Abstract
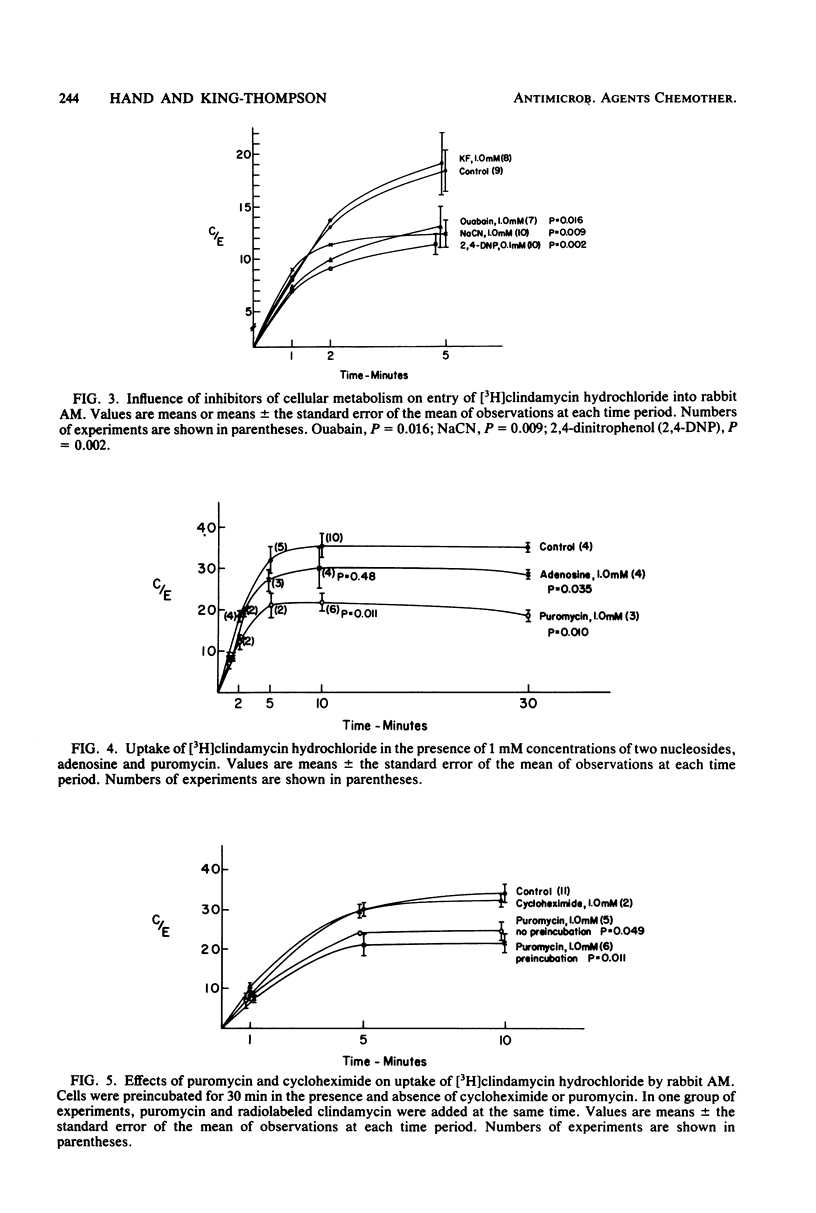
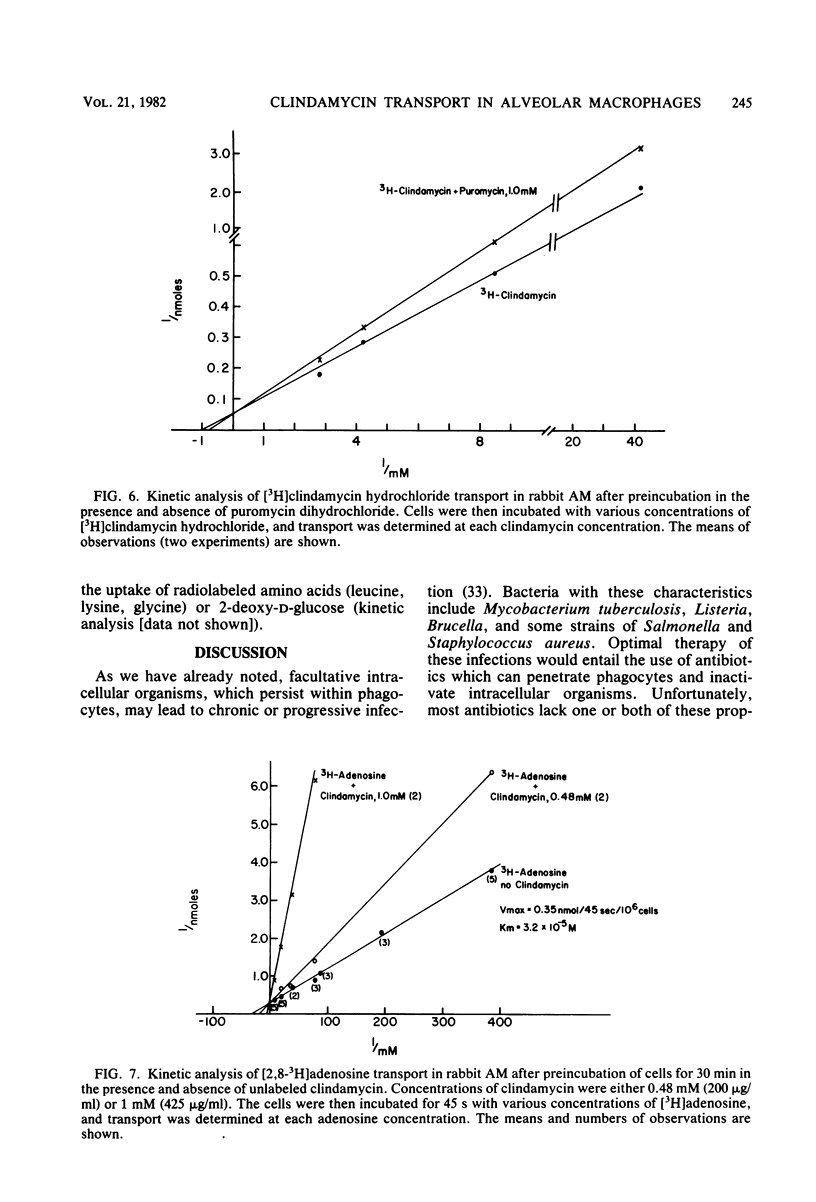
The use of antibiotics which can penetrate phagocytic cells and kill intracellular organisms is desirable in the treatment of chronic facultative bacterial infections. Recently, we reported that several antibiotics were selectively concentrated by rabbit alveolar macrophages. Clindamycin accumulation was especially marked. In the present study we evaluated the plasma membrane transport (initial uptake) of clindamycin in alveolar macrophages. The transport of clindamycin is an active process, as documented by requirements for cellular viability, elevated environmental temperature, metabolic energy, and establishment of the 40- to 50-fold cellular/extracellular gradient. Energy for membrane transport of the drug depended at least in part upon mitochondrial oxidative respiration and cell membrane Na-K pump activity. Kinetic analysis of active clindamycin transport revealed it to be saturable, with a high binding affinity (Km = 1 mM) and a high velocity of uptake (Vmax = 15.8 nmol/45 s per 10(6) cells). Clindamycin uptake was not influenced by the presence of hexose or amino acids, but was inhibited by nucleosides (adenosine, puromycin). Decreased clindamycin transport in the presence of puromycin was typical of competitive inhibition (increased Km, unchanged Vmax). Conversely, competitive inhibition of adenosine transport by clindamycin was documented. Thus, clindamycin is transported into alveolar macrophages via the nucleoside system. The potential biological consequences of this unique antibiotic transport mechanism are of interest.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander J. W., Good R. A. Effect of antibiotics on the bactericidal activity of human leukocytes. J Lab Clin Med. 1968 Jun;71(6):971–983. [PubMed] [Google Scholar]
- Berlin R. D. Temperature dependence of nucleoside membrane transport of rabbit alveolar macrophages and polymorphonuclear leukocytes. J Biol Chem. 1973 Jul 10;248(13):4724–4730. [PubMed] [Google Scholar]
