Abstract
Rap1 has emerged as an important regulator of adhesion in multicellular organisms. In the immune system, Rap1 functions as an inside-out signalling molecule for leucocyte integrins following stimulation with chemokines or antigens. Regulator of adhesion and cell polarization enriched in lymphoid tissues (RAPL) is a novel Rap1 effector molecule that mediates Rap1 signalling to integrins. The Rap1–RAPL complex regulates the spatial distribution of the integrin lymphocyte function-associated antigen-1 as well as cell polarization. The linking of inside-out signalling with polarization synergistically promotes highly efficient lymphocyte trafficking. Targeted deletion of RAPL in mice has demonstrated multiple indispensable roles for this protein in lymphocyte and dendritic cell trafficking critical for immunosurveillance.
Keywords: Rap1, RAPL, LFA-1, lymphocytes, dendritic cells
Introduction
Immune cells are the most motile cells in the body. Multiphoton microscopy has been used to generate a vivid picture of the robust motility that occurs during lymphocyte interactions with dendritic cells in peripheral lymphoid tissues.1,2 The dynamic regulation of immune cell adhesive interactions is fundamentally integrated into immunological responses, and the integrin adhesion receptors play critical roles in this process.3 Integrins constitute a large family of surface glycoproteins, and they are composed of α and β subunits. In particular, leucocyte integrins, such as lymphocyte function-associated antigen (LFA-1) (αL/β2) and the α4 integrins are important in lymphocyte trafficking through binding to the endothelial ligands intercellular adhesion molecule (ICAM)-1 and ICAM-2 for LFA-1 and vascular cell adhesion molecule (VCAM)-1 and mucosal addressin cell adhesion molecule (MAdCAM)-1 for α4 integrins.4,5 LFA-1 and α4 integrins mediate firm attachment of lymphocytes to high endothelial venules and facilitate subsequent migration into tissues. After entering lymphoid tissues, the high motility of naive T cells enables them to encounter the rare population of antigen-presenting dendritic cells (APC) that have migrated into the lymph node from peripheral tissues. T cells undergo distinct changes of adhesive behaviour once they have encountered APC-presented cognate antigens in vivo:1,2,6 initial short-lived contacts with APC followed by the formation of stable T cell–APC conjugates, which eventually lead to autonomous T-cell migration and cell division. LFA-1 is also the critical adhesion molecule in the immunological synapse, a specialized adhesion complex between T cells and antigen-presenting cells.7 Integrins and intracellular signalling from receptors for chemokines and antigens likely regulate these changes in T-cell behaviour after activation, but it is poorly understood how they orchestrate intricate lymphocyte adhesion patterns.
The ability of cells to modulate the strength of integrin adhesion in response to extracellular stimuli such as antigen or chemokines is essential to proper immune function. This activation process, referred to as ‘inside-out signalling’8 ultimately modulates integrin adhesiveness through affinity modulation, in which ligand-binding affinity is altered9 and avidity modulation, in which integrin cell surface diffusion and clustering are modified.10 Recently, our understanding of these phenomena has been facilitated by three-dimensional structures of integrins. Distinct conformational changes in the integrin extracellular domains are clearly associated with affinity changes upon ligand binding or cell activation.11,12 Although integrin activation following physiological adhesion has been documented, the molecules that relay the inside-out signal and the mechanism(s) by which affinity and avidity modulation are regulated are unknown.
To search for inside-out signalling molecules with important roles in lymphocyte migration and antigen-recognition, we designed a system to detect signalling molecules that activate the binding of LFA-1 to ICAM-1. Using this system, we identified the small guanosine triphosphatase (GTPase) Rap1 as a potent activator of LFA-1 activity.13 Other groups also found Rap1 involvement in integrin regulation.14,15 Rap1 was originally characterized as a Ras antagonist that sequestered downstream Ras effector molecules such as the Raf kinase.16 However, it was later reported that Rap1 did not antagonize Ras signalling under physiologically relevant conditions.17 Thus, Rap1 was thought to function in cell adhesion in a Ras-independent manner.17 To more fully examine this hypothesis, we isolated a novel Rap1 effector, regulator of adhesion and cell polarization enriched in lymphoid tissues (RAPL), and investigated its role in regulating integrin adhesion in vitro and in vivo. Here, we review the mechanism(s) by which the Rap1–RAPL system regulates integrin adhesion and the physiological importance of this system in immunity.
Rap1–RAPL signalling to integrins
Rap1 as an inside-out signal
Initial studies of Rap1 function in integrin signalling used constitutively active Rap1 mutants. Active mutants of Rap1A and Rap1B induced LFA-1-mediated cell adhesion13,14 whereas constitutively active forms of other Ras/Rho family members including Ras, R-Ras, Rac1, Cdc42, and Rho either did not stimulate adhesion or induced only mild adhesion. Additionally, active Rap1 mutants augmented integrin affinity and avidity. In the mouse proB cell line BAF reconstituted with human LFA-1, the active Rap1 mutant (Rap1V12) up-regulated the NKI-L16 activation epitope and affinity of LFA-1 for soluble ICAM-1-Fc chimeric proteins.13 The NKI-L16 antibody recognizes the ‘genu’ of the αL extracellular domain, which is exposed in the extended, intermediate affinity conformation of LFA-1.18 Active Rap1 most likely induced LFA-1 to adopt its extended, intermediate affinity conformation.9 The ability of Rap1V12 to stimulate LFA-1 was confirmed in vivo using transgenic mice in which Rap1V12 was expressed in the T-cell lineage.19 Active Rap1 increased the overall ‘adhesiveness’ of LFA-1, very late antigen-4 (VLA-4), and VLA-5. Additionally, Rap1V12 expression spontaneously induced LFA-1 clustering on primary T cells, but affinity changes could not be detected in these cells.19 A possible explanation for this could be the rather low concentrations of soluble ICAM-1 used. Rap1V12 also augmented the affinity modulation of αIIbβ3 in megakaryocytes.20 These studies clearly indicate that Rap1 is capable of activating integrins.
