Abstract
The local immune response is characterized by an increase in the rate of entry of lymphocytes from the blood into regional lymph nodes and changes in the output of cells in lymph. While significant data are available regarding the role of inflammation-induced vascular adhesion processes in regulating lymphocyte entry into inflamed tissues and lymph nodes, relatively little is known about the molecular processes governing lymphocyte exit into efferent lymph. We have defined a novel role for lymphatic endothelial cells in the regulation of lymphocyte exit during a delayed type hypersensitivity (DTH) response to mycobacterial purified protein derivative (PPD). Soluble, pro-adhesive factors were identified in efferent lymph concomitant with reduced lymphocyte output in lymph, which significantly increased lymphocyte binding to lymphatic endothelial cells. While all lymphocyte subsets were retained, CD4+ T cells appeared less susceptible than others. Among a panel of cytokines in inflammatory lymph plasma, interferon (IFN)-γ alone appeared responsible for this retention. In vitro adhesion assays using physiological levels of IFN-γ confirmed the interaction between recirculating lymphocytes and lymphatic endothelium. These data demonstrate a new level of immune regulation, whereby the exit of recirculating lymphocytes from lymph nodes is selectively and sequentially regulated by cytokines in a manner equally as complex as lymphocyte recruitment.
Keywords: delayed hypersensitivity, lymph node, lymphatics, lymphocyte recirculation
Introduction
Lymphocytes recirculate continuously between blood, tissues and the lymphatic system.1 While lymphocytes are capable of recirculating through virtually any vascularized tissue, 90% of traffic occurs through lymph nodes.2 Although these processes were described originally at the whole-animal and organ level, the advent of molecular technologies has allowed the cellular processes regulating the blood-to-tissue migration of lymphocytes to be better defined. It is now clear that lymphocytes, like neutrophils, exit the blood through a regulated, multistep molecular interaction between vascular endothelial cells and lymphocytes.3 However, it is important to remember that the full process of lymphocyte recirculation involves not only blood-to-tissue migration, but also their return to blood via efferent lymph.4 Large animal models offer unique surgical advantages for the study of lymphocyte traffic, in that it is possible to directly cannulate individual lymphatic vessels draining defined lymphoid and non-lymphoid tissues. It is therefore possible to analyse quantitatively and qualitatively the traffic of lymph cells through lymph nodes under physiological conditions for extended periods of time, and as a result more is known about the traffic of cells through lymph nodes in sheep than in any other species.5
Under normal conditions, the output of cells in efferent lymph is remarkably stable, and can in fact be quantified based on organ weight such that 30 × 106 lymphocytes exit per gram of lymph node each hr.2 However, antigen stimulation causes marked, well-documented changes in the exit of recirculating lymphocytes in the efferent lymph of stimulated nodes.6 Virtually all antigens are capable of inducing an increase in cellular output from lymph nodes, but some antigens, including purified protein derivative (PPD) in bacille Calmette–Guérin (BCG)-sensitized animals, also evoke a period of decreased lymphocyte release within hours of antigen injection, which has been termed ‘shut-down’.7,8 Intriguingly, it has been demonstrated clearly that the shut-down response occurs concomitantly with an increased rate of entry of lymphocytes from the blood7 indicating that lymphocytes are retained specifically within the lymph node at this early time-point. Following shut-down, there is a marked increase in the output of lymphocytes from the stimulated lymph node and includes increased numbers of blast cells and antigen specific cells.9 It is hypothesized that increasing lymphocyte traffic into the node enhances the probability of specific lymphocytes encountering their antigen during an immune response, but the precise role and mechanism of shut-down remains unclear.
The mechanisms involved in lymph node shut-down are unresolved; however, it seems likely that exit regulation may parallel entry processes and involve adhesion molecules, chemokines and cytokines. A recent report showed that endothelia isolated from different tissues differentially up-regulated chemokines and adhesion molecules in response to tumour necrosis factor (TNF)-α, IFN-γ and transforming growth factor (TGF)-β.10 A similar effect may be seen in vivo within an antigen-stimulated lymph node, as cytokines are produced and may induce the expression of lymphocyte-specific adhesion molecules on both high endothelial venules and lymphatic endothelium. In culture, TNF-α increases the ability of lymphatic endothelial cells to bind lymphocytes.11 Furthermore, local injection of TNF-α will induce the expression of the endothelial inflammatory adhesion receptor VCAM-1 on lymphatic vessels within the lymph node that may serve to preferentially sequester γδ-T cells.12 Therefore it appears that cytokines, chemokines and endothelia work in concert to increase lymphocyte traffic and retention within lymph nodes during antigen stimulation.
In an effort to uncover potential mediators of shut-down in a physiological model of T cell immunity, we collected efferent lymph from a lymph node draining a PPD-induced delayed type hypersensitivity reaction (DTH). This antigen was chosen as previous reports have demonstrated that PPD reproducibly induces lymph node shut-down.7,8 In order to isolate and identify soluble mediators of shut-down, the effects of cell-free lymph plasma obtained at various stages of lymph node shut-down were screened using an in vitro assay of lymphocyte binding to cultured lymphatic endothelium and levels of soluble cytokines were measured. In an effort to investigate specificity in the shut-down response, we have examined the effects of shut-down on the migration of blood (PBL) and efferent lymph lymphocytes (ELL) through the stimulated lymph node.
