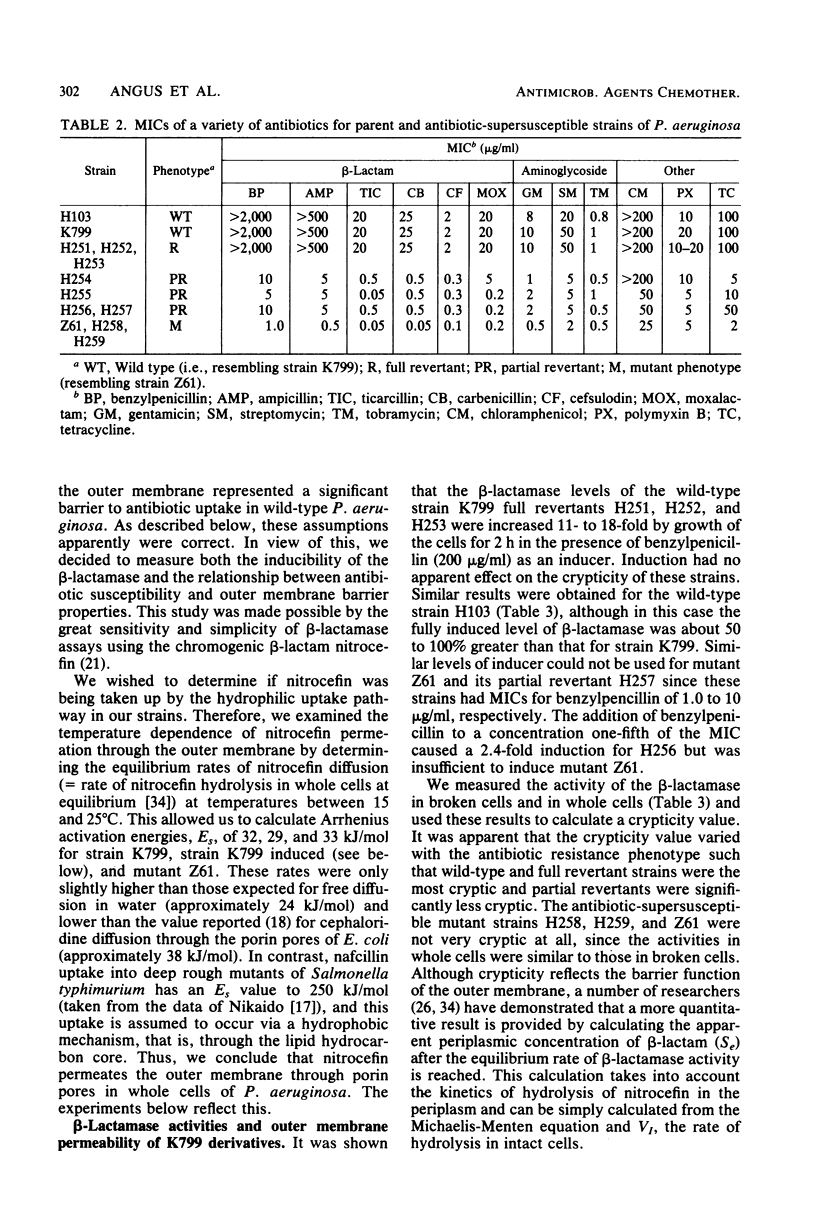
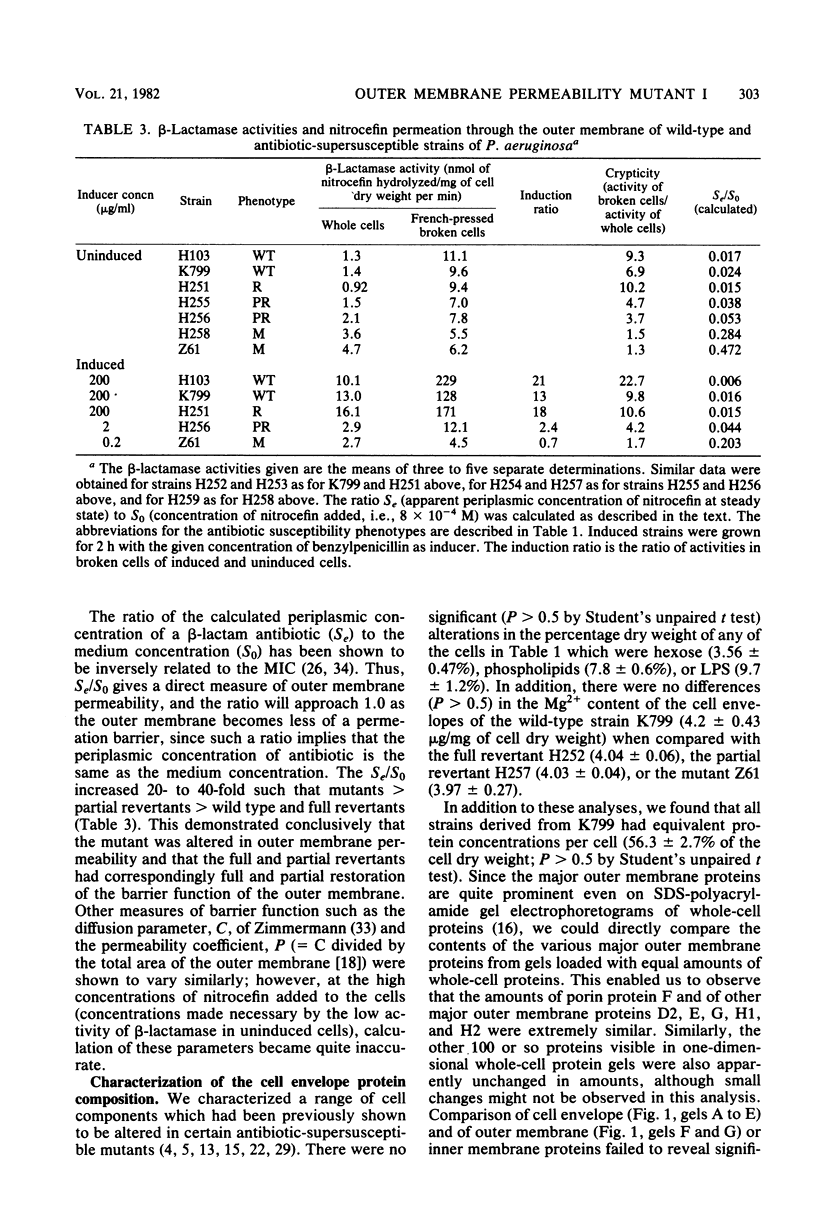
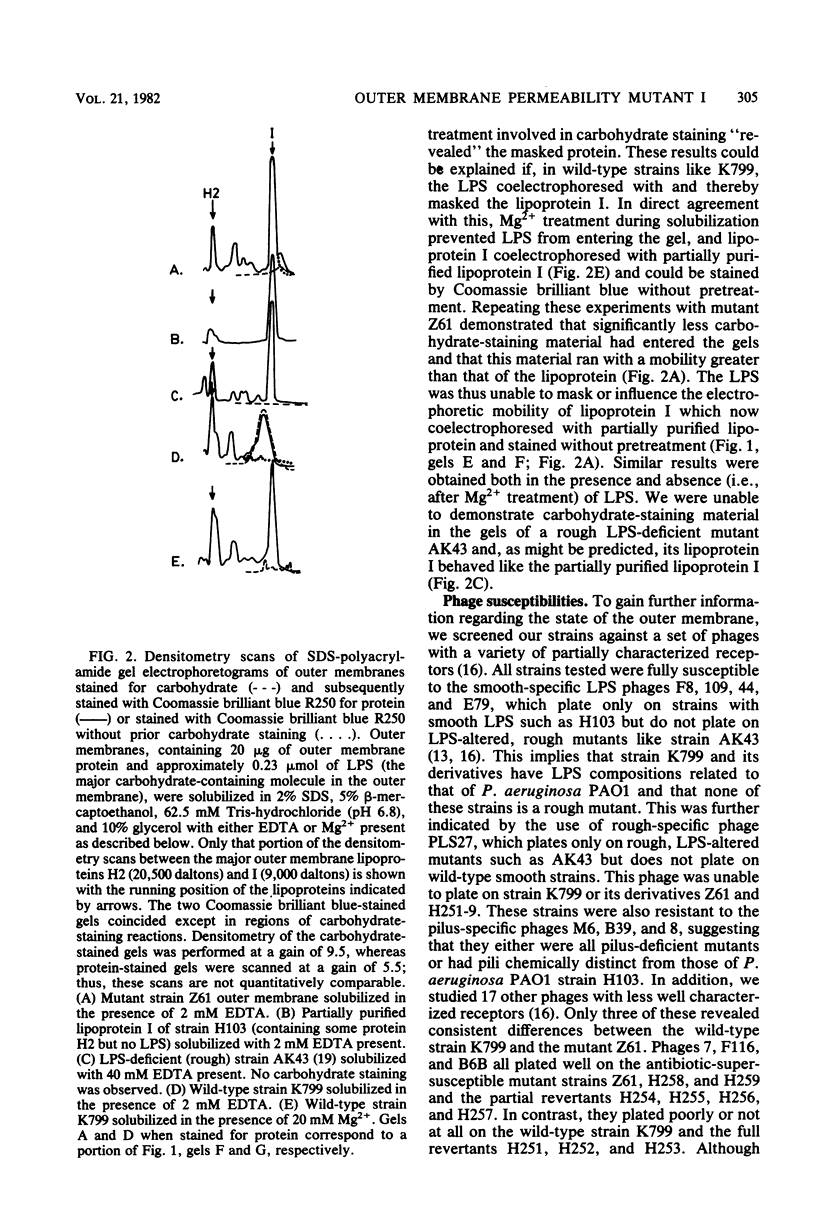
Abstract
The Pseudomonas aeruginosa mutant Z61 has been shown to be highly supersusceptible to a wide range of antibiotics, including beta-lactams, aminoglycosides, rifampin, tetracycline, and chloramphenicol (W. Zimmerman, Int. J. Clin. Pharmacol. Biopharm. 17:131-134, 1979). Spontaneous revertants were isolated, using gentamicin or carbenicillin as selective agents, and shown to have two patterns of susceptibility to a group of 12 antibiotics. Partial revertants had 2- to 10-fold greater resistance to these antibiotics than mutant Z61, whereas full revertants had antibiotic susceptibilities indistinguishable from those of the wild-type strain K799, from which mutant Z61 had been derived. Uptake of a chromogenic beta-lactam nitrocefin was studied in both uninduced and induced cells of all strains by measuring the steady-state rate of nitrocefin hydrolysis by the inducible, periplasmic beta-lactamase in both whole and broken cells. This demonstrated that outer membrane permeability decreased as antibiotic resistance increased in the series mutant Z61, partial revertants, wild type, and full revertants. The data were consistent with the idea of low outer membrane permeability being caused by a low proportion of open functional porins in the outer membrane as the reason for the high natural antibiotic resistance of wild-type P, aeruginosa strains. In addition, it was observed that levels of benzylpenicillin below the minimal inhibitory concentration for mutant Z61 failed to induce beta-lactamase production. The possibility that this was related to the observed increase in outer membrane permeability is discussed. Preliminary evidence is presented that the pore-forming outer membrane porin protein F is not altered in mutant Z61.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benz R., Hancock R. E. Properties of the large ion-permeable pores formed from protein F of Pseudomonas aeruginosa in lipid bilayer membranes. Biochim Biophys Acta. 1981 Aug 20;646(2):298–308. doi: 10.1016/0005-2736(81)90336-9. [DOI] [PubMed] [Google Scholar]
