Abstract
We report that osteopenia is a prominent and previously unappreciated clinical feature of patients with X-linked hyper-IgM syndrome, an inherited immune deficiency disorder caused by mutations in the gene encoding CD40 ligand (CD40L). We therefore conducted studies to determine the relationship between CD40L and osteoclastogenesis. Recognizing that activated T cells express surface receptor activator of NF-κB ligand (RANKL) and can induce osteoclast differentiation of myeloid cells expressing RANK, we assessed the capacity of wild-type T cells and CD40L−/− T cells to induce osteoclastogenesis in vitro. Relative to wild-type T cells, activated CD40L−/− T cells from both humans and mice promoted robust osteoclast differentiation of myeloid cells. Whereas activated CD40L−/− T cells had normal expression of RANKL, they were deficient in IFN-γ production. In subsequent studies, we cultured activated CD40L−/− T cells in the presence of IFN-γ, and we found that the osteoclastic capacity of CD40L−/− T cells could be greatly diminished. These results show that CD40L can influence RANKL signaling through T cell priming, and thus they demonstrate a regulatory role for CD40L in bone mineralization that is absent in patients with X-linked hyper-IgM syndrome.
Keywords: osteoclast, IFN-γ, receptor activator of NF-κB ligand (RANKL), bone homeostasis, T cells
Bone is subject to continuous remodeling by the coupled activity of two different cells types: osteoblasts that synthesize new bone matrix and osteoclasts that lead to its resorption (1). The discovery of receptor activator of nuclear factor (NF)-κB ligand (RANKL; also known as TRANCE), a member of the TNF superfamily of cytokines, has led to a deeper understanding of osteoclastogenesis, the process of osteoclast differentiation and activation. RANKL is synthesized by bone marrow stromal cells, osteoblasts, and activated T cells (2–5). Its interaction with RANK on the surface of myeloid precursor cells induces their differentiation into osteoclasts. In addition, stimulation of mature osteoclasts by RANKL leads to their activation and bone resorption in vivo (4–7). The majority of skeletal diseases associated with low BMD are related to excessive osteoclast activity (8).
The RANK signaling pathway has several levels of regulation. Osteoprotegerin (OPG) is an osteoclastogenesis-inhibitory factor that is secreted by osteoblasts and functions as a soluble decoy receptor for RANKL (9). RANK stimulation leads to the recruitment of the tumor necrosis factor receptor-associated factor 6 (TRAF6), which then regulates the downstream activation of the IκB kinase complex and the c-Jun N-terminal kinase signaling pathways. IFN-γ signaling leads to the proteosomal degradation of TRAF6, and it can therefore have an inhibitory effect on RANK intracellular signaling and osteoclast activity (10). In contrast, IL-1, TNF-α, and TGF-β can enhance osteoclastogenesis in vitro (11, 12). IL-1 and TNF-α are members of the TNF family of proteins, and increased signaling by these soluble factors may have a synergistic effect on the expression of target genes that mediate osteoclast activation.
CD40 ligand (CD40L or CD154) is expressed on the surface of activated CD4+ T cells. The gene encoding CD40L lies on the X chromosome, and alterations in CD40L cause X-linked hyper-IgM syndrome (XHIM), which is a rare immune deficiency disorder that is inherited as an X-linked inherited trait and is usually only found in males (13–15). As a consequence of deficiency in CD40L, patient CD4+ T cells are unable to stimulate CD40-expressing B cells to switch Ig production from IgM to IgG, or IgA. Thus, XHIM patients have skewed IgM antibody responses and a markedly diminished or absent IgG response to protein antigens (16).
CD40L stimulation of antigen-presenting cells (APCs) induces up-regulation of CD80/86 and IL-12 secretion. IL-12 secretion by APCs leads to the differentiation of naïve T cells into TH1 effector cells capable of producing IFN-γ. The importance of this interaction has been shown in both humans and mice with CD40L deficiency, where the population of antigen-primed memory T cells is markedly diminished, and activated T cells fail to secrete IFN-γ or induce IL-12 secretion in APCs (17, 18). Clinically, such impairments in T cell immunity predispose XHIM patients to opportunistic infections with Pneumocystis carinii and Cryptosporidium (19–21). In addition, reports of hepatobiliary cancer, carcinoid tumor, and other neoplasms in patients with XHIM suggest defective immune surveillance (22). The immune defects in patients with XHIM have consequences early in life. In the only large retrospective study of 56 patients, the Kaplan–Meier survival rate was 20% by the age of 25 years despite i.v. Ig therapy and antibiotic prophylaxis (19).
We noted that clinic patients with XHIM had spontaneous rib fractures without antecedent trauma. This clinical observation prompted us to investigate whether CD40L deficiency may contribute to an imbalance in bone mineral homeostasis. In this work we show that, compared with age- and sex-matched normal controls, XHIM patients have significantly lower bone mineral density (BMD) and have elevated levels of N-terminal telopeptides of type I collagen (NTX), a urinary marker indicative of osteoclast activity (23–25). We further demonstrate that activated CD4+ CD40L−/− T cells from either humans or mice have normal expression of RANKL and promote marked osteoclastogenesis of myeloid cells because of their defect in T cell priming and IFN-γ production. Taken together, these findings suggest an important regulatory role for CD40L in osteoclastogenesis, which is impaired in patients with XHIM.
