |
CGRP, calcitonin gene-related peptide; CNS, central nervous system; NPY, neuropeptide y; POMC, pro-opiomelanocortin; PNS, peripheral nervous system; PLC, phospholipase C; SDF-1, stromal cell derived factor-1; TNF-α, tumour necrosis factor-α.
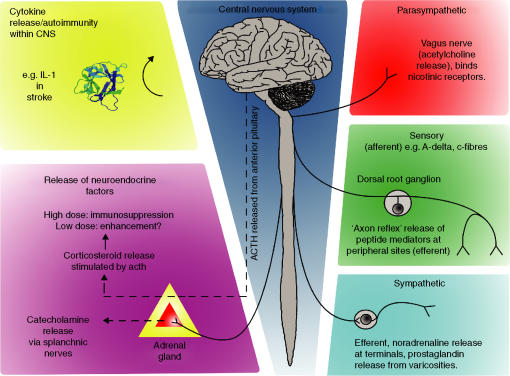
A second, broad category includes the study of ‘appropriate’ or homeostatic neuroendocrine mechanisms, the core of which is an adaptive effect of short-term (acute) stress-activation of the hypothalamus–pituitary–adrenal (HPA) axis5,6 that is sufficient to terminate inflammatory responses and steer T-helper cytokine balance. Immunological status appears to be coupled to homeostatic endocrine control via cytokine levels, including interleukin (IL)-1, which mediate activation of the HPA axis.7–9 Mediators in this axis involve hypothalamic corticotropin-releasing hormone (CRH) evoking adrenocorticotrophic hormone (ACTH) release from the anterior pituitary, which, in turn, stimulates corticosteroid release from the adrenal cortex (Fig. 1).
Figure 1.
Immunomodulation by the nervous system. Adrenocorticotrophic hormone (ACTH) released from the pituitary (centre) acts upon the adrenal cortex to stimulate the release of glucocorticoids (bottom left), which typically suppress immune function, especially biasing towards T helper 2 (Th2) cells. The adrenal medulla receives sympathetic innervation from the splanchnic nerves, which can provoke release of catecholamines into the circulation. Cytokine release and autoimmune processes within the central nervous system (top left) are also considered to be a form of neuroimmunomodulation. Sympathetic innervation (bottom right) is present in all lymphoid organs, as well as tertiary sites. Sensory innervation (centre right) is similarly pervasive and is characterized by peptide neurotransmitter release and capsaicin sensitivity. Parasympathetic innervation, (top right) principally from the vagus nerve, releases acetylcholine. Leucocytes have been shown to harbour receptors for and respond to all of these chemical mediators. CNS, central nervous system; IL-1, interleukin-1.
Another difficulty in understanding ‘neuroimmune’ mediation is that generalizations of function cannot easily be ascribed to specific mediators. For example, corticosteroids are not simply immunosuppressive; at extremes, low-level corticosteroid release may sustain immune system function,10 while chronic stress can elevate susceptibility to disease via secondary immune deficiency.11–14
A third category involves homeostatic activity, mediated not by neuroendocrine outflow, but by direct, ‘hard-wired’ interactions between the nervous and immune systems that are the focus of the present article.
Since we last reviewed this subject,15 every major category of peripheral nerve has been shown to be involved in the modification of an immunological activity (Fig. 1). We therefore provide a brief review of the ability of peripheral nerve fibres and specific cells of the host defence system to engage in cross-talk at the cellular level, followed by two examples, from our own work, on the involvement of peptidergic nerves on:
haemopoietic cell differentiation and release from bone marrow; and
the availability of hapten-specific memory in contact sensitivity responses in the skin.
Furthermore, we develop a theory concerning the wider role of c-fibres in the co-ordination of immune function.
Defining and establishing functional connectivity (synaptic or otherwise) between cells
To ‘prove the principle’ that productive interactions at a cellular level between peripheral nerves and the immune system take place, evidence needs to be accumulated that the criteria established for synaptic connectivity are met. Not only must a mediator be synthesized by a cell, it must be released, be detected by a receptor expressed by another cell, and induce a functional alteration within that cell as a result. Past research has clearly established that the immune and nervous systems share common mediators (Table 1). Not only can cells in both systems synthesize and release these mediators, they both show physiological responses based on the presence of specific receptors. This presents both the evidence for physiological cross talk between these systems and a major problem for neuroimmunologists, as to establish in vivo connectivity it must demonstrate that the source of a mediator is cells of one system and the target is the other system. Experiments utilizing pharmacological agents in vivo therefore present problems for the isolation of neuroimmune interactions, as shared mediators could be affecting either one or both systems. Similarly, tools previously thought to be highly specific for one system may eventually be shown to affect both systems; capsaicin being a case in point. This molecule (an extract of chilli peppers) had been thought to exclusively target specific vanilloid receptors on small-diameter, unmyelinated sensory nerve fibres, but these same receptors have recently been shown to exist on a range of cells in the skin and immune system,16–19 requiring a re-evaluation of data from experiments using capsaicin. It can be appreciated, then, that the design of experiments is critical for establishing connectivity between these two systems that share so many chemical mediators. Moreover, while in vitro experiments can establish the presence of functional receptors and physiological responses, they cannot define the in vivo source and target of the specific chemical mediator, or whether the elements for signalling pathways demonstrated in vitro may in fact be utilized in vivo. Although imperfect, the neurotoxic actions of capsaicin have improved our understanding of functional connectivity between nervous and immune systems in vivo.
Co-ordination of host defence mediated by c-fibres
Although primarily recognized as afferent nerve fibres for the sensation of damaging or painful stimuli, c-fibres have been identified to have important roles in conveying sensibility to immunological stimuli as well as providing efferent drive for inflammatory processes and homing cues for dendritic cells (DCs) in most peripheral sites and organs of the body. Previously thought to have ‘free’ nerve endings, c-fibres are associated with a variety of specialized cells in the organ systems, notably with modified macrophages [e.g. Langerhans' cells (LCs), DCs and stromal cells]16 and mast cells.17
Current dogma states that the immune system is widely regarded as autonomous and self-regulating. While at the cellular level there is little doubt that immune cells are capable of regulating each others' activities, recent experimental evidence suggests that neural pathways are essential for the co-ordination of immune activity in vivo. In an attempt to resolve the function of c-fibres in neuroimmune co-ordination, we must first propose a regulatory loop and gather evidence to test whether the elements of this circuit are connected and regulate immune function. If c-fibres are to exert any direct influence over an immune response, the first requirement is that they are present and functional throughout the host defence system, including in the bone marrow and secondary lymphoid organs, as well as at the sites of potential antigen exposure (the skin, the surfaces of the airways and digestive tracts, for instance), such that the static nervous system has as many contacts as possible with a highly mobile immune system through its more static, regulatory components. Second, immune cells must be capable of responding to neurotransmitters released by these nerve fibres, to products of neurotransmission or to factors generated by the stimulation of other non-neural cells. Third, there must be sensory monitoring of host challenge and immune activity such that the nervous system is ‘informed’ of host status and/or host response to challenge so that it can effectively co-ordinate host defence activity. Below we give examples of data that support the presence of such an integrated and co-ordinated host defence system.
Neurogenic inflammation
It was first documented by Bayliss, in 1901, that stimulation of neurones in dorsal root ganglia results in vasodilation18 (reviewed in refs 19 and 20), suggesting that these afferent (sensory) nerves also had an efferent (motor) function. The role of neural afferents and efferents in the inflammatory response, and in specific inflammatory conditions, has received a great deal of attention, with the identification of substance P (SP), calcitonin gene-related peptide (CGRP) and nitric oxide as key neurotransmitters. SP is synthesized in dorsal root ganglion neurones21 and is also found in the hypothalamus, substantia nigra, the dorsal horn of the spinal cord and in small Aδ and unmyelinated c-fibres in the periphery22,23 (reviewed in ref. 24). It has long been accepted in clinical dermatology and gastroenterology that peptide mediators released from cutaneous c-fibres are potent proinflammatory agents, giving rise to neurogenic inflammation and underlying a range of conditions, including dermatitis, psoriasis, eczema, Crohn's disease and colitis. Collateral c-fibre axons provide an efferent route for inflammatory cues to spread laterally from afferent terminals in the form of a local reflexive arc, termed the ‘axon reflex’ (Fig. 1). A major component of allergic reactions both in the airways25,26 and in the skin,27–30 neurogenic inflammation may be initiated by the release of SP and CGRP. Additionally, and probably more important in promoting vasodilation and vascular permeability,31–34 leading to plasma extravasation, sympathetic nerve fibres release potent inflammatory mediators, including norepinephrine, from varicosities along the nerve fibre as well as from the nerve terminals.28 Nowhere is this more evident than in synovial joints.35 c-Fibres are also intimately associated with mast cells throughout the body (see below) and stimulate the release of histamine, further aggravating inflammatory conditions. These observations indicate that both direct and indirect interactions between nerve fibres and immune cells, as well as independent neural effects on peripheral targets, are activated in inflammatory conditions.
Anatomical and functional evidence of lymphoid innervation
Studies of lymph node innervation were conducted as long ago as 1899, by Tonkoff,36 that hinted at the presence of sensory nerves in lymph nodes. Similar conclusions were arrived at in the late 1980s, when electron microscopy enabled the definition of partially myelinated axons entering the node, as well as potential sensory nerve terminals associated principally with the vasculature37 but also branching into cortical and paracortical regions, terminating among lymphocytes.38,39 Dense plexuses of adrenergic fibres in association with blood vessels and the surrounding periarterial lymphatic sheath in lymph nodes have been demonstrated, as have occasional fibres with no apparent vascular association in the cortical, paracortical and medullary regions of lymph nodes.40 Staining for a number of peptide mediators has proven positive, showing potential co-localization between SP and CGRP, among others, reflecting the association seen in the skin.41,42 In contrast to noradrenergic fibres that are mainly associated with the vasculature and perivascular areas, SP/CGRP-positive nerves are often seen in the cortical regions of lymph nodes, as well as in the vasculature and lymphatics. Some evidence already exists to suggest that the activity of these and other sympathetic nerve fibres is immunomodulatory, both in lymph nodes and skin. For example, rats that had undergone sympathetic ganglionectomy prior to immune challenge showed decreased lymphocyte proliferation in response to concanavalin A (Con A).43 Co-cultures of neonatal superior cervical ganglia and adult murine tissue revealed significant outgrowth of neurites from the ganglia towards lymphoid tissue, which was diminished by administering anti-nerve growth factor (NGF).44 Interestingly, neurite outgrowth to mesenteric lymph nodes was enhanced in this study when the lymphoid tissue was taken from mice infected with nematode worms. This observation is consistent with evidence that showed an increase in the density of lymph node innervation following antigenic stimulation;45 increases in the numbers of axonal profiles within the medulla were observed 4 months after the subcutaneous injection of bovine serum. In a contact sensitivity (CS) model, antigenic challenge induced an increase in the length of cutaneous protein gene product 9·5-reactive nerve fibres and their NGF content.46 Similarly, nerve fibre density has been found to be enhanced in psoriatic lesions.47 The spleen is invested with a similarly dense innervation, where release of IL-6 and tumour necrosis factor-α (TNF-α) is ‘fine-tuned’ by sympathetic neurotransmission.48 Consistent with a close functional relationship between peripheral nerves and key immunocytes within lymphoid tissue, including the gut, which are proposed to mediate homeostatic benefit, the closeness of sympathetic innervation to follicular DCs has been correlated with their rate of neuroinvasion by prions.49
Sympathetic and neuroendocrine influences on leucocytosis
A number of studies have shown that acute psychological stress induces the mobilization of lymphocytes into the blood50 and that catecholamines, namely epinephrine, play a critical role in the induction of this stress-induced redistribution of lymphocytes, known as lymphocytosis. First described 100 years ago,51,52 catecholamine-induced lymphocytosis is characterized by a rapid (10-min) increase in lymphocyte numbers in the circulation; the numbers of ‘large granular lymphocytes’, which would come to be known as natural killer (NK) cells, were found to increase to a greater extent, by ≈ 400%.53 This reproducible lymphocytosis is typically followed by a more variable increase in granulocyte numbers.50 There exist several possibilities to explain the source of this rapid increase in immune cell numbers. As the effect is too rapid and too great in magnitude to be explained by proliferation, cells loosely adherent to venule walls (the marginal pool), the lungs, spleen, bone marrow and lymph nodes, are all possible reservoirs50 and are all served by adrenergic and, indeed, peptidergic nerve fibres.42 Neuropeptide Y and catecholamines can be secreted simultaneously in these regions, consistent with neuropeptide Y (NPY)-mediated leucocyte mobilization.54,55 Cortisol secretion in response to stressful stimuli also prompts a redistribution of lymphocytes.56 In a wider context, sympathetic leucocytosis can be thought of as a less-recognized facet of the ‘fight-or-flight’ response.57 Mobilization of immune cells into the tissues readies the organism to deal with physical trauma and any potential infection that may result.50 Catecholamine and cortisol-induced leucocytosis is a well-documented phenomenon, but it seems that this is not the sole level of neural control over immune-cell trafficking; an indication of more widespread neural control comes from studies of the effects of anaesthetics.
