Abstract
We previously reported that compared with a non-deprivation state, overnight abstinence from cigarette smoking was associated with higher brain activity in the left dorsolateral prefrontal cortex (L-DLPFC) during a low demanding working memory challenge, and little increase beyond this activity level during more taxing working memory conditions. In the present study, we aimed to assess how recent smoking (overnight abstinence Vs smoking ad libitum) influenced the effect of smoking a cigarette on brain activity related to a working memory challenge. Six smokers performed the N-Back working memory task during functional magnetic resonance imaging (fMRI) both before and after smoking a cigarette in each of two test sessions: one following overnight abstinence from smoking (>13 h) and the other following ad libitum smoking.
Task-related activity in L-DLPFC showed a significant interaction between the effects of acute smoking, test session, and task load. After overnight abstinence, post-smoking brain activity in L-DLPFC was lower than before smoking at low task-load and higher at high task-load; corresponding activity on a day of ad libitum smoking was higher at low load and lower at high task-load after smoking during the session. These data suggest that the effect of acute smoking on working-memory processing depends on recent prior smoking and task-load. In particular, they provide preliminary evidence that functional efficiency of working memory is improved by smoking a cigarette during abstinence, while the effect of a cigarette in a non-deprived state varies with the nature and difficulty of the working memory challenge. This interaction merits further examination in larger studies specifically designed to consider this issue.
Keywords: Functional magnetic resonance imaging, Tobacco, Nicotine, Working Memory
1. INTRODUCTION
Among nicotine-dependent individuals, abrupt initiation of abstinence from smoking produces both psychological and somatic withdrawal symptoms (DSM-IV). Impairment of cognitive functioning is among these symptoms, and smokers may continue to smoke in part to relieve it (Snyder, Davis, & Henningfield, 1989; Snyder & Henningfield, 1989; Pineda, Herrera, Kang, & Sandler, 1998; al‘Absi, Amunrud, & Wittmers, 2002; Mendrek et al., 2005). Indeed behavioral testing indicates that after acute abstinence, resumption of smoking does improve cognitive performance. For example, after resuming smoking, abstinent smokers showed reduced latency during a serial-probe working memory task (Pineda et al., 1998) and during a Sternberg-type working memory test (Houlihan, Pritchard, & Robinson, 2001).
Neuroimaging has been used to assess the effects of smoking and of nicotine per se on working memory (Kumari et al., 2003; Jacobsen et al., 2004), but with inconsistent findings. In one study, abstinent smokers performing a 2-back task showed less activation in the frontoparietal cortex and shorter response latency after chewing nicotine gum relative to placebo. In another study, abstinent smokers showed increased activation of the right occipitotemporal cortex and decreased activation of the right globus pallidus with reduced accuracy on an auditory 2-back task after receiving transdermal nicotine (Jacobsen et al., 2004). In a third study, after subcutaneous injection of nicotine, non-smokers showed increased activation in frontoparietal cortex at 1-, 2-, and 3-back condition, and decreased activation in the right parietal cortex at 3-back with decreased response latency and increased accuracy (Kumari et al., 2003).
In a fourth study, we tested the effect of smoking abstinence (> 13 h) on working memory using a parametric N-Back task (including 0-, 1-, 2-, and 3-back conditions). On a day that smokers had smoked ad libitum, task-related activity in the left dorsolateral prefrontal cortex (L-DLPFC) was relatively low for an easy task condition (1-back), and increased as task difficulty increased; but when smokers were abstinent overnight, activity in the L-DLPFC was approximately as high at low task level as it was at more difficult levels (Xu et al., 2005). We interpreted this pattern of task-related activity in abstinent smokers as reflecting decreased functional efficiency of working memory-related brain function.
