Abstract
The intraperitoneal injection of lipopolysaccharide (LPS) (400 µg/kg body weight) induced the expression of mRNAs of inflammatory cytokines such as interleukin (IL)-1β, IL-6 and tumour necrosis factor (TNF)-α in the submandibular gland (SMG) of C3H/HeN mice but not that of C3H/HeJ mice, a mutant strain for Toll-like receptor-4 (TLR-4– mutant). The mRNA levels of these cytokines in the SMG of the wild-type mice increased as early as 3 hr after injection, peaked at 3–6 hr, and had decreased again by 24 hr. In this study, we particularly focused on IL-1β, and induction by this endotoxin was investigated in detail. Denervation of the superior cervical trunk and chorda tympani nerve did not diminish the LPS-induced elevation of IL-1β mRNA in the SMG, indicating the irrelevance of the central nervous system in this induction. TLR-4 mRNA and protein were shown to be strongly expressed in the SMG, suggesting the direct action of LPS on this gland. IL-1β proteins were localized in the secretory granules of granular convoluted tubular (GCT) cells, and their molecular weights in the gland were 17·5 and 20 kDa. IL-1β of the same size appeared in the saliva 6 hr after LPS injection in C3H/HeN but not in C3H/HeJ mice. The present study thus suggests that IL-1β, an inflammation cytokine, is induced and secreted into the saliva in response to endotoxin injected intraperitoneally.
Keywords: interleukin-1β, lipopolysaccharide, Toll-like receptor-4, submandibular gland
Introduction
The salivary glands secrete water, electrolytes, various proteins, glycoproteins and mucins to aid digestion, cleansing and protection of the oral cavity. In addition to these proteins, many other species of proteins and peptides related to growth promotion, wound healing and immune/inflammation regulation are also contained in saliva. Among the three major salivary glands, the submandibular gland (SMG) of rodents produces significant quantities of nerve growth factor (NGF), epidermal growth factor (EGF), transforming growth factor (TGF)-β, renin and kallikreins. These proteins are secreted into the saliva, regulate immune/inflammatory responses in the mucosal tissue, and participate in regeneration or healing of wound tissue.1,2
The SMG has also been reported to produce antimicrobial proteins and secretory immunoglobulin A (IgA) antibodies,3,4 all of which proteins have been shown to be present in the saliva. Of these, cystatins,5 mucins6 and defensins7,8 have both antibacterial and antiviral activities; peroxidases and amylase have antibacterial activity; and histatins have antifungal activity.9,10 These antimicrobial proteins are essential factors in host defence in the mucosa, and protect the host against infectious micro-organisms. Other salivary gland proteins that belong to the immune/inflammation system are C-reactive protein (CRP),11 which is known as an acute-phase protein,12 and interleukins [interleukin (IL)-1α and β], tumour necrosis factors (TNFs), and interferon (IFN)-γ, which are known as inflammatory cytokines.13 The SMG therefore appears to interact with the mucosal and systemic compartments of the immune system1 via its secretion, saliva.
IL-1β is a polypeptide that is produced upon infection, injury or antigenic challenge; and a wide variety of cells in the epidermal, epithelial, lymphoid and vascular tissues synthesize this protein. Recently, the Toll-like receptor (TLR) superfamily has been defined, and continues to expand. Its members are known to participate in host responses to injury and infection,14,15 indicating their strong linkage with the cytokines mentioned above. TLRs for innate immunity to pathogens play an important role and are the most important class of pattern-recognition receptors in mammals. For example, TLR-4 recognizes molecules present in the cell walls of Gram-negative bacteria (LPS16–18), and TLR-2 binds to components of Gram-positive bacteria, mycobacteria and fungi.19–22 TLRs recognize pathogen-associated molecular patterns (PAMPs) and activate a number of signalling pathways that stimulate the production of certain cytokines, such as IL-1β, IL-6 and TNF-α.23,24
Although the oral cavity is one of the first barriers to the entry of bacteria and viruses into the body25 and most saliva is secreted by the SMG as well as the parotid gland (PG), the role of TLRs in the initiation of host responses in the SMG is unclear. In the present study, therefore, the role of TLRs in the SMG during host defence against LPS-bearing bacteria was investigated.
Materials and methods
Reagents
LPS from Escherichia coli (serotype 0111:B4), propidium iodide, RNase A and Tri Reagent™ were obtained from Sigma-Aldrich (St. Louis, MO). Superscript™ One-Step RT-PCR System was obtained from Invitrogen (Carlsbad, CA), whereas NuSieve GTG agarose and SeaKem GTG agarose were from Cambrex Bio Science (Rockland, ME). Nembutal (pentobarbital sodium) was a product of Abbott Laboratories (North Chicago, IL), and the Historesin Plus kit was bought from Leica Microsystems (Heidel-Berger, Germany). Goat polyclonal antibodies raised against a carboxy terminal peptide of mouse IL-1β and mouse TLR-4, donkey anti-goat IgG (H + b) conjugated with fluorescein isothiocyanate (FITC), and donkey anti-goat IgG-HRP were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). 3-Aminopropyltriethoxysilane (APS)-coated micro slide glasses and micro cover glasses were purchased from Matsunami Glass Ind. Ltd. (Osaka, Japan). Recombinant mouse IL-1β and goat anti-mouse recombinant IL-1β IgG were products of Peprotech EC Ltd (London, UK) and Genzyme Techne (Cambridge, MA), respectively. Phenylmethylsulphonyl fluoride (PMSF) and aprotinin were procured from Wako Pure Chemicals Ind. Ltd (Osaka, Japan). Complete™ EDTA-free protease inhibitor cocktail tablets were obtained from Boehringer Mannheim GmbH (Mannheim, Germany). The Bio-Rad protein assay kit used was purchased from Bio-Rad Laboratories (Hercules, CA), and the Enhanced Chemical Luminescence (ECL) Detection Kit™, from Amersham Biosciences UK Ltd. (Buckinghamshire, UK). Fuji RX X-ray film was a product of Fuji Film Co. (Kanagawa, Japan).
Animals and endotoxin treatments
Male C3H/HeN and C3H/HeJ mice aged 7–8 weeks were purchased from Nippon SLC (Shizuoka, Japan) and housed under standard conditions (12 : 12 hr light/dark cycle at 22–25°) with free access to food and water. They were killed for experiments at the age of 8–9 weeks.
