Abstract
IFN-γ induces its immunoregulatory activities by activating genes mainly through the Jak-STAT signaling pathway. Here we show that what was considered to be intrinsic IFN-γ activities depend largely on the basal level of NF-κB, which is maintained by constitutively expressed IL-1α. The IL-1 receptor antagonist and antibodies to IL-1α, but not to IL-1β, inhibited the antiviral activity of IFN-γ by 90%, whereas no inhibition of type I IFN activity was observed. Similarly, the induction of many genes by IFN-γ, including HLA-DR, ICAM-1, IL-18BP, and genes mediating its antiviral activity, greatly depended on basal IL-1α. Furthermore, IFN-γ induced serum IL-18 binding protein in wild-type mice but not in IL-1α/β double-deficient mice. Thus, constitutively expressed IL-1α is critical for numerous IFN-γ activities.
Keywords: IFN regulatory factor-1, NF-κB, transcription regulation
Initially identified by its antiviral activity in nonimmune cells (1), IFN-γ was found later to regulate a remarkable range of immune responses and is a master regulator of Th1 response (2–4). Most cell types express the IFN-γ receptor (5), and its binding activates STAT1, which translocates as a dimer to the nucleus, activating genes whose promoter contains the γ-activated sequence (GAS) (6). Some of these IFN-γ-induced genes are transcription regulators, such as IFN regulatory factor (IRF)-1, which induces other genes, including those mediating the antiviral action of IFN-γ (7).
TNF and IL-1 enhance the transcriptional activity of IFN-γ. Additive or even synergistic induction of several proinflammatory genes has been reported, including ICAM-1, iNOS, various chemokine genes, and hepatocyte growth factor (8–15). The additive and synergistic induction of genes by IFN-γ and IL-1/TNF is largely attributable to independent activation of the GAS and κB response elements, respectively. These two response elements are juxtaposed in many promoters of the above-mentioned genes (16).
The importance of basal NF-κB in IFN-γ action has been studied to a limited extent. Induction of the chemokine genes CXCL9 and CXCL10 by IFN-γ depends on constitutive activation of NF-κB, yet the trigger of this basal activation was not identified (17). Because most cell types express constitutively low levels of IL-1α (18), we studied here its role in induction of genes by IFN-γ.
Results
IL-1α Inhibitors Diminish the Antiviral Activity of IFN-γ.
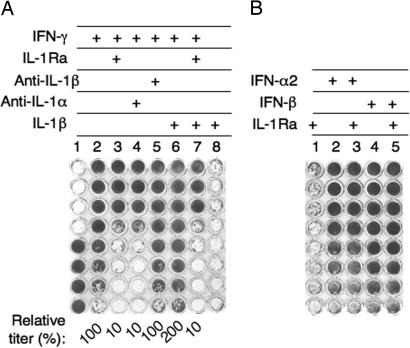
We first studied the role of endogenous IL-1α in the antiviral activity of various IFNs. Human WISH epithelial cells were incubated with various IFNs in the presence or absence of IL-1 inhibitors. The cultures were then challenged with vesicular stomatitis virus, and the ability of IFN-γ to protect from cell death was scored. When IFN-γ (serially 2-fold diluted) was added to the cells, there was 50% protection in well 7, corresponding to a titer of 4,000 units/ml (Fig. 1A, column 2). Addition of either IL-1 receptor antagonist (IL-1Ra) or anti-IL-1α antibodies together with the IFN-γ reduced its antiviral titer by 90% to 400 units/ml (Fig. 1A, columns 3 and 4, respectively). In contrast, anti-IL-1β antibodies had no effect on the antiviral titer of IFN-γ (column 5). Exogenous IL-1α (data not shown) or IL-1β increased the antiviral potency of IFN-γ by almost 2-fold, and IL-1Ra blocked the antiviral activity augmented by both exogenous and endogenous IL-1α (Fig. 1A, columns 6 and 7). IL-1α (data not shown) and IL-1β (column 8) had no antiviral activity in the absence of IFN-γ. In contrast, IL-1 inhibitors had no effect on the antiviral activity of type I IFNs. (Fig. 1B). Nevertheless, 2 ng/ml IL-1β increased the antiviral activity of both IFN-α2 and IFN-β by ≈2-fold, confirming a previous study (data not shown) (19). These results indicate that endogenous IL-1α is critical for the antiviral activity of IFN-γ but not for type I IFNs.
Fig. 1.
The effect of IL-1 inhibitors on the antiviral activity of IFNs. The antiviral titer of various type I and II human IFNs was determined by a viral (vesicular stomatitis virus) cytopathic effect inhibition assay. (A) Column 1 shows control WISH cells (lower four wells) and the complete cytopathic effect of the same cells after vesicular stomatitis virus challenge (upper four wells). WISH epithelial cells were incubated with a serial 2-fold dilution of 4,000 units/ml IFN-γ, either alone or in the presence of exogenous 2 ng/ml IL-1β or IL-1 inhibitors (10 μg/ml IL-1Ra, 2 μg/ml anti-IL-1α antibodies, or 2 μg/ml anti- IL-1β antibodies). The titer relative to IFN-γ alone (column 2) is given as a percentage below the wells. (B) IFN-α2 (4,000 units/ml) or IFN-β (8,000 units/ml) was added to cells either alone or together with IL-1Ra, and the antiviral titer was determined as in A.