Effects of anaesthetics and vasoactive mediators in leucocyte trafficking
It has long been suspected that general anaesthesia has a deleterious effect on the immune system, both in the suppression of resistance to infection and enhanced tumour growth,58 an idea supported by more recent evidence suggesting that NK cell number and activity can be depressed by general anaesthesia.59 Studies with cannulated ovine lymph nodes show that the lymphocyte output from this node into the efferent vessel is reduced by over 99% by barbiturate or halothane (i.e. general) anaesthesia. Similar findings were reported with local (lidocaine) anaesthetic.58 Confirming a neural involvement in this effect, stimulation of the sympathetic chain prompted an increase in lymphocyte and lymph output from the node.60 These experimental findings suggest that there is control of leucocyte retention and/or trafficking through this sympathetic neural pathway. Although peripheral lymphatic vessels are little more than an endothelial cell monolayer, larger collecting lymphatic vessels often contain a layer of lymphatic smooth muscle along with connective tissue and nerve terminals, which enable contractility to the point where the lumen can transiently disappear,61 propelling lymph along the vessels. However, the control over lymphocyte retention in nodes is likely to be based more on the modulation of adhesive mechanisms than on mechanical gates.
The list of vasoactive substances that have been found to alter lymphocyte flow has steadily grown. Serotonin (5-HT), SP (Met)enkephalin and phenylephrine prompt an approximate doubling in lymphocyte flow over 1–4 hr, whereas neurotensin, vasoactive intestinal peptide (VIP) and carbachol all reduce lymphocyte numbers by ≥ 50% over a similar timescale (see Table 1).62,63
The exposure of peripheral blood lymphocytes to physiological concentrations of somatostatin, CGRP, NPY or dopamine specifically increases integrin-mediated adhesion of these lymphocytes to fibronectin by 10–30 times above background levels.64–66 By contrast, SP inhibits leucocyte adhesion, regardless of whether it has been induced by a ‘pro-adhesive’ peptide mediator, a number of chemokines, or stimulation with phorbol ester.64,65
Leucocyte trafficking is determined by the expression of adhesion molecules, not only by leucocytes but also by endothelia. A series of studies using human dermal microvascular endothelial cells have shown that SP, acting primarily through a neurokinin receptor (NK-1R), induces an increase in intercellular adhesion molecule type 1 (ICAM-1) mRNA and ICAM-1 surface expression, as well as vascular cell adhesion molecule-1 (VCAM-1).67–69 Similar SP-induced ICAM-1 expression has been reported in keratinocytes.70 It would appear that such an increase is of immunological significance, as levels of neutrophil adhesion to human umbilical vein endothelial cells rise to ≈ 250% above background with exposure of both cell types to 10−15 or 10−10m SP.71 SP also induces neutrophil chemotaxis in inflammatory responses, presenting a potential means of control over one of the major events in cell-mediated inflammation.22,72,73 This idea was elaborated upon with the finding that SP (or a NK-1 receptor agonist) cannot induce neutrophil accumulation in skin in the normal state, but it can potentiate neutrophil accumulation when inflammation is induced by IL-1β.74
Peptide mediators and cytokine shift
VIP inhibits the proliferative T-cell response normally seen with exposure to mitogens, such as ConA,75 and it also decreases the level and delays the onset of IL-2 production by these cells.76 It now seems that VIP promotes T helper 2 (Th2) cells and suppresses the development of T helper 1 (Th1) cells through at least two different mechanisms. The pretreatment of macrophages in vitro with VIP enhances their ability to induce Th2-type cytokines in CD4+ cells and inhibits the induction of Th1 cytokines.77,78 Accordingly, it appears that Th2 effectors are the sole source of lymphocyte-derived VIP,79 suggesting that this forms part of a Th2-polarizing loop. VIP is also present in nerve fibres present in lymphoid organs, providing a potential neural pathway for stimulation of this cytokine shift.
Parasympathetic influences
The majority of neurotransmitters implicated in the modulation of inflammation are neuropeptides, but a role does seem to exist in this regard for the parasympathetic neurotransmitter, acetylcholine (ACh). In human macrophage cultures stimulated with lipopolysaccharide, nanomolar concentrations of ACh dramatically decrease the secretion of the pro-inflammatory cytokines TNF, IL-1β, IL-6 and IL-18, without affecting the levels of the anti-inflammatory cytokine, IL-10.80 This would fit with the expression of cholinergic receptors by keratinocytes and the functional downstream signalling that ACh induces in these cells.81 Furthermore, electrical stimulation of the vagus nerve (within which ACh is the principal efferent neurotransmitter) was found to inhibit levels of TNF both in the liver and serum of rats, preventing the manifestation of endotoxic shock.80 Some of these immunomodulatory effects may be exerted indirectly by CGRP, as administration of ACh at lower or higher concentrations to isolated rat mucosa could inhibit or potentiate CGRP release, respectively.82 The expression of α7 nicotinic receptors by DCs has also been reported.83 Although high-dose immunosuppressive effects of nicotine are known, Aicher et al. showed nicotine (10−7m) to be proinflammatory by three measures:
Nicotine enhanced antigen-presenting cell (APC) or DC expression of surface molecules involved in inflammation.
Nicotine promoted the homing of DC to atherosclerotic plaques.
Nicotine-stimulated APCs showed a greater capacity to stimulate T-cell proliferation and cytokine secretion.
Their findings included the stimulation of CD40, CD86, major histocompatibility complex class II (MHC II), CD54 and IL-12 expression through this nicotinic pathway. Such findings ought to encourage a closer look at the effects of parasympathetic innervation on adaptive immune response. In contrast to sympathetic nerve fibres, parasympathetic, cholinergic nerve fibres have not been demonstrated in many anatomical associations with immune cells, presenting another case questioning source and target of chemical mediator in vivo.
Peptide mediators in the skin
Nanomolar concentrations of SP (as well as VIP and somatostatin) induce mast cell degranulation and the release of histamine from serosal and connective tissue mast cells,22,84 as well as inducing the release of TNF-α.85 The release of peptides such as SP (or other proinflammatory mediators, e.g. serotonin86 see Table 1) from nerves might initiate or enhance inflammation through contact with mast cells in the skin,87 and in the gastrointestinal and respiratory tracts.88 Experiments have shown that peptidergic and/or sympathetic neurones growing in tissue culture are ‘attracted’ towards a mast cell-like cell line and can form sustained contacts with these cells.17 This is followed by an inhibition of mitotic activity in these mast cell-like cells and an increase in the number of granules that they harbour. Significantly, this phenomenon was only observed with cells directly contacting a nerve membrane; cells directly adjacent to, but not in contact with, a neurone exhibited neither reduced mitotic activity nor reduced granule numbers.89 An intriguing interaction was exposed when it emerged that mast cell degranulation up-regulates integrin expression on the surface of LCs,90 hinting that mast cell activation (by nerves or otherwise) might contribute towards LC migration and subsequent antigen presentation.
It has been reported that SP has no direct effect on the antigen-presenting capacity of epidermal LCs16,91 and that LC frequency within the epidermis is not altered by SP.92 However, nanomolar concentrations of neurokinin receptor agonists or antagonists enhance or suppress the CS response, respectively, when injected into murine skin 30 min prior to sensitization,92 suggesting an indirect route for SP immunomodulation. By contrast, CGRP has been shown to directly inhibit antigen presentation by macrophages through the down-regulation of MHC II and CD86/B7-2 expression,91,93–96 an observation consistent with the anatomical association between sensory nerves and 70–80% of LCs in the skin16,97 (reviewed in ref. 98), as well as the induction of tolerance in a CS model by the intracutaneous injection of CGRP.99 CGRP can also inhibit T-cell proliferation and IL-2 production,100 as well as peripheral blood mononuclear cell proliferation; the response to tetanus toxin antigen was reduced by as much as 50% by preincubation with CGRP, for example. Interestingly, these immunosuppressive effects can be mitigated by neutralizing IL-10, suggesting this as a channel through which CGRP exerts its effects.101 This may also explain the apparent production of CGRP by rat and human lymphocytes themselves, in response to mitogenic stimuli,102,103 as a self-regulatory loop to restrain proliferation. CGRP does induce cAMP production in keratinocytes, although basal levels of cAMP and the increases seen in response to CGRP are both higher in LCs.91 LCs also contain transcripts of both pituitary adenylate cyclase-activating peptide (PACAP) receptors, VPAC1 and VPAC2.104 Accordingly, intradermal injection of 5 µm PACAP 15 min prior to hapten sensitization can significantly inhibit ear swelling upon challenge 1 week later.105 This implies that PACAP, like CGRP, can inhibit antigen presentation by LCs.
Both SP and the neurokinin-1 receptor are expressed by human monocytes and macrophages.106 SP down-regulates membrane-bound enzymes on monocytes, as well as inducing the release of lipo-oxygenase and cyclo-oxygenase pathway products (i.e. leukotrienes and prostaglandins)22 and TNF-α.107 The production of IL-1, IL-6, IL-12 and TNF-α by macrophages is induced by nanomolar concentrations of SP,108,109 as is the production of keratinocyte IL-1.110 Significant T-lymphocyte proliferation is provoked by the specific binding of SP at nanomolar levels;21,111–115 increases in DNA and protein synthesis in such cells are indicative of this proliferative effect116 (reviewed in ref. 117). Owing to the presence of SP and NK-1 receptors in both immune and nervous systems, the question again arises as to source and target in in vivo functions. This question was directly addressed in a series of elegant experiments by Chavolla-Calderón and colleagues who deleted the PPT-A gene (from which the SP transcript is derived) or sensory nerve fibres and found that the immune complex-mediated hypersensitivity response in murine lung tissue was abolished and could not be overcome by reconstituting such mice with wild-type bone marrow.118 These experiments lend strong support to the contention that the requirement for the PPT-A gene in inflammation does not lie within immune cells but requires a neural source.
Perhaps in agreement with the finding that mechanical denervation increases the time that skin takes to heal119 and reduces macrophage and T-cell infiltrates into the wounded site,120 platelets have been shown to express neurokinin receptors 1 and 3 and aggregate in response to SP.121 The numbers of SP-positive fibres associated with blood vessels increase in the skin following burns,122 implying that this peptide is involved in the recruitment of immunity and the repair of tissue following damage caused by inflammation or injury. Tissue lymphocyte populations have a higher percentage of peptide mediator receptor-positive cells than blood lymphocytes;123 fluorescent somatostatin conjugates are bound by one-third to one-half of T and B cells from lymphoid sites, this decreases to less than one-quarter in blood lymphocytes.124 It is unclear whether this reflects induction of this phenotype at a certain site, or selective homing of such a population to the tissues.
Local neuroendocrine modulation of skin function
Cells within the skin are capable not only of responding to neuroendocrine mediators, but can, in many cases, synthesize and process the same mediators themselves, generating an additional, local level of fine-tuning of immune responses.125–132 Corticotropin-releasing hormone (CRH) and the pro-opiomelanocortin (POMC)-derived peptides α-melanocyte-stimulating hormone (α-MSH), ACTH and β-endorphins can all be produced by cells in cutaneous compartments (see Table 1).
Along with resident macrophages, keratinocytes harbour β-adrenoreceptors and can also synthesize and produce catecholamines; defects in catecholamine responsiveness have been implicated in vitiligo and atopic eczema.133,134 Treatment of LCs in vitro with epinephrine or norepinephrine inhibits their antigen-presentation capability and diminishes CS responses when administered locally or at a remote site in vivo,135 implying that catecholamines released either from cutaneous nerves or the adrenal gland are capable of reducing LC activity. The same suppressive effect may also occur elsewhere, as β2-agonist treatment inhibits mast cell TNF-α release.136 These observations may explain the inhibition of cutaneous norepinephrine turnover observed in the hours following oxazolone sensitization,137 effectively relieving cutaneous leucocytes of inhibition in order to initiate a response to the antigen.