To study the effect of LPS on the SMG, LPS dissolved in saline at 1 mg/ml was stored at −84° for later use. It was injected intraperitoneally (i.p.) into mice at a concentration of 100 µg/ml and at a dose of 400 µg/kg body weight. Mice were killed at 3, 6, 12 and 24 hr after the injection. Control mice were injected with saline i.p. and killed at 0, 6 and 24 hr after injection. For all LPS experiments, each group consisted of four mice and the results for two animals are shown.
Preparation of total RNA and reverse transcriptase–polymerase chain reaction (RT-PCR)
Total RNA was isolated from the SMG and liver using Tri-Reagent™ following a standard protocol, as described previously.26 The mRNAs for IL-1β, IL-6, TNF-α and TLR-4 were reverse-transcribed followed by cDNA amplification using the Superscript™ One-Step RT-PCR System. The cDNA was synthesized by incubating the RNA at 45° for 30 min and then denatured by heating at 94° for 2 min. The cDNA amplification by PCR was subsequently carried out for 35 cycles, with each cycle consisting of denaturation at 94° for 15 s, primer annealing at 55° for 30 s, and extension at 72° for 1·5 min. The reaction was finally incubated for one cycle at 72° for 5 min. The reaction mixture without RNA was run simultaneously as a negative control. These reactions were sequentially conducted in a TaKaRa PCR Thermal Cycler MP, Model TP3000 (Shiga, Japan). All RT-PCR products were resolved by electrophoresis in 3% agarose gel (NuSieve:SeaKem = 3 : 1) following the standard procedure. The primer sequences for amplification of mRNAs are listed in Table 1.
Table 1.
Sequences of primers used for amplification of mRNAs for inflammatory cytokines, Toll-like receptor (TLR)- and β-actin
| mRNAs | Primer sequences | Product size (bp) |
|---|---|---|
| IL-1β | Sense, 5′-CTTCATCTTTGAAGAAGAGCCC-3′ | 418 |
| Anti-sense, 5′-CTCTGCAGACTCAAACTCCAC-3′ | ||
| IL-6 | Sense, 5′-CCAAACTGGATATAATCAGGAAAT-3′ | 337 |
| Anti-sense, 5′-CTAGGTTTGCCGAGTAGATCTC-3′ | ||
| TNF-α | Sense, 5′-ATGAGCACAGAAAGCATGATC-3′ | 305 |
| Anti-sense, 5′-TCCACTTGGTGGTTTGCTACG-3′ | ||
| TLR-4 | Sense, 5′-AGCAGAGGAGAAAGCATCTATGATGC-3′ | 524 |
| Anti-sense, 5′-GGTTTAGGCCCCAGAGTTTTGTTCTCC-3′ | ||
| β-actin | Sense, 5′-ACCCACACTGTGCCCATCTA-3′ | 289 |
| Anti-sense, 5′-CGGAACCGCTCATTGCC-3′ |
IL, interleukin; TNF, tumour necrosis factor.
Denervation
Mice at the age of 7 weeks were anaesthetized by injection with Nembutal (pentobarbital sodium; 50 mg/ml; 60 mg/kg body weight; i.p.) and the parasympathetic and sympathetic nerves were surgically bisected. For chorda tympani denervation (CTD), mice were held with their right side down and the auditory ossicles containing chorda tympani were removed by approaching from the left external auditory canal.27 For sympathetic denervation, the anterior neck was opened and the right cervical sympathetic nerve was exposed. The cervical sympathetic trunk between the stellate and superior cervical ganglia was bisected28 (cervical sympathetic trunk denervation (CSTD)). Completion of sympathectomy was recognized by the inability of the animal to lift its eyelid on the denervated side (right). To confirm that the denervation was completed successfully, we measured the weight of the SMG on the experimental and control sides after the animals had been killed for experiments. Usually the gland weight was reduced by 10–20% at 7 days after the operation.
Immunohistochemistry
The SMG was cut into 5-mm3 cubes and fixed for 3 hr in freshly prepared periodate-lysin-paraformaldehyde (PLP) fixative containing 4% paraformaldehyde29 at 4°, after which the cubes were washed at 4° overnight in phosphate-buffered saline (PBS) (pH 7·4) containing 6·8% sucrose. The fixed tissues were dehydrated in 100% acetone at 4° for 1 hr; during the first 5 min, acetone was replaced twice or more until the solution became clear, and then the tissues were embedded in Historesin Plus. Tissue sections were cut at 2-µm thickness, mounted on APS-coated slides, and dried at 37° for 2 hr, and then these sections were stored at −20° until used for immunostaining. Prior to immunostaining, the sections were incubated in 0·01% trypsin in PBS for 10 min and washed with PBS three times, each for 5 min. The sections were blocked with 5% normal donkey serum at room temperature for 2 hr, followed by incubation with 0·2 µg/ml goat anti-IL-1β (Santa Cruz Biotechnology) at 4° overnight, after which the sections were rinsed in PBS three times, each for 5 min. The washed sections were incubated with 200-times-diluted, affinity-purified donkey anti-goat IgG (H + L) conjugated with FITC and subsequently washed with PBS. For control staining, sections were incubated with the same concentration of primary antibody pre-absorbed with blocking peptide supplied with the antibody. One volume of anti-IL-1β (0·2 µg/µl) and 10 volumes of blocking peptide (0·2 µg/µl) were mixed and incubated at 4° overnight, after which the mixture was diluted so that the antibody concentration became the same as that used for staining of experimental sections (0·2 µg/ml). After immunostaining and washing, the sections were reacted with PBS containing 0·1 µg/ml propidium iodide and 20 µg/ml RNase A for 15 min at room temperature for nuclear staining. The stained sections were examined under a fluorescence microscope (Nikon, Tokyo, Japan).
Collection of saliva
Saliva was collected by the cotton pellet procedure from C3H/HeN and C3H/HeJ mice at 0, 6 and 24 hr after the animals had been injected with LPS (400 µg/kg body weight). A cotton pellet (approximately 5 mg, washed with sterile water and dried) was placed in the oral cavity of the animal, and was allowed to absorb saliva for 30 min. It was then placed in a 0·5-ml Eppendorf tube with a pinhole at the bottom, and this tube was then inserted into another Eppendorf tube (1·5 ml). These tubes were then centrifuged at 12 000 g for 5 min to recover the saliva, which was then mixed with acetic acid [at a final concentration of 5% (v/v)] and centrifuged at 12 000 g for 10 min to remove mucin. The saliva was then stored at −84° prior to use.
