Abstract
The potential utility of plasmid DNA as an HIV-1 vaccination modality currently is an area of active investigation. However, recent studies have raised doubts as to whether plasmid DNA alone will elicit immune responses of sufficient magnitude to protect against pathogenic AIDS virus challenges. We therefore investigated whether DNA vaccine-elicited immune responses in rhesus monkeys could be augmented by using either an IL-2/Ig fusion protein or a plasmid expressing IL-2/Ig. Sixteen monkeys, divided into four experimental groups, were immunized with (i) sham plasmid, (ii) HIV-1 Env 89.6P and simian immunodeficiency virus mac239 Gag DNA vaccines alone, (iii) these DNA vaccines and IL-2/Ig protein, or (iv) these DNA vaccines and IL-2/Ig plasmid. The administration of both IL-2/Ig protein and IL-2/Ig plasmid induced a significant and sustained in vivo activation of peripheral T cells in the vaccinated monkeys. The monkeys that received IL-2/Ig plasmid generated 30-fold higher Env-specific antibody titers and 5-fold higher Gag-specific, tetramer-positive CD8+ T cell levels than the monkeys receiving the DNA vaccines alone. IL-2/Ig protein also augmented the vaccine-elicited immune responses, but less effectively than IL-2/Ig plasmid. Augmentation of the immune responses by IL-2/Ig was evident after the primary immunization and increased with subsequent boost immunizations. These results demonstrate that the administration of IL-2/Ig plasmid can substantially augment vaccine-elicited humoral and cellular immune responses in higher primates.
The worldwide spread of HIV-1 (1) will be controlled only by the development of an effective HIV-1 vaccine. The recognition of the limitations of traditional vaccination modalities for preventing HIV-1 infection has led to the development of a number of novel vaccination strategies, including recombinant live vectors and plasmid DNA (2). Intramuscular injection of purified plasmid DNA has been shown to transfect cells in mice (3) and induce antigen-specific antibody and cytotoxic T lymphocyte (CTL) responses (4–7). In particular, plasmids encoding HIV-1 and simian immunodeficiency virus (SIV) proteins have been shown to elicit specific humoral and cellular immune responses in both mice (8–10) and rhesus monkeys (11–16).
The immune responses elicited by DNA vaccination have afforded a degree of protection in nonhuman primates against challenges with nonpathogenic AIDS viruses (17–20), but these immune responses have not been of a magnitude sufficient to protect against pathogenic viral challenges (21). We therefore were interested in exploring strategies for augmenting DNA vaccine-elicited immune responses. Augmentation of vaccine-elicited antibody and CTL responses has been demonstrated in mice by using cytokine administration and by triggering of costimulatory signaling. However, such approaches, to date, have not been applied successfully in nonhuman primates.
Augmentation of DNA vaccine-elicited immune responses using plasmid IL-2 has been reported in several murine disease models (22–25). We sought to build on this observation by exploring the utility of IL-2/Ig as a vaccine adjuvant. IL-2/Ig is a fusion protein that has IL-2 functional activity and the advantages of divalent avidity and a long in vivo half-life (26, 27). We have reported previously that an IL-2/Ig plasmid was able to augment the antibody and CTL responses elicited by an HIV-1 gp120 DNA vaccine in mice (28). In fact, IL-2/Ig was significantly more effective than native IL-2 as a vaccine adjuvant, and augmentation was most marked when the plasmid cytokine was delivered 2 days after the DNA vaccine (28). The present study was performed to evaluate the ability of plasmid-encoded IL-2/Ig to augment DNA vaccine-elicited HIV-1 and SIV-specific immune responses in rhesus monkeys.
Materials and Methods
Construction of IL-2/Ig Plasmids.
Human IgG2 cDNA was prepared by reverse transcription–PCR (Stratagene) from an IgG2-expressing myeloma cell line. Human IL-2 and human IgG2 Fc were amplified by PCR using Pfu polymerase (Stratagene) and synthetic primers with engineered BglII and PvuI restriction sites (Operon Technologies, Alameda, CA) (IL-2 forward, 5′-cgc aga tct atg tac agg atg caa ctc ctg tct tg-3′; IL-2 reverse, 5′-cgc cga tcg gtc agt gtt gag atg atg ctt tg-3′; IgG2 Fc forward, 5′-cgc cga tcg caa atg ttg tgt cga gtg ccc acc-3′; IgG2 Fc reverse, 5′-cgc aga tct tat cat tta ccc gga gac agg gag agg ctc- 3′). The PCR products were restriction-digested to generate a BglII/PvuI IL-2 fragment and a PvuI/BglII Ig fragment. The pV1J and pCMV (Invitrogen) expression vectors were digested with BglII, and the pV1J-IL-2/Ig and pCMV-IL-2/Ig plasmids were constructed by a triple ligation using the vector and both inserts.
Production of IL-2/Ig Protein.
