Abstract
Dendritic cells produce cytokines that regulate the class of the adaptive immune response. Microbial recognition is mediated, at least in part, by pattern recognition receptors such as Toll-like receptors, which influence dendritic cell maturation. In humans it is not yet clear how intact pathogens modulate the developing immune response. To address the effects of intact pathogens on the maturation and effector functions of human dendritic cells, we investigated their responses to a number of microbial pathogens. We studied a range of micro-organisms including Gram-negative bacteria (Escherichia coli and Salmonella enterica sv. typhimurium), Gram-positive cocci (Staphylococcus aureus) and atypical bacteria (Mycobacterium tuberculosis and Mycoplasma hominis) as well as the human protozoal parasite Trichomonas vaginalis. The micro-organisms were fixed in formaldehyde to prevent replication whilst preserving surface morphology. All the pathogens induced similar up-regulation of dendritic cell activation-associated cell surface markers but there was a profound difference in the patterns of cytokines produced by the stimulated dendritic cells. Some pathogens (E. coli, Salmonella enterica sv. typhimurium and S. aureus) induced interleukin-12 (IL-12), IL-10 and interferon-α whereas others (M. tuberculosis, Mycoplasma hominis and T. vaginalis) induced only IL-10. This differential effect was not altered by costimulation of the dendritic cells through CD40. These results support the notion that human dendritic cells are plastic in their response to microbial stimuli and that the nature of the pathogen dictates the response of the dendritic cell.
Keywords: dendritic cells, interleukin-10, interleukin-12, pathogens, CD40
Introduction
The immune system has evolved to protect individuals from potentially dangerous antigens, such as invading pathogens. The generation of an appropriate response is essential and higher animals have evolved complex recognition systems to ensure that relevant responses are induced.1,2 Dendritic cells (DCs) are potent, professional antigen-presenting cells (APCs) that recognize pathogens, either directly or indirectly, by sensing changes in the environment such as cell damage or inflammation.3,4 Following activation by appropriate ‘danger signals’, peripheral DCs begin to mature and migrate from the periphery to local lymph nodes where maturation is completed following interaction with the appropriate T cells.5,6 Tissue-resident immature DCs can identify pathogens directly by recognizing pathogen-associated molecular patterns (PAMPs) using highly conserved pattern recognition receptors (PRRs) such as the Toll-like receptors (TLRs).7 The responsiveness to a given PAMP is linked to the expression of its relevant TLR – for example TLR4 is necessary for the recognition of lipopolysaccharide (LPS)8 and TLR2 identifies peptidoglycan from Gram-positive bacteria and lipoprotein from different bacteria.9–14 The different TLRs interact to form homo- or heterodimers; TLR6, in combination with TLR2, recognizes zymosan and peptidoglycan15–17 and TLR9 and TLR5 have been shown to recognize bacterially derived CpG DNA motifs18 and flagellin,19 respectively. Following ligand binding, the receptors activate intracellular signalling pathways via a variety of mediators including MyD88 and NFκB.20 The net result of TLR activation is the expression of a variety of genes that have direct and indirect effects on the developing immune response.7,21
DC maturation involves enhanced expression of major histocompatibility complex (MHC) class I and class II molecules, increased formation of MHC–peptide complexes and their delivery to the cell surface, in combination with up-regulation of costimulatory molecules such as CD80, CD86 and CD40.22–25 It is now clear that the interaction between TLRs and PAMPs plays a key role in enhancing the release of cytokines, such as interleukin-10 (IL-10), IL-12 and type I interferons. Production of IL-12 and IL-10 by mature murine DCs can be elicited by many pathogens or their products.22,25–27 In the human system, Gram-negative bacteria, but not Gram-positive bacteria, prime DCs to produce the IL-12 family of cytokines.28–30 In contrast, Mycobacterium tuberculosis blocks DC maturation and induces IL-10 release by targeting DC-SIGN.31 DCs have also been shown to produce type I interferons in response to micro-organisms as well as in response to viral infection or following interaction with T cells.32–38 In mice, enhanced IL-12 or IL-10 production by DCs requires two signals, one from the encounter with the pathogen and the second from the interaction with T cells,39–41 suggesting that the role of the pathogen in mice is to ‘sensitize’ DCs such that they can be fully activated following encounter with T cells. Similar studies in human-derived DC have not been reported.
It is now well established that DCs are both the initiators and the regulators of the developing immune response.27,42 The signals that instruct naive T-cell development are not yet fully understood but the cytokine milieu plays a central role with IL-12 driving T helper type 1 (Th1) responses whereas IL-10 inhibits them, favouring Th2-type responses instead.22 Type I interferons have been shown to regulate T-cell differentiation, but their role has not yet been clearly resolved.43,44 Since DCs control the developing T-cell response, the pattern of cytokines that they release when activated is believed to play an important role in determining the ultimate nature of the T-cell response. However, it is not yet clear how cytokine production is regulated in DCs. One possible explanation is that there are different DC subsets that are preprogrammed to produce different cytokine profiles and selective activation of these different subsets dictates the fate of the developing immune response. An alternative model is that each individual DC is pluripotential and the nature of the maturation signal determines the pattern of the cytokines that are produced during subsequent encounters with T cells.4,26,45
To examine the effects of the binding of intact pathogens on human DCs, we studied three pathogens associated with acute sepsis, Escherichia coli, Salmonella enterica sv. typhimurium, Staphylococcus aureus and three that are more associated with chronic infections, Mycoplasma hominis, Mycobacterium tuberculosis and Trichomonas vaginalis. Here we show that monocyte-derived human DCs possess significant plasticity and that different pathogens induce the production of different cytokines by a single subset of DCs. These results suggest that the same subset of DC has the potential to have different effects dependent on the primary encounter with intact pathogens. Specifically our results suggest that the pathogens typically associated with acute infections and sepsis induce IL-12 and type I interferons, whereas those that are more often associated with chronic infections gave rise to IL-10-secretion only.
Materials and methods
Pathogens and their preparation
The micro-organisms used in this study were E. coli, Salmonella enterica sv. typhimurium (hereafter S. enterica sv. typh.), Staphylococcus aureus (hereafter Staph. aureus), Mycoplasma hominis, Mycobacterium tuberculosis and T. vaginalis.