- Bonventre P. F., Imhoff J. G. Uptake of h-dihydrostreptomycin by macrophages in culture. Infect Immun. 1970 Jul;2(1):89–95. doi: 10.1128/iai.2.1.89-95.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonventre P. F., Mukkada A. J. Augmentation of glucose transport in macrophages after particle ingestion. Infect Immun. 1974 Dec;10(6):1391–1396. doi: 10.1128/iai.10.6.1391-1396.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonventre P. F., Straus D., Baughn R. E., Imhoff J. Enhancement of carrier-mediated transport after immunologic activation of peritoneal macrophages. J Immunol. 1977 May;118(5):1827–1835. [PubMed] [Google Scholar]
- Cantey J. R., Hand W. L. Cell-mediated immunity after bacterial infection of the lower respiratory tract. J Clin Invest. 1974 Nov;54(5):1125–1134. doi: 10.1172/JCI107856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Easmon C. S. The effect of antibiotics on the intracellular survival of Staphylococcus aureus in vitro. Br J Exp Pathol. 1979 Feb;60(1):24–28. [PMC free article] [PubMed] [Google Scholar]
- Ezer G., Soothill J. F. Intracellular bactericidal effects of rifampicin in both normal and chronic ganulomatous disease polymorphs. Arch Dis Child. 1974 Jun;49(6):463–466. doi: 10.1136/adc.49.6.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes B., Quie P. G., Windhorst D. B., Pollara B., Good R. A. Protection of phagocytized bacteria from the killing action of antibiotics. Nature. 1966 Jun 11;210(5041):1131–1132. doi: 10.1038/2101131a0. [DOI] [PubMed] [Google Scholar]
- Johnson J. D., Hand W. L., Francis J. B., King-Thompson N., Corwin R. W. Antibiotic uptake by alveolar macrophages. J Lab Clin Med. 1980 Mar;95(3):429–439. [PubMed] [Google Scholar]
- Lobo M. C., Mandell G. L. The effect of antibiotics on Escherichia coli ingested by macrophages. Proc Soc Exp Biol Med. 1973 Mar;142(3):1048–1050. doi: 10.3181/00379727-142-37173. [DOI] [PubMed] [Google Scholar]
- MACKANESS G. B. The action of drugs on intracellular tubercle bacilli. J Pathol Bacteriol. 1952 Jul;64(3):429–446. doi: 10.1002/path.1700640302. [DOI] [PubMed] [Google Scholar]
- MYRVIK Q., LEAKE E. S., FARISS B. Studies on pulmonary alveolar macrophages from the normal rabbit: a technique to procure them in a high state of purity. J Immunol. 1961 Feb;86:128–132. [PubMed] [Google Scholar]
