Abstract
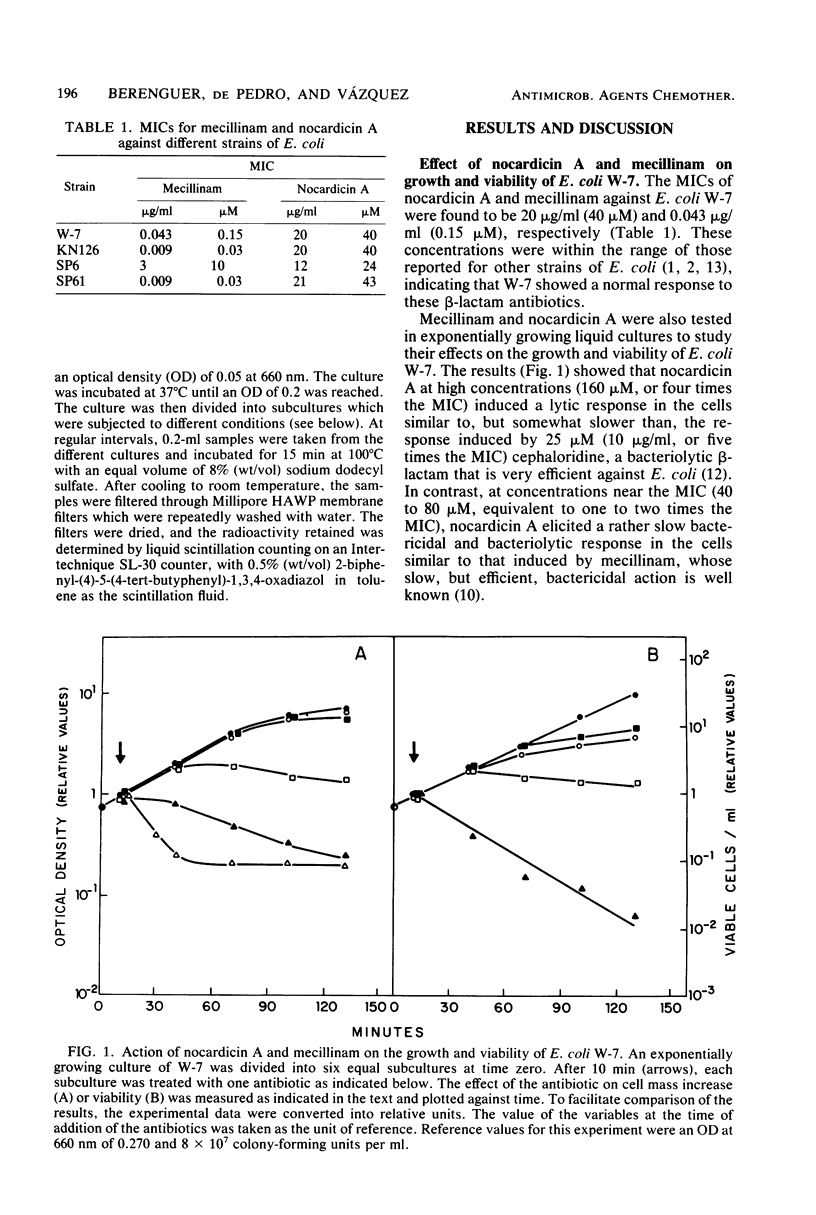
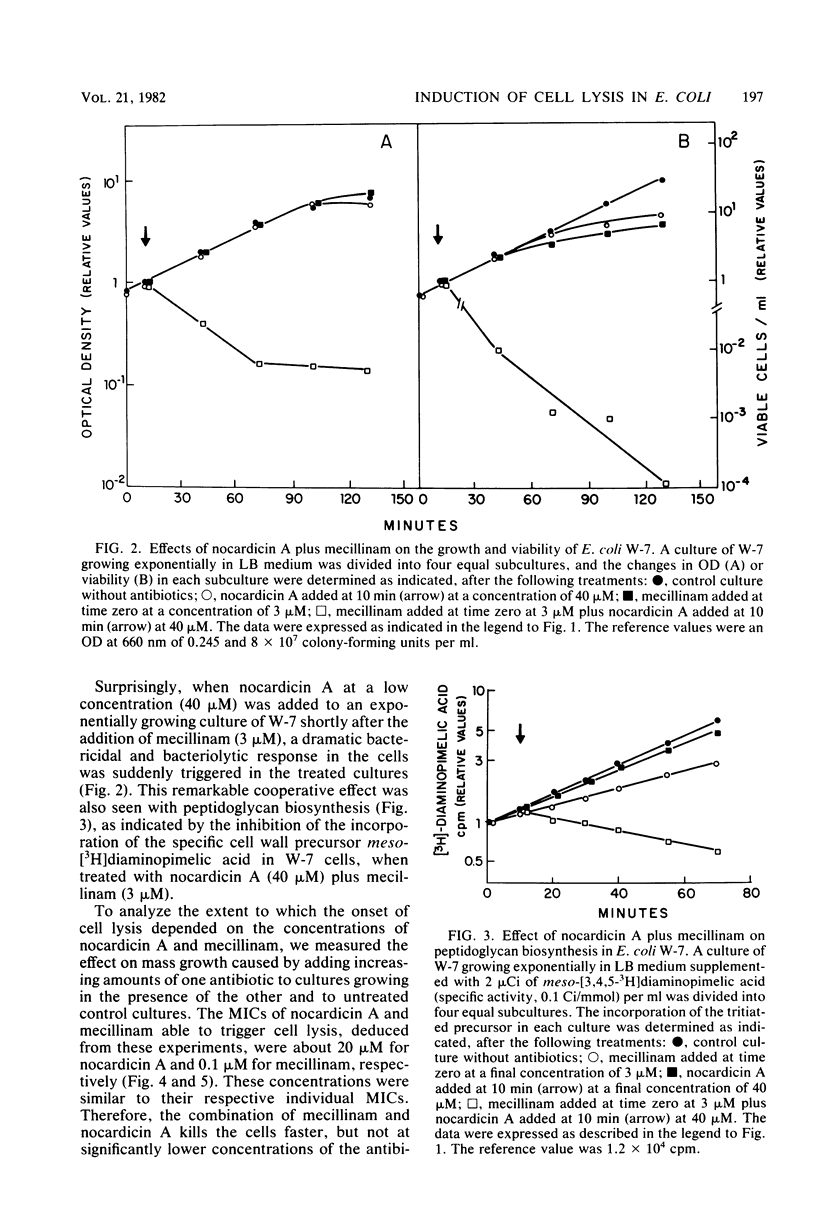
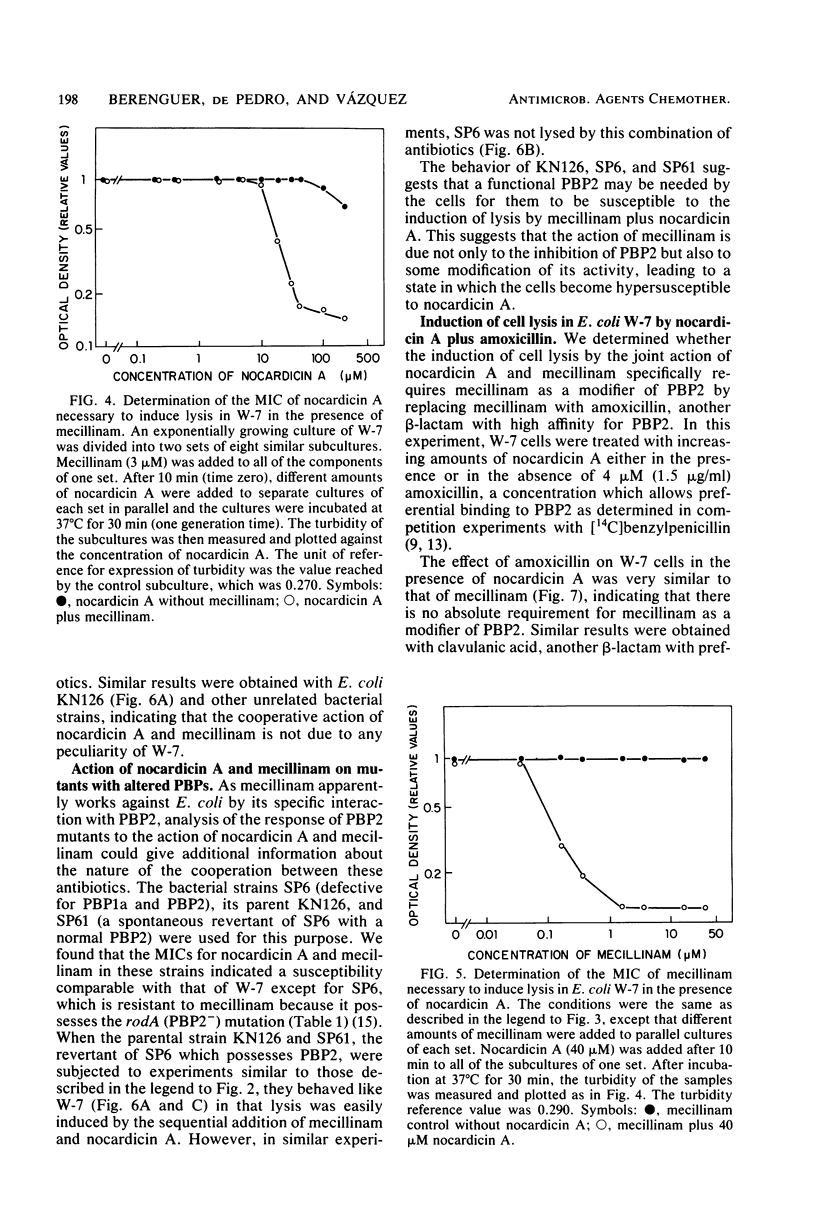
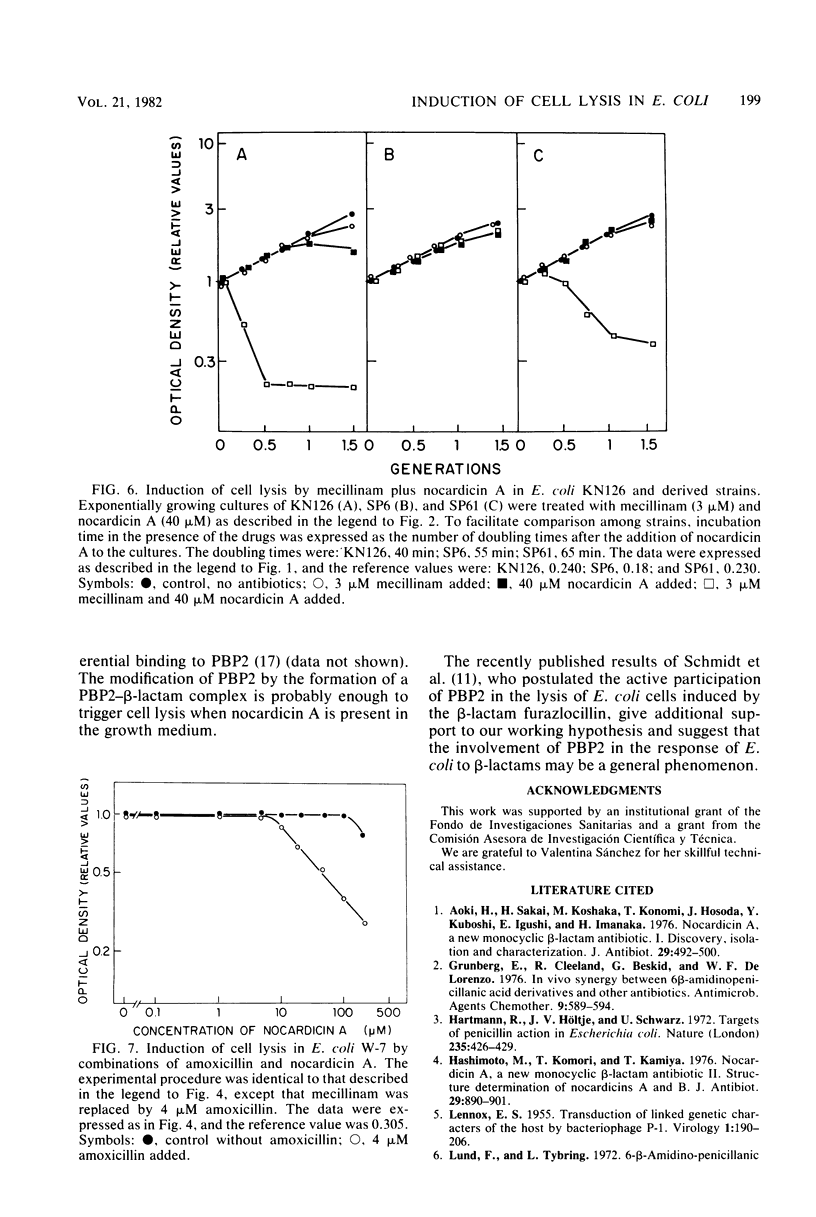
Nocardicin A and mecillinam are two beta-lactam antibiotics with poor bacteriolytic activity against Escherichia coli. However, the combined use of these drugs resulted in the induction of a fast lytic response in E. coli cells. For this cooperative effect to take place, the formation of a complex between penicillin-binding protein 2 and mecillinam is apparently necessary. This suggests that penicillin-binding protein 2 might be actively involved in the response of E. coli to bacteriolytic beta-lactam antibiotics.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aoki H., Sakai H., Kohsaka M., Konomi T., Hosoda J. Nocardicin A, a new monocyclic beta-lactam antibiotic. I. Discovery, isolation and characterization. J Antibiot (Tokyo) 1976 May;29(5):492–500. doi: 10.7164/antibiotics.29.492. [DOI] [PubMed] [Google Scholar]
- Grunberg E., Cleeland R., Beskid G., DeLorenzo W. F. In vivo synergy between 6 beta-amidinopenicillanic acid derivatives and other antibiotics. Antimicrob Agents Chemother. 1976 Apr;9(4):589–594. doi: 10.1128/aac.9.4.