Requirement of the integrin α-subunit cytoplasmic region
How does Rap1 modulate integrin adhesion? Mutation of the LFA-1 cytoplasmic domain identified two amino acids, K1097/K1099, in the αL tail that are critical for Rap1-dependent LFA-1 activation.21 Alanine replacement of K1097/K1099 inhibited Rap1V12-induced up-regulation of LFA-1 affinity for soluble ICAM-1 as well as the generation of the NKI-L16 activation epitope. The requirement for the αL cytoplasmic domain in receiving Rap1-induced adhesion was unexpected because previous studies identified the β2 cytoplasmic region as essential for inside-out signalling.22 However, most of these studies used adherent cells (Cos or Chinese hamster ovary cells) and examined the effects of mutations on constitutive adhesion. These conditions are not optimal for the examination of activation-dependent adhesion, however. Residues K1097/K1099 are also important in the adhesive response to physiologically relevant stimuli.21 Expression of the K1097/1099 mutant in αL-deficient Jurkat T cells led to severely reduced T-cell receptor (TCR)-triggered adhesion to ICAM-1 compared to wild-type LFA-1. Adhesion in response to stromal derived factor (SDF-1, CXCL12) was also impaired in BAF proB cells expressing the K1097/K1099 mutant.21 These results are consistent with studies showing a critical role for Rap1 in adhesive responses of lymphocyte following exposure to chemokines and TCR crosslinking. Although the β2 cytoplasmic region is not required for Rap1-induced adhesion, tyrosine 735 in the β2 tail plays an essential role in LFA-1 internalization and detachment from ICAM-1.21 Alanine substitution of tyrosine 735 impaired detachment of the trailing edge of the cell, resulting in an elongated cell shape. Thus, the αL and β2 cytoplasmic tails play distinct roles in Rap1-mediated attachment and detachment.
Cell polarization and motility
In addition to integrin activation, Rap1 induces cell polarization, affects cell surface receptor distribution, and facilitates cell migration. Rap1V12 expression polarizes lymphocytes and generates a leading edge and uropod. The acquisition of front-rear polarity suggested a role for Rap1 in cell migration. As expected, expression of Rap1V12 in lymphocytes stimulated robust random migration over ICAM-1 and VCAM-1.23 Furthermore, Rap1V12 expression alone induced lymphocyte transmigration through the endothelium under flow as efficiently as chemokine stimulation. Cell polarization induced by chemokines and Rap1V12 is indistinguishable in cell morphology and the distribution of cell surface molecules such as CD44 and CXCR4, which are known to localize to the uropod and the leading edge, respectively. Thus, Rap1 is critical for cell polarization and integrin activation. Rap1-mediated cell polarization appears to be intrinsic in lymphocytes because it occurs independently of spatial cues such as adhesion or chemokine gradients. Although the Rho family GTPases play important roles in cell shape and migration24 the ability of Rap1 to induce integrin activation, cell polarization, and migration seems to be unique, as the constitutively active mutants of other Ras/Rho family members examined did not show this property.23
Rap1 regulates chemokine-stimulated cell migration
Rapid integrin activation by chemokines is a critical step for the firm attachment to and the subsequent transmigration through the vascular endothelium. Rap1 plays a critical role in chemokine-triggered firm lymphocyte attachment to the endothelium under shear flow. In primary lymphocytes, Rap1 is activated by the chemokines SLC (CCL21) and SDF-1 (CXCL12) within seconds and is rapidly inactivated within a few minutes23 unlike the sustained Rap1 activation observed following TCR stimulation.25,26 Rap1 activation by SLC (CXCL21) is Gi-dependent and increases adhesion mediated by both LFA-1 and VLA-4.23 Additionally, Spa1, a Rap1-specific GTPase activating protein27 efficiently suppressed Rap1 activation by SLC and SDF-1, and it inhibited the shear-resistant attachment to ICAM-1, VCAM-1, and the vascular endothelium. In contrast, Spa-1 did not affect phorbol 12-myristate 13-acetate (PMA)-stimulated adhesion under the same conditions, indicating that Rap1 activation is required for chemokine-induced adhesion, but not PMA, although Rap1 activation was shown to be activated by PMA.28 It is likely that PMA-activated PKC compensate for adhesion.13
We recently identified defective Rap1 regulation in EBV-transformed lymphocytes derived from patients with variant leucocyte adhesion deficiency (LAD) type 1.29 Unlike LAD type1, integrin expression levels and functions are normal in this case. Both basal and SDF-1-stimulated Rap1 activation were severely reduced in LAD lymphocytes. Additionally, basal Rap1 activity plays an important role in the stabilization of VLA-4 weakly bound to VCAM-1 under shear flow. Several cases have reported that integrin activation appears to be similarly impaired in cells isolated from LAD variant patients, and it has been proposed that these cases be categorized as LAD-III.30