To further confirm the critical role of endogenous IL-1 in the antiviral activity of IFN-γ, this activity was assessed in wild-type murine embryonic fibroblasts (MEFs) and in IL-1α/β double-knockout MEFs. Similarly to WISH cells, IFN-γ was only 10% active in MEFs from one double-knockout mouse compared with wild-type C/57BL MEFs and 33% active in MEFs from another double-knockout mouse.
Analysis of IFN-γ-Induced Genes by Expression Arrays.
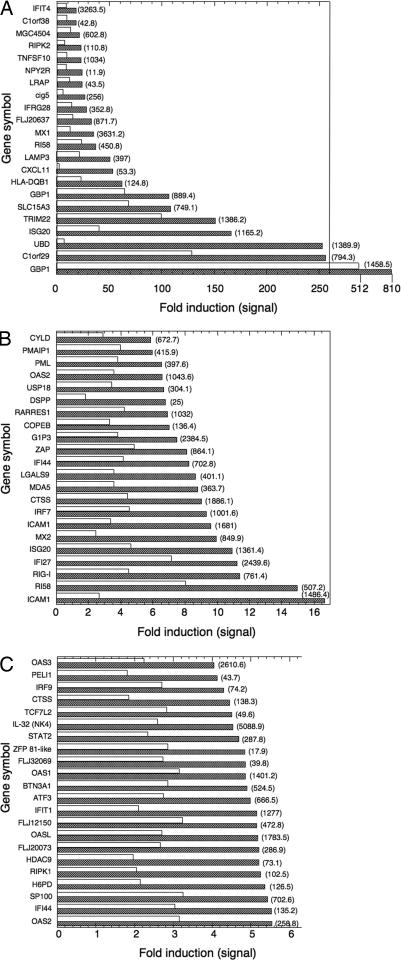
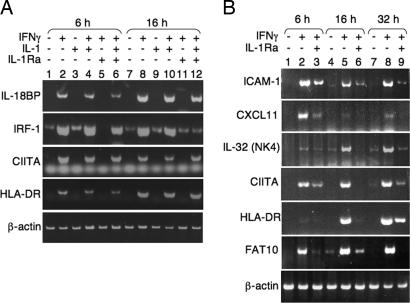
Because the antiviral state is induced at the transcriptional level, we performed expression-array analysis to gain insight on the role of IL-1α in the transcriptional activity induced by IFN-γ. WISH cells were treated with IFN-γ, IL-1Ra, or their combination for different times, and total RNA was subjected to expression array analysis. The effect of IL-1Ra on gene expression by IFN-γ was minimal at 3 h, increased at 6 h, and was maximal at 17 h. Of 370 genes induced at least 4-fold at 17 h, we found a group of 65 genes whose induction was inhibited by at least one-third in the presence of IL-1Ra. Among these genes were some of the major IFN-γ-induced transcripts associated with the antiviral response, including Mx1, Mx2, 2′-5′ oligoA synthetase, ISG20, and GBP1 (Fig. 2) (20–23). Additional known IFN-γ-induced genes unrelated to the antiviral activity of IFN-γ were found to be IL-1-dependent as well, including diubiquitin (UBD/FAT10), ICAM1, and MHCII (Fig. 2) (3, 24, 25). However, other known IFN-γ-induced genes were not modulated by IL-1Ra, including complement components C4A and C4B, IL-15 receptor α chain, IFI 16b, IFI 41, and tryptophanyl synthetase (data not shown). Semiquantitative RT-PCR of RNA from IFN-γ-treated WISH cells (Fig. 3A) as well as HaCaT keratinocytes (Fig. 3B) confirmed the expression array data for many of these transcripts, including IRF-1, CIITA, HLA-DR, ICAM1, CXCL11, IL-32 (NK4) (26), and FAT10 (UBD). Addition of exogenous IL-1β reversed the inhibition of IL-1Ra (Fig. 3A, lanes 6 and 12). Taken together, these results establish the existence of a group of genes whose induction by IFN-γ largely depends on endogenously expressed IL-1α.
Fig. 2.
Expression array analysis of IFN-γ-treated human WISH cells. Human WISH cells were treated with either medium, 100 units/ml IFN-γ, 10 μg/ml IL-1Ra, or their combination. Total RNA was isolated at 17 h and subjected to expression-array analysis (Affymetrix U133A chip). Using Excel software, the Affymetrix data files were sorted by the algorithm described in Materials and Methods. Shown is the fold induction of genes in cells treated with IFN-γ for 17 h in the absence (hatched bars) or presence (open bars) of IL-1Ra. The values in parentheses are the average signal of two replicate assays. All signals and fold inductions were considered significant by the Affymetrix software (P < 0.05). Gene induction and array analyses were performed twice with very similar results.
Fig. 3.
RT-PCR of select genes after induction with IFN-γ. Human WISH cells (A) or HaCaT keratinocytes (B) were treated with 100 units/ml IFN-γ, 2 ng/ml IL-1β, 10 μg/ml IL-1Ra, or their combination. RNA was isolated at the indicated times and subjected to semiquantitative RT-PCR with specific primers. The number of cycles was adjusted to show maximal differences if any. RT-PCR of β-actin was used to control for sample loading.
IL-1Ra Inhibits the Induction of IL-18 Binding Protein (IL-18BP) by IFN-γ.