- Brown M. R., Wood S. M. Relation between cation and lipid content of cell walls of Pseudomonas aeruginosa, Proteus vulgaris and Klebsiella aerogenes and their sensitivity to polymyxin B and other antibacterial agents. J Pharm Pharmacol. 1972 Mar;24(3):215–218. doi: 10.1111/j.2042-7158.1972.tb08967.x. [DOI] [PubMed] [Google Scholar]
- Gmeiner J., Schlecht S. Molecular composition of the outer membrane of Escherichia coli and the importance of protein-lipopolysaccharide interactions. Arch Microbiol. 1980 Sep;127(2):81–86. doi: 10.1007/BF00428010. [DOI] [PubMed] [Google Scholar]
- Grundström T., Normark S., Magnusson K. E. Overproduction of outer membrane protein suppresses envA-induced hyperpermeability. J Bacteriol. 1980 Dec;144(3):884–890. doi: 10.1128/jb.144.3.884-890.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock R. E., Carey A. M. Outer membrane of Pseudomonas aeruginosa: heat- 2-mercaptoethanol-modifiable proteins. J Bacteriol. 1979 Dec;140(3):902–910. doi: 10.1128/jb.140.3.902-910.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock R. E., Decad G. M., Nikaido H. Identification of the protein producing transmembrane diffusion pores in the outer membrane of Pseudomonas aeruginosa PA01. Biochim Biophys Acta. 1979 Jul 5;554(2):323–331. doi: 10.1016/0005-2736(79)90373-0. [DOI] [PubMed] [Google Scholar]
- Hancock R. E., Irvin R. T., Costerton J. W., Carey A. M. Pseudomonas aeruginosa outer membrane: peptidoglycan-associated proteins. J Bacteriol. 1981 Jan;145(1):628–631. doi: 10.1128/jb.145.1.628-631.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock R. E., Raffle V. J., Nicas T. I. Involvement of the outer membrane in gentamicin and streptomycin uptake and killing in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1981 May;19(5):777–785. doi: 10.1128/aac.19.5.