Abstract
In some regions of the developing rat brain such as the nucleus accumbens (Acb), mu opioid (MOP) receptor specific binding in the perinatal period exceeds that in the adult. To investigate the significance of these developmental changes, MOP and nociceptin/orphanin FQ (NOP) receptor binding and G protein coupling as determined by GTPγS binding experiments were examined in mesolimbic regions of postnatal day 2 (P2) pups and compared to those of their dams. Acb of the P2 pup exhibited 2-fold greater MOP receptor specific binding than that of the dam. In the ventral tegmental area (VTA), NOP specific binding was about 2-fold higher in the P2 pup. A correlation was found between MOP and NOP binding and their coupling to G protein on dam and P2 pup brain sections. However, the magnitude of increases in MOP and NOP receptor G protein coupling to G protein in P2 pups exceeded the 2-fold differences in binding between pups and dams. Furthermore, the amplitude of the MOP receptor G protein coupling in female P2 Acb was greater than increases in male P2 pup Acb. Differences in MOP and NOP binding and G protein coupling in other mesolimbic regions between P2 pups and dams were rarely observed. The data indicate that greater binding and G protein coupling of MOP and NOP receptors occur in discrete, mesolimbic regions of P2 pups when compared to their dams. It may be of significance that these brain regions, Acb and VTA, are undergoing maturation on P2.
Keywords: Development, Nociceptin, Mu opioid receptor, Mesolimbic region, Cortex, Nociceptin receptor
Opioid receptors and their endogenous ligands have been detected in rodent brain beginning as early as the second week of gestation [4,5,10,15,21,22,25,26]. In experiments where fore-brain opioid receptor binding densities were measured by in vitro binding assays, it was determined that the number of opioid receptor binding sites increases with age. When selective ligands were used in such studies, a differential ontogeny for μ (MOP), δ (DOP) and κ (KOP) receptors was discovered. Forebrain MOP and KOP binding was detectable in the prenatal period, whereas DOP binding was detectable only in the second postnatal week [10,15,22,25,26,28]. Interestingly, when MOP binding density in rodent forebrain was measured with respect to protein instead of wet tissue weight, its binding density was higher in the prenatal interval [15,22], but declined in the postnatal period [25,26].
Autoradiographic localization also revealed a transient increase in opioid receptor binding in some regions of perinatal rat brain [10,12,27]. Substantial declines in MOP binding as measured by 3H-DAMGO autoradiography during the postnatal period occurred in the nucleus accumbens (Acb), olfactory bulb, cerebellum, central gray, midbrain tegmentum and globus pallidus [12]. These regions displayed varying ontogenic profiles with peaks and troughs at different times as would be expected if the brain develops in an anatomically specific manner. KOP binding was also transient in some regions, but DOP binding differed from MOP and KOP receptors by appearing later in development and progressively increasing in the postnatal period. In a few regions, such as the human cerebellum, a complete loss of OR binding was observed in the adult, which suggested that opioids may have a role in regulating brain development [11,29]. In support of this possibility, DAMGO stimulation of GTPγS binding has been detected in mouse brain as early as embryonic day 12.5, a time at which MOP receptor is also detected [19,22].
The newest member of the opioid receptor family is the NOP receptor. NOP receptor binding and G protein coupling are abundant in the cerebral cortex and some limbic regions of the adult rodent and primate brain as shown by autoradiographic methods [3,6,9,13,23,24]. Although the ontogeny of NOP receptor mRNA expression has been studied [9,17], its binding and G protein coupling capacity in brain during development has not been reported. Here we relate an examination of NOP and MOP receptor binding as well as G protein coupling activity in mesolimbic brain regions of P2 rat pups and their dams.
[D-Ala2,N-Me-Phe4,Gly5-ol] enkephalin (DAMGO) [D-pen2,D-pen5]enkephalin (DPDPE), β-endorphin [125I]-β-endorphin (2000 Ci/mmol) and nociceptin/orphanin FQ were obtained from Multiple Peptide Systems (San Diego, CA); (5α,7α,8β)-(−)-N-methyl-N-(7-1-pyrrolidinyl)-1-oxaspiro(4,5)dec-8-yl-benzeneacetamide (U69,593) and naloxone were from NIDA Drug Supply (Research Triangle, NC). [35S]-GTPγS (46.3 TBQ, 1250 Ci/mmol) and [125I]-nociceptin 74 TBQ (2000 Ci/mmol) were purchased from Perkin Elmer Life Sciences (Boston, MA). 125I and 14C microscales were from Amersham (Arlington Heights, IL). The NOP receptor antagonist [Nphe1]nociceptin(1–13)NH2, was from Tocris Cookson (Ellisville, MO). Most of the other chemicals were purchased from Sigma (St. Louis, MO).
