Abstract
Lipopolysaccharide (LPS) tolerance is a state of refractoriness towards a second stimulation by LPS after a preceding stimulation. LPS is recognized by Toll-like receptor-4 (TLR-4), which belongs to a group of pattern recognition receptors mediating activation of innate immunity by microbial components. To date, it is not known in detail to what extent other TLR-dependent stimuli also induce tolerance and whether preceding and challenging stimuli are interchangeable. We have examined tolerance induction in detail for lipoteichoic acid (LTA), LPS and CpG-DNA, which are recognized by TLR-2, -4 and -9, respectively. In RAW264·7 macrophages, all three stimuli induced tolerance towards a subsequent challenge with the same stimulus used for priming, as well as cross-tolerance towards subsequent challenge with other stimuli signalling via different TLRs. However, whereas LPS/LTA cross-tolerance was also functional in an in vivo model of galactosamine (GalN)-primed liver damage, pretreatment with CpG only protected against GalN/CpG challenge and failed to induce cross-tolerance for LPS and LTA. CpG-DNA pretreatment even enhanced tumour necrosis factor (TNF)-α production and liver damage upon subsequent challenge with LPS or LTA. Stimulation with CpG-DNA resulted in a peculiar sensitization for interferon (IFN)-γ secretion. The data indicate that, in contrast to in vitro macrophage desensitization, the in vivo consequences of repeated TLR stimulation greatly differ amongst different TLR ligands.
Keywords: CpG-DNA, lipopolysaccharide, lipoteichoic acid, tolerance, Toll-like receptors
Introduction
Lipopolysaccharide (LPS) tolerance, which is hyporesponsiveness to LPS stimulation after a preceding low-dose treatment with LPS, is a phenomenon1,2 that reproduces certain aspects of the immunosuppression observed frequently in patients during late-phase sepsis.3,4 In recent years, our knowledge of the mechanisms of LPS tolerance and macrophage desensitization by repeated LPS exposure has increased considerably as a result of novel insights into the LPS signalling pathways. LPS signal transduction is initiated by Toll-like receptor 4 (TLR-4),5,6 which belongs to the group of pattern recognition receptors.7 These receptors mediate activation of the innate immune system by microbial compounds.8 To date, 13 TLRs have been identified, most of which have been studied in detail and have had specific ligands identified.8,9 Thus, TLR-2 mediates recognition of such microbial components as lipoproteins, lipopeptides (e.g. macrophage-activating lipopeptide (MALP)-2) and lipoteichoic acid (LTA).8 TLR-9 has been shown to be activated by bacterial CpG-containing DNA, some double-stranded DNA viruses and synthetic CpG-oligodesoxynucleotides (ODN).8,10–12
In light of the finding that cellular responses to different microbial stimuli are mediated by different TLRs, studies were initiated to determine whether, in analogy to LPS tolerance, pretreatment with microbial non-LPS stimuli also induces hyporesponsiveness to subsequent restimulation. Indeed, it has been reported that stimulation with prototypical ligands for TLR-2 (+TLR-1 or -6),13–18 TLR-4, TLR-519 and TLR-920,21 also induces this state of hyporesponsiveness towards subsequent stimulation with the same ligand. Moreover, stimuli signalling via TLR-2 and TLR-4,14,15 as well as TLR-4 and TLR-9,20 can substitute for each other, mediating cross-tolerance in vitro as well as in vivo. In contrast to the above findings of cross-tolerance for different TLRs, the TLR-2-dependent stimuli Porphyromonas gingivalis LPS,17 LTA16 and PAM3CSK422 do not induce cross-tolerance to LPS in different experimental systems. It becomes obvious that tolerance development is dependent on the cell type, the respective TLR ligand and the experimental conditions and cannot be extrapolated from one TLR to another.
A second layer of complexity was added when it became clear that TLRs differ in their requirements for signal transduction molecules.8,23 All TLRs seem to use a common signalling pathway which involves the recruitment of the adaptor protein myeloid differentiation factor 88 (MyD88). However, additional adaptor proteins have been identified which mediate TLR-specific signal transduction.24–29 Thus, in TLR-4 signalling, toll-interleukin 1 receptor domain containing adaptor protein (TIRAP)/MyD88 adaptor like protein (Mal) can partly substitute for MyD88. Furthermore, TLR-3 and TLR-4 make use of toll-interleukin 1 receptor domain containing adaptor inducing interferon beta (TRIF)/toll-interleukin 1 receptor domain (TIR) containing adaptor molecule (TICAM)-1 and TRIF related adaptor molecule (TRAM)/TICAM-2, thereby inducing interferon (IFN)-β, which in turn activates a set of IFN-inducible genes in a paracrine mode. In the light of this knowledge, Sato et al. observed that MALP-2, while inducing cross-tolerance for MyD88-dependent LPS signalling, was not able to confer tolerance for the MyD88-independent LPS-specific signalling pathway.30 Also, TLR-7 only induced tolerance for MyD88-dependent LPS genes, while TLR-3-mediated cross-tolerance was restricted to MyD88-independent signalling. More generally, it was stated that tolerance affects more genes than cross-tolerance.22 Thus, tolerance as well as cross-tolerance can be differentially developed depending on the specific TLR-activating compound.
Our understanding of the mechanisms underlying tolerance induction and cellular hyporesponsiveness is still incomplete. It seems that tolerance induction is an interplay of altered conditions at several steps of signal transduction. Whereas initial findings in human cells stressed the importance of inhibitory cytokines such as interleukin (IL)-10 or transforming growth factor (TGF)-β,31 experiments using knockout mice14,32 and co-culture experiments15 did not support a major contribution of these mediators. Also, while down-regulation of TLR-4 has been postulated to contribute to tolerance,33 other reports demonstrated cellular refractoriness independent of TLR-4 regulation,13 and no similar findings of an involvement of receptor down-regulation in cellular refractoriness were obtained for other TLRs. Next, tolerant cells seem to have a very proximal failure in signalling, as reported for recruitment of respective adaptor proteins.34 Of special interest is the finding that interleukin-1 receptor associated kinase (IRAK)-1 is altered at least in some aspects of TLR signalling, a result that has been confirmed by different groups.16,20,34 Without claiming completeness for the various findings of tolerance experiments, it has to be stated that different modes of action seem to be operative and that TLR ligands seem to make different use of them.