A subset of the participants in the aforementioned study repeated the N-Back Task after smoking one cigarette during both test sessions: one that began after ad libitum smoking and another began after overnight abstinence, and these data were not included in our earlier report. Here we report on this assessment, which was made to gather preliminary data on the possible contribution of deprivation state to the effect of smoking a cigarette on brain activity related to working memory. Given our previous finding that the effect of smoking abstinence on brain activity varied with task difficulty (load), we anticipated that the effects of acute smoking might vary both with task load and recent smoking. In particular, we expected that smoking a cigarette would produce a greater decrease in task-related activity at low task loads after abstinence than during a non-deprived state. Such a difference would suggest a normalization of the pattern of activity observed in abstinent smokers.
2. METHODS
2. 1. Study Participants
As described previously (Xu et al., 2005), healthy participants provided written informed consent after receiving a detailed explanation of the research protocol, which was approved by the Institutional Review Board of University of California Los Angeles. They completed questionnaires covering medical and smoking histories, childhood attention deficit hyperactivity disorder, and depressive symptoms.
Data were acquired from six smokers (20–40 years of age, 3 women), who were among the eight participants in the study we reported previously (Xu et al., 2005). They smoked 13–20 cigarettes/day, having smoked regularly for 2–26 yr (mean = 10.8 yr, SD = 9.4). Their scores on the Fagerström Test for Nicotine Dependence (Heatherton, Kozlowski, Frecker, & Fägerström, 1991), ranged from 3–7 (mean = 4.2, SD=1.3), indicating moderate dependence.
2.2. Experimental design
Each participant completed four test sessions of the N-Back working memory task. Of the four test sessions, two involved behavioral testing and two involved fMRI. One of each type of session (i.e., behavioral or fMRI) began after > 13 h abstinence from smoking, and the other began after smoking ad libitum. In both behavioral and fMRI testing, the abstinence session was first for three participants, and the session following ad libitum smoking was first for the other three participants. Each test session was conducted in two blocks, with participants always smoking one cigarette before beginning the second block. The period during which each participant finished the four test sessions ranged from 5 to 14 days (mean = 9 days, SD = 3.3). The period between the two sessions that followed overnight abstinence ranged from 2–8 days (mean = 4.3 day, SD = 2.5).
On each fMRI day, two fMRI testing blocks were administered with a 15-min break between the two blocks. During the break, each participant exited the scanner and smoked one cigarette of his/her usual brand. On the test day that allowed ad libitum smoking, 20 – 45 min elapsed between smoking and the beginning of first fMRI scanning block. On each of the fMRI test days, the time between acute smoking and the beginning of second fMRI scanning test block was about 20 min. Before each test block, the participants completed the Shiffman/Jarvik Withdrawal Scale (Jarvik et al., 2000). The score of each subscale of SJWS ranges from “1” to “7”, with scores of “1”, “4”, and “7”, corresponding to “definitely no withdrawal or craving symptom”, “possibly withdrawal or craving symptom”, and “definitely withdrawal or craving symptom”, respectively.
2.3. Task design
We used a 4-step letter version of the N-Back Task. During fMRI, stimuli were displayed through video goggles (Resonance Technology Corporation, Northridge, CA). The test stimuli were letters, presented one at a time. Each letter appeared for 400 ms, and the inter-stimulus interval (ISI) was 1600 ms. In the 0-back condition, subjects pressed a “target key” when the letter “X” appeared, and the “non-target key” when any other letter appeared. In the 1-back, 2-back, and 3-back conditions, participants pressed the “target key” when the letter presented was identical to the letter 1, 2, or 3 letters preceding it, respectively. Otherwise, they were to press the “non-target key.”
The N-Back conditions were presented individually in 42-sec blocks. In each block, 21 trials were presented, each containing 7 (33%) targets and 14 non-targets. There was a 3-sec instruction screen before each task block and a 15-sec block of rest after each task block. During rest, subjects fixed their eyes on a cross that was displayed at the center of the screen. The task blocks were programmed into four scripts. In each script, each of the four N-Back conditions was presented twice in one of four pseudorandom orders, resulting in an 8-min run of the entire task. Each subject completed two test blocks (before and after the break) on each day, with two 8-min runs in each block. The sequence of presentation of the scripts was counterbalanced among subjects. Performance data were not acquired during scanning due to a programming error. We therefore report on the N-Back performance data collected outside the scanner.