Evidence of neural involvement in the CS response
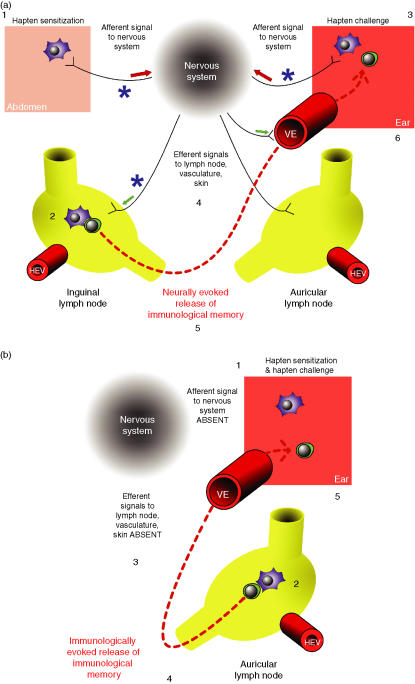
Given the presence of small, unmyelinated c-fibres in the skin, their involvement in neurogenic inflammation,138 and their functional association with epidermal LCs and mast cells, it was hypothesized that their removal would impact upon a CS model, a primarily Th1-mediated response to the hapten antigen 2,4-dinitrochlorobenzene (DNCB).139 In this model, sensitizing hapten is given to the abdomen, or another site, and a challenge with the same hapten given 4 days later to an ear results in a CS response measured as increased ear thickness 24 hr after challenge. This presents a useful model for using to test the role of cutaneous nerve fibres in a specific immune response. Chemical deletion of vanilloid receptor-1 (VR-1)-positive nerve fibres can be achieved with a sufficiently high dose of capsaicin.140–143 Denervation of the site of sensitization by topical capsaicin application could abolish the response to DNCB at the site of challenge, provided denervation was carried out before sensitization.139 A similar outcome was observed, regardless of whether all nerve fibres were destroyed by application of a dilute phenol solution, or if sensory nerve fibres alone were destroyed by capsaicin application. The same outcome was observed following denervation of both the sensitization and challenge sites (abdomen and right ear, respectively) (Fig. 2). No evidence of a systemic effect of topical capsaicin application was found, as a sensitizing application of DNCB to the back of a mouse on which the abdomen had been denervated produced a normal CS response measured at the ear. The CS response measured at the ear was also lost if the capsaicin was administered systemically, destroying all sensory nerve fibres. LC migration, and vascular and lymphatic integrity, all appeared to be unaffected in denervated tissue. A separate study carried out some years previously showed that in rats systemically denervated with capsaicin, there was no difference in neutrophil chemotaxis towards SP,72 suggesting that treatment with capsaicin is not toxic to the immune system and that these cells retain their responsiveness to this mediator, despite its depletion from the skin. However, VR-1 receptors have been found on a range of skin and immune cells, meaning that the possibility of direct effects of capsaicin treatment on immune function remains.144–147 The time course of denervation experiments suggests that any effects of capsaicin on immune function would need to be non-toxic and very long lasting, something that would be unusual for this chemical agent at the doses used.
Figure 2.
Effect of sensory denervation on the manifestation of contact sensitivity (CS). (a) Challenge at a site remote to sensitization: nerves are required to relay activation signals for CS between distant sites. Upon hapten sensitization of the abdomen (1), a signal is generated in the c-fibre associated with the Langerhans' cells (LC). The LC migrate to the draining lymph node (2) and interact with naïve, hapten-specific T lymphocytes. Upon ear challenge 4 days later (3), a second signal reaches the nervous system, prompting efferent signalling (4) to the inguinal lymph node (which harbours memory T cells from sensitization), as well as the vasculature and skin cells at the site of challenge. This stimulus evokes the release of memory T cells into the circulation (5), whereupon they migrate to the site of challenge and secrete a variety of pro-inflammatory mediators, contributing to contact sensitivity (6). When the site of sensitization is denervated with capsaicin 48 hr beforehand (top left asterisk), hapten sensitization of the abdomen does not generate a nervous system signal. However, LC migration to the local (inguinal) node and memory T-cell generation is unaffected. There is now a discrepancy in the system; the animal has immunological memory of exposure to the hapten, but no neural memory was formed. Upon challenge of an innervated site (ear), the nervous system receives the appropriate signal, but fails to mobilize memory T cells from the remote (abdominal–inguinal) lymph node. This, in turn, means that no recordable CS response occurs. Denervation of the site of challenge (top right asterisk) does not affect the propagation of a sensitization signal or formation of immunological memory, but a signal is not received from the site of challenge, meaning that no elicitation of the neural memory occurs, efferent signals are not generated and immunological memory is not mobilized, rendering the CS response absent. Denervation of the lymph node draining the site of sensitization (bottom asterisk) does not prevent the formation of neural or immunological memory, but the signal to the lymph node following challenge cannot be delivered. Consequently, mobilization of immunological memory fails and no CS response takes place. Loss of the CS response is reversed by the artificial infusion of substance P (SP) to the denervated node (data not shown). (b) Sensitization and challenge at the same site: local mechanisms exist for the activation of CS that are independent of nerves. Denervation of the site of sensitization or the draining lymph node does not preclude the formation of immunological memory. Following sensitization (1), a population of memory T cells, specific for hapten, are formed and remain in the draining lymph node (2). If the same site is used for challenge 4 days later, LC harbouring antigen will migrate to this same node, prompting effector T-cell release and migration to the site of challenge (3,4), initiating the response independently of nervous system input (5). The site of challenge and/or the draining lymph node can be denervated in this situation with no visible effect on the CS response. HEV, high endothelial venule; VE, vascular endothelia.
In a study of adjuvant-induced arthritis, it was discovered that capsaicin injection into the draining lymph node did not affect manifestation of the disease in the region served by the denervated node, but it did preclude the spread of inflammation to the contralateral limb.148 The role of lymph node innervation has also been investigated in CS reactions.149 Denervation of the lymph node draining the site of sensitization similarly compromises the CS response measured at the ear, reducing ear swelling to the same extent as complete removal of the node. Consistent with the anti-adhesive properties of SP,64,65 subcutaneous injection of the peptide over the node before challenge rescues the response in a dose-dependent manner. It appears, from these experiments, that cutaneous nerve fibres and nerve fibres supplying lymph nodes are required for the normal process of eliciting a CS response. Indeed, cutting off sensitization or challenge sites, or draining lymph nodes from their nerve supply, results in a failure to release specific (memory) cells for the hapten. Immunological theory states that memory cells traffic from the draining lymph node, and memory is eventually present in all lymph nodes, but these experiments suggest that this phenomenon may require neural involvement. Controversially, perhaps, they also suggest that there might be a neural memory for host challenge, as well as an immunological memory for specific antigen, and that this is required to release memory on subsequent challenge by the same antigen. The memory for hapten-antigen from the lymph node draining the site of sensitization is made available to distant (naive) sites of subsequent challenge with hapten via a neural mechanism sensitive to interruption by neurotoxins (capsaicin and phenol), which can be replaced by the administration of SP.
Evidence of neural involvement in leucocyte release from bone marrow
Bone marrow is innervated with both myelinated and non-myelinated nerve fibres,42 many of which have been shown anatomically to associate with stromal cells, specifically perivascular cells.150 As stromal cells are a key source of growth factors and regulatory adhesion molecules for haematopoietic cells, it was hypothesized that this association represents a site of neural control of haemopoiesis151–153 and consequently of immune responses.
It has been suggested that nerve fibres present in the marrow regulate the mobility of haematopoietic cells and the permeability of the stromal cells that surround the vascular network. Severing the femoral nerve hat supplies the bone marrow in the femur, or chemical sympathectomy with 6-hydroxydopamine causes a marked and prolonged reduction in leucocyte numbers in the bone marrow (by approximately one-third) and a concomitant increase in the circulation.151 Interestingly, chemical sympathectomy did not provoke mobilization of colony-forming progenitor cells from the marrow, whereas mechanical denervation did, ruling out adrenergic input and implicating other transmitters in the control of adhesion of these cells in the marrow. These findings have been further confirmed by surgical removal of sympathetic ganglia supplying the femur (J. Miyan, unpublished data) indicating that the effects are probably a result of the loss of neural control, rather than direct or indirect effects on immune cells. The authors proposed that nerve fibres can alter the adhesive properties of perivascular cells and perhaps even the haematopoietic cells themselves, controlling the mobilization of such cells into the periphery. Treatment with capsaicin produced a dramatic change in bone marrow haemopoiesis, as measured in in vitro colony-forming assays,152 suggesting a direct role of CGRP, a suggestion confirmed by the finding that this peptide had direct access to haemopoietic progenitors to induce changes in proliferation, while other mediators required indirect pathways, presumably via stromal cells to mediate changes in activity.152 Neural control of haemopoiesis through c-fibres may underlie the complications seen in burns patients where leucocyte profiles in the peripheral blood are abnormally high and include immature and progenitor cells. Furthermore, spinal injury patients show a loss of bone marrow activity below the level of the spinal lesion, suggesting that this loss of function may result from loss of neural input.154–156 The indications from all these lines of evidence point to significant neural involvement in bone marrow function and leucocyte mobilization to the circulation. Coupled with the data on neural involvement in peripheral host defence and immune functions, there is convincing evidence supporting a case for neural co-ordination, something that only comes to light in in vivo investigations where neural elements are targeted.
Immune system to nervous system communication
The guinea-pig superior cervical ganglion, located in the neck and the most likely source of the sympathetic innervation of the ears and auricular lymph nodes, contains around 1500 mast cells which degranulate upon antigenic stimulation of the ganglion in vitro, releasing histamine, leukotrienes and prostaglandins.157,158 Following as little as 5 min of exposure to ovalbumin, compound action potentials have been recorded from the nerve trunk leaving the ganglion. This reflects an increase in the number of postganglionic neurones induced to fire, possibly mediated by products of mast cell degranulation.157 This may represent a feedback loop of neural activity, a notion consistent with pro-inflammatory cytokines as sensitizers of sensory neurone depolarization and of autonomic neuronal involvement in negative feedback control of inflammation. However, it is unclear how and why mast cells should populate the ganglion, or how antigen would be delivered to this site in vivo. In different experiments, treatment with TNF-α, IL-1β or IL-6 elevated the release of peptide mediators from isolated sensory neurones in response to low-dose capsaicin,159 providing evidence that the function of not only sympathetic, but also sensory, nerves can be modified by inflammatory cytokine levels. Several other studies have demonstrated that these same cytokines can inhibit the release of norepinephrine from sympathetic neurones in the myenteric plexus and spleen, whereas ACTH stimulates norepinephrine release.48
In patients with minor stroke, a delayed-type hypersensitivity response was markedly weaker when elicited on the side of the body affected by the stroke.160 This may connect with findings that neocortical stimulation in rats can increase T-lymphocyte output from the thymus, apparently by glucocorticoid-independent means.161 Using viral trans-synaptic tracing methods in rats, a pathway from the bone marrow, spleen and lymph nodes to the spinal cord, medulla, hypothalamus and insular cortex has been described (ref. 162; Krisztina Kovács, personal communication), reinforcing the hypothesis that bone marrow and immune system function can be brought under the control of the autonomic nervous system. These examples suggest that alternative central and local neural reflexive pathways are associated with immune system function and also illustrate a model of a hierarchy of candidate levels of regulation by nerve fibres. Regulatory loops could be entirely local, depending only on nerve fibres in the periphery, and decisions could be made at the level of the sympathetic ganglia, the spinal cord, or even the brain itself, echoing the theories proposed by Besedovsky & del Rey.132
Conclusions
Integrated host monitoring and defence
Evidence of peripheral nerve fibres showing close anatomical association and functional effects upon immune cells has not only been documented for lymphoid organs, but also for tertiary sites, such as the skin and mucosal surfaces.136
From our own experiments (summarized above) ‘memory’ of antigen exposure (wherever and in whatever form that memory may reside) is formed in both immune and nervous systems. Second exposure to that antigen at another site elicits the release of peptides from neurones in the draining lymph node, stimulating the release of hapten-specific T lymphocytes (and perhaps any other lymphocytes in the node) into efferent lymphatics and the circulation. In animals with a denervated lymph node, this neural input is missing and the lymphocytes are not mobilized, a situation reversed by the artificial infusion of SP. We speculate that the nervous system retains a memory for prior challenge, although the level of specification of this neural memory is not yet clear. Immune specificity arises from the T-cell receptor for antigen, but such specific receptors are neither present nor required on sensory nerves for antigen to produce neural memory. The mechanism, in this case, is the modality-specific sensing cell (e.g. a photoreceptor, hair cell, chemosensor or, in this case, an APC) that produces a response to the stimulation that is transmitted to the sensory nerve. The pattern of activity in the sensory nerve codes for the pattern produced in the sensing cell (e.g. in a frequency of nerve potentials). In this way the sensory nerve would need no specific mechanism to detect the antigen, relying instead on the membrane changes of the APC as it processes antigen, to produce a characteristic signature for the antigen. Clearly, this requires further experimental investigation, but it is not an unreasonable suggestion given the operation of other sensory mechanisms. We therefore propose that sensory nerves, in conjunction with LCs/DCs, ‘sense’ exposure to antigen. Speculative although this may seem, we are far from the first to propose that this neuroimmune network be regarded as an additional sensory system.163
When theories this controversial are presented, they rightly undergo an extraordinary degree of scrutiny. Although there may always be alternative, non-neural interpretations of some data, the collective body of evidence reviewed here, and other evidence we have previously reviewed15 represents a strong case for the existence of a neural co-ordination of host defence activity. As mentioned above, the presence of common mediators and receptors across these two systems, and also shared with the neuroendocrine and endocrine systems, present serious problems in the design of experiments and interpretation of data. The utilization of transgenic models, as well as new techniques to selectively affect one or other systems, may aid in the resolution of these issues.