Having established a role for endogenous IL-1α in modulating the expression of IFN-γ-induced transcripts, we examined its effect at the protein level. IL-18BP is an IFN-γ-induced gene whose expression is significantly elevated in various inflammatory diseases (27). Its basal expression is very low in nonhematopoietic cells and so far, only IFN-γ was identified as an IL-18BP inducer (28, 29). Unexpectedly, the expression array analysis did not detect any induction of IL-18BP mRNA by IFN-γ. However, RT-PCR confirmed a robust induction of IL-18BP mRNA by IFN-γ in WISH cells and in HaCaT keratinocytes. Using RT-PCR, we found that IL-18BP induction in WISH cells was significantly dependent on endogenous IL-1 (Fig. 3). At the protein level, control cultures of HaCaT keratinocytes contained 0.39 ± 0.03 ng/ml IL-18BP as determined by ELISA. Upon induction with IFN-γ, the level of IL-18BP rose to 2.77 ± 0.024 ng/ml (P = 0.0003, n = 9), whereas no induction by IFN-γ was obtained in the presence of IL-1Ra (0.35 ± 0.003 ng/ml).
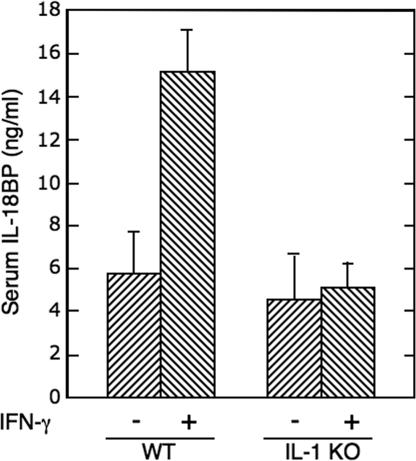
We then determined the role of IL-1α in the induction of IL-18BP in vivo by comparing serum IL-18BP in IL-1α/β double-deficient mice and wild-type C57BL/6 mice. Although both groups of mice had a similar basal level of circulating IL-18BP, significant induction of IL-18BP was obtained after IFN-γ administration only in the wild-type mice (P = 0.0004, n = 8) (Fig. 4). Taken together, these results indicate that endogenous IL-1α is essential for the induction of IL-18BP by IFN-γ, as determined at the mRNA and protein levels in vitro and in vivo.
Fig. 4.
Serum IL-18BP in wild-type and IL-1α/β double-knockout mice after administration of IFN-γ. Wild-type and IL-1α/β double-deficient C57BL/6 mice (n = 8 per group) were injected i.p. with murine 50,000 units of IFN-γ per mouse. Serum IL-18BP was determined before IFN-γ administration and 24 h after administration.
The Role of NF-κB in the IFN-γ-Induced Gene Activation.
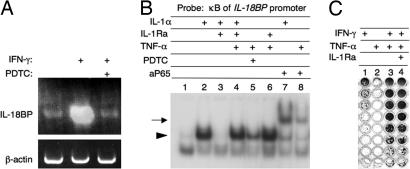
IFN-γ signals through the Jak-STAT pathway and does not activate NF-κB directly. We hypothesized that endogenous IL-1α was critical for IFN-γ action by providing a basal level of NF-κB activity. Indeed, ammonium pyrrolidinedithiocarbamate (PDTC), a specific inhibitor of NF-κB translocation to the nucleus, completely abrogated the induction of IL-18BP mRNA by IFN-γ (Fig. 5A). Furthermore, EMSA with a probe corresponding to the κB site of the IL-18BP promoter [bases −396 to −387 relative to the transcription start site (28)] revealed the formation of a complex with nuclear extracts of IL-1α-treated WISH cells, which was abolished by IL-1Ra (Fig. 5B, lanes 1–3). This complex contained NF-κB, as shown by supershifting with anti-p65 antibodies (Fig. 5B, lane 7).
Fig. 5.
The role of IL-1α-induced NF-κB in IFN-γ activities. (A) Human WISH cells were treated with ammonium PDTC at 300 μM for 30 min, followed by 100 units/ml IFN-γ for 17 h. Total RNA was isolated and subjected to semiquantitative RT-PCR with primers specific for human IL-18BP. (B) Human WISH cells were treated for 30 min with the indicated combinations of PDTC (300 μM), IL-1α (2 ng/ml), IL-1Ra (10 μg/ml), and TNF-α (10 ng/ml). Nuclear extracts were subjected to EMSA with a probe corresponding to the κB site of the IL-18BP promoter. Supershifting was performed with anti-NF-κB p65 antibodies (lanes 7 and 8). (C) Serial 2-fold-diluted IFN-γ (100 units/ml) mixed with either 10 ng/ml TNF-α with or without 10 μg/ml IL-1Ra was added to WISH cells. Protection from viral cytopathic effect was scored as in Fig. 1.
To further establish the role of NF-κB as a coactivator of IFN-γ signaling, we used exogenous TNF-α as an alternative inducer of NF-κB. As expected, nuclear extracts of WISH cells treated with 10 ng/ml TNF-α for 30 h also formed a complex with the κB probe, which was inhibited by PDTC, but not by IL-1Ra (Fig. 5B, lanes 4–6). This TNF-α-induced complex was identified as NF-κB by supershifting with anti-p65 antibodies (Fig. 5B, lane 8). We then tested whether exogenously added TNF-α could replace the endogenous IL-1α in establishing the antiviral state by IFN-γ. Indeed, TNF-α (10 ng/ml), which was devoid of intrinsic antiviral activity, enhanced the antiviral activity of IFN-γ by 32-fold (Fig. 5C), a result consistent with previous reports (30). The TNF-induced enhancement of the antiviral titer was not inhibited by IL-1Ra (Fig. 5C, column 4), demonstrating that TNF-α exerts its enhancing effect on IFN-γ independently of IL-1. Taken together, these data suggest that IL-1α-activated NF-κB has a critical role in the expression of a set of IFN-γ-induced genes, including those mediating its antiviral action.