Seven-day pregnant Sprague–Dawley rats were purchased from Harlan (Westbury, NY). Upon arrival, animals were weighed, housed individually in maternity cages, and maintained in a temperature-controlled colony room with free access to food and water. Animals were handled in accordance with the Guide for the Care and Use of Laboratory Animals as adopted and promulgated by the National Institutes of Health.
Pups were decapitated and their dams were sacrificed by ketamine/xylazine anesthetic overdose. Brains were rapidly removed, immediately frozen in isopentane at −30 °C and stored at −80 °C prior to sectioning. Twenty micrometers of coronal sections were cut at −20 °C in a cryostat, thaw-mounted onto gelatin and polylysine double-coated slides. Sections were vacuum desiccated overnight at 4 °C, then stored at −80 °C until use for in situ [35S]-GTPγS or ligand binding.
After thawing at room temperature, slides were preincubated for 15 min in buffer 50 mM Tris–HCl, 150 mM NaCl, 0.1% BSA (pH 7.4) and then incubated for 90 min in the same buffer containing 0.1 nM [125I]-β-endorphin specific activity (2000 Ci/mmol) in the presence (for specific binding) or absence (for total and non-specific binding) of 0.1 μM unlabeled β-endorphin at room temperature. In addition, μ specific binding was determined in the presence of 100 nM DPDPE (δ agonist) and 100 nM U69,593 (κ agonist). In our prior studies this radioligand generated suitable Scatchard plots and non-specific binding with rat brain cell membranes. Slides were then washed with 50 mM Tris–HCl, pH 7.4, buffer four times at 4 °C, followed by a brief rinse in cold distilled water. After they were dried under a stream of air, slides were apposed to 125I sensitive film Kodak Biomax (Rochester, NY) together with 125I microscales for 48–72 h. NOP receptor binding was measured with 0.1 nM [125I]-nociceptin in the presence or absence of unlabeled 0.1 μM nociceptin. Specific binding is expressed as nCi/g wet weight tissue.
The protocol for in situ [35S]-GTPγS binding was adapted from Martin et al. [16]. After thawing at room temperature for 10 min, sections were rinsed for 10 min in assay buffer 50 mM Tris–HCl, 0.3 mM MgCl2, 0.2 mM EGTA, and 100 mM NaCl (pH 7.4) followed by 30 min preincubation in fresh assay buffer + 1 mM GDP at room temperature. Basal binding was assessed by incubating sections for 90 min at room temperature in fresh assay buffer with 1 mM GDP and 40 pM [35S]-GTPγS and values obtained were similar to those previously reported [8]. Agonist-stimulated binding was conducted by incubating slides in assay buffer with 1 mM GDP and 40 pM [35S]-GTPγS + DAMGO (10 μM) or nociceptin (1 μM) for 90 min at room temperature. Sections were then washed twice for 2 min each in ice-cold 50 mM Tris–HCl buffer, pH 7.4, followed by a brief rinse in distilled water. Although present in many mesolimbic rat brain regions, KOP binding density proved to be less than 10% of MOP. Due to this paucity of KOP receptors in rat pup brain, the GTPγS binding assay is not sufficiently sensitive to measure their coupling to G protein ([8], Hou et al., unpublished observations).
Slides were dried under a stream of air and apposed to 35S or 125I sensitive film (Kodak Biomax) together with 14C or 125I microscales for 48–72 h. Films were analyzed using the NIH IMAGE program and quantified with the microscales. Regions were identified by reference to the atlas of Paxinos and Watson [20] and the same regional delineations were used in measuring basal and stimulated [35S]-GTPγS binding in serial sections taken from the same animal. Basal and stimulated [35S]-GTPγS binding was determined on adjacent sections. All measurements reflect the average from three sections for each region from three to six animals. See figure legends for n values for each experiment. A percentage of basal [35S]-GTPγS binding (percentage = [stimulated − basal]/basal × 100) was estimated. The percentage stimulation ± S.E. values or specific binding ± S.E. are shown in the figures. Statistical data were analyzed with a one-way ANOVA followed by a post hoc Dunnett’s test.