Murine myeloma NS-1 cells were stably transfected with the human IL-2/Ig fusion gene. One microgram of linearized pCMV-IL-2/Ig expression plasmid containing the neomycin resistance gene was added to 107 washed NS-1 cells in PBS and electroporated at 1.5 kV and 3 μF with a Bio-Rad Gene Pulser System. Transfectants were selected in R10 medium containing 1.5 mg/ml G418 (Geneticin; Life Technologies, Gaithersburg, MD) and cloned twice by limiting dilution in 96-well plates. Large-scale cultures of transfected NS-1 cells were grown in UltraDOMA medium (BioWhittaker) with 1% low IgG-containing FCS (HyClone). Culture supernatants were filtered through a 0.2-μm filtration apparatus, and purification of 10 liters was performed by using a 2-ml protein A-Sepharose column (Amersham Pharmacia) at a flow rate of 5 ml/min. The column then was washed with 50 ml of PBS, eluted with 0.1 M citrate, pH 4.0, and immediately neutralized with 0.3 vol of 1 M Tris⋅HCl, pH 8. Fractions containing protein were pooled and dialyzed extensively against PBS. The final yield of IL-2/Ig fusion protein was 0.5–1.0 mg/liter of culture supernatant. Analysis of the final IL-2/Ig fusion protein was performed by SDS/PAGE and gel-filtration HPLC, and activity was measured by an IL-2 ELISA (BioSource International, Camarillo, CA) and cellular proliferation assays.
Selection and Vaccination of Monkeys.
To select adult rhesus monkeys (Macaca mulatta) that expressed the Mamu-A*01 MHC class I allele, a PCR-based assay was utilized (29). Briefly, DNA was extracted from peripheral blood lymphocytes (PBL) by using a QIAmp Blood Kit (Qiagen, Chatsworth, CA). PCR then was performed using Mamu-A*01-specific primers (forward, 5′ gac agc gac gcc gcg agc caa 3′; reverse, 5′ cgc tgc agc gtc tcc ttc ccc 3′). Two additional primers specific for a conserved MHC class II sequence also were included as internal positive controls (forward, 5′ gcc tcg agt gtc ccc cca gca cgt ttc 3′; reverse, 5′ gca agc ttt cac ctc gcc gct g 3′). Electrophoresis on a 2% agarose gel yielded a 685-bp band in Mamu-A*01-positive samples and a 260-bp control band in all samples. Verification of positive samples was achieved by complete DNA sequence analysis and comparison with the published Mamu-A*01 sequence (30). Monkeys were housed at Southern Research Institute, Frederick, MD. The animals were maintained in accordance with Henry M. Jackson Foundation and Harvard Medical School guidelines.
Maxipreparations of plasmids were carried out by alkaline lysis followed by double-CsCl gradient banding as described (28). Twelve monkeys were vaccinated by separate intramuscular injections of 5 mg of HIV-1 89.6P Env (KB9) DNA and 5 mg of SIV mac239 Gag DNA in sterile saline without adjuvant. Half the dose was delivered to each quadriceps muscle, and each injection was delivered in a 0.5-ml volume by using a needle-free Biojector apparatus and a no. 3 syringe (Bioject, Portland, OR). Four additional monkeys received 10 mg of sham plasmid DNA. Of the 12 vaccinated monkeys, 4 received 5 mg of IL-2/Ig plasmid on day 2 after DNA vaccination, 4 received 5 mg of sham plasmid on day 2 after DNA vaccination, and 4 received 0.5 mg/day IL-2/Ig protein as twice-daily intramuscular injections on days 1–14 after DNA vaccination.
CD25 Staining of Lymphocytes.
Phycoerythrin–Texas red (ECD)-labeled anti-human CD8αβ (2ST8–5H7; Beckman Coulter), allophycocyanin (APC)-labeled anti-rhesus monkey CD3 (FN18; gift from D. M. Neville, Jr., National Institutes of Health, Bethesda, MD), and phycoerythrin (PE)-labeled anti-human CD25 (M-A251; PharMingen) mAbs were used to stain fresh PBL from vaccinated rhesus monkeys. Antibodies were added to 100 μl of whole blood, and red blood cells were lysed by using an Immunoprep Reagent Q-Prep Workstation (Beckman Coulter). Stained PBL were washed with 3 ml of PBS, resuspended in 0.5 ml of PBS containing 1.5% paraformaldehyde, and analyzed by using a Coulter EPICS flow cytometry system.
Anti-Env Antibody ELISA.
A direct ELISA was used to measure plasma titers of anti-gp120 antibodies. Ninety-six-well Maxisorp ELISA plates (Nunc) were coated overnight at 4°C with 100 μl of PBS containing 0.5 μg/ml HIV-1 Env 89.6 gp140 (gift from Robert Doms, University of Pennsylvania). The remainder of the ELISA was carried out at room temperature. After a wash with PBS containing 0.05% Tween-20, the wells were blocked for 2 hr with a solution containing 2% BSA (Sigma) and 0.05% Tween-20 in PBS. Plasma samples were serially diluted in 2% BSA/0.05% Tween-20 and added to the ELISA wells. After a 1-hr incubation, the plate was washed three times and then incubated with a 1:3,000 dilution of a peroxidase-conjugated anti-human IgG + IgM secondary antibody (The Jackson Laboratory) for 1 hr. The plate was washed three times, developed with 3,3′,5,5′-tetramethylbenzidine (TMB) (K&P Laboratories, Gaithersburg, MD), stopped with 1% HCl, and analyzed at 450 nm with a Dynatech MR5000 ELISA plate reader.