Results
Patient Characteristics.
Fourteen patients from 13 unrelated families (ages 6–33 years) were enrolled into the study. The diagnosis of XHIM was established in each patient by medical and family history, Ig profile, and the presence of the CD40L mutation and/or activated T cells that do not express CD40L. All patients presented in childhood with recurrent bacterial sinorespiratory infections and some experienced opportunistic infections. All patients after diagnosis received i.v. gamma globulin replacement therapy. During routine clinic visits, two of the patients were noted to have spontaneous rib fractures without a history of antecedent trauma. No significant abnormality was found in biochemical indices of calcium homeostasis. Specifically, serum calcium, serum phosphorus, 1,25-dihydroxyvitamin D3, 25-dihydroxyvitamin D3, and intact parathyroid hormone remained within the normal range.
Bone Density, Histomorphometry, and NTX Measurements.
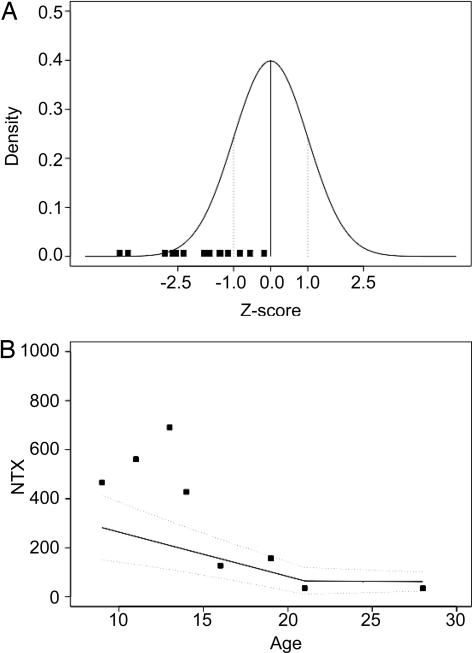
BMD was analyzed by dual-energy x-ray absorptiometry (DXA) (26) at several skeletal locations in all 14 XHIM patients. The L1–L4 location in the posterior–anterior position was chosen for statistical evaluation because pediatric reference values are available at this location and it allows the patients' results to be compared with age-matched male controls. Our group of XHIM patients exhibited a Z score (number of SD away from the mean) distribution that was markedly shifted to the negative values compared with the distribution expected in a normal population (Fig. 1A). The mean Z score was −1.92 instead of the 0 value that would be expected in the distribution of a normal group. This difference is statistically significant (P < 0.001; Wilcoxon test on Z scores). Eleven of 14 patients were noted to have BMD (Z score) ≤ −1 SD below normal controls, and four of the patients had Z scores ≤ −2.5, which is below the first percentile in age-matched male controls. Low BMD was apparent at 6 years of age, and it was evident at all ages. Taken together, these findings indicate that low BMD is common in patients with XHIM.
Fig. 1.
BMD and biochemical measurements of bone metabolism. (A) Reduced BMD in XHIM patients. The BMD (g/cm2) at the spinal L1–L4 position in the posterior–anterior projection for each patient was compared with an age- and sex-matched control group. In each patient, the number of standard deviations of the BMD value compared with the mean value for the control group (Z score; filled squares) is represented over the Gaussian distribution of the normal population. (B) Measurements of NTXs in 24-h urine samples of eight XHIM patients. The filled squares represent the values after adjusting to the creatinine level (nmol of bone collagen equivalents/mmol of creatinine). The values of an age- and sex-matched control group (27) are represented by a regression method where the thick line represents the mean value for the different ages and the dashed lines the mean value ± 1 SD.
The majority of skeletal diseases result from increased bone resorption. However, abnormalities in the morphogenesis of bone are also known to affect bone mass. We therefore performed histomorphometric analyses on decalcified iliac bone samples from two XHIM patients. The bone volume/tissue volume ratio in a 15-year-old patient was 20.9%, and in a 19-year-old it was 22.3%. Both values were within the 95% confidence interval for age-matched controls (mean value ± SD for controls between 14 and 17 years old, 25.7 ± 5.3; between 17 and 23 years old, 27.8 ± 4.5). These patients were noted to have BMD Z scores of −3.5 and −2.3, respectively. It should be noted that the bone densitometry measurement in the 15-year-old was concurrent with the bone biopsy, but the DXA scan in the 19-year-old patient was performed 10 years after the bone biopsy. Osteoblast quantification was possible in the sample from the 19-year-old patient. The result of 12.9% was also within the 95% confidence interval for osteoblast surface/bone surface ratio in age-matched controls (mean value ± SD for controls between 17 and 23 years old, 7.9 ± 4.1). These limited histomorphometric results are compatible with the hypothesis that trabecular bone volume and bone formation are normal in XHIM.
NTX is a well validated static measure of bone resorption, and it serves as a relative index of current osteoclastic activity. Urinary NTX measurements were made in 24-h urine samples in eight patients. The results were compared with a control population by the generation of a D score calculated as the SD for each value compared with a control group (27). The NTX values in the older patients were not significantly different from the control group (Fig. 1B). For the youngest children, however, all NTX measurements were >1 SD larger than the mean for normal controls, suggesting that the reduced BMD in XHIM could be the result of enhanced osteoclastogenesis.