Materials and methods
Animals, immunization and antigen stimulation
Randomly bred, adult ewes were obtained from Versuchsbetrieb Sennweid (Olsberg, Switzerland). Handling and treatment of the animals was conducted according to protocols approved by the regional government authority, the Kantonales Veterinäramt. The sheep were immunized with a single injection of BCG equivalent to five times the normal human dose (Institut Serotherapique et Vaccinal Suisse Berne) at least 21 days prior to surgery. To provoke a delayed hypersensitivity lesion, either 50 mg or 100 mg PPD (Statens Seruminstitut) was suspended in 1 ml of saline with 2% Evan's blue and injected intradermally in multiple sites in the drainage area of the cannulated lymph node, as described previously.8 The appearance of Evan's Blue dye in the draining efferent lymph was evidence that the lesion was provoked in the drainage area of the cannulated lymph node.
Surgical procedures
All surgical procedures were performed under aseptic conditions and analgesic was administered as needed postoperatively. Cannulation of prescapular efferent lymphatics was carried out as described previously.13 During surgery, a roentgenography catheter vein with a three-way stopcock attached (Becton Dickinson, Franklin Lakes, NJ) was inserted into the jugular vein, as described previously.13 Lymph was collected into sterile bottles sutured to the skin of the animal, which contained penicillin and heparin as described previously.13 All animals were allowed to recuperate for at least 2 days before injection of PPD.
Cell collection and labelling
Efferent lymph lymphocytes (ELLs) were labelled with either of the lipophilic dyes PKH-2 (Sigma, St. Louis, MO)14 or DiI-DS (Molecular Probes, Eugene, OR)15 as described previously. Mononuclear cells were harvested using centrifugation over Percoll and labelled with carboxyfluorescein diacetate succinimydl ester (CFSE) as described previously.14 Both blood and lymph cells were resuspened in saline for intravenous injection. After re-injection, the concentration of labelled cells was allowed to stabilize in lymph and blood for at least 48 hr before experiments were started.
Cell tracking and immunophenotyping
Lymphocytes were harvested from lymph by centrifugation at 450 g and washed twice with phosphate buffered saline (PBS). Blood cells were harvested by hypotonic lysis using distilled water and washed with PBS. Immunophenotyping was performed as described previously using well-characterized antibodies14 against ovine CD4 [monoclonal antibody (mAb) 17D], CD8 (mAb 7C2), CD21 (mAb 2–87) and γδ T cells (mAb 86D) using a goat-anti-mouse allophycocyanin conjugated secondary antibody (Molecular Probes, Eugene, OR). Labelled cells were analysed using a FACScalibur (Becton Dickinson). The phenotype of lymphocytes present in lymph nodes during lymph node shut-down was also examined. The right popliteal lymph node was stimulated with PPD as described and the contralateral lymph node treated with an equal volume of Evan's Blue saline. After 15 hr (which was determined to be the period of maximum shut-down) the sheep were killed and the lymph nodes removed. Half the lymph node was embedded in optimal cooling temperature (OCT) and frozen for immunohistochemistry, while the remainder was minced, the mononuclear cells purified using a Percoll gradient, and immunophenotyped as above.
Lymphocyte/endothelial binding assay
Ovine lymphatic endothelial cells were cultured and the binding assay performed as described previously.11 Care was taken during the dissection of the lymphatic to exclude any overlaying tissue so that blood vessels were not present. The cultures had the typical cobblestone appearance of lymphatic endothelial cells. Lymph plasma was harvested at various times both before and after PPD stimulation by harvesting cells at 450 g for 7 min. The cell-free supernatant was removed and stored at −70° until analysis. Lymphatic endothelium was digested from intact mesenteric efferent lymphatics, cultured until confluence and binding assays carried out between passages 6 and 15. Endothelial cells were characterized by their ability to uptake DiI-acetylated LDL according to the manufacturer's instructions (Molecular Probes). The endothelium was stimulated with lymph plasma, cytokines or media for 17 hr, after which the monolayers were washed extensively with PBS. In later experiments, CD4+ T cells were purified from efferent lymph or peripheral blood mononuclear cells by negative selection panning. Briefly, large, sterile Petri dishes were coated with 12–15 ml of a solution of sheep-anti-mouse IgG antisera (Cappel no. 55466) in PBS and incubated overnight at 4C. The antibody solution was removed and the plates washed extensively with PBS. The plates were then blocked with 12–15 ml of PBS containing 5% fetal calf serum (FCS) for 15 min at room temperature. Cells were stained with appropriate primary antibodies against CD8+ T cells, γδ-T cells, a pan-granulocyte/myeloid marker analogous to murine CD66, and B cells (CD8: mAb 6–87; γδ-TcR mAb 127·5; CD66 mAb 1-44-19; B cells mAb 2–10412,14,15), and washed three times to remove unbound antibody; 108 total cells were then dissolved in 12–15 ml PBS containing 2% FCS, and added to the plate. Plates were incubated for 1 h at 4°, and the non-adherent CD4+ T cell population collected in a final volume of 30 ml. Selected lymphocytes were labelled with 111-Indium oxine (111-In) and 1 × 106 were added to the stimulated endothelium. After 1 hr non-adherent cells were removed by vigorous washing, the adherent cells lysed, and total radioactivity determined in a gamma counter. The raw counts were divided by the total radioactivity added and expressed as the percentage bound.