MOP specific binding was measured in discrete mesolimbic regions and sub-regions by receptor autoradiography. These included the Acb shell (AcbSh) and core (AcbC), VTA, lateral septum (LS), cingulate cortex (CG), insular cortex (IC) and amygdala (Amyg) except in the incompletely developed P2 Acb, where it was not possible to distinguish shell and core regions. In situ receptor autoradiography experiments on sections from dams and their pups were performed with [125I]-radioligands because of the shorter exposure times, the unavailability of suitable film to detect [3H]-radioligands and greater sensitivity. The Acb in P2 male and female pups had 1.7-fold higher β-endorphin specific binding values than did the Acb in their dams (0.96 ± 0.04 nCi/g tissue in male and female P2 pups versus 0.55 ± 0.03 nCi/g tissue in their dams, n = 5–6, P < 0.01). However, in the VTA, LS, CG, IG, Amyg and caudate putamen, specific binding in dams were several fold higher than those in P2 pups (data not shown). These results are consistent with previously published MOP binding data on the Acb, cortex, LS, Amyg and caudate putamen of P1 rat pups and adults using [3H]-DAMGO as the radioligand [12].
Initial [35S]-GTPγS binding experiments on dam and pup brain sections were performed to investigate the effects of gestational opiates on MOP and NOP receptor coupling to G proteins [8]. The first evidence for age dependent differences in GTPγS binding in mesolimbic regions was detected in water- (vehicle) treated control dams and P2 and P7 pups. The unusual finding of greater GTPγS binding in P2 compared to the vehicle-treated dam Acb and VTA gained in our original studies prompted these investigations on both receptor binding and G protein coupling in untreated pregnant rats. Optimal resolution of autoradiographs was achieved by varying a number of parameters including agonist and GDP concentrations. In previous studies, DAMGO stimulation of GTPγS binding was abolished by preincubation with corresponding selective antagonists in dam brain [8]. Densitometry revealed that antagonists 1 μM naloxone or 1 μM [Nphe1]nociceptin(1–13)NH2 for MOP or NOP receptor (resp.) diminished agonist stimulation to basal levels in all of the regions under investigation. Similar findings were obtained for P2 pup brain mesolimbic regions. DAMGO-stimulated GTPγS binding was also performed on dams and their pups (Fig. 1). In accordance with the MOP binding data, MOP receptor G protein coupling in Acb of P2 female and male pups was 5.1- and 2.9-fold greater than in the dams, respectively. Thus, a correlation between the increases in MOP binding and G protein coupling was observed. The amplitude of this increase in MOP receptor G protein coupling in Acb of P2 pups was also gender-specific. Similar gender differences were seen in their CG and IC regions (Fig. 1). The percentage stimulation of DAMGO-induced GTPγS binding in Acb of dams was comparable to the values reported for Acb of adult male rats eliminating the possibility that hormonal differences in pregnant females may be responsible for the decrease in coupling [16]. Moreover, the percentage stimulation of DAMGO-induced GTPγS binding in Acb of P2 pups were similar to values obtained using P2 pups from dams treated with water during gestation [8]. Little or no increases in MOP receptor G protein coupling of P2 pups over that of dams were seen in the other brain regions examined (Fig. 1). Instead, values for net DAMGO-stimulated GTPγS binding in all but one of these regions of dams were similar or less than that of P2 pups (data not shown).
Fig. 1.
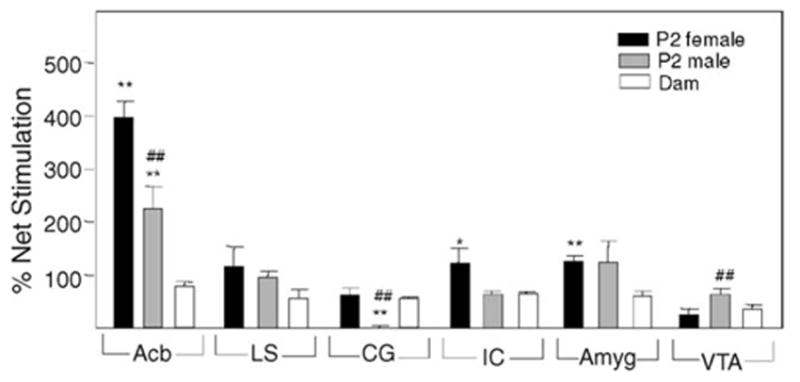
DAMGO-stimulated GTPγS binding in mesolimbic regions of P2 pups and their dams. Brain sections from P2 rats and their dams were subjected to in situ [35S]-GTPγS autoradiography in the presence and absence of 10 μM DAMGO (n = 4 for both pups and dams). *Significantly different from dams (*P < 0.05 and **P < 0.01). ##Significantly different from female P2 pups (P < 0.01).