Scanning parameters
Functional images were acquired on a 3T MRI scanner (GE, Signa with an EPI upgrade from Advanced NMR Systems), using T2* weighted gradient-recalled EPI, with blood oxygen level-dependent (BOLD) contrast (TR = 3000 ms, TE = 42 ms, flip angle = 80°, slice thickness = 4 mm with a 1-mm inter-slice interval, matrix of 64 x 64, in-plane resolution = 3.12 x 3.12 mm2). One hundred sixty images were acquired for each of 16 axial slices through the brain during the presentation of N-Back script. High-resolution T2-weighted EPI anatomical images of the whole brain (23 – 25 slices, 4-mm thick) were acquired in each scanning session to help define the locations of the BOLD signal changes.
2.4. Statistical analysis
2.4.1. Behavioral data
Repeated measures analyses of variance (ANOVAs) were conducted in which N-Back level (0-, 1-, 2-, and 3-back), test session (during smoking deprivation or following ad libitum smoking) and test block (pre- or post-smoking) were each within-subject independent factors. Separate analyses were conducted for errors and response times (RTs).
2.4.2. Imaging data
The functional images of each subject were corrected for motion. We used 4 mm, the thickness of imaging slices, as the maximum motion allowed, and none of the data from the six subjects exceeded this threshold during any functional scanning. There also was no significant difference in maximum motion among the four test blocks (One-way ANOVA, df = 3, F = 1.588, p = 0.224).
After motion correction, functional series were co-registered with the high-resolution T2-weighted EPI anatomical image, spatially normalized with the study-specific template, and smoothed with a 10-mm FWHM Gaussian filter using SPM2 (Welcome Department of Cognitive Neurology, London). We constructed model time courses for each task level by convolving a boxcar waveform representing the times of onset and duration of each task level with the canonical hemodynamic response function offered with SPM2.
Given our small sample size and the multiple comparisons in fMRI analysis, we limited the search volume in the statistical analysis. The ROI (region of interest) was defined in our earlier report (Xu et al., 2005). To define this ROI, we combined the imaging data acquired during pre-smoking test block of the two test sessions together. We then used a parametric contrast (i.e., -2 -1 1 2) to identify brain regions showing significant increase in BOLD signal with increasing task load. Clusters in the left dorsal lateral prefrontal cortex (DLPFC), and bilateral pre-supplementary motor cortex and parietal cortex showed significant changes in BOLD signal at this contrast. All these significant clusters were defined as one ROI by using the SPM2-compatible ROI analysis tool Marsbar (Brett et al 2002). The size of this ROI was 12992 m3.
Considering our previous finding of a 2-way interaction (test session x task load) only at the contrast of 3-back minus 1-back, we used this contrast to test for a 3-way interaction in changes of BOLD signal between task load, test session, and test block. We first used the contrast of “3-back minus 1-back” to create a SPM{T} map for each test block (i.e., pre- and post-smoking) of each test session of each participant. Then we entered these SPM{T} maps into second level (one-way ANOVA, within-subject) for random effect analysis at the group level. The contrast [(pre-smoking test block minus post-smoking test block during the session following ad libitum smoking) minus or plus (pre-smoking test block minus post-smoking test block during the session that followed abstinence)] was used to assess the task load X test session X test block 3-way interaction of changes in BOLD signal. Only voxels that survived the voxel-level threshold of p < .001 (uncorrected) and were within the a priori ROI, were further analyzed, with multiple comparison correction using the small volume correction (SVC) tool in SPM2. The threshold for cluster significance was p < .05 (after correction). Relative to baseline (0-back), the mean signal change of significant voxels within the ROI at 1-back, 2-back, and 3-back were extracted with the Marsbar tool (Brett et al., 2002) for presentation purposes only.