Enteropathogenic E. coli (EPEC strain, characterized by the presence of eaeA and bfpA genes by polymerase chain reaction) and Staph. aureus were obtained, respectively, from patients affected by enteritis and acute cystitis. Escherichia coli, Staph. aureus and S. enterica sv. typh. (reference strain ATCC, 14028s) were cultured in Lauria Bertani (LB) broth at 37°. Mycoplasma hominis reference strain PG21 was cultured in BEA medium.46Mycobacterium tuberculosis reference strain H37RV, was cultured in liquid medium Middlebrook 7H9 medium and T. vaginalis strain SS-22, was obtained from a patient affected by acute trichomoniasis. After axenization and before conducting the experiments, these protozoa were tested by polymerase chain reaction to detect any bacterial presence using universal bacterial primers. The T. vaginalis were cultured by daily 1 : 16 passages in fresh Diamond's trypticase yeast maltose (TYM) medium with 10% fetal bovine serum. The absence of symbiont mycoplasmas was assessed by both cultural and molecular methods, as described elsewhere.47,48
The numbers of all bacteria were evaluated as CFU (colony-forming units) in specific solid media. The T. vaginalis were counted using a haemocytometric chamber. All micro-organisms were extensively washed in LPS-free phosphate-buffered saline (PBS) then fixed for 4 hr in 4% paraformaldehyde. After fixation, cells were washed in PBS.
Generation of DCs and pathogen stimulation
DCs were generated from peripheral blood mononuclear cells separated from buffy coats obtained from the National Blood Transfusion Centre (Colindale, UK) as previously described.32 DCs were cultured with RPMI-1640 (Invitrogen, Paisley, UK), supplemented with 10% fetal calf serum (FCS, SeraQ, Sussex, UK), penicillin, streptomycin and l-glutamine, which contained 10 ng/ml IL-4 (First Link, West Midlands, UK) and 20 ng/ml granulocyte–macrophage colony-stimulating factor (GM-CSF; kindly donated by Dr S. Brett, GlaxoSmithKline, Stevenage, UK). Purity of DC preparations was evaluated by fluorescence-activate cell sorter (FACS) analysis using anti-CD11c and anti-CD3 monoclonal antibodies (mAbs) and was always more than 95%. On day 5 of DC culture, the Gram-negative bacteria (E. coli, S. enterica sv. typh.), Gram-positive cocci (Staph. aureus), the atypical bacteria (Mycobacterium tuberculosis, Mycoplasma hominis) and the human protozoal parasite (T. vaginalis) were added at different doses and then incubated at 37°. The following pathogens, E. coli, S. enterica sv. typh., Staph. aureus and Mycoplasma hominis were used at between 105 and 108 cells/ml, T. vaginalis was used at the maximal concentration of 107 cells/ml to account for its larger size, and Mycobacterium tuberculosis was used at a concentration between 104 and 107 because this was considered to be more representative of the concentrations present during infection. DCs were also cultured alone (iDCs) or in the presence of LPS (E. coli strain 026:B6, Sigma, St Louis, MO) at a concentration of 50 ng/ml. DCs were used for FACS analysis 48 hr after the exposure to the different pathogens. Supernatants were harvested after 24 hr and 48 hr and were stored at −20° until analysis.
Cell surface staining
After 48 hr of incubation with pathogens, DCs were harvested, counted and plated at a concentration of 105 per staining condition. Mouse anti-human mAbs, directly conjugated to fluorescein isothiocyanate (FITC) or phycoerythrin (PE), specific for the following molecules were added: HLA-DR (Sigma), CD40 (Caltag, Burlingame, CA), CD83 (Caltag) and CD86 (Caltag). After a 1-hr incubation the cells were washed, fixed in 1% paraformaldehyde (PFA; Merck, Letterworth, UK) and analysed using a FACScalibur flow cytometer and cellquest software (Becton Dickinson, San Jose, CA).
CD40 ligation of DCs
DCs were cocultured with CD40L-expressing L cells (CD40L) either simultaneously, or 48 hr after pathogen addition. Supernatants were harvested after 24 hr or 48 hr from the beginning of the coculture and stored at −20°.
Enzyme-linked immunosorbent assay (ELISA)
Supernatants collected from DCs incubated with pathogens in the presence or in the absence of CD40L transfectants were analysed by ELISA. IL-12p70 and IL-10 were measured using a standard indirect sandwich ELISA. The following pairs of purified and biotinylated antibodies were used: anti-IL-10 and anti-IL-12 (BD PharMingen, San Diego, CA). Optical density (OD) was measured at 450 nm on an ELISA plate reader (Titertek Multiscan PLUS, Helsinki, Finland).
Anti-viral assay for type I interferon detection
The production of interferon-α (IFN-α) was measured using an antiviral assay as previously published.32 Briefly, duplicate, serial three-fold dilutions of supernatants from DCs cultured with pathogens were added to confluent cultures of A549. The cells were then incubated for 24 hr after which supernatants were discarded and replaced with murine encephalomyelitis (EMC) virus for 1 hr. The virus was then removed and cell viability was assessed by staining with 0·25% methyl violet (Sigma) in 2% PFA (Merck) and the OD was measured by spectrophotometry using an automated plate reader. The concentration of type I interferon was determined by comparison with a standard curve prepared from IFN-α2a (Roferon) at known concentrations and the antiviral activity of the original sample calculated.
Results
Maturation of human DC by pathogens
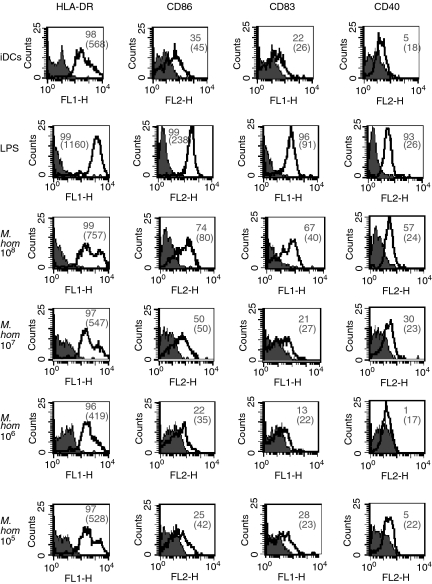
To determine how different pathogens affect DC maturation, immature DCs were prepared from human monocytes (see Materials and methods section). At day 5 DCs were homogeneous with 100% expression of CD11c (data not shown). DCs were incubated for 48 hr in the presence of a variety of intact, paraformaldehyde-fixed pathogens. Surface expression of activation/maturation markers (HLA-DR, CD86, CD40 and CD83) was assessed by flow cytometric analysis using unstimulated immature DCs (iDCs) as a negative control and DCs stimulated by E. coli-derived LPS (LPS-DCs) as a positive control. We compared different doses of intact E. coli, S. enterica sv. typh., Staph. aureus, Mycoplasma hominis, Mycobacterium tuberculosis and T. vaginalis and Fig. 1 shows a representative result with Mycoplasma hominis. There was a dose-dependent increase in the expression of all of the activation/maturation markers studied.