In this study, we addressed the question of whether and to what extent tolerance and cross-tolerance phenomena could be induced via TLR-2, -4 and -9. Therefore, we intended to perform tolerance experiments under defined in vitro and in vivo conditions. Our findings demonstrate robust in vitro and in vivo induction of cross-tolerance against LPS, LTA and CpG-ODN after initial TLR-2 (LTA) or TLR-4 (LPS) engagement. In contrast, depending on the experimental setting, pretreatment with the TLR-9 ligand CpG-ODN either suppressed or even enhanced responses to subsequent challenge with LPS or LTA, suggesting non-redundant signalling of different TLRs.
Materials and methods
Reagents
Completely phosphorothioate-modified CpG-ODN #1668 (TCC ATG ACG TTC CTG ATG CT) was purchased from TIB Molbiol (Berlin, Germany). LTA had been purified from Staphylococcus aureus and had previously been characterized as being essentially dependent on TLR-2.15 Highly purified LPS from Salmonella minnesota (smooth form) was kindly provided by U. Seydel (Borstel, Germany). It was solely recognized by TLR-4.
Mice
Details of the generation of IL-18–/– mice backcrossed to C57Bl/6 mice have been published.35 Balb/c and C57Bl/6 mice were purchased from Harlan-Winkelmann (Borchen, Germany), while C3H/HeN mice were from Charles-River (Sulzfeld, Germany). All animal experiments were approved by the local authorities.
Cells and culture conditions
Cells were cultured in Clicks/RPMI 1640 supplemented with 5–10% fetal calf serum (FCS), 50 µmβ-mercaptoethanol and antibiotics (penicillin G and streptomycin). Peritoneal macrophages were obtained by intraperitoneal (i.p.) thioglycollate injection for 3 days. Cells were further purified by overnight adherence. Spleen cells were obtained by passing removed spleens through a mesh to obtain single cell suspensions followed by lysis of erythrocytes. RAW264·7 cells, a murine macrophage cell line, were a kind gift from Dr R. Schumann (Institute for Med. Microbiology, Humboldt University, Berlin, Germany).
Cell stimulation experiments
Macrophages (0·15 × 106) or spleen cells (0·5 × 106) were prestimulated in 96-well plates in a total volume of 300 µl medium as indicated in the respective experiments. Subsequently, cells were washed thoroughly three times and then re-challenged by addition of the appropriate stimulus. Supernatant was harvested after 6–8 hr and cytokine levels were determined by commercially available enzyme-linked immunosorbent assay (ELISA) (OptEIA sets; BD Pharmingen, Heidelberg, Germany) in duplicates.
In vivo tolerance experiments
C3H/HeN mice (six mice in each group) were pretreated with either pyrogen-free saline, 2 µg/kg LPS (Salmonella abortus equi; Metalon, Aidenbach, Germany), 100 mg/kg LTA or 500 nmol/kg CpG-ODN #1668 via i.p. injection and starved overnight. Twenty-four hours later, the animals were challenged i.p. with galactosamine (GalN; 0·75 g/kg; Roth Chemicals, Karlsruhe, Germany) together with CpG-ODN, LPS or LTA at the above doses to induce systemic tumour necrosis factor (TNF)-α production and inflammatory liver damage. Tail blood was obtained after 90 min for determination of TNF-α plasma levels by ELISA (OptEIA; BD Pharmingen). Seven hours post-challenge, mice were killed and heparin blood samples were drawn by cardiac puncture. Plasma alanine aminotransferase (ALT) activities were determined with an EPOS 5060 analyzer (Netheler & Hinz, Hamburg, Germany), and cytokine levels of IFN-γ and IL-18 were determined by ELISA (OptEIA).
Quantitative reverse transcriptase–polymerase chain reaction (RT-PCR)
Total RNA from 1 × 106 cells was isolated using the HighPureTM RNA kit (Roche, Mannheim, Germany) which included DNaseI digestion. A cDNA synthesis kit (MBI Fermentas, St. Leon-Rot, Germany) was used to reverse-transcribe 1 µg of the total RNA preparation. cDNA diluted 1 : 4 was used as template in the quantitative PCR mix according to the manufacturer's standard protocol (Eurogentec, Seraign, Belgium) (ABI Prism 7700; Applied Biosystems, Darmstadt, Germany). Primer sequences were: β-actin primer (sense: CCC TGT GCT GCT CAC CGA, antisense: ACA GTG TGG GTG ACC CCG TC), IRAK-M primer (sense: CAC AGT TGC TGC TCT TCG AC, antisense: CCC AGG ACC AGA GCA ATT C), MyD88spliced primer (sense: TCG CGC ATC GGA CAA ACG, antisense: GCA ATG GAC CAG ACA CAG GT). MyD88 primers were used that only amplified the published inhibitory splice variant of MyD88.36 Quantifications were performed by means of SYBR-Green (Eurogentec, Seraing, Belgium) with melting curve analysis. The specificity of RT-PCR was controlled using no template and no RT controls. PCR efficiencies for all reactions were determined and were similar (0·93–0·98). Threshold values were normalized to expression of β-actin. Quantitative PCR results are expressed as relative induction towards the housekeeping gene β-actin (1/2ΔCt).
Statistics
Data are presented as mean + standard error of the mean (SEM). Statistical differences were determined by one-way analysis of variance (ANOVA) followed by Dunnett's multiple comparison test of all groups versus the control group. P < 0·05 was considered significant.