NOP receptor autoradiography on sections from rat brains revealed that the VTA of P2 pups displayed 1.8- to 2.0-fold higher specific [125I]-nociceptin binding than that of their dams (Fig. 2). In the other mesolimbic regions [125I]-nociceptin binding in the dams was greater than that in P2 pups. The gender differences seen for MOP binding were not observed for nociceptin binding in mesolimbic regions of the P2 pups.
Fig. 2.
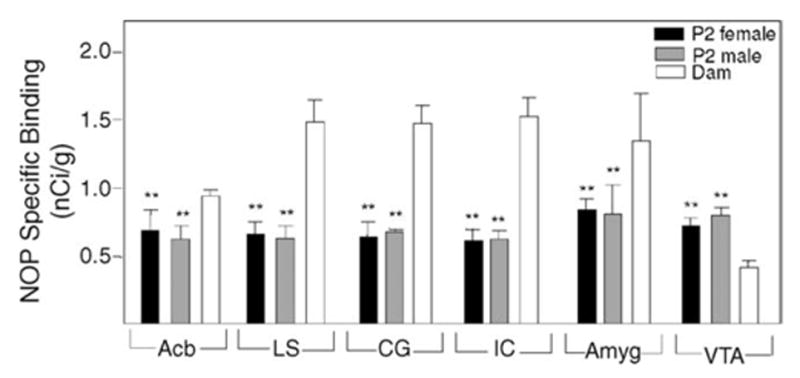
Nociceptin binding in mesolimbic regions of P2 pups and their dams. Brain sections from P2 rats and their dams were subjected to receptor autoradiography using [125I]-nociceptin as radioligand. Specific binding is expressed as nCi/g tissue wet weight (n = 3–4 for both pups and dams). **Significantly different from dams (P < 0.01).
When nociceptin-induced GTPγS binding was measured, percentage stimulation of VTA from P2 pups was 15- to 20-fold higher than that of their mothers (Fig. 3). These data correlate with the increase in NOP binding. In the CG and IC of P2 pups, there were no age dependent differences, whereas in the Acb, LS and Amyg there were lesser increases in nociceptin-induced GTPγS binding values of P2 pups in comparison to those of dams. Thus, despite the greater amounts of NOP binding in dams when compared to pups, its GTPγS binding values were similar or less than those of the pups.
Fig. 3.
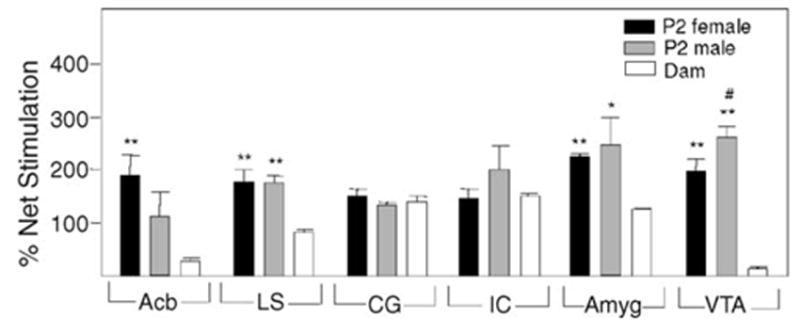
Nociceptin-stimulated GTPγS binding in mesolimbic regions of P2 pups and their dams. Brain sections from P2 rats and their dams were subjected to in situ [35S]-GTPγS autoradiography in the presence and absence of 1 μM nociceptin (n = 4 for both pups and dams). *Significantly different from dams (*P < 0.05 and **P < 0.01). #Significantly different from female P2 pups (P < 0.05).
In contrast to the MOP receptor G protein coupling in P2 Acb where female values were higher than males, male P2 VTA displayed a greater nociceptin stimulation of GTPγS binding than females (Fig. 3). Gender differences in nociceptin-induced GTPγS binding were not detected in the other mesolimbic regions examined in P2 pups.