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock R. E., Reeves P. Bacteriophage resistance in Escherichia coli K-12: general pattern of resistance. J Bacteriol. 1975 Mar;121(3):983–993. doi: 10.1128/jb.121.3.983-993.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haque H., Russell A. D. Effect of chelating agents on the susceptibility of some strains of gram-negative bacteria to some antibacterial agents. Antimicrob Agents Chemother. 1974 Aug;6(2):200–206. doi: 10.1128/aac.6.2.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kropinski A. M., Chan L., Milazzo F. H. Susceptibility of lipopolysaccharide-defective mutants of Pseudomonas aeruginosa strain PAO to dyes, detergents, and antibiotics. Antimicrob Agents Chemother. 1978 Mar;13(3):494–499. doi: 10.1128/aac.13.3.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kropinski A. M., Kuzio J., Angus B. L., Hancock R. E. Chemical and chromatographic analysis of lipopolysaccharide from an antibiotic-supersusceptible mutant of Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1982 Feb;21(2):310–319. doi: 10.1128/aac.21.2.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills B. J., Holloway B. W. Mutants of Pseudomonas aeruginosa that show specific hypersensitivity to aminoglycosides. Antimicrob Agents Chemother. 1976 Sep;10(3):411–416. doi: 10.1128/aac.10.3.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicas T. I., Hancock R. E. Outer membrane protein H1 of Pseudomonas aeruginosa: involvement in adaptive and mutational resistance to ethylenediaminetetraacetate, polymyxin B, and gentamicin. J Bacteriol. 1980 Aug;143(2):872–878. doi: 10.1128/jb.143.2.872-878.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikaido H. Outer membrane of Salmonella typhimurium. Transmembrane diffusion of some hydrophobic substances. Biochim Biophys Acta. 1976 Apr 16;433(1):118–132. doi: 10.1016/0005-2736(76)90182-6. [DOI] [PubMed] [Google Scholar]