Cytokine immunoassays
Matched pairs of ovine cytokine specific antibodies for IL-1β, IL-6, and IL-8 were obtained from Serotec (Raleigh, NC). Antibodies against ovine TNF were obtained from the Center for Animal Biotechnology (Melbourne, Australia). Each pair was composed of a monoclonal murine antibody and a polyclonal rabbit antiserum. The immunoassays were performed as described previously using 0·1% alpha casein as the blocking agent.16 The murine antibody was used as the capture antibody, followed by the rabbit serum, which was detected by horseradish peroxidase-conjugated goat anti-rabbit IgG and visualized using tetramethylbenzidine substrate (Sigma). IFN-γ was detected using the BOVIGAM commercial kit obtained from CSL Veterinary according to the manufacturer's instructions (Victoria, Australia). Recombinant bovine IFN-γ was a kind gift from Dr Stephen Jones, CSL Veterinary. The BOVIGAM kit cross-reacts with ovine IFN-γ (product insert and our own unpublished observations). All samples from each experiment were run in duplicate for the respective cytokine on the same day and included a standard curve and controls.
Immunohistochemistry
Immunohistochemistry was performed on the lymph nodes harvested during the shut-down period. Monoclonal murine antibodies against IL-6, IL-1β, IL-8, TNF-α, CD4, CD8, CD21 and γδ T cells were used (sources as above). The staining was performed as described previously with the optimal dilution of primary antibody determined by previous titration.8 Diaminobenzidine was used to visualize the reaction and Giemsa used as a light counterstain.
Statistics
To pool the data from multiple animals, the values obtained during the baseline period were averaged. All values were then divided by this baseline value, to give a relative change over the course of the experiment. A value of more than 1 indicates an increase over the baseline value, while a number lower than 1 indicates a decrease. Where applicable, an analysis of variance (anova) with appropriate post-hoc tests and two-tailed t-test with a P < 0·05 was used to determine significance.
Results
PPD-induced shut-down preferentially affects recirculating lymphocytes
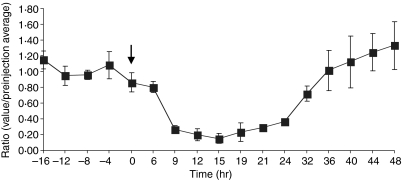
The injection of 50 µg of PPD into the drainage area of the lymph node caused the cell output in efferent lymph to decrease followed by an increase (Fig. 1). The shut-down response reached its nadir approximately 15 hr post-injection, and started to increase by approximately 32 hr post-injection. This is similar to numerous previous reports using different antigens.6,8,17
Figure 1.
The output of lymphocytes in the efferent lymph decreases significantly over the first 24 hr after initiation of a DTH response: there was a substantial decrease in the hourly output of lymphocytes in regional efferent lymph after the injection of 50 µg of PPD into the drainage area of a prescapular lymph node (n = 3). Ratios for individual experiments were determined by dividing measured cell output over a given experimental period by the average output obtained during the baseline period. These ratios were then pooled for three animals. The arrow indicates the time of PPD injection (results presented as mean ± SEM).
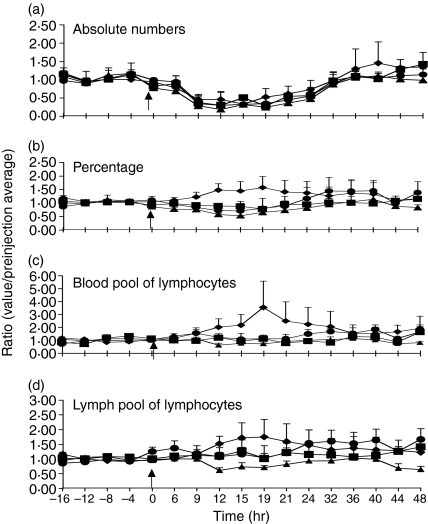
In three separate experiments the phenotype of lymphocytes exiting during lymph node shut-down was investigated. Although the absolute number of CD4, CD8, γδ T cells and B cells was decreased uniformly during shut-down, the percentage of CD4 cells in the efferent lymph increased relative to other subsets (Fig. 2). Therefore, while the total number of exiting CD4 lymphocytes decreased during the shut-down period, they appeared to form a greater percentage of exiting lymphocytes. This implied that CD4+ T cells may have been less susceptible to the retaining effects of PPD stimulation.
Figure 2.
Blood CD4+ T cells are retained to a lesser degree than other subsets during DTH-induced shut-down. The number of all measured lymphocyte subsets decreases during the period of lymph node shut-down. (a) CD8 (▪), γδ (▴) and CD21 (•) percentages also decrease during lymph node shut-down but the percentage of CD4 (♦) increases (b). To determine which of the pools of lymphocytes was responsible, three-colour FACs analysis was performed. The CFSE-labelled blood pool of lymphocytes (c) shows an increase in the CD4 subset. The recirculating lymph pool of lymphocytes labelled with DiI-DS also shows an increase in the percentage of CD4 cells (d), but not as large as the blood pool of lymphocytes. Ratios are calculated as in Fig. 1, and are the means of three animals. Arrow indicates time of PPD injection (results presented as mean ± SEM).