Rap1 as a critical inside-out signalling molecule triggered by TCR stimulation
Activation of LFA-1 by the TCR helps facilitates stable T cell interactions with antigen-presenting cells (APC) via ICAM-1; this interaction sustains TCR engagement with peptide–major histocompatibility complex (MHC) complexes to promote T-cell growth and differentiation. However, the inside-out signalling molecule leading to LFA-1 activation following TCR stimulation is unknown. Rap1 is persistently activated by TCR crosslinking and exposure to antigen-loaded APCs, and it relocates to the site of T cell–APC interactions.25 A complex of the Rap1 exchange factor C3G, the adaptor protein CrkL, and Cbl may be involved in Rap1 activation following TCR stimulation.31 Both Rap1 activation and LFA-1 dependent adhesion to ICAM-1 were defective in phospholipase C (PLC)-γ1-deficient Jurkat cells following TCR crosslinking.26 Introduction of PLC-γ1 fully restored these defects. Members of the CalDAG-GEF family act as Rap1 exchange factors, and they may contribute to Rap1 activation in T cells. The requirement for Rap1 in TCR-triggered LFA-1 activation was directly demonstrated using the Rap1-specific GTPase activating protein Spa1.25 Expression of Spa1 abrogated Rap1 activation downstream of the TCR without affecting Ras and Rac activation. Spa1 expression further inhibited LFA-1-mediated adhesion of T cells to ICAM-1 and antigen-loaded APCs and the subsequent production of interleukin-2. Conversely, introduction of wild-type Rap1 resulted in increased Rap1 activity and enhanced T–APC interactions concomitant with accelerated TCR engagement, increased Erk1/2 activation, and interleukin-2 production, which was followed by apoptosis.25 This is a typical T-cell response, termed activation-induced cell death, which occurs when T cells are stimulated too strongly. Thus, Rap1 affects T-cell activation by regulating the strength of interaction with APCs and, accordingly, TCR interactions with peptide–MHC complexes. Interestingly, expression of a 10-fold excess of Rap1V12 rendered T cells unresponsive to antigen stimulation.25 Anergy was also observed in Spa1-deficient T cells with an accumulation of Rap1-GTP.32 In addition, Spa-1 deficient mice developed myeloproliferative disorders.33 The accumulation of active Rap1 was able to perturb cell growth and differentiation, although the relationship of these observed phenomena with integrin-related functions is not clear.
RAPL, a novel Rap1 effector molecule controlling integrin activation
RAPL was isolated in a yeast two-hybrid screen using Rap1V12 as bait. RAPL is a 265 amino acid protein containing a central Rap1-binding domain (RBD)34 (Fig. 1). RAPL is expressed predominantly in lymphoid tissues. RAPL is an alternative spliced form of Nore1 (Rassf5) and belongs to the Rassf (Ras suppressor factor) family.35 RAPL binds to active Rap1-GTP much more strongly than inactive Rap1-GDP.34 Mutagenesis studies demonstrated that both the RBD and N-terminal regions contribute to Rap1-GTP binding. RAPL associated with Rap1 in vivo upon TCR or SDF-1stimulation. RAPL overexpression in lymphocytes increased the adhesiveness of LFA-1 to ICAM-1, and this was accompanied by increased LFA-1 clustering at the cell surface and affinity for ICAM-1. The N-terminal deletion mutant, which is incapable of binding to Rap1-GTP, acted as a dominant negative and suppressed TCR- and chemokine-triggered adhesion to ICAM-1 and antigen-loaded APCs.34 RAPL-deficient mice have been generated by gene-targeting of the exon1 of the RAPL locus. The RAPL, but Nore1 expression was deficient in these mice. Studies using RAPL-deficient lymphocytes demonstrate that RAPL is indispensable for chemokine-stimulated adhesion via LFA-1 and VLA-4.36 RAPL-deficient T cells also poorly adhered to ICAM-1 when stimulated by TCR crosslinking (unpublished data). In addition to integrin activation, RAPL induced cell polarization, as is seen in chemokine-stimulated lymphocytes. RAPL-deficient lymphocytes did not become polarized following SLC stimulation.26 These results suggest that RAPL is essential for Rap1 function in mediating integrin adhesion and cell polarization, although it is possible that certain chemokines have other signalling pathways to affect adhesion in an integrin subfamily specific manner.37
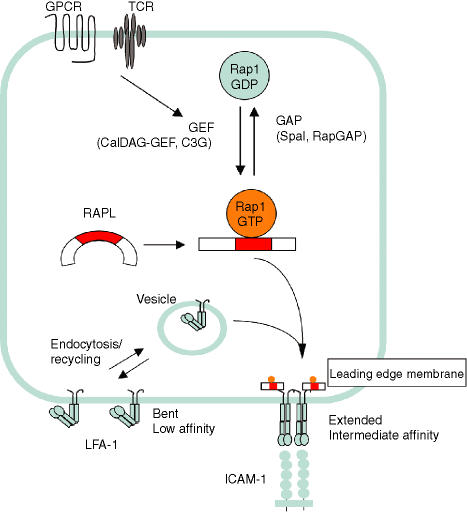
Figure 1.
Rap1-RAPL signalling to integrins. Rap1 cycles between a GDP-bound inactive form and a GTP-bound active form. Guanine nucleotide exchange factors (GEF) convert the GDP-bound form to the GTP-bound form. Conversely, Spa1 or RapGAP, a GTPase activating protein (GAP), accelerates GTP hydrolysis to inactivate Rap1. LFA-1 is internalized and recycled between the plasma membrane and intracellular pools. Upon stimulation with chemokines or antigen, Rap1 is rapidly activated by CalDAG GEF family members or C3G. Activated Rap1 bind to RAPL, which forms a larger complex with LFA-1 molecules via the αL cytoplasmic region, this complex is translocated to the leading edge, resulting in clustering of LFA-1 (avidity modulation), accompanied by conformational changes and affinity modulation.