Figure 1.
Pathogens induce maturation of DCs in a dose-dependent manner. DCs were prepared from human monocytes. After 5 days of culture in GM-CSF and IL-4, they were cultured either alone (iDCs), or with LPS (LPS-DCs) or with different doses of Mycoplasma hominis. The DCs were then stained with mAbs specific for the indicated molecules. Filled histograms represent isotype controls while the white ones are the relevant mAbs. The numbers are the percentage of positive cells and the mean fluorescence intensity (MFI) is indicated in brackets. Data are representative of more than three experiments.
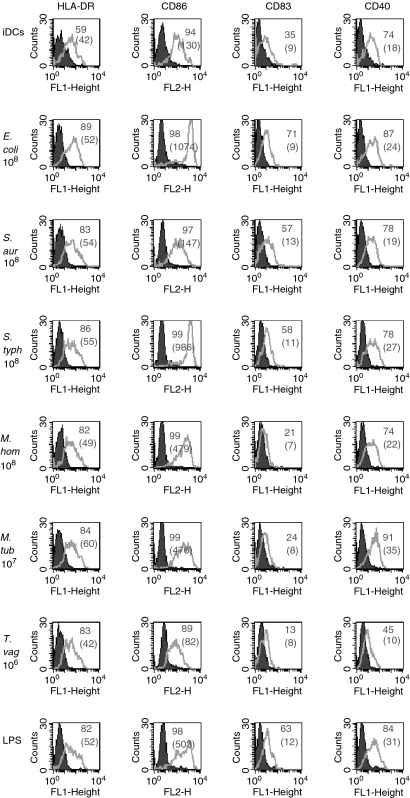
Figure 2 shows the effects of a single, optimal concentration of all the pathogens tested on the expression of MHC class II, CD86, CD40 and CD83. All the pathogens induced changes in cell surface expression of DC markers. The data obtained from 18 experiments are summarized in Table 1. Intact pathogens induced maturation/activation of DCs, with some markers such as CD86 being more consistently up-regulated by most of the pathogens tested. However, T. vaginalis had little effect on the expression of all cell surface markers.
Figure 2.
Up-regulation of molecules on DCs is similar among the different pathogens. Day 5 DCs were cultured for 2 days either alone (iDC) or with the optimal concentration of pathogen (as indicated) and then stained with the mAbs specific for the indicated molecules. Filled histograms represent isotype controls and the white ones are the relevant mAb. The numbers represent the percentage of positive cells and the MFI is indicated in brackets. Data are representative of more than three experiments for each pathogen.
Table 1.
Effects of different pathogenic stimuli on DC maturation
| HLADR | CD86 | CD83 | CD40 | |
|---|---|---|---|---|
| LPS | 9/17 (53%) | 15/18 (83%) | 12/16 (75%) | 13/16 (93%) |
| Escherichia coli | 6/8 (75%) | 9/9 (100%) | 4/8 (50%) | 7/8 (87%) |
| Staphylococcusaureus | 3/6 (50%) | 7/7 (100%) | 4/6 (57%) | 4/6 (67%) |
| Salmonella enterica sv. typhimurium | 9/11 (82%) | 11/12 (92%) | 3/10 (30%) | 10/11 (91%) |
| Mycoplasma hominis | 6/9 (67%) | 8/10 (80%) | 4/9 (44%) | 8/9 (89%) |
| Mycobacterium tuberculosis | 4/7 (57%) | 7/8 (87%) | 5/7 (71%) | 5/7 (71%) |
| Trichomonas vaginalis | 6/14 (43%) | 3/14 (21%) | 5/12 (42%) | 3/14 (21%) |
DCs were cultured with the different stimuli for 48 hr and stained for the markers indicated. Results are expressed as the proportion (and percentage) of experiments where the mean fluorescence intensity (MFI) of the marker was up-regulated by more than 10% as compared with the MFI of the marker on immature DCs.
Production of IL-10 and IL-12 from human DCs exposed to different pathogens
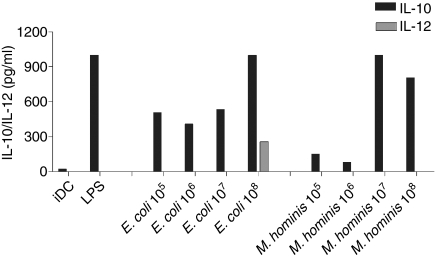
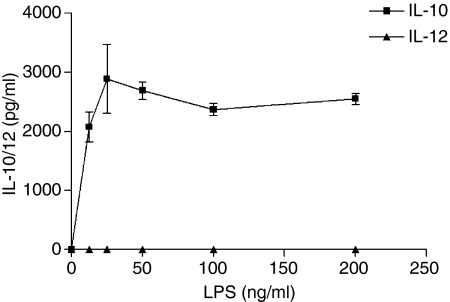
To assess whether the different pathogens had differential effects on the cytokine profile of activated DCs, iDCs were cultured with various concentrations of the pathogens and cytokine production (IL-12 and IL-10) was measured in the supernatants by ELISA after 24 and 48 hr of culture. A dose-dependent increase in IL-10 secretion was induced by both E. coli andMycoplasma hominis after 48 hr of culture. Only E. coli led to measurable IL-12 release, and this was only seen at the highest concentration of the organism. In contrast, LPS derived from E. coli led to the release of IL-10 only (Fig. 3). The data from 48 hr of culture are shown and similar cytokine levels were seen after 24 hr incubation with these pathogens (data not shown).
Figure 3.
Production of cytokines by DCs in a dose-dependent manner. Different doses of pathogens were added to DCs cultured for 5 days. Supernatants were harvested after 48 hr and IL-10/IL-12 were measured by ELISA. Data are representative of more than five experiments for each pathogen.