Functional CTL Assays.
PBL from rhesus monkeys expressing the Mamu-A*01 MHC class I allele (30) were isolated and washed in Hanks' balanced salt solution containing 2% FCS. PBL (5 × 106) in 2 ml of RPMI 1640 medium containing 12% FCS (R12) were cultured in the presence of 10 μg/ml optimal p11C peptide from SIV Gag (amino acids 181–189; CTPYDINQM) (30, 31). This Mamu-A*01-restricted nonamer peptide previously has been referred to as p11C, C-M (32). On day 3 of culture, 2 ml of 40 units/ml human recombinant IL-2 (Hoffman–La Roche) was added. On day 12 of culture, peptide-stimulated PBL were centrifuged over Ficoll (Ficoll/Paque) and assessed as effectors in standard 51Cr-release assays by using U-bottomed, 96-well plates containing 104 target cells per well. Autologous B lymphoblastoid cell lines pulsed with 1 μg/ml p11C peptide or p11B control peptide (ALSEGCTPYDIN) and labeled overnight with 100 μCi/ml 51Cr were used as targets. All wells were assayed in duplicate. After incubating effector and target cells together for 4 hr at 37°C, supernatants were harvested, mixed with scintillation fluid, and measured by using a Wallac 1450 Microbeta liquid scintillation counter. To measure spontaneous release of 51Cr, target cells were incubated with 100 μl of medium, and for maximum release target cells were incubated with 100 μl of 2% Triton X-100. Percent lysis was calculated as: (experimental release − spontaneous release)/(maximum release − spontaneous release).
Tetramer Staining of Peptide-Specific CD8+ T Cells.
Soluble tetrameric Mamu-A*01/p11C complexes were prepared as described (32, 33). One microgram of phycoerythrin-labeled tetrameric Mamu-A*01/p11C complexes in conjunction with FITC-labeled anti-human CD8α (Leu2a; Becton Dickinson), ECD-labeled anti-human CD8αβ (2ST8–5H7; Beckman Coulter), and APC-labeled anti-rhesus CD3 (FN18) mAbs was used to stain p11C-specific CD8+ T cells as described (32). One hundred microliters of whole blood from the vaccinated monkeys was directly stained with these reagents, lysed on an Immunoprep Reagent Q-Prep Workstation (Coulter), washed in 3 ml of PBS, and fixed in 0.5 ml of PBS containing 1.5% paraformaldehyde. Alternatively, 5 × 105 cultured PBL from the 12-day, peptide-stimulated cultures were similarly stained, washed, and fixed. Samples were analyzed by four-color flow cytometry on a Coulter EPICS Elite ESP system. Gated CD3+CD8+ T cells were examined for staining with tetrameric Mamu-A*01/p11C complexes.
Statistical Analysis.
To determine whether immune responses among the groups of monkeys were significantly different from each other, ANOVA was performed by using a 95% confidence interval (Microsoft excel 97 statistical package). A value of P < 0.05 was considered significant.
Results
Production of IL-2/Ig Plasmid and IL-2/Ig Protein.
The cytokine/Ig fusion protein selected for evaluation in this study consisted of human IL-2 fused to the Fc portion of human IgG2. We utilized a construct based on human sequences because rhesus monkey and human IL-2 and Ig amino acid sequences are >99% identical and because human IL-2 is capable of driving the proliferation of rhesus monkey T lymphocytes. The IgG2 isotype was chosen because of its limited capacity to facilitate antibody-dependent, cell-mediated cytotoxicity and complement fixation. The plasmid-encoded IL-2/Ig used in this study was a pV1J vector (13, 34, 35) expressing IL-2/Ig under control of a strong cytomegalovirus (CMV) promoter and enhancer. Expression was confirmed by transient transfection of COS cells followed by analysis of culture supernatants by using an IL-2 ELISA (data not shown). To produce IL-2/Ig fusion protein, a pCMV vector expressing IL-2/Ig was constructed and used to transfect murine NS-1 myeloma cells as described (36). Stable transfectants were selected, cloned, and screened for high levels of IL-2/Ig expression. The IL-2/Ig protein then was purified from large volumes of tissue culture supernatant by using protein A-Sepharose columns. The purified IL-2/Ig protein was a single, 45-kDa band as analyzed by SDS/PAGE, a 90-kDa dimer as determined by gel-filtration chromatography, readily detected by using an IL-2 ELISA, and fully biologically active as determined by CTLL proliferation assays (data not shown). The endotoxin level of the final product was less than 10 units/ml (Sigma E-Toxate assay).
Vaccine Trial Design.
SIV Gag-specific CTL in rhesus monkeys can be quantitated using both functional and tetramer-binding assays through analysis of CD8+ T lymphocyte recognition of the optimal Gag p11C epitope (amino acids 181–189) restricted by the HLA-A homolog allele Mamu-A*01 (29, 30). A colony of rhesus monkeys therefore was screened for the presence of the Mamu-A*01 gene by PCR using Mamu-A*01-specific primers. Eleven Mamu-A*01-positive monkeys were identified by PCR and confirmatory DNA sequencing. These monkeys, as well as 5 Mamu-A*01-negative monkeys, were included in the vaccination trial.