Results
Tolerance and cross-tolerance for TLR-2, -4 and -9-dependent stimuli
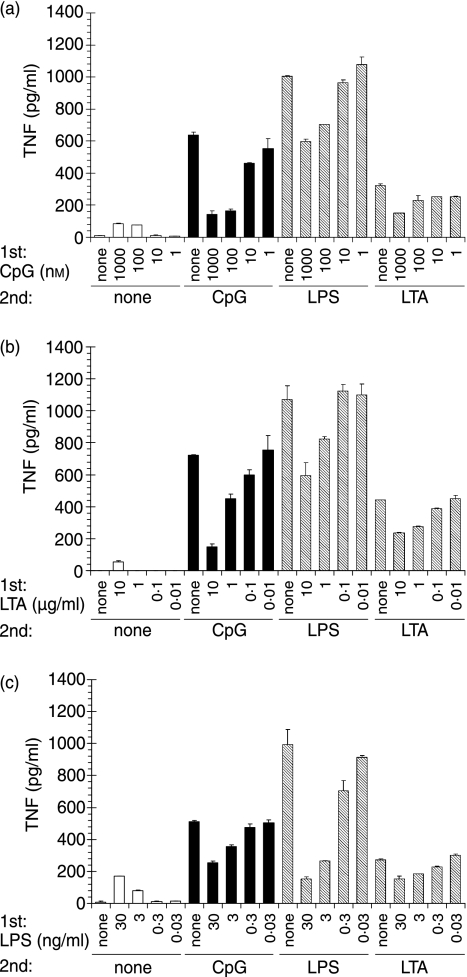
We first assessed to what extent tolerance and cross-tolerance could be observed for TLR-2, -4 or -9-dependent, highly purified microbial or synthetic stimuli. We pretreated RAW264·7 macrophages with differing amounts of the respective stimuli and subsequently re-challenged them with the same panel of TLR ligands (Fig. 1). The results show that tolerance as well as cross-tolerance induction was operative in each of the tested combinations of stimuli. However, only LPS pretreatment nearly completely abolished TNF-α production upon subsequent LPS re-challenge; pretreatment with either LTA or CpG-ODN was less effective. Similarly, TNF-α production upon LTA re-challenge was only slightly diminished after LTA and CpG-ODN pretreatment, suggesting different potencies of the employed stimuli.
Figure 1.
Induction of tolerance and cross-tolerance by Toll-like receptor (TLR) stimuli. RAW264·7 macrophages were prestimulated for 20 hr with (a) CpG-oligodesoxynucleotides (ODN), (b) lipoteichoic acid (LTA) or (c) lipopolysaccharide (LPS) at the indicated concentrations. Subsequently cells were washed and challenged with 100 nm CpG-ODN, 30 ng/ml LPS or 10 µg/ml LTA for 8 hr. Tumour necrosis factor (TNF)-α concentrations were determined in the supernatant. Displayed are mean values of duplicates + standard deviation for one of three independent experiments.
Differential effects of CpG-DNA in cross-tolerance induction in peritoneal macrophages
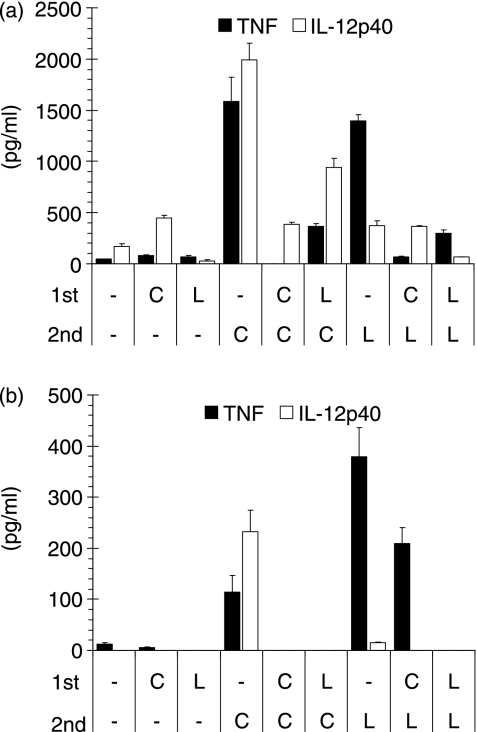
Next, we intended to verify the novel results of TLR-2 and -9 cross-tolerance with isolated primary cells. In naïve spleen cells, LTA and CpG-ODN induced comparable levels of TNF-α, while CpG-ODN induced significantly greater amounts of IL-12p40 (Fig. 2a). Consistent with the data from RAW macrophages, CpG-ODN prestimulation rendered cells refractory towards subsequent restimulation with CpG-ODN or LTA. Interestingly, LTA was able to partly inhibit CpG-ODN-induced IL-12p40 secretion, although alone it activated the secretion of IL-12p40 only weakly. Next we resorted to induced peritoneal macrophages for the analysis of CpG-ODN-mediated effects (Fig. 2b). While CpG-ODN but not LTA induced IL-12p40, the latter was more effective in the induction of TNF-α. Consistent with the above findings, LTA prestimulation resulted in the induction of complete refractoriness towards subsequent LTA or CpG-ODN administration in both TNF-α and IL-12p40 secretion. Prestimulation with CpG-ODN also completely abrogated subsequent CpG-ODN stimulation (tolerance). However, CpG-ODN prestimulation inhibited subsequent LTA only partially. Thus, TNF-α secretion upon LTA administration was only reduced by 43%, while with CpG-ODN as a second stimulus there was 100% inhibition. Peritoneal macrophages from C3H/HeN and C57Bl/6 mice gave similar results (data not shown).
Figure 2.
Differential effects of CpG-DNA in cross-tolerance induction in vitro. (a) Spleen cells or (b) thioglycollate-induced peritoneal macrophages from Balb/c mice were stimulated with either 100 nm CpG-oligodesoxynucleotides (ODN) (C) or 3 µg/ml lipoteichoic acid (LTA) (L) for 18 or 28 hr, respectively. Subsequently, cells were washed twice in phosphate-buffered saline and once in medium and then were restimulated with either CpG-ODN or LTA as above. Eight hours later, tumour necrosis factor (TNF)-α and interleukin (IL)-12p40 concentrations were measured in the supernatant. Mean values of duplicates + standard deviation for one of two experiments are shown.
Mechanisms of TLR-mediated tolerance/cross-tolerance
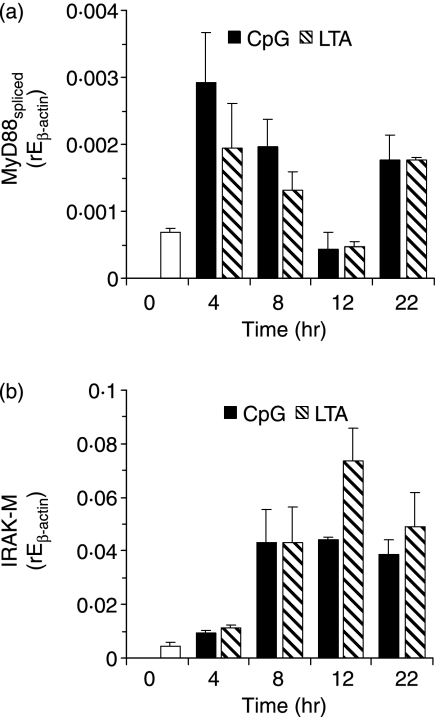
Next we asked which mechanisms are involved in either CpG-ODN- or LTA-mediated tolerance/cross-tolerance. Induction of an inhibitory splice variant of MyD8836 was observed at equal levels upon LTA or CpG-DNA stimulation (Fig. 3a). This showed a biphasic course, being induced after 4–8 hr and again after 22 hr of stimulation. Expression of full-length MyD88, which was about 50-fold higher than that of the splice variant in non-stimulated cells, did not change significantly over time (data not shown). In addition, we examined the inhibitory IRAK-M molecule.37 This was also equally induced by LTA and CpG-DNA (Fig. 3b) and increased in expression over 4–22 hr after stimulation. Furthermore, we examined expression of ST2, which has recently been reported to be a regulator of LPS tolerance.38 However, ST2 expression was not altered by CpG-DNA, LPS or LTA stimulation (data not shown).