Preparation of the tissue extract
The SMG and PG tissues from C3H/HeN and/or C3H/HeJ mice were isolated at 0, 6 and 24 hr after injection of LPS. These tissues were homogenized with 9 volumes (w/v) of ice-cold homogenization buffer using a glass mortar homogenizer fitted with a Teflon pestle. The buffer consisted of 50 mm Tris-HCl, pH 7·5, 20 mm ethylenediaminetetraacetic acid (EDTA), 10 mm phenylmethylsulphonyl fluoride (PMSF), 2 µg/ml aprotinin, and one tablet of Complete™ EDTA-free protease inhibitor cocktail per 25 ml of buffer. The homogenate was centrifuged at 800 g for 10 min at 4° to remove the nuclei and cell debris, and then the supernatant was recovered and centrifuged again at 150 000 g for 30 min at 4°. The protein concentration of the final supernatant thus obtained was determined, and the extract was stored at −84° prior to the IL-1β analysis. Protein concentrations were determined with a Bio-Rad protein assay kit using bovine serum albumin as a standard.
Western blotting
Aliquots of 10 µg of SMG homogenate proteins, 10 µg of cytosol proteins or 10 µg of saliva were mixed with 2 × sodium dodecyl sulphate (SDS) sample buffer, incubated at 40° for 1 hr, and applied to 12% polyacrylamide slab gels containing 0·1% SDS. After electrophoresis, the proteins separated on the gel were transferred onto a nitrocellulose filter by means of a Mini Trans Blot apparatus (Bio-Rad) according to the procedure reported by Towbin et al.30 The blotted filter was blocked at room temperature for 2 hr with 3% horse serum in 0·2% T-TBS (10 mm Tris-HCl, pH 8·0, containing 150 mm NaCl and 0·2% Tween) and then incubated overnight at 4° with 0·1 µg/ml of goat antirecombinant mouse IL-1β IgG (Genzyme Techne) in 0·2% T-TBS containing 1% horse serum. For the control reaction, the filter was incubated with the same concentration of antibody preabsorbed with the recombinant mouse IL-1β used for immunization; i.e. 1 volume of anti IL-1β (0·1 µg/µl) was mixed with 10 volumes of recombinant IL-1β (0·1 µg/µl) and incubated at 4° overnight, after which the mixture was diluted so that the antibody concentration became the same as that used for the staining of the experimental blot (0·1 µg/ml). The filter was washed with 0·2% T-TBS, incubated with 3000-times-diluted donkey antigoat IgG-HRP, and washed with 0·2% T-TBS. The filter was then reacted with ECL reagents, and exposed to an X-ray film for the appropriate time.
Results
LPS-induced elevation of cytokine mRNAs in the SMG
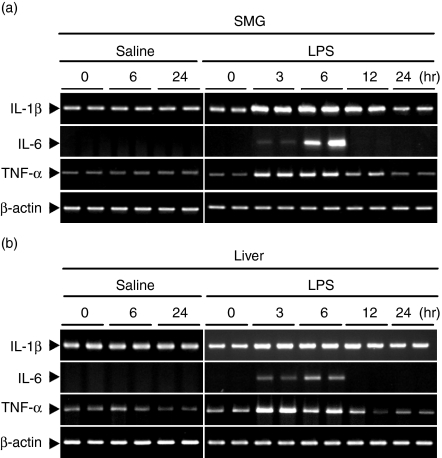
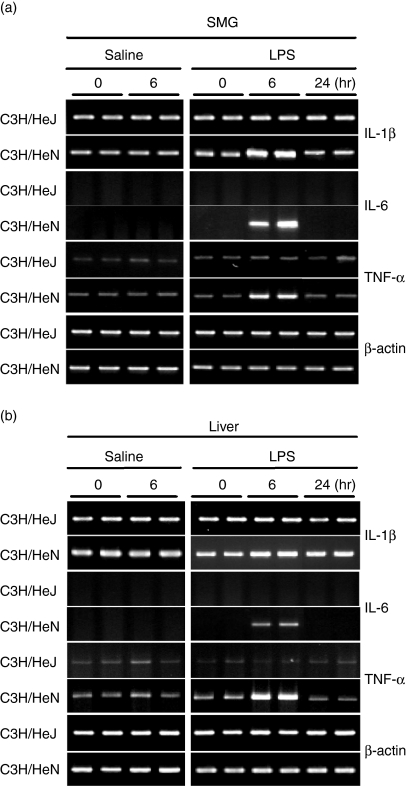
The induction of IL-1β, IL-6 and TNF-α mRNAs was first examined in the liver and SMG of normal C3H/HeN mice (Fig. 1). Strong expression of IL-1β, IL-6 and TNF-α was observed not only in the liver but also in the SMG after injection of LPS. In all of these studies, all cytokine mRNAs examined began to increase 3 hr after the injection of LPS, reached a maximum level at 3–6 hr, and had decreased again by 24 hr. Mice injected with saline did not show any increase at 6 or 24 hr. As seen in Fig. 2, injection of LPS or saline did not induce any mRNA expression for these cytokines (IL-1β, IL-6 and TNF-α) in the SMGs or livers of C3H/HeJ mice, which are known to have defective TLR-4 as a result of a point mutation.18
Figure 1.
Time course of changes in cytokine mRNA expression in (a) the submandibular gland (SMG) and (b) the liver of C3H/HeN mice after injection of lipopolysaccharide (LPS). LPS at a concentration of 100 µg/ml of saline was injected intraperitoneally at a dose of 400 µg/kg body weight. Mice injected with saline were regarded as controls. Total RNA (1 µg) isolated from the SMG and liver after injection of LPS at 0, 3, 6, 12 and 24 hr or saline at 0, 6 and 24 hr was reverse-transcribed and PCR-amplified (see Materials and methods). IL, interleukin; TNF, tumour necrosis factor.
Figure 2.
Reverse transcriptase–polymerase chain reaction (RT-PCR) analysis of cytokine mRNAs expressed in the submandibular gland (SMG) and liver of C3H/HeN mice and C3H/HeJ mice. Experimental conditions were the same as those described in Fig. 1. IL, interleukin; TNF, tumour necrosis factor.