DNA vaccines expressing HIV-1 89.6P Env gp140 (KB9) or SIV mac239 Gag in the pV1R backbone were used as immunogens. The HIV-1 89.6 envelope gene, which was derived from a primary patient R5/X4 dual-tropic HIV-1 isolate, was used with the SIVmac239 backbone to construct a simian-HIV (SHIV) chimera (37). In vivo passage of this virus yielded the pathogenic virus SHIV89.6P. The KB9 envelope clone was derived from this pathogenic viral isolate (38).
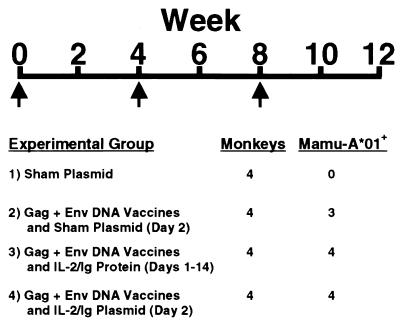
Four groups of rhesus monkeys, each composed of four animals, were vaccinated as outlined in Fig. 1. The 11 available Mamu-A*01-positive monkeys were placed into the groups receiving the experimental DNA vaccines. Ten milligrams of a sham plasmid was injected into the quadriceps muscles of one group of rhesus monkeys by using a needle-free Biojector apparatus. Five milligrams of the env DNA vaccine and 5 mg of the gag DNA vaccine were administered as separate injections to the other three groups by the same method of inoculation. Of these vaccinated animals, one group also received 5 mg of sham plasmid on day 2 after vaccination, one group received 5 mg of IL-2/Ig plasmid on day 2 after vaccination, and one group received 0.5 mg/day IL-2/Ig protein on days 1–14 after vaccination. The IL-2/Ig protein was injected intramuscularly in two divided doses per day. The monkeys received a similar immunization with cytokine administration at week 4 and another immunization without cytokine administration at week 8. The vaccination and cytokine regimens were well tolerated.
Figure 1.
Vaccine trial design. Sixteen monkeys were divided into four experimental groups and were immunized with: 1) 10 mg of sham plasmid; 2) 5 mg of HIV-1 Env 89.6P and 5 mg of SIV mac239 Gag DNA vaccines; 3) DNA vaccines and IL-2/Ig protein; or 4) DNA vaccines and IL-2/Ig plasmid. Five milligrams of sham plasmid was administered to the animals that received the DNA vaccines alone on day 2 after the week 0 and week 4 immunizations. IL-2/Ig protein (0.5 mg/day) was administered by twice-daily i.m. injections on days 1–14 after the week 0 and week 4 immunizations. Five milligrams of IL-2/Ig plasmid was administered on day 2 after the week 0 and week 4 immunizations. The week 8 immunization was performed without cytokine administration.
Plasma IL-2/Ig levels in the vaccinated animals were determined by ELISA. Peak levels, measured 3 hr after the first IL-2/Ig protein injection, ranged from 41 to 102 ng/ml. Trough levels ranged from 13 to 23 ng/ml. Comparable trough cytokine levels were detected on days 5 and 14, suggesting that no functionally significant neutralizing antibody response against the cytokine fusion protein developed during this period of cytokine administration. Plasma IL-2/Ig levels in the animals that received the IL-2/Ig plasmid or in the animals that received the DNA vaccines alone were <0.1 ng/ml.
CD25 Expression on Peripheral T Cells of the Vaccinated Monkeys.
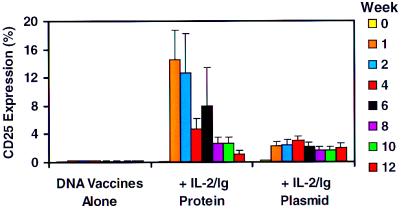
CD25 expression on peripheral T cells served as a marker for activation and in vivo responsiveness to IL-2/Ig. As shown in Fig. 2, CD3+ lymphocytes from the animals that received the DNA vaccines alone did not demonstrate significant levels of CD25 expression. In contrast, CD3+ lymphocytes from the animals that received IL-2/Ig protein showed a dramatic increase in CD25 expression during the time of cytokine/Ig treatment, peaking at 1 week after the first inoculation and then declining. The percentage of CD3+ lymphocytes that expressed CD25 in the monkeys that received IL-2/Ig plasmid was initially low, but the level of CD25 expression in these monkeys remained significant and sustained through week 12 at a level comparable to the T cells of the monkeys that received IL-2/Ig protein. CD25 expression on CD4+ T cells and CD8+ T cells showed similar kinetic trends, although slightly higher levels of CD25 expression were evident on CD4+ T cells and slightly lower levels on CD8+ T cells (data not shown).
Figure 2.
