Abstract
Protein kinase (PK) C comprises a family of isoenzymes that play key roles in downstream signalling and cell functions. We studied PKC ζ participation in the effector functions of human eosinophils stimulated with platelet-activating factor (PAF) or complement (C) 5a. After pretreating eosinophils with a myristoylated specific PKC ζ inhibitor; bisindlolylmaleimide I (BisI), an inhibitor of conventional and novel PKCs; or rottlerin, a PKC δ inhibitor, we examined PAF- and C5a-evoked functions. Induced PKC translocation was characterized by confocal laser scanning microscopy. The PKC ζ inhibitor blocked PAF- or C5a-induced eosinophil superoxide anion generation as effectively as BisI or rottlerin. The PKC ζ inhibitor also attenuated PAF- or C5a-induced eosinophil degranulation and adhesion. In contrast, the PKC ζ inhibitor did not affect PAF- or C5a-induced CD11b expression. Finally, both eosinophil shape changes and the translocation of PKC ζ and p47phox, a component of nicotinamide adenine dinucleotide phosphate (NADPH) oxidase, to the plasma membrane induced by PAF or C5a were completely inhibited by the PKC inhibitor. Thus, the atypical PKC ζ regulates human eosinophil adhesion and effector functions.
Keywords: eosinophils, protein kinase C, superoxide anion, degranulation, adhesion, platelet-activating factor, complement 5a
Introduction
Eosinophils are involved in the pathogenesis of allergic diseases such as bronchial asthma, pollinosis and atopic dermatitis, and in the inflammatory response to parasitic infections.1 When eosinophils are activated by appropriate stimuli including immunoglobulins, cytokines and lipid mediators, these cells release inflammatory mediators, toxic cationic granule proteins and oxygen radicals, all resulting in tissue damage at inflammatory sites.2
Platelet-activating factor (PAF) and complement 5a (C5a) are among the most potent activators of eosinophil effector functions.2,3 These effectors act as agonists at leucocyte surface seven-transmembrane receptors that are coupled to G proteins.4,5 We have shown that cellular adhesion-mediated β2 integrins, especially αMβ2 (CD11b/CD18, Mac-1), are critical to the effector functions of eosinophils occurring in response to these receptor–ligand interactions.6–8 This receptor-mediated stimulation induces a variety of functions, including αMβ2 expression on the cell surface and conformational changes of integrin molecules via a process termed ‘inside-out’ signalling.9 Once clustering or multimerization of αMβ2 occurs at the site of focal adhesion, this stimulation of αMβ2 triggers activation of protein tyrosine kinases, phospholipase (PL) C and phosphatidylinostol 3-kinase (PI3K); calcium mobilization; and activation of protein kinase (PK) C, resulting in promotion of eosinophil effector functions. This phase is called ‘outside-in’ signalling.8,9 In both signalling processes, PKC comprises a family of isoenzymes that play key roles in downstream signalling events and cell functions.10–12 Activation of PKCs in intact cells generally is associated with translocation of the enzyme from the cytosol to the cell membrane.13–15
PKCs have been classified into three groups based on molecular structure and mode of activation: (1) ‘conventional’ PKCs (α, βΙ, βΙΙ and γ), which require phosphatidylserine (PS) and are activated by calcium and diacylglycerol (DAG); (2) ‘novel’ PKCs (δ, ε, µ, θ and η), which require PS and DAG but not calcium for activation; and (3) ‘atypical’ PKCs (ζ and τ/λ), which are calcium-independent and are not activated by DAG.10–12 Among the atypical PKCs, PKC ζ can be stimulated by phospholipids including phosphatidic acid (PA), phosphatidylinositol triphosphates and ceramides.
A recent report demonstrated expression of at least eight PKC isoforms in human eosinophils (PKC α, βΙ, βΙΙ, ζ, δ, ε, ι and µ), and indicated that PKC ζ expression was induced after antigen challenge.16 Another study indicated that PKC α, βΙ, βΙΙ, γ, δ and ζ are expressed in human eosinophils, and that PKC δ makes an important contribution to agonist-induced nicotinamide adenine dinucleotide phosphate (NADPH) oxidase activity.17 However, the role of atypical PKC ζ in mediating eosinophil effector functions has not been documented to date. In the present study, we sought to determine how PKC ζ modulates the effector functions of human eosinophils stimulated with PAF or C5a.
Materials and methods
Reagents
Platelet-activating factor (PAF) C-16 was obtained from Biomol Research Laboratories (Plymouth Meeting, PA). Complement (C) 5a was purchased from Novabiochem (San Diego, CA). Globulin-free human serum albumin (HSA, A3782), cytochrome c, superoxide dismutase (SOD) and dimethyl sulfoxide (DMSO) were purchased from Sigma (St. Louis, MO). Myristoylated peptide PKC ζ and peptide η inhibitors, a myristoylated peptide 20-28 PKC inhibitor, or bisindolylmaleimide I (2-[1-(3-dimethylaminopropyl)-1H-indol-3-yl]-3-(1H-indol-3-yl)-maleimide; BisI), and rottlerin were purchased from Calbiochem (San Diego, CA) and dissolved at 100 mm in distilled water or DMSO, respectively. The solutions were diluted in reaction medium immediately before use. A chemiluminescence probe for superoxide radicals, the Cypridina luciferin analogue (2-methyl-6-(p-methoxyphenyl)-3,7-dihydroimidazo-[1,2-a] pyrazin-3-one; MCLA), obtained from Tokyo Kasei (Tokyo, Japan), was dissolved in doubly distilled water. The concentration of MCLA was based on ε430 nm = 9600 m−1cm−1, as previously described.18,19 The solutions were diluted in reaction medium immediately before use. Alexa Fluor 532 phalloidin, Alexa Fluor 488 rabbit anti-goat immunoglobulin G (IgG) (H + l), and 4′,6-diamidino-2-phenylindole (DAPI) were obtained from Molecular Probes (Eugene, OR). p47phox-specific or PKC ζ-specific goat polyclonal antibody and goat IgG were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). All of these reagents were diluted in each reaction medium at optimal concentration immediately before the experiments. Phycoerythrin (PE)-conjugated anti-CD11b mouse monoclonal antibody (mAb; ICRF44) and mouse IgG2a control immunoglobulin were purchased from Becton Dickinson (San Jose, CA).