It is now clear that not all lymphocytes are equally adept at recirculation. To investigate if shut-down had a selective effect on highly mobile lymphocytes of the recirculating lymphocyte pool, we compared the ability of labelled PBLs (poorly recirculating) and labelled ELLs (rapidly recirculating) to transit stimulated lymph nodes.
Irrespective of the source of labelled cells, the proportion of CD4 cells from both labelled pools were increased in efferent lymph during peak shut-down. However, retention appeared to be much more effective on CD4 positive T cells of the recirculating lymphocyte pool. Labelled blood CD4 cells increased 300% over baseline values and lymph CD4 cells by only 50% (Fig. 2). B cells, CD8 and γδ T cells from either pool were sequestered within the lymph node to a similar extent. These results confirm previous reports that CD4+ T cells are differentially retained during lymph node shut-down compared to B cells, CD8 and γδ T cells. However, it seems clear that subsets of CD4+ T cells are differentially affected, depending upon their recirculation capacity.
Pro-adhesive factors are present in efferent lymph during shut-down
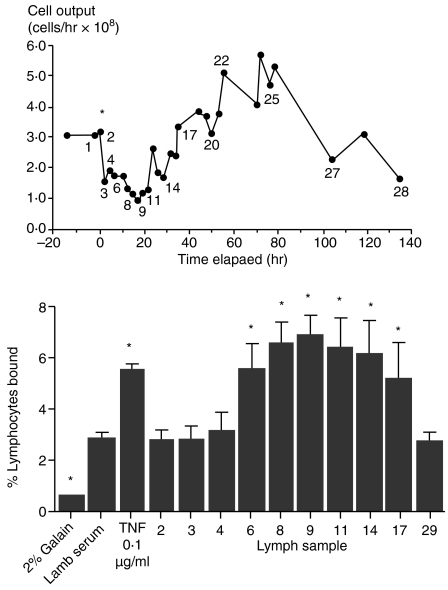
Factors, including cytokines, produced locally within the lymph node, can be detected in efferent lymph. To determine if soluble factors in efferent lymph were capable of inducing lymphocyte binding to lymphatic endothelium, lymph plasma was collected prior to and during the maximal shut-down response and tested in an in vitro model. Lymph plasma harvested either before PPD stimulation or during the first 6 hr post-injection did not increase lymphocyte binding to lymphatic endothelial monolayers in four separate experiments (Fig. 3). In contrast, lymph samples taken during maximal lymph node shut-down caused a significant increase in the binding of lymphocytes to lymphatic endothelium. This ability to increase lymphocyte binding gradually decreased as lymph node output increased following shut-down. Both carotid artery and jugular vein vascular endothelial cells were examined in parallel and also exhibited increased binding when stimulated with cytokines (data not shown). In control experiments, both TNF-α and IFN-γ increased lymphocyte binding to a similar degree as efferent lymph samples. These results indicated that soluble substances were present in lymph plasma, which acted on lymphatic endothelial cells to increase adhesive processes for ELL. We therefore decided to test for the presence of immunoactive cytokines in the lymph plasma draining a stimulated lymph node.
Figure 3.
Efferent lymph plasma collected during shut-down significantly increases lymphocytes binding to lymphatic endothelial cells in vitro. Lymph plasma was collected from the efferent lymph draining a DTH-stimulated lymph node, and cells removed by centrifugation. Individual samples were incubated with confluent cultures of lymphatic endothelium in the well-established binding assay. Numbered samples in the upper panel correspond to the lymph sample number in the lower panel. Samples obtained during lymph node shut-down significantly increased the binding of lymphocytes to lymphatic endothelium relative to other lymph samples (*P < 0·05, anova).
Bioactivity of efferent lymph corresponds to the presence of IL-6 and IFN-γ
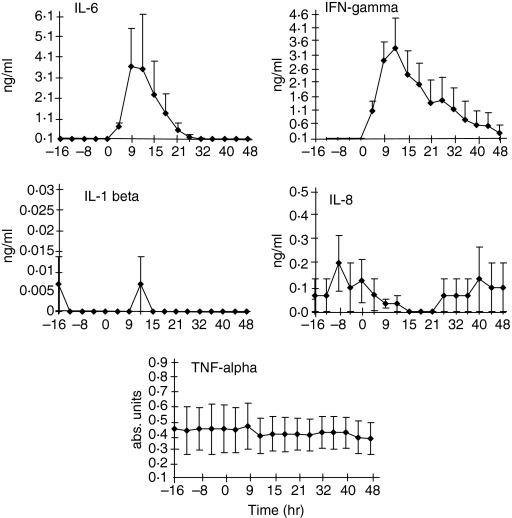
The levels of TNF-α, IFN-γ, IL-1β, IL-6 and IL-8 in efferent lymph plasma were measured in three separate experiments. The limit of detection for the enzyme-linked immunosorbent assays (ELISAs) was 0·1 ng/ml for all cytokines except the TNF-α assay. Unfortunately, no ovine TNF-α of a known concentration was available, therefore a standard curve was not constructed. None the less, it was possible to establish variation from baseline values for this assay.
In normal unstimulated lymph the levels of these cytokines were not consistently detectable. There was no increase in TNF-α, IL-1β and IL-8 in the lymph during antigen stimulation (Fig. 4). In contrast, IFN-γ and IL-6 were increased beginning 6 hr after the PPD injection. The peak concentration of IL-6 and IFN-γ corresponded to the period of maximum lymph node shut-down (Fig. 1). IL-1β did not reach the detection limit of the ELISA assay (0·1 ng/ml) at any time tested.
