Abstract
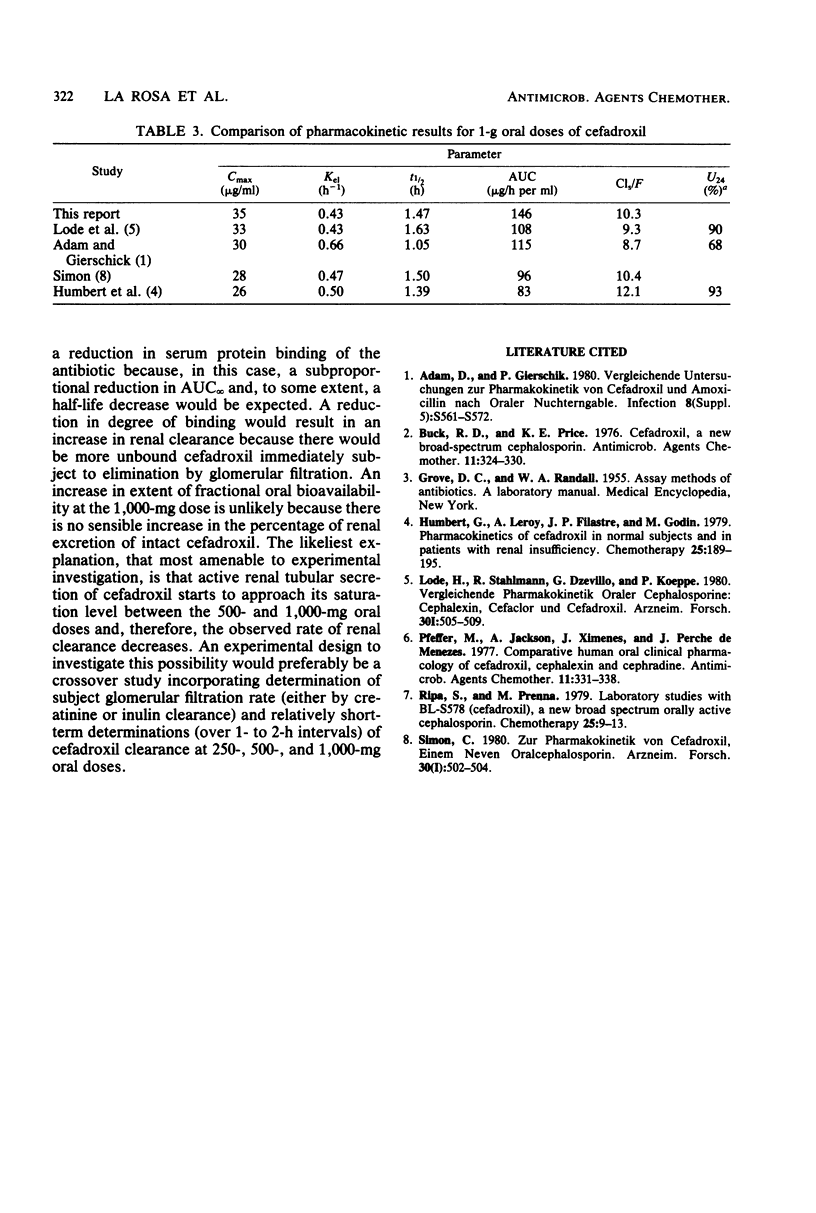
The human oral pharmacokinetics of cefadroxil were studied in parallel at doses of 250, 500, and 1,000 mg in three groups of 10 healthy young male volunteers. Renal excretion of intact cefadroxil, accounted for 82, 79, and 77% of the above doses. Mean peak serum levels were dose linear: 9, 18, and 35 microgram/ml at 250, 500, and 1,000 mg, respectively. However, overall pharmacokinetics were linear only in the 250- to 500-mg dose range; apparent serum clearances were 10 liters/h, and true renal clearances were 9 and 8 liters/h at 250 and 500 mg. At 1,000 mg, apparent serum clearance dropped to about 7 liters/h, true renal clearance, dropped to 6 liters/h, and the area under the curve increased disproportionately. At 250 and 500 mg, mean half-life was about 1.2 h; at 1,000 mg, however, it was 1.6h. The nonlinear decrease in clearance could be related to saturation of active renal tubular secretion of cefadroxil between the 500- and 1,000-mg doses. Previous results indicating that cefadroxil has greater persistence than other oral cephalosporins such as cephalexin, cephradine, cefaclor were confirmed.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Buck R. E., Price K. E. Cefadroxil, a new broad-spectrum cephalosporin. Antimicrob Agents Chemother. 1977 Feb;11(2):324–330. doi: 10.1128/aac.11.2.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humbert G., Leroy A., Fillastre J. P., Godin M. Pharmacokinetics of cefadroxil in normal subjects and in patients with renal insufficiency. Chemotherapy. 1979;25(4):189–195. doi: 10.1159/000237839. [DOI] [PubMed] [Google Scholar]
- Lode H., Stahlmann R., Dzwillo G., Koeppe P. Vergleichende Pharmakokinetik oraler Cephalosporine: Cephalexin, Cefaclor und Cefadroxil. Arzneimittelforschung. 1980;30(3):505–509. [PubMed] [Google Scholar]
- Pfeffer M., Jackson A., Ximenes J., de Menezes J. P. Comparative human oral clinical pharmacology of cefadroxil, cephalexin, and cephradine. Antimicrob Agents Chemother. 1977 Feb;11(2):331–338. doi: 10.1128/aac.11.2.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ripa S., Prenna M. Laboratory studies with BL-S 578 (Cefadroxil) a new broad-spectrum orally active cephalosporin. Chemotherapy. 1979;25(1):9–13. doi: 10.1159/000237816. [DOI] [PubMed] [Google Scholar]
- Simon C. Zur Pharmakokinetik von Cefadroxil, einem neuen oral-cephalosporin. Arzneimittelforschung. 1980;30(3):502–504. [PubMed] [Google Scholar]