Cell isolation
Eosinophils were purified by a previously described method with minor modifications, using a magnetic cell separation (MACS; Becton Dickinson, San Jose, CA) system.19,20 Briefly, heparinized blood was obtained from healthy donors and diluted with an equal volume of phosphate-buffered saline (PBS). Diluted blood was layered over Histopaque solution (density, 1·083 g/ml; Sigma) and centrifuged at 1000 g for 30 min. Then the supernatant was removed and erythrocytes in pellets were subjected to two cycles of hypotonic water lysis. Isolated granulocytes were washed with piperazine-N,N′-bis (2-ethanesulfonic acid) (PIPES; Sigma) buffer containing 1% fetal bovine serum (FBS; JRH Biosciences, Lenexa, KS), and an equal volume of anti-CD16 antibody-conjugated magnetic particles (Miltenyi Biotec GmbH, Bergish Gladbach, Germany) was added to the cell pellet. After 45 min on ice, cells were resuspended and loaded onto a separation column positioned in a strong magnetic field for MACS. Cells were eluted three times. The purity of eosinophils determined by counting Randolph's stain was more than 98%. Cell viability always exceeded 98% as determined by trypan blue exclusion and propidium iodide staining.
Superoxide anion (O2–) generation
O2– release by eosinophils was measured by MCLA-dependent chemiluminescence using a luminescence reader (BLR-102, 301; Aloka, Tokyo, Japan) as described previously.18,19 Polyethylene test tubes (Aloka) were coated with 250 µl of 2·5% human serum albumin (HSA) dissolved in PBS at pH 7·40 and kept overnight at 4°. The next day, the tubes were washed three times with PBS and then used immediately. Eosinophils were washed with Hanks' balanced salt solution (HBSS) and resuspended in the same medium at 106 cells/ml. Stimulants were diluted in the same medium at the desired concentration. Assay mixtures contained 2·5 × 105 cells, the various PKC inhibitors, 3 µm MCLA, PAF or C5a, and HBSS in a total volume of 2·0 ml. Briefly, cell suspensions (250 µl) and the various PKC inhibitors were added to the HSA-coated tubes, incubated for 10 min at 37°, and then prewarmed at 37° for 5 min with MCLA. After incubation, the reaction was then started by adding PAF or C5a as a stimulant. The amount of O2– release (counts) was calculated as the area under the chemiluminescence curve (AUC) and corrected by subtracting control readings.
Eosinophil degranulation
Eosinophil degranulation was assessed by a slight modification of a previously described method.20–22 Briefly, in HSA-coated, flat-bottomed, 96-well, flat-bottomed tissue culture plates, freshly isolated human eosinophils were suspended at 5 × 104 cells/well in RPMI 1640 with the addition of 25 mm HEPES. After a PKC inhibitor was added to each well and the plate was incubated for 15 min at 37°, the reactions were initiated by adding a stimulant (PAF or C5a at a final concentration of 1 µm or 100 ng/ml, respectively). After incubation for 4 hr, the supernatants were collected and stored at −20° until radioimmunoassay (RIA) for eosinophil protein X (EPX; Pharmacia-Upjohn, Tokyo, Japan) content to quantify eosinophil degranulation.20–22 Total cellular EPX content was measured simultaneously in supernatants from cells lysed with 0·5% Nonidet P-40 (NP-40) detergent. All experiments were performed in duplicate.
Eosinophil adhesion assay
Numbers of adherent eosinophils were determined by measuring the content of EPX in adherent cells by RIA, as previously reported.21,22 Briefly, in the HSA-coated wells of 96-well, flat-bottomed tissue culture plates, freshly isolated human eosinophils were suspended at 5 × 104 cells/well in RPMI 1640 including 25 mm HEPES. Then a PKC inhibitor was added to each well, and the plate was incubated at 37° for 15 min. After incubation, reactions were initiated by adding the stimulant, PAF or C5a, at respective concentrations of 1 µm or 100 ng/ml. After 60 min the supernatants were collected, and the plate was rinsed gently with warm RPMI 1640 to remove non-adherent cells. Adherent cells were then lysed with 0·5% NP-40 detergent, and EPX content in the lysate was measured by RIA. All experiments were performed in duplicate. Per cent adhesion was calculated as the ratio of EPX content in adherent eosinophils to total available EPX after incubation according to the following equation:
Expression of CD11b
Purified eosinophils were suspended in RPMI 1640 with 1% FBS at 106 cells/ml, preincubated with each PKC inhibitor for 15 min, and then stimulated with 1 µm PAF or 100 ng/ml C5a for 15 min at 37°. The reaction was then stopped on ice, and cells were resuspended in 50 µl of RPMI 1640 for incubation with an anti-CD11b mAb or isotype-matched immunoglobulin, mouse IgG2a, for 30 min at 4°. Cells were resuspended in PBS, and expression of CD11b was determined using a flow cytometer (Epics XLII; Beckman Coulter, Tokyo, Japan) and reported as mean fluorescence intensity (MFI).21,22 The viability of cells was determined simultaneously by staining with propidium iodide (1 µg/ml).
