Abstract
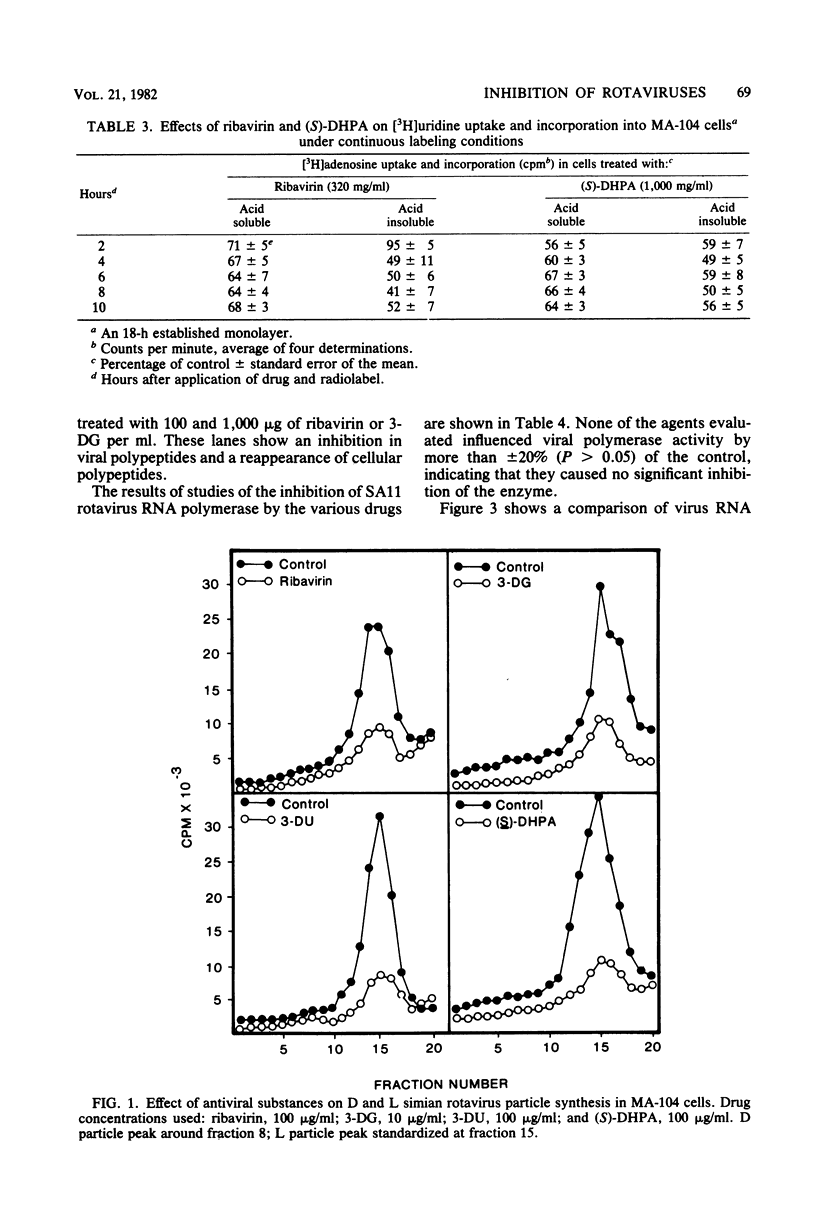
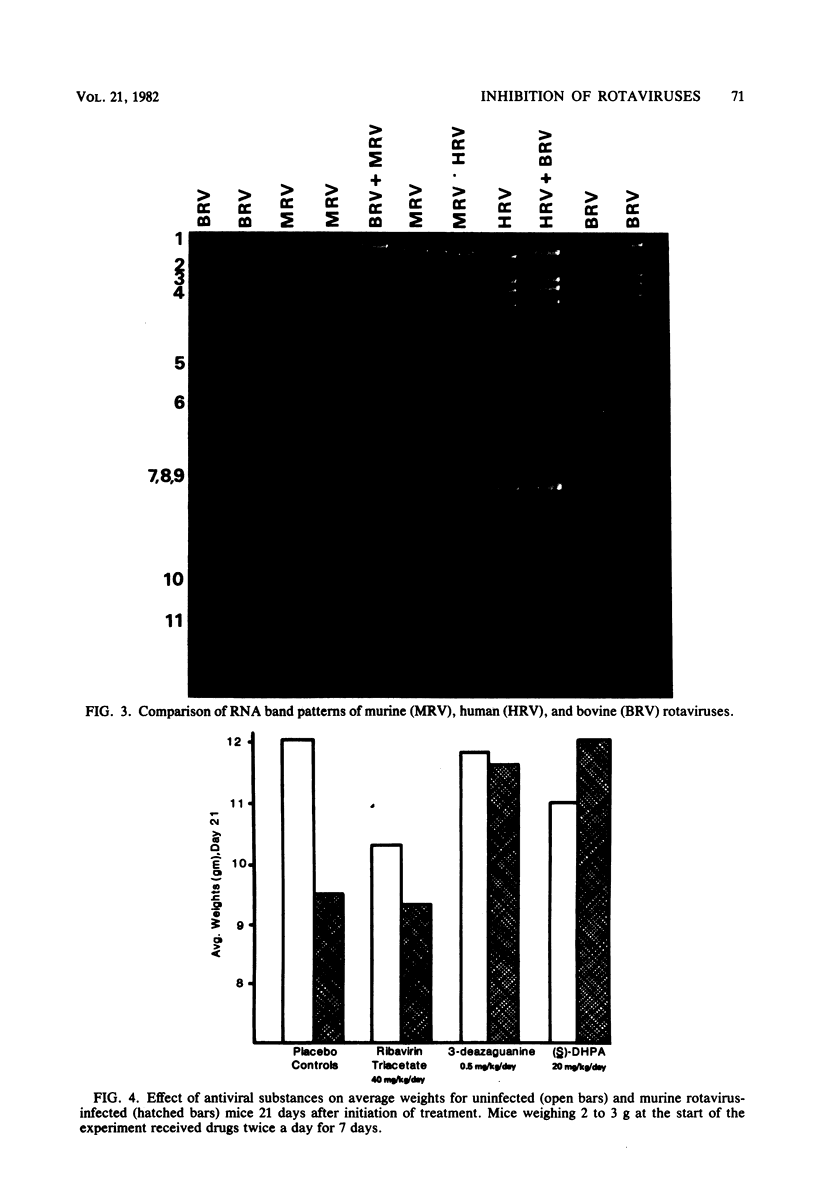
Several RNA virus inhibitors were evaluated against simian (SA11) rotavirus infections in vitro and murine rotavirus gastroenteritis in vivo. Test compounds included 1-beta-D-ribofuranosyl-1,2,4-triazole-3-carboxamide (ribavirin), 3-deazaguanine (3-DG), 3-deazauridine, and 9-(S)-(2,3-dihydroxypropyl)adenine [(S)-DHPA]. All drugs inhibited total infectious SA11 virus yields in MA-104 cells. Ribavirin, 3-DG, and (S)-DHPA affected [3H]uridine uptake into uninfected MA-104 cells in both the acid-soluble and -insoluble fractions. All drugs reduced the levels of dense (precursor) and light (complete) SA11 particle yields compared with control but did not alter the relative amounts of dense compared with light particles, suggesting that the agents did not interfere with virus assembly. Ribavirin and 3-DG inhibited SA11 polypeptide synthesis, as determined by polyacrylamide gel electrophoresis studies. None of the agents or mono- and triphosphate derivatives of ribavirin inhibited SA11 RNA polymerase activity. In murine rotavirus studies, oral therapy with ribavirin-2',3',5'-triacetate and (S)-DHPA increased mean survival time, but no increase in survivor rate was observed. 3-DG- and (S)-DHPA-treated mice had a more rapid weight gain than controls, suggesting a probable lessening of the severity of the disease.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnett B. B., Egbert L. N., Spendlove R. S. Characteristics of neonatal calf diarrhea virus ribonucleic acid. Can J Comp Med. 1978 Jan;42(1):46–53. [PMC free article] [PubMed] [Google Scholar]
- Barnett B. B., Spendlove R. S., Peterson M. W., Hsu L. Y., LaSalle V. A., Egbert L. N. Immunofluorescent cell assay of neonatal calf diarrhea virus. Can J Comp Med. 1975 Oct;39(4):462–465. [PMC free article] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Clark S. M., Spendlove R. S., Barnett B. B. Role of two particle types in bovine rotavirus morphogenesis. J Virol. 1980 Apr;34(1):272–276. doi: 10.1128/jvi.34.1.272-276.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DE Clercq E., Descamps J., DE Somer P., Holyacute A. (S)-9-(2,3-Dihydroxypropyl)adenine: An Aliphatic Nucleoside Analog with Broad-Spectrum Antiviral Activity. Science. 1978 May 5;200(4341):563–565. doi: 10.1126/science.200.4341.563. [DOI] [PubMed] [Google Scholar]