3. RESULTS
3.1. Subjective self-reports of Shiffman-Jarvik Withdrawal Scale
A significant interaction between acute smoking and test session was observed on reported cigarette craving (F(1,5)=45.47, p < .05) and psychological withdrawal symptoms (F(1,5) = 9.19, p < .05). These interactions reflected decreases in the self-ratings of symptoms after acute smoking during the abstinence session but not in the non-abstinent session (Table 1).
Table 1.
Withdrawal Scale of Shiffman/Jarvik: Mean Scores (Standard Error) of Craving and Withdrawal
| Satiety Session | Abstinence Session | |||
|---|---|---|---|---|
| Pre-Smoking | Post-Smoking | Pre-Smoking | Post-Smoking | |
| Craving* | 3.67(0.4) | 4.31(0.3) | 6.36(.5) | 4.17(0.6) |
| Psychological Symptoms* | 3.08(0.34) | 3.10(0.6) | 4.49(0.6) | 3.38(0.46) |
| Physical Symptoms | 1.67(0.4) | 2.11(0.8) | 2.72(0.9) | 2.33(0.8) |
| Sedation | 3.25(0.7) | 3.79(0.8) | 3.42(0.3) | 3.88(0.8) |
| Appetite | 4.0(0.2) | 4.0(0.2) | 4.42(0.75) | 4.67(0.8) |
| Total score | 15.67(1.0) | 17.3(2.2) | 21.41(2.0) | 18.44(3.4) |
Repeated measures analyses of variance (ANOVAs) was used to assess the overall significance of differences.
p<0.05, interaction between acute smoking and test session.
3.2. Behavioral performance
N-Back performance data (sessions combined) showed no significant effects on reaction time RT with this small sample size, and a nonsignificant trend towards decreases in error rate from the pre- to the post-smoking test (F(1,5)= 5.85, p = .06). Error rates were significantly lower after smoking at the 3-back condition in both the abstinent session (t = 4.0, p < .05, df = 5) and the non-abstinent session (t = 2.6, p < .05, df = 5, Fig. 1). No interaction was observed between test session and test block as predictors of either error rates or RT.
Fig. 1.
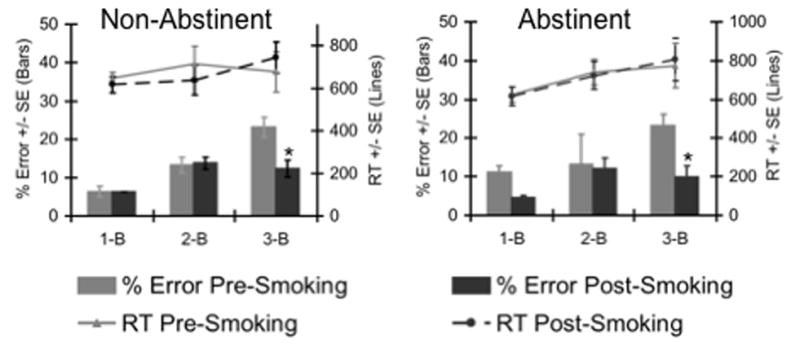
N-Back performance of smokers in sessions following either smoking ad libitum or smoking abstinence (> 13 h). Lines indicate mean RT’s and bars indicate mean percent of errors, both at each N-Back level. Left panel, session following smoking ad libitum, right panel, session following smoking abstinence. In both sessions, subjects showed significantly fewer errors at the 3-back level after acute smoking.
3.3. Task-related fMRI signal change
Group level, within subject, one-way ANOVA analysis (SPM2) found a significant interaction between acute smoking, test session, and task load in the left DLPFC (1176 mm3, MNI x, y, z coordinates: −38, 6, 34 p < .001, Fig. 2A). This cluster was within the a priori ROI, and survived the correction for multiple comparisons within the ROI (SPM2 SVC, p < .05). The extracted % signal change from this cluster indicating that task-related BOLD signal change decreased at the 1-back condition and increased at the 3-back condition in the test session following smoking abstinence (> 13 h); and increased at the 1-back condition and decreased at the 3-back condition in the test session following ad libitum smoking (Fig. 2B,C).