Visualization of PKCs
A suspension of 1–3 × 105 eosinophils in phenol red-free RPMI 1640 medium with 1 mm HEPES was incubated with or without 1 or 3 µm PKC ζ inhibitor for 15 min in 37° in an HSA-coated 35-mm glass-bottomed dish (Matsunami, Osaka, Japan). After incubation, PAF or C5a was added at a final concentration of 1 µm or 100 ng/ml, respectively. After 15 min at 37°, the mixture in each dish was gently aspirated, and 2 ml of 3·7% formaldehyde in PBS (v/v) was added carefully to each dish to fix attached cells for 5 min at room temperature. Then cells were washed gently with PBS and permeabilized with 2 ml of 0·1% Triton X-100 in PBS in each dish for 10 min. After permeabilization, cells were washed twice with PBS and blocked with BSA for 30 min at room temperature. Cells were then washed once, and incubated for 30 min at 4° with primary antibody (p47phox-specific or PKC ζ-specific antibody, or goat IgG as a negative control; all dilutions 1 : 500). Then cells were washed three times and incubated for 30 min in darkness at room temperature with a mixture of Alexa Fluor 532 phalloidin (1 unit/dish) and secondary antibody (Alexa Fluor 488 rabbit anti-goat IgG; dilution 1 : 1000). Finally, after five washes with PBS, DAPI was mounted on the samples, and the fluorescent signal was observed with a confocal laser scanning microscope system (MRC-1024; Bio-Rad, Tokyo, Japan) as previously described with minor modifications.22
Statistical analysis
All data were normalized to the increased values in response to PAF or C5a without the drugs (considered 100%) and presented as standard errors of means (SEMs) of three to seven experiments. The statistical significance of the differences from the increased values obtained without preincubation of the drugs in response to PAF or C5a was assessed using paired or unpaired Student t-tests. Differences associated with a P-value of < 0·05 were considered significant.
Results
Effects of PKC inhibitors on superoxide anion (O2–) generation by human eosinophils
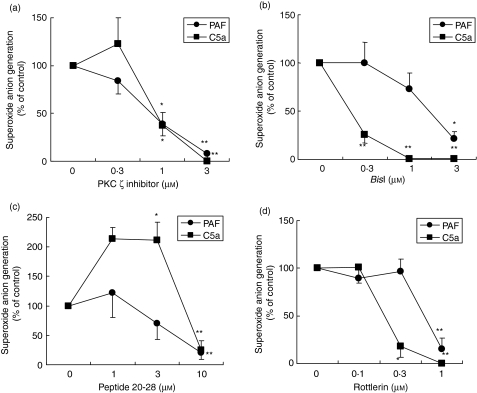
As shown in Fig. 1(a), myristoylated PKC ζ inhibitor peptide significantly suppressed PAF- or C5a-induced O2– generation in a dose-dependent manner. We compared this effect with those of other PKC inhibitors such as BisI (a conventional and novel PKC inhibitor), PKC inhibitor peptide 20-28 (a PKC α, β inhibitor) and rottlerin (a PKC δ inhibitor). As shown in Figs 1(b) and (d), BisI, PKC inhibitor peptide 20-28 and rottlerin blocked PAF- or C5a-induced O2– generation. However, a significant difference in the inhibitory effects of BisI, peptide 20-28 and rottlerin was observed between PAF- and C5a-induced eosinophil superoxide generation. A summary of the inhibitory effects of PKC inhibitors, including IC50values, is presented in Table 1; the IC50 of the specific PKC ζ inhibitor with respect to PAF- or C5a-induced O2– generation was very similar to that of rottlerin. The IC50 value for the effect of BisI on C5a-induced O2– generation was much smaller than that on PAR-induced O2– generation. Preliminary studies demonstrated that concentrations of DMSO used in these experiments did not affect eosinophil viability or stimulus-dependent O2– production, and each PKC inhibitor, by itself, did not affect spontaneous O2– generation and cell viability (data not shown).
Figure 1.
Effects of protein kinase C (PKC) inhibitors on superoxide anion (O2–) generation by human eosinophils. Purified human eosinophils in human serum albumin-coated wells (2·5 × 105 cells/tube) were stimulated with platelet-activating factor (PAF) (1 µm) or complement 5a (C5a) (100 ng/ml) after preincubation with each PKC inhibitor [ (a) PKC ζ inhibitor; (b) bisindlolylmaleimide I (BisI); (c) PKC inhibitor peptide 20-28; (d) rottlerin] for 10 min at 37° and then prewarmed at 37° for 5 min with 2-methyl-6-(p-methoxyphenyl)-3,7-dihydroimidazo-[1,2-a] pyrazin-3-one (MCLA). The O2– generated for 30 min after stimulation by PAF or C5a is presented as the mean ± standard error of the mean (SEM) for four to 11 experiments. *P < 0·05; **P < 0·01; significant differences compared with cells stimulated with PAF or C5a in the absence of PKC inhibitors. O2– production in the absence of drug was as follows: PAF, 354·5 ± 41·1; C5a, 120·5 ± 23·9 (× 104 counts; mean ± SEM; n = 11–14).
Table 1.
IC50 values of protein kinase C (PKC) inhibitors for superoxide anion generation induced by platelet-activating factor (PAF) or complement 5a (C5a)
| [IC50 (µm)] | ||
|---|---|---|
| PAF | C5a | |
| PKC ζ inhibitor | 0·8 | 0·9 |
| BisI | 1·5 | 0·1 |
| Peptide 20-28 | 5·1 | 7·5 |
| Rottlerin | 0·5 | 0·2 |
Purified human eosinophils in human serum albumin-coated wells (5 ×104 cells/well) were stimulated with PAF (1 µm) or C5a (100 ng/ml) after preincubation with each PKC inhibitor for 15 min. IC50 values of PKC inhibitors (µm) were calculated from the values of superoxide anion generation for 30 min after stimulation by PAF or C5a.
BisI, bisindlolylmaleimide I.
Effects of PKC inhibitors on eosinophil degranulation
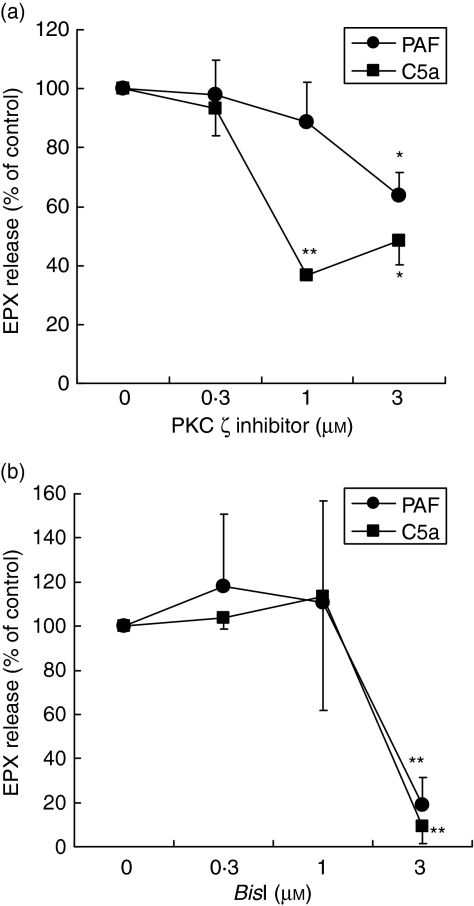
We next examined effects of PKC inhibitors on another major eosinophil effector function, degranulation, by quantifying release of a granule protein, EPX, into the supernatants by eosinophils. As shown in Fig. 2(a), the PKC ζ inhibitor suppressed PAF- or C5a-induced degranulation as well as BisI (Fig. 2b). PKC ζ inhibitor or BisI by itself did not affect the spontaneous degranulation response (data not shown).