Integral Membrane IL-1α Plays a Role in Gene Activation by IFN-γ.
IFN-γ was reported to induce membrane-associated IL-1α in various cells (31, 32). We found that the basal level of cell-associated IL-1α was 10.5 ± 1.6 and 2.9 ± 0.4 pg/μg protein in WISH cells and HaCaT keratinocytes, respectively. At 24 h, IFN-γ induced IL-1α in these cells to 30.8 ± 6.5 and 34.9 ± 6.3 pg/μg, respectively (P = 0.004 and 0.001, respectively; n = 9). In contrast, the level of IL-1α in culture supernatants of WISH cells and HaCaT keratinocytes, either before or after 24 h of treatment with IFN-γ, was below the limit of detection (≈2 pg/ml).
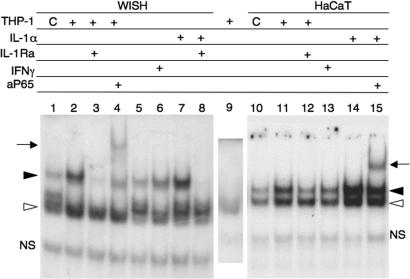
Because most of the basal and IFN-γ-induced IL-1α was cell-associated, we used coculturing experiments to determine whether it was active as an integral-membrane protein. IL-1α was induced in human macrophage-like THP-1 (nonadherent) cells by treatment with IFN-γ for 1–17 h. The cells were washed, fixed with 1% paraformaldehyde for 4 h, washed again, and incubated for 24 h at 37°C in growth medium. This procedure has been shown to induce membrane-associated IL-1α and to prevent leakage of biologically active pro-IL-1α from intracellular pools (33). The washed THP-1 cells were coincubated for 6 h with WISH cells in the presence or absence of IL-1Ra. After removal of the THP-1 cells, the extent of NF-κB activation in the WISH cells was evaluated by EMSA with a γ-32P-labeled κB probe. Basal NF-κB activation was observed in WISH cells that were cocultured with untreated THP-1 cells, and it was greatly induced when the WISH cells were coincubated with THP-1 cells that were pretreated with IFN-γ for 1–17 h (17 h shown, Fig. 6, compare lanes 1 and 2). Formation of NF-κB p65-containing complexes was reduced when the coculturing was performed in the presence of IL-1Ra (Fig. 6, compare lanes 1 and 3), thereby identifying integral-membrane IL-1α of the THP-1 cells as an important NF-κB inducer in WISH cells upon coculturing. The same results were seen upon coculturing of the THP-1 cells with WISH cells for 24 h instead of 6 h (data not shown). Practically identical results were observed upon coculturing the THP-1 cells with HaCaT keratinocytes instead of WISH cells (Fig. 6, lanes 10–12). Importantly, these data demonstrate that basal and IFN-γ-induced integral membrane IL-1α can act on neighboring cells to elicit biological activities in a juxtacrine manner.
Fig. 6.
IFN-γ induces active integral membrane IL-1α. Human THP1 macrophage-like cells were either not treated (C) or pretreated with 100 units/ml IFN-γ for 17 h (+) to induce membrane-associated IL-1α. The cells were then fixed as described (33) and cocultured for 6 h with either WISH cells (lanes 1–4) or HaCaT keratinocytes (lanes 10–12) in medium alone or together with 10 μg/ml IL-1Ra. Other WISH and HaCaT cultures (lanes 5–8 and 13–15, respectively) were treated with the indicated combinations of IL-1α, IL-1Ra, and IFN-γ. After coincubation, the THP-1 cells were removed and the WISH and HaCaT cells were washed, and their extracts were subjected to EMSA by using a κB probe. A control extract of fixed THP-1 cells was subjected to EMSA as well (lane 9). The bands marked with an arrowhead represent nuclear proteins binding specifically to the κB probe. The open arrowhead indicates the faster migrating complex that mainly contained NF-κB p50, and the closed arrowhead indicates the slower migrating complex that contained both NF-κB p50 and p65. Anti-NF-κB (p65) antibody was used for supershift (arrow, lanes 4 and 15).
IL-1 Accelerates and Elevates the Induction of IRF-1 by IFN-γ.
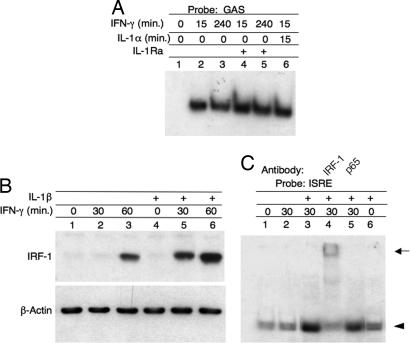
To gain insight on the mechanism by which endogenous IL-1α augmented the activity of IFN-γ, we tested whether IL-1α modulated the Jak-STAT signaling pathway. WISH cells were incubated for 15 min or 4 h with IFN-γ or IL-1α, alone or in combination, in the presence or absence of IL-1Ra. EMSA of nuclear extracts using a γ-32P-labeled GAS dsDNA probe revealed rapid IFN-γ-dependent induction of a complex between nuclear proteins and the GAS probe (Fig. 7A, lane 2). Formation of this complex lasted at least 4 h (lane 3) and was not affected by either IL-1Ra or IL-1α (lanes 4–6). In a control study, IL-1Ra abrogated the formation of IL-1α-induced complex of nuclear proteins with a κB probe (data not shown). Therefore, it can be concluded that the GAS and NF-κB pathways are independent and, hence, that the effect of endogenous IL-1α on the antiviral response of IFN-γ is not mediated through the Jak-STAT1 signaling pathway.