This review has emphasized neural involvements in the co-ordination of multiple immune activities. It demonstrates the importance of neural signalling in either the promotion or the limitation of innate and adaptive immune responses present in vivo. It offers a physiological and pharmacological understanding for therapeutic opportunity in immune modulation.
Acknowledgments
The authors thank The Wellcome Trust and the Medical Research Council for past and current funding, as well as past and current colleagues for working with us on the experiments and for discussion of data.
References
- 1.Lubahn CL, Schaller JA, Bellinger DL, Sweeney S, Lorton D. The importance of timing of adrenergic drug delivery in relation to the induction and onset of adjuvant-induced arthritis. Brain Behav Immun. 2004;18:563–71. doi: 10.1016/j.bbi.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 2.Harle P, Mobius D, Carr DJJ, Scholmerich J, Straub RH. An opposing time-dependent immune-modulating effect of the sympathetic nervous system conferred by altering the cytokine profile in the local lymph nodes and spleen of mice with type II collagen-induced arthritis. Arthritis Rheumatism. 2005;52:1305–13. doi: 10.1002/art.20987. [DOI] [PubMed] [Google Scholar]
- 3.Sospedra M, Martin R. Immunology of multiple sclerosis. Annu Rev Immunol. 2005;23:683–747. doi: 10.1146/annurev.immunol.23.021704.115707. [DOI] [PubMed] [Google Scholar]
- 4.Gibson RM, Rothwell NJ, Le Feuvre RA. CNS injury: the role of the cytokine IL-1. Vet J. 2004;168:230–7. doi: 10.1016/j.tvjl.2003.10.016. [DOI] [PubMed] [Google Scholar]
- 5.Berczi I. Neuroimmune biology – an introduction. In: Berczi I, Gorczynski RM, editors. New Foundation of Biology. Oxford: Elsevier Science; 2001. pp. 1–46. [Google Scholar]
- 6.Neylan TC. Hans Selye and the Field of Stress Research. J Neuropsychiatry Clin Neurosci. 1998;10:230–1. doi: 10.1176/jnp.10.2.230a. [DOI] [PubMed] [Google Scholar]
- 7.Berkenbosch F, van Oers J, del Rey A, Tilders F, Besedovsky HO. Corticotropin-releasing factor-producing neurons in the rat activated by interleukin-1. Science. 1987;238:524–6. doi: 10.1126/science.2443979. [DOI] [PubMed] [Google Scholar]
- 8.Berkenbosch F, de Goeij DEC, Del Rey A, Besedovsky HO. Neuroendocrine, sympathetic and metabolic responses induced by interleukin-1. Neuroendocrinology. 1989;50:570–6. doi: 10.1159/000125283. [DOI] [PubMed] [Google Scholar]
- 9.Sapolsky R, Rivier C, Yamamoto G, Plotsky P, Vale W. Interleukin-1 stimulates the secretion of hypothalamic corticotropin-releasing factor. Science. 1987;238:522–4. doi: 10.1126/science.2821621. [DOI] [PubMed] [Google Scholar]
- 10.McEwen BS, Biron CA, Brunson KW, et al. The role of adrenocorticoids as modulators of immune function in health and disease: neural, endocrine and immune interactions. Brain Res Brain Res Rev. 1997;23:79–133. doi: 10.1016/s0165-0173(96)00012-4. [DOI] [PubMed] [Google Scholar]
- 11.Fukui Y, Sudo N, Yu X-N, Nukina H, Sogawa H, Kubo C. The restraint stress-induced reduction in lymphocyte cell number in lymphoid organs correlates with the suppression of in vivo antibody production. J Neuroimmunol. 1997;79:211–7. doi: 10.1016/s0165-5728(97)00126-4. [DOI] [PubMed] [Google Scholar]
- 12.Dhabhar FS, Mcewen BS. Acute stress enhances while chronic stress suppresses cell-mediated immunity in vivo: a potential role for leukocyte trafficking. Brain Behav Immun. 1997;11:286–306. doi: 10.1006/brbi.1997.0508. [DOI] [PubMed] [Google Scholar]
- 13.Cohen S, Tyrrell DA, Smith AP. Psychological stress and susceptibility to the common cold. N Engl J Med. 1991;325:606–12. doi: 10.1056/NEJM199108293250903. [DOI] [PubMed] [Google Scholar]
- 14.Glaser R, Rice J, Sheridan J, et al. Stress-related immune suppression: health implications. Brain Behav Immun. 1987;1:7–20. doi: 10.1016/0889-1591(87)90002-x. [DOI] [PubMed] [Google Scholar]
- 15.Downing JEG, Miyan JA. Neural immunoregulation: emerging roles for nerves in immune homeostasis and disease. Immunol Today. 2000;21:281–9. doi: 10.1016/s0167-5699(00)01635-2. [DOI] [PubMed] [Google Scholar]
- 16.Hosoi J, Murphy GF, Egan CL, Lerner EA, Grabbe S, Asahina A, Granstein RD. Regulation of Langerhans' cell function by nerves containing calcitonin gene-related peptide. Nature. 1993;363:159–63. doi: 10.1038/363159a0. [DOI] [PubMed] [Google Scholar]
- 17.Blennerhassett MG, Tomioka M, Bienenstock J. Formation of contacts between mast cells and sympathetic neurons in vitro. Cell Tissue Res. 1991;265:121–8. doi: 10.1007/BF00318146. [DOI] [PubMed] [Google Scholar]
- 18.Bayliss WM. On the origin from the spinal cord of the vaso-dilator fibres of the hind-limb and on the nature of these fibres. J Physiol (Lond) 1901;26:173–209. doi: 10.1113/jphysiol.1901.sp000831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Foreman JC. Peptides and neurogenic inflammation. Br Med Bull. 1987;43:386–400. doi: 10.1093/oxfordjournals.bmb.a072189. [DOI] [PubMed] [Google Scholar]
- 20.Foreman JC. Neuropeptides and the pathogenesis of allergy. Allergy. 1987;42:1–11. doi: 10.1111/j.1398-9995.1987.tb02180.x. [DOI] [PubMed] [Google Scholar]
- 21.Kim KH, Park KC, Chung JH, Choi HR. The effect of substance P on peripheral blood mononuclear cells in patients with atopic dermatitis. J Dermatol Sci. 2003;32:115–24. doi: 10.1016/s0923-1811(03)00070-7. [DOI] [PubMed] [Google Scholar]
- 22.McGillis JP, Mitsuhashi M, Payan DG. Immunologic Properties of Substance PPsychoneuroimmunology. New York: Academic Press, Inc.; 1991. pp. 209–23. [Google Scholar]
- 23.Ansel JC, Kaynard AH, Armstrong CA, Olerud J, Bunnett N, Payan D. Skin–nervous system interactions. J Invest Dermatol. 1996;106:198–204. doi: 10.1111/1523-1747.ep12330326. [DOI] [PubMed] [Google Scholar]
- 24.Mantyh PW. Neurobiology of Substance P and the NK1 receptor. J Clin Psychiatry. 2002;63(Suppl. 11):6–10. [PubMed] [Google Scholar]
- 25.Belvisi MG. Sensory nerves and airway inflammation. Role of Aδ and C-fibres. Pulm Pharmacol Ther. 2003;16:1–7. doi: 10.1016/S1094-5539(02)00180-3. [DOI] [PubMed] [Google Scholar]
- 26.Auais A, Adkins B, Napchan G, Piedimonte G. Immunomodulatory effects of sensory nerves during respiratory syncytial virus infection in rats. Am J Physiol Lung Cell Mol Physiol. 2003;285:L105–13. doi: 10.1152/ajplung.00004.2003. [DOI] [PubMed] [Google Scholar]
- 27.Darsow U, Ring J. Neuroimmune interactions in the skin. Curr Opin Allergy Clin Immunol. 2001;5:435–9. doi: 10.1097/01.all.0000011057.09816.61. [DOI] [PubMed] [Google Scholar]
- 28.Saade NE, Massaad CA, Ochoa-Chaar CI, Jabbur SJ, Safieh-Garabedian B, Atweh SF. Upregulation of proinflammatory cytokines and nerve growth factor by intraplantar injection of capsaicin in rats. J Physiol (Lond) 2002;545:241–53. doi: 10.1113/jphysiol.2002.028233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Veronesi B, Sailstad DM, Doerfler DL, Selgrade M. Neuropeptide modulation of chemically induced skin irritation. Toxicol Appl Pharmacol. 1995;135:258–67. doi: 10.1006/taap.1995.1232. [DOI] [PubMed] [Google Scholar]
- 30.Pernow B. Role of tachykinins in neurogenic inflammation. J Immunol. 1985;135:812S–5S. [PubMed] [Google Scholar]
- 31.Luger TA. Neuromediators – a crucial component of the skin immune system. J Dermatol Sci. 2002;30:87–93. doi: 10.1016/s0923-1811(02)00103-2. [DOI] [PubMed] [Google Scholar]
- 32.Steinhoff M. Modern aspects of cutaneous neurogenic inflammation. Arch Dermatol. 2003;139:1479–88. doi: 10.1001/archderm.139.11.1479. [DOI] [PubMed] [Google Scholar]
- 33.Luger TA, Lotti T. Neuropeptides: role in inflammatory skin diseases. J Eur Acad Dermatol Venerol. 1998;10:207–11. [PubMed] [Google Scholar]
- 34.Brain SD. Sensory neuropeptides: their role in inflammation and wound healing. Immunopharmacology. 1997;37:133–52. doi: 10.1016/s0162-3109(97)00055-6. [DOI] [PubMed] [Google Scholar]
- 35.Miao FJ, Janig W, Levine J. Role of sympathetic postganglionic neurons in synovial plasma extravasation induced by bradykinin. J Neurophysiol. 1996;75:715–24. doi: 10.1152/jn.1996.75.2.715. [DOI] [PubMed] [Google Scholar]
- 36.Tonkoff W. Zur Kenntnis der Nerven der Lymph-drüsen. ActaAnz. 1899;16:456–459. [Google Scholar]
- 37.Novotny GEK, Kliche KO. Innervation of lymph nodes: a combined silver impregnation and electron-microscopic study. Acta Anat. 1986;127:243–8. doi: 10.1159/000146293. [DOI] [PubMed] [Google Scholar]
- 38.Felten DL, Felten SY, Carlson SL, Olschowka JA, Livnat S. Noradrenergic and peptidergic innervation of lymphoid tissue. J Immunol. 1985;135:755S–65S. [PubMed] [Google Scholar]
- 39.Villaro AC, Sesma MP, Vazquez JJ. Innervation of mouse lymph nodes: nerve endings on muscular vessels and reticular cells. Am J Anat. 1987;179:175–85. doi: 10.1002/aja.1001790210. [DOI] [PubMed] [Google Scholar]
- 40.Panuncio AL, De La Pena S, Gualco G, Reissenweber N. Adrenergic innervation in reactive human lymph nodes. J Anat. 1998;194:143–6. doi: 10.1046/j.1469-7580.1999.19410143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bellinger DL, Lorton D, Horn L, Brouxhon S, Felten SY, Felten DL. Vasoactive intestinal polypeptide (VIP) innervation of rat spleen, thymus, and lymph nodes. Peptides. 1997;18:1139–49. doi: 10.1016/s0196-9781(97)00075-2. [DOI] [PubMed] [Google Scholar]
- 42.Felten SY, Felten DL. Innervation of Lymphoid TissuePsychoneuroimmunology. New York: Academic Press, Inc.; 1991. pp. 27–69. [Google Scholar]
- 43.Esquifino AI, Castrillon PO, Chacon F, Cutrera R, Cardinali DP. Effect of local sympathectomy on 24-h changes in mitogenic responses and lymphocyte subset populations in rat submaxillary lymph nodes during the preclinical phase of Freund's adjuvant arthritis. Brain Res. 2001;888:227–34. doi: 10.1016/s0006-8993(00)03060-2. [DOI] [PubMed] [Google Scholar]