IL-2/Ig protein and IL-2/Ig plasmid administration lead to significant CD25 expression on CD3+ PBL of the vaccinated monkeys. The monkeys were bled at week 0, week 1, week 2, and every 2 weeks thereafter. Gated CD3+ lymphocytes from whole blood were examined for staining with an anti-CD25 mAb by flow cytometry. The monkeys that received IL-2/Ig protein demonstrated a striking rise and rapid fall of CD25 expression. The monkeys that received IL-2/Ig plasmid showed a less dramatic but more sustained increase in CD25 expression. Means and SE for each group are shown.
Humoral Immune Responses.
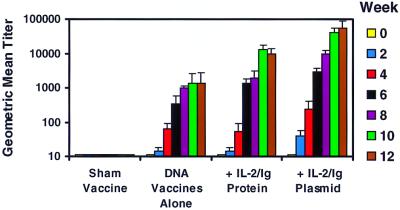
Plasma samples from the vaccinated monkeys were analyzed for anti-Env antibodies by a direct ELISA using purified Env 89.6 protein. Fig. 3 shows that anti-Env antibodies were detected in the vaccinated monkeys by week 2 after primary immunization. All the DNA-vaccinated animals seroconverted by week 4 and had higher anti-Env antibody titers after the boost immunizations. In contrast, the sham-vaccinated animals did not develop detectable anti-Env antibodies. By week 12, the group that received IL-2/Ig protein had a 10-fold higher antibody titer when compared with the group that received the DNA vaccines alone. More strikingly, the group that received IL-2/Ig plasmid had more than a 30-fold higher antibody titer when compared with the group that received the DNA vaccines alone (P < 0.001). These antibodies were not capable of neutralizing free virus.
Figure 3.
IL-2/Ig protein and IL-2/Ig plasmid administration augment vaccine-elicited anti-Env antibody responses in monkeys. Plasma was obtained from the vaccinated animals every 2 weeks and analyzed for anti-Env antibodies by ELISA. Sham-vaccinated monkeys did not have detectable anti-Env antibody titers. The most striking and consistent augmentation of anti-Env antibody titers was observed in the animals that received IL-2/Ig plasmid. Geometric mean titers and SE for each group are shown.
Cellular Immune Responses.
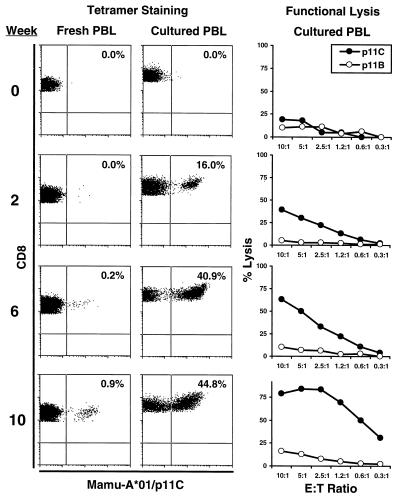
Staining with tetrameric MHC class I/peptide complexes and analysis by flow cytometry recently has proven to be an accurate method for quantitating epitope-specific CTL in both whole-blood and peptide-stimulated cell cultures (32, 33). Both tetramer staining and functional cytotoxicity assays documented the rapid emergence of SIV Gag-specific CTL in the vaccinated animals. CTL specific for the Mamu-A*01-restricted immunodominant SIV Gag p11C epitope (CTPYDINQM) (30, 31) were monitored in the 11 Mamu-A*01-positive monkeys by tetramer staining of fresh PBL, tetramer staining of PBL stimulated in vitro with the p11C peptide, and functional 51Cr release assays using peptide-stimulated PBL as effector cells. Fig. 4 shows an example of the tetramer staining and functional lysis data generated in monkey 772, a representative animal that received the DNA vaccines and IL-2/Ig protein. Tetramer-positive CD3+CD8+ cells were detected in peptide-stimulated PBL 2 weeks after the primary immunization and in freshly isolated PBL 2 weeks after the second immunization. The level of functional cytotoxicity correlated well with the level of tetramer staining in these lymphocyte populations. These data suggest that the tetramer-positive CD3+CD8+ cells represent functional CTL.
Figure 4.
Evolution of the SIV Gag p11C-specific CTL response detected by tetramer staining and functional cytotoxicity assays in a representative vaccinated monkey. Tetramer staining of fresh PBL, tetramer staining of p11C-stimulated PBL, and functional lysis of p11C-pulsed targets by epitope peptide-stimulated PBL are shown for the following time points: week 0 (preimmune), week 2 (2 weeks after the primary immunization), week 6 (2 weeks after the second immunization), and week 10 (2 weeks after the third immunization). Staining with the Mamu-A*01/p11C tetramer was assessed by four-color flow cytometry on gated CD3+CD8+ lymphocytes. Functional lysis was determined by using p11C epitope peptide- and p11B control peptide-pulsed target cells. Tetramer staining was evident in peptide-stimulated PBL after the primary immunization and in fresh PBL after the second immunization. Functional lysis correlated well with tetramer staining. This particular monkey (772) received the DNA vaccines and IL-2/Ig protein.