Analysis of RANKL Expression and IFN-γ Production by Activated T Cells.
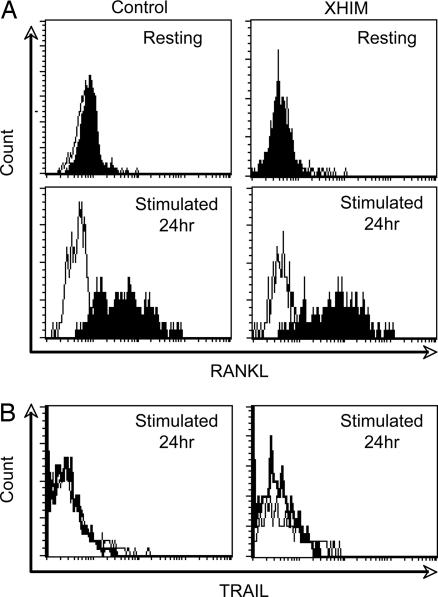
To address whether the RANKL/RANK signaling pathway might be involved in the low mineral density that is observed in XHIM, we first determined the expression of RANKL on the surface of activated CD4+ T cells. We stimulated the T cells in PBMC with anti-CD3 in vitro, and we determined the expression of RANKL by flow cytometry using OPG-Fc fusion protein. Activated CD4+ T cells from XHIM patients up-regulate expression of RANKL at levels comparable with those seen in normal controls (Fig. 2A). In certain experimental conditions, OPG can also bind to TNF-related apoptosis-inducing ligand (TRAIL) and thus lead to a false-positive assessment of RANKL expression (28). However, only minimal TRAIL expression was detected at 24 h after stimulation with a TRAIL-specific antibody in both patient and control T cells (Fig. 2B), indicating that the surface protein recognized by OPG-Fc was indeed RANKL. These findings demonstrate that under physiologic stimulation conditions, activated T cells from XHIM patients express RANKL, and the level of RANKL expression is comparable with that seen in normal controls.
Fig. 2.
RANKL and TRAIL expression by T cells. (A) (Upper) Resting CD4+ T cells do not express RANKL. (Lower) CD4+ T cells from the XHIM patient up-regulate RANKL expression with an intensity similar to that of a normal control 24 h after anti-CD3ϵ stimulation. The result of one experiment representative of three is shown. (B) TRAIL expression was minimally detected at 24 h after stimulation (thin line). The staining by an isotype-matched control antibody (thick line) is overlaid for each condition.
Humans and mice with CD40L deficiency have a diminished capacity to prime T cells and induce the memory T cell phenotype (17, 18). Antigen priming of T cells is associated with a change in the T cell CD45 isoform: human naïve T cells express the CD45RA isoform, whereas antigen-primed human memory T cells express the CD45RO isoform (29). We evaluated the expressions of CD45 isoforms by the CD4+ T cells of 13 patients with XHIM and compared them with age-matched controls by flow cytometry. In keeping with previously published results, the average value of CD4+ T cells expressing CD45RO in all patients was significantly reduced (P < 0.0001) compared with normal volunteers [supporting information (SI) Fig. 5a]. One conse-quence of reduced T cell priming is decreased T cell cytokine production. To verify this possibility in the present context, we cultured peripheral blood mononuclear cells (PBMCs) with anti-CD3 for 36 h and then measured IFN-γ secretion into the culture fluid by specific ELISA. In agreement with previous published results, patients with XHIM produce markedly reduced levels of IFN-γ compared with controls (SI Fig. 5). These findings demonstrate that XHIM patients have a diminished capacity to prime T cells and thus manifest impaired production of IFN-γ.
CD4+ CD40L−/− T Cells Strongly Promote Osteoclastogenesis in Vitro.
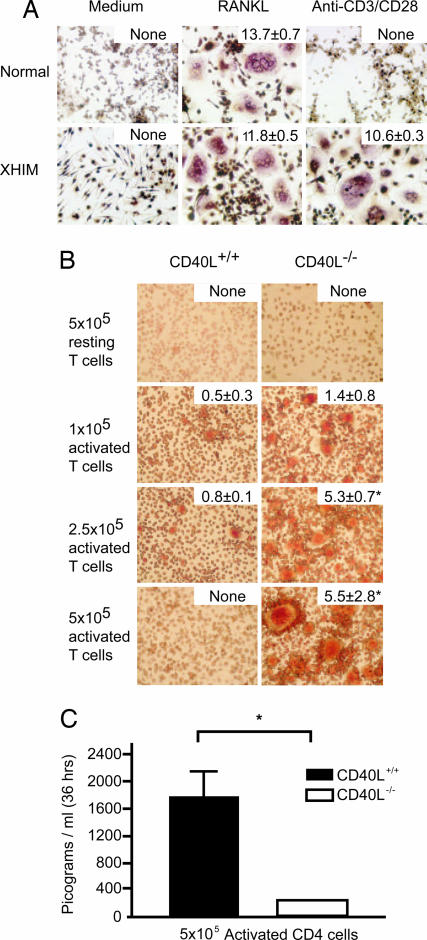
We next determined the capacity of activated T cells from XHIM patients to mediate osteoclast formation in vitro. Peripheral blood monocytes from XHIM patients or normal controls were cultured with recombinant macrophage colony-stimulating factor (M-CSF) and recombinant RANKL. Then, after 7 days, nonadherent cells were removed, and osteoclasts, identified as giant multinucleated tartrate-resistant acid phosphatase (TRAP)-positive cells, were counted. Recombinant RANKL induced osteoclast formation in M-CSF-treated monocytes from both normal control and XHIM patients (Fig. 3A), which indicates that XHIM monocytes are normal with respect to RANKL-induced osteoclastogenesis. However, when M-CSF-treated monocytes were stimulated with activated CD4+ T cells from XHIM patients or normal controls, the monocytes from XHIM patients exhibited robust differentiation into osteoclasts, whereas treated monocytes from normal controls exhibited reduced differentiation into osteoclasts. These data indicate that CD4+ T cells from XHIM patients differ from controls in their capacity to induce osteoclastogenesis.