- 44.Kannan Y, Bienenstock J, Ohta M, Stanisz AM, Stead RH. Nerve growth factor and cytokines mediate lymphoid tissue-induced neurite outgrowth from mouse superior cervical ganglia in vitro. J Immunol. 1996;156:313–20. [PubMed] [Google Scholar]
- 45.Novotny GEK, Heuer T, Schottelndreier A, Fleisgarten C. Plasticity of innervation of the medulla of axillary lymph nodes in the rat after antigenic stimulation. Anat Record. 1994;238:213–24. doi: 10.1002/ar.1092380208. [DOI] [PubMed] [Google Scholar]
- 46.Kinkelin I, Mötzing S, Koltzenburg M, Bröcker E-B. Increase in NGF content and nerve fiber sprouting in human allergic contact eczema. Cell Tissue Res. 2000;302:31–7. doi: 10.1007/s004410000202. [DOI] [PubMed] [Google Scholar]
- 47.Jiang W-Y, Raychaudhuri SP, Farber EM. Double-labeled immunofluorescence study of cutaneous nerves in psoriasis. Int J Dermatol. 1998;37:572–4. doi: 10.1046/j.1365-4362.1998.00533.x. [DOI] [PubMed] [Google Scholar]
- 48.Straub RH. Complexity of the bi-directional neuroimmune junction in the spleen. Trends Pharmacol Sci. 2004;25:640–6. doi: 10.1016/j.tips.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 49.Prinz M, Heikenwalder M, Junt T, et al. Positioning of follicular dendritic cells within the spleen controls prion neuroinvasion. Nature. 2003;425:957–62. doi: 10.1038/nature02072. [DOI] [PubMed] [Google Scholar]
- 50.Benschop RJ, Rodriguez-Feuerhahn M, Schedlowski M. Catecholamine-induced leukocytosis: early observations, current research, and future directions. Brain Behav Immun. 1996;10:77–91. doi: 10.1006/brbi.1996.0009. [DOI] [PubMed] [Google Scholar]
- 51.Elenkov IJ, Wilder RL, Chrousos GP, Vizi ES. The sympathetic nerve – an integrative interface between two supersystems. The brain and the immune system. Pharmacol Rev. 2000;52:595–638. [PubMed] [Google Scholar]
- 52.Ottaway CA, Husband AJ. The influence of neuroendocrine pathways on lymphocyte migration. Immunol Today. 1994;15:511–7. doi: 10.1016/0167-5699(94)90206-2. [DOI] [PubMed] [Google Scholar]
- 53.Benschop RJ, Schedlowski M, Wienecke H, Jacobs R, Schmidt RE. Adrenergic control of natural killer cell circulation and adhesion. Brain Behav Immun. 1997;11:321–32. doi: 10.1006/brbi.1997.0499. [DOI] [PubMed] [Google Scholar]
- 54.Bedoui S, Miyake S, Straub RH, von Horsten S, Yamamura T. More sympathy for autoimmunity with neuropeptide Y? Trends Immunol. 2004;25:508–12. doi: 10.1016/j.it.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 55.Bedoui S, Kawamura N, Straub RH, Pabst R, Yamamura T, Von Horsten S. Relevance of neuropeptide Y for the neuroimmune crosstalk. J Neuroimmunol. 2003;134:1–11. doi: 10.1016/s0165-5728(02)00424-1. [DOI] [PubMed] [Google Scholar]
- 56.Hennig J, Netter P, Voigt K-H. Cortisol mediates redistribution of CD8+ but not of CD56+ cells after the psychological stress of public speaking. Psychoneuroendocrinology. 2001;26:673. doi: 10.1016/s0306-4530(01)00020-8. [DOI] [PubMed] [Google Scholar]
- 57.Chrousos GP, Gold PW. The concepts of stress and stress system disorders. Overview of physical and behavioral homeostasis. JAMA. 1992;267:1244–52. [PubMed] [Google Scholar]
- 58.Moore TC. Anesthesia-associated depression in lymphocyte traffic and its modulation. Am J Surg. 1984;147:807–12. doi: 10.1016/0002-9610(84)90207-1. [DOI] [PubMed] [Google Scholar]
- 59.Melamed R, Bar-Yosef S, Shakhar G, Shakhar K, Ben-Eliyahu S. Suppression of natural killer cell activity and promotion of tumor metastasis by ketamine, thiopental, and halothane, but not by propofol: mediating mechanisms and prophylactic measures. Anesth Analg. 2003;97:1331–9. doi: 10.1213/01.ANE.0000082995.44040.07. [DOI] [PubMed] [Google Scholar]
- 60.McHale NG, Thornbury KD. Sympathetic stimulation causes increased output of lymphocytes from the popliteal node in anaesthetized sheep. Exp Physiol. 1990;75:847–50. doi: 10.1113/expphysiol.1990.sp003467. [DOI] [PubMed] [Google Scholar]
- 61.von der Weid P-Y, Zawieja DC. Lymphatic smooth muscle: the motor unit of lymph drainage. Int J Biochem Cell Biol. 2004;36:1147–53. doi: 10.1016/j.biocel.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 62.Moore TC. Modification of lymphocyte traffic by vasoactive neurotransmitter substances. Immunology. 1984;52:511–8. [PMC free article] [PubMed] [Google Scholar]
- 63.Moore TC, Lami JL, Spruck CH. Substance P increases lymphocyte traffic and lymph flow through peripheral lymph nodes of sheep. Immunology. 1989;67:109–14. [PMC free article] [PubMed] [Google Scholar]
- 64.Levite M, Cahalon L, Hershkoviz R, Steinman L, Lider O. Neuropeptides, via specific receptors, regulate T cell adhesion to fibronectin. J Immunol. 1998;160:993–1000. [PubMed] [Google Scholar]
- 65.Levite M. Nerve-driven immunity. The direct effects of neurotransmitters on T-cell function. Ann NY Acad Sci. 2000;917:307–21. doi: 10.1111/j.1749-6632.2000.tb05397.x. [DOI] [PubMed] [Google Scholar]
- 66.Levite M, Chowers Y, Ganor Y, Besser M, Hershkovits R, Cahalon L. Dopamine interacts directly with its D3 and D2 receptors on normal human T cells, and activates α1 integrin function. Eur J Immunol. 2001;31:3504–12. doi: 10.1002/1521-4141(200112)31:12<3504::aid-immu3504>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 67.Lindsey KQ, Caughman SW, Olerud JE, Bunnett NW, Armstrong CA, Ansel JC. Neural regulation of endothelial cell-mediated inflammation. J Invest Dermatol Symp Proc. 2000;5:74–8. doi: 10.1046/j.1087-0024.2000.00013.x. [DOI] [PubMed] [Google Scholar]
- 68.Quinlan KL, Song I-S, Bunnett NW, et al. Neuropeptide regulation of human dermal microvascular endothelial cell ICAM-1 expression and function. Am J Physiol – Cell Physiol. 1998;275:C1580–90. doi: 10.1152/ajpcell.1998.275.6.C1580. [DOI] [PubMed] [Google Scholar]
- 69.Quinlan KL, Song I-S, Naik SM, et al. VCAM-1 expression on human dermal microvascular endothelial cells is directly and specifically up-regulated by Substance P. J Immunol. 1999;162:1656–61. [PubMed] [Google Scholar]
- 70.Viac J, Gueniche A, Doutremepuich JD, Reichert U, Claudy A, Schmitt D. Substance P and keratinocyte activation markers: an in vitro approach. Arch Dermatol Res. 1996;288:85–90. doi: 10.1007/BF02505049. [DOI] [PubMed] [Google Scholar]
- 71.Dianzani C, Collino M, Lombardi G, Garbarino G, Fantozzi R. Substance P increases neutrophil adhesion to human umbilical vein endothelial cells. Br J Pharmacol. 2003;139:1103–10. doi: 10.1038/sj.bjp.0705344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Helme RD, Eglezos A, Hosking CS. Substance P induces chemotaxis of neutrophils in normal and capsaicin-treated rats. Immunol Cell Biol. 1987;65:267–9. doi: 10.1038/icb.1987.30. [DOI] [PubMed] [Google Scholar]
- 73.Harvath L, Robbins JD, Russell AA, Seamon KB. cAMP and human neutrophil chemotaxis. Elevation of cAMP differentially affects chemotactic responsiveness. J Immunol. 1991;146:224–32. [PubMed] [Google Scholar]
- 74.Cao T, Pinter E, Al-Rashed S, Gerard N, Hoult JR, Brain SD. Neurokinin-1 receptor agonists are involved in mediating neutrophil accumulation in the inflamed, but not normal, cutaneous microvasculature: an in vivo study using neurokinin-1 receptor knockout mice. J Immunol. 2000;164:5424–9. doi: 10.4049/jimmunol.164.10.5424. [DOI] [PubMed] [Google Scholar]
- 75.Ottaway CA, Greenberg GR. Interaction of vasoactive intestinal peptide with mouse lymphocytes: specific binding and the modulation of mitogen responses. J Immunol. 1984;132:417–23. [PubMed] [Google Scholar]
- 76.Ottaway CA. Selective effects of vasoactive intestinal peptide on the mitogenic response of murine T cells. Immunology. 1987;62:291–7. [PMC free article] [PubMed] [Google Scholar]
- 77.Delgado M. VIP: a very important peptide in T helper differentiation. Trends Immunol. 2003;24:221–4. doi: 10.1016/s1471-4906(03)00069-3. [DOI] [PubMed] [Google Scholar]
- 78.Delgado M, Leceta J, Gomariz RP, Ganea D. Vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide stimulate the induction of Th2 responses by up-regulating B7.2 expression. J Immunol. 1999;163:3629–35. [PubMed] [Google Scholar]
- 79.Delgado M, Ganea D. Cutting edge: is vasoactive intestinal peptide a type 2 cytokine? J Immunol. 2001;166:2907–12. doi: 10.4049/jimmunol.166.5.2907. [DOI] [PubMed] [Google Scholar]
- 80.Borovikova LV, Ivanova S, Zhang M, et al. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature. 2000;405:458–62. doi: 10.1038/35013070. [DOI] [PubMed] [Google Scholar]
- 81.Grando SA. Biological functions of keratinocyte cholinergic receptors. J Invest Dermatol Symp Proc. 1997;2:41–8. doi: 10.1038/jidsymp.1997.10. [DOI] [PubMed] [Google Scholar]
- 82.Dussor GO, Helesic G, Hargreaves KM, Flores CM. Cholinergic modulation of nociceptive responses in vivo and neuropeptide release in vitro at the level of the primary sensory neuron. Pain. 2004;107:22–32. doi: 10.1016/j.pain.2003.09.022. [DOI] [PubMed] [Google Scholar]
- 83.Aicher A, Heeschen C, Mohaupt M, Zeiher AM, Dimmeler S. Nicotine strongly activates dendritic cell-mediated adaptive immunity. Potential role for progression of atherosclerotic lesions. Circulation. 2003;107:604–11. doi: 10.1161/01.cir.0000047279.42427.6d. [DOI] [PubMed] [Google Scholar]
- 84.Church MK, el-Lati S, Caulfield JP. Neuropeptide-induced secretion from human skin mast cells. Int Arch Allergy Appl Immunol. 1991;94:310–8. doi: 10.1159/000235393. [DOI] [PubMed] [Google Scholar]
- 85.Ansel JC, Brown JR, Payan DG, Brown MA. Substance P selectively activates TNF-alpha gene expression in murine mast cells. J Immunol. 1993;150:4478–85. [PubMed] [Google Scholar]
- 86.Gordon J, Barnes NM. Lymphocytes transport serotonin and dopamine: agony or ecstasy? Trends Immunol. 2003;24:438–43. doi: 10.1016/s1471-4906(03)00176-5. [DOI] [PubMed] [Google Scholar]
- 87.Botchkarev VA, Eichmuller S, Peters EMJ, Pietsch P, Johansson O, Maurer M, Paus R. A simple immunofluorescence technique for simultaneous visualization of mast cells and nerve fibers reveals selectivity and hair cycle-dependent changes in mast cell–nerve fiber contacts in murine skin. Arch Dermatol Res. 1997;289:292–302. doi: 10.1007/s004030050195. [DOI] [PubMed] [Google Scholar]
