Abstract
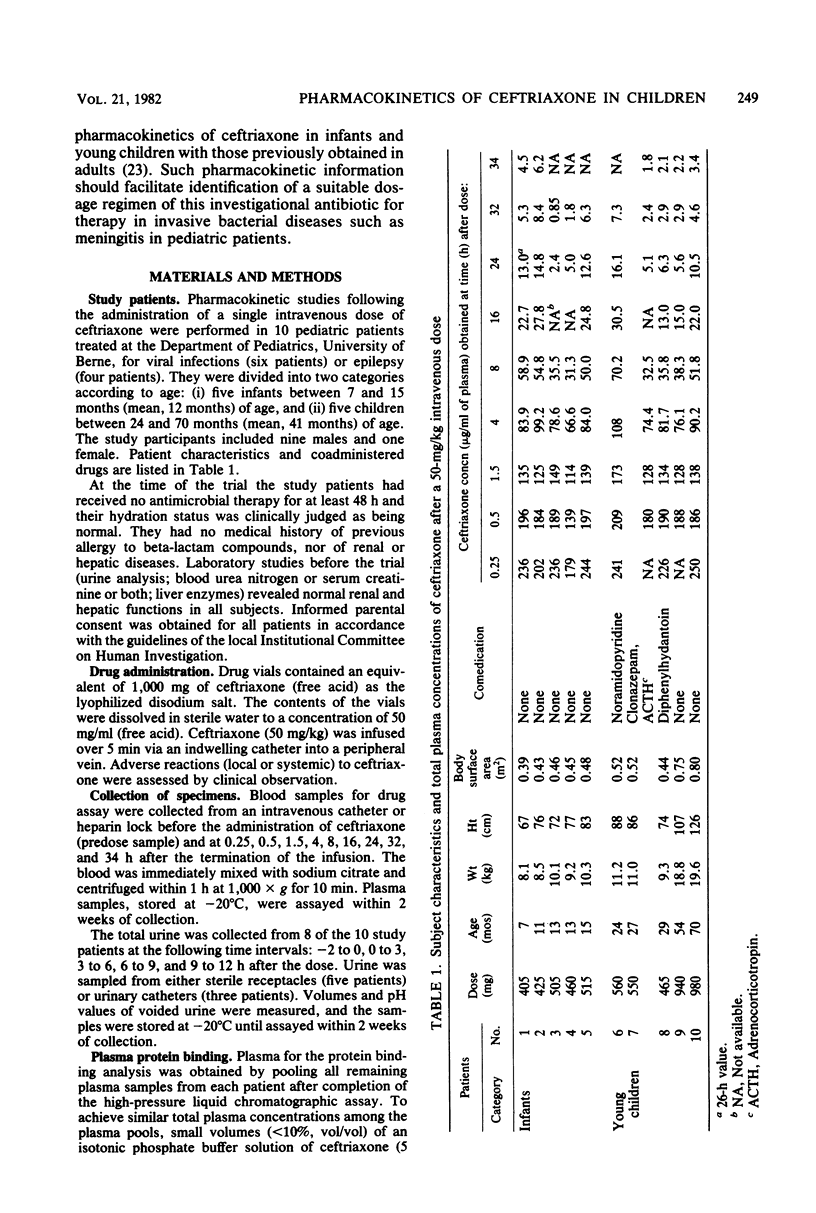
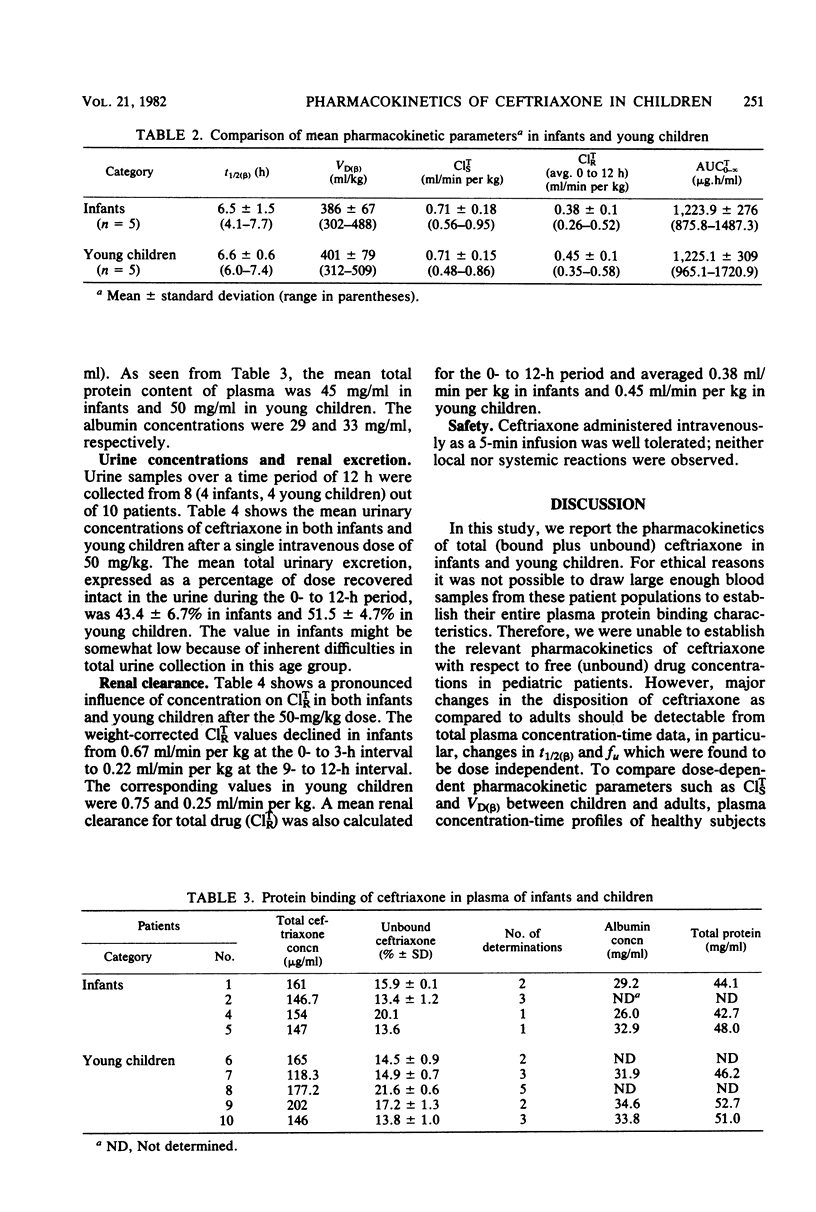
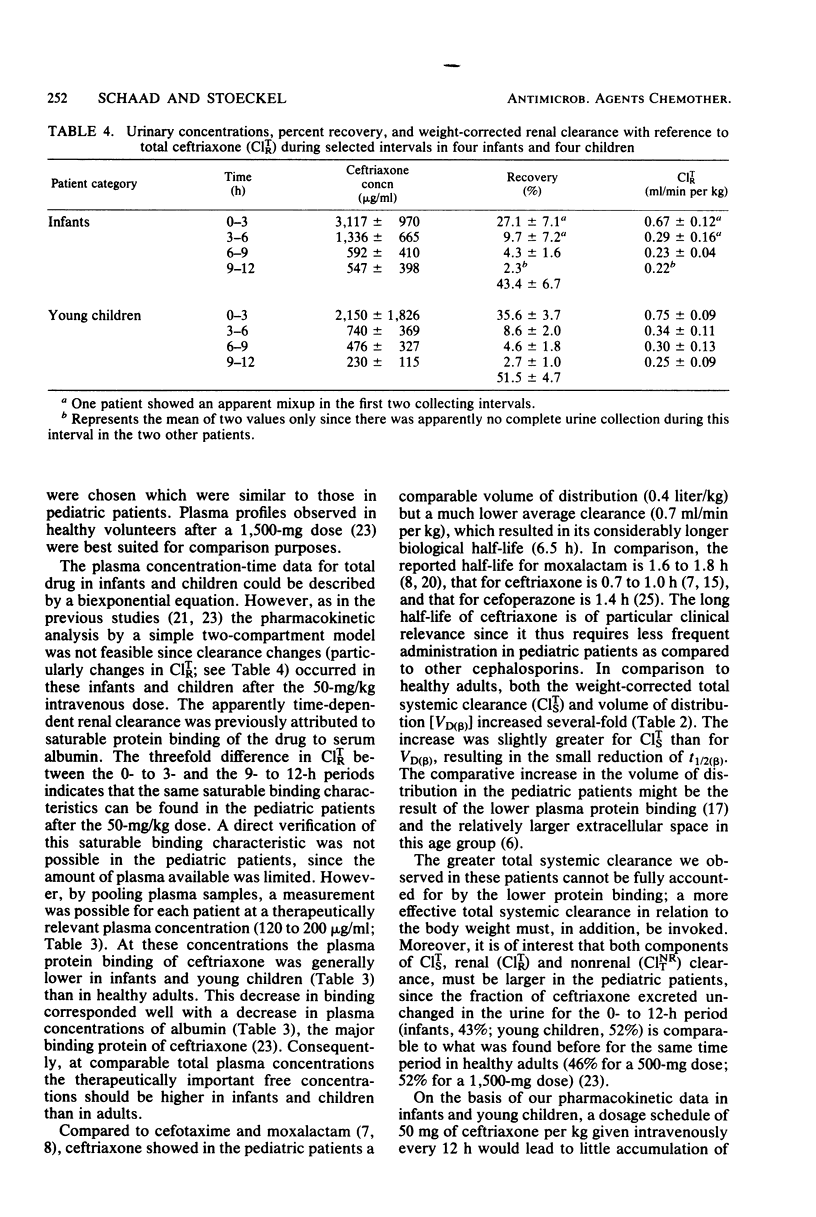
The pharmacokinetics of ceftriaxone were studied in five infants (7 to 15 months old) and five young children (24 to 70 months old). Both groups received a single 50-mg/kg dose in an intravenous infusion over 5 min. No major pharmacokinetic differences were observed between the two populations. The total (bound plus unbound) plasma concentration-versus-time data could be described in each case by a biexponential equation. Changes in renal clearance indicated time- and dose- dependent pharmacokinetic behavior. The fraction excreted unchanged in the urine (fu) and the biological half-life (t 1/2 (beta)) were, however, dose independent. The average values were 47% for fu (0 to 12 h) and 6.5 for T 1/2 (beta). Weight-corrected total systemic clearance was C1TS = 0.71 ml/min per kg; volume of distribution was VD (beta) = 394 mg/kg. The data support intravenous administration of 50 mg of ceftriaxone per kg of body weight every 12 h in assessing its activity against Haemophilus influenzae, Streptococcus pneumoniae, and Neisseria meningitidis in postneonatal-stage pediatric patients.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahronheim G. A., Reich B., Marks M. I. Penicillin-insensitive pneumococci. Case report and review. Am J Dis Child. 1979 Feb;133(2):187–191. doi: 10.1001/archpedi.1979.02130020079017. [DOI] [PubMed] [Google Scholar]
- Anderson K. C., Maurer M. J., Dajani A. S. Pneumococci relatively resistant to penicillin: a prevalence survey in children. J Pediatr. 1980 Dec;97(6):939–941. doi: 10.1016/s0022-3476(80)80426-4. [DOI] [PubMed] [Google Scholar]