Fig. 3.
CD4+ T cell-induced osteoclastogenesis. (A) T cells from XHIM patients induce robust osteoclastogenesis. Purified monocytes (5 × 105) were cultured alone, with recombinant RANKL (100 ng/ml), or with positively selected autologous CD4+ T cells (1 × 105) activated by plate-bound anti-CD3 and soluble anti-CD28. The total number of osteoclasts (as defined by TRAP-staining giant cells with more than three nuclei) was counted in each well after 7 days of culture. These values are expressed as the number of cells per mm2 ± SEM, and they are represented in the top right of each image. One representative example of two independent experiments is shown. (B) CD4+ CD40L−/− mouse T cells induce marked osteoclast differentiation of BMMs. Nonadherent mouse BMM cells were cocultured with CD4+ CD40+/+ (Left) or CD4+ CD40L−/− (Right) T cells. T cells were activated with anti-CD3ϵ antibody (10 μg/ml), and the number of cells added to the cocultures is specified on the left side of the figure. The total number of osteoclasts in each well was counted after 72 h of culture, and it is expressed as the number of cells per mm2. These values are expressed in top right of each image. One representative example of three independent experiments is shown. ∗, P = statistically significant. (C) IFN-γ levels in mouse coculture supernatants were analyzed 36 h after the addition and activation of T cells (the columns represent the median ± SEM for three independent assays measured by specific ELISA). ∗, P = statistically significant.
Further insight into the role of CD40L in the induction of osteoclastogenesis was obtained by using a murine coculture system of osteoclastogenesis. In this system, bone marrow monocyte/macrophage precursors (BMMs) from wild-type mice were cultured with recombinant M-CSF for 48 h and then cocultured with syngenic purified CD4+ CD40L+/+ or CD4+ CD40L−/− T cells activated with anti-CD3ε-specific antibody. Then, after 72 h, nonadherent cells were removed, and osteoclasts, identified as TRAP-positive cells, were counted. In contrast to CD4+ CD40L+/+ cells, CD4+ CD40L−/− cells strongly induce the appearance of giant, highly complex multinucleated TRAP+ cells (Fig. 3B). Furthermore, the number of osteoclasts depends directly on the number of activated T cells added to the coculture. We also measured the IFN-γ concentration in culture fluids at 36 h after the addition and stimulation of T cells by specific ELISA. In accordance with our finding with human T cells, the concentration of IFN-γ was significantly lower in the CD40L−/− cocultures than in the CD40L wild-type cultures (Fig. 3C). These findings suggested to us that the reduced production of IFN-γ by CD40L−/− T cells may account for their increased capacity to induce osteoclast formation.
RANK-Fc or IFN-γ Inhibit in Vitro Osteoclastogenesis Mediated by CD40L−/− T Cells.
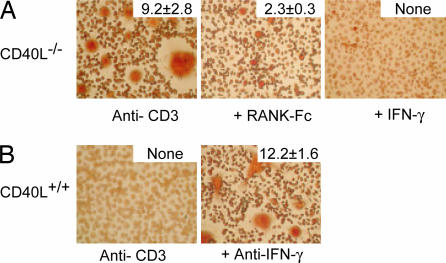
To assess the relative contribution of RANKL expressed by activated CD40L−/− T cells on in vitro osteoclastogenesis, we added RANK-Fc, a molecule that binds to RANKL thus prevents signaling via RANK. The number and complexity of osteoclasts were greatly decreased in the presence of RANK-Fc, but they were not completely absent (Fig. 4A). Several factors could account for this finding, including the possibility that recombinant RANK-Fc may not completely block RANK–RANKL interaction in vitro or the presence of osteoclastogenesis-promoting cytokines in the culture supernatant such as IL-1 or TNF-α. To provide further support to our hypothesis that the impairment of IFN-γ secretion by activated CD40L−/− T cells is responsible for enhanced osteoclastogenesis in vitro, we added recombinant mouse IFN-γ to the CD40L−/− cocultures and a neutralizing anti-IFN-γ antibody to the wild-type cocultures. In cocultures containing CD40L−/− T cells and recombinant IFN-γ, osteoclastogenesis was completely absent (Fig. 4A), whereas in cocultures containing wild-type T cells and anti-IFN-γ, osteoclastogenesis was strongly enhanced (Fig. 4B). Taken together, these IFN-γ and RANKL blockade data indicate that IFN-γ regulates RANKL-induced osteoclastogenesis and that CD40L−/− T cells promote osteoclast differentiation because of defective T cell priming and IFN-γ production.