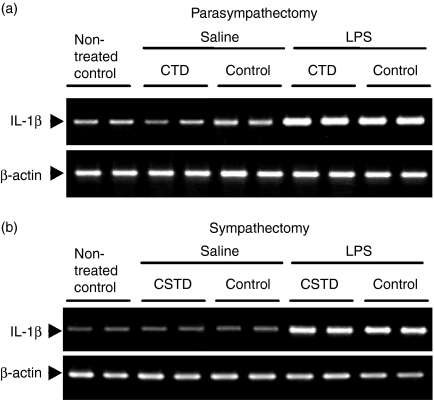
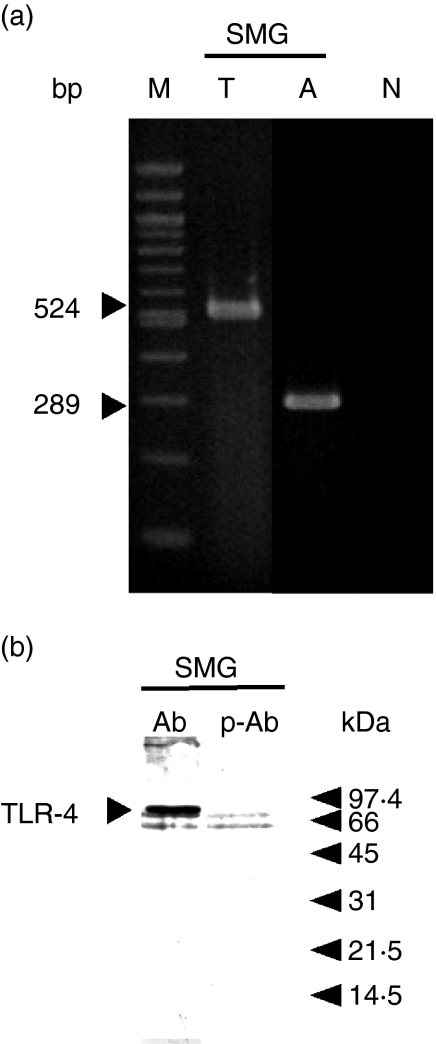
To investigate whether the induction of cytokine mRNAs by LPS was produced by direct action of the endotoxin on the SMG or produced indirectly via the central nervous system, we conducted two types of experiment: the effect of LPS was examined in mice in which the left chorda tympani had been denervated (CTD) and in those in which the right cervical sympathetic trunk had been denervated (CSTD). Compared with that of the contralateral gland, the weight of the SMG was decreased by 20·16% (n = 8, P < 0·005) after CTD and by 12·26% (n = 6, P < 0·005) after CSTD, suggesting that both operations had been completed successfully. mRNA levels for IL-1β were then examined in the SMG on the denervation side and control side after the injection of saline or LPS (Figs 3a and b). Even after CTD, the mRNA level for IL-1β in the SMG on the experimental side was found to be increased 6 hr after the injection of LPS, and was about the same as that of the innervation-intact control gland (Fig. 3a). Injection of saline did not increase the level of IL-1β mRNA in the gland on either side. Similarly, IL-1β mRNA was induced by LPS in the SMG on the CSTD side (Fig. 3b). These results suggest that the central nervous system is not involved in the LPS-induced elevation of IL-1β mRNA in the SMG. LPS thus appears to have stimulated the SMG directly to induce the IL-1β mRNA. In fact, the expression of mRNA and protein for TLR-4 was observed in the SMG of mice (Fig. 4). The molecular weight of TLR-4 of the SMG was between 66 and 97 kDa which was close to that reported recently.31
Figure 3.
Effects of (a) chorda tympani denervation and (b) cervical sympathetic trunk denervation on lipopolysaccharide (LPS)-induced interleukin (IL)-1β elevation in the submandibular gland (SMG) of C3H/HeN mice. The left chorda timpani or right cervical sympathetic trunk was denervated and LPS or saline was injected on the 7th day after the operation. At 6 hr after injection of LPS or saline, total RNA was isolated. CTD, chorda tympani denervation; CSTD, cervical sympathetic trunk denervation.
Figure 4.
Reverse transcriptase–polymerase chain reaction (RT-PCR) and Western blot analyses of submandibular gland (SMG) RNAs and proteins to verify the expression of Toll-like receptor-4 (TLR-4). Total RNA or homogenate proteins were subjected to RT-PCR and Western blot analyses. Other experimental conditions are described in the text. (a) RT-PCR; (b) Western blotting. Sizes of DNA markers in (a) are 1500, 1200, 1000, 900, 800, 700, 600, 500, 400, 300, 200 and 100 bp. T, TLR-4 mRNA amplification; A, β-actin amplification; N, no added RNA; M, DNA markers; Ab, anti-TLR-4 polyclonal antibody; p-Ab, peptide-preabsorbed anti-TLR-4 polyclonal antibody.
Expression of the IL-1β protein in the SMG and saliva
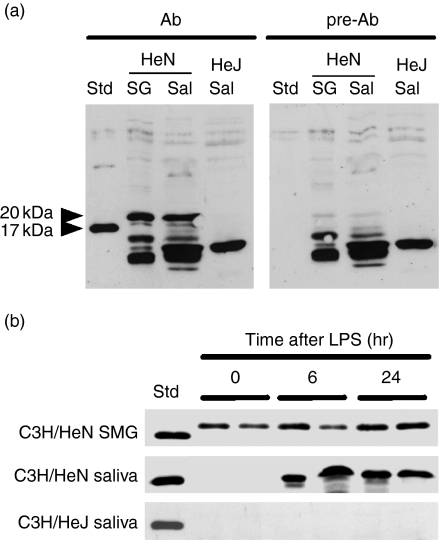
We next examined the effects of LPS on the IL-1β protein in the SMG, the PG and whole saliva by Western blotting in both C3H/HeN and C3H/HeJ mice (Figs 5 and 6). As shown in the left panel of Fig. 5(a), the antibody we used detected 20- and 17·5-kDa IL-1β bands in the SMG and saliva of LPS-stimulated C3H/HeN mice, which were not seen when the antibody had been pre-absorbed with the immunogen peptide (Fig. 5a, right panel). No positive band corresponding to 20- and 17·5-kDa IL-1β was detected in the saliva from LPS-stimulated C3H/HeJ mice with either antibody (Fig. 5a, both panels). Although the antibody bound non-specifically to proteins ranging from 10 to 15 kDa, this experiment indicated that IL-1β was detected by this antibody (Genzyme-Techne). IL-1β was secreted into the saliva when C3H/NeN mice were injected with LPS (Figs 5a and b). Salivary IL-1β appeared as early as 6 hr after LPS injection. Such secretion was not observed in C3H/HeJ mice, although a small amount of IL-1β protein was detected in the SMG of these mice (data not shown). These results suggest the possibility that LPS induced not only the synthesis but also the secretion of IL-1β from the SMG, although further experiments are required to verify this.