Figure 4.
IL-6 and IFN-γ are increased in the efferent lymph concomitant with maximal shut-down. Lymph, collected as in Fig. 3, was tested in ELISA as described. IL-6 and IFN-γ are both increased over baseline measurements during the period of maximal lymphocyte retention in the lymph node. IL-8 and TNF-α are not increased during this time. IL-1β results were below the limits of detection for the assay (mean results from three animals ± SEM).
Evan's Blue dye (used as a marker in the PPD injections) was present in significant amounts in some of the lymph samples collected soon after injection. To test if Evans Blue affected the cytokine ELISAs it was added to lymph plasma and cytokine levels were determined. The addition of Evan's Blue dye did not cause a deviation in the cytokine levels. To determine if inhibitory substances were present in lymph, cytokine was added exogenously to known negative lymph. The added cytokine was detected in expected concentrations, indicating that no interfering substances were present (data not shown).
Lymphatic endothelial cells can be stimulated by physiological levels of IFN-γ to bind ELL
To determine if preferential binding of recirculating CD4+ T cells within the lymph node could be accounted for by the increase in IFN-γ production, in vitro binding assays were performed using labelled blood or lymph CD4+ T cells. Using similar concentrations of IFN-γ to those found in efferent lymph, we found a significant increase in lymphocyte binding to endothelial cells. The baseline binding of PBL CD4 cells was significantly greater then ELL CD4 T cells. However, ELL CD4 cells increased their binding to IFN-γ stimulated lymphatic endothelium to a greater extent compared to blood CD4 cells (Table 1). Therefore, it appears that the ELL CD4+ T cells recovered in lymph are better able to increase their binding even at low concentrations of IFN-γ. This effect appeared to be saturable, as 100 ng/ml of IFN-γ was no more effective in stimulating the endothelium then 10 ng/ml.
Table 1.
Differential binding of blood versus lymph CD4 cells to lymphatic endothelium1
| Experimental conditions | Blood CD4 cells | Lymph CD4 cells |
|---|---|---|
| EC† + media | 6·15 ± 0·49* | 2·85 ± 0·26 |
| EC + 1 ng IFN-γ | 8·96 ± 1·14 | 6·01 ± 0·49‡ |
| EC + 10 ng IFN-γ | 11·58 ± 1·49‡ | 7·00 ± 0·71‡ |
| EC + 100 ng IFN-γ | 12·08 ± 1·16‡ | 6·24 ± 0·77‡ |
| EC + 100 ng TNF | 10·2 ± 2·99 | 4·04 ± 0·49 |
PBL and ELL were separated by standard panning methods and radiolabelled. Lymphatic endothelium was stimulated with cytokines for 17 hr, washed and radiolabelled lymphocytes added. After 1 hr non-adherent lymphocytes were removed by washing and the remaining radioactivity determined.
Average ± SEM of the percentage bound of the added lymphocytes in four experiments.
EC = lymphatic endothelial cell.
Statistically significant (P < 0·05) compared to media alone.
No difference in lymphocyte subsets in the lymph node during lymph node shut-down
To examine whether shut-down would transiently expand the numbers of T or B cell subsets within the local lymph node, regional lymph nodes draining either PPD-injected or saline-injected skin were excised during the maximal shut-down phase and the percentage of CD4+, CD8+, CD21+ and γδ-TcR+ cells determined by flow cytometry. There was no significant difference in lymphocyte phenotypes between the PPD-stimulated lymph node and the contralateral NaCl-stimulated lymph node with respect to CD4, CD8, CD21 and γδ T cells. These data show that while shut-down induces significant changes in the efferent lymph cell populations, there is no measurable change in the distribution of lymphocyte subsets in stimulated lymph nodes as a whole during shut-down (Table 2).
Table 2.
Subset-specific retention of lymphocyte subsets is restricted to the recirculating lymphocyte pool1
| Lymphocyte subset | PPD stimulated | Saline control |
|---|---|---|
| CD4 | 41·5* (34·5, 48·5) | 37·1 (34·2, 40·0) |
| CD8 | 18·9 (19·5, 18·2) | 18·9 (19·5, 18·2) |
| γδ | 4·6 (5·8, 3·3) | 4·5 (6·2, 2·8) |
| CD21 | 45·7 (43·0, 48·4) | 42·7 (39·2, 32·0) |
PPD or saline was infused into the drainage area of prefemoral lymph nodes, which were removed 19 hr later (during the time of maximum lymph node shut-down as measured in the efferent lymph). Cell suspensions were prepared, and the phenotype of cells in the normal and stimulated lymph node compared. No significant differences were evident between the PPD and the saline injected lymph node
average of two experiments. Numbers in parentheses give the measured proportion of each subset in each of the two sheep.
Discussion
In these experiments we investigated mechanisms involved in the sequestration of lymphocytes in lymph nodes during antigen-induced lymph node shut-down. We simultaneously examined cytokine production, the migration of the blood/lymph pools of lymphocytes and their phenotype during a PPD induced DTH response.