- 88.Artico M, Cavallotti C, Cavallotti D. Adrenergic nerve fibres and mast cells: correlation in rat thymus. Immunol Lett. 2002;84:69–76. doi: 10.1016/s0165-2478(02)00145-1. [DOI] [PubMed] [Google Scholar]
- 89.Blennerhassett MG, Bienenstock J. Sympathetic nerve contact causes maturation of mast cells in vitro. J Neurobiol. 1998;35:173–82. doi: 10.1002/(sici)1097-4695(199805)35:2<173::aid-neu4>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 90.Ioffreda MD, Whitaker D, Murphy GF. Mast cell degranulation upregulates alpha 6 integrins on epidermal Langerhans' cells. J Invest Dermatol. 1993;101:150–4. doi: 10.1111/1523-1747.ep12363632. [DOI] [PubMed] [Google Scholar]
- 91.Asahina A, Moro O, Hosoi J, Lerner E, Xu S, Takashima A, Granstein R. Specific induction of cAMP in Langerhans' cells by calcitonin gene-related peptide: relevance to functional effects. Proc Natl Acad Sci USA. 1995;92:8323–7. doi: 10.1073/pnas.92.18.8323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Niizeki H, Kurimoto I, Streilein JW. A Substance P agonist acts as an adjuvant to promote hapten-specific skin immunity. J Invest Dermatol. 1999;112:437–42. doi: 10.1046/j.1523-1747.1999.00534.x. [DOI] [PubMed] [Google Scholar]
- 93.Asahina A, Hosoi J, Beissert S, Stratigos A, Granstein RD. Inhibition of the induction of delayed-type and contact hypersensitivity by calcitonin gene-related peptide. J Immunol. 1995;154:3056–61. [PubMed] [Google Scholar]
- 94.Carucci JA, Ignatius R, Wei Y, Cypess AM, Schaer DA, Pope M, Steinman RM, Mojsov S. Calcitonin gene-related peptide decreases expression of HLA-DR and CD86 by human dendritic cells and dampens dendritic cell-driven T cell-proliferative responses via the Type I calcitonin gene-related peptide receptor. J Immunol. 2000;164:3494–9. doi: 10.4049/jimmunol.164.7.3494. [DOI] [PubMed] [Google Scholar]
- 95.Torii H, Tamaki K, Granstein RD. The effect of neuropeptides/hormones on Langerhans' cells. J Dermatol Sci. 1999;20:21–8. doi: 10.1016/s0923-1811(99)00004-3. [DOI] [PubMed] [Google Scholar]
- 96.Nong YH, Titus RG, Ribeiro JM, Remold HG. Peptides encoded by the calcitonin gene inhibit macrophage function. J Immunol. 1989;143:45–9. [PubMed] [Google Scholar]
- 97.Gaudillere A, Misery L, Souchier C, Claudy A, Schmitt D. Intimate associations between PGP9.5-positive nerve fibres and Langerhans' cells. Br J Dermatol. 1996;135:343–4. doi: 10.1111/j.1365-2133.1996.tb01191.x. [DOI] [PubMed] [Google Scholar]
- 98.Misery L. Langerhans' cells in the neuro-immuno-cutaneous system. J Neuroimmunol. 1998;89:83–7. doi: 10.1016/s0165-5728(98)00117-9. [DOI] [PubMed] [Google Scholar]
- 99.Kitazawa T, Streilein JW. Hapten-specific tolerance promoted by calcitonin gene-related peptide. J Invest Dermatol. 2000;115:942–8. doi: 10.1046/j.1523-1747.2000.00155.x. [DOI] [PubMed] [Google Scholar]
- 100.Wang F, Millet I, Bottomly K, Vignery A. Calcitonin gene-related peptide inhibits interleukin 2 production by murine T lymphocytes. J Biol Chem. 1992;267:21052–7. [PubMed] [Google Scholar]
- 101.Fox FE, Kubin M, Cassin M, et al. Calcitonin gene-related peptide inhibits proliferation and antigen presentation by human peripheral blood mononuclear cells: effects on B7, interleukin 10, and interleukin 12. J Invest Dermatol. 1997;108:43–8. doi: 10.1111/1523-1747.ep12285627. [DOI] [PubMed] [Google Scholar]
- 102.Wang H, Xing L, Li W, Hou L, Guo J, Wang X. Production and secretion of calcitonin gene-related peptide from human lymphocytes. J Neuroimmunol. 2002;130:155–62. doi: 10.1016/s0165-5728(02)00221-7. [DOI] [PubMed] [Google Scholar]
- 103.Xing L, Guo J, Wang X. Induction and expression of α-calcitonin gene-related peptide in rat T lymphocytes and its significance. J Immunol. 2000;165:4359–66. doi: 10.4049/jimmunol.165.8.4359. [DOI] [PubMed] [Google Scholar]
- 104.Torii H, Yan Z, Hosoi J, Granstein RD. Expression of neurotrophic factors and neuropeptide receptors by Langerhans' cells and the Langerhans' cell-like cell line XS52: further support for a functional relationship between Langerhans' cells and epidermal nerves. J Invest Dermatol. 1997;109:586–91. doi: 10.1111/1523-1747.ep12337516. [DOI] [PubMed] [Google Scholar]
- 105.Kodali S, Friedman I, Ding W, Seiffert K, Wagner JA, Granstein RD. Pituitary adenylate cyclase-activating polypeptide inhibits cutaneous immune function. Eur J Immunol. 2003;33:3070–9. doi: 10.1002/eji.200324085. [DOI] [PubMed] [Google Scholar]
- 106.Ho W-Z, Lai JP, Zhu XH, Uvaydova M, Douglas SD. Human monocytes and macrophages express substance P and neurokinin-1 receptor. J Immunol. 1997;159:5654–60. [PubMed] [Google Scholar]
- 107.Ho W-Z, Stavropoulos G, Lai J-P, Hu B-F, Magafa V, Anagnostides S, Douglas SD. Substance P C-terminal octapeptide analogues augment tumor necrosis factor-[alpha] release by human blood monocytes and macrophages. J Neuroimmunol. 1998;82:126–32. doi: 10.1016/s0165-5728(97)00175-6. [DOI] [PubMed] [Google Scholar]
- 108.Lotz M, Vaughan JH, Carson DA. Effect of neuropeptides on production of inflammatory cytokines by human monocytes. Science. 1988;241:1218–21. doi: 10.1126/science.2457950. [DOI] [PubMed] [Google Scholar]
- 109.Kincy-Cain T, Bost KL. Substance P-induced IL-12 production by murine macrophages. J Immunol. 1997;158:2334–9. [PubMed] [Google Scholar]
- 110.Song I-S, Bunnett NW, Olerud JE, et al. Substance P induction of murine keratinocyte PAM 212 interleukin 1 production is mediated by the neurokinin 2 receptor (NK-2R) Exp Dermatol. 2000;9:42–52. doi: 10.1034/j.1600-0625.2000.009001042.x. [DOI] [PubMed] [Google Scholar]
- 111.Payan DG, Brewster DR, Missirian-Bastian A, Goetzl EJ. Substance P recognition by a subset of human T lymphocytes. J Clin Invest. 1984;74:1532–9. doi: 10.1172/JCI111567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Stanisz AM, Befus D, Bienenstock J. Differential effects of vasoactive intestinal peptide, substance P, and somatostatin on immunoglobulin synthesis and proliferations by lymphocytes from Peyer's patches, mesenteric lymph nodes, and spleen. J Immunol. 1986;136:152–6. [PubMed] [Google Scholar]
- 113.Lai J-P, Douglas SD, Ho W-Z. Human lymphocytes express substance P and its receptor. J Neuroimmunol. 1998;86:80–6. doi: 10.1016/s0165-5728(98)00025-3. [DOI] [PubMed] [Google Scholar]
- 114.Lai J-P, Douglas SD, Shaheen F, Pleasure DE, Ho W-Z. Quantification of Substance P mRNA in human immune cells by real-time reverse transcriptase PCR assay. Clin Diagn Lab Immunol. 2002;9:138–43. doi: 10.1128/CDLI.9.1.138-143.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lai J-P, Douglas SD, Zhao M, Ho W-Z. Quantification of substance P mRNA in human mononuclear phagocytes and lymphocytes using a mimic-based RT-PCR. J Immunol Methods. 1999;230:149–57. doi: 10.1016/s0022-1759(99)00120-9. [DOI] [PubMed] [Google Scholar]
- 116.Payan DG, Brewster DR, Goetzl EJ. Specific stimulation of human T lymphocytes by Substance P. J Immunol. 1983;131:1613–5. [PubMed] [Google Scholar]
- 117.Payan DG, Goetzl EJ. Modulation of lymphocyte function by sensory neuropeptides. J Immunol. 1985;135:783S–6S. [PubMed] [Google Scholar]
- 118.Chavolla-Calderón M, Bayer MK, Perez Fontan JJ. Bone marrow transplantation reveals an essential synergy between neuronal and hemopoietic cell neurokinin production in pulmonary inflammation. J Clin Invest. 2003;111:973–80. doi: 10.1172/JCI17458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Richards AM, Mitsou J, Floyd DC, Terenghi G, McGrouther DA. Neural innervation and healing. Lancet. 1997;350:339–40. doi: 10.1016/s0140-6736(05)63391-0. [DOI] [PubMed] [Google Scholar]
- 120.Richards AM, Floyd DC, Terenghi G, McGrouther DA. Cellular changes in denervated tissue during wound healing in a rat model. Br J Dermatol. 1999;140:1093–9. doi: 10.1046/j.1365-2133.1999.02908.x. [DOI] [PubMed] [Google Scholar]
- 121.Graham GJ, Stevens JM, Page NM, Grant AD, Brain SD, Lowry PJ, Gibbins JM. Tachykinins regulate the function of platelets. Blood. 2004;104:1058–65. doi: 10.1182/blood-2003-11-3979. [DOI] [PubMed] [Google Scholar]
- 122.Hermanson A, Dalsgaard CJ, Bjorklund H, Lindblom U. Sensory reinnervation and sensibility after superficial skin wounds in human patients. Neurosci Lett. 1987;74:377–82. doi: 10.1016/0304-3940(87)90327-2. [DOI] [PubMed] [Google Scholar]
- 123.Goetzl EJ, Turck CW, Sreedharan SP. Production and Recognition of Neuropeptides by Cells of the Immune SystemPsychoneuroimmunology. New York: Academic Press, Inc.; 1991. pp. 263–82. [Google Scholar]
- 124.Scicchitano R, Dazin P, Bienenstock J, Payan DG, Stanisz AM. Distribution of somatostatin receptors on murine spleen and Peyer's patch T and B lymphocytes. Brain Behav Immun. 1987;1:173–84. doi: 10.1016/0889-1591(87)90019-5. [DOI] [PubMed] [Google Scholar]
- 125.Slominski A, Roloff B, Curry J, Dahiya M, Szczesniewski A, Wortsman J. The skin produces urocortin. J Clin Endocrinol Metab. 2000;85:815–23. doi: 10.1210/jcem.85.2.6381. [DOI] [PubMed] [Google Scholar]
- 126.Slominski A, Wortsman J. Neuroendocrinology of the skin. Endocr Rev. 2000;21:457–87. doi: 10.1210/edrv.21.5.0410. [DOI] [PubMed] [Google Scholar]
- 127.Slominski A, Wortsman J, Luger T, Paus R, Solomon S. Corticotropin releasing hormone and proopiomelanocortin involvement in the cutaneous response to stress. Physiol Rev. 2000;80:979–1020. doi: 10.1152/physrev.2000.80.3.979. [DOI] [PubMed] [Google Scholar]
- 128.Slominski A, Wortsman J, Pisarchik A, Zbytek B, Linton EA, Mazurkiewicz JE, Wei ET. Cutaneous expression of corticotropin-releasing hormone (CRH), urocortin, and CRH receptors. FASEB J. 2001;15:1678–93. doi: 10.1096/fj.00-0850rev. [DOI] [PubMed] [Google Scholar]
