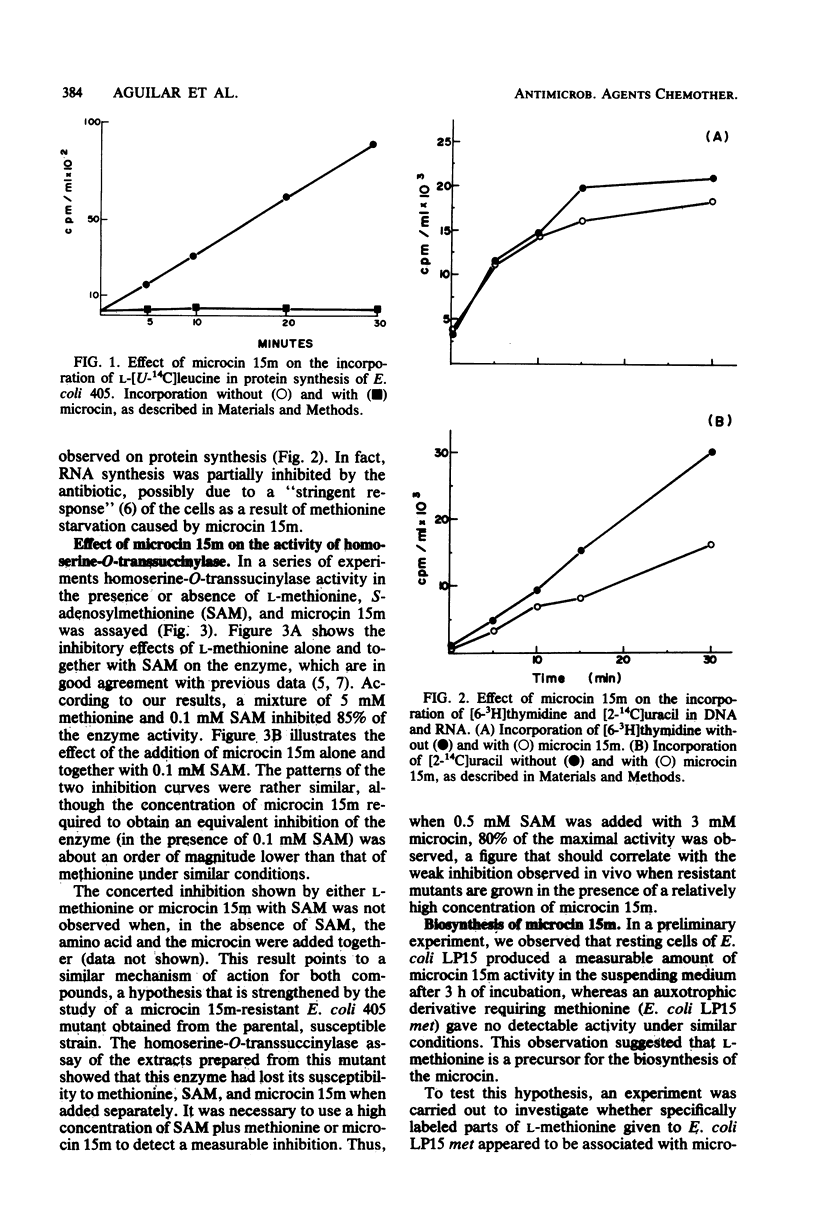
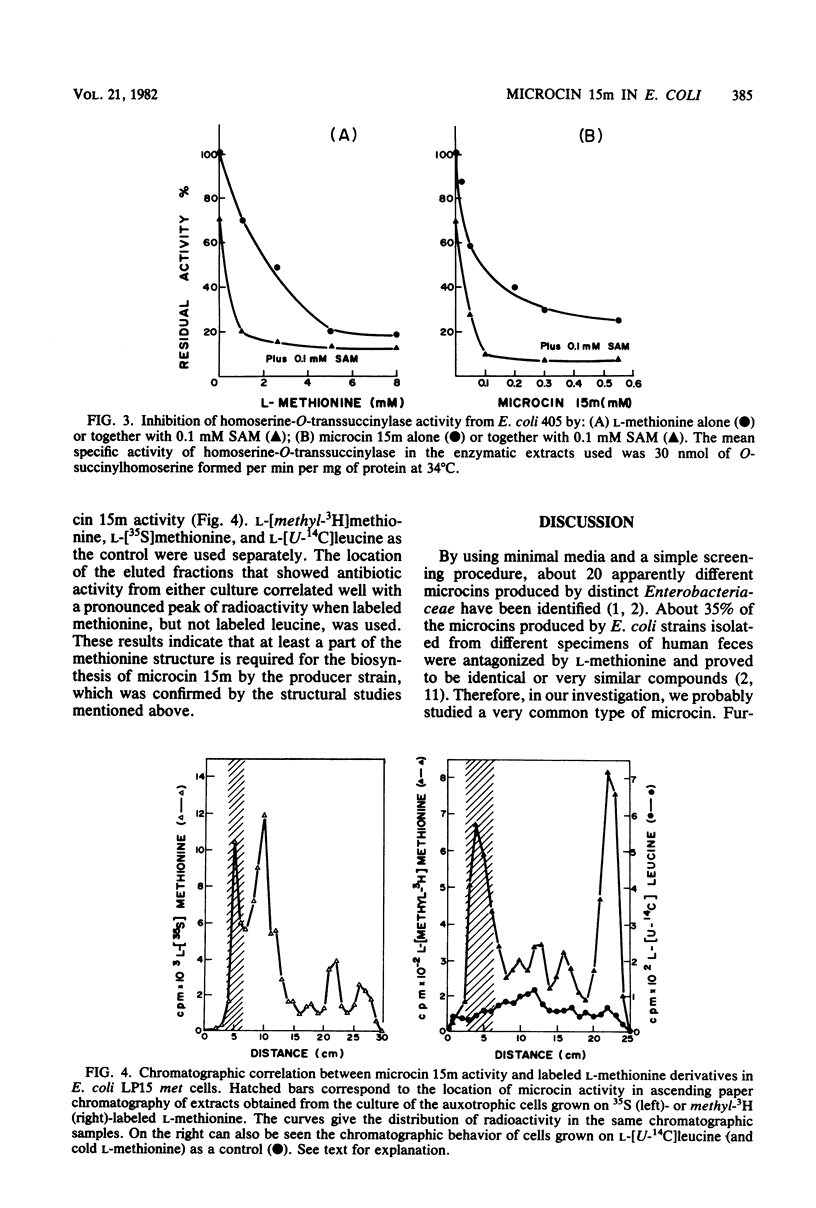
Abstract
It has been previously established that a high proportion of enterobacteria isolated from the intestinal content of humans, mostly Escherichia coli strains, secrete into the culture media antibiotic substances of low molecular weight, which have been called microcins. It was also found that the synthesis of these antibiotics is determined by plasmids. In this paper, a method for the purification of microcin 15m is described, and experimental data are given on the mechanism of its action. The data indicate that this action is based mainly on the inhibition of the first enzyme of the methionine biosynthetic pathway, homoserine-O-transsuccinylase, a similar way to the allosteric inhibition caused by this amino acid.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asensio C., Pérez-Díaz J. C. A new family of low molecular weight antibiotics from enterobacteria. Biochem Biophys Res Commun. 1976 Mar 8;69(1):7–14. doi: 10.1016/s0006-291x(76)80264-1. [DOI] [PubMed] [Google Scholar]
- Baquero F., Bouanchaud D., Martinez-Perez M. C., Fernandez C. Microcin plasmids: a group of extrachromosomal elements coding for low-molecular-weight antibiotics in Escherichia coli. J Bacteriol. 1978 Aug;135(2):342–347. doi: 10.1128/jb.135.2.342-347.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brush A., Paulus H. The enzymic formation of O-acetylhomoserine in Bacillus subtilis and its regulation by methionine and S-adenosylmethionine. Biochem Biophys Res Commun. 