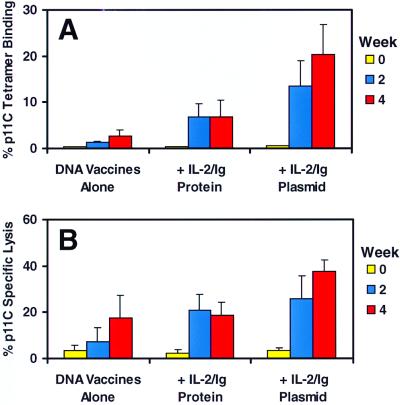
Fig. 5 summarizes the tetramer staining and functional cytotoxicity data of p11C-stimulated PBL from the Mamu-A*01-positive vaccinated monkeys after the primary immunization. No p11C-specific CTL were detected in the preimmune bleeds from the monkeys. By week 4, tetramer-positive CD8+ T cells and functional CTL were detected in peptide-stimulated PBL from all the vaccinated monkeys. Background tetramer staining of the week 4 peptide-stimulated PBL using a control Mamu-A*01/Pol p68A (STPPLVRLV) tetramer was <0.1% (data not shown). Fig. 5A depicts the mean percentage of Mamu-A*01/Gag p11C tetramer staining of p11C-stimulated PBL for each group of monkeys, demonstrating that IL-2/Ig plasmid administration significantly augmented the Gag p11C-specific CD8+ T cell response and that IL-2/Ig protein administration had less of an enhancing effect. Fig. 5B shows that the mean functional cytotoxicity of these peptide-stimulated PBL correlated with the levels of tetramer staining.
Figure 5.
IL-2/Ig protein and IL-2/Ig plasmid administration augment SIV Gag p11C-specific CTL responses in monkeys after primary immunization. p11C-specific CTL responses were analyzed every 2 weeks by tetramer staining (A) and functional cytotoxicity assays (B) using peptide-stimulated PBL. Staining with the Mamu-A*01/p11C tetramer was assessed by four-color flow cytometry on gated CD3+CD8+ lymphocytes. Specific functional lysis was determined by using chromium-release cytotoxicity assays at effector-to-target ratios of 10:1. The most consistent and significant augmentation was observed in the monkeys that received IL-2/Ig plasmid. Means and SE for each group are shown.
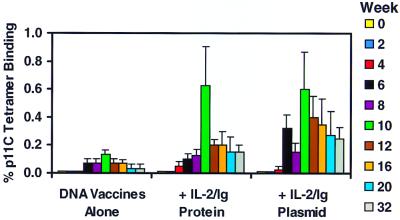
The IL-2/Ig-mediated augmentation of the p11C-specific CTL responses persisted after the boost immunizations in the Mamu-A*01-positive monkeys, as shown in Fig. 6. Tetramer-positive CD8+ T cells were detected in freshly isolated PBL by week 6, 2 weeks after the second immunization. The animals that received IL-2/Ig plasmid had significantly higher levels of circulating tetramer-positive CD8+ T cells as compared with the animals that received DNA vaccines alone (P < 0.05). The tetramer-positive CD8+ T cell responses peaked 2 weeks after each boost immunization and then declined. At week 10, 2 weeks after the third immunization, p11C-specific CD8+ T cells accounted for an average of 0.6% of circulating T cells in both groups of monkeys that received IL-2/Ig, a level 5-fold higher than in the animals that received the DNA vaccines alone.
Figure 6.
IL-2/Ig protein and IL-2/Ig plasmid administration augment SIV Gag p11C-specific CTL responses in fresh PBL from the vaccinated monkeys. p11C-specific CTL responses were analyzed every 2 weeks by tetramer staining of fresh PBL. Staining with the Mamu-A*01/p11C tetramer was assessed by four-color flow cytometry on gated CD3+CD8+ lymphocytes. The most consistent and significant augmentation was observed in the monkeys that received IL-2/Ig plasmid. Means and SE for each group are shown.
Discussion
Cytokines have been shown in a number of murine models to enhance virus-specific immune responses elicited by DNA vaccines. Coinoculation of a plasmid expressing granulocyte/macrophage colony-stimulating factor (GM-CSF) with a rabies virus DNA vaccine was demonstrated to increase the rabies-specific antibody response in mice (39). Administration of plasmids expressing IL-2 or GM-CSF also has been reported to augment cellular immune responses generated by hepatitis B and C virus DNA vaccines (22–24). We previously have reported that an IL-2/Ig fusion protein can augment DNA vaccine-elicited immune responses in mice more effectively than native IL-2 (28). The present study demonstrates clear-cut, enhanced, vaccine-elicited humoral and cellular immune responses through cytokine administration in nonhuman primates. The augmentation of tetramer-positive CD8+ T cell responses was observed 2 weeks after vaccination and persisted over time for at least 32 weeks.