- 129.Slominski A, Zbytek B, Semak I, Sweatman T, Wortsman J. CRH stimulates POMC activity and corticosterone production in dermal fibroblasts. J Neuroimmunol. 2005;162:97–102. doi: 10.1016/j.jneuroim.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 130.Slominski A, Zbytek B, Szczesniewski A, Semak I, Kaminski J, Sweatman T, Wortsman J. CRH stimulation of corticosteroids production in melanocytes is mediated by ACTH. Am J Physiol Endocrinol Metab. 2005;288:E701–6. doi: 10.1152/ajpendo.00519.2004. [DOI] [PubMed] [Google Scholar]
- 131.Slominski AT, Botchkarev V, Choudhry M, et al. Cutaneous expression of CRH and CRH-R. Is there a ‘skin stress response system?’. Ann NY Acad Sci. 1999;885:287–311. doi: 10.1111/j.1749-6632.1999.tb08686.x. [DOI] [PubMed] [Google Scholar]
- 132.Besedovsky H, del Rey A. Immune–neuro–endocrine interactions: facts and hypotheses. Endocr Rev. 1996;17:64–102. doi: 10.1210/edrv-17-1-64. [DOI] [PubMed] [Google Scholar]
- 133.Schallreuter KU, Pittelkow MR, Swanson NN, Beazley WD, Korner C, Ehrke C, Buttner G. Altered catecholamine synthesis and degradation in the epidermis of patients with atopic eczema. Arch Dermatol Res. 1997;289:663–6. doi: 10.1007/s004030050258. [DOI] [PubMed] [Google Scholar]
- 134.Schallreuter KU. Epidermal adrenergic signal transduction as part of the neuronal network in the human epidermis. J Invest Dermatol Symp Proc. 1997;2:37–40. doi: 10.1038/jidsymp.1997.9. [DOI] [PubMed] [Google Scholar]
- 135.Seiffert K, Hosoi J, Torii H, Ozawa H, Ding W, Campton K, Wagner JA, Granstein RD. Catecholamines inhibit the antigen-presenting capability of epidermal Langerhans' cells. J Immunol. 2002;168:6128–35. doi: 10.4049/jimmunol.168.12.6128. [DOI] [PubMed] [Google Scholar]
- 136.Bissonnette EY, Befus AD. Anti-inflammatory effect of beta 2-agonists: inhibition of TNF-alpha release from human mast cells. J Allergy Clin Immunol. 1997;100:825–31. doi: 10.1016/s0091-6749(97)70280-x. [DOI] [PubMed] [Google Scholar]
- 137.Maestroni GJM. Modulation of skin norepinephrine turnover by allergen sensitization: impact on contact hypersensitivity and T helper priming. J Invest Dermatol. 2004;122:119–24. doi: 10.1046/j.0022-202X.2003.22132.x. [DOI] [PubMed] [Google Scholar]
- 138.Durnett Richardson J, Vasko MR. Cellular mechanisms of neurogenic inflammation. J Pharmacol Exp Ther. 2002;302:839–45. doi: 10.1124/jpet.102.032797. [DOI] [PubMed] [Google Scholar]
- 139.Beresford L, Orange O, Bell EB, Miyan JA. Nerve fibres are required to evoke a contact sensitivity response in mice. Immunology. 2004;111:118–25. doi: 10.1111/j.1365-2567.2003.01786.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Simone DA, Nolano M, Johnson T, Wendelschafer-Crabb G, Kennedy WR. Intradermal injection of capsaicin in humans produces degeneration and subsequent reinnervation of epidermal nerve fibers: correlation with sensory function. J Neurosci. 1998;18:8947–59. doi: 10.1523/JNEUROSCI.18-21-08947.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Burks TF, Buck SH, Miller MS. Mechanisms of depletion of substance P by capsaicin. Federation Proc. 1985;44:2531–4. [PubMed] [Google Scholar]
- 142.Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–24. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- 143.Julius D, Basbaum AI. Molecular mechanisms of nociception. Nature. 2001;413:203–10. doi: 10.1038/35093019. [DOI] [PubMed] [Google Scholar]
- 144.Bodo E, Kovacs I, Telek A, Varga A, Paus R, Kovacs L, Biro T. Vanilloid receptor-1 (VR1) is widely expressed on various epithelial and mesenchymal cell types of human skin. J Invest Dermatol. 2004;123:410–3. doi: 10.1111/j.0022-202X.2004.23209.x. [DOI] [PubMed] [Google Scholar]
- 145.Southall MD, Li T, Gharibova LS, Pei Y, Nicol GD, Travers JB. Activation of epidermal vanilloid receptor-1 induces release of proinflammatory mediators in human keratinocytes. J Pharmacol Exp Ther. 2003;304:217–22. doi: 10.1124/jpet.102.040675. [DOI] [PubMed] [Google Scholar]
- 146.Inoue K, Koizumi S, Fuziwara S, Denda S, Inoue K, Denda M. Functional vanilloid receptors in cultured normal human epidermal keratinocytes. Biochem Biophys Res Commun. 2002;291:124–9. doi: 10.1006/bbrc.2002.6393. [DOI] [PubMed] [Google Scholar]
- 147.Biro T, Maurer M, Modarres S, et al. Characterization of functional vanilloid receptors expressed by mast cells. Blood. 1998;91:1332–40. [PubMed] [Google Scholar]
- 148.Lorton D, Lubahn C, Engan C, Schaller J, Felten DL, Bellinger DL. Local application of capsaicin into the draining lymph nodes attenuates expression of adjuvant-induced arthritis. Neuroimmunomodulation. 2000;7:115–25. doi: 10.1159/000026429. [DOI] [PubMed] [Google Scholar]
- 149.Shepherd AJ, Beresford L, Bell EB, Miyan JA. Mobilisation of specific T cells from lymph nodes in contact sensitivity requires substance P. J Neuroimmunol. 2005 doi: 10.1016/j.jneuroim.2005.04.008. in press. [DOI] [PubMed] [Google Scholar]
- 150.Yamazaki K, Allen TD. Ultrastructural morphometric study of efferent nerve terminals on murine bone marrow stromal cells, and the recognition of a novel anatomical unit: the ‘neuro-reticular complex’. Am J Anat. 1990;187:261–76. doi: 10.1002/aja.1001870306. [DOI] [PubMed] [Google Scholar]
- 151.Afan AM, Broome CS, Nicholls SE, Whetton AD, Miyan JA. Bone marrow innervation regulates cellular retention in the murine haemopoietic system. Br J Haematol. 1997;98:569–77. doi: 10.1046/j.1365-2141.1997.2733092.x. [DOI] [PubMed] [Google Scholar]
- 152.Broome CS, Whetton AD, Miyan JA. Neuropeptide control of bone marrow neutrophil production is mediated by both direct and indirect effects on CFU-GM. Br J Haematol. 2000;108:140–50. doi: 10.1046/j.1365-2141.2000.01808.x. [DOI] [PubMed] [Google Scholar]
- 153.Miyan JA, Broome CS, Whetton AD. Neural regulation of bone marrow. Blood. 1998;92:2971–3. [PubMed] [Google Scholar]
- 154.Iversen PO, Hjeltnes N, Holm B, Flatebo T, Strom-Gundersen I, Ronning W, Stanghelle J, Benestad HB. Depressed immunity and impaired proliferation of hematopoietic progenitor cells in patients with complete spinal cord injury. Blood. 2000;96:2081–3. [PubMed] [Google Scholar]
- 155.Livingston DH, Anjaria D, Wu J, Hauser CJ, Chang V, Deitch EA, Rameshwar P. Bone marrow failure following severe injury in humans. Ann Surg. 2003;238:748–53. doi: 10.1097/01.sla.0000094441.38807.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Iversen PO, Nicolaysen A, Hjeltnes N, Nja A, Benestad HB. Preserved granulocyte formation and function, as well as bone marrow innervation, in subjects with complete spinal cord injury. Br J Haematol. 2004;126:870–7. doi: 10.1111/j.1365-2141.2004.05085.x. [DOI] [PubMed] [Google Scholar]
- 157.Undem BJ, Myers AC, Weinreich D. Antigen-induced modulation of autonomic and sensory neurons in vitro. Int Arch Allergy Appl Immunol. 1991;94:319–24. doi: 10.1159/000235394. [DOI] [PubMed] [Google Scholar]
- 158.Weinreich D, Undem BJ. Immunological regulation of synaptic transmission in isolated guinea pig autonomic ganglia. J Clin Invest. 1987;79:1529–32. doi: 10.1172/JCI112984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Opree A, Kress M. Involvement of the proinflammatory cytokines tumor necrosis factor-alpha, IL-1beta, and IL-6, but not IL-8, in the development of heat hyperalgesia: effects on heat-evoked calcitonin gene-related peptide release from rat skin. J Neurosci. 2000;20:6289–93. doi: 10.1523/JNEUROSCI.20-16-06289.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Tarkowski E, Naver H, Wallin BG, Blomstrand C, Tarkowski A. Lateralization of T-lymphocyte responses in patients with stroke: effect of sympathetic dysfunction? Stroke. 1995;26:57–62. doi: 10.1161/01.str.26.1.57. [DOI] [PubMed] [Google Scholar]
- 161.Moshel YA, Durkin HG, Amassian VE. Lateralized neocortical control of T lymphocyte export from the thymus. I. Increased export after left cortical stimulation in behaviorally active rats, mediated by sympathetic pathways in the upper spinal cord. J Neuroimmunol. 2005;158:3–13. doi: 10.1016/j.jneuroim.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 162.Dénes A, Boldogkoi Z, Uhereczky G, Hornyak A, Rusvai M, Palkovits M, Kovacs KJ. Central autonomic control of the bone marrow: multisynaptic tract tracing by recombinant pseudorabies virus. Neuroscience. 2005 doi: 10.1016/j.neuroscience.2005.03.060. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 163.Blalock JE. The immune system as the sixth sense. J Intern Med. 2005;257:126–38. doi: 10.1111/j.1365-2796.2004.01441.x. [DOI] [PubMed] [Google Scholar]
- 164.Levite M. Neuropeptides, by direct interaction with T cells, induce cytokine secretion and break the commitment to a distinct T helper phenotype. Proc Natl Acad Sci USA. 1998;95:12544–9. doi: 10.1073/pnas.95.21.12544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Blum AM, Metwali A, Elliott DE, Weinstock JV. T cell substance P receptor governs antigen-elicited IFN-gamma production. Am J Physiol Gastrointest Liver Physiol. 2003;284:G197–204. doi: 10.1152/ajpgi.00271.2002. [DOI] [PubMed] [Google Scholar]
- 166.van der Kleij HPM, Ma D, Redegeld FAM, Kraneveld AD, Nijkamp FP, Bienenstock J. Functional expression of neurokinin 1 receptors on mast cells induced by IL-4 and stem cell factor. J Immunol. 2003;171:2074–9. doi: 10.4049/jimmunol.171.4.2074. [DOI] [PubMed] [Google Scholar]
- 167.Goode T, O'Connell J, Ho W-Z, O'Sullivan GC, Collins JK, Douglas SD, Shanahan F. Differential expression of neurokinin-1 receptor by human mucosal and peripheral lymphoid cells. Clin Diagn Lab Immunol. 2000;7:371–6. doi: 10.1128/cdli.7.3.371-376.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Goode T, O'Connor T, Hopkins A, et al. Neurokinin-1 receptor (NK-1R) expression is induced in human colonic epithelial cells by proinflammatory cytokines and mediates proliferation in response to substance P. J Cell Physiol. 2003;197:30–41. doi: 10.1002/jcp.10234. [DOI] [PubMed] [Google Scholar]