Figure 3.
Mechanisms of tolerance induction. mRNA induction of an inhibitory splice variant of (a) myeloid differentiation factor 88 (MyD88) (b) interleukin-1 receptor associated kinase (IRAK)-M upon stimulation with 1000 nm CpG-oligodesoxynucleotides (ODN) or 3 µg/ml lipoteichoic acid (LTA) was determined by quantitative reverse transcriptase–polymerase chain reaction (RT-PCR). Shown is the relative expression normalized to β-actin for one of three experiments with RAW264·7 cells.
Induction of tolerance but not of cross-tolerance by CpG-DNA in vivo
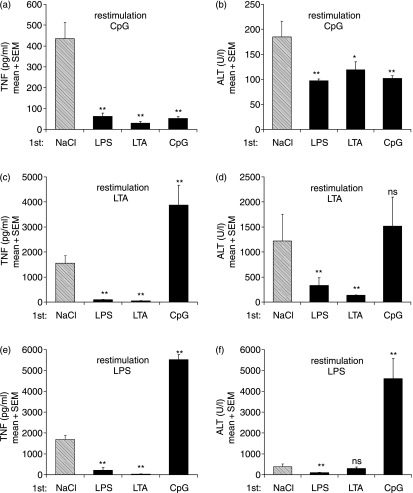
We then addressed the question of tolerance and cross-tolerance in an in vivo model of TLR ligand/GalN-induced liver injury in mice (Fig. 4). In saline-pretreated animals, challenge with any of the three TLR ligands (LPS, LTA or CpG-ODN) in combination with GalN induced increased plasma TNF-α levels at 90 min post-challenge and resulted in significant liver damage (Fig. 4; NaCl pretreatment), as measured by increased ALT levels 7 hr after challenge. Control mice without restimulation showed no significant ALT or TNF-α release. As predicted from the in vitro data, pretreatment with any of the three TLR stimuli induced refractoriness towards CpG-ODN re-challenge in terms of both ALT and TNF-α induction (Figs 4a and b). Upon challenge with either LPS or LTA (Figs 4c–f), protection was also observed in animals pretreated with either LTA or LPS, indicating the establishment of tolerance and cross-tolerance via TLR-2 and TLR-4. However, CpG-ODN pretreatment, surprisingly, was not able to induce cross-tolerance to either LPS or LTA challenge and even enhanced TNF-α secretion and liver damage. Thus, pretreatment with the TLR-9 stimulus CpG-ODN, instead of inducing the refractory state of cross-tolerance, led to an enhanced sensitivity in the case of restimulation of TLR-2 and -4 but not of TLR-9.
Figure 4.
Differences in the induction of tolerance and cross-tolerance in vivo. Mice were pretreated intraperitoneally (i.p.) with NaCl, lipopolysaccharide (LPS) (2 µg/kg), lipoteichoic acid (LTA) (100 mg/kg) or CpG-oligodesoxynucleotides (ODN) (500 nmol/kg) 24 hr prior to i.p. challenge with galactosamine (GalN) (0·75 g/kg) and the indicated Toll-like receptor (TLR) stimulus at the same dose used for pretreatment. Circulating blood levels of tumour necrosis factor (TNF)-α (a, c, e) were determined 90 min later and alanine aminotransferase (ALT) plasma activities (b, d, f) were measured after 7 hr. Displayed are mean values + standard error of the mean (six mice in each group). *P < 0·05; **P < 0·01; ns, not significant (Dunnett's multiple comparison test).
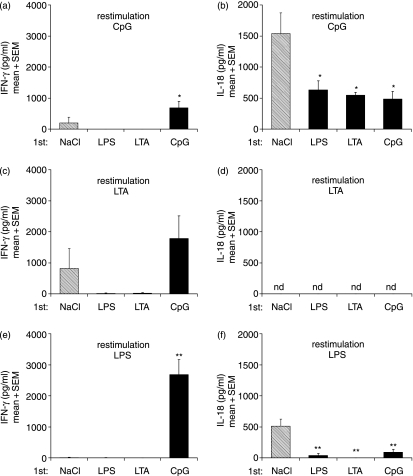
IL-18 and IFN-γ are differentially affected in CpG-ODN-induced tolerance/cross-tolerance
IFN-γ has been shown to overcome tolerance.39 In our experiments, challenge with CpG-ODN and LTA but not with LPS (at this dose) was able to induce detectable IFN-γ levels in the plasma of animals without prestimulation (Fig. 5). The failure of LPS to induce detectable amounts of IFN-γ was attributable to the low LPS dose employed in the GalN model but did not represent a general lack of activity (data not shown). LPS and LTA pretreatment conferred complete tolerance and cross-tolerance towards subsequent CpG-ODN or LTA administration in terms of IFN-γ production. In contrast, CpG-ODN pretreatment failed to induce either tolerance or cross-tolerance and even boosted IFN-γ levels in LPS-restimulated animals. Because of the chosen experimental setting, the elevated levels of IFN-γ might also be attributable at least partly to the prestimulation with CpG-ODN.
Figure 5.
Differential induction of interleukin (IL)-18 and interferon (IFN)-γ by Toll-like receptor (TLR) stimulation in vivo. Mice were pretreated intraperitoneally (i.p.) with NaCl, lipopolysaccharide (LPS) (2 µg/kg), lipoteichoic acid (LTA) (100 mg/kg) or CpG-oligodesoxynucleotides (ODN) (500 nmol/kg) 24 hr prior to i.p. challenge with galactosamine (GalN) (0·75 g/kg) and the indicated TLR stimulus at the same dose used for pretreatment. Circulating blood levels of IFN-γ (a, c, e) and IL-18 (b, d, f) were determined after 7 hr of restimulation. Displayed are mean values + standard error of the mean (six mice in each group). *P < 0·05; **P < 0·01; nd, not detectable (Dunnett's multiple comparison test).