Fig. 2.
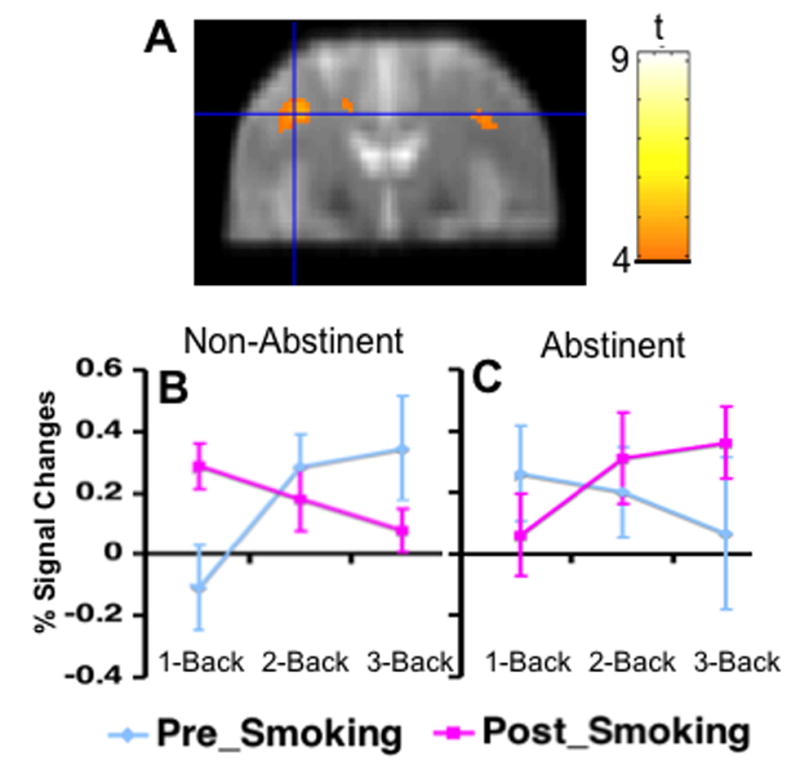
A cluster in the left dorsal lateral prefrontal cortex (DLPFC) showed a 3-way interaction among acute smoking, test session, and task load on task-related activity. Colors superimposed on the gray scale image, from the study-specific structural brain template, indicate values of t according to the color bar. The line graph indicates the mean percent signal changes from the 0-back and standard deviation at each level of the N-Back Task. B. data from session after smoking ad libitum, C: data from session after overnight abstinence.
4. DISCUSSION
The findings suggest that the effect of acute smoking on activation within the left DLPFC related to working memory challenge depends on the state of abstinence. In particular, the effect observed in the left DLPFC was roughly opposite when the participants were smoking-deprived or relatively non-deprived: during abstinence, smoking was followed by decrease in activity at low task load and increased activity at high load; on the day of ad libitum smoking, activity increased at low task load and decreased at high task load. Notably, smoking a cigarette after overnight abstinence changed the pattern of activity in the left DLPFC to resemble the relationship between task-related activity and task difficulty that was observed in healthy nonsmokers and non-deprived smokers (i.e., low activity at low load and high activity at high load, (Jansma, Ramsey, Coppola, & Kahn, 2000; Xu et al., 2005).