In this study, a number of differences were found between MOP and NOP receptor binding and G protein coupling when comparing P2 pups with their dams. The β-endorphin specific binding in the Acb of P2 rat pups was 1.7-fold higher than in the Acb of their dams, consistent with earlier evidence showing that 3H-DAMGO binding to MOP receptor is 2-fold higher in Acb of P1 pups than in that of P7 pups and 4-fold greater than in that of P30 adult rats [12]. A similar increase in nociceptin specific binding in the VTA of P2 pups compared to that of their dams was also observed (Fig. 2). It is known that the Acb undergoes development in the perinatal period ([30] and references cited therein). The development of the rat VTA extends into the postnatal period as well [14].The fact that the higher levels of MOP and NOP binding is accompanied by greater MOP and NOP G protein coupling, respectively, indicates that an increase in opioid receptor that is capable of signaling has occurred. This increase in MOP and NOP signaling capacity may be required for a transient functionality relevant to development. Opioids have been implicated in various postnatal events including mother–infant social interactions [18]. Another attractive possibility is suggested by earlier studies on a regulatory role of opioid signaling in cell division of germinal regions of developing brain ([7,21] and references cited therein others).
Transient functional increases in MOP and NOP receptors during development may also be explained by age-dependent differential distribution of MOP and NOP receptors in neurons, glia and progenitor cells of the Acb and VTA. Upon maturation of the brain region, the cells may be reduced or disappear as seen for rodent cerebellar opioid receptors [1,11,29]. Programmed neuronal cell death that occurs during development may be responsible for this loss.
Another explanation for the transient increases seen in P2 pups is that protein levels of MOP receptor in Acb and NOP receptor in VTA do not change but that more receptors become coupled to G proteins thereby enhancing specific binding of agonists to the opioid receptors. Accordingly, the extent of the increase in P2 MOP and NOP receptor G protein coupling in Acb and VTA, respectively, over that in the dam was greater than the corresponding increase in binding. However, in most of the other mesolimbic regions examined, dams possessed greater opioid receptor binding compared to their P2 pups, while their GTPγS binding values were similar or less than P2 pups. The latter may be explained by our earlier findings that P1 rat forebrain has a greater proportion of intracellular MOP receptor than the adult and the intracellular MOP receptor is more coupled to G protein than that of the adult rat [2]. In this study, P1 rat forebrain contained more Go than that of the adult. Go is a GTP binding regulatory protein that has been shown to couple with opioid receptors. It has been postulated that the intracellular MOP receptor in P1 rat brain is newly synthesized receptor along with G protein en route to the cell surface. Such coupled MOP receptors would be more abundant in pups than adults.
MOP receptor G protein coupling in the Acb, IC and CG of P2 females were more robust than that in male P2 pups (Fig. 1). In contrast, NOP receptor G protein coupling showed no gender differences in the mesolimbic regions tested except for the VTA in P2 pups (Fig. 3). In the VTA, MOP and NOP receptor G protein coupling in male P2 pups were greater than in females. Little or no chronic opiate-elicited changes were observed in MOP-induced GTPγS binding in Acb of P2 and P7 females [8]. Perhaps, the stronger MOP receptor G protein coupling response in Acb seen in untreated P2 females makes their OR signaling more resistant to the inhibitory actions of chronic opiates than that of males. Alternatively, MOP receptor in the female Acb may differ with respect to their properties or those of factors that may influence their signaling.
In conclusion, these novel initial findings may reflect the compelling possibility of an age-dependent role of opioids in cell proliferation suggested by recent discoveries [7,21]. It will be of interest in future studies to focus on this opioid function in other brain regions at time points during their ontogeny when their maturation is proceeding optimally.
Acknowledgments
Supported in part by National Institutes of Health grant DA13475. We thank Dr. James Pauly (Division of Pharmaceutical Sciences, University of Kentucky, Lexington) for many helpful discussions on the preparation of brain sections and their autoradiography.