Figure 2.
Effects of protein kinase C (PKC) inhibitors on eosinophil degranulation. Purified human eosinophils (5 × 104 cells/well) were stimulated with platelet-activating factor (PAF) (1 µm) or complement 5a (C5a) (100 ng/ml) in the presence or absence of PKC in a human serum albumin-coated plate at 37°. After 4 hr, concentrations of eosinophil protein X (EPX) in the cell-free supernatants were analysed by radioimmunoassay (RIA) as an indicator of eosinophil degranulation. Values are expressed as mean ± standard error of the mean (SEM) for four to seven independent experiments. EPX content in the absence of drug was as follows: medium alone, 92·9 ± 13·9; PAF, 512·9 ± 103·4; and C5a, 417·6 ± 91·9 ng/106 cells (mean ± SEM; n = 8–11). Total cellular EPX content was 2771·7 ± 332·76 ng/106 cells (mean ± SEM; n = 11). *P < 0·05; **P < 0·01; significant differences compared with cells stimulated with PAF or C5a in the absence of inhibitor.
Effects of PKC inhibitors on eosinophil adhesion
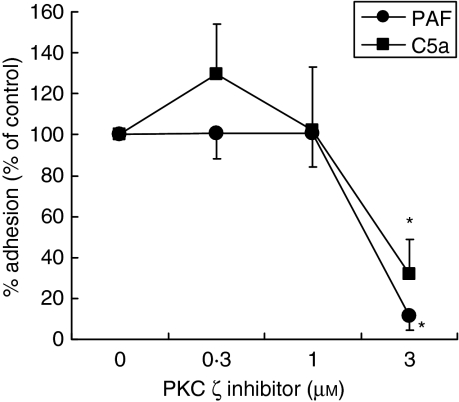
Because cellular adhesion is considered critical to the effector functions of human eosinophils, we examined the effect of the PKC ζ inhibitor on adhesion of eosinophils to HSA-coated plates at 1 hr after stimulation using an adhesion assay. As shown in Fig. 3, the PKC ζ inhibitor suppressed PAF- or C5a-induced cellular adhesion. The PKC ζ inhibitor did not affect the spontaneous adhesion response (data not shown).
Figure 3.
Effects of protein kinase C (PKC) inhibitors on eosinophil adhesion. Purified human eosinophils (5 × 104 cells/well) were stimulated with platelet-activating factor (PAF) (1 µm) or complement component 5a (C5a) (100 ng/ml) in the presence or absence of PKC inhibitor in a human serum albumin-coated plate at 37°. After 1 hr, percentages of eosinophils adherent to the wells of the plate were determined. Data are expressed as mean ± standard error of the mean (SEM) for four independent experiments. Percentage adhesion of control cells in the absence of drugs was as follows: medium alone, 4·3 ± 0·9%; PAF, 14·1 ± 1·7%; and C5a, 15·9 ± 1·9% (mean ± SEM; n = 4). Total cellular EPX content was 2817·7 ± 235·8 ng/106 cells (mean ± SEM; n = 4). *P < 0·05; significant differences compared with cells stimulated with PAF or C5a in the absence of inhibitor.
Effects of PKC inhibitors on CD11b expression in eosinophils
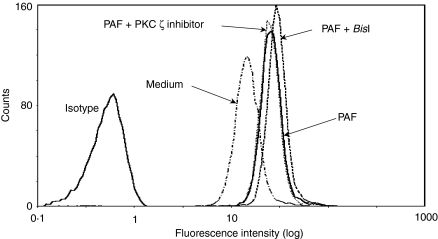
We tested the effect of PKC inhibitors on CD11b expression on the surfaces of eosinophils, an indicator of ‘inside-out’ signalling involving β2 integrin-mediated effector functions. As shown in Table 2 and Fig. 4, the PKC ζ inhibitors did not have as great an effect on spontaneous or PAF- or C5a-induced CD11b expression in eosinophils as other PKC inhibitors, while only BisI enhanced PAF-induced CD11b expression, as previously reported.21,34
Table 2.
Effects of protein kinase C (PKC) inhibitors on CD11b expression on eosinophils
| Mean fluorescence intensity (MFI) | ||
|---|---|---|
| PAF | C5a | |
| PKC ζ inhibitor | 91·6 ± 3·1 | 103·3 ± 4·4 |
| BisI | 145·9 ± 7·4** | 125·5 ± 12·3 |
| Peptide 20-28 | 101·9 ± 9·6 | 118·0 ± 7·2 |
| Rottlerin | 88·1 ± 6·5 | 127·8 ± 16·4 |
Purified eosinophils (2·5 ×105 cells/sample) were stimulated with or without 1 µm platelet-activating factor (PAF) or 100 ng/ml complement 5a (C5a) after pretreatment with PKC inhibitor (3 µm PKC ζinhibitor, 1 µmBisI, 10 µm peptide 20-28 or 10 µm rottlerin) or medium alone for 15 min at the concentrations indicated. Expression of CD11b was determined by flow cytometric analysis as mean fluorescence intensity (MFI). The MFI in the absence of inhibitors was as follows: medium alone, 14·3 ± 1·1; PAF, 22·8 ± 1·7; and C5a, 24·2 ± 0·6 (mean ± standard error of the mean (SEM); n = 5–9).
P < 0·01; significant differences compared with the cells stimulated with PAF or C5a in the absence of inhibitors.
BisI, bisindlolylmaleimide I.
Figure 4.