- Noguchi H., Fukasawa M., Komatsu T., Iyobe S., Mitsuhashi S. Isolation of two types of Pseudomonas aeruginosa mutants highly sensitive to a specific group of beta-lactam antibiotics and with defect in penicillin-binding proteins. J Antibiot (Tokyo) 1980 Dec;33(12):1521–1526. doi: 10.7164/antibiotics.33.1521. [DOI] [PubMed] [Google Scholar]
- Nordström K., Sykes R. B. Induction kinetics of beta-lactamase biosynthesis in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1974 Dec;6(6):734–740. doi: 10.1128/aac.6.6.734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Callaghan C. H., Morris A., Kirby S. M., Shingler A. H. Novel method for detection of beta-lactamases by using a chromogenic cephalosporin substrate. Antimicrob Agents Chemother. 1972 Apr;1(4):283–288. doi: 10.1128/aac.1.4.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohmori H., Azuma A., Suzuki Y., Hashimoto Y. Isolation and properties of beta-lactamase-less mutants from clinically important gram-negative bacteria. J Antibiot (Tokyo) 1977 Mar;30(3):267–269. doi: 10.7164/antibiotics.30.267. [DOI] [PubMed] [Google Scholar]
- Richmond M. H., Sykes R. B. The beta-lactamases of gram-negative bacteria and their possible physiological role. Adv Microb Physiol. 1973;9:31–88. doi: 10.1016/s0065-2911(08)60376-8. [DOI] [PubMed] [Google Scholar]
- Rosselet A., Zimmermann W. Mutants of Pseudomonas aeruginosa with impaired -lactamase inducibility and increased sensitivity to -lactam antibiotics. J Gen Microbiol. 1973 Jun;76(2):455–457. doi: 10.1099/00221287-76-2-455. [DOI] [PubMed] [Google Scholar]
- Sandermann H., Jr, Strominger J. L. Purification and properties of C 55 -isoprenoid alcohol phosphokinase from Staphylococcus aureus. J Biol Chem. 1972 Aug 25;247(16):5123–5131. [PubMed] [Google Scholar]