Fig. 7.
The effect of IL-1 on the induction and activation of IRF-1 by IFN-γ. (A) EMSA of GAS–STAT complexes. WISH cells were treated for the indicated times with the indicated combinations of IFN-γ (100 units/ml), IL-1Ra (10 μg/ml), and IL-1α (2 ng/ml). Nuclear extracts were prepared and reacted with a GAS probe. (B) Immunoblots of IRF-1. WISH cells were treated with 2 ng/ml IL-1β for 1 h, followed by 100 units/ml IFN-γ. Total cell extract was obtained at the indicated times after IFN-γ addition and subjected to SDS/PAGE and immunoblotting with IRF-1 and β-actin antibodies. (C) EMSA of IRF-1–ISRE complexes. WISH cells were treated with 2 ng/ml IL-1β for 1 h, followed by 100 units/ml IFN-γ for 30 min. Total cell extract was subjected to EMSA with an ISRE probe. The identity of the complex was confirmed by supershifting with the indicated antibodies. The arrowhead identifies the ISRE complex, and the arrow identifies the supershifted complexes.
IRF-1 is an IFN-γ-induced transcription factor, which mediates many late IFN-γ–induced transcriptional activities. Previously, we demonstrated that induction of IL-18BP by IFN-γ is abolished in IRF-1-deficient mice (28). We evaluated the effect of exogenous IL-1β on IRF-1 induction by IFN-γ in WISH and HaCaT cells. Treatment with IFN-γ alone induced IRF-1 only after 1 h as detected by immunoblotting of total cell extract. Preincubation of the cells with IL-1β for 1 h before addition of IFN-γ not only accelerated IRF-1 induction, resulting in detectable IRF-1 after only 30 min, but also increased its level (Fig. 7B). The activity of this elevated IRF-1 was verified by the observation of increased formation of IRF-1 nuclear complexes after treatment with 100 units/ml IFN-γ for 30 min in cell cultures supplemented with IL-1, as determined by EMSA with an IFN-stimulated response element (ISRE) probe (Fig. 7C, compare lanes 2 and 3). Because induction of IRF-1 mRNA by IFN-γ was inhibited by IL-1Ra (Fig. 3A) and augmented by exogenous IL-1, we conclude that it requires activation of both the GAS and κB elements. This conclusion is consistent with the reported presence of κB elements in the IRF-1 promoter (34).
Discussion
In the present study, we demonstrate that constitutively expressed IL-1α is critical for the induction of many genes by IFN-γ, including HLA-DR, ICAM-1, IL-18BP, and genes mediating the antiviral activity of IFN-γ. As a result, specific inhibitors of IL-1α reduce the antiviral activity of IFN-γ by 90% in human WISH cells and to a similar extent in embryonic fibroblasts derived from two IL-1 knockout mice as compared with fibroblasts from wild-type mice. We further show that IL-1α inhibition and IL-1 deficiency abolished the induction of IL-18BP in vitro and in vivo, respectively. It is remarkable that the dependence of the antiviral state on basal IL-1α was unique to IFN-γ and was not observed with type I IFNs (α and β).
We found that NF-κB activation by basal IL-1α was required for the induction of these genes and for the establishment of the antiviral state by IFN-γ. We further found that IFN-γ increased the expression of membrane-associated IL-1α, thereby further enhancing gene activation by the IFN-γ/IL-1α pair. The observation that a biological activity or gene induction may depend on more than one cue is well documented. Such dual and even multiple signaling in parallel probably provide a safety measure, typical to many immune responses, such as T cell receptor signaling (35).
Many studies have demonstrated that TNF synergizes with IFN-γ, probably by eliciting the complementary NF-κB pathway. Here we show that NF-κB activation not only enhances IFN-γ activity, but is in fact essential for its basal activity. A clue to this dependency was provided in two earlier studies. Induction of CD40 in macrophages and microglial cells by IFN-γ was attenuated upon inhibition of constitutively expressed TNF (36). The role of constitutively expressed TNF in microglial cells is probably similar to that of IL-1α in the cells that we studied, because TNF and IL-1 share the ability to activates NF-κB. In accordance with the present study, it is likely that constitutively expressed IL-1α maintained the basal level of NF-κB reported to be required for induction of CXCL9 and CXCL10 by IFN-γ (17).
The critical role of basal IL-1 in IFN-γ action, combined with the ability of IFN-γ to increase the production of IL-1α, raises the possibility that cells such as keratinocytes, which express high levels of IL-1α, may enhance the responsiveness of infiltrating macrophages or dendritic cells to T cell-derived IFN-γ. Such a cascade of events may be the underlying mechanism of chronic skin inflammations. A role for IL-1 in psoriasis was confirmed recently by the finding of high levels of the IL-1-inducible chemokine IL-8 in psoriatic plaques as compared with nonlesional skin (37).