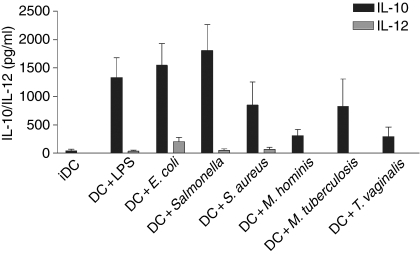
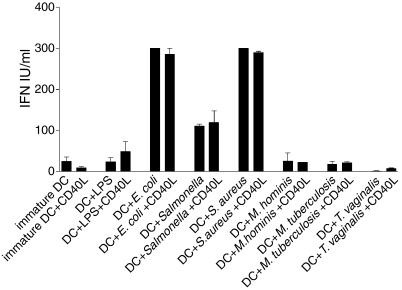
Figure 4 shows the cytokine profile in response to all the pathogens tested after 48 hr of coculture with DCs. Two different response patterns were observed –E. coli, S. enterica sv. typh. and Staph. aureus (all at 108 cells/ml), induced production of both IL-10 (> 90% of experiments) and IL-12. However, IL-12 was not produced in all experiments – from 12 separate experiments IL-12 was induced by E. coli in seven (58%), S. enterica sv. typh. in four (33%) and Staph. aureus in five (42%). LPS from E. coli induced IL-10 production by DCs in all the experiments; however, IL-12 release was detected in only two out of 14 experiments (14%). This was not a consequence of the dose of LPS used. In fact, while IL-10 was induced in a dose-dependent manner, IL-12 was undetectable (Fig. 5). Mycobacterium tuberculosis (107 cells/ml) promoted the release of IL-10 in the absence of any detectable IL-12 and both Mycoplamsa hominis (107 cells/ml) and T. vaginalis (106 cells/ml) induced IL-10 production alone, although the levels detected were always less than those seen with other pathogens (Fig. 4). Similar cytokine levels and profiles were seen after 24 hr of culture (data not shown). Hence, although all of the pathogens induced the maturation of DCs and IL-10 secretion, some pathogens did not promote the release of IL-12.
Figure 4.
Differential production of cytokines by DCs cultured with different pathogens. Optimal doses of each pathogen were added to DCs cultured for 5 days. Supernatants were harvested after 48 hr and IL-10/IL-12 were measured by ELISA. Means and SEMs are shown for at least eight experiments for each pathogen.
Figure 5.
LPS induces IL-10 in a dose-dependent manner. Day 5 DCs were cultured for 48 hr with different doses of LPS and supernatants were collected. IL-10 and IL-12 were measured by ELISA and the data shown are representative of three experiments.
Production of type I interferons from human dendritic cells exposed to different pathogens
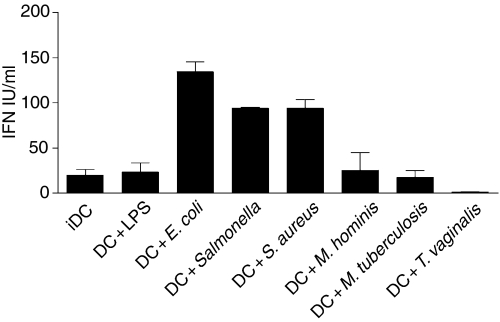
Type I interferons are potent antiviral cytokines that play a central role in the development of Th1 cells in humans.44 We therefore examined their production in supernatants from stimulated DCs. Figure 6 shows that interferon was detected in DCs cultured in the presence of E. coli, S. enterica sv. typh. and Staph. aureus which correlated with pathogens that also induced IL-12 production for one representative experiment. Those that did not stimulate IL-12 release –Mycoplasma hominis, T. vaginalis and Mycobacterium tuberculosis– did not induce IFN-αβ production when compared to unstimulated, immature DCs. To ensure that these pathogens did not induce the production of an IFN-α subtype that had limited antiviral activity (e.g. IFN-α1) supernatants from Mycobacterium tuberculosis-stimulated DCs were analysed with a commercial ELISA (PBL Laboratories, New Jersey, NJ) that detects all IFN-α subtypes. No significant IFN-α was detected in this assay (data not shown) confirming that paraformaldehyde-fixed Mycobacterium tuberculosis do not induce the production of IFN-α.
Figure 6.
Differential production of type I interferon by DCs cultured with pathogens. The type I interferon was measured using an antiviral assay from supernatants collected after 48 hr. Each supernatant was analysed in duplicate and means + SEM are shown. Data are representative of more than six experiments for each pathogen.
Again there was some heterogeneity in the response of different DC preparations from different donors and not all preparations induced type I interferon – for E. coli the interferon was detected in 10 of 12 experiments (83%), for S. enterica sv. typh., eight out of 13 experiments tested positively (62%) and Staph. aureus induced interferon secretion in eight out of 11 experiments (72·7%). As previously shown for IL-12 production, LPS induced IFN-α release in only two out of nine experiments at these time-points (22%).
Effects of CD40 ligation on production of different cytokines from pathogen-stimulated human DCs
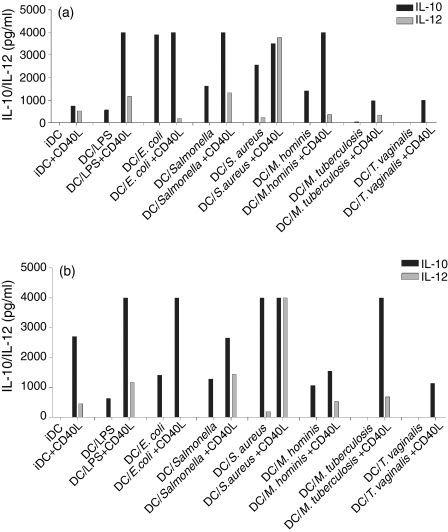
In view of reports in murine models showing that DCs stimulated with bacteria do not release cytokines unless they are activated by contact with CD40L expressed on the surface of T cells, we examined cytokine production in our pathogen-stimulated DCs after ligation of CD40. To determine whether those donors who did not induce IL-12 when stimulated with E. coli could be induced to express this cytokine after ligation of CD40, three donors who were ‘poor IL-12 producers’ were simultaneously exposed to pathogens and CD40L-expressing transfectants. Results from a representative experiment are shown in Fig. 7(a). There was a marked increase in IL-10, with all pathogens tested and a slight up-regulation in IL-12 release with some pathogens (S. enterica sv. typh. and Staph. aureus) compared with immature DCs (iDCs). However, in the presence of the CD40L transfectants, the pathogens that failed to induce IL-12 secretion when used in isolation did not cause any increase in IL-12 release above the baseline (immature DCs). When CD40L transfectants were added to the DCs 48 hr after the addition of the pathogens (Fig. 7b) a similar phenomenon was seen with an increase in IL-10 production and a modest increase in IL-12 with some organisms. However, CD40L stimulation did not modify the clear difference between those pathogens that induce large amounts of IL-12 and those that did not do so, suggesting that ligation of CD40 amplifies the response to the pathogens but does not fundamentally modify the nature of the response.
Figure 7.
CD40 cross-linking amplified the cytokines produced in pathogen-exposed DCs. Murine fibroblasts expressing human CD40 ligand (CD40L) were added to DCs along with the indicated pathogens. The pathogens were added either simultaneously (a) or 48 hr before the addition of the CD40L-expressing fibroblasts (b) Supernatants were harvested after 48 hr and IL-10/IL-12 production was measured by ELISA. Data shown are representative of more than three experiments for each pathogen.