We have shown effectiveness of cytokine administration in monkeys by using both IL-2/Ig protein and IL-2/Ig plasmid. Because IL-2 has been shown in vitro to activate T cells and to induce the expression of the IL-2 receptor subunit CD25, we monitored these effects in vivo on circulating T lymphocytes in the monkeys that received IL-2/Ig. Administration of either IL-2/Ig protein or IL-2/Ig plasmid led to a significantly increased expression of CD25 on peripheral T cells, suggesting that both methods of cytokine administration induced IL-2 receptor expression on T cells in vivo and led to a state of systemic immune stimulation. The animals treated with IL-2/Ig protein showed a dramatic rise followed by a rapid fall in CD25 expression, whereas those injected with IL-2/Ig plasmid showed a lower but sustained level of CD25 expression. Interestingly, by week 8, the two groups of IL-2/Ig-treated animals had comparable levels of CD25 expression on peripheral T cells. Because the monkeys that received the IL-2/Ig plasmid had undetectable levels of plasma IL-2/Ig (<0.1 ng/ml), the IL-2/Ig plasmid likely functions by expressing a low but persistent level of functional cytokine in vivo.
The temporal sequence of administering the IL-2/Ig plasmid after the DNA vaccine in the present study was based on a protocol devised in our previous experiments optimizing the delivery of these constructs in mice (28). We have not carried out an analogous study to optimize such a protocol in monkeys. In the present study, the IL-2/Ig plasmid was more effective than the IL-2/Ig protein in augmenting vaccine-elicited humoral and cellular immune responses, suggesting that a sustained release of low-dose IL-2 may be more effective than shorter courses of high-dose IL-2 as a vaccine adjuvant. In addition, administering single injections of IL-2/Ig plasmid has significant practical and economic advantages over frequent dosing of purified IL-2/Ig protein. The IL-2/Ig plasmid also may prove useful in augmenting immune responses elicited by other vaccine modalities.
Acknowledgments
We acknowledge Meryl A. Forman, Robert W. Doms, Keith A. Reimann, Wenyu Lin, and Frederick Vogel for generous advice, assistance, and reagents. We acknowledge support from Grants AI/GF-41521 (T.B.S.), AI-42298 (T.B.S.), NO1-AI-65301 (M.G.L.), AI-85343 (N.L.L.), and CA-50139 (N.L.L).
Abbreviations
- CTL
cytotoxic T lymphocyte
- SIV
simian immunodeficiency virus
- SHIV
simian HIV
- PBL
peripheral blood lymphocytes
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.050417697.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.050417697
References
- 1.United Nations Programme on HIV/AIDS (UNAIDS) Report on the global HIV/AIDS epidemic. 1998. [Google Scholar]
- 2.Letvin N L. Science. 1998;280:1875–1880. doi: 10.1126/science.280.5371.1875. [DOI] [PubMed] [Google Scholar]
- 3.Wolff J A, Malone R W, Williams P, Chong W, Acsadi G, Jani A, Felgner P L. Science. 1990;247:1465–1468. doi: 10.1126/science.1690918. [DOI] [PubMed] [Google Scholar]
- 4.Tang D C, Devit M, Johnson S A. Nature (London) 1992;356:152–154. doi: 10.1038/356152a0. [DOI] [PubMed] [Google Scholar]
- 5.Ulmer J B, Donnelly J J, Parker S E, Rhodes G H, Felgner P L, Dwarki V J, Gromkowski S H, Deck R R, DeWitt C M, Friedman A, et al. Science. 1993;259:1745–1749. doi: 10.1126/science.8456302. [DOI] [PubMed] [Google Scholar]
- 6.McDonnell W M, Askari F K. New Engl J Med. 1996;334:42–45. doi: 10.1056/NEJM199601043340110. [DOI] [PubMed] [Google Scholar]
- 7.Donnelly J J, Ulmer J B, Shiver J W, Liu M A. Annu Rev Immunol. 1997;15:617–648. doi: 10.1146/annurev.immunol.15.1.617. [DOI] [PubMed] [Google Scholar]
- 8.Wang B, Boyer J, Srikantan V, Coney L, Carrano R, Phan C, Merva M, Dang K, Agadjanyan M, Gilbert L, et al. DNA Cell Biol. 1993;12:799–805. doi: 10.1089/dna.1993.12.799. [DOI] [PubMed] [Google Scholar]
- 9.Wang B, Ugen E K, Srikantan V, Agadjanyan M, Dang K, Refaeli Y, Sato A I, Boyer J D, Williams W V, Weiner D B. Proc Natl Acad Sci USA. 1993;90:4156–4160. doi: 10.1073/pnas.90.9.4156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lu S, Santoro J C, Fuller D H, Haynes J R, Robinson H L. Virology. 1995;209:147–154. doi: 10.1006/viro.1995.1238. [DOI] [PubMed] [Google Scholar]