- Cadoz M., Denis F., Félix H., Diop Mar I. Treatment of purulent meningitis with a new cephalosporin-Rocephin (Ro 13-9904). Clinical, bacteriological and pharmacological observations in 24 cases. Chemotherapy. 1981;27 (Suppl 1):57–61. doi: 10.1159/000238030. [DOI] [PubMed] [Google Scholar]
- Eickhoff T. C., Ehret J. Comparative in vitro studies of Ro 13-9904, a new cephalosporin derivative. Antimicrob Agents Chemother. 1981 Mar;19(3):435–442. doi: 10.1128/aac.19.3.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRIIS-HANSEN B. The extracellular fluid volume in infants and children. Acta Paediatr. 1954 Sep;43(5):444–458. doi: 10.1111/j.1651-2227.1954.tb04052.x. [DOI] [PubMed] [Google Scholar]
- Feldman W. E., Zweighaft T. Effect of ampicillin and chloramphenicol against Streptococcus pneumoniae and Neisseria meningitidis. Antimicrob Agents Chemother. 1979 Feb;15(2):240–242. doi: 10.1128/aac.15.2.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan S. L., Mason E. O., Jr, Garcia H., Kvernland S. J., Loiselle E. M., Anderson D. C., Mintz A. A., Feigin R. D. Pharmacokinetics and cerebrospinal fluid penetration of moxalactam in children with bacterial meningitis. J Pediatr. 1981 Jan;98(1):152–157. doi: 10.1016/s0022-3476(81)80562-8. [DOI] [PubMed] [Google Scholar]
- Kenny J. F., Isburg C. D., Michaels R. H. Meningitis due to Haemophilus influenzae type b resistant to both ampicillin and chloramphenicol. Pediatrics. 1980 Jul;66(1):14–16. [PubMed] [Google Scholar]
- Marchou B., Tran Van Tho, Armengaud M. Diffusion of ceftriaxone (Ro 13-9004/001) in the cerebrospinal fluid. Comparison with other beta-lactam antibiotics in dogs with healthy meninges and in dogs with experimental meningitis. Chemotherapy. 1981;27 (Suppl 1):37–41. doi: 10.1159/000238027. [DOI] [PubMed] [Google Scholar]