Mackay et al. demonstrated an increase in the percentage of CD4 cells during lymph node shut-down8 similar to the results reported in this study. They also noted a decrease in the number of exiting CD4 cells, although not as marked as reported here. Bujdoso et al.7 reported an increase in the percentage of CD4 cells 24 hr after the injection of PPD, but the number of cells was not determined. These slight differences may be due to differences in vaccination schedules. Nevertheless, in all three studies a difference in the migration of CD4 cells during lymph node shut-down was noted while CD8, γδ T cells and B cells all decreased uniformly. This retention is due probably to an up-regulation or increased avidity of adhesion molecules on lymphocytes, endothelium and other lymph node stromal cells. These adhesion molecules have yet to be investigated fully but may involve VCAM, which is increased on lymphatics of TNF-α-stimulated lymph nodes.12 The recently described common lymphatic endothelium and vascular receptor-1 (CLEVER-1) may also have a role in lymphocyte sequestration, as it is found on lymphatic endothelium and acts as an adhesion molecule.18 As well, the role of lymphatic endothelium and adhesion molecules in the exit of lymphocytes from lymph nodes is still incompletely understood during normal exit and especially during the enhanced efflux seen after lymph node shut-down. It is possible that different adhesion molecules or chemokines are up-regulated to allow greater migration of lymphocytes out of the lymph node. Thus it appears that adhesion molecules may have an important role in both pathological conditions such as lymph node shut-down and during normal lymphocyte traffic through lymph nodes, but further research is required to delineate their role.
The recently described poorly recirculating blood pool of lymphocytes (PBL)14,19 and the recirculating lymph pool (ELL) were examined for their migration through an antigen-stimulated lymph node. An increase in the percentage of both PBL and ELL CD4+ T cells in the efferent lymph was observed, with the PBL CD4 cells increasing to a greater extent. We hypothesized that this may be due to PBL CD4+ lymphocytes not binding to the lymphatic endothelium in the hilum of the lymph node to the same extent as ELL CD4+ cells. The in vitro binding assay confirms this, as PBL CD4 cells do not increase their binding to IFN-γ-stimulated lymphatic endothelium, while ELL CD4+ cells bind avidly. We have reported that the PBL and ELL pools of lymphocytes migrated differentially into cerebrospinal fluid after an intracerebroventricular infusion of TNF-α.20 Together with the present study, this indicates that these two pools may behave differently during inflammation.
Two explanations could account for the increased percentage of CD4 cells in efferent lymph during lymph node shut-down. One possibility is that there was a reduced retention of CD4 cells within the lymph node compared to other subsets. Alternatively, CD4 lymphocytes may have been selectively migrating into the lymph node and hence exiting in larger numbers. There was no significant difference between excised lymph nodes injected with PPD or saline during lymph node shut-down, suggesting that CD4 cells were not preferentially migrating into the lymph node. This argues that CD4 lymphocytes, especially blood CD4 cells, were not sequestered during lymph node shut-down to the same degree as other lymphocyte subsets. It is intriguing that CD4+ T cells, which are found in high numbers at the site of DTH, were actually less likely to be retained within the local lymph node. We did not measure directly the afferent lymph draining the DTH lesions, to determine if CD4+ T cells were retained preferentially in the skin lesions. Such a situation has been reported in the rejection of kidney allografts, where donor-specific reactivity disappears from the afferent lymph draining the graft but not the regional lymph node.21 These data demonstrate the importance of the lymph node in initiating the response, but it is necessary for lymphocytes to exit the lymph node in the efferent lymph, migrate to the site of inflammation and perform their effector function.
Lymph collected during the shut-down phase of the immune response resulted in a dramatic increase in lymphocyte binding to cultured lymphatic endothelium, similar to that induced by IFN-γ and TNF-α. This pro-adhesive characteristic of cell-free lymph is the result of soluble substances and may include cytokines, chemokines and other uncharacterized factors. IL-1β, IL-6, IL-8, IFN-γ and TNF-α were not detected in appreciable amounts in prestimulated lymph; however, IFN-γ and IL-6 were selectively increased during lymph node shut-down. Both of these molecules activate vascular endothelium to up-regulated known adhesion molecules and increase lymphocyte adhesion in vitro and in vivo.22,23 A recent report has demonstrated that IL-6 has chemotactic abilities on human T cells,24 which suggests that IL-6 may be acting to increase adhesion molecules but also recruit lymphocytes directly into the lymph node. IFN-γ applied at similar levels to that found in lymph during shut-down, increased lymphocyte binding to cultured lymphatic endothelial cells. We have demonstrated that an in vivo injection of TNF-α12 or IFN-α25 may cause lymph node shut-down. These results imply that cytokines have a role in mediating lymphocyte retention within the lymph node.
The mechanism and importance of lymph node shut-down has remained unresolved to date. There are currently no monoclonal antibodies specific for ovine lymphatic endothelium to confirm the colocalization of lymphatic endothelium with sequestered lymphocytes using immunohistochemistry (IHC). However, we have previously reported a role for lymphatic endothelium in the retention of γδ-T lymphocytes during TNF-α stimulation.12 Recent data suggest that lymphatic endothelium plays an active role in lymphocyte exit from lymph nodes. The mannose receptor on lymphatic endothelium can mediate lymphocyte exit from lymph nodes using l-selectin expressed on lymphocytes.26 As well, CLEVER-1 mediates lymphocyte binding to lymphatic endothelium.18 Interestingly, the immunosuppressant FTY720 inhibits peripheral immunity by inducing the accumulation of recirculating lymphocytes on the abluminal side of endothelial cells lining lymphatic sinuses.27 Altogether, these observations plus those from the current study suggest that the lymphatic endothelium plays a dynamic role in lymphocyte egress from the lymph node.