Fig. 4.
RANKL and IFN-γ mediate in vitro osteoclastogenesis. (A) Nonadherent BMM cells were cocultured with activated CD4+ CD40−/− T cells in the presence a RANK-Fc (10 μg/ml) fusion protein or recombinant IFN-γ (100 units/ml). The total number of osteoclasts per mm2 is on the top right of each image. One representative of three independent experiments is shown. (B) Nonadherent BMM cells were cocultured with activated CD4+ CD40+/+ T cells. Blocking anti-IFN-γ antibody (2 μg/ml) was added to the activated CD4+ CD40+/+ T cell coculture. The total number of osteoclasts/mm2 is on the top right of each image. One representative of three independent experiments is shown.
Discussion
In this work, we show that osteopenia is a common clinical feature of XHIM in the absence of definable metabolic and endocrine abnormalities. We demonstrate that activated CD40L−/− CD4+ T cells are poor producers of INF-γ because of defective T cell–APC interaction, but they express RANKL normally. As a consequence, activated CD40L−/− T cells from both humans and mice induce marked osteoclast differentiation of myeloid cells through RANKL signaling in vitro, and this differentiation can be reversed by the addition of INF-γ. Taken together, these findings offer support for the hypothesis that in the face of continual exposure to environmental antigens, activated T cells expressing RANKL but deficient in INF-γ contribute to the profound generalized osteopenia found in patients with XHIM.
Preservation of bone mass is achieved by the concerted action of osteoblasts that synthesize bone matrix and osteoclasts that lead to its resorption. The model of bone loss that we are proposing predicts that reduced BMD in XHIM is the result of increased osteoclastic activity. NTX is a validated measure of bone resorption, and it serves as relative index of current osteoclastic activity (26). Five of eight XHIM patients had urinary NTX values 1 SD or higher than the mean value for age-matched controls, thus confirming an increase in bone degradation in XHIM. It is interesting to note that the elevated NTX levels were primarily observed in younger patients with XHIM. We found no difference in the expression of RANKL on the surface of activated T cells between children (age <8) or young adults (age >16) with XHIM (E.L.-G. and A.J., unpublished observation), which suggests that the enhanced osteoclastic activity observed in the younger XHIM patients is not a result of increased RANKL expression by activated T cells. During childhood, bones undergo rapid transformation because of processes leading to both bone formation and resorption. Osteoblasts and bone marrow stromal cells express RANKL and coordinate bone remodeling by stimulating local osteoclasts, which in turn stimulate bone synthesis by nearby osteoblasts. Reflective of age-related bone remodeling, urinary NTX peaks in adolescence and declines with age (27). We propose that in healthy individuals, INF-γ secretion by T cells that are under continual stimulation from environmental antigens may limit the extent of osteoclast activity during childhood. The absence of this regulatory mechanism may make younger XHIM patients susceptible to marked bone loss. Osteoporosis, long-bone fractures, and defects in TH1 T cell differentiation have been observed in patients with Job's syndrome (30, 31). Although the precise mechanism for reduced BMD in these patients awaits further investigation, it may relate to the selective deficiency of IFN-γ secretion by activated T cells.
Prospective studies in the elderly have shown that a 1 SD reduction in BMD can be associated with an increase of 50–100% in the incidence of fractures (32). In children and young adults, the diagnosis of osteoporosis cannot be made on the basis of bone density alone because body size and normal variation of bone density must be taken into consideration. However, the fact that four of our XHIM patients had bone density values >2.5 SD below the average for their age and two patients experienced spontaneous rib fractures without a history of trauma suggests that their low bone density was clinically important.
The decision on whether and how to treat bone loss in XHIM is difficult. Although IFN-γ can have an inhibitory effect on osteoclastogenesis in vitro, IFN-γ therapy is expensive, and it is not free of side effects. However, IFN-γ treatment should not be dismissed out of hand because it has the potential to improve other manifestations of the disease such the frequency of opportunistic and bacterial infections. Blocking RANKL interactions with an anti-RANK antibody has entered into early clinical development. In the absence of CD40L stimulation, RANKL interaction with RANK on the surface of dendritic cells has been shown to mediate immunity to certain viral infections in the CD40L−/− mice. Thus, the use of RANKL antagonist in XHIM patients may lead to an increased predisposition to infection. Oral bisphosphonates target osteoclast-mediated bone loss, and they have been shown to enhance bone mineralization in adults (33, 34). Although the majority of XHIM patients are children, and well controlled studies using bisphosphonates in younger patients are lacking, this class of drugs could be of value for some XHIM patients with marked bone loss.
In summary, our results identify a mechanism for CD40L in regulating bone mass that is absent in patients with XHIM. Several CD40 agonists are currently in preclinical development. Whether such biological agents can reverse bone loss in XHIM patients through immunological reconstitution awaits further investigation.