The opposite effect on changes of BOLD signal after acute smoking on a day of ad libitum smoking was unexpected. Since there were no changes in performance at the 1-back level and improved performance at the 3-back level (decreased error rates), this effect suggested that cigarette smoking decreased the functional efficiency of left DLPFC at the 1-back level and increased it at the 3-back level. Although not easily explained, the inconsistencies related to the state of abstinence underscore previous assertions that cigarette smoking and/or nicotine administration have complex effects on performance of cognitive tasks and related brain activity (Gilbert, Robinson, Chamberlin, & Spielberger, 1989; Lambe, Picciotto, & Aghajanian, 2003; Pritchard, 1991). For example, electroencephalography (EEG) revealed that cigarette smoking increased EEG dimensional complexity (DCx) in smokers with low pre-smoking DCx, and decreased EEG DCx in smokers with high pre-smoking DCx (Houlihan et al., 2001).
Our findings are also consistent with the theory that neurochemical optima in the brain vary for different cognitive tasks (Clark, Cools, & Robbins, 2004; Lambe et al., 2003; Robbins, 2005). The 1-back task is certainly easier than 3-back task, and the two tasks arguably involve qualitatively different functions. While the 1-back requires maintenance alone, success on the 3-back requires extensive manipulation of working memory content (Ragland et al., 2002). These two task conditions, accordingly, may have different neurochemical optima. Nicotine stimulates the release of dopamine (DA), serotonin (5-HT), and norepinephrine (NE) in multiple brain regions (George, Verrico, & Roth, 1998; Rossi, Singer, Shearman, Sershen, & Lajtha, 2005). The neurochemical effects of cigarette smoking (following ad libitum smoking throughout the test day) could be optimal for the 3-back condition, but not for the 1-back condition.
This preliminary report has several limitations, including the small sample size, lack of plasma nicotine levels, and failure to record task performance during fMRI. The absence of behavioral data prevented the analysis of relation between performance and changes in BOLD signal, and also prevented the analysis of the data in an event-related fashion. It is possible that performance differences across conditions contributed to observed differences in task-related activity. Another caveat is that CO and nicotine have complex effects on cerebral perfusion (Ghatan et al., 1998; Rose et al., 2003; Domino et al., 2004), possibly complicating interpretation of differences in BOLD signal. There is evidence, however, that nicotine does not alter the coupling between the BOLD signal and the activity of the visual cortex in response to photic stimulation (Jacobsen et al., 2002). Finally, it is important to consider that smoking a cigarette was confounded with test order in the present study; therefore, differences in performance and/or task-related activity before Vs. after acute smoking may reflect more than an effect of cigarette smoking alone. Order effects related to factors such as fatigue, or learning may have contributed to the differences observed on either test-day. It is more reasonable to interpret interactions between smoking state and changes in performance after acute smoking as evidence that the effect of smoking a cigarette is influenced by recent smoking. As order effects may interact with smoking state (e.g., overnight abstinence vs. ad libitum smoking), however, conclusions regarding the effect of smoking manipulations should be tentative.
In conclusion, this report presents the first fMRI evidence that the effects of acute smoking on the function of the left DLPC depend on the smoking status of smokers (non-abstinent vs. abstinent), in conjunction with the working memory load. The data suggest in particular that acute smoking improves the functional efficiency of the DLPFC in abstinent smokers performing a working memory task; but the effect of acute smoking on the function of DLPFC in non-abstinent smokers may vary with the tasks. This conclusion merits further examination in larger studies specifically designed to consider this issue.
Acknowledgments
The authors thank Dr. Russell A. Poldrack (Dept. Psychology, UCLA) for advice on data analysis. The image data were collected at the Ahmanson Lovelace Brain Mapping Center, which is supported by the Brain Mapping Medical Research Organization, the Brain Mapping Support Foundation, the Pierson-Lovelace Foundation, the Ahmanson Foundation, the Tamkin Foundation, the Jennifer Jones-Simon Foundation, the Capital Group, the Companies Charitable Foundation, the Robson Family, and the Northstar Fund.