In conclusion, this study shows that the cellular response to a given cue is determined not only by its repertoire of receptors and transcription factors but also by its collection of constitutively expressed cytokines and their associated signaling molecules.
Materials and Methods
Cells, Mice, and Reagents.
Human amniotic WISH cells (CCL 25) and human monocytic THP-1 cells (TIB-202) were obtained from the American Type Culture Collection (Manassas, VA). Human HaCaT keratinocytes were from P. Boukamp (German Cancer Research Center, Heidelberg, Germany) (38). Homozygous IL-1α/β double-deficient C57BL/6 mice (39) were kindly provided by Y. Iwakura (University of Tokyo, Tokyo, Japan). Humane animal care was overseen by The Weizmann Institute Animal Care and Use Committee, according to guidelines set by Israeli laws. Rabbit polyclonal antibodies to IL-1α, IL-1β, IRF-1, NF-κB, p65, and HLA-DR were from Santa Cruz Biotechnology (Santa Cruz, CA). Recombinant human cytokines were from Cytolab (Rehovot, Israel). Chemicals and growth media were from Sigma (Rehovot, Israel).
ELISA of IL-18BP and IL-1α.
Human IL-18BP was measured in culture supernatants by a double-antibody ELISA as described (29). Murine IL-18BP was determined by ELISA based on rabbit polyclonal antibodies (Cytolab). IL-1α was measured in whole-cell extracts by a double antibody ELISA purchased from R & D Systems (Minneapolis, MN).
RNA Isolation and RT-PCR.
WISH cells (106) in minimum essential medium (2 ml) were plated in six-well plates for 17 h. Various cytokines were added for the indicated times; the cells were then harvested, and total RNA was extracted by using TRI reagent (Sigma). cDNA was prepared from RNA by using random hexamers and SuperscriptII (Invitrogen, Leek, The Netherlands). PCR was performed with the following primers: IL-18BP, 5′ CACGTCGTCACTCTCCTGG and 5′-CGACGTGACGCTGGACAAC; ICAM-1, 5′-CAATGCCCAGACATCTGTGTC and 5′-CAGTGCGGCACGAGAAATTG; CXCL11, 5′-GCTGTGATATTGTGTGCTAC and 5′-TCGATTTGGGATTTAGGCATC; IL-32 (NK4), 5′-TAATGCTCCTCCCTACTTC and 5′-CAGCAGAAACTCTGGAACTG; FAT10, 5′-GATGGCTCCCAATGCTTCCTGC and 5′-GTCACCCTCCAATACAATAAGATGC; CIITA, 5′-CTGAAGGATGTGGAAGACCTGGGAAAGC and 5′-GTCCCCGATCTTGTTCTCACTC; HLA-DR, 5′-GAGTTTGATGCTCCAAGCCCTCTCCCA and 5′-CAGAGGCCCCCTGCGTTCTGCTGCATT; Mx1, 5′-CATCGACCTCATTGACTCCC and 5′-TCCACATTACTGGGGACCAC; Mx2, 5′-TCCAAAAATCGCTCCCGTTG and 5′-GACGTTTGCTGGTTTCCAAG; ISG20, 5′-GTTGCAGCCTCGTGAACGTC and 5′-CTGTGTCCAAGCAGGCTGTTC; β-Actin, 5′-GTGGGGCGCCCCAGGCACCA and 5′-CTCCTTAATGTCACGCACGATTTC.
Amplifications were done by initial denaturation at 92°C for 2 min and 28 cycles of denaturation at 92°C for 30 sec, annealing at 62°C for 45 sec, extension at 72°C for 45 sec, and final extension at 72°C for 10 min. The resulting PCR products were resolved by agarose (1%) gel electrophoresis.
DNA Array Analysis.
Human WISH cells were treated with 100 units/ml IFN-γ, 10 μg/ml IL-1Ra, or their combination for 3, 6, and 17 h. RNA was isolated and subjected to expression array analysis, employing Affymetrix U133A gene chips. The resulting Excel data files were sorted by the following algorithm: genes that were present and induced ≥4-fold by IFN-γ were selected. Of these genes, those whose signal after combined treatment with IFN-γ and IL-1Rα was reduced by ≤2/3 when compared with the signal from cells treated with IFN-γ alone made the final selection. Genes whose basal level was reduced by IL-1Ra alone by ≤2/3 were excluded as well.
Antiviral Assay.
WISH cells (40,000/ml) were plated in 100 μl of MEM in 96-well plates. Cells were incubated with serially 2-fold-diluted stocks of IFN-α2, IFN-β, or IFN-γ for 17 h. IL-1β, TNF-α, IL-1Ra, anti-IL-1α, or anti-IL-β antibodies were added together with the IFNs. Vesicular stomatitis virus was added at 17 h, and protection from viral cytopathic effect was scored in crystal-violet-stained wells at 40 h.
Luciferase Reporter Vectors.
The luciferase reporter vector pGL3 (656), comprising the plasmid pGL3-Basic vector (Promega, Madison, WI) into which a 656-bp section of the human IL-18BP promoter has been ligated, was described previously (28). The κB-response element was mutated to give vector pmGL3 (656) by a PCR-based mutagenesis method (40) that used the following sense and its complement primers: 5′-CAACTTGGAAAATTTTAGTGCCTAGGAAAG. Mutated regions are in italic type.