The mechanism by which Rap1–RAPL regulate integrin activation appears to be linked with the induction of cell polarization. Microscopic studies have demonstrated that RAPL moves dynamically from the cytoplasm near the perinuclear region to the leading edge upon stimulation by chemokines and the TCR and colocalizes with LFA-1.34 In the presence of Rap1V12, RAPL coprecipitated with LFA-1, but it did not interact with the Rap1-unresponsive LFA-1 mutant. These results indicate that RAPL recruits LFA-1 to the leading edge by forming a K1097/K1099 motif dependent complex with LFA-1.34 Consistent with a RAPL function as a spatial regulator of LFA-1, RAPL colocalized with the ring of LFA-1 surrounding the central TCR complex in mature immunological synapses, which supports the stable association with APCs.34 The spatiotemporal regulation of LFA-1 by Rap1–RAPL is consistent with previous studies suggesting that Rap1 functions in intracellular vesicle transport and cell polarity.17 Rap1 is found in exocytic and endocytic vesicles as well as on the plasma membrane. Electron microscopic studies demonstrated that RAPL localizes primarily in vesicular structures in lymphoid cells (unpublished data). Rap1 and RAPL might be involved in the loading and direction of cargo molecules destined for the front of the membrane.
RAPL functions in vivo
Biochemical and functional characterizations of RAPL suggest important roles in lymphocyte adhesion and migration in vivo. Studies of RAPL-deficient mice provide unique opportunities to investigate integrin regulation by RAPL in the immune system (Fig. 2).36
Figure 2.
In vivo roles of Rap1–RAPL. RAPL knockout mice exhibit multiple defects in immune cell trafficking. RAPL deficiency impairs trafficking of both B and T cells into peripheral lymph nodes, likely due to defective firm attachment and transmigration of lymphocytes to high endothelial venules (HEV). From in vitro studies of lymphocytes inhibiting Rap1-RAPL signalling, RAPL-deficient lymphocytes are likely impaired in interstitial migration and formation of immune synapses with DCs. RAPL-deficient lymphocytes also inefficiently migrate to the spleen, resulting in hypoplastic B-cell follicles and T cell areas in the white pulp. Marginal zone B cell (MZB) populations are also deficient in RAPL-knockout mice. In addition to lymphocytes, dendritic cells abundantly express RAPL. RAPL-deficient DCs exhibit defective migration from the skin to draining lymph nodes and T-cell areas in the splenic white pulp upon inflammatory stimuli. Finally, RAPL-deficient thymocytes inefficiently migrate out of the thymus, resulting in the accumulation of mature thymocytes.
Lymphocyte trafficking to peripheral lymphoid organs
RAPL-deficient B and T cells are defective in chemokine-stimulated adhesion to immobilized ICAM-1 mediated by LFA-1. VLA-4-dependent adhesion to VCAM-1 is also severely reduced.36 Following chemokine stimulation, RAPL-deficient lymphocytes exhibit reduced cell motility as well as an inability to adopt a polarized morphology. To examine the cellular trafficking of these cells, short-term homing assays were used. Labelled wild-type and RAPL-deficient lymphocytes were mixed at equal ratios and injected into wild-type mice, and the migration to peripheral lymph nodes and spleen was examined after 1 hr. Compared to wild-type cells, RAPL-deficient lymphocytes entered the spleen and lymph nodes in much lower numbers. The reduced trafficking of RAPL-deficient lymphocytes to peripheral lymph nodes is comparable to that of LFA-1-deficient lymphocytes.38 In contrast to RAPL-deficient lymphocytes, migration of LFA-1-deficient lymphocytes to the spleen was increased. This difference could arise from the effects of RAPL deficiency on integrins other than LFA-1 such as α4 integrins as well as inefficient cell motility in these cells.36
Consistent with these results, secondary lymphoid organs became hypoplastic in RAPL-deficient mice. The numbers of CD4+ and CD8+ T cells and B220+ B cells in RAPL–/– LN were reduced by 20 to 30% compared to control animals. Additionally, the density of B-cell follicles and T-cell zones within the lymphoid organs were reduced as assessed by immunohistology. The total and lymphocyte subset numbers in the spleen were reduced by half in RAPL–/– animals, but only marginal effects were seen in RAPL+/– littermates. In the RAPL–/– spleens, B-cell follicles were hypoplastic with sparse T cells in the white pulp. Reciprocal increases in the number of T and B cells were found in the red pulp. Furthermore, CD21hi CD23lo marginal zone B cells (MZB) were notably reduced from 5·6% in wild-type to 0·8% in RAPL-deficient spleens. Previous studies identified roles for LFA-1 and VLA-4 in lymphocyte entry into splenic lymphoid follicles39 and MZB cells localize to the marginal zone in an LFA-1 and VLA-4 dependent manner.40 While B-cell entry into lymphoid follicles depends on CXCL13, the sphingosin-1 phosphate receptor 1 was recently found to be responsible for MZB retention.41 Our results suggest that RAPL signalling is also actively involved in the integrin-mediated compartmentalization of the spleen (Fig. 2).
B-cell maturation defects
Although there were no apparent changes in the B-cell lineages in the bone marrow, RAPL-deficient mice exhibited a decrease in immunoglobulin M (IgM)lo IgDhi mature B cells in the spleen and peripheral blood, as well as a reciprocal increase in newly formed B cells in the blood. Newly formed B cells enter the periphery and pass through distinct transitional stages before forming mature B-cell subsets.42 Where in the periphery B cells mature is not clear, but it is thought to occur in the spleen, where the major circulating IgMlo IgDhi subset is generated.43 These findings suggest that the migration defects of newly formed B cells into the spleen impair B-cell maturation.