1971 Nov 5;45(3):735–741. doi: 10.1016/0006-291x(71)90478-5. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lee L. W., Ravel J. M., Shive W. Multimetabolite control of a biosynthetic pathway by sequential metabolites. J Biol Chem. 1966 Nov 25;241(22):5479–5480. [PubMed] [Google Scholar]
- Nagai S., Flavin M. Acetylhomoserine. An intermediate in the fungal biosynthesis of methionine. J Biol Chem. 1967 Sep 10;242(17):3884–3895. [PubMed] [Google Scholar]
- Perez-Diaz J. C., Clowes R. C. Physical characterization of plasmids determining synthesis of a microcin which inhibits methionine synthesis in Escherichia coli. J Bacteriol. 1980 Mar;141(3):1015–1023. doi: 10.1128/jb.141.3.1015-1023.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ron E. Z., Shani M. Growth rate of Escherichia coli at elevated temperatures: reversible inhibition of homoserine trans-succinylase. J Bacteriol. 1971 Aug;107(2):397–400. doi: 10.1128/jb.107.2.397-400.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SISTROM W. R. On the physical state of the intracellularly accumulates substrates of beta-galactoside-permease in Escherichia coli. Biochim Biophys Acta. 1958 Sep;29(3):579–587. doi: 10.1016/0006-3002(58)90015-5. [DOI] [PubMed] [Google Scholar]
- Yabu K., Takahashi S. Protoplast formation of selected Mycobacterium smegmatis mutants by lysozyme in combination with methionine. J Bacteriol. 1977 Mar;129(3):1628–1631. doi: 10.1128/jb.129.3.1628-1631.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]