References
- 1.Abeyta A, Dettmer TS, Barnes A, Vega D, Carta M, Gallegos N, Raymond-Stintz M, Savage DD, Valenzuela CF, Saland LC. Delta opioid receptor localization in the rat cerebellum. Brain Res. 2002;931:100–105. doi: 10.1016/s0006-8993(02)02248-5. [DOI] [PubMed] [Google Scholar]
- 2.Bem WT, Yeung SJ, Belcheva M, Barg J, Coscia CJ. Age-dependent changes in the subcellular distribution of rat brain muopioid receptors and GTP binding regulatory proteins. J Neurochem. 1991;57:1470–1477. doi: 10.1111/j.1471-4159.1991.tb06340.x. [DOI] [PubMed] [Google Scholar]
- 3.Bridge KE, Wainwright A, Reilly K, Oliver KR. Autoradiographic localization of 125I[Tyr14] nociceptin/orphanin FQ binding sites in macaque primate CNS. Neuroscience. 2003;118:513–523. doi: 10.1016/s0306-4522(02)00927-2. [DOI] [PubMed] [Google Scholar]
- 4.Clendeninn NJ, Petraitis M, Simon EJ. Ontological development of opiate receptors in rodent brain. Brain Res. 1976;118:157–160. doi: 10.1016/0006-8993(76)90852-0. [DOI] [PubMed] [Google Scholar]
- 5.Coyle JT, Pert CB. Ontogenetic development of [3H]naloxone binding in rat brain. Neuropharmacology. 1976;159:555–560. doi: 10.1016/0028-3908(76)90107-6. [DOI] [PubMed] [Google Scholar]
- 6.Florin S, Lerouxnicollet I, Meunier JC, Costentin J. Autoradiographic localization of [H-3]nociceptin binding sites from telencephalic to mesencephalic regions of the mouse brain. Neurosci Lett. 1997;230:33–36. doi: 10.1016/s0304-3940(97)00470-9. [DOI] [PubMed] [Google Scholar]
- 7.Hauser KF, Mangoura D. Diversity of the endogenous opioid system in development. Novel signal transduction translates multiple extracellular signals into neural cell growth and differentiation. Perspect Dev Neurobiol. 1998;5:437–449. [PubMed] [Google Scholar]
- 8.Hou YN, Tan Y, Belcheva MM, Clark AL, Zahm DS, Coscia CJ. Differential effects of gestational buprenorphine, naloxone, and methadone on mesolimbic mu opioid and ORL1 receptor G protein coupling. Dev Brain Res. 2004;151:149–157. doi: 10.1016/j.devbrainres.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 9.Ikeda K, Watanabe M, Ichikawa TT, Kobayashi T, Yano R, Kumanishi T. Distribution of prepronociceptin/orphanin FQ mRNA and its receptor mRNA in developing and adult mouse central nervous systems. J Comp Neurol. 1998;399:139–151. doi: 10.1002/(sici)1096-9861(19980914)399:1<139::aid-cne11>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 10.Kent JL, Pert CB, Herkenham M. Ontogeny of opiate receptors in rat forebrain: visualization by in vitro autoradiography. Brain Res. 1981;254:487–504. doi: 10.1016/0165-3806(81)90018-3. [DOI] [PubMed] [Google Scholar]
- 11.Kinney HC, White WF. Opioid receptors localize to the external granular cell layer of the developing human cerebellum. Neuroscience. 1991;45:13–21. doi: 10.1016/0306-4522(91)90099-a. [DOI] [PubMed] [Google Scholar]
- 12.Kornblum HI, Hurlbut DE, Leslie FM. Postnatal development of multiple opioid receptors in rat brain. Brain Res. 1987;465:21–41. doi: 10.1016/0165-3806(87)90226-4. [DOI] [PubMed] [Google Scholar]
- 13.Letchworth SR, Mathis JP, Rossi GC, Bodnar RJ, Pasternak GW. Autoradiographic localization of I-125[Tyr14]orphanin FQ/nociceptin, I-125[Tyr10]orphanin FQ/nociceptin1-11 binding sites in rat brain. J Comp Neurol. 2000;423:319–329. [PubMed] [Google Scholar]
- 14.Lieb K, Andersen C, Lazarov N, Zienecker R, Urban I, Reisert I, Pilgrim C. Pre- and postnatal development of dopaminergic neuron numbers in the male and female mouse midbrain. Dev Brain Res. 1996;94:37–43. doi: 10.1016/0165-3806(96)00063-6. [DOI] [PubMed] [Google Scholar]
- 15.Loh YP, Rius RA, Elkabes S, Bem W, Coscia CJ. Prenatal expression of pro-opiomelanocortin mRNA, POMC-derived peptides, and mu-opiate receptors in the mouse embryo. NIDA Res Monogr. 1991;111:96–112. [PubMed] [Google Scholar]