According to recent findings by Gould et al.,40 enhanced LPS-induced release of IFN-γ in CpG-ODN-pretreated animals critically depends on IL-18. However, in our model, preconditioning with CpG-ODN equally attenuated IL-18 release upon GalN/CpG challenge, as observed for animals pretreated with either LPS or LTA (Fig. 5b). In addition, CpG-ODN pretreatment suppressed LPS-induced IL-18 production (Fig. 5f), which is in contrast to the observed enhancement of LPS-induced TNF-α production and liver damage by CpG-ODN pretreatment. Although no IL-18 was induced in any of the LTA-challenged animals (Fig. 5d), pretreatment with LTA at the same dose potently suppressed CpG-ODN- and LPS-induced IL-18 (Figs 5b and f).
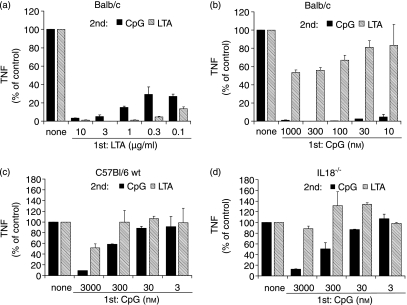
IL-18 is not required for the differential CpG-DNA effects on tolerance induction
To further clarify whether the poor cross-desensitization activity of CpG to LTA restimulation in vivo and in peritoneal macrophages depended on endogenous IL-18 production, we examined IL-18 knockout mice (Fig. 6). For better comparison, the cytokine levels induced by either CpG-ODN or LTA in non-pretreated peritoneal macrophages were set to 100%. CpG-DNA induced complete tolerance for CpG re-challenge while only partly desensitizing for LTA (compare Figs 6a and b) in wild-type Balb/c mice.
Figure 6.
Differences in Toll-like receptor (TLR)-9/TLR-2 cross-tolerance are independent of interleukin (IL)-18. Thioglycolate-induced peritoneal macrophages from Balb/ c (a, b), C57Bl/6 (c) or IL-18–/– (d) mice were pretreated with either lipoteichoic acid (LTA) (a) or CpG-oligodesoxynucleotides (ODN) (b–d) for 24 hr and re-challenged with either 1000 nm (a, b) or 3000 nm CpG-ODN (c, d) or 3 µg/ml LTA for 8 hr. Tumour necrosis factor (TNF)-α concentration was measured in the supernatant. Data for LTA and CpG-ODN restimulation without pretreatment were each set at 100% for better comparison. Shown are results from one of three experiments in each case (mean + standard deviation).
We then studied whether the poor cross-desensitization activity of CpG to subsequent LTA restimulation depended on endogenously produced IL-18. Comparison of peritoneal macrophages from IL-18 knockout and C57Bl/6 wild-type mice (Figs 6c and d) revealed no qualitative differences between the strains. Again, pretreatment with CpG-ODN efficiently desensitized cells to subsequent restimulation with CpG-ODN, whereas only a partial attenuation of TNF-α release in response to LTA restimulation was observed (similar to the results with Balb/c macrophages). However, cytokine production was a little lower in IL-18 knockout mice as compared to the wild type (2196 pg/ml versus 3004 pg/ml of TNF-α for CpG-ODN restimulation and 1158 pg/ml versus 1624 pg/ml for LTA). The results do not support a major role for CpG-ODN-induced IL-18 in the poor cross-desensitization potency of CpG to LTA.
Discussion
To our knowledge, this is the first comprehensive study of cross-tolerance of TLR-2, -4 and -9 in vitro and in vivo. Within the in vitro system of RAW264·7 macrophages, we observed induction of tolerance as well as cross-tolerance in terms of suppressed TNF-α secretion in response to restimulation by any of the three TLR ligands. No qualitative differences could be observed. Thus our findings support and add to the results of other groups showing in vitro cross-tolerance for TLR-2/TLR-414 and TLR-4/TLR-9.20,21 However, contrasting results have been reported for TLR-2/TLR-4 using either P. gingivalis LPS (PgLPS)17 or LTA16 within human THP-1 cells. In addition, another report showed that PgLPS/Escherichia coli LPS tolerance was operative but not vice versa, and Pam3CSK4 did not induce tolerance to LPS.22 These contrasting results are difficult to interpret. However, there were differences in cell type (human THP-1 versus murine RAW264·7) as well as in the stimuli and stimulus concentrations employed in the two studies. In murine macrophages, we observed that 1–10 µg/ml LTA was required to induce tolerance as well as cross-tolerance. Some of the contrasting studies, however, used maximum concentrations of 1 µg/ml, and thus a potential inhibitory effect might have been overlooked.
Macrophage desensitization with a suppression of cytokine release is often used to explain the protective effect of LPS tolerance in vivo. In a model of GalN + CpG/LPS/LTA-induced inflammatory liver damage, we assessed to what extent the observed (cross-) desensitization potency of CpG-ODN, LPS and LTA in RAW macrophages also translated into in vivo (cross-)tolerance induction. Pretreatment of mice with either LPS or LTA potently suppressed TNF-α production and liver damage in response to LPS and LTA challenge, confirming previous results.26 In addition, both stimuli similarly conferred protection to subsequent GalN/CpG challenge, suggesting that common signalling cascades shared by different TLRs are suppressed. Our finding that TLR-9 engagement also suppressed TNF-α production and liver damage in response to subsequent GalN/CpG challenge accords well with the view of macrophage desensitization as a central mechanism of in vivo ‘TLR tolerance’. However, no protection and even an enhancement of TNF-α release and liver damage were observed when CpG-ODN-preconditioned mice were challenged with GalN + LPS/LTA. This indicates that in certain settings CpG-DNA might induce sensitization instead of tolerance.
