Abstract
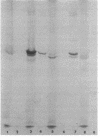
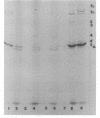
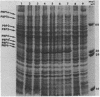
A mutant of Pseudomonas aeruginosa strain PAO503 was isolated after ethane-methane-sulfonate mutagenesis and selection of ticarcillin. The mutant, PCC17, displayed reduced affinity for [14C] penicillin G at all of its penicillin-binding proteins as well as a general increase in resistance to all the beta-lactam antibiotics tested. The mutation designated pbpA has been mapped by FP-2-mediated conjugation and was located distal to the proA locus and 33% linked to it. The two loci were not cotransducible with phage F116L. PCC17 and exconjugants produced from it had similar phenotypes, displayed the reduced affinity for [14C] penicillin G, had similar resistance profiles, and had an increased amount of protein corresponding to penicillin-binding protein 6. On back mutation the pbpA locus reverted to the PAO503 phenotype.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blumberg P. M. Penicillin binding components of bacterial cells and their relationship to the mechanism of penicillin action. Ann N Y Acad Sci. 1974 May 10;235(0):310–325. doi: 10.1111/j.1749-6632.1974.tb43274.x. [DOI] [PubMed] [Google Scholar]
- Blumberg P. M., Strominger J. L. Interaction of penicillin with the bacterial cell: penicillin-binding proteins and penicillin-sensitive enzymes. Bacteriol Rev. 1974 Sep;38(3):291–335. doi: 10.1128/br.38.3.291-335.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis N. A., Brown C., Boxall M., Boulton M. G. Modified peptidoglycan transpeptidase activity in a carbenicillin-resistant mutant of Pseudomonas aeruginosa 18s. Antimicrob Agents Chemother. 1978 Aug;14(2):246–251. doi: 10.1128/aac.14.2.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis N. A., Orr D., Boulton M. G., Ross G. W. Penicillin-binding proteins of Pseudomonas aeruginosa. Comparison of two strains differing in their resistance to beta-lactam antibiotics. J Antimicrob Chemother. 1981 Feb;7(2):127–136. doi: 10.1093/jac/7.2.127. [DOI] [PubMed] [Google Scholar]
- Georgopapadakou N. H., Liu F. Y. Penicillin-binding proteins in bacteria. Antimicrob Agents Chemother. 1980 Jul;18(1):148–157. doi: 10.1128/aac.18.1.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godfrey A. J., Bryan L. E., Rabin H. R. beta-Lactam-resistant Pseudomonas aeruginosa with modified penicillin-binding proteins emerging during cystic fibrosis treatment. Antimicrob Agents Chemother. 1981 May;19(5):705–711. doi: 10.1128/aac.19.5.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutmann L., Williamson R., Tomasz A. Physiological properties of penicillin-binding proteins in group A streptococci. Antimicrob Agents Chemother. 1981 May;19(5):872–880. doi: 10.1128/aac.19.5.872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakenbeck R., Tarpay M., Tomasz A. Multiple changes of penicillin-binding proteins in penicillin-resistant clinical isolates of Streptococcus pneumoniae. Antimicrob Agents Chemother. 1980 Mar;17(3):364–371. doi: 10.1128/aac.17.3.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holloway B. W. Genetics of Pseudomonas. Bacteriol Rev. 1969 Sep;33(3):419–443. doi: 10.1128/br.33.3.419-443.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen-Distèche M., Ghuysen J. M., Pollock J. J., Reynolds P., Perkins H. R., Coyette J., Salton M. R. Enzymes involved in wall peptide crosslinking in Escherichia coli K12, strain 44. Eur J Biochem. 1974 Feb 1;41(3):447–455. doi: 10.1111/j.1432-1033.1974.tb03286.x. [DOI] [PubMed] [Google Scholar]
- Nguyen-Distèche M., Pollock J. J., Ghuysen J. M., Puig J., Reynolds P., Perkins H. R., Coyette J., Salton M. R. Sensitivity to ampicillin and cephalothin of enzymes involved in wall peptide crosslinking in Escherichia coli K12, strain 44. Eur J Biochem. 1974 Feb 1;41(3):457–463. doi: 10.1111/j.1432-1033.1974.tb03287.x. [DOI] [PubMed] [Google Scholar]
- Noguchi H., Matsuhashi M., Mitsuhashi S. Comparative studies of penicillin-binding proteins in Pseudomonas aeruginosa and Escherichia coli. Eur J Biochem. 1979 Oct;100(1):41–49. doi: 10.1111/j.1432-1033.1979.tb02031.x. [DOI] [PubMed] [Google Scholar]
- Ohya S., Yamazaki M., Sugawara S., Matsuhashi M. Penicillin-binding proteins in Proteus species. J Bacteriol. 1979 Jan;137(1):474–479. doi: 10.1128/jb.137.1.474-479.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Percheson P. B., Bryan L. E. Penicillin-binding components of penicillin-susceptible and -resistant strains of Streptococcus pneumoniae. Antimicrob Agents Chemother. 1980 Sep;18(3):390–396. doi: 10.1128/aac.18.3.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royle P. L., Matsumoto H., Holloway B. W. Genetic circularity of the Pseudomonas aeruginosa PAO chromosome. J Bacteriol. 1981 Jan;145(1):145–155. doi: 10.1128/jb.145.1.145-155.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabath L. D., Jago M., Abraham E. P. Cephalosporinase and penicillinase activities of a beta-lactamase from Pseudomonas pyocyanea. Biochem J. 1965 Sep;96(3):739–752. doi: 10.1042/bj0960739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd S. T., Chase H. A., Reynolds P. E. The separation and properties of two penicillin-binding proteins from Salmonella typhimurium. Eur J Biochem. 1977 Sep;78(2):521–523. doi: 10.1111/j.1432-1033.1977.tb11765.x. [DOI] [PubMed] [Google Scholar]
- Spratt B. G. Distinct penicillin binding proteins involved in the division, elongation, and shape of Escherichia coli K12. Proc Natl Acad Sci U S A. 1975 Aug;72(8):2999–3003. doi: 10.1073/pnas.72.8.2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spratt B. G. Properties of the penicillin-binding proteins of Escherichia coli K12,. Eur J Biochem. 1977 Jan;72(2):341–352. doi: 10.1111/j.1432-1033.1977.tb11258.x. [DOI] [PubMed] [Google Scholar]
- Stanisich V., Holloway B. W. Conjugation in Pseudomonas aeruginosa. Genetics. 1969 Feb;61(2):327–339. doi: 10.1093/genetics/61.2.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suginaka H., Ichikawa A., Kotani S. Penicillin-resistant mechanisms in Pseudomonas aeruginosa: binding of penicillin to Pseudomonas aeruginosa KM 338. Antimicrob Agents Chemother. 1975 May;7(5):629–635. doi: 10.1128/aac.7.5.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann W. Penetration of beta-lactam antibiotics into their target enzymes in Pseudomonas aeruginosa: comparison of a highly sensitive mutant with its parent strain. Antimicrob Agents Chemother. 1980 Jul;18(1):94–100. doi: 10.1128/aac.18.1.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann W., Rosselet A. Function of the outer membrane of Escherichia coli as a permeability barrier to beta-lactam antibiotics. Antimicrob Agents Chemother. 1977 Sep;12(3):368–372. doi: 10.1128/aac.12.3.368. [DOI] [PMC free article] [PubMed] [Google Scholar]