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann R., Höltje J. V., Schwarz U. Targets of penicillin action in Escherichia coli. Nature. 1972 Feb 25;235(5339):426–429. doi: 10.1038/235426a0. [DOI] [PubMed] [Google Scholar]
- Hashimoto M., Komori T., Kamiya T. Nocardicin A, a new monocyclic beta-lactam antibiotic II. Structure determination of nocardicins A and B. J Antibiot (Tokyo) 1976 Sep;29(9):890–901. doi: 10.7164/antibiotics.29.890. [DOI] [PubMed] [Google Scholar]
- LENNOX E. S. Transduction of linked genetic characters of the host by bacteriophage P1. Virology. 1955 Jul;1(2):190–206. doi: 10.1016/0042-6822(55)90016-7. [DOI] [PubMed] [Google Scholar]
- Lund F., Tybring L. 6 -amidinopenicillanic acids--a new group of antibiotics. Nat New Biol. 1972 Apr 5;236(66):135–137. doi: 10.1038/newbio236135a0. [DOI] [PubMed] [Google Scholar]
- Neu H. C. Mecillinam, a novel penicillanic acid derivative with unusual activity against gram-negative bacteria. Antimicrob Agents Chemother. 1976 May;9(5):793–799. doi: 10.1128/aac.9.5.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neu J. C. Synergy of mecillinam, a beta-amidinopenicillanic acid derivative, combined with beta-lactam antibiotics. Antimicrob Agents Chemother. 