Materials and Methods
Study Subjects.
Fourteen patients with XHIM were studied at the Clinical Center, National Institute of Allergy and Infectious Diseases, National Institutes of Health (NIAID/NIH; protocol 89-I-006). Unaffected family members, other normal volunteers, and immunologically normal individuals enrolled on other NIH protocols served as controls. The Institutional Review Board of the NIAID approved the open protocol, and informed consent was obtained from all of the patients or parents before the enrollment in the study.
Endocrine and Bone Metabolism Evaluation.
Serum levels of calcium, phosphorus, magnesium, albumin, alkaline phosphatase, intact parathyroid hormone, osteocalcitonin, 25-dihydroxyvitamin D3, and 1,25-vitamin D were measured in all study patients. When possible, 24-h urine samples were collected, and the levels of calcium, creatinine clearance, and NTX (Ostex, Seattle, WA) were determined according to the manufacturer's instructions. BMD was measured by DXA with a Delphi-A densitometer (Hologic, Inc., Bedford, MA) at different skeletal locations using Hologic, Inc. software.
Flow Cytometry.
For RANKL expression analysis, PBMCs from healthy volunteers or XHIM patients were stimulated with OKT3 antibody (gift of Ortho Biotech, Bridgewater, NJ) at a concentration of 5 μg/ml. Cellular expression markers were determined with the following antibodies: anti-CD3, anti-CD8, anti-TRAIL, anti-CD40L, and anti-CD69 (BD Biosciences Pharmingen, San Diego, CA). RANKL expression was determined by incubation with human OPG-IgG Fc fusion protein (R&D Systems, Inc., Minneapolis, MN).
Cell Preparation and Cell Culture Conditions.
For in vitro osteoclastogenesis, BMMs were harvested from the femurs of C57BL/6J wild-type mice and cultured in α-MEM 10% FCS medium with 10 ng/ml recombinant M-CSF (R&D Systems, Inc.) for 48 h at 37°C. CD4+ T cells from CD40L−/− or wild-type animals were isolated from splenocyte suspensions by high-affinity negative-selection columns (R&D Systems, Inc.) according to the manufacturer's instructions, and they were added to the BMMs and stimulated with 10 mg/ml anti-CD3ε-specific antibody or an unspecific isotype control (BD Biosciences Pharmingen). After 3 days in culture, the medium and the nonadherent cells were removed. The remaining cells were washed, fixed with 10% (vol/vol) glutaraldehyde solution, and then stained by the TRAP method (Sigma, St. Louis, MO) according to the manufacturer's instructions. The nuclei were counterstained with the hematoxylin/eosin method (Sigma). To evaluate osteoclast differentiation, the number of giant, multinucleated (more than three nuclei) TRAP+ cells was counted in each well over a grid covering the entire surface. The results were expressed as the total number of cells per mm2 of well surface. The concentration of INF-γ in coculture supernatants was determined at 36 h after stimulation by using a commercially obtained ELISA kit (BD Biosciences Pharmingen) according to the manufacturer's indications. Mouse recombinant IFN-γ (100 units/ml) (R&D Systems, Inc.), mouse RANK-human Fc fusion protein (5 μg/ml) (Sigma), or anti-IFN-γ Ab (2 μg/ml) (a gift from Carl June, Bethesda Naval Research Institute, Bethesda, MD) were added to some culture conditions with T cells. The number of osteoclasts in these conditions was evaluated at 3 days.
Statistical Analysis.
The bone density measured at the L1–L4 vertebral position in the posterior–anterior projection was expressed in each patient as the number of standard deviations (Z score) away from the mean value in a population of age- and sex-matched healthy controls (software data base from Hologic, Inc.). If the values in these XHIM patients would follow a normal distribution, the group of Z scores would have a median 0 and a SD of 1. We performed a Wilcoxon signed-rank test to determine whether the median Z score of the patients with XHIM was different from 0. For NTXs, a similar strategy was used; however, age-specific means and SD values were constructed by using regression methods based on previously published information for pediatric normal controls using the same ELISA-based commercial assay (Ostex) (27). A D score for NTX was constructed according with the formula D = (NTX − M)/S, where NTX was the value for an individual, M was the age-adjusted mean, and S was the age-adjusted standard deviation. Analytical quantitative results were compared by a t test with two-tailed P values. Histomorphometric analyses were performed on decalcified iliac bone samples from two patients with XHIM. These samples had been obtained for medically indicated reasons. Measurements were performed in trabecular bone as described in detail in ref. 35. Trabecular bone volume/tissue volume corresponds to the percentage of the trabecular compartment that is taken up by bone. Osteoblasts were defined as cells directly apposed to osteoid and exhibiting a definite Golgi apparatus. Measurements were carried out at a magnification of 200 by using a digitizing table with Osteomeasure software (Osteometrics, Inc., Atlanta, GA). Nomenclature and abbreviations follow the recommendations of the American Society for Bone and Mineral Research (36).