TLR ligands, although inducing an overlapping set of genes, are able to elicit some specific responses, as observed in gene array experiments.41 In the case of TLR-9, a peculiarity is the induction of large amounts of IL-12,42,43 at least in mice, and subsequently strong Th1 polarization.44 Moreover, CpG-DNA is a potent inducer of the pro-inflammatory cytokines IL-18 and IFN-γ which have been shown to reverse LPS tolerance and macrophage desensitization.39 Toxicity of either LPS or LTA can be markedly enhanced by concomitant administration of exogenous IFN-γ.45 In addition, recently published data demonstrated enhanced release of IFN-γ in CpG-DNA-preconditioned mice upon subsequent challenge with either CpG or LPS.40 Our findings show that CpG-ODN-preconditioned animals challenged with CpG, LTA or LPS displayed increased plasma IFN-γ levels compared with saline-pretreated mice. This provides a possible clue to the contrasting effects of TLR-9 and TLR-2/-4 preconditioning on immune responsiveness. The findings support the concept that CpG-ODN-induced IFN-γ release accounts for the observed lack of TLR-9/TLR-2 and TLR-9/TLR-4 cross-tolerance. Indeed, it could be shown that in vivo administration of CpG-DNA followed 4 hr later by LPS increased LPS toxicity in an IFN-γ-dependent manner.46 As there is no induction of IFN-γ in isolated RAW264·7 macrophages in vitro, it is conceivable that we did not observe the sensitizing effects of CpG-DNA as compared with the in vivo situation. However, if CpG-DNA induces tolerance for subsequent CpG-DNA stimulation also in vivo, then additional factors must be present.
Release of IL-18 in response to secondary CpG-ODN or LPS challenge was equally suppressed in CpG-ODN-, LPS- and LTA-pretreated animals, suggesting IL-18-independent up-regulation of IFN-γ in CpG-preconditioned animals in our model. However, Gould et al. showed a 10-fold increase in LPS-induced IL-18 release in CpG-DNA-pretreated mice.40 This discrepancy is probably related to the different treatment schedule and the higher doses used for preconditioning and challenge as compared with our study, as it has been shown that in many settings tolerance can be overcome by increasing the dose of the challenge.
The role of IL-18 was further studied in an in vitro setting of repeated stimulation of peritoneal macrophages. However, following CpG-ODN preconditioning, cells from IL-18-deficient mice displayed no difference in their responses to restimulation with either CpG-ODN or LTA, as compared with cells from wild-type mice, supporting the view that IL-18-independent mechanisms are responsible for the different consequences of CpG and LPS/LTA pretreatment.
Concerning the differences between in vivo and in vitro experiments, additional factors have to be acknowledged. TLRs differ in their expression profiles on various immune cells. Plasmacytoid dendritic cells (pDCs) have been reported to express TLR-9 but not TLR-2 and -447 and equivalent cells have been identified in mice.48 Given the possibility that CpG-DNA especially activates a subset of cells like pDCs, it seems feasible that these cells do not confer tolerance to LPS- and LTA-responsive myeloid DCs. Also, it has to be noted that TLR-9 is expressed intracellularly while TLR-2 and -4 are thought to initiate activation from the cell surface.49
Taken together, the results of our experiments demonstrate interchangeable potency of TLR-2, -4 and -9 ligands to induce tolerance as well as cross-tolerance in vitro. Whereas LTA/LPS cross-tolerance could also be observed in an in vivo model of GalN + TLR-ligand liver damage, CpG-DNA preconditioning not only failed to induce cross-tolerance to either LPS or LTA but even enhanced liver damage. This suggests non-redundant biological responses of TLR-9 and TLR-2/-4 stimulation in orchestrating the immune response.
Acknowledgments
We thank Helene Bykow, Christine Barett and Margarete Kreuer-Ullmann for excellent technical support. This work was supported by the Deutsche Forschungsgemeinschaft Da 592/1 and He 1452/2, He1452/4, He1452/5.
Abbreviations
- IRAK
interleukin-1 receptor associated kinase
- Mal
MyD88 adaptor like protein
- MALP
macrophage-activating lipopeptide
- MyD88
myeloid differentiation factor 88
- TICAM
toll-interleukin 1 receptor domain (TIR) containing adaptor molecule
- TIRAP
toll-interleukin 1 receptor domain containing adaptor protein
- TRAM
TRIF related adaptor molecule
- TRIF
toll-interleukin 1 receptor domain containing adaptor inducing interferon beta
References
- 1.Greisman SE, Young EJ, Woodward WE. Mechanisms of endotoxin tolerance. IV. Specificity of the pyrogenic refractory state during continuous intravenous infusions of endotoxin. J Exp Med. 1966;124:983–1000. doi: 10.1084/jem.124.5.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Granowitz EV, Porat R, Mier JW, et al. Intravenous endotoxin suppresses the cytokine response of peripheral blood mononuclear cells of healthy humans. J Immunol. 1993;151:1637–45. [PubMed] [Google Scholar]
- 3.Angele MK, Faist E. Clinical review: immunodepression in the surgical patient and increased susceptibility to infection. Crit Care. 2002;6:298–305. doi: 10.1186/cc1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lehner MD, Hartung T. Endotoxin tolerance – mechanisms and beneficial effects in bacterial infection. Rev Physiol. 2002;144:95–141. doi: 10.1007/BFb0116586. [DOI] [PubMed] [Google Scholar]
- 5.Poltorak A, He X, Smirnova I, et al. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 1998;282:2085–8. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- 6.Hoshino K, Takeuchi O, Kawai T, et al. Cutting edge: Toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: evidence for TLR4 as the Lps gene product. J Immunol. 1999;162:3749–52. [PubMed] [Google Scholar]