A typical result for CD11b expression on eosinophils. The histograms show a typical result for CD11b expression on eosinophils stimulated with platelet-activating factor (PAF). Purified eosinophils (2·5 × 105 cells/sample) were stimulated with or without 1 µm PAF after pretreatment with 3 µm protein kinase C (PKC) ζ inhibitor, 1 µm bisindlolylmaleimide I (BisI), or medium alone for 15 min.
Effect of PKC ζ inhibitor on stimulus-induced translocation of p47phox or PKC ζ in eosinophils
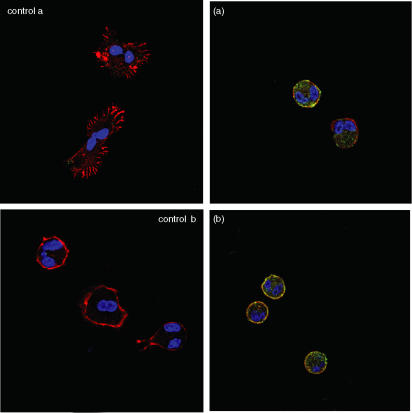
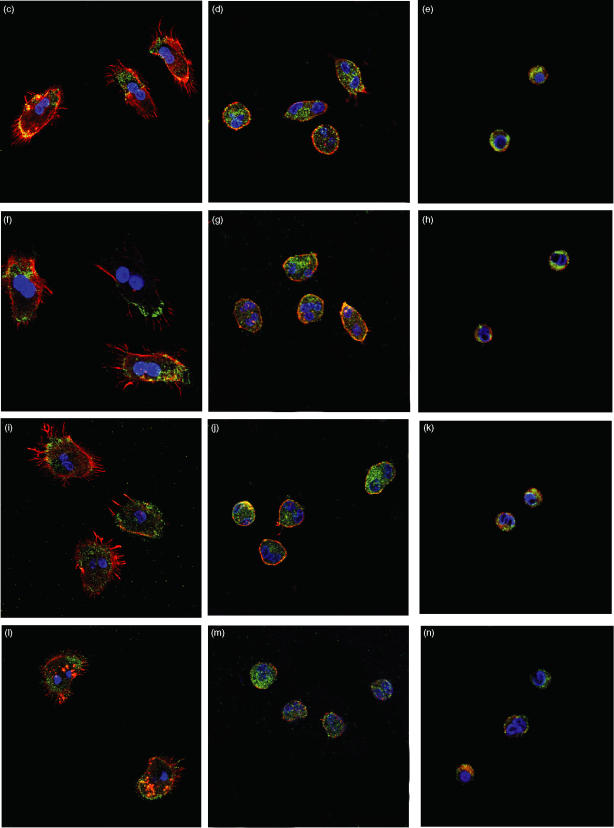
Finally, to confirm the above results suggesting that PKC ζ modulates eosinophil effector functions, we examined the effect of the PKC ζ inhibitor on PAF- or C5a-induced translocation of p47phox or PKC ζ in eosinophils. Because these molecules translocate from the cytosol to just beneath the plasma membrane upon activation, we assessed the intracellular locarization of these molecules 15 min after stimulation by PAF or C5a. Immunofluorescent staining was used to mark the locarization of the molecules, as determined by confocal laser scanning microscopy. As shown in Fig. 5, p47phox (Figs 5c and i) or PKC ζ (Figs 5f and l), marked with green fluorescence, translocated from the cytosol to the plasma membrane upon stimulation by PAF (Figs 5b, c and f) or C5a (Figs 5c, i and l), but did not do so in medium alone (Figs 5a and b). Fluorescent phalloidin staining (F-actin), displayed in red, indicated that upon activation eosinophils changed shape dramatically, with extension of filopodia. Both the change in the cell shape and translocation of p47phox (Figs 5d, e, j and k) or PKC ζ (Figs 5g, h, m and n) were blocked by 1 or 3 µm PKC ζ inhibitor, while the effect of the pretreatment with 0·3 µm PKC ζ inhibitor was negligible (data not shown).
Figure 5.
Effect of protein kinase C (PKC) ζ inhibitor on (b) platelet-activating factor (PAF)- or (c) complement 5a (C5a)-induced translocation of p47phox and PKC ζ in human eosinophils. Eosinophils were treated with medium alone (a and b), 1 µm PAF (c–h; control a) or 100 ng/ml C5a (i–n; control, b) in a human serum albumin-coated glass-bottomed dish without (a, b, c, f, i and l) or with 1 µ m (d, g, j and m) and 3 µm (e, h, k and n) PKC ζ inhibitor. After adding PAF, C5a or medium, cells were fixed and stained using p47phox-specific (a, c, d, e, i, j and k) and PKC ζ-specific (b, f, g, h, l, m and n) goat polyclonal antibody (primary antibody). Cells then were exposed to a staining cocktail containing Alexa Fluor 532 phalloidin and Alexa Fluor 488 rabbit anti-goat immunoglobulin G (IgG) as labelled second ligands. DAPI was mounted on each sample and fluorescence was observed with a confocal laser scanning microscope. The original magnification of all figures is the same (×600). The red colour shows fluorescence induced by Alexa Fluor 532 phalloidin (F-actin). The green colour shows fluorescence induced by Alexa Fluor 488 rabbit anti-goat IgG (p47phox or PKC ζ). The blue colour shows fluorescence induced by DAPI (nucleus). The negative control (goat IgG used instead of primary antibody) was shown as control a or b. The experiments were repeated three to five times with essentially identical results.
Discussion
In the present study, a PKC ζ inhibitor blocked PAF- or C5a-induced eosinophil O2– generation, as did BisI or rottlerin. This inhibitor also attenuated PAF- or C5a-induced degranulation and adhesion. In contrast, the PKC ζ inhibitor did not affect PAF- or C5a-induced CD11b expression. The PKC ζ inhibitor completely blocked PAF- or C5a-induced eosinophil shape changes as well as the translocation of PKC ζ and p47phox to the plasma membrane.