We also examined the effects of CD40 ligation on the production of type I interferons. In a series of eight experiments, no significant differences were observed between CD40L-stimulated and non-stimulated DCs after they were exposed to the pathogens. Figure 8 shows a typical experiment when the pathogens were added simultaneously with the CD40L transfectants. Similar results were obtained when the pathogens were added before ligation of CD40 on DCs (data not shown).
Figure 8.
CD40 cross-linking does not modify the production of type I interferon in pathogen-exposed DCs. Murine fibroblasts expressing human CD40 ligand (CD40L) were added to DCs along with the indicated pathogens. The pathogens were added simultaneously and supernatants were harvested after 24 hr and type I interferon production was measured by an antiviral assay. Data are representative of more than three experiments for each pathogen.
Discussion
We have examined the effects of different pathogens on the maturation and activation of human monocyte-derived DCs. Paraformaldehyde-fixed intact pathogens were used to enable us to study the role of the entire micro-organism and to evaluate the effects of microbiological structures without the influence of secreted metabolic products. Paraformaldehyde has been suggested to preserve most of the structural features on the organism concerned. Furthermore, the epitopes of interest are not normally obscured.49,50 An additional benefit of fixed pathogens is that variability induced by microbe replication and changes in the number of microbes during the course of the experiment are eliminated. All of the organisms tested had an effect on DC maturation. In addition, the pathogens had a wide range of effects on the cytokine profile of activated DCs. These differences are of physiological significance as the specific conditioning stimuli caused DCs to produce very different cytokine profiles. On the basis of these effects we can divide the pathogens studied into two groups – group 1 includes pathogens such as E. coli, S. enterica sv. typh. and Staph. aureus (which can induce DCs to release IL-12 and type I interferon in addition to IL-10) and group 2 includes Mycoplasma hominis, Mycobacterium tuberculosis and T. vaginalis (which induce DCs to produce IL-10 alone). In addition, Mycoplasma hominis and T. vaginalis seem to have a reduced ability to induce cytokine release by DCs. Notably, these two pathogens are structurally very different from the group 1 pathogens. For example, Mycoplasma hominis lacks a cell wall and T. vaginalis is a protozoa as opposed to a bacterium. It is noteworthy that the pathogens that induced both IL-12 and type I interferons are normally associated with acute, severe infections and sepsis whereas pathogens that induce IL-10 alone are usually associated with chronic indolent disease. Further experiments in animal models will be required before we can definitively conclude that DC induction of IL-12 and type I interferons is associated with acute sepsis while IL-10 release is associated with chronic infections. Nevertheless, our in vitro experiments indicate that this may be the case. Our experiments support the notion of DC plasticity and show, for the first time, that intact micro-organisms can condition DCs to release cytokines that direct the phenotype of T cells.
In general DCs respond to foreign particles by up-regulating both cell surface expression of a variety of maturation markers and releasing a wide range of cytokines.22,24,25,27 Some heterogeneity among the different DC preparations has been observed, mainly for the up-regulation of CD83 following the interaction with pathogens. The variability observed is a common finding in experiments involving human DC51–53 and presumably reflects genetic heterogeneity that is absent in experiments using inbred mice. We found a clear difference between the production of cytokines and the induction of maturation markers – all pathogens tested led to similar changes in DC phenotype, however, only some induced IL-12 and interferon production by DCs. Hence it seems likely that specific intracellular signalling pathways are responsible for these different responses. Type I interferons are usually regarded as antiviral cytokines but it is now clear that they play a role in a wide variety of host defence systems. We have previously shown that IFN-α is produced following the interaction between DCs and naive T cells, suggesting that these cytokines play a role during the initiation of the immune response and it has been shown that they are important for the development of a Th1 response.32,44 Studies with LPS and whole bacteria have shown that they too can induce production of this key initiator of immune responses.33–37 Previously published work using live Mycobacterium tuberculosis and human DCs37,54 suggest that in this experimental model, large amounts of IFN-α are produced, but in our assay using fixed bacteria this was not seen, suggesting that surface ligation of extracellular receptors may have effects that are different from those seen during intracellular replication.
Previous studies have examined the effects of isolated TLR ligands on the maturation/activation of DCs and have provided valuable information relating to the key events in early pathogen recognition.55 We have chosen to use intact pathogens that, presumably, activate a number of different TLRs and we believe that this experimental system mimics more closely the situation in vivo where DCs are exposed to micro-organisms rather than to their components in isolation. The micro-organisms were inactivated to avoid the presence of metabolites. It would be of interest to repeat the experiments presented in this paper with live pathogens. Our experiments indicate that there are significant differences in the response to isolated bacterial products and intact pathogens. For example, intact E. coli induced IL-12 and IFN-α production in addition to IL-10, whereas the purified LPS from E. coli induced predominantly IL-10 release in our experimental system, suggesting that LPS is not the only component involved in the effect seen with E. coli. We also found marked differences between the responses in human cells and reports from other groups in murine models. For example, LPS from E. coli has been shown to induce both IL-10 and IL-12 production in the murine models.56–60 In the human system, the production of IL-12 in response to LPS has been shown to be variable and is affected by the way that DCs are generated61 and by the amount of serum used in the culture media.62 In addition, the amount of IL-12 produced in response to LPS can be affected by the amount of IL-10 endogenously produced. As previously described, LPS induced only small amounts of IL-12 that were increased by neutralizing IL-10.63 Furthermore, it has been shown that bacterial interactions with murine DCs can have a very limited effect and CD40L engagement is required before significant cytokine release occurs.4,26,45 By contrast, in our human model we find that bacterial engagement per se can induce cytokine release although this is amplified by costimulation with CD40L. These data suggest that there are profound differences in the responses in humans and mice and extrapolations from murine studies with bacterial components to human responses with intact pathogens may not be straightforward.
It has been previously shown that there is an inverse relationship between IL-10 and IL-12 production by activated DCs.64–66 Although the autocrine effect of IL-10 in suppression of IL-12 production by DCs has been reported,63,66–68 it is not known whether this autocrine effect is involved in the inverse relationship reported by others. In our experimental system we found that both IL-10 and IL-12 can be produced simultaneously although we have been unable to determine whether the same DCs simultaneously produce both cytokines. It therefore seems likely that the reported ‘reciprocal’ arrangement between IL-10 and IL-12 is not as straightforward as is currently supposed and further work will be required to understand the nature of the relationship between these two cytokines.