Footnotes
Present address: Dept. Cognitive Science, University of California Irvine, Irvine, CA
Supported by NIH grants RO1 DA014093.03 (EDL), RO1 DA015059 (ALB), R21 DA 13627 and DA13637 (MSC), MOI RR 00865, NCRR R12169 and RR08655; UC Tobacco-Related Disease Research Program awards 10RT-0091 (EDL) and 11RT-0024 (ALB), VA Merit Review Type I Award (ALB), and Philip Morris USA contract 02066286 (EDL).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- al‘Absi M, Amunrud T, Wittmers LE. Psychophysiological effects of nicotine abstinence and behavioral challenges in habitual smokers. Pharmacology Biochemistry and Behavior. 2002;72:707–716. doi: 10.1016/s0091-3057(02)00739-6. [DOI] [PubMed] [Google Scholar]
- Brett M, Anton JL, Valabregue R, Poline JB. Region of interest analysis using an SPM toolbox. Neuroimage. 2002;16(2):1140–1141. [Google Scholar]
- Clark L, Cools R, Robbins TW. The neuropsychology of ventral prefrontal cortex: Decision-making and reversal learning. Brain and Cognition. 2004;55:41–53. doi: 10.1016/S0278-2626(03)00284-7. [DOI] [PubMed] [Google Scholar]
- Domino EF, Ni L, Xu Y, Koeppe RA, Guthrie S, Zubieta JK. Regional cerebral blood flow and plasma nicotine after smoking tobacco cigarettes. Prog Neuropsychopharmacol Biol Psychiatry. 2004;28:319–327. doi: 10.1016/j.pnpbp.2003.10.011. [DOI] [PubMed] [Google Scholar]
- Ernst M, Matochik JA, Heishman SJ, Van Horn JD, Jons PH, Henningfield JE, et al. Effect of nicotine on brain activation during performance of a working memory task. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:4728–4733. doi: 10.1073/pnas.061369098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George TP, Verrico CD, Roth RH. Effects of repeated nicotine pre-treatment on mesoprefrontal dopaminergic and behavioral responses to acute footshock stress. Brain Research. 1998;801:36–49. doi: 10.1016/s0006-8993(98)00537-x. [DOI] [PubMed] [Google Scholar]
- Ghatan PH, Ingvar M, Eriksson L, Stone-Elander S, Serrander M, Ekberg K, et al. Cerebral effects of nicotine during cognition in smokers and non-smokers. Psychopharmacology (Berlin) 1998;136:179–189. doi: 10.1007/s002130050554. [DOI] [PubMed] [Google Scholar]
- Gilbert DG, Robinson JH, Chamberlin CL, Spielberger CD. Effects of smoking/nicotine on anxiety, heart rate, and lateralization of EEG during a stressful movie. Psychophysiology. 1989;26:311–320. doi: 10.1111/j.1469-8986.1989.tb01924.x. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fägerström KO. The Fägerström Test for Nicotine Dependence: a revision of the Fägerström Tolerance Questionnaire. British Journal of Addiction. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Houlihan ME, Pritchard WS, Robinson JH. Effects of smoking/nicotine on performance and event-related potentials during a short-term memory scanning task. Psychopharmacology (Berlin) 2001;156:388–396. doi: 10.1007/s002130100751. [DOI] [PubMed] [Google Scholar]
- Jacobsen LK, Cyril D‘Souza D, Einar Mencl W, Pugh KR, Skudlarski P, Krystal JH. Nicotine effects on brain function and functional connectivity in schizophrenia. Biological Psychiatry. 2004;55:850–858. doi: 10.1016/j.biopsych.2003.12.023. [DOI] [PubMed] [Google Scholar]
- Jacobsen LK, Gore JC, Skudlarski P, Lacadie CM, Jatlow P, Krystal JH. Impact of intravenous nicotine on BOLD signal response to photic stimulation. Magn Reson Imaging. 2002;20:141–145. doi: 10.1016/s0730-725x(02)00494-0. [DOI] [PubMed] [Google Scholar]