1976 Sep;10(3):535–542. doi: 10.1128/aac.10.3.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguchi H., Matsuhashi M., Mitsuhashi S. Comparative studies of penicillin-binding proteins in Pseudomonas aeruginosa and Escherichia coli. Eur J Biochem. 1979 Oct;100(1):41–49. doi: 10.1111/j.1432-1033.1979.tb02031.x. [DOI] [PubMed] [Google Scholar]
- Park J. T., Burman L. FL-1060: a new penicillin with a unique mode of action. Biochem Biophys Res Commun. 1973 Apr 16;51(4):863–868. doi: 10.1016/0006-291x(73)90006-5. [DOI] [PubMed] [Google Scholar]
- Schmidt L. S., Botta G., Park J. T. Effects of furazlocillin, a beta-lactam antibiotic which binds selectively to penicillin-binding protein 3, on Escherichia coli mutants deficient in other penicillin-binding proteins. J Bacteriol. 1981 Jan;145(1):632–637. doi: 10.1128/jb.145.1.632-637.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spratt B. G. Comparison of the binding properties of two 6 beta-amidinopenicillanic acid derivatives that differ in their physiological effects on Escherichia coli. Antimicrob Agents Chemother. 1977 Jan;11(1):161–166. doi: 10.1128/aac.11.1.