Figure 5.
Western blotting for detection of interleukin (IL)-1β in the submandibular gland (SMG) and saliva of C3H/HeN and C3H/HeJ mice. A 10-µg aliquot of SMG sample or 10-µl salivary sample was subjected to electrophoresis. (a) Western blot analysis of IL-1β protein in the SMG and saliva of C3H/HeN and C3H/HeJ mice. Ab, anti-IL-1β antibody; pre-Ab, peptide-preabsorbed anti-IL-1β antibody; Std, standard (recombinant mouse IL-1β); SG, SMG from untreated mice; Sal, saliva collected 6 hr after lipopolysaccharide (LPS) injection. (b) Time course of IL-1β protein expression of C3H/HeN mice after injection of LPS. The SMG extract and saliva of C3H/HeN mice and C3H/HeJ mice were analysed 0, 6 and 24 hr after the injection of LPS.
Figure 6.
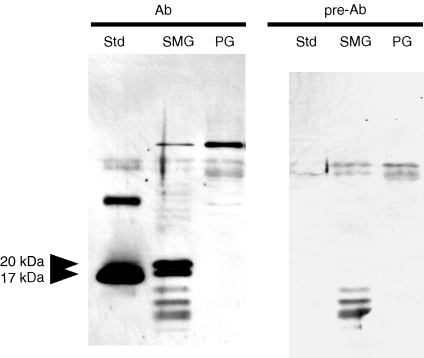
Western blotting for detection of interleukin (IL)-1β in the submandibular gland (SMG) and parotid gland (PG) of untreated C3H/HeN mice. Std, standard (recombinant mouse IL-1β); Ab, anti-recombinant mouse IL-1β antibody; pre-Ab, antigen-pre-absorbed anti-recombinant mouse IL-1β antibody. SMG or PG samples (10 µg) were subjected to electrophoresis.
Saliva is mainly produced by the SMG and PG. Although we assumed that all salivary IL-1β was from the SMG, we needed to determine the percentage ratio of IL-1β from the two glands. We therefore analysed the same amounts of SMG and PG total protein by Western blotting. As shown in Fig. 6, the results indicate that no detectable IL-1β was present in the extract of the PG. These data suggest that most, if not all, of the salivary IL-1β originated from the SMG.
Pro-IL-1β is known to be 35 kDa in size in both humans and mice.32 Because no positive band of this size was detected when the SMG extract was examined by Western blotting, it was not clear whether such a precursor protein was present in the SMG. It was thus also not clear whether there were processing enzymes for pro-IL-1β in the SMG. We therefore investigated whether mRNA for pro-IL-1β was present in the SMG, and found that such a large mRNA existed in the SMG as well as in the LPS-stimulated macrophages (C. Yao, unpublished data). Although this is not direct evidence for the presence of 35-kDa pro-IL-1β protein in the SMG, synthesis of such precursor proteins in the SMG is strongly suggested by the results of this experiment.
Immunostaining for IL-1β in the SMG
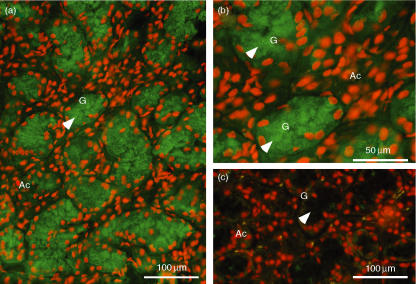
As large amounts of IL-1β mRNA and protein were detected in the SMG, cellular localization of the IL-1βprotein was investigated immunohistochemically. As shown in Figs 7(a) and (b), secretory granules of granular convoluted tubule (GCT) cells showed a positive immunoreaction, which disappeared when the primary antibody was preabsorbed with the immunogen peptide (Fig. 7c), indicating the specificity of the staining. No other cells, such as acinar cells, showed a positive reaction. These results suggest that IL-1β was exclusively expressed in the GCT cells.
Figure 7.
Immunohistochemical staining for interleukin (IL)-1β in the submandibular gland (SMG) of lipopolysaccharide (LPS)-injected C3H/HeN mice. The SMG was removed 6 hr after LPS injection. (a, c) Low-power magnification. (b) High-power magnification. (a, b) Staining with anti-IL-1β antibody immunoglobulin (IgG). (c) Staining with antigen-pre-absorbed antibody IgG. Arrows indicate secretory granules of granular convoluted tubule (GCT) cells. Red staining indicates the location of the nucleus, whereas green staining indicates the location of IL-1β. The GCT cells (G) filled with immunopositive secretory granules have nuclei in the basal area. No positive reaction was seen in acini (Ac).
Discussion
In the present study, it was found for the first time that inflammatory cytokines (i.e. IL-1β, IL-6 and TNF-α) were induced by LPS stimulation via TLR-4 in the SMG of C3H/HeN mice. The first line of evidence is that the mRNAs for these cytokines were significantly induced in the SMG after LPS injection. The second is that such LPS-induced cytokine elevations were not seen in C3H/HeJ mice, a TLR-4− mutant strain. The third is that increased expression of IL-1β mRNA after injection of LPS was also observed in the sympatho-denervated and/or parasympatho-denervated SMG. Lastly, mRNA for TLR-4 was expressed strongly in the SMG. These results demonstrate that LPS induces inflammatory cytokines in the SMG via TLR-4 by direct action on this gland.