PKCs are being elucidated as an increasingly diverse family of enzymes involved in signal transduction in various cells, including eosinophils.10–12 Studies using a phorbol ester, phorbol 12-myristate 13-acetate (PMA), that activates PKC suggest that this enzyme is involved in eosinophil functions such as cell adhesion,23 degranulation,6,24 and O2– generation.6,25 Non-specific PKC inhibitors also modulate eosinophil functions stimulated with PAF or C5a that do not involve motility.26–28 For example, staurosporine blocked a C5a- or PMA-induced respiratory burst,26,27 and staurosporine or calphostin C inhibited PAF- or C5a-enhanced eosinophil cationic protein (ECP) release.28 However, in contrast to eosinophil functions not involving motility, PAF-induced chemotaxis is independent of PKC activation,29 while PKC negatively regulates PAF-induced aggregation.30 In guinea pig eosinophils, PKC has been suggested to negatively regulate PAF-induced calcium influx and degranulation.31 A previous study in human eosinophils demonstrated that PKC negatively modulated PAF-induced arachidonic acid metabolite production,32 while augmenting sustained intracellular calcium increase and O2– generation induced by PAF.32,33 We also reported that PKC modulated PAF-activated eosinophil functions in two ways; PKC inhibition enhanced CD11b expression and adhesion, while suppressing O2– generation and degranulation.21,34 Furthermore, in this study, a significant difference was observed in the effects of each PKC inhibitor on PAF- and C5a-induced eosinophil superoxide generation. Although the exact mechanism by which differences were produced between PAF and C5a in the IC50 values of each inhibitor is unknown, the involvement of the PKC isoform might differ between individual stimulant and eosinophil functions.
To date, 11 PKC isoforms have been identified: α, βΙ, βΙΙ, δ, ε, γ, η, ι, θ, µ and ζ. On the basis of molecular structure and biochemical properties, the PKC family can be divided into three groups: conventional PKCs (α, βΙ, βΙΙ and γ isoforms); the novel PKCs (δ, ε, η and θ isoforms); and the atypical PKCs (ι, µ and ζ isoforms).10–12 At present, the biologic significance of heterogeneity as well as the function of individual isoenzymes largely remains unknown. Further, specific roles of PKC isozymes in eosinophil function remain to be investigated. In contrast to the many reports concerning other cells, such as neutrophils, little has been revealed about atypical, conventional or novel PKCs in eosinophils. A recent study demonstrated PKC α, βΙ, βΙΙ, γ, δ and ζ expression as well as important regulation by PKC δ of interleukin (IL)-5- or leukotriene (LT)B4-evoked NADPH oxidase activity in human eosinophils.17 We also showed that PKC δ is involved in O2– generation but not in CD11b expression in PAF-stimulated eosinophils.33 Another report demonstrated expression of at least eight isoforms of PKC (PKC α, βΙ, βΙΙ, ζ, δ, ε, ι and µ) in human eosinophils, in which PKC ζ but not PKC α, βI or βII showed increased translocation to the membrane in vitro 24 hr after antigen challenge in the asthmatic patients whose cells were sampled.16 Very recently, we have shown that translocation of PKC βΙΙ, δ and ζ modulates eosinophil O2– generation and that an actin polymerization inhibitor, cytochalasin B, inhibits eosinophil shape change and the translocation of these PKCs and a NADPH oxidase component, p47phox, suggesting that the actin cytoskeleton and the translocation of PKCs and p47phox play an important role in O2– generation in human adherent eosinophils.22 In this study we further demonstrated that the PKC ζ inhibitor completely blocked both the eosinophil shape change and the translocation of p47phox. This result is to a large extent comparable to that of Dang et al., who showed that p47phox is a substrate for PKC ζ and participates in the signalling cascade between the receptor and NADPH oxidase activation in human neutrophils stimulated with N-formyl-methionyl-leucyl-phenylalanine (FMLP).35
In the context of these observations, the present study is the first to show that PKC ζ modulates human eosinophil adhesion and effector functions in vitro. In human neutrophils, PKC ζ but no conventional PKC was found to participate in integrin-dependent adhesion.36 This is comparable to our finding in eosinophils that at least PKC ζ modulated integrin-dependent adhesion and effector functions but did not affect CD11b expression. With respect to integrin activation, cellular events preceding adhesion are referred to as ‘inside-out’ signalling, a view supported by observation of CD11b expression before adhesion occurred. In contrast, most effector functions of eosinophils, such as O2– generation, require adhesion through integrins (‘outside-in’ signalling). In this study, treatment with a myristoylated PKC inhibitor (20-28 peptide), rottlerin, and the PKC ζ inhibitor did not affect PAF- or C5a-induced CD11b expression, while only BisI enhanced PAF-induced CD11b expression. This indicates that PKC isoforms other than PKC α, β, δ and ζ are involved in PAF-induced CD11b expression, representing inside-out signalling. In contrast, at high concentrations, BisI up-regulated PAF- or C5a-induced eosinophil adhesion, while it decreased PAF- or C5a-induced superoxide generation and degranulation (reference21 and data not shown). Furthermore, rottlerin or peptide 20-28 inhibited the adhesion response stimulated by PAF or C5a (data not shown). These results suggest that conventional and novel PKCs are diversely involved in both cell adhesion and effector functions of eosinophils, representing ‘outside-in’ signalling.
Recently, we have found that PAF activates two distinct effector pathways leading to O2– generation: one is pertussis toxin (PTX)-sensitive with immediate and transient activation, and the other is PTX-resistant with late and extended activation. We have discovered that the latter pathway evokes substantial O2– production and is mediated by PI3K.20 In addition, previous reports have shown that PI3K is essential for PKC δ37 and PKC ζ activation.38 Taken together, these observations and our present results suggest that substantial stimulus-induced O2– generation, one of the functions mediated by outside-in signalling, is modulated by PKC ζ and δ, which might be activated by PI3K. In marked contrast, inhibition of PI3K did not notably reduce PAF-induced CD11b expression (Y. Motegi, M. Kato et al., unpublished data), which suggests that CD11b expression is mediated by a signalling pathway distinct from O2– generation, and independent of PI3K activation and PKC ζ and δ.