- Mandell G. L. Interaction of intraleukocytic bacteria and antibiotics. J Clin Invest. 1973 Jul;52(7):1673–1679. doi: 10.1172/JCI107348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandell G. L., Vest T. K. Killing of intraleukocytic Staphylococcus aureus by rifampin: in-vitro and in-vivo studies. J Infect Dis. 1972 May;125(5):486–490. doi: 10.1093/infdis/125.5.486. [DOI] [PubMed] [Google Scholar]
- Norton J. M., Munck A. Glucose transport in murine macrophages: in vitro characterization of the monosaccharide transport system of the thioglycollate-elicited mouse peritoneal macrophage. J Immunol. 1980 Jul;125(1):252–258. [PubMed] [Google Scholar]
- Pesanti E. L. Protection of staphylococci ingested by macrophages from the bactericidal action of rifampin. Antimicrob Agents Chemother. 1980 Jul;18(1):208–209. doi: 10.1128/aac.18.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes M. W., Hsu H. S. Effect of kanamycin on the fate of Salmonella enteritidis within cultured macrophages of guinea pigs. J Reticuloendothel Soc. 1974 Jan;15(1):1–12. [PubMed] [Google Scholar]
- Robin E. D., Smith J. D., Tanser A. R., Adamson J. S., Millen J. E., Packer B. Ion and macromolecular transport in the alveolar macrophage. Biochim Biophys Acta. 1971 Jul 6;241(1):117–128. doi: 10.1016/0005-2736(71)90310-5. [DOI] [PubMed] [Google Scholar]
- SHAFFER J. M., KUCERA C. J., SPINK W. W. The protection of intracellular brucella against therapeutic agents and the bactericidal action of serum. J Exp Med. 1953 Jan;97(1):77–90. doi: 10.1084/jem.97.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SUTER E. Multiplication of tubercle bacilli within phagocytes cultivated in vitro, and effect of streptomycin and isonicotinic acid hydrazide. Am Rev Tuberc. 1952 Jun;65(6):775–776. doi: 10.1164/art.1952.65.6.775. [DOI] [PubMed] [Google Scholar]
- Solberg C. O. Protection of phagocytized bacteria against antibiotics. A new method for the evaluation of neutrophil granulocyte functions. Acta Med Scand. 1972 May;191(5):383–387. [PubMed] [Google Scholar]