Thymocyte migration defects
In 7–9 week old RAPL–/– mice, total thymocyte numbers decreased to about 50–60% the levels seen in littermate controls.36 The total number of CD4+ CD8+ thymocytes was reduced, but CD4+ and CD8+ single positive thymocytes were increased relatively by approximately twofold. The RAPL–/– single positive thymocytes contained the HSAlo and l-selectinhi mature T-cell population, indicating an accumulation of mature thymocytes. Furthermore, the boundary of the cortex and medulla in the RAPL–/– thymus was ambiguous, and the medulla frequently had a spotty appearance. Additionally, RAPL–/– thymocytes emigrated out of the thymus with only 10% the efficiency of the controls in response to CCL19. Thus, RAPL-deficient thymocytes are defective in emigration from the thymus (Fig. 2), which likely leads to the accumulation of mature thymocytes in the thymus. Thymocyte emigration is a Gi-dependent process44 and is regulated by CCR7 in neonatal mice45 and the shingosine-1 phosphate receptor1 in adult mice.46 Integrins are thought to be involved in thymocyte migration in the thymus.47 Our results suggest that RAPL signalling is involved in efficient emigration through an integrin-dependent process. The reason why the number of immature, double-positive thymocytes was reduced in RAPL-deficient mice is not clear; however, this pattern has also been observed in pertussis-toxin-expressing transgenic mice.44 This may indicate that Gi-dependent intrathymic thymocyte migration affects T-cell selection. Indeed, Dock2-deficient thymi also have poorly demarcated cortices and medullae48 and thymocyte emigration as well as positive and negative selection are impaired in these animals.49 Because Rap1 is activated by TCR cross-linking in thymocytes as well as in mature T cells50 and the presence of an activated Rap1 mutant enhanced positive selection19 RAPL deficiency could impair T-cell growth and selection in the thymus.
Defects in dendritic cell adhesion and migration
Dendritic cells (DC) reside in peripheral tissues and lymphoid organs and act as antigen-presenting cells to induce immunity or tolerance.51 RAPL is highly expressed in CD11c+ dendritic cells (DC) in the spleen and lymph nodes, Langerhans cells in the skin, and cultured bone-marrow derived DC (BM-DC).36 Isolated CD11c+ splenic DCs from RAPL-deficient mice inefficiently bound ICAM-1 or fibronectin via LFA-1 and VLA-4, respectively.36 RAPL-deficient BM-DC also are unable to bind fibronectin via VLA-4 and VLA-5 when stimulated with CCL21 (unpublished data). Because the expression of integrins and the chemokine receptor CCR7 are normal in RAPL-deficient DCs, these results indicate that RAPL is an indispensable inside-out signalling molecule.
In RAPL-deficient animals, the total number of CD11c+ splenic DCs was reduced to less than 40% the number seen in control animals; the major CD8– (CD8– CD4+, CD8– CD4–) DC subset was affected more severely than the minor CD8+ DC population.36 The CD11c+ DC lineage usually distributes diffusely in the macrophage-free regions at the marginal zone border and extends into the red pulp and T-cell areas. This population was scare in these regions in the RAPL–/– spleens, however. Our findings suggest that RAPL is required for DC precursor migration where integrins are involved. In addition, RAPL-deficient CD11c+ DCs and Langerhans cells inefficiently migrated into the white pulp and draining lymph nodes following exposure to inflammatory stimuli. This migration requires chemokines, as demonstrated by the study of plt mice deficient in the production of CCL19 and CCL21,52 or CCR7 knock-out mice.53 The migration of Langerhans cells into lymph nodes reportedly utilized α6 integrins54 and ICAM-1.55 Therefore, RAPL is likely to be involved in the chemokine-controlled migratory activity of activated DCs (Fig. 2).
Future perspective
Within the immune system, Rap1–RAPL modulates the adhesive interactions and cell polarization that promote multiple, essential immune processes. The biochemical coupling of these two processes is critical for immune cell trafficking and antigen recognition. Analysis of the functions of RAPL in vivo using gene targeting demonstrates that RAPL is essential for proper lymphocyte and DC trafficking, processes required for the generation of an effective immune response. There are important issues still to be addressed regarding RAPL functions in the immune system, however. Importantly, the details of the interaction of RAPL with the cytoplasmic domain of LFA-1 and other integrins are not known. Additionally, the mechanism by which RAPL links integrin adhesion and cell polarization has yet to be identified. Further molecular studies are particularly needed to identify the relationship of intracellular vesicle transport and cytoskeletal structures that determine cell polarity. With respect to lymphocyte trafficking, it is possible that RAPL regulates the rapid (within a second) activation of LFA-1 triggered by chemokines. Conversely, it may act at a later stage in the stabilization of transient attachments and migration through the endothelium. It has not yet been demonstrated that integrins are required for robust lymphocyte interstitial motility and interactions with DCs in vivo. Intravital and multiphoton microscopy could dissect these processes and reveal roles for RAPL. The effects of RAPL deficiency on the development of antigen-specific immune responses should be investigated. These should include analysis of DC functions and trafficking, immunological synapse formation and T-cell antigen recognition, T-cell growth and differentiation, T helper 1/T helper 2 polarity, and antibody production. Finally, the altered behaviour of thymocytes in RAPL-deficient animals needs to be further characterized especially with respect to positive and negative selection. It will be interesting to examine how RAPL deficiency affects selection using TCR transgenic mice models.
Rap1 is reportedly involved in morphogenesis,56 epithelial polarity,57 endothelial junctions58 and dendrite development in neurons.59 Additionally, Rap1 is associated with myeloproliferative diseases32 and tumorgenesis.60 The Rap1-interacting proteins responsible for mediating these events are not known, however. The identification of RAPL as a key regulator of integrins in immune-cells will facilitate the identification of the molecular factors regulating cell adhesion, and provide unique insights into Rap1 function on other adhesion-related, but physiologically distinct processes.