It was noteworthy that some DC preparations failed to secrete both IL-12 and IFN-α in response to group 1 pathogens. It is not yet clear whether this failure was related to preparation artefacts, introduced during the separation and maturation of the intact DC, or whether some individuals are predisposed to produce different cytokines in response to the same pathogen. Studies to address this possibility and to determine the clinical relevance of this observation are under way.
Thus the host response to invading micro-organisms is orchestrated by the APCs that identify and present antigenic fragments to the lymphocytes in association with a cytokine milieu that dictates the subsequent course of the immune response. We found that the cytokine response of the DCs is profoundly influenced by the nature of the pathogen. Our experiments indicate that studies of TLR engagement in isolation may be poorly representative of the situation when entire organisms are studied and further work will be required to determine how the activated DC integrates the signals that arise when multiple TLRs are engaged by intact pathogens.
Acknowledgments
This work was financed at least in part by the Wellcome Trust.
References
- 1.Abbas AK, Murphy KM, Sher A. Functional diversity of helper T lymphocytes. Nature. 1996;383:787–93. doi: 10.1038/383787a0. [DOI] [PubMed] [Google Scholar]
- 2.Romagnani S. Lymphokine production by human T cells in disease states. Annu Rev Immunol. 1994;12:227–57. doi: 10.1146/annurev.iy.12.040194.001303. [DOI] [PubMed] [Google Scholar]
- 3.Janeway CA, Jr, Medzhitov R. Innate immune recognition. Annu Rev Immunol. 2002;20:197–216. doi: 10.1146/annurev.immunol.20.083001.084359. [DOI] [PubMed] [Google Scholar]
- 4.Reis e Sousa C. Dendritic cells as sensors of infection. Immunity. 2001;14:495–8. doi: 10.1016/s1074-7613(01)00136-4. [DOI] [PubMed] [Google Scholar]
- 5.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–52. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 6.Sallusto F, Lanzavecchia A. Mobilizing dendritic cells for tolerance, priming, and chronic inflammation. J Exp Med. 1999;189:611–14. doi: 10.1084/jem.189.4.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Medzhitov R, Preston-Hurlburt P, Janeway CA., Jr A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature. 1997;388:394–7. doi: 10.1038/41131. [DOI] [PubMed] [Google Scholar]
- 8.Poltorak A, He X, Smirnova I, et al. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 1998;282:2085–8. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- 9.Brightbill HD, Libraty DH, Krutzik SR, et al. Host defense mechanisms triggered by microbial lipoproteins through toll-like receptors. Science. 1999;285:732–6. doi: 10.1126/science.285.5428.732. [DOI] [PubMed] [Google Scholar]
- 10.Hirschfeld M, Kirschning CJ, Schwandner R, et al. Cutting edge: inflammatory signaling by Borrelia burgdorferi lipoproteins is mediated by toll-like receptor 2. J Immunol. 1999;163:2382–6. [PubMed] [Google Scholar]
- 11.Schwandner R, Dziarski R, Wesche H, Rothe M, Kirschning CJ. Peptidoglycan- and lipoteichoic acid-induced cell activation is mediated by toll-like receptor 2. J Biol Chem. 1999;274:17406–9. doi: 10.1074/jbc.274.25.17406. [DOI] [PubMed] [Google Scholar]
- 12.Takeuchi O, Hoshino K, Kawai T, et al. Differential roles of TLR2 and TLR4 in recognition of Gram-negative and Gram-positive bacterial cell wall components. Immunity. 1999;11:443–51. doi: 10.1016/s1074-7613(00)80119-3. [DOI] [PubMed] [Google Scholar]
- 13.Thoma-Uszynski S, Stenger S, Takeuchi O, et al. Induction of direct antimicrobial activity through mammalian toll-like receptors. Science. 2001;291:1544–7. doi: 10.1126/science.291.5508.1544. [DOI] [PubMed] [Google Scholar]
- 14.Yoshimura A, Lien E, Ingalls RR, Tuomanen E, Dziarski R, Golenbock D. Cutting edge. recognition of Gram-positive bacterial cell wall components by the innate immune system occurs via Toll-like receptor 2. J Immunol. 1999;163:1–5. [PubMed] [Google Scholar]
- 15.Hajjar AM, O'Mahony DS, Ozinsky A, et al. Cutting edge: functional interactions between toll-like receptor (TLR) 2 and TLR1 or TLR6 in response to phenol-soluble modulin. J Immunol. 2001;166:15–19. doi: 10.4049/jimmunol.166.1.15. [DOI] [PubMed] [Google Scholar]
- 16.Ozinsky A, Underhill DM, Fontenot JD, et al. The repertoire for pattern recognition of pathogens by the innate immune system is defined by cooperation between toll-like receptors. Proc Natl Acad Sci USA. 2000;97:13766–71. doi: 10.1073/pnas.250476497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Takeuchi O, Kawai T, Muhlradt PF, et al. Discrimination of bacterial lipoproteins by Toll-like receptor 6. Int Immunol. 2001;13:933–40. doi: 10.1093/intimm/13.7.933. [DOI] [PubMed] [Google Scholar]
- 18.Hemmi H, Takeuchi O, Kawai T, et al. A Toll-like receptor recognizes bacterial DNA. Nature. 2000;408:740–5. doi: 10.1038/35047123. [DOI] [PubMed] [Google Scholar]
- 19.Hayashi F, Smith KD, Ozinsky A, et al. The innate immune response to bacterial flagellin is mediated by Toll-like receptor 5. Nature. 2001;410:1099–103. doi: 10.1038/35074106. [DOI] [PubMed] [Google Scholar]
- 20.O'Neill LA. The role of MyD88-like adapters in Toll-like receptor signal transduction. Biochem Soc Trans. 2003;31:643–7. doi: 10.1042/bst0310643. [DOI] [PubMed] [Google Scholar]
- 21.Muzio M, Natoli G, Saccani S, Levrero M, Mantovani A. The human toll signaling pathway. divergence of nuclear factor kappaB and JNK/SAPK activation upstream of tumor necrosis factor receptor-associated factor 6 (TRAF6) J Exp Med. 1998;187:2097–101. doi: 10.1084/jem.187.12.2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Banchereau J, Briere F, Caux C, et al. Immunobiology of dendritic cells. Annu Rev Immunol. 2000;18:767–811. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- 23.Reis e Sousa Yap G, Schulz O, et al. Paralysis of dendritic cell IL-12 production by microbial products prevents infection-induced immunopathology. Immunity. 1999;11:637–47. doi: 10.1016/s1074-7613(00)80138-7. [DOI] [PubMed] [Google Scholar]