The SMG tissue comprises six major epithelial compartments: acinar cells, intercalated ducts, GCT cells, striated duct cells, excretory duct cells and myoepithelial cells.33 By immunohistochemistry, the IL-1β protein was detected in the secretory granules present in the apical area of GCT cells in the SMG of C3H/HeN mice. Together with the Western blotting data, this finding suggests that the IL-1β protein expressed and localized in the GCT of the SMG may be secreted into the saliva. Indeed, a 20-kDa IL-1β protein was present not only in the SMG but also in saliva from LPS-stimulated C3H/HeN mice. Although it was reported previously that the mouse PG produces IL-1β,34,35 in our experiments most of this cytokine present in the saliva appeared to originate from the SMG. In addition, the level of salivary IL-1β was increased after LPS injection, suggesting that LPS induced secretion of IL-1β in C3H/HeN mice, although the precise mechanism is not clear. These data therefore suggest that the SMG plays an important role as a defence system in the oral cavity, prohibiting the entry of micro-organisms from the mouth.
As the GCT of mouse SMG is known to be androgen-dependent,36 we also examined the LPS response of IL-1β expression in mice with different hormonal status. Thus, expression levels of mRNA and protein for IL-1β were examined in females, castrated males, and mice injected with testosterone propionate (0·4 mg/mouse, every other day for 9 days). mRNA levels for IL-1β were almost the same in all of these mice, and were increased by LPS injection similarly as in normal males. However, the 17·5- and 20-kDa IL-1β proteins in both tissues and saliva of androgen-deficient mice (females and castrated males) were hardly detected, suggesting a difference in activity for either translational or post-translational steps between mice with high and low androgen levels (data not shown). A similar sexual difference has been reported for many important biologically active peptides such as NGF and EGF.36 Although the reason for, and the physiological meaning of, such sexual differences are not clear, the present finding regarding the LPS response of IL-1β induction and secretion may provide new insights in this field of research.
In the present experiments, elevation of cytokine mRNA expression was detected following the injection of 400 µg LPS per kg body weight. Both in the liver and in the SMG, inflammatory cytokines (IL-1β, IL-6 and TNF-α) were strongly induced. These cytokine mRNAs were induced as early as 3 hr after the LPS injection, reached peak levels between 3 and 6 hr after injection, and had returned to their original levels at 24 hr after injection. These data agree well with a previous report by Wei et al.11 in which rats were used as experimental animals. Earlier, Purves et al.27 and Mathison et al.37 reported that immunomodulatory factors such as TGF-β and EGF were derived from the SMG, and their synthesis and release were controlled by cervical sympathetic nerves. In contrast, our data suggest that the expression of IL-1β mRNA is controlled by the direct stimulation of the SMG by LPS, because denervation of the cervical sympathetic trunk between the stellate and superior cervical ganglion or destruction of the chorda tympani nerve did not affect the increase in expression of IL-1β mRNA by LPS (at 6 hr). These data suggest the existence of a LPS-provoked TLR-4 signalling pathway in the SMG by which the IL-1β elevation is induced.
Like other SMG bioactive polypeptides, such as EGF and TGF-β,38,39 IL-1β was detected in the secretory granules in the apical area of GCT cells in C3H/HeN mice. In murine macrophages, Beuscher et al.40 reported that the mature 20-kDa IL-1β is produced by processing with a LPS-inducible protease and that this small IL-1β is secreted. In our experiments, we also detected a 20-kDa IL-1β in both the SMG and saliva by Western blotting. Although the IL-1β precursor protein with a molecular weight of 35 kDa was not detected in this tissue, a large mRNA for pro-IL-1β was detected (data not shown), indicating that all IL-1β precursor is processed rapidly to 17·5- and 20-kDa IL-1β as soon as it is formed in the SMG. A positive band with an approximate molecular weight of 100 kDa appeared in both SMG and PG. This band disappeared after pre-absorption of antibody with peptide, suggesting its specificity. However, the nature of this protein is not clear at present and needs to be determined in future studies.
In the SMG, it is well known that many species of bioactive polypeptides and growth factors, such as NGF and EGF, are produced by proteolytic processing from their precursor proteins.41 Some or many of these processing enzymes belong to a kallikrein family and co-localize with precursor proteins in the secretory granules of the GCT cells.42 The processing enzyme for pro-IL-1β is interleukin-1β converting enzyme (ICE); it is not yet clear whether it is present in the SMG. These findings, together with the present results, suggest that the 17·5- and 20-kDa IL-1β proteins are produced from a large precursor protein in the SMG by the action of processing enzymes, such as caspase, kallikrein or other proteases. As the presence of a LPS-inducible protease in dendritic cells has been reported,43 it would be worthwhile to investigate whether the processing enzyme responsible for the production of 17·5- and 20-kDa IL-1β is indeed present and regulated by LPS in the SMG.
Generally, the molecular size of active/mature IL-1β is thought to be 17·5 kDa,44 which size of band was also detected in the SMG or saliva in this study. However, from band intensity, the 20-kDa IL-1β appears to be more abundant in both the SMG and saliva than the 17·5-kDa IL-1β. A 20-kDa IL-1β is secreted from macrophages and also reported to be active.40 Thus, IL-1β of the same size as that secreted by macrophages appeared in the saliva and is also presumed to be active. All these data suggest the existence of a salivary IL-1β response to infection, by which secreted IL-1β, by acting on oral and gastrointestinal mucosal tissues, may induce the synthesis of mucin or inflammatory proteins such as defensin, as their genes have an nuclear factor-κB (NF-κB) responsive element in their promoter regions.45 Actually, we have detected mRNA expression for IL-1 receptors type I and II and accessory protein in the cells of the oral mucosa by RT-PCR (data not shown). Thus, the possible existence of an axis, the salivary IL-1β–oral mucosa response, is now being investigated using an in vitro culture system.