- Straus D. C., Imhoff J. G., Bonventre P. F. Membrane transport by guinea pig peritoneal exudate leukocytes: effect of phagocytosis on hexose and amino acid transport. J Cell Physiol. 1977 Oct;93(1):105–116. doi: 10.1002/jcp.1040930114. [DOI] [PubMed] [Google Scholar]
- Straus D. C., Imhoff J., Bonventre P. F. Membrane transport of amino acids and hexose by guinea pig and mouse phagocytes. J Reticuloendothel Soc. 1977 Nov;22(5):403–416. [PubMed] [Google Scholar]
- Strauss P. R., Berlin R. D. Effects of serum on membrane transport. I. Separation and preliminary characterization of factors which depress lysine or stimulate adenosine transport in rabbit alveolar macrophages. J Exp Med. 1973 Feb 1;137(2):359–368. doi: 10.1084/jem.137.2.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsan M. F., Berlin R. D. Effect of phagocytosis on membrane transport of nonelectrolytes. J Exp Med. 1971 Oct 1;134(4):1016–1035. doi: 10.1084/jem.134.4.1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsan M. F., Berlin R. D. Membrane transport in the rabbit alveolar macrophage. The specificity and characteristics of amino acid transport systems. Biochim Biophys Acta. 1971 Jul 6;241(1):155–169. doi: 10.1016/0005-2736(71)90313-0. [DOI] [PubMed] [Google Scholar]
- Tsan M. F. Effect of phagocytosis by human polymorphonuclear leukocytes and rabbit alveolar macrophages on 2-deoxyglucose transport. J Cell Physiol. 1979 Apr;99(1):23–30. doi: 10.1002/jcp.1040990104. [DOI] [PubMed] [Google Scholar]
- Ukena T. E., Berlin R. D. Effect of colchicine and vinblastine on the topographical separation of membrane functions. J Exp Med. 1972 Jul 1;136(1):1–7. doi: 10.1084/jem.136.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaudaux P., Waldvogel F. A. Gentamicin antibacterial activity in the presence of human polymorphonuclear leukocytes. Antimicrob Agents Chemother. 1979 Dec;16(6):743–749. doi: 10.1128/aac.16.6.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuckerman S. H., Douglas S. D. Dynamics of the macrophage plasma membrane. Annu Rev Microbiol. 1979;33:267–307. doi: 10.1146/annurev.mi.33.100179.001411. [DOI] [PubMed] [Google Scholar]