- 16.Martin TJ, Sim LJ, Selley DE, deMontis MG, Childers SR. Effects of intracerebroventricular administration of beta-funaltrexamine on DAMGO-stimulated [35S] GTP-gamma-S binding in rat brain sections. Synapse. 1997;27:177–182. doi: 10.1002/(SICI)1098-2396(199711)27:3<177::AID-SYN3>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 17.Neal CR, Akil H, Watson SJ. Expression of orphanin Fq and the opioid receptor-like ORL1 receptor in the developing human and rat brain. J Chem Neurol. 2001;224:219–249. doi: 10.1016/s0891-0618(01)00135-1. [DOI] [PubMed] [Google Scholar]
- 18.Nelson EE, Panksepp J. Brain substrates of infant–mother attachment: contributions of opioids, oxytocin, and norepinephrine. Neurosci Biobehav Rev. 1998;22:437–452. doi: 10.1016/s0149-7634(97)00052-3. [DOI] [PubMed] [Google Scholar]
- 19.Nitsche JF, Pintar JE. Opioid receptor-induced GTPgamma S-35 binding during mouse development. Dev Biol. 2003;253:99–108. doi: 10.1006/dbio.2002.0855. [DOI] [PubMed] [Google Scholar]
- 20.Paxinos G, Watson C, editors. The Rat Brain in Stereotaxic Coordinates. 4. Academic Press; New York: 1998. p. 256. [Google Scholar]
- 21.Reznikov K, Hauser KF, Nazarevskaja G, Trunova Y, Derjabin V, Bakalkin G. Opioids modulate cell division in the germinal zone of the late embryonic neocortex. Eur J Neurosci. 1999;11:2711–2719. doi: 10.1046/j.1460-9568.1999.00680.x. [DOI] [PubMed] [Google Scholar]
- 22.Rius RA, Barg J, Bem WT, Coscia CJ, Loh YP. The prenatal development profile of expression of opioid peptides and receptors in the mouse brain. Dev Brain Res. 1991;58:237–241. doi: 10.1016/0165-3806(91)90010-G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sim LJ, Xiao RY, Childers SR. Identification of opioid receptor-like ORL1 peptide-stimulated. [35S]GTPgammaS binding in rat brain. Neuroreport. 1996;73:729–733. doi: 10.1097/00001756-199602290-00012. [DOI] [PubMed] [Google Scholar]
- 24.Sim-Selley LJ, Vogt LJ, Childers SR, Vogt BA. Distribution of ORL-1 receptor binding and receptor-activated G-proteins in rat fore-brain and their experimental localization in anterior cingulate cortex. Neuropharmacology. 2003;45:220–230. doi: 10.1016/s0028-3908(03)00155-2. [DOI] [PubMed] [Google Scholar]
- 25.Spain JW, Roth BL, Coscia CJ. Differential ontogeny of multiple opioid receptors (mu, delta, and kappa) J Neurosci. 1985;5:584–588. doi: 10.1523/JNEUROSCI.05-03-00584.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tavani A, Robson LE, Kosterlitz HW. Differential postnatal development of mu-, delta- and chi-opioid binding sites in mouse brain. Brain Res. 1985;3552:306–309. doi: 10.1016/0165-3806(85)90056-2. [DOI] [PubMed] [Google Scholar]
- 27.Unnerstall JR, Molliver ME, Kuhar MJ, Palacios JM. Ontogeny of opiate binding sites in the hippocampus, olfactory bulb and other regions of the rat forebrain by autoradiographic methods. Brain Res. 1983;283:157–169. doi: 10.1016/0165-3806(83)90172-4. [DOI] [PubMed] [Google Scholar]
- 28.Wohltmann M, Roth BL, Coscia CJ. Differential postnatal development of mu and delta opiate receptors. Brain Res. 1982;255:679–684. doi: 10.1016/0165-3806(82)90066-9. [DOI] [PubMed] [Google Scholar]
- 29.Zagon IS, Gibo DM, McLaughlin PJ. Adult and developing human cerebella exhibit different profiles of opioid binding sites. Brain Res. 1990;523:62–68. doi: 10.1016/0006-8993(90)91635-t. [DOI] [PubMed] [Google Scholar]
- 30.Zahm DS, Williams EA, Latimer MP, Winn P. Ventral mesopontine projections of the caudomedial shell of the nucleus accumbens and extended amygdala in the rat: double dissociation by organization and development. J Comp Neurol. 2001;436:111–125. [PubMed] [Google Scholar]