In conclusion, we have demonstrated that PKC ζ modulates eosinophil effector functions. Although much about the mechanism of PKC ζ participation in eosinophil functions requires further elucidation, our results provide evidence that an atypical PKC, PKC ζ, as well as a novel PKC, PKC δ, might be therapeutic targets for decreasing eosinophilic inflammation in clinical states such as asthma.
Abbreviations
- C5a
complement 5a
- DAPI
4′,6-diamidino-2-phenylindole
- DMSO
dimethyl sulfoxide
- EPX
eosinophil protein X
- HSA
human serum albumin
- NADPH
nicotinamide adenine dinucleotide phosphate
- p47phox
p47 phagocyte oxidase
- PAF
platelet-activating factor
- PI3K
phosphatidylinostol 3-kinase
- PKC
protein kinase C
- PTX
pertussis toxin
References
- 1.Gleich GJ. Mechanisms of eosinophil-associated inflammation. J Allergy Clin Immunol. 2000;105:651–63. doi: 10.1067/mai.2000.105712. [DOI] [PubMed] [Google Scholar]
- 2.Kita H, Adolphson CR, Gleich GJ. Biology of eosinophils. In: Middleton E Jr, Reed CE, Ellis EF Jr, Adkinson NF, Yunginger JW, Busse WW, editors. Allergy: Principles and Practice. 5. St Louis: Mosby; 1998. pp. 242–60. [Google Scholar]
- 3.Fujiu T, Kato M, Kimura H, Tachibana A, Suzuki M, Nako Y, Morikawa A. Cellular adhesion is required for effector functions of human eosinophils via G-protein coupled receptors. Ann Allergy Asthma Immunol. 2002;89:90–8. doi: 10.1016/S1081-1206(10)61917-5. [DOI] [PubMed] [Google Scholar]
- 4.Nakamura M, Honda Z, Izumi T, et al. Molecular cloning and expression of platelet-activating factor receptor from human leukocytes. J Biol Chem. 1991;266:20400–5. [PubMed] [Google Scholar]
- 5.Gerard NP, Gerard C. The chemotactic receptor for human C5a anaphylatoxin. Nature. 1991;349:614–9. doi: 10.1038/349614a0. [DOI] [PubMed] [Google Scholar]
- 6.Horie S, Kita H. CD11b/CD18 (Mac-1) is required for degranulation of human eosinophils induced by human recombinant granulocyte-macrophage colony-stimulating factor and platelet-activating factor. J Immunol. 1994;152:5457–67. [PubMed] [Google Scholar]
- 7.Kaneko M, Horie S, Kato M, Gleich GJ, Kita H. A crucial role for β2 integrin in the activation of eosinophils stimulated by IgG. J Immunol. 1995;155:2631–41. [PubMed] [Google Scholar]
- 8.Kato M, Abraham RT, Okada S, Kita H. Ligation of the β2 integrin triggers activation and degranulation of human eosinophils. Am J Respir Cell Mol Biol. 1998;18:675–86. doi: 10.1165/ajrcmb.18.5.2885. [DOI] [PubMed] [Google Scholar]
- 9.Lub M, van Kooyk Y, Figdor CG. Ins and outs of LFA-1. Immunol Today. 1995;16:479–83. doi: 10.1016/0167-5699(95)80031-x. [DOI] [PubMed] [Google Scholar]
- 10.Nishizuka Y. Intracellular signaling by hydrolysis of phospholipids and activation of protein kinase C. Science. 1992;258:607–14. doi: 10.1126/science.1411571. [DOI] [PubMed] [Google Scholar]
- 11.Nishizuka Y. Protein kinase C and lipid signaling for sustained cellular responses. FASEB J. 1995;9:484–96. [PubMed] [Google Scholar]
- 12.Dekker LV, Parker PJ. Protein kinase C – a question of specificity. Trends Biochem Sci. 1994;19:73–7. doi: 10.1016/0968-0004(94)90038-8. [DOI] [PubMed] [Google Scholar]
- 13.Dang PM, Hakim J, Perianin A. Immunochemical identification and translocation of protein kinase C zeta in human neutrophils. FEBS Lett. 1994;349:338–42. doi: 10.1016/0014-5793(94)00700-4. [DOI] [PubMed] [Google Scholar]
- 14.Kent JD, Sergeant S, Burns J, McPhail LC. Identification and regulation of protein kinase C-δ in human neutrophils. J Immunol. 1996;157:4641–7. [PubMed] [Google Scholar]
- 15.Sergeant S, McPhail LC. Opsonized zymosan stimulates the redistribution of protein kinase C isoforms in human neutrophils. J Immunol. 1997;159:2877–85. [PubMed] [Google Scholar]
- 16.Evans DJ, Lindsay MA, Webb BL, Kankaanranta H, Giembycz MA, O'Connor BJ, Barnes PJ. Expression and activation of protein kinase C-ζ in eosinophils after allergen challenge. Am J Physiol. 1999;277:L233–9. doi: 10.1152/ajplung.1999.277.2.L233. [DOI] [PubMed] [Google Scholar]
- 17.Bankers-Fulbright JL, Kita H, Gleich GJ, O'Grady SM. Regulation of human eosinophil NADPH oxidase activity: a central role for PKCδ. J Cell Physiol. 2001;189:306–15. doi: 10.1002/jcp.10022. [DOI] [PubMed] [Google Scholar]
- 18.Nakano M. Determination of superoxide radical and singlet oxygen based on chemiluminescence of luciferin analogs. Meth Enzymol. 1990;186:585–91. doi: 10.1016/0076-6879(90)86154-n. [DOI] [PubMed] [Google Scholar]
- 19.Kato M, Kimura H, Motegi Y, Tachibana A, Minakami H, Morikawa A, Kita H. Platelet-activating factor activates two distinct effector pathways in human eosinophils. J Immunol. 2002;169:5252–9. doi: 10.4049/jimmunol.169.9.5252. [DOI] [PubMed] [Google Scholar]
- 20.Kato M, Abraham RT, Kita H. Tyrosine phosphorylation is required for eosiniophil degranulation induced by immobilized immunoglobulins. J Immunol. 1995;155:357–66. [PubMed] [Google Scholar]