- 7.Medzhitov R, Janeway CA. Innate immunity: the virtues of a nonclonal system of recognition. Cell. 1997;91:295–8. doi: 10.1016/s0092-8674(00)80412-2. [DOI] [PubMed] [Google Scholar]
- 8.Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- 9.Tabeta K, Georgel P, Janssen E, et al. Toll-like receptors 9 and 3 as essential components of innate immune defense against mouse cytomegalovirus infection. Proc Natl Acad Sci USA. 2004;101:3516–21. doi: 10.1073/pnas.0400525101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hemmi H, Takeuchi O, Kawai T, et al. A Toll-like receptor recognizes bacterial DNA. Nature. 2000;408:740–5. doi: 10.1038/35047123. [DOI] [PubMed] [Google Scholar]
- 11.Krug A, French AR, Barchet W, et al. TLR9-dependent recognition of MCMV by IPC and DC generates coordinated cytokine responses that activate antiviral NK cell function. Immunity. 2004;21:107–19. doi: 10.1016/j.immuni.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 12.Bauer S, Kirschning CJ, Hacker H, et al. Human TLR9 confers responsiveness to bacterial DNA via species-specific CpG motif recognition. Proc Natl Acad Sci USA. 2001;98:9237–42. doi: 10.1073/pnas.161293498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Medvedev AE, Henneke P, Schromm A, et al. Induction of tolerance to lipopolysaccharide and mycobacterial components in Chinese hamster ovary/CD14 cells is not affected by overexpression of Toll-like receptors 2 or 4. J Immunol. 2001;167:2257–67. doi: 10.4049/jimmunol.167.4.2257. [DOI] [PubMed] [Google Scholar]
- 14.Sato S, Nomura F, Kawai T, Takeuchi O, Muhlradt PF, Takeda K, Akira S. Synergy and cross-tolerance between toll-like receptor (TLR) 2- and TLR4-mediated signaling pathways. J Immunol. 2000;165:7096–101. doi: 10.4049/jimmunol.165.12.7096. [DOI] [PubMed] [Google Scholar]
- 15.Lehner MD, Morath S, Michelsen KS, Schumann RR, Hartung T. Induction of cross-tolerance by lipopolysaccharide and highly purified lipoteichoic acid via different Toll-like receptors independent of paracrine mediators. J Immunol. 2001;166:5161–7. doi: 10.4049/jimmunol.166.8.5161. [DOI] [PubMed] [Google Scholar]
- 16.Jacinto R, Hartung T, McCall C, Li L. Lipopolysaccharide- and lipoteichoic acid-induced tolerance and cross-tolerance: distinct alterations in IL-1 receptor-associated kinase. J Immunol. 2002;168:6136–41. doi: 10.4049/jimmunol.168.12.6136. [DOI] [PubMed] [Google Scholar]
- 17.Martin M, Katz J, Vogel SN, Michalek SM. Differential induction of endotoxin tolerance by lipopolysaccharides derived from porphyromonas gingivalis and Escherichia coli. J Immunol. 2001;167:5278–85. doi: 10.4049/jimmunol.167.9.5278. [DOI] [PubMed] [Google Scholar]
- 18.Wang JH, Doyle M, Manning BJ, et al. Cutting edge: bacterial lipoprotein induces endotoxin-independent tolerance to septic shock. J Immunol. 2003;170:14–8. doi: 10.4049/jimmunol.170.1.14. [DOI] [PubMed] [Google Scholar]
- 19.Mizel SB, Snipes JA. Gram-negative flagellin-induced self-tolerance is associated with a block in interleukin-1 receptor-associated kinase release from toll-like receptor 5. J Biol Chem. 2002;277:22414–20. doi: 10.1074/jbc.M201762200. [DOI] [PubMed] [Google Scholar]
- 20.Yeo SJ, Yoon JG, Hong SC, Yi AK. CpG DNA induces self and cross-hyporesponsiveness of RAW264.7 cells in response to CpG DNA and lipopolysaccharide: alterations in IL-1 receptor-associated kinase expression. J Immunol. 2003;170:1052–61. doi: 10.4049/jimmunol.170.2.1052. [DOI] [PubMed] [Google Scholar]
- 21.Crabtree TD, Jin L, Raymond DP, et al. Preexposure of murine macrophages to CpG oligonucleotide results in a biphasic tumor necrosis factor alpha response to subsequent lipopolysaccharide challenge. Infect Immun. 2001;69:2123–9. doi: 10.1128/IAI.69.4.2123-2129.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dobrovolskaia MA, Medvedev AE, Thomas KE, et al. Induction of in vitro reprogramming by Toll-like receptor (TLR) 2 and TLR4 agonists in murine macrophages: effects of TLR ‘homotolerance’ versus ‘heterotolerance’ on NF-κB signaling pathway components. J Immunol. 2003;170:508–19. doi: 10.4049/jimmunol.170.1.508. [DOI] [PubMed] [Google Scholar]
- 23.O'Neill LA, Fitzgerald KA, Bowie AG. The Toll-IL-1 receptor adaptor family grows to five members. Trends Immunol. 2003;24:286–90. doi: 10.1016/s1471-4906(03)00115-7. [DOI] [PubMed] [Google Scholar]
- 24.Fitzgerald KA, Palsson-McDermott EM, Bowie AG, et al. Mal (MyD88-adapter-like) is required for Toll-like receptor-4 signal transduction. Nature. 2001;413:78–83. doi: 10.1038/35092578. [DOI] [PubMed] [Google Scholar]
- 25.Horng T, Barton GM, Medzhitov R. TIRAP: an adapter molecule in the Toll signaling pathway. Nat Immunol. 2001;2:835–41. doi: 10.1038/ni0901-835. [DOI] [PubMed] [Google Scholar]
- 26.Yamamoto M, Sato S, Mori K, Hoshino K, Takeuchi O, Takeda K, Akira S. Cutting edge: a novel Toll/IL-1 receptor domain-containing adapter that preferentially activates the IFN-β promoter in the Toll-like receptor signaling. J Immunol. 2002;169:6668–72. doi: 10.4049/jimmunol.169.12.6668. [DOI] [PubMed] [Google Scholar]
- 27.Yamamoto M, Sato S, Hemmi H, et al. TRAM is specifically involved in the Toll-like receptor 4-mediated MyD88-independent signaling pathway. Nat Immunol. 2003;4:1144–50. doi: 10.1038/ni986. [DOI] [PubMed] [Google Scholar]
- 28.Oshiumi H, Matsumoto M, Funami K, Akazawa T, Seya T. TICAM-1, an adaptor molecule that participates in Toll-like receptor 3-mediated interferon-beta induction. Nat Immunol. 2003;4:161–7. doi: 10.1038/ni886. [DOI] [PubMed] [Google Scholar]