Lymphocyte sequestration within lymphoid tissue is not unique to antigen-induced lymph node shut-down, and has been found to be a characteristic of numerous pathological situations. Specifically, human immunodeficiency virus has been demonstrated to cause enhanced migration into and retention of lymphocytes within lymph nodes.28,29 This altered migration has been correlated with increased levels of IFN-γ,30 which indicated that physiological levels of IFN-γ influence lymphocyte migration in vivo. It seems probable that this virus-induced sequestration of recirculating lymphocytes contributes at least in part to the marked immunosuppression observed in AIDS patients. Furthermore, FTY720 induces sequestration of T and B cells within lymph nodes without affecting their function in vitro.31 Therefore, while lymphocyte retention within lymph nodes is a normal component of the acute immune response, extended periods appear to inhibit systemic immunity.
In conclusion, we have demonstrated that the traffic of cells through stimulated lymph nodes is regulated independently of lymphocyte entry. Furthermore, it appears clear that lymphatics and lymphatic endothelium play an active role in this process, potentially through cytokine-reactive expression of pro-adhesive molecules. Although all lymphocyte subsets migrated into the lymph node during the shut-down period, PBL CD4+ lymphocytes were the least retained. These data suggest, for the first time, a mechanism for the normal shut-down response observed in acute immunity, and may indicate novel therapeutic targets for immunomodulation in vivo.
Acknowledgments
The authors wish to acknowledge Drs Wayne Hein, Steven Mentzer, Mei Su, Chufa He and Peter Borgs for helpful discussion in the preparation of the manuscript.
Abbreviations
- CFSE
carboxyfluorescein diacetate succinimydl ester
- IHC
immunohistochemistry
- OCT
optimal cooling temperature
References
- 1.Young AJ, Hay JB, Mackay CR. Lymphocyte recirculation and life span in vivo. Curr Top Microbiol Immunol. 1993;184:161–73. doi: 10.1007/978-3-642-78253-4_13. [DOI] [PubMed] [Google Scholar]
- 2.Young AJ. The physiology of lymphocyte migration through the single lymph node in vivo. Semin Immunol. 1999;11:73–83. doi: 10.1006/smim.1999.0163. [DOI] [PubMed] [Google Scholar]
- 3.Butcher EC, Williams M, Youngman K, Rott L, Briskin M. Lymphocyte trafficking and regional immunity. Adv Immunol. 1999;72:209–53. doi: 10.1016/s0065-2776(08)60022-x. [DOI] [PubMed] [Google Scholar]
- 4.Seabrook T, Au B, Dickstein J, Zhang X, Ristevski B, Hay JB. The traffic of resting lymphocytes through delayed hypersensitivity and chronic inflammatory lesions: a dynamic equilibrium. Semin Immunol. 1999;11:115–23. doi: 10.1006/smim.1999.0167. [DOI] [PubMed] [Google Scholar]
- 5.Hay JB, Young AJ. Lymphocyte recirculation. In: Reed RK, McHale NG, Bert GA, Winlove JL, Laine CP, editors. Interstitium, Connective Tissue and Lymphatics. London: Portland Press; 1995. pp. 245–54. [Google Scholar]
- 6.Cahill RN, Frost H, Trnka Z. The effects of antigen on the migration of recirculating lymphocytes through single lymph nodes. J Exp Med. 1976;143:870–88. doi: 10.1084/jem.143.4.870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bujdoso R, Young P, Hopkins J, Allen D, McConnell I. Non-random migration of CD4 and CD8 T cells: changes in the CD4:CD8 ratio and interleukin 2 responsiveness of efferent lymph cells following in vivo antigen challenge. Eur J Immunol. 1989;19:1779–84. doi: 10.1002/eji.1830191003. [DOI] [PubMed] [Google Scholar]
- 8.Mackay CR, Marston W, Dudler L. Altered patterns of T cell migration through lymph nodes and skin following antigen challenge. Eur J Immunol. 1992;22:2205–10. doi: 10.1002/eji.1830220904. [DOI] [PubMed] [Google Scholar]
- 9.Cahill R, Hay JB, Frost H, Trnka Z. Changes in lymphocyte circulation after administration of antigen. Haematologia (Budapest) 1974;8:321–34. [PubMed] [Google Scholar]
- 10.Hillyer P, Mordelet E, Flynn G, Male D. Chemokines, chemokine receptors and adhesion molecules on different human endothelia: discriminating the tissue-specific functions that affect leucocyte migration. Clin Exp Immunol. 2003;134:431–41. doi: 10.1111/j.1365-2249.2003.02323.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Borron P, Hay JB. Characterization of ovine lymphatic endothelial cells and their interactions with lymphocytes. Lymphology. 1994;27:6–13. [PubMed] [Google Scholar]