- 169.Torii H, Hosoi J, Asahina A, Granstein RD. Calcitonin gene-related peptide and Langerhans' cell function. J Invest Dermatol SP. 1997;2:82–6. doi: 10.1038/jidsymp.1997.16. [DOI] [PubMed] [Google Scholar]
- 170.Delgado M, Munoz-Elias EJ, Gomariz RP, Ganea D. Vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide enhance IL-10 production by murine macrophages: in vitro and in vivo studies. J Immunol. 1999;162:1707–16. [PubMed] [Google Scholar]
- 171.Delgado M, Sun W, Leceta J, Ganea D. Vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide differentially regulate the costimulatory activity of resting and activated macrophages through the modulation of B7.1 and B7.2 expression. J Immunol. 1999;163:4213–23. [PubMed] [Google Scholar]
- 172.Lin Q, Zou X, Ren Y, Wang J, Fang L, Willis WD. Involvement of peripheral neuropeptide y receptors in sympathetic modulation of acute cutaneous flare induced by intradermal capsaicin. Neuroscience. 2004;123:337–47. doi: 10.1016/j.neuroscience.2003.09.017. [DOI] [PubMed] [Google Scholar]
- 173.Nair MPN, Schwartz SA, Wu KS, Kronfol Z. Effect of neuropeptide y on natural killer activity of normal human lymphocytes. Brain Behav Immun. 1993;7:70–8. doi: 10.1006/brbi.1993.1007. [DOI] [PubMed] [Google Scholar]
- 174.Schwarz H, Villiger PM, von Kempis J, Lotz M. Neuropeptide Y is an inducible gene in the human immune system. J Neuroimmunol. 1994;51:53–61. doi: 10.1016/0165-5728(94)90128-7. [DOI] [PubMed] [Google Scholar]
- 175.Bedoui S, Kawamura N, Straub RH, Pabst R, Yamamura T, von Horsten S. Relevance of Neuropeptide Y for the neuroimmune crosstalk. J Neuroimmunol. 2003;134:1–11. doi: 10.1016/s0165-5728(02)00424-1. [DOI] [PubMed] [Google Scholar]
- 176.Kimata H, Yoshida A, Fujimoto M, Mikawa H. Effect of vasoactive intestinal peptide, somatostatin, and substance P on spontaneous IgE and IgG4 production in atopic patients. J Immunol. 1993;150:4630–40. [PubMed] [Google Scholar]
- 177.Helyes Z, Szabo A, Nemeth J, et al. Antiinflammatory and analgesic effects of somatostatin released from capsaicin-sensitive sensory nerve terminals in a Freund's adjuvant-induced chronic arthritis model in the rat. Arthritis Rheum. 2004;50:1677–85. doi: 10.1002/art.20184. [DOI] [PubMed] [Google Scholar]
- 178.Lygren I, Revhaug A, Burhol PG, Giercksky KE, Jenssen TG. Vasoactive intestinal polypeptide and somatostatin in leucocytes. Scand J Clin Lab Invest. 1984;44:347–51. doi: 10.3109/00365518409083818. [DOI] [PubMed] [Google Scholar]
- 179.Chen A, Ganor Y, Rahimipour S, Ben-Aroya N, Koch Y, Levite M. The neuropeptides GnRH-II and GnRH-I are produced by human T cells and trigger laminin receptor gene expression, adhesion, chemotaxis and homing to specific organs. Nat Med. 2002;8:1421–6. doi: 10.1038/nm1202-801. [DOI] [PubMed] [Google Scholar]
- 180.Lipton JM, Catania A. Anti-inflammatory actions of the neuroimmunomodulator alpha-MSH. Immunol Today. 1997;18:140–5. doi: 10.1016/s0167-5699(97)01009-8. [DOI] [PubMed] [Google Scholar]
- 181.Grabbe S, Bhardwaj RS, Mahnke K, Simon MM, Schwarz T, Luger TA. alpha-Melanocyte-stimulating hormone induces hapten-specific tolerance in mice. J Immunol. 1996;156:473–8. [PubMed] [Google Scholar]
- 182.Luger TA, Brzoska T, Scholzen TE, Kalden D-H, Sunderkotter C, Armstrong C, Ansel J. The role of alpha-MSH as a modulator of cutaneous inflammation. Ann NY Acad Sci. 2000;917:232–8. doi: 10.1111/j.1749-6632.2000.tb05388.x. [DOI] [PubMed] [Google Scholar]
- 183.Luger TA, Scholzen TE, Brzoska T, Becher E, Slominski A, Paus R. Cutaneous immunomodulation and coordination of skin stress responses by alpha-melanocyte-stimulating hormone. Ann NY Acad Sci. 1998;840:381–94. doi: 10.1111/j.1749-6632.1998.tb09577.x. [DOI] [PubMed] [Google Scholar]
- 184.Bhardwaj R, Becher E, Mahnke K, Hartmeyer M, Schwarz T, Scholzen T, Luger TA. Evidence for the differential expression of the functional alpha-melanocyte-stimulating hormone receptor, MC-1, on human monocytes. J Immunol. 1997;158:3378–84. [PubMed] [Google Scholar]
- 185.Luger TA, Scholzen T, Grabbe S. The role of alpha-melanocyte-stimulating hormone in cutaneous biology. J Invest Dermatol SP. 1997;2:87–93. doi: 10.1038/jidsymp.1997.17. [DOI] [PubMed] [Google Scholar]
- 186.Becher E, Mahnke K, Brzoska T, Kalden DH, Grabbe S, Luger TA. Human peripheral blood-derived dendritic cells express functional melanocortin receptor MC-1R. Ann NY Acad Sci. 1999;885:188–95. doi: 10.1111/j.1749-6632.1999.tb08676.x. [DOI] [PubMed] [Google Scholar]
- 187.Slominski A, Tobin DJ, Shibahara S, Wortsman J. Melanin pigmentation in mammalian skin and its hormonal regulation. Physiol Rev. 2004;84:1155–228. doi: 10.1152/physrev.00044.2003. [DOI] [PubMed] [Google Scholar]
- 188.Maloof PB, Joshi DD, Qian J, Gascon P, Singh D, Rameshwar P. Induction of preprotachykinin-I and neurokinin-1 by adrenocorticotropin and prolactin. Implication for neuroendocrine-immune-hematopoietic axis. J Neuroimmunol. 2001;112:188–96. doi: 10.1016/s0165-5728(00)00405-7. [DOI] [PubMed] [Google Scholar]
- 189.Lyons PD, Blalock JE. The kinetics of ACTH expression in rat leukocyte subpopulations. J Neuroimmunol. 1995;63:103–12. doi: 10.1016/0165-5728(95)00133-6. [DOI] [PubMed] [Google Scholar]
- 190.Slominski A, Wortsman J, Tobin DJ. The cutaneous serotoninergic/melatoninergic system: securing a place under the sun. FASEB J. 2005;19:176–94. doi: 10.1096/fj.04-2079rev. [DOI] [PubMed] [Google Scholar]
- 191.Smith EM, Harbour-McMenamin D, Blalock JE. Lymphocyte production of endorphins and endorphin-mediated immunoregulatory activity. J Immunol. 1985;135:779S–82S. [PubMed] [Google Scholar]
- 192.van Epps DE, Saland L. Beta-endorphin and met-enkephalin stimulate human peripheral blood mononuclear cell chemotaxis. J Immunol. 1984;132:3046–53. [PubMed] [Google Scholar]
- 193.Hosoi J, Ozawa H, Granstein RD. beta-Endorphin binding and regulation of cytokine expression in Langerhans' cells. Ann NY Acad Sci. 1999;885:405–13. doi: 10.1111/j.1749-6632.1999.tb08700.x. [DOI] [PubMed] [Google Scholar]
- 194.Gilmore W, Weiner LP. Beta-endorphin enhances interleukin-2 (IL-2) production in murine lymphocytes. J Neuroimmunol. 1988;18:125–38. doi: 10.1016/0165-5728(88)90061-6. [DOI] [PubMed] [Google Scholar]
- 195.Egeling K, Muller H, Karschunke B, Tschischka M, Spennemann V, Hain B, Teschemacher H. Specific binding sites for {beta}-endorphin on keratinocytes. Ann NY Acad Sci. 1999;885:464–5. doi: 10.1111/j.1749-6632.1999.tb08713.x. [DOI] [PubMed] [Google Scholar]
- 196.Madden KS. Catecholamines, sympathetic innervation, and immunity. Brain Behav Immun. 2003;17:5–10. doi: 10.1016/s0889-1591(02)00059-4. [DOI] [PubMed] [Google Scholar]
- 197.Madden KS, Sanders VM, Felten DL. Catecholamine influences and sympathetic neural modulation of immune responsiveness. Annu Rev Pharmacol Toxicol. 1995;35:417–48. doi: 10.1146/annurev.pa.35.040195.002221. [DOI] [PubMed] [Google Scholar]
- 198.Panina-Bordignon P, Mazzeo D, Lucia PD, D'Ambrosio D, Lang R, Fabbri L, Self C, Sinigaglia F. beta 2-Agonists prevent Th1 development by selective inhibition of interleukin 12. J Clin Invest. 1997;100:1513–9. doi: 10.1172/JCI119674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Avella J, Madsen JE, Binder HJ, Askenase PW. Effect of histamine H2-receptor antagonists on delayed hypersensitivity. Lancet. 1978;311:624–6. doi: 10.1016/s0140-6736(78)91134-0. [DOI] [PubMed] [Google Scholar]
- 200.Elenkov IJ, Webster E, Papanicolaou DA, Fleisher TA, Chrousos GP, Wilder RL. Histamine potently suppresses human IL-12 and stimulates IL-10 production via H2 receptors. J Immunol. 1998;161:2586–93. [PubMed] [Google Scholar]
- 201.Jutel M, Watanabe T, Akdis M, Blaser K, Akdis CA. Immune regulation by histamine. Curr Opin Immunol. 2002;14:735–40. doi: 10.1016/s0952-7915(02)00395-3. [DOI] [PubMed] [Google Scholar]
- 202.Ohtani T, Aiba S, Mizuashi M, Mollah ZU, Nakagawa S, Tagami H. H1 and H2 histamine receptors are absent on Langerhans' cells and present on dermal dendritic cells. J Invest Dermatol. 2003;121:1073–9. doi: 10.1046/j.1523-1747.2003.12570.x. [DOI] [PubMed] [Google Scholar]
- 203.Rinner I, Kawashima K, Schauenstein K. Rat lymphocytes produce and secrete acetylcholine in dependence of differentiation and activation. J Neuroimmunol. 1998;81:31–7. doi: 10.1016/s0165-5728(97)00155-0. [DOI] [PubMed] [Google Scholar]
- 204.Musso NR, Brenci S, Indiveri F, Lotti G. Acetylcholine-induced, calcium-dependent norepinephrine outflow from peripheral human lymphocytes. J Neuroimmunol. 1998;87:82–7. doi: 10.1016/s0165-5728(98)00057-5. [DOI] [PubMed] [Google Scholar]
- 205.Maestroni GJ, Conti A, Pierpaoli W. Role of the pineal gland in immunity. Circadian synthesis and release of melatonin modulates the antibody response and antagonizes the immunosuppressive effect of corticosterone. J Neuroimmunol. 1986;13:19–30. doi: 10.1016/0165-5728(86)90047-0. [DOI] [PubMed] [Google Scholar]
- 206.Kuhlwein E, Irwin M. Melatonin modulation of lymphocyte proliferation and Th1/Th2 cytokine expression. J Neuroimmunol. 2001;117:51–7. doi: 10.1016/s0165-5728(01)00325-3. [DOI] [PubMed] [Google Scholar]
- 207.Granstein RD. The skinny on CD39 in immunity and inflammation. Nat Med. 2002;8:336–8. doi: 10.1038/nm0402-336. [DOI] [PubMed] [Google Scholar]
- 208.Levite M. Nervous immunity: neurotransmitters, extracellular K+ and T-cell function. Trends Immunol. 2001;22:2–5. doi: 10.1016/s1471-4906(00)01799-3. [DOI] [PubMed] [Google Scholar]
- 209.Levite M, Cahalon L, Peretz A, et al. Extracellular K+ and opening of voltage-gated potassium channels activate T cell integrin function: physical and functional association between Kv1.3 channels and {beta}1 integrins. J Exp Med. 2000;191:1167–76. doi: 10.1084/jem.191.7.1167. [DOI] [PMC free article] [PubMed] [Google Scholar]