- Espejo R. T., López S., Arias C. Structural polypeptides of simian rotavirus SA11 and the effect of trypsin. J Virol. 1981 Jan;37(1):156–160. doi: 10.1128/jvi.37.1.156-160.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goswami B. B., Borek E., Sharma O. K., Fujitaki J., Smith R. A. The broad spectrum antiviral agent ribavirin inhibits capping of mRNA. Biochem Biophys Res Commun. 1979 Aug 13;89(3):830–836. doi: 10.1016/0006-291x(79)91853-9. [DOI] [PubMed] [Google Scholar]
- Graham D. Y., Estes M. K. Proteolytic enhancement of rotavirus infectivity: biology mechanism. Virology. 1980 Mar;101(2):432–439. doi: 10.1016/0042-6822(80)90456-0. [DOI] [PubMed] [Google Scholar]
- Kalica A. R., Garon C. F., Wyatt R. G., Mebus C. A., van Kirk D. H., Chanock R. M., Kapikian A. Z. Differentiation of human and calf reoviruslike agents associated with diarrhea using polyacrylamide gel electrophoresis of RNA. Virology. 1976 Oct 1;74(1):86–92. doi: 10.1016/0042-6822(76)90131-8. [DOI] [PubMed] [Google Scholar]
- Kapikian A. Z., Yolken R. H., Wyatt R. G., Kalica A. R., Chanock R. M., Kim H. W. Viral diarrhea. Etiology and control. Am J Clin Nutr. 1978 Dec;31(12):2219–2236. doi: 10.1093/ajcn/31.12.2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kára J., Vácha P., Holý A. 9-(S)-(2,3-Dihydroxypropyl)adenine inhibits the transformation of chick embryo fibroblasts infected with Rous sarcoma virus: Evidence for inhibition of enzymatic activity of isolated cellular protein kinases by the drug. FEBS Lett. 1979 Nov 1;107(1):187–192. doi: 10.1016/0014-5793(79)80492-5. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- McClain M. E., Spendlove R. S., Lennette E. H. Infectivity assay of Reoviruses: comparison of immunofluorescent cell count and plaque methods. J Immunol. 1967 Jun;98(6):1301–1308. [PubMed] [Google Scholar]