Transient Transfection Assays.
HaCaT or WISH cells in six-well plates (5 × 105 cells per well) were transfected with FuGene 6 (Roche Molecular Biochemicals, Mannheim, Germany), the indicated luciferase reporter vector (0.5 μg per well), and pSV40 βGAL (0.2 μg per well; Promega). After 16 h, cells were treated with 100 units/ml IFN-γ, 10 μg/ml IL-Ra, or their combination in serum-free medium for 24 h. Cells were then collected and lysed, and luciferase activity was measured. All results were normalized against β-galactosidase activity.
EMSAs.
The following oligonucleotides were used for generating probes: 5′-GATCCGTCGACATTTCCCGTAAATCG and 5′-AGCTTGATTTACGGGAAATGTCGACG, corresponding to GAS, and 5′-AGTTGAGGGGACTTTCCCAGGC and 5′-CCTGGGAAAGTCCCCTCAACT, corresponding to the κB binding site. dsDNA (10 pmol) was labeled with [γ-32P]ATP and used in EMSA as previously described (28).
Statistics.
The significance of differences between groups showing similar variance was evaluated by the Student t test.
Acknowledgments
We thank Sara Barak for excellent technical assistance, Shirley Horn Saban for performing the Affymetrix gene array analysis, and Yoichiro Iwakura for providing the IL-1α/β double-knockout mice. This study was supported by the Serono Group.
Abbreviations
- IRF
IFN regulatory factor
- GAS
γ-activated sequence
- MEF
murine embryonic fibroblast
- IL-1Ra
IL-1 receptor antagonist
- IL-18BP
IL-18 binding protein
- PDTC
pyrrolidinedithiocarbamate
- ISRE
IFN-stimulated response element.
Footnotes
The authors declare no conflict of interest.
References
- 1.Wheelock EF. Science. 1965;149:310–311. [PubMed] [Google Scholar]
- 2.Billiau A. Adv Immunol. 1996;62:61–130. doi: 10.1016/s0065-2776(08)60428-9. [DOI] [PubMed] [Google Scholar]
- 3.Boehm U, Klamp T, Groot M, Howard JC. Annu Rev Immunol. 1997;15:749–795. doi: 10.1146/annurev.immunol.15.1.749. [DOI] [PubMed] [Google Scholar]
- 4.Schroder K, Hertzog PJ, Ravasi T, Hume DA. J Leukoc Biol. 2004;75:163–189. doi: 10.1189/jlb.0603252. [DOI] [PubMed] [Google Scholar]
- 5.Valente G, Ozmen L, Novelli F, Geuna M, Palestro G, Forni G, Garotta G. Eur J Immunol. 1992;22:2403–2412. doi: 10.1002/eji.1830220933. [DOI] [PubMed] [Google Scholar]
- 6.Darnell JE., Jr Science. 1997;277:1630–1635. doi: 10.1126/science.277.5332.1630. [DOI] [PubMed] [Google Scholar]
- 7.Sims SH, Cha Y, Romine MF, Gao PQ, Gottlieb K, Deisseroth AB. Mol Cell Biol. 1993;13:690–702. doi: 10.1128/mcb.13.1.690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yohn JJ, Critelli M, Lyons MB, Norris DA. J Invest Dermatol. 1990;95:233–237. doi: 10.1111/1523-1747.ep12478093. [DOI] [PubMed] [Google Scholar]
- 9.Geller DA, Nussler AK, Di Silvio M, Lowenstein CJ, Shapiro RA, Wang SC, Simmons RL, Billiar TR. Proc Natl Acad Sci USA. 1993;90:522–526. doi: 10.1073/pnas.90.2.522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ohmori Y, Hamilton TA. J Immunol. 1995;154:5235–5244. [PubMed] [Google Scholar]
- 11.Visser CE, Tekstra J, Brouwer-Steenbergen JJ, Tuk CW, Boorsma DM, Sampat-Sardjoepersad SC, Meijer S, Krediet RT, Beelen RH. Clin Exp Immunol. 1998;112:270–275. doi: 10.1046/j.1365-2249.1998.00592.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Struyf S, Van Collie E, Paemen L, Put W, Lenaerts JP, Proost P, Opdenakker G, Van Damme J. J Leukoc Biol. 1998;63:364–372. doi: 10.1002/jlb.63.3.364. [DOI] [PubMed] [Google Scholar]
- 13.Man L, Lewis E, Einbinder T, Rogachev B, Chaimovitz C, Douvdevani A. Kidney Int. 2003;64:2064–2071. doi: 10.1046/j.1523-1755.2003.00338.x. [DOI] [PubMed] [Google Scholar]
- 14.Hiroi M, Ohmori Y. J Biol Chem. 2003;278:651–660. doi: 10.1074/jbc.M204544200. [DOI] [PubMed] [Google Scholar]
- 15.Takami Y, Motoki T, Yamamoto I, Gohda E. Biochem Biophys Res Commun. 2005;327:212–217. doi: 10.1016/j.bbrc.2004.11.144. [DOI] [PubMed] [Google Scholar]