- 12.Young AJ, Seabrook TJ, Marston WL, Dudler L, Hay JB. A role for lymphatic endothelium in the sequestration of recirculating gamma delta T cells in TNF-alpha-stimulated lymph nodes. Eur J Immunol. 2000;30:327–34. doi: 10.1002/1521-4141(200001)30:1<327::AID-IMMU327>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 13.Young A, Hein WR, Hay JB. Cannulation of lymphatic vessels and its use in the study of lymphocyte traffic. In: Lefkovitz I, editor. Immunology Methods Manual. London: Academic Press; 1997. pp. 2039–59. [Google Scholar]
- 14.Young AJ, Marston WL, Dessing M, Dudler L, Hein WR. Distinct recirculating and non-recirculating B-lymphocyte pools in the peripheral blood are defined by coordinated expression of CD21 and 1-selectin. Blood. 1997;90:4865–75. [PubMed] [Google Scholar]
- 15.Seabrook TJ, Hein WR, Dudler L, Young AJ. Splenectomy selectively affects the distribution and mobility of the recirculating lymphocyte pool. Blood. 2000;96:1180–3. [PubMed] [Google Scholar]
- 16.Nash A, Andrews A, Martin M, Colditz I, Wood PR. Methods for the detection and assay of ovine cytokines. In: Lefkovitz I, editor. Immunology Methods Manual. London: Academic Press; 1997. pp. 2066–78. [Google Scholar]
- 17.Hay JB, Cahill RN, Trnka Z. The kinetics of antigen-reactive cells during lymphocyte recruitment. Cell Immunol. 1974;10:145–53. doi: 10.1016/0008-8749(74)90158-0. [DOI] [PubMed] [Google Scholar]
- 18.Irjala H, Elima K, Johansson EL, et al. The same endothelial receptor controls lymphocyte traffic both in vascular and lymphatic vessels. Eur J Immunol. 2003;33:815–24. doi: 10.1002/eji.200323859. [DOI] [PubMed] [Google Scholar]
- 19.Andrade WN, Johnston MG, Hay JB. The relationship of blood lymphocytes to the recirculating lymphocyte pool. Blood. 1998;91:1653–61. [PubMed] [Google Scholar]
- 20.Seabrook T, Hay J. Intracerebroventricular infusions of TNF-alpha preferentially recruit blood lymphocytes and induce a perivascular leukocyte infiltrate. J Neuroimmunol. 2001;113:81–8. doi: 10.1016/s0165-5728(00)00429-x. [DOI] [PubMed] [Google Scholar]
- 21.Hay JB, Morris B. Generation and selection of specific reactive cells by antigen. Br Med Bull. 1976;32:135–40. doi: 10.1093/oxfordjournals.bmb.a071345. [DOI] [PubMed] [Google Scholar]
- 22.de Vries HE, Moor AC, Blom-Roosemalen MC, et al. Lymphocyte adhesion to brain capillary endothelial cells in vitro. J Neuroimmunol. 1994;52:1–8. doi: 10.1016/0165-5728(94)90155-4. [DOI] [PubMed] [Google Scholar]
- 23.Cartwright JE, Whitley GS, Johnstone AP. The expression and release of adhesion molecules by human endothelial cell lines and their consequent binding of lymphocytes. Exp Cell Res. 1995;217:329–35. doi: 10.1006/excr.1995.1094. [DOI] [PubMed] [Google Scholar]
- 24.Weissenbach M, Clahsen T, Weber C, et al. Interleukin-6 is a direct mediator of T cell migration. Eur J Immunol. 2004;34:2895. doi: 10.1002/eji.200425237. [DOI] [PubMed] [Google Scholar]
- 25.Kalaaji AN, Abernethy NJ, McCullough K, Hay JB. Recombinant bovine interferon-alpha I 1 inhibits the migration of lymphocytes from lymph nodes but not into lymph nodes. Reg Immunol. 1988;1:56–61. [PubMed] [Google Scholar]
- 26.Irjala H, Johansson EL, Grenman R, Alanen K, Salmi M, Jalkanen S. Mannose receptor is a novel ligand for 1-selectin and mediates lymphocyte binding to lymphatic endothelium. J Exp Med. 2001;194:1033–42. doi: 10.1084/jem.194.8.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mandala S, Hajdu R, Bergstrom J, et al. Alteration of lymphocyte trafficking by sphingosine-1-phosphate receptor agonists. Science. 2002;296:346–9. doi: 10.1126/science.1070238. [DOI] [PubMed] [Google Scholar]
- 28.Rosenberg YJ, Anderson AO, Pabst R. HIV-induced decline in blood CD4/CD8 ratios: viral killing or altered lymphocyte trafficking? Immunol Today. 1998;19:10–7. doi: 10.1016/s0167-5699(97)01183-3. [DOI] [PubMed] [Google Scholar]
- 29.Rosenberg YJ, Janossy G. The importance of lymphocyte trafficking in regulating blood lymphocyte levels during HIV and SIV infections. Semin Immunol. 1999;11:139–54. doi: 10.1006/smim.1999.0169. [DOI] [PubMed] [Google Scholar]
- 30.Rosenberg YJ, Cafaro A, Brennan T, et al. Virus-induced cytokines regulate circulating lymphocyte levels during primary SIV infections. Int Immunol. 1997;9:703–12. doi: 10.1093/intimm/9.5.703. [DOI] [PubMed] [Google Scholar]
- 31.Pinschewer DD, Ochsenbein AF, Odermatt B, Brinkmann V, Hengartner H, Zinkernagel RM. FTY720 immunosuppression impairs effector T cell peripheral homing without affecting induction, expansion, and memory. J Immunol. 2000;164:5761–70. doi: 10.4049/jimmunol.164.11.5761. [DOI] [PubMed] [Google Scholar]