- Jansma JM, Ramsey NF, Coppola R, Kahn RS. Specific versus nonspecific brain activity in a parametric n-back task. Neuroimage. 2000;12:688–697. doi: 10.1006/nimg.2000.0645. [DOI] [PubMed] [Google Scholar]
- Jarvik M, Madsen D, Olmstead R, Iwamoto-Schaap P, Elins J, Eisenberger N, et al. Blood nicotine levels and subjective craving for cigarettes. Pharmacology Biochemistry & Behavior. 2000;66:553–558. doi: 10.1016/s0091-3057(00)00261-6. [DOI] [PubMed] [Google Scholar]
- Kumari V, Gray JA, ffytche DH, Mitterschiffthaler MT, Das D, Zachariah E, et al. Cognitive effects of nicotine in humans: an fMRI study. Neuroimage. 2003;19:1002–1013. doi: 10.1016/s1053-8119(03)00110-1. [DOI] [PubMed] [Google Scholar]
- Lambe EK, Picciotto MR, Aghajanian GK. Nicotine induces glutamate release from thalamocortical terminals in prefrontal cortex. Neuropsychopharmacology. 2003;28:216–225. doi: 10.1038/sj.npp.1300032. [DOI] [PubMed] [Google Scholar]
- Mendrek A, Monterosso JR, Simon SL, Jarvik M, Brody AL, Olmstead R, et al. Working memory in cigarette smokers: Comparison to non-smokers and effects of abstinence. 2005 doi: 10.1016/j.addbeh.2005.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pineda JA, Herrera C, Kang C, Sandler A. Effects of cigarette smoking and 12-h abstention on working memory during a serial-probe recognition task. Psychopharmacology (Berlin) 1998;139:311–321. doi: 10.1007/s002130050722. [DOI] [PubMed] [Google Scholar]
- Pritchard WS. Electroencephalographic effects of cigarette smoking. Psychopharmacology (Berlin) 1991;104:485–490. doi: 10.1007/BF02245654. [DOI] [PubMed] [Google Scholar]
- Ragland JD, Turetsky BI, Gur RC, Gunning-Dixon F, Turner T, Schroeder L, et al. Working memory for complex figures: An fMRI comparison of letter and fractal n-back tasks. Neuropsychology. 2002;16:370–379. [PMC free article] [PubMed] [Google Scholar]
- Robbins TW. Chemistry of the mind: neurochemical modulation of prefrontal cortical function. J Comp Neurol. 2005;493:140–146. doi: 10.1002/cne.20717. [DOI] [PubMed] [Google Scholar]
- Rose JE, Behm FM, Westman EC, Mathew RJ, London ED, Hawk TC, et al. PET studies of the influences of nicotine on neural systems i cigarette smokers. Am J Psychiatry. 2003;160:323–333. doi: 10.1176/appi.ajp.160.2.323. [DOI] [PubMed] [Google Scholar]
- Rossi S, Singer S, Shearman E, Sershen H, Lajtha A. Regional heterogeneity of nicotine effects on neurotransmitters in rat brains in vivo at low doses. Neurochemical Research. 2005;30:91–103. doi: 10.1007/s11064-004-9690-7. [DOI] [PubMed] [Google Scholar]
- Snyder FR, Davis FC, Henningfield JE. The tobacco withdrawal syndrome: Performance decrements assessed on a computerized test battery. Drug and Alcohol Dependence. 1989;23:259–266. doi: 10.1016/0376-8716(89)90090-2. [DOI] [PubMed] [Google Scholar]
- Snyder FR, Henningfield JE. Effects of nicotine administration following 12 h of tobacco deprivation: Assessment on computerized performance tasks. Psychopharmacology (Berlin) 1989;97:17–22. doi: 10.1007/BF00443406. [DOI] [PubMed] [Google Scholar]
- Xu J, Mendrek A, Cohen MS, Monterosso J, Rodriguez P, Simon S, et al. Brain activity in cigarette smokers performing a working memory task: Effects of smoking. Biol Psychiatry. 2005;58:143–50. doi: 10.1016/j.biopsych.2005.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]