- 24.Rescigno M, Granucci F, Ricciardi-Castagnoli P. Molecular events of bacterial-induced maturation of dendritic cells. J Clin Immunol. 2000;20:161–6. doi: 10.1023/a:1006629328178. [DOI] [PubMed] [Google Scholar]
- 25.Verhasselt V, Buelens C, Willems F, De Groote D, Haeffner-Cavaillon N, Goldman M. Bacterial lipopolysaccharide stimulates the production of cytokines and the expression of costimulatory molecules by human peripheral blood dendritic cells: evidence for a soluble CD14-dependent pathway. J Immunol. 1997;158:2919–25. [PubMed] [Google Scholar]
- 26.Edwards AD, Manickasingham SP, Sporri R, et al. Microbial recognition via Toll-like receptor-dependent and -independent pathways determines the cytokine response of murine dendritic cell subsets to CD40 triggering. J Immunol. 2002;169:3652–60. doi: 10.4049/jimmunol.169.7.3652. [DOI] [PubMed] [Google Scholar]
- 27.Reis e Sousa C, Sher A, Kaye P. The role of dendritic cells in the induction and regulation of immunity to microbial infection. Curr Opin Immunol. 1999;11:392–9. doi: 10.1016/S0952-7915(99)80066-1. [DOI] [PubMed] [Google Scholar]
- 28.Dreher D, Kok M, Cochand L, et al. Genetic background of attenuated Salmonella typhimurium has profound influence on infection and cytokine patterns in human dendritic cells. J Leukoc Biol. 2001;69:583–9. [PubMed] [Google Scholar]
- 29.Karlsson H, Larsson P, Wold AE, Rudin A. Pattern of cytokine responses to Gram-positive and Gram-negative commensal bacteria is profoundly changed when monocytes differentiate into dendritic cells. Infect Immun. 2004;72:2671–8. doi: 10.1128/IAI.72.5.2671-2678.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smits HH, van Beelen AJ, Hessle C, et al. Commensal Gram-negative bacteria prime human dendritic cells for enhanced IL-23 and IL-27 expression and enhanced Th1 development. Eur J Immunol. 2004;34:1371–80. doi: 10.1002/eji.200324815. [DOI] [PubMed] [Google Scholar]
- 31.Geijtenbeek TB, Van Vliet SJ, Koppel EA, et al. Mycobacteria target DC-SIGN to suppress dendritic cell function. J Exp Med. 2003;197:7–17. doi: 10.1084/jem.20021229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Foster GR, Germain C, Jones M, Lechler RI, Lombardi G. Human T cells elicit IFN-alpha secretion from dendritic cells following cell to cell interactions. Eur J Immunol. 2000;30:3228–35. doi: 10.1002/1521-4141(200011)30:11<3228::AID-IMMU3228>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 33.Cella M, Facchetti F, Lanzavecchia A, Colonna M. Plasmacytoid dendritic cells activated by influenza virus and CD40L drive a potent TH1 polarization. Nat Immunol. 2000;1:305–10. doi: 10.1038/79747. [DOI] [PubMed] [Google Scholar]
- 34.Diebold SS, Montoya M, Unger H, et al. Viral infection switches non-plasmacytoid dendritic cells into high interferon producers. Nature. 2003;424:324–8. doi: 10.1038/nature01783. [DOI] [PubMed] [Google Scholar]
- 35.Ito T, Amakawa R, Inaba M, Ikehara S, Inaba K, Fukuhara S. Differential regulation of human blood dendritic cell subsets by IFNs. J Immunol. 2001;166:2961–9. doi: 10.4049/jimmunol.166.5.2961. [DOI] [PubMed] [Google Scholar]
- 36.Montoya M, Schiavoni G, Mattei F, et al. Type I interferons produced by dendritic cells promote their phenotypic and functional activation. Blood. 2002;99:3263–71. doi: 10.1182/blood.v99.9.3263. [DOI] [PubMed] [Google Scholar]
- 37.Remoli ME, Giacomini E, Lutfalla G, et al. Selective expression of type I IFN genes in human dendritic cells infected with Mycobacterium tuberculosis. J Immunol. 2002;169:366–74. doi: 10.4049/jimmunol.169.1.366. [DOI] [PubMed] [Google Scholar]
- 38.Muller U, Steinhoff U, Reis LF, et al. Functional role of type I and type II interferons in antiviral defense. Science. 1994;264:1918–21. doi: 10.1126/science.8009221. [DOI] [PubMed] [Google Scholar]
- 39.Cella M, Scheidegger D, Palmer-Lehmann K, Lane P, Lanzavecchia A, Alber G. Ligation of CD40 on dendritic cells triggers production of high levels of interleukin-12 and enhances T cell stimulatory capacity: T-T help via APC activation. J Exp Med. 1996;184:747–52. doi: 10.1084/jem.184.2.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Koch F, Stanzl U, Jennewein P, et al. High level IL-12 production by murine dendritic cells: upregulation via MHC class II and CD40 molecules and downregulation by IL-4 and IL-10. J Exp Med. 1996;184:741–6. doi: 10.1084/jem.184.2.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schulz O, Edwards AD, Schito M, et al. CD40 triggering of heterodimeric IL-12 p70 production by dendritic cells in vivo requires a microbial priming signal. Immunity. 2000;13:453–62. doi: 10.1016/s1074-7613(00)00045-5. [DOI] [PubMed] [Google Scholar]
- 42.Moser M, Murphy KM. Dendritic cell regulation of TH1-TH2 development. Nat Immunol. 2000;1:199–205. doi: 10.1038/79734. [DOI] [PubMed] [Google Scholar]
- 43.Levings MK, Sangregorio R, Galbiati F, Squadrone S, de Waal MR, Roncarolo MG. IFN-alpha and IL-10 induce the differentiation of human type 1 T regulatory cells. J Immunol. 2001;166:5530–9. doi: 10.4049/jimmunol.166.9.5530. [DOI] [PubMed] [Google Scholar]
- 44.Rogge L, Barberis-Maino L, Biffi M, et al. Selective expression of an interleukin-12 receptor component by human T helper 1 cells. J Exp Med. 1997;185:825–31. doi: 10.1084/jem.185.5.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lanzavecchia A, Sallusto F. Regulation of T cell immunity by dendritic cells. Cell. 2001;106:263–6. doi: 10.1016/s0092-8674(01)00455-x. [DOI] [PubMed] [Google Scholar]
- 46.Andersen H, Birkelund S, Christiansen G, Freundt EA. Electrophoretic analysis of proteins from Mycoplasma hominis strains detected by SDS-PAGE, two-dimensional gel electrophoresis and immunoblotting. J Gen Microbiol. 1987;133:181–91. doi: 10.1099/00221287-133-1-181. [DOI] [PubMed] [Google Scholar]