Abbreviations
- APS
aminopropyltriethoxysilane
- CSTD
cervical sympathetic trunk denervation
- ECL
Enhanced Chemical Luminescence
- EDTA
ethylenediaminetetraacetic
- EGF
epidermal growth factor
- GCT
granular convoluted tubular
- NGF
nerve growth factor
- PAMP
pathogen-associated molecular pattern
- PLP
periodate-lysin-paraformaldehyde
- PMSF
phenylmethylsulphonyl fluoride
- TGF
transforming growth factor
- TLR
toll-like receptor
- TNF
tumour necrosis factor
References
- 1.Sabbadini E, Berczi I. The submandibular gland: a key organ in the neuro-immuno-regulatory network? Neuroimmunomodulation. 1995;2:184–202. doi: 10.1159/000097197. [DOI] [PubMed] [Google Scholar]
- 2.Donald IH, William HB. The functions of salivary proteins. In: Edgar WM, O'Mullane DM, editors. Saliva and Oral Health. 2. London: British Dental Association; 1996. pp. 105–22. [Google Scholar]
- 3.Mestecky J. Saliva as a manifestation of the common mucosal immune system. Ann NY Acad Sci. 1993;694:184–94. doi: 10.1111/j.1749-6632.1993.tb18352.x. [DOI] [PubMed] [Google Scholar]
- 4.Jackson DE, Lally ET, Nakamura MC, Montgomery PC. Migration of IgA-bearing lymphocytes into salivary glands. Cell Immunol. 1981;63:203–9. doi: 10.1016/0008-8749(81)90042-3. [DOI] [PubMed] [Google Scholar]
- 5.Matsuura S, Hand AR. Quantitative immunocytochemistry of rat submandibular secretory proteins during chronic isoproterenol administration and recovery. J Histochem Cytochem. 1991;39:945–54. doi: 10.1177/39.7.1865112. [DOI] [PubMed] [Google Scholar]
- 6.Jaskoll T, Chen H, Denny PC, Denny PA, Melnick M. Mouse submandibular gland mucin: embryo-specific mRNA and protein species. Mech Dev. 1998;74:179–83. doi: 10.1016/s0925-4773(98)00062-8. [DOI] [PubMed] [Google Scholar]
- 7.Bonass WA, High AS, Owen PJ, Devine DA. Expression of beta-defensin genes by human salivary glands. Oral Microbiol Immunol. 1999;14:371–4. doi: 10.1034/j.1399-302x.1999.140607.x. [DOI] [PubMed] [Google Scholar]
- 8.Mizukawa N, Sugiyama K, Kamio M, Yamachika E, Ueno T, Fukunaga J, Takagi S, Sugahara T. Immunohistochemical staining of human alpha-defensin-1 (HNP-1), in the submandibular glands of patients with oral carcinomas. Anticancer Res. 2000;20:1125–7. [PubMed] [Google Scholar]
- 9.Ruissen AL, Groenink J, Van't Hof W, et al. Histatin 5 and derivatives. Their localization and effects at the ultra-structural level. Peptides. 2002;23:1391–9. doi: 10.1016/s0196-9781(02)00076-1. [DOI] [PubMed] [Google Scholar]
- 10.Edgerton M, Koshlukova SE. Salivary histatin 5 and its similarities to the other antimicrobial proteins in human saliva. Adv Dent Res. 2000;14:16–21. doi: 10.1177/08959374000140010201. [DOI] [PubMed] [Google Scholar]
- 11.Wei W, Parvin N, Tsumura K, Akamatsu T, Tada J, Kanamori N, Hosoi K. Induction of C-reactive protein, serum amyloid P component, and kininogens in the submandibular and lacrimal glands of rats with experimentally induced inflammation. Life Sci. 2001;69:359–68. doi: 10.1016/s0024-3205(01)01129-8. [DOI] [PubMed] [Google Scholar]
- 12.Hamano H, Saito I, Haneji N, Mitsuhashi Y, Miyasaka N, Hayashi Y. Expressions of cytokine genes during development of autoimmune sialadenitis in MRL/lpr mice. Eur J Immunol. 1993;23:2387–91. doi: 10.1002/eji.1830231002. [DOI] [PubMed] [Google Scholar]
- 13.Yamano S, Atkinson JC, Baum BJ, Fox PC. Salivary gland cytokine expression in NOD and normal BALB/c mice. Clin Immunol. 1999;92:265–75. doi: 10.1006/clim.1999.4759. [DOI] [PubMed] [Google Scholar]
- 14.Lynn WA, Golenbock DT. Lipopolysaccharide antagonists. Immunol Today. 1992;13:271–6. doi: 10.1016/0167-5699(92)90009-V. [DOI] [PubMed] [Google Scholar]
- 15.Hla T, Lee MJ, Ancellin N, Paik JH, Kluk MJ. Lysophospholipids-receptor revelations. Science. 2001;294:1875–8. doi: 10.1126/science.1065323. [DOI] [PubMed] [Google Scholar]
- 16.Wang X, Moser C, Louboutin JP, Lysenko ES, Weiner DJ, Weiser JN, Wilson JM. Toll-like receptor 4 mediates innate immune responses to Haemophilus influenzae infection in mouse lung. J Immunol. 2002;168:810–5. doi: 10.4049/jimmunol.168.2.810. [DOI] [PubMed] [Google Scholar]
- 17.Chow JC, Young DW, Golenbock DT, Christ WJ, Gusovsky F. Toll-like receptor-4 mediates lipopolysaccharide-induced signal transduction. J Biol Chem. 1999;274:10689–92. doi: 10.1074/jbc.274.16.10689. [DOI] [PubMed] [Google Scholar]
- 18.Poltorak A, He X, Smirnova I, et al. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 1998;282:2085–8. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- 19.Yoshimura A, Lien E, Ingalls RR, Tuomanen E, Dziarski R, Golenbock D. Cutting edge: recognition of Gram-positive bacterial cell wall components by the innate immune system occurs via Toll-like receptor 2. J Immunol. 1999;163:1–5. [PubMed] [Google Scholar]
- 20.Takeuchi O, Hoshino K, Kawai T, Sanjo H, Takada H, Ogawa T, Takeda K, Akira S. Differential roles of TLR2 and TLR4 in recognition of gram-negative and gram-positive bacterial cell wall components. Immunity. 1999;11:443–51. doi: 10.1016/s1074-7613(00)80119-3. [DOI] [PubMed] [Google Scholar]
- 21.Means TK, Wang S, Lien E, Yoshimura A, Golenbock DT, Fenton MJ. Human toll-like receptors mediate cellular activation by Mycobacterium tuberculosis. J Immunol. 1999;163:3920–7. [PubMed] [Google Scholar]