- Meissner H. C., Smith A. L. The current status of chloramphenicol. Pediatrics. 1979 Sep;64(3):348–356. [PubMed] [Google Scholar]
- Nelson J. D. The increasing frequency of beta-lactamase-producing Haemophilus influenzae B. JAMA. 1980 Jul 18;244(3):239–239. [PubMed] [Google Scholar]
- Neu H. C., Meropol N. J., Fu K. P. Antibacterial activity of ceftriaxone (Ro 13-9904), a beta-lactamase-stable cephalosporin. Antimicrob Agents Chemother. 1981 Mar;19(3):414–423. doi: 10.1128/aac.19.3.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadatos C. J., Kafetzis D. A., Kanarios J. Cefotaxime in the treatment of severe paediatric infections. J Antimicrob Chemother. 1980 Sep;6 (Suppl A):243–248. doi: 10.1093/jac/6.suppl_a.243. [DOI] [PubMed] [Google Scholar]
- Patel I. H., Miller K., Weinfeld R., Spicehandler J. Multiple intravenous dose pharmacokinetics of ceftriaxone in man. Chemotherapy. 1981;27 (Suppl 1):47–56. doi: 10.1159/000238029. [DOI] [PubMed] [Google Scholar]
- Sack C. M., Koup J. R., Smith A. L. Chloramphenicol pharmacokinetics in infants and young children. Pediatrics. 1980 Oct;66(4):579–584. [PubMed] [Google Scholar]
- Schaad U. B., McCracken G. H., Jr, Loock C. A., Thomas M. L. Pharmacokinetics and bacteriologic efficacy of moxalactam, cefotaxime, cefoperazone, and rocephin in experimental bacterial meningitis. J Infect Dis. 1981 Feb;143(2):156–163. doi: 10.1093/infdis/143.2.156. [DOI] [PubMed] [Google Scholar]
- Schaad U. B., McCracken G. H., Jr, Threlkeld N., Thomas M. L. Clinical evaluation of a new broad-spectrum oxa-beta-lactam antibiotic, moxalactam, in neonates and infants. J Pediatr. 1981 Jan;98(1):129–136. doi: 10.1016/s0022-3476(81)80559-8. [DOI] [PubMed] [Google Scholar]