- McNulty M. S. Rotaviruses. J Gen Virol. 1978 Jul;40(1):1–18. doi: 10.1099/0022-1317-40-1-1. [DOI] [PubMed] [Google Scholar]
- McSharry J. J., Caliguiri L. A., Eggers H. J. Inhibition of uncoating of poliovirus by arildone, a new antiviral drug. Virology. 1979 Sep;97(2):307–315. doi: 10.1016/0042-6822(79)90342-8. [DOI] [PubMed] [Google Scholar]
- Rada B., Dragún M., Votruba I., Holý A. Characteristics of the antiviral effect of 9-(S)-(2, 3-dihydroxypropyl)adenine. Acta Virol. 1980 Dec;24(6):433–438. [PubMed] [Google Scholar]
- Scheline R. R. Metabolism of foreign compounds by gastrointestinal microorganisms. Pharmacol Rev. 1973 Dec;25(4):451–523. [PubMed] [Google Scholar]
- Schoub B. D., Prozesky O. W. Antiviral activity of ribavirin in rotavirus gastroenteritis of mice. Antimicrob Agents Chemother. 1977 Oct;12(4):543–544. doi: 10.1128/aac.12.4.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snodgrass D. R., Fahey K. J., Wells P. W., Campbell I., Whitelaw A. Passive immunity in calf rotavirus infections: maternal vaccination increases and prolongs immunoglobulin G1 antibody secretion in milk. Infect Immun. 1980 May;28(2):344–349. doi: 10.1128/iai.28.2.344-349.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streeter D. G., Koyama H. H. Inhibition of purine nucleotide biosynthesis by 3-deazaguanine, its nucleoside and 5'-nucleotide. Biochem Pharmacol. 1976 Nov 1;25(21):2413–2415. doi: 10.1016/0006-2952(76)90041-1. [DOI] [PubMed] [Google Scholar]
- Streeter D. G., Witkowski J. T., Khare G. P., Sidwell R. W., Bauer R. J., Robins R. K., Simon L. N. Mechanism of action of 1- -D-ribofuranosyl-1,2,4-triazole-3-carboxamide (Virazole), a new broad-spectrum antiviral agent. Proc Natl Acad Sci U S A. 1973 Apr;70(4):1174–1178. doi: 10.1073/pnas.70.4.1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thouless M. E. Rotavirus polypeptides. J Gen Virol. 1979 Jul;44(1):187–197. doi: 10.1099/0022-1317-44-1-187. [DOI] [PubMed] [Google Scholar]
- Wells P. W., Snodgrass D. R., Herring J. A., Dawson A. M. Antibody titres to lamb rotavirus in colostrum and milk of vaccinated ewes. Vet Rec. 1978 Jul 15;103(3):46–48. doi: 10.1136/vr.103.3.46. [DOI] [PubMed] [Google Scholar]