Supplementary Material
Acknowledgments
We thank the patients studied for the invaluable contribution to this work; Ronald Hornung, Thomas Fleisher, and Margaret Brown for immunological evaluations; William Dougall, Bill Fanslow, and Diane Wara for helpful discussions; and Harry Malech and Warren Strober for careful review of the manuscript. This work was supported by the Intramural Research Program of the National Institute of Allergy and Infectious Diseases, National Institutes of Health. E.L.-G. was supported by a Primary Immunodeficiency Association Center of Excellence Award.
Abbreviations
- APC
antigen-presenting cell
- BMD
bone mineral density
- BMM
bone marrow monocyte/macrophage precursor
- CD40L
CD40 ligand
- DXA
dual-energy x-ray absorptiometry
- M-CSF
macrophage colony-stimulating factor
- NTX
N-terminal telopeptide of type I collagen
- OPG
osteoprotegerin
- PBMC
peripheral blood mononuclear cell
- RANKL
receptor activator of NF-κB ligand
- TRAF6
tumor necrosis factor receptor-associated factor 6
- TRAP
tartrate-resistant acid phosphatase
- XHIM
X-linked hyper-IgM syndrome.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS direct submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0605715104/DC1.
References
- 1.Boyle WJ, Simonet WS, Lacey DL. Nature. 2003;423:337–342. doi: 10.1038/nature01658. [DOI] [PubMed] [Google Scholar]
- 2.Anderson DM, Maraskovsky E, Billingsley WL, Dougall WC, Tometsko ME, Roux ER, Teepe MC, DuBose RF, Cosman D, Galibert L. Nature. 1997;390:175–179. doi: 10.1038/36593. [DOI] [PubMed] [Google Scholar]
- 3.Wong BR, Josien R, Lee SY, Sauter B, Li HL, Steinman RM, Choi Y. J Exp Med. 1997;186:2075–2080. doi: 10.1084/jem.186.12.2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yasuda H, Shima N, Nakagawa N, Yamaguchi K, Kinosaki M, Mochizuki S, Tomoyasu A, Yano K, Goto M, Murakami A, et al. Proc Natl Acad Sci USA. 1998;95:3597–3602. doi: 10.1073/pnas.95.7.3597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lacey DL, Timms E, Tan HL, Kelley MJ, Dunstan CR, Burgess T, Elliott R, Colombero A, Elliott G, Scully S, et al. Cell. 1998;93:165–176. doi: 10.1016/s0092-8674(00)81569-x. [DOI] [PubMed] [Google Scholar]
- 6.Burgess TL, Qian Y, Kaufman S, Ring BD, Van G, Capparelli C, Kelley M, Hsu H, Boyle WJ, Dunstan CR, et al. J Cell Biol. 1999;145:527–538. doi: 10.1083/jcb.145.3.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kong YY, Yoshida H, Sarosi I, Tan HL, Timms E, Capparelli C, Morony S, Oliveira-dos-Santos AJ, Van G, Itie A, et al. Nature. 1999;397:315–323. doi: 10.1038/16852. [DOI] [PubMed] [Google Scholar]
- 8.Rodan GA, Martin TJ. Science. 2000;289:1508–1514. doi: 10.1126/science.289.5484.1508. [DOI] [PubMed] [Google Scholar]
- 9.Simonet WS, Lacey DL, Dunstan CR, Kelley M, Chang MS, Luthy R, Nguyen HQ, Wooden S, Bennett L, Boone T, et al. Cell. 1997;89:309–319. doi: 10.1016/s0092-8674(00)80209-3. [DOI] [PubMed] [Google Scholar]
- 10.Takayanagi H, Ogasawara K, Hida S, Chiba T, Murata S, Sato K, Takaoka A, Yokochi T, Oda H, Tanaka K, et al. Nature. 2000;408:600–605. doi: 10.1038/35046102. [DOI] [PubMed] [Google Scholar]
- 11.Zhang YH, Heulsmann A, Tondravi MM, Mukherjee A, Abu-Amer Y. J Biol Chem. 2001;276:563–568. doi: 10.1074/jbc.M008198200. [DOI] [PubMed] [Google Scholar]
- 12.Ma T, Miyanishi K, Suen A, Epstein NJ, Tomita T, Smith RL, Goodman SB. Cytokine. 2004;26:138–144. doi: 10.1016/j.cyto.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 13.Xu J, Foy TM, Laman JD, Elliott EA, Dunn JJ, Waldschmidt TJ, Elsemore J, Noelle RJ, Flavell RA. Immunity. 1994;1:423–431. doi: 10.1016/1074-7613(94)90073-6. [DOI] [PubMed] [Google Scholar]
- 14.Han S, Hathcock K, Zheng B, Kepler TB, Hodes R, Kelsoe G. J Immunol. 1995;155:556–567. [PubMed] [Google Scholar]