- 21.Takizawa T, Kato M, Kimura H, et al. Inhibition of protein kinases A and C demonstrates dual modes of response in human eosinophils stimulated with platelet-activating factor. J Allergy Clin Immunol. 2002;110:241–8. doi: 10.1067/mai.2002.126303. [DOI] [PubMed] [Google Scholar]
- 22.Suzuki M, Kato M, Hanaka H, Izumi T, Morikawa A. Actin assembly is a crucial factor for superoxide anion generation from adherent human eosinophils. J Allergy Clin Immunol. 2003;112:126–33. doi: 10.1067/mai.2003.1515. [DOI] [PubMed] [Google Scholar]
- 23.Dobrina A, Menegazzi R, Carlos TM, et al. Mechanisms of eosinophil adherence to cultured vascular endothelial cells. Eosinophils bind to the cytokine-induced ligand vascular cell adhesion molecule-1 via the very late activation antigen-4 integrin receptor. J Clin Invest. 1991;88:20–6. doi: 10.1172/JCI115278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kroegel C, Yukawa T, Dent G, Venge P, Chung KF, Barnes PJ. Stimulation of degranulation from human eosinophils by platelet-activating factor. J Immunol. 1989;142:3518–26. [PubMed] [Google Scholar]
- 25.Sedgwick JB, Geiger KM, Busse WW. Superoxide generation by hypodense eosinophils from patients with asthma. Am Rev Respir Dis. 1990;142:120–5. doi: 10.1164/ajrccm/142.1.120. [DOI] [PubMed] [Google Scholar]
- 26.Wymann MP, Kernen P, Von Tscharner V, Tai PC, Spry CJ, Baggiolini M. Activation of the respiratory burst in eosinophil leucocytes – a transduction sequence decoupled from cytosolic Ca2+ rise. Eur J Clin Invest. 1995;25:25–31. doi: 10.1111/j.1365-2362.1995.tb01521.x. [DOI] [PubMed] [Google Scholar]
- 27.Elsner J, Hochstetter R, Kimmig D, Kapp A. Human eotaxin represents a potent activator of the respiratory burst of human eosinophils. Eur J Immunol. 1996;26:1919–25. doi: 10.1002/eji.1830260837. [DOI] [PubMed] [Google Scholar]
- 28.Egesten A, Malm J. Eosinophil leukocyte degranulation in response to serum-opsonized beads. C5a and platelet-activating factor enhance ECP release, with roles for protein kinases A and C. Allergy. 1998;53:1066–73. doi: 10.1111/j.1398-9995.1998.tb03816.x. [DOI] [PubMed] [Google Scholar]
- 29.Schweizer RC, van Kessel-Welmers BA, Warringa RA, Maikoe T, Raaijmakers JA, Lammers JW, Koenderman L. Mechanisms involved in eosinophil migration. Platelet-activating factor-induced chemotaxis and interleukin-5-induced chemokinesis are mediated by different signals. J Leukoc Biol. 1996;59:347–56. doi: 10.1002/jlb.59.3.347. [DOI] [PubMed] [Google Scholar]
- 30.Teixeira MM, Giembycz MA, Lindsay MA, Hellewell PG. Pertussis toxin shows distinct early signalling events in platelet-activating factor-, leukotriene B4-, and C5a-induced eosinophil homotypic aggregation in vitro and recruitment in vivo. Blood. 1997;89:4566–73. [PubMed] [Google Scholar]
- 31.Kroegel C, Giembycz MA, Matthys H, Westwick J, Barnes PJ. Modulatory role of protein kinase C on the signal transduction pathway utilized by platelet-activating factor in eosinophil activation. Am J Respir Cell Mol Biol. 1994;11:593–9. doi: 10.1165/ajrcmb.11.5.7946388. [DOI] [PubMed] [Google Scholar]
- 32.Dent G, Munoz NM, Ruhlmann E, Zhu X, Leff AR, Magnussen H, Rabe KF. Protein kinase C inhibition enhances platelet-activating factor-induced eicosanoid production in human eosinophils. Am J Respir Cell Mol Biol. 1998;18:136–44. doi: 10.1165/ajrcmb.18.1.2817. [DOI] [PubMed] [Google Scholar]
- 33.Oshiro T, Kakuta Y, Shimura S, Nara M, Shirato K. Characterization of platelet-activating factor-induced cytosolic calcium mobilization in human eosinophils. Clin Exp Allergy. 2000;30:699–705. doi: 10.1046/j.1365-2222.2000.00786.x. [DOI] [PubMed] [Google Scholar]
- 34.Takizawa T, Kato M, Suzuki M, et al. Distinct isoforms of protein kinase C are involved in human eosinophil functions induced by platelet-activating factor. Int Arch Allergy Immunol. 2003;131(Suppl. 1):5–9. doi: 10.1159/000070476. [DOI] [PubMed] [Google Scholar]
- 35.Dang PM, Fontayne A, Hakim J, El Benna J, Perianin A. Protein kinase C ζ phosphorylates a subset of selective sites of the NADPH oxidase component p47phox and participates in formyl peptide-mediated neutrophil respiratory burst. J Immunol. 2001;166:1206–13. doi: 10.4049/jimmunol.166.2.1206. [DOI] [PubMed] [Google Scholar]
- 36.Laudanna C, Mochly-Rosen D, Liron T, Constantin G, Butcher EC. Evidence of zeta protein kinase C involvement in polymorphonuclear neutrophil integrin-dependent adhesion and chemotaxis. J Biol Chem. 1998;273:30306–15. doi: 10.1074/jbc.273.46.30306. [DOI] [PubMed] [Google Scholar]
- 37.Le Good JA, Ziegler WH, Parekh DB, Alessi DR, Cohen P, Parker PJ. Protein kinase C isotypes controlled by phosphoinositide 3-kinase through the protein kinase PDK1. Science. 1998;281:2042–5. doi: 10.1126/science.281.5385.2042. [DOI] [PubMed] [Google Scholar]
- 38.Kim MS, Lim WK, Cha JG, et al. The activation of PI3-K and PKC zeta in PMA-induced differentiation of HL-60 cells. Cancer Lett. 2001;171:79–85. doi: 10.1016/s0304-3835(01)00505-5. [DOI] [PubMed] [Google Scholar]