- Seddon M., Wise R., Gillett A. P., Livingston R. Pharmacokinetics of Ro 13-9904, a broad-spectrum cephalosporin. Antimicrob Agents Chemother. 1980 Aug;18(2):240–242. doi: 10.1128/aac.18.2.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelton S., Nelson J. D., McCracken G. H., Jr In vitro susceptibility of gram-negative bacilli from pediatric patients to moxalactam, cefotaxime, Ro 13-9904, and other cephalosporins. Antimicrob Agents Chemother. 1980 Sep;18(3):476–479. doi: 10.1128/aac.18.3.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoeckel K., McNamara P. J., Brandt R., Plozza-Nottebrock H., Ziegler W. H. Effects of concentration-dependent plasma protein binding on ceftriaxone kinetics. Clin Pharmacol Ther. 1981 May;29(5):650–657. doi: 10.1038/clpt.1981.90. [DOI] [PubMed] [Google Scholar]
- Syriopoulou V., Scheifele D., Smith A. L., Perry P. M., Howie V. Increasing incidence of ampicillin resistance in Hemophilus influenzae. J Pediatr. 1978 Jun;92(6):889–892. doi: 10.1016/s0022-3476(78)80354-0. [DOI] [PubMed] [Google Scholar]
- Toyonaga Y., Kurosu Y., Yoshikawa H., Kumagai K., Takahashi T., Hori M. [Experimental and clinical studies on cefoperazone (author's transl)]. Jpn J Antibiot. 1980 Aug;33(8):820–840. [PubMed] [Google Scholar]
- Uchiyama N., Greene G. R., Kitts D. B., Thrupp L. D. Meningitis due to Haemophilus influenzae type b resistant to ampicillin and chloramphenicol. J Pediatr. 1980 Sep;97(3):421–424. doi: 10.1016/s0022-3476(80)80193-4. [DOI] [PubMed] [Google Scholar]
- Verbist L., Verhaegen J. In vitro activity of Ro 13-9904, a new beta-lactamase-stable cephalosporin. Antimicrob Agents Chemother. 1981 Feb;19(2):222–225. doi: 10.1128/aac.19.2.222. [DOI] [PMC free article] [PubMed] [Google Scholar]