- 15.Renshaw BR, Fanslow WC, III, Armitage RJ, Campbell KA, Liggitt D, Wright B, Davison BL, Maliszewski CR. J Exp Med. 1994;180:1889–1900. doi: 10.1084/jem.180.5.1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Notarangelo LD, Hayward AR. Clin Exp Immunol. 2000;120:399–405. doi: 10.1046/j.1365-2249.2000.01142.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grewal IS, Xu J, Flavell RA. Nature. 1995;378:617–620. doi: 10.1038/378617a0. [DOI] [PubMed] [Google Scholar]
- 18.Jain A, Atkinson TP, Lipsky PE, Slater JE, Nelson DL, Strober W. J Clin Invest. 1999;103:1151–1158. doi: 10.1172/JCI5891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Levy J, Espanol-Boren T, Thomas C, Fischer A, Tovo P, Bordigoni P, Resnick I, Fasth A, Baer M, Gomez L, et al. J Pediatr. 1997;131:47–54. doi: 10.1016/s0022-3476(97)70123-9. [DOI] [PubMed] [Google Scholar]
- 20.Soong L, Xu JC, Grewal IS, Kima P, Sun J, Longley BJ, Jr, Ruddle NH, McMahon-Pratt D, Flavell RA. Immunity. 1996;4:263–273. doi: 10.1016/s1074-7613(00)80434-3. [DOI] [PubMed] [Google Scholar]
- 21.Campbell KA, Ovendale PJ, Kennedy MK, Fanslow WC, Reed SG, Maliszewski CR. Immunity. 1996;4:283–289. doi: 10.1016/s1074-7613(00)80436-7. [DOI] [PubMed] [Google Scholar]
- 22.Hayward AR, Levy J, Facchetti F, Notarangelo L, Ochs HD, Etzioni A, Bonnefoy JY, Cosyns M, Weinberg A. J Immunol. 1997;158:977–983. [PubMed] [Google Scholar]
- 23.Apone S, Lee MY, Eyre DR. Bone. 1997;21:129–136. doi: 10.1016/s8756-3282(97)00105-1. [DOI] [PubMed] [Google Scholar]
- 24.Hanson DA, Weis MA, Bollen AM, Maslan SL, Singer FR, Eyre DR. J Bone Miner Res. 1992;7:1251–1258. doi: 10.1002/jbmr.5650071119. [DOI] [PubMed] [Google Scholar]
- 25.Bollen AM, Eyre DR. Bone. 1994;15:31–34. doi: 10.1016/8756-3282(94)90888-5. [DOI] [PubMed] [Google Scholar]
- 26.Genant HK, Engelke K, Fuerst T, Gluer CC, Grampp S, Harris ST, Jergas M, Lang T, Lu Y, Majumdar S, et al. J Bone Miner Res. 1996;11:707–730. doi: 10.1002/jbmr.5650110602. [DOI] [PubMed] [Google Scholar]
- 27.Mora S, Prinster C, Proverbio MC, Bellini A, de Poli SC, Weber G, Abbiati G, Chiumello G. Calcif Tissue Int. 1998;63:369–374. doi: 10.1007/s002239900542. [DOI] [PubMed] [Google Scholar]
- 28.Emery JG, McDonnell P, Burke MB, Deen KC, Lyn S, Silverman C, Dul E, Appelbaum ER, Eichman C, DiPrinzio R, et al. J Biol Chem. 1998;273:14363–14367. doi: 10.1074/jbc.273.23.14363. [DOI] [PubMed] [Google Scholar]
- 29.Fujii Y, Okumura M, Inada K, Nakahara K, Matsuda H. Eur J Immunol. 1992;22:1843–1850. doi: 10.1002/eji.1830220725. [DOI] [PubMed] [Google Scholar]
- 30.Kirchner SG, Sivit CJ, Wright PF. Radiology. 1985;156:362. doi: 10.1148/radiology.156.2.4011897. [DOI] [PubMed] [Google Scholar]
- 31.Grimbacher B, Holland SM, Gallin JI, Greenberg F, Hill SC, Malech HL, Miller JA, O'Connell AC, Puck JM. N Engl J Med. 1999;340:692–702. doi: 10.1056/NEJM199903043400904. [DOI] [PubMed] [Google Scholar]
- 32.Johnston CC, Jr, Slemenda CW, Melton LJ., III N Engl J Med. 1991;324:1105–1109. doi: 10.1056/NEJM199104183241606. [DOI] [PubMed] [Google Scholar]
- 33.Saag KG, Emkey R, Schnitzer TJ, Brown JP, Hawkins F, Goemaere S, Thamsborg G, Liberman UA, Delmas PD, Malice MP, et al. N Engl J Med. 1998;339:292–299. doi: 10.1056/NEJM199807303390502. [DOI] [PubMed] [Google Scholar]
- 34.Hosking D, Chilvers CE, Christiansen C, Ravn P, Wasnich R, Ross P, McClung M, Balske A, Thompson D, Daley M, Yates AJ. N Engl J Med. 1998;338:485–492. doi: 10.1056/NEJM199802193380801. [DOI] [PubMed] [Google Scholar]
- 35.Glorieux FH, Travers R, Taylor A, Bowen JR, Rauch F, Norman M, Parfitt AM. Bone. 2000;26:103–109. doi: 10.1016/s8756-3282(99)00257-4. [DOI] [PubMed] [Google Scholar]
- 36.Parfitt AM, Drezner MK, Glorieux FH, Kanis JA, Malluche H, Meunier PJ, Ott SM, Recker RR. J Bone Miner Res. 1987;2:595–610. doi: 10.1002/jbmr.5650020617. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.