- 29.Oshiumi H, Sasai M, Shida K, Fujita T, Matsumoto M, Seya T. TICACM-2: a bridging adapter recruiting to Toll-like receptor 4 TICAM-1 that induces interferon-beta. J Biol Chem. 2003;278:49751–62. doi: 10.1074/jbc.M305820200. [DOI] [PubMed] [Google Scholar]
- 30.Sato S, Takeuchi O, Fujita T, Tomizawa H, Takeda K, Akira S. A variety of microbial components induce tolerance to lipopolysaccharide by differentially affecting MyD88-dependent and -independent pathways. Int Immunol. 2002;14:783–91. doi: 10.1093/intimm/dxf046. [DOI] [PubMed] [Google Scholar]
- 31.Randow F, Syrbe U, Meisel C, Krausch D, Zuckermann H, Platzer C, Volk HD. Mechanism of endotoxin desensitization: involvement of interleukin 10 and transforming growth factor beta. J Exp Med. 1995;181:1887–92. doi: 10.1084/jem.181.5.1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Medvedev AE, Kopydlowski KM, Vogel SN. Inhibition of lipopolysaccharide-induced signal transduction in endotoxin-tolerized mouse macrophages: dysregulation of cytokine, chemokine, and toll-like receptor 2 and 4 gene expression. J Immunol. 2000;164:5564–74. doi: 10.4049/jimmunol.164.11.5564. [DOI] [PubMed] [Google Scholar]
- 33.Nomura F, Akashi S, Sakao Y, et al. Cutting edge: endotoxin tolerance in mouse peritoneal macrophages correlates with down-regulation of surface toll-like receptor 4 expression. J Immunol. 2000;164:3476–9. doi: 10.4049/jimmunol.164.7.3476. [DOI] [PubMed] [Google Scholar]
- 34.Medvedev AE, Lentschat A, Wahl LM, Golenbock DT, Vogel SN. Dysregulation of LPS-induced Toll-like receptor 4-MyD88 complex formation and IL-1 receptor-associated kinase 1 activation in endotoxin-tolerant cells. J Immunol. 2002;169:5209–16. doi: 10.4049/jimmunol.169.9.5209. [DOI] [PubMed] [Google Scholar]
- 35.Hochholzer P, Lipford GB, Wagner H, Pfeffer K, Heeg K. Role of interleukin-18 (IL-18) during lethal shock: decreased lipopolysaccharide sensitivity but normal superantigen reaction in IL-18-deficient mice. Infect Immun. 2000;68:3502–8. doi: 10.1128/iai.68.6.3502-3508.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Janssens S, Burns K, Tschopp J, Beyaert R. Regulation of interleukin-1- and lipopolysaccharide-induced NF-kappaB activation by alternative splicing of MyD88. Curr Biol. 2002;12:467–71. doi: 10.1016/s0960-9822(02)00712-1. [DOI] [PubMed] [Google Scholar]
- 37.Kobayashi K, Hernandez LD, Galan JE, Janeway CA, Medzhitov R, Flavell RA. IRAK-M is a negative regulator of Toll-like receptor signaling. Cell. 2002;110:191–202. doi: 10.1016/s0092-8674(02)00827-9. [DOI] [PubMed] [Google Scholar]
- 38.Sweet MJ, Leung BP, Kang D, et al. A novel pathway regulating lipopolysaccharide-induced shock by ST2/T1 via inhibition of toll-like receptor 4 expression. J Immunol. 2001;166:6633–9. doi: 10.4049/jimmunol.166.11.6633. [DOI] [PubMed] [Google Scholar]
- 39.Bundschuh DS, Barsig J, Hartung T, Randow F, Docke WD, Volk HD, Wendel A. Granulocyte-macrophage colony-stimulating factor and IFN-gamma restore the systemic TNF-alpha response to endotoxin in lipopolysaccharide-desensitized mice. J Immunol. 1997;158:2862–71. [PubMed] [Google Scholar]
- 40.Gould MP, Greene JA, Bhoj V, DeVecchio JL, Heinzel FP. Distinct modulatory effects of LPS and CpG on IL-18-dependent IFN-γ synthesis. J Immunol. 2004;172:1754–62. doi: 10.4049/jimmunol.172.3.1754. [DOI] [PubMed] [Google Scholar]
- 41.Gao JJ, Diesl V, Wittmann T, Morrison DC, Ryan JL, Vogel SN, Follettie MT. Regulation of gene expression in mouse macrophages stimulated with bacterial CpG-DNA and lipopolysaccharide. J Leukoc Biol. 2002;72:1234–45. [PubMed] [Google Scholar]
- 42.Albrecht I, Tapmeier T, Zimmermann S, Frey M, Heeg K, Dalpke A. Toll-like receptors differentially induce nucleosome remodelling at the IL-12p40 promoter. EMBO Rep. 2004;5:172–7. doi: 10.1038/sj.embor.7400078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cowdery JS, Boerth NJ, Norian LA, Myung PS, Koretzky GA. Differential regulation of the IL-12 p40 promoter and of p40 secretion by CpG DNA and lipopolysaccharide. J Immunol. 1999;162:6770–5. [PubMed] [Google Scholar]
- 44.Zimmermann S, Egeter O, Hausmann S, Lipford GB, Roecken M, Wagner H, Heeg K. Cutting edge: CpG oligodeoxynucleotides trigger protective and curative Th1 responses in lethal murine leishmaniasis. J Immunol. 1998;160:3627–30. [PubMed] [Google Scholar]
- 45.Hermann C, Spreitzer I, Schroder NW, et al. Cytokine induction by purified lipoteichoic acids from various bacterial species – role of LBP, sCD14, CD14 and failure to induce IL-12 and subsequent IFN-γ release. Eur J Immunol. 2002;32:541–51. doi: 10.1002/1521-4141(200202)32:2<541::AID-IMMU541>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 46.Cowdery JS, Chace JH, Yi AK, Krieg AM. Bacterial DNA induces NK cells to produce IFN-gamma in vivo and increases the toxicity of lipopolysaccharides. J Immunol. 1996;156:4570–5. [PubMed] [Google Scholar]
- 47.Hornung V, Rothenfusser S, Britsch S, et al. Quantitative expression of Toll-like receptor 1–10 mRNA in cellular subsets of human peripheral blood mononuclear cells and sensitivity to CpG oligodeoxynucleotides. J Immunol. 2002;168:4531–7. doi: 10.4049/jimmunol.168.9.4531. [DOI] [PubMed] [Google Scholar]
- 48.Nakano H, Yanagita M, Gunn MD. CD11c+B220+Gr-1+ cells in mouse lymph nodes and spleen display characteristics of plasmacytoid dendritic cells. J Exp Med. 2001;194:1171–8. doi: 10.1084/jem.194.8.1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Latz E, Schoenemeyer A, Visintin A, et al. TLR9 signals after translocating from the ER to CpG DNA in the lysosome. Nat Immunol. 2004;5:190–8. doi: 10.1038/ni1028. [DOI] [PubMed] [Google Scholar]