- 47.Rappelli P, Addis MF, Carta F, Fiori PL. Mycoplasma hominis parasitism of Trichomonas vaginalis. Lancet. 1998;352:1286. doi: 10.1016/S0140-6736(98)00041-5. [DOI] [PubMed] [Google Scholar]
- 48.Rappelli P, Carta F, Delogu G, et al. Mycoplasma hominis and Trichomonas vaginalis symbiosis: multiplicity of infection and transmissibility of M. hominis to human cells. Arch Microbiol. 2001;175:70–4. doi: 10.1007/s002030000240. [DOI] [PubMed] [Google Scholar]
- 49.Lanier LL, Warner NL. Paraformaldehyde fixation of hematopoietic cells for quantitative flow cytometry (FACS) analysis. J Immunol Meth. 1981;47:25–30. doi: 10.1016/0022-1759(81)90253-2. [DOI] [PubMed] [Google Scholar]
- 50.Newman GR, Jasani B, Williams ED. The preservation of ultrastructure and antigenicity. J Microsc. 1982;127:RP5–RP6. [Google Scholar]
- 51.Fedele G, Frasca L, Palazzo R, Ferrero E, Malavasi F, Ausiello CM. CD38 is expressed on human mature monocyte-derived dendritic cells and is functionally involved in CD83 expression and IL-12 induction. Eur J Immunol. 2004;34:1342–50. doi: 10.1002/eji.200324728. [DOI] [PubMed] [Google Scholar]
- 52.Gomez J, Borras FE, Singh R, et al. Differential up-regulation of HLA-DM, invariant chain, and CD83 on myeloid and plasmacytoid dendritic cells from peripheral blood. Tissue Antigens. 2004;63:149–57. doi: 10.1111/j.1399-0039.2004.00159.x. [DOI] [PubMed] [Google Scholar]
- 53.Zhou LJ, Tedder TF. Human blood dendritic cells selectively express CD83, a member of the immunoglobulin superfamily. J Immunol. 1995;154:3821–35. [PubMed] [Google Scholar]
- 54.Giacomini E, Iona E, Ferroni L, et al. Infection of human macrophages and dendritic cells with Mycobacterium tuberculosis induces a differential cytokine gene expression that modulates T cell response. J Immunol. 2001;166:7033–41. doi: 10.4049/jimmunol.166.12.7033. [DOI] [PubMed] [Google Scholar]
- 55.Akira S, Takeda K, Kaisho T. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat Immunol. 2001;2:675–80. doi: 10.1038/90609. [DOI] [PubMed] [Google Scholar]
- 56.Haller D, Blum S, Bode C, Hammes WP, Schiffrin EJ. Activation of human peripheral blood mononuclear cells by nonpathogenic bacteria in vitro. Evidence of NK cells as primary targets. Infect Immun. 2000;68:752–9. doi: 10.1128/iai.68.2.752-759.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hirschfeld M, Weis JJ, Toshchakov V, et al. Signaling by toll-like receptor 2 and 4 agonists results in differential gene expression in murine macrophages. Infect Immun. 2001;69:1477–82. doi: 10.1128/IAI.69.3.1477-1482.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pulendran B, Kumar P, Cutler CW, Mohamadzadeh M, Van Dyke T, Banchereau J. Lipopolysaccharides from distinct pathogens induce different classes of immune responses in vivo. J Immunol. 2001;167:5067–76. doi: 10.4049/jimmunol.167.9.5067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Qi H, Denning TL, Soong L. Differential induction of interleukin-10 and interleukin-12 in dendritic cells by microbial toll-like receptor activators and skewing of T-cell cytokine profiles. Infect Immun. 2003;71:3337–42. doi: 10.1128/IAI.71.6.3337-3342.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Macatonia SE, Hosken NA, Litton M, et al. Dendritic cells produce IL-12 and direct the development of Th1 cells from naive CD4+ T cells. J Immunol. 1995;154:5071–9. [PubMed] [Google Scholar]
- 61.Elkord E, Williams PE, Kynaston H, Rowbottom AW. Human monocyte isolation methods influence cytokine production from in vitro generated dendritic cells. Immunology. 2005;114:204–12. doi: 10.1111/j.1365-2567.2004.02076.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jakobsen MA, Moller BK, Lillevang ST. Serum concentration of the growth medium markedly affects monocyte-derived dendritic cells' phenotype, cytokine production profile and capacities to stimulate in MLR. Scand J Immunol. 2004;60:584–91. doi: 10.1111/j.0300-9475.2004.01515.x. [DOI] [PubMed] [Google Scholar]
- 63.Koski GK, Lyakh LA, Rice NR. Rapid lipopolysaccharide-induced differentiation of CD14(+) monocytes into CD83(+) dendritic cells is modulated under serum-free conditions by exogenously added IFN-gamma and endogenously produced IL-10. Eur J Immunol. 2001;31:3773–81. doi: 10.1002/1521-4141(200112)31:12<3773::aid-immu3773>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 64.Hessle C, Andersson B, Wold AE. Gram-positive bacteria are potent inducers of monocytic interleukin-12 (IL-12) while Gram-negative bacteria preferentially stimulate IL-10 production. Infect Immun. 2000;68:3581–6. doi: 10.1128/iai.68.6.3581-3586.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kariminia A, Kavoossy G, Khatami S, Zowghi E, Ardestani SK. Study of interleukin-10 and interleukin-12 productions in response to lipopolysaccharides extracted from two different Brucella strains. Comp Immunol Microbiol Infect Dis. 2002;25:85–93. doi: 10.1016/s0147-9571(01)00029-7. [DOI] [PubMed] [Google Scholar]
- 66.Xia CQ, Kao KJ. Suppression of IL-12 production through endogenously secreted IL-10 in activated dendritic cells: involvement of activation of extracellular signal-regulated protein kinase. Scand J Immunol. 2003;58:23–32. doi: 10.1046/j.1365-3083.2003.01268.x. [DOI] [PubMed] [Google Scholar]
- 67.Demangel C, Bertolino P, Britton WJ. Autocrine IL-10 impairs dendritic cell (DC) -derived immune responses to mycobacterial infection by suppressing DC trafficking to draining lymph nodes and local IL-12 production. Eur J Immunol. 2002;32:994–1002. doi: 10.1002/1521-4141(200204)32:4<994::AID-IMMU994>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 68.Zhou L, Nazarian AA, Smale ST. Interleukin-10 inhibits interleukin-12 p40 gene transcription by targeting a late event in the activation pathway. Mol Cell Biol. 2004;24:2385–96. doi: 10.1128/MCB.24.6.2385-2396.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]