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spratt B. G. Distinct penicillin binding proteins involved in the division, elongation, and shape of Escherichia coli K12. Proc Natl Acad Sci U S A. 1975 Aug;72(8):2999–3003. doi: 10.1073/pnas.72.8.2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spratt B. G., Jobanputra V. Mutants of Escherichia coli which lack a component of penicillin-binding protein 1 are viable. FEBS Lett. 1977 Jul 15;79(2):374–378. doi: 10.1016/0014-5793(77)80824-7. [DOI] [PubMed] [Google Scholar]
- Spratt B. G., Jobanputra V., Zimmermann W. Binding of thienamycin and clavulanic acid to the penicillin-binding proteins of Escherichia coli K-12. Antimicrob Agents Chemother. 1977 Sep;12(3):406–409. doi: 10.1128/aac.12.3.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spratt B. G. Properties of the penicillin-binding proteins of Escherichia coli K12,. Eur J Biochem. 1977 Jan;72(2):341–352. doi: 10.1111/j.1432-1033.1977.tb11258.x. [DOI] [PubMed] [Google Scholar]
- Tybring L., Melchior N. H. Mecillinam (FL 1060), a 6beta-amidinopenicillanic acid derivative: bactericidal action and synergy in vitro. Antimicrob Agents Chemother. 1975 Sep;8(3):271–276. doi: 10.1128/aac.8.3.271. [DOI] [PMC free article] [PubMed] [Google Scholar]