References
- 1.Miller MJ, Safrina O, Parker I, Cahalan MD. Imaging the single cell dynamics of CD4+ T cell activation by dendritic cells in lymph nodes. J Exp Med. 2004;200:847. doi: 10.1084/jem.20041236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mempel TR, Henrickson SE, Von Andrian UH. T-cell priming by dendritic cells in lymph nodes occurs in three distinct phases. Nature. 2004;427:154. doi: 10.1038/nature02238. [DOI] [PubMed] [Google Scholar]
- 3.Springer TA. Adhesion receptors of the immune system. Nature. 1990;346:425. doi: 10.1038/346425a0. [DOI] [PubMed] [Google Scholar]
- 4.Springer TA. Traffic signals on endothelium for lymphocyte recirculation and leukocyte emigration. Annu Rev Physiol. 1995;57:827. doi: 10.1146/annurev.ph.57.030195.004143. [DOI] [PubMed] [Google Scholar]
- 5.Butcher EC, Williams M, Youngman K, Rott L, Briskin M. Lymphocyte trafficking and regional immunity. Adv Immunol. 1999;72:209. doi: 10.1016/s0065-2776(08)60022-x. [DOI] [PubMed] [Google Scholar]
- 6.Bousso P, Robey E. Dynamics of CD8+ T cell priming by dendritic cells in intact lymph nodes. Nat Immunol. 2003;4:579. doi: 10.1038/ni928. [DOI] [PubMed] [Google Scholar]
- 7.Grakoui A, Bromley SK, Sumen C, Davis MM, Shaw AS, Allen PM, Dustin ML. The immunological synapse: a molecular machine controlling T cell activation. Science. 1999;285:221. doi: 10.1126/science.285.5425.221. [DOI] [PubMed] [Google Scholar]
- 8.Dustin ML, Springer TA. T-cell receptor cross-linking transiently stimulates adhesiveness through LFA-1. Nature. 1989;341:619. doi: 10.1038/341619a0. [DOI] [PubMed] [Google Scholar]
- 9.Carman CV, Springer TA. Integrin avidity regulation: are changes in affinity and conformation underemphasized? Curr Opin Cell Biol. 2003;15:547. doi: 10.1016/j.ceb.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 10.van Kooyk Y, Figdor CG. Avidity regulation of integrins: the driving force in leukocyte adhesion. Curr Opin Cell Biol. 2000;12:542. doi: 10.1016/s0955-0674(00)00129-0. [DOI] [PubMed] [Google Scholar]
- 11.Shimaoka M, Takagi J, Springer TA. Conformational regulation of integrin structure and function. Annu Rev Biophys Biomol Struct. 2002;485:485. doi: 10.1146/annurev.biophys.31.101101.140922. [DOI] [PubMed] [Google Scholar]
- 12.Takagi J, Petre BM, Walz T, Springer TA. Global conformational rearrangements in integrin extracellular domains in outside-in and inside-out signaling. Cell. 2002;110:599. doi: 10.1016/s0092-8674(02)00935-2. [DOI] [PubMed] [Google Scholar]
- 13.Katagiri K, Hattori M, Minato N, Irie S, Takatsu K, Kinashi T. Rap1 is a potent activation signal for leukocyte function-associated antigen 1 distinct from protein kinase C and phosphatidylinositol-3-kinase. Mol Cell Biol. 2000;20:1956. doi: 10.1128/mcb.20.6.1956-1969.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reedquist KA, Ross E, Koop EA, et al. The small GTPase, Rap1, mediates CD31-induced integrin adhesion. J Cell Biol. 2000;148:1151. doi: 10.1083/jcb.148.6.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Caron E, Self AJ, Hall A, The GT. Pase Rap1 controls functional activation of macrophage integrin αMβ2 by LPS and other inflammatory mediators. Curr Biol. 2000;10:974. doi: 10.1016/s0960-9822(00)00641-2. [DOI] [PubMed] [Google Scholar]
- 16.Kitayama H, Sugimoto Y, Matsuzaki T, Ikawa Y, Noda M. A ras-related gene with transformation suppressor activity. Cell. 1989;56:77. doi: 10.1016/0092-8674(89)90985-9. [DOI] [PubMed] [Google Scholar]
- 17.Bos JL, de Rooij J, Reedquist KA. Rap1 signaling: Adhering to new models. Nat Rev. 2001;2:369. doi: 10.1038/35073073. [DOI] [PubMed] [Google Scholar]
- 18.Xie C, Shimaoka M, Xiao T, Schwab P, Klickstein LB, Springer TA. The integrin alpha-subunit leg extends at a Ca2+-dependent epitope in the thigh/genu interface upon activation. Proc Natl Acad Sci USA. 2004;101:15422. doi: 10.1073/pnas.0406680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sebzda E, Bracke M, Tugal T, Hogg N, Cantrell DA. Rap1A positively regulates T cells via integrin activation rather than inhibiting lymphocyte signaling. Nat Immunol. 2002;3:251. doi: 10.1038/ni765. [DOI] [PubMed] [Google Scholar]
- 20.Bertoni A, Tadokoro S, Eto K, Pampori N, Parise LV, White GC, Shattil SJ. Relationships between Rap1b, affinity modulation of integrin αIIbβ3, and the actin cytoskeleton. J Biol Chem. 2002;277:25715. doi: 10.1074/jbc.M202791200. [DOI] [PubMed] [Google Scholar]