- Sawai T., Matsuba K., Yamagishi S. A method for measuring the outer membrane-permeability of beta-lactam antibiotics in gram-negative bacteria. J Antibiot (Tokyo) 1977 Dec;30(12):1134–1136. doi: 10.7164/antibiotics.30.1134. [DOI] [PubMed] [Google Scholar]
- Sykes R. B., Matthew M. The beta-lactamases of gram-negative bacteria and their role in resistance to beta-lactam antibiotics. J Antimicrob Chemother. 1976 Jun;2(2):115–157. doi: 10.1093/jac/2.2.115. [DOI] [PubMed] [Google Scholar]
- Tamaki S., Sato T., Matsuhashi M. Role of lipopolysaccharides in antibiotic resistance and bacteriophage adsorption of Escherichia coli K-12. J Bacteriol. 1971 Mar;105(3):968–975. doi: 10.1128/jb.105.3.968-975.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZIERDT C. H., FOX F. A., NORRIS G. F. A multiple-syringe bacteriophage applicator. Am J Clin Pathol. 1960 Mar;33:233–237. doi: 10.1093/ajcp/33.3.233. [DOI] [PubMed] [Google Scholar]
- Zacharius R. M., Zell T. E., Morrison J. H., Woodlock J. J. Glycoprotein staining following electrophoresis on acrylamide gels. Anal Biochem. 1969 Jul;30(1):148–152. doi: 10.1016/0003-2697(69)90383-2. [DOI] [PubMed] [Google Scholar]
- Zimmermann W. Penetration of beta-lactam antibiotics into their target enzymes in Pseudomonas aeruginosa: comparison of a highly sensitive mutant with its parent strain. Antimicrob Agents Chemother. 1980 Jul;18(1):94–100. doi: 10.1128/aac.18.1.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann W. Penetration through the gram-negative cell wall: a co-determinant of the efficacy of beta-lactam antibiotics. Int J Clin Pharmacol Biopharm. 1979 Mar;17(3):131–134. [PubMed] [Google Scholar]
- Zimmermann W., Rosselet A. Function of the outer membrane of Escherichia coli as a permeability barrier to beta-lactam antibiotics. Antimicrob Agents Chemother. 1977 Sep;12(3):368–372. doi: 10.1128/aac.12.3.368. [DOI] [PMC free article] [PubMed] [Google Scholar]