- 16.Sizemore N, Agarwal A, Das K, Lerner N, Sulak M, Rani S, Ransohoff R, Shultz D, Stark GR. Proc Natl Acad Sci USA. 2004;101:7994–7998. doi: 10.1073/pnas.0401593101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hiroi M, Ohmori Y. Biochem J. 2003;376:393–402. doi: 10.1042/BJ20030842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lonnemann G, Engler-Blum G, Muller GA, Koch KM, Dinarello CA. Kidney Int. 1995;47:845–854. doi: 10.1038/ki.1995.127. [DOI] [PubMed] [Google Scholar]
- 19.Ichikawa T, Nakao K, Nakata K, Yamashita M, Hamasaki K, Shigeno M, Abiru S, Ishikawa H, Ishii N, Eguchi K. Biochem Biophys Res Commun. 2002;294:414–422. doi: 10.1016/S0006-291X(02)00502-8. [DOI] [PubMed] [Google Scholar]
- 20.Anderson SL, Carton JM, Lou J, Xing L, Rubin BY. Virology. 1999;256:8–14. doi: 10.1006/viro.1999.9614. [DOI] [PubMed] [Google Scholar]
- 21.Espert L, Rey C, Gonzalez L, Degols G, Chelbi-Alix MK, Mechti N, Gongora C. Oncogene. 2004;23:4636–4640. doi: 10.1038/sj.onc.1207586. [DOI] [PubMed] [Google Scholar]
- 22.Aebi M, Fah J, Hurt N, Samuel CE, Thomis D, Bazzigher L, Pavlovic J, Haller O, Staeheli P. Mol Cell Biol. 1989;9:5062–5072. doi: 10.1128/mcb.9.11.5062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Coccia EM, Marziali G, Stellacci E, Perrotti E, Ilari R, Orsatti R, Battistini A. Virology. 1995;211:113–122. doi: 10.1006/viro.1995.1384. [DOI] [PubMed] [Google Scholar]
- 24.Hou J, Baichwal V, Cao Z. Proc Natl Acad Sci USA. 1994;91:11641–11645. doi: 10.1073/pnas.91.24.11641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Raasi S, Schmidtke G, de Giuli R, Groettrup M. Eur J Immunol. 1999;29:4030–4036. doi: 10.1002/(SICI)1521-4141(199912)29:12<4030::AID-IMMU4030>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 26.Kim SH, Han SY, Azam T, Yoon DY, Dinarello CA. Immunity. 2005;22:131–142. doi: 10.1016/j.immuni.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 27.Novick D, Kim SH, Fantuzzi G, Reznikov LL, Dinarello CA, Rubinstein M. Immunity. 1999;10:127–136. doi: 10.1016/s1074-7613(00)80013-8. [DOI] [PubMed] [Google Scholar]
- 28.Hurgin V, Novick D, Rubinstein M. Proc Natl Acad Sci USA. 2002;99:16957–16962. doi: 10.1073/pnas.262663399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Novick D, Schwartsburd B, Pinkus R, Suissa D, Belzer I, Sthoeger Z, Keane WF, Chvatchko Y, Kim SH, Fanuzzi G, et al. Cytokine. 2001;14:334–342. doi: 10.1006/cyto.2001.0914. [DOI] [PubMed] [Google Scholar]
- 30.Mestan J, Brockhaus M, Kirchner H, Jacobsen H. J Gen Virol. 1988;69:3113–3120. doi: 10.1099/0022-1317-69-12-3113. [DOI] [PubMed] [Google Scholar]
- 31.Inamura N, Sone S, Okubo A, Kunishige E, Nakanishi M, Ogura T. Cancer Immunol Immunother. 1989;28:164–170. doi: 10.1007/BF00204984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gueniche A, Viac J, Charveron M, Schmitt D. Exp Dermatol. 1994;3:113–118. doi: 10.1111/j.1600-0625.1994.tb00268.x. [DOI] [PubMed] [Google Scholar]
- 33.Kaplanski G, Farnarier C, Kaplanski S, Porat R, Shapiro L, Bongrand P, Dinarello CA. Blood. 1994;84:4242–4248. [PubMed] [Google Scholar]
- 34.Imanishi D, Yamamoto K, Tsushima H, Miyazaki Y, Kuriyama K, Tomonaga M, Matsuyama T. J Immunol. 2000;165:3907–3916. doi: 10.4049/jimmunol.165.7.3907. [DOI] [PubMed] [Google Scholar]
- 35.Davis SJ, van der Merwe PA. Nat Immunol. 2006;7:803–809. doi: 10.1038/ni1369. [DOI] [PubMed] [Google Scholar]
- 36.Nguyen VT, Benveniste EN. J Biol Chem. 2002;277:13796–13803. doi: 10.1074/jbc.M111906200. [DOI] [PubMed] [Google Scholar]
- 37.Mee JB, Cork MJ, di Giovine FS, Duff GW, Groves RW. Cytokine. 2006;33:72–78. doi: 10.1016/j.cyto.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 38.Boukamp P, Petrussevska RT, Breitkreutz D, Hornung J, Markham A, Fusenig NE. J Cell Biol. 1988;106:761–771. doi: 10.1083/jcb.106.3.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yamada H, Mizumo S, Horai R, Iwakura Y, Sugawara I. Lab Invest. 2000;80:759–767. doi: 10.1038/labinvest.3780079. [DOI] [PubMed] [Google Scholar]
- 40.Meza R, Nunez-Valdez ME, Sanchez J, Bravo A. FEMS Microbiol Lett. 1996;145:333–339. doi: 10.1111/j.1574-6968.1996.tb08597.x. [DOI] [PubMed] [Google Scholar]