- 21.Tohyama Y, Katagiri K, Pardi R, Lu C, Springer TA, Kinashi T. The critical cytoplasmic regions of the αL/β2 integrin in Rap1-induced adhesion and migration. Mol Biol Cell. 2003;14:2570. doi: 10.1091/mbc.E02-09-0615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hibbs ML, Jakes S, Stacker SA, Wallace RW, Springer TA. The cytoplasmic domain of the integrin lymphocyte-function-associated antigen 1 β subunit: Sites required for binding to intercellular adhesion molecules 1 and the phorbol ester-stimulated phosphorylation site. J Exp Med. 1991;174:1227. doi: 10.1084/jem.174.5.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shimonaka M, Katagiri K, Nakayama T, Fujita N, Tsuruo T, Yoshie O, Kinashi T. Rap1 translates chemokine signals to integrin activation, cell polarization, and motility across vascular endothelium under flow. J Cell Biol. 2003;161:417. doi: 10.1083/jcb.200301133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sanchez-Madrid F, del Pozo MA. Leukocyte polarization in cell migration and immune interactions. EMBO J. 1999;18:501. doi: 10.1093/emboj/18.3.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Katagiri K, Hattori M, Minato N, Kinashi T. Rap1 functions as a key regulator of T-cell and antigen-presenting cell interactions and modulates T-cell responses. Mol Cell Biol. 2002;22:1001. doi: 10.1128/MCB.22.4.1001-1015.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Katagiri K, Shimonaka M, Kinashi T. Rap1-mediated lymphocyte function-associated antigen-1 activation by the T cell antigen receptor is dependent on phospholipase C-γ1. J Biol Chem. 2004;279:11875. doi: 10.1074/jbc.M310717200. [DOI] [PubMed] [Google Scholar]
- 27.Kurachi H, Wada Y, Tsukamoto N, Maeda M, Kubota H, Hattori M, Iwai K, Minato N. Human SPA-1 gene product selectively expressed in lymphoid tissues is a specific GTPase-activating protein for Rap1 and Rap2. Segregate expression profiles from a rap1GAP gene product. J Biol Chem. 1997;272:28081. doi: 10.1074/jbc.272.44.28081. [DOI] [PubMed] [Google Scholar]
- 28.Liu L, Schwartz BR, Tupper J, Lin N, Winn RK, Harlan JM. The GTPase Rap1 regulates phorbol 12-myristate 13-acetate-stimulated but not ligand-induced beta 1 integrin-dependent leukocyte adhesion. J Biol Chem. 2002;277:40893. doi: 10.1074/jbc.M206208200. [DOI] [PubMed] [Google Scholar]
- 29.Kinashi T, Aker M, Sokolovsky-Eisenberg M, et al. LAD-III, a leukocyte adhesion deficiency syndrome associated with defective Rap1 activation and impaired stabilization of integrin bonds. Blood. 2004;103:1033. doi: 10.1182/blood-2003-07-2499. [DOI] [PubMed] [Google Scholar]
- 30.Alon R, Etzioni A. LAD-III, a novel group of leukocyte integrin activation deficiencies. Trends Immunol. 2003;24:561. doi: 10.1016/j.it.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 31.Boussiotis VA, Freeman GJ, Berezovskaya A, Barber DL, Nadler LM. Maintenance of human T cell anergy. blocking of IL-2 gene transcription by activated Rap1. Science. 1997;278:124. doi: 10.1126/science.278.5335.124. [DOI] [PubMed] [Google Scholar]
- 32.Ishida D, Kometani K, Yang H, et al. Myeloproliferative stem cell disorders by deregulated Rap1 activation in SPA-1-deficient mice. Cancer Cell. 2003;4:55. doi: 10.1016/s1535-6108(03)00163-6. [DOI] [PubMed] [Google Scholar]
- 33.Ishida D, Yang H, Masuda K, Uesugi K, Kawamoto H, Hattori M, Minato N. Antigen-driven T cell anergy and defective memory T cell response via deregulated Rap1 activation in SPA-1-deficient mice. Proc Natl Acad Sci USA. 2003;100:10919. doi: 10.1073/pnas.1834525100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Katagiri K, Maeda A, Shimonaka M, Kinashi T. RAPL, a Rap1-binding molecule that mediates Rap1-induced adhesion through spatial regulation of LFA-1. Nature Immunol. 2003;4:741. doi: 10.1038/ni950. [DOI] [PubMed] [Google Scholar]
- 35.Tommasi S, Dammann R, Jin SG, Zhang XF, Avruch J, Pfeifer GP. RASSF3 and NORE1: identification and cloning of two human homologues of the putative tumor suppressor gene RASSF1. Oncogene. 2002;21:2713. doi: 10.1038/sj.onc.1205365. [DOI] [PubMed] [Google Scholar]
- 36.Katagiri K, Ohnishi N, Kabashima K, Iyoda T, Takeda N, Shinkai Y, Inaba K, Kinashi T. Crucial roles of Rap1 effector molecule RAPL in lymphocyte and dendritic cell trafficking. Nat Immunol. 2004;5:1045. doi: 10.1038/ni1111. [DOI] [PubMed] [Google Scholar]
- 37.Weber C, Katayama J, Springer TA. Differential regulation of β1 and β2 integrin avidity by chemoattractants in eosinophils. Proc Natl Acad Sci USA. 1996;93:10939. doi: 10.1073/pnas.93.20.10939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Berlin-Rufenach C, Otto F, Mathies M, Westermann J, Owen MJ, Hamann A, Hogg N. Lymphocyte migration in lymphocyte function-associated antigen (LFA)-1-deficient mice. J Exp Med. 1999;189:1467. doi: 10.1084/jem.189.9.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lo CG, Lu TT, Cyster JG. Integrin-dependence of lymphocyte entry into the splenic white pulp. J Exp Med. 2003;197:353. doi: 10.1084/jem.20021569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lu TT, Cyster JG. Integrin-mediated long-term B cell retention in the splenic marginal zone. Science. 2002;297:409. doi: 10.1126/science.1071632. [DOI] [PubMed] [Google Scholar]