- 22.Schwandner R, Dziarski R, Wesche H, Rothe M, Kirschning CJ. Peptidoglycan- and lipoteichoic acid-induced cell activation is mediated by toll-like receptor 2. J Biol Chem. 1999;274:17406–9. doi: 10.1074/jbc.274.25.17406. [DOI] [PubMed] [Google Scholar]
- 23.Nair SP, Meghji S, Wilson M, Reddi K, White P, Henderson B. Bacterially induced bone destruction: mechanisms and misconceptions. Infect Immun. 1996;64:2371–80. doi: 10.1128/iai.64.7.2371-2380.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Verghese MW, Snyderman R. Differential anti-inflammatory effects of LPS in susceptible and resistant mouse strains. J Immunol. 1981;127:288–93. [PubMed] [Google Scholar]
- 25.Tenovuo J. Antimicrobial agents in saliva-protection for the whole body. J Dent Res. 2002;81:807–9. doi: 10.1177/154405910208101202. [DOI] [PubMed] [Google Scholar]
- 26.Chomczynski P. A reagent for the single-step simultaneous isolation of RNA, DNA and proteins from cell and tissue samples. Biotechniques. 1993;15:532–4. [PubMed] [Google Scholar]
- 27.Purves DA. Trophic Theory of Neural Connections, Body and Brain. Cambridge: Harvard University Press; 1988. [DOI] [PubMed] [Google Scholar]
- 28.Branica S, Sprem N, Mihelic D, Zobundzija M, Cvoriscec D, Cacic M, Dawidowsky K. Biochemical changes in the sublingual and submandibular glands after interruption of chorda tympani. Acta Med Croatica. 2002;56:11–5. [PubMed] [Google Scholar]
- 29.McLean IW, Nakane PK. Periodate-lysine-paraformaldehyde fixative. A new fixation for immunoelectron microscopy. J Histochem Cytochem. 1974;22:1077–83. doi: 10.1177/22.12.1077. [DOI] [PubMed] [Google Scholar]
- 30.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–435. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hollestelle SC, De Vries MR, Van Keulen JK, et al. Toll-like receptor 4 is involved in outward arterial remodeling. Circulation. 2004;109:393–8. doi: 10.1161/01.CIR.0000109140.51366.72. [DOI] [PubMed] [Google Scholar]
- 32.Dinarello CA. Interleukin-1 beta, interleukin-18, and the interleukin-1 beta converting enzyme. Ann NY Acad Sci. 1998;856:1–11. doi: 10.1111/j.1749-6632.1998.tb08307.x. [DOI] [PubMed] [Google Scholar]
- 33.Penschow JD, Coghlan JP. Secretion of glandular kallikrein and renin from the basolateral pole of mouse submandibular duct cells: an immunocytochemical study. J Histochem Cytochem. 1993;41:95–103. doi: 10.1177/41.1.8417114. [DOI] [PubMed] [Google Scholar]
- 34.Tanda N, Ohyama H, Yamakawa M, et al. IL-1 beta and IL-6 in mouse parotid acinar cells: characterization of synthesis, storage, and release. Am J Physiol. 1998;274:G147–56. doi: 10.1152/ajpgi.1998.274.1.G147. [DOI] [PubMed] [Google Scholar]
- 35.Yamakawa M, Weinstein R, Tsuji T, McBride J, Wong DT, Login GR. Age-related alterations in IL-1beta, TNF-alpha, and IL-6 concentrations in parotid acinar cells from BALB/c and non-obese diabetic mice. J Histochem Cytochem. 2000;48:1033–42. doi: 10.1177/002215540004800802. [DOI] [PubMed] [Google Scholar]
- 36.Barka T. Biologically active polypeptides in submandibular glands. J Histochem. 1980;28:836–59. doi: 10.1177/28.8.7003006. [DOI] [PubMed] [Google Scholar]
- 37.Mathison R, Davison JS, Befus AD. Neuroendocrine regulation of inflammation and tissue repair by submandibular gland factors. Immunol Today. 1994;15:527–32. doi: 10.1016/0167-5699(94)90209-7. [DOI] [PubMed] [Google Scholar]
- 38.Gresik E, Barka T. Immunocytochemical localization of epidermal growth factor in mouse submandibular gland. J Histochem Cytochem. 1977;25:1027–35. doi: 10.1177/25.9.333017. [DOI] [PubMed] [Google Scholar]
- 39.Amano O, Tsuji T, Nakamura T, Iseki S. Expression of transforming growth factor beta 1 in the submandibular gland of the rat. J Histochem Cytochem. 1991;39:1707–11. doi: 10.1177/39.12.1940322. [DOI] [PubMed] [Google Scholar]
- 40.Beuscher HU, Gunther C, Rollinghoff M. IL-1 beta is secreted by activated murine macrophages as biologically inactive precursor. J Immunol. 1990;144:2179–83. [PubMed] [Google Scholar]
- 41.Zabel BU, Eddy RL, Lalley PA, Scott J, Bell GI, Shows TB. Chromosomal locations of the human and mouse genes for precursors of epidermal growth factor and the beta subunit of nerve growth factor. Proc Natl Acad Sci USA. 1985;82:469–73. doi: 10.1073/pnas.82.2.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kikkawa Y, Yamanaka N, Tada J, Kanamori N, Tsumura K, Hosoi K. Prorenin processing and restricted endoproteolysis by mouse tissue kallikrein family enzymes (mK1, mK9, mK13, and mK22) Biochim Biophys Acta. 1998;1382:55–64. doi: 10.1016/s0167-4838(97)00144-1. [DOI] [PubMed] [Google Scholar]
- 43.Kitajima T, Ariizumi K, Mohamadazadeh M, Edelbaum D, Bergstresser PR, Takashima A. T cell-dependent secretion of IL-1 beta by a dendritic cell line (XS52) derived from murine epidermis. J Immunol. 1995;155:3794–800. [PubMed] [Google Scholar]
- 44.Kim NG, Lee H, Son E, et al. Hypoxic induction of caspase-11/caspase-1/interleukin-1beta in brain microglia. Brain Res Mol Brain Res. 2003;114:107–14. doi: 10.1016/s0169-328x(03)00135-9. [DOI] [PubMed] [Google Scholar]
- 45.Liu L, Wang L, Jia HP, Zhao C, Heng HH, Schutte BC, McCray PB, Jr, Ganz T. Structure and mapping of the human beta-defensin HBD-2 gene and its expression at sites of inflammation. Gene. 1998;222:237–44. doi: 10.1016/s0378-1119(98)00480-6. [DOI] [PubMed] [Google Scholar]