- 41.Cinamon G, Matloubian M, Lesneski MJ, Xu Y, Low C, Lu T, Proia RL, Cyster JG. Sphingosine 1-phosphate receptor 1 promotes B cell localization in the splenic marginal zone. Nat Immunol. 2004;5:713. doi: 10.1038/ni1083. [DOI] [PubMed] [Google Scholar]
- 42.Pillai S. The chosen few? Positive selection and the generation of naive B lymphocytes. Immunity. 1999;10:493. doi: 10.1016/s1074-7613(00)80049-7. [DOI] [PubMed] [Google Scholar]
- 43.Loder F, Mutschler B, Ray RJ, Paige CJ, Sideras P, Torres R, Lamers MC, Carsetti RB. cell development in the spleen takes place in discrete steps and is determined by the quality of B cell receptor-derived signals. J Exp Med. 1999;190:75. doi: 10.1084/jem.190.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chaffin KE, Perlmutter RMA. pertussis toxin-sensitive process controls thymocyte emigration. Eur J Immunol. 1991;21:2565. doi: 10.1002/eji.1830211038. [DOI] [PubMed] [Google Scholar]
- 45.Ueno T, Hara K, Willis MS, et al. Role for CCR7 ligands in the emigration of newly generated T lymphocytes from the neonatal thymus. Immunity. 2002;16:205. doi: 10.1016/s1074-7613(02)00267-4. [DOI] [PubMed] [Google Scholar]
- 46.Matloubian M, Lo CG, Cinamon G, et al. Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature. 2004;427:355. doi: 10.1038/nature02284. [DOI] [PubMed] [Google Scholar]
- 47.Petrie HT. Cell migration and the control of post-natal T-cell lymphopoiesis in the thymus. Nat Rev Immunol. 2003;3:859. doi: 10.1038/nri1223. [DOI] [PubMed] [Google Scholar]
- 48.Fukui Y, Hashimoto O, Sanui T, et al. Haematopoietic cell-specific CDM family protein DOCK2 is essential for lymphocyte migration. Nature. 2001;412:826. doi: 10.1038/35090591. [DOI] [PubMed] [Google Scholar]
- 49.Sanui T, Inayoshi A, Noda M, Iwata E, Oike M, Sasazuki T, Fukui Y. DOCK2 is essential for antigen-induced translocation of TCR and lipid rafts, but not PKC-theta and LFA-1, in T cells. Immunity. 2003;19:119. doi: 10.1016/s1074-7613(03)00169-9. [DOI] [PubMed] [Google Scholar]
- 50.Amsen D, Kruisbeek A, Bos JL, Reedquist K. Activation of the Ras-related GTPase Rap1 by thymocyte TCR engagement and during selection. Eur J Immunol. 2000;30:2832. doi: 10.1002/1521-4141(200010)30:10<2832::AID-IMMU2832>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 51.Steinman RM, Pack M, Inaba K. Dendritic cells in the T-cell areas of lymphoid organs. Immunol Rev. 1997;156:25. doi: 10.1111/j.1600-065x.1997.tb00956.x. [DOI] [PubMed] [Google Scholar]
- 52.Gunn MD, Kyuwa S, Tam C, Kakiuchi T, Matsuzawa A, Williams LT, Nakano H. Mice lacking expression of secondary lymphoid organ chemokine have defects in lymphocyte homing and dendritic cell localization. J Exp Med. 1999;189:451. doi: 10.1084/jem.189.3.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Forster R, Schubel A, Breitfeld D, Kremmer E, Renner-Muller I, Wolf E, Lipp M. CCR7 coordinates the primary immune response by establishing functional microenvironments in secondary lymphoid organs. Cell. 1999;99:23. doi: 10.1016/s0092-8674(00)80059-8. [DOI] [PubMed] [Google Scholar]
- 54.Price AA, Cumberbatch M, Kimber I, Ager A. α6 integrins are required for Langerhans cell migration from the epidermis. J Exp Med. 1997;186:1725. doi: 10.1084/jem.186.10.1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Xu H, Guan H, Zu G, Bullard D, Hanson J, Slater M, Elmets CA. The role of ICAM-1 molecule in the migration of Langerhans cells in the skin and regional lymph node. Eur J Immunol. 2001;31:3085. doi: 10.1002/1521-4141(2001010)31:10<3085::AID-IMMU3085>3.0.CO;2-B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Asha H, de Ruiter ND, Wang MG, Hariharan IK. The Rap1 GTPase functions as a regulator of morphogenesis in vivo. EMBO J. 1999;18:605. doi: 10.1093/emboj/18.3.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Knox AL, Brown NH. Rap1 GTPase regulation of adherens junction positioning and cell adhesion. Science. 2002;295:1285. doi: 10.1126/science.1067549. [DOI] [PubMed] [Google Scholar]
- 58.Wittchen ES, Worthylake RA, Kelly P, Casey PJ, Quilliam LA, Burridge K. Rap1 GTPase inhibits leukocyte transmigration by promoting endothelial barrier function. J Biol Chem. 2005;280:11675. doi: 10.1074/jbc.M412595200. [DOI] [PubMed] [Google Scholar]
- 59.Chen Y, Wang PY, Ghosh A. Regulation of cortical dendrite development by Rap1 signaling. Mol Cell Neurosci. 2005;28:215. doi: 10.1016/j.mcn.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 60.Yajnik V, Paulding C, Sordella R, et al. DOCK4, a GTPase activator, is disrupted during tumorigenesis. Cell. 2003;112:673. doi: 10.1016/s0092-8674(03)00155-7. [DOI] [PubMed] [Google Scholar]