- 11.Wang B, Boyer J, Srikantan V, Ugen K, Gilbert L, Phan C, Dang K, Merva M, Agadjanyan M, Newman M, et al. Virology. 1995;221:102–112. doi: 10.1006/viro.1995.1383. [DOI] [PubMed] [Google Scholar]
- 12.Shiver J W, Perry H C, Davies M-E, Freed D C, Liu M A. Ann N Y Acad Sci. 1995;772:198–208. doi: 10.1111/j.1749-6632.1995.tb44745.x. [DOI] [PubMed] [Google Scholar]
- 13.Shiver J W, Davies M-E, Perry H C, Freed D C, Liu M A. J Pharm Sci. 1996;85:1317–1323. doi: 10.1021/js9600991. [DOI] [PubMed] [Google Scholar]
- 14.Liu M A, Yasutomi Y, Davies M-E, Perry H C, Letvin N L, Shiver J W. Antibiot Chemother. 1996;48:100–104. doi: 10.1159/000425163. [DOI] [PubMed] [Google Scholar]
- 15.Yasutomi Y, Robinson H L, Lu S, Mustafa F, Lekutis C, Arthos J, Mullins J I, Voss G, Manson K, Wyand M, et al. J Virol. 1996;70:678–681. doi: 10.1128/jvi.70.1.678-681.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lekutis C, Shiver J W, Liu M A, Letvin N L. J Immunol. 1997;158:4471–4477. [PubMed] [Google Scholar]
- 17.Letvin N L, Montefiori D C, Yasutomi Y, Perry H C, Davies M-E, Lekutis C, Alroy M, Freed D C, Lord C I, Handt L K, et al. Proc Natl Acad Sci USA. 1997;94:9378–9383. doi: 10.1073/pnas.94.17.9378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boyer J D, Ugen K E, Wang B, Agadjanyan M, Gilbert L, Bagarazzi M L, Chattergoon M, Frost P, Javadian A, Williams W V, et al. Nat Med. 1997;3:526–532. doi: 10.1038/nm0597-526. [DOI] [PubMed] [Google Scholar]
- 19.Kent S J, Zhao A, Best S J, Chandler J D, Boyle D B, Ramshaw I A. J Virol. 1998;72:10180–10188. doi: 10.1128/jvi.72.12.10180-10188.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Robinson H L, Montefiori D C, Johnson R P, Manson K H, Kalish M L, Lifson J D, Rizvi T A, Lu S, Hu S-L, Mazzara G P, et al. Nat Med. 1999;5:526–534. doi: 10.1038/8406. [DOI] [PubMed] [Google Scholar]
- 21.Lu S, Arthos J, Montefiori D C, Yasutomi Y, Manson K, Mustafa F, Johnson E, Santoro J C, Wissink J, Mullins J I, et al. J Virol. 1996;70:3978–3991. doi: 10.1128/jvi.70.6.3978-3991.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Geissler M, Gesien A, Tokushige K, Wands J R. J Immunol. 1997;158:1231–1237. [PubMed] [Google Scholar]
- 23.Geissler M, Gesien A, Wands J R. J Immunol. 1997;159:5107–5113. [PubMed] [Google Scholar]
- 24.Chow Y-H, Huang W-L, Chi W-K, Chu Y-D, Tao M-H. J Virol. 1997;71:169–178. doi: 10.1128/jvi.71.1.169-178.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xin K-Q, Hamajima K, Sasaki S, Honsho A, Tsuji T, Ishii N, Cao X-R, Lu Y, Fukushima J, Shapshak P, et al. Immunology. 1998;94:438–444. doi: 10.1046/j.1365-2567.1998.00533.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Landolfi N F. J Immunol. 1991;146:915–919. [PubMed] [Google Scholar]
- 27.Nickerson P, Zheng X X, Steiger J, Steele A W, Steurer W, Roy-Chaudhury P, Muller W, Strom T B. Transplant Immunol. 1996;4:81–85. doi: 10.1016/s0966-3274(96)80043-8. [DOI] [PubMed] [Google Scholar]
- 28.Barouch D H, Santra S, Steenbeke T D, Zheng X X, Perry H C, Davies M-E, Freed D C, Craiu A, Strom T B, Shiver J W, et al. J Immunol. 1998;161:1875–1882. [PubMed] [Google Scholar]
- 29.Knapp L A, Lehmann E, Piekarczyk M S, Urvater J A, Watkins D I. Tissue Antigens. 1997;50:657–661. doi: 10.1111/j.1399-0039.1997.tb02927.x. [DOI] [PubMed] [Google Scholar]
- 30.Miller M D, Yamamoto H, Hughes A H, Watkins D I, Letvin N L. J Immunol. 1991;147:320–329. [PubMed] [Google Scholar]
- 31.Allen T M, Sidney J, del Guercio M-F, Glickman R L, Lensmeyer G L, Wiebe D A, DeMars R, Pauza C D, Johnson R P, Sette A, et al. J Immunol. 1998;160:6062–6071. [PubMed] [Google Scholar]
- 32.Kuroda M J, Schmitz J E, Barouch D H, Craiu A, Allen T M, Sette A, Watkins D I, Forman M A, Letvin N L. J Exp Med. 1998;187:1373–1381. doi: 10.1084/jem.187.9.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Altman J D, Moss P A H, Goulder P J R, Barouch D H, McHeyzer-Williams M G, Bell J I, McMichael A J, Davis M M. Science. 1996;274:94–96. [PubMed] [Google Scholar]
- 34.Chapman B S, Thayer R M, Vincent K A, Haigwood N L. Nucleic Acids Res. 1991;19:3979–3986. doi: 10.1093/nar/19.14.3979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Montgomery D L, Shiver J W, Leander K R, Perry H C, Friedman A, Martinez D, Ulmer J B, Donnelly J J, Liu M A. DNA Cell Biol. 1993;12:777–783. doi: 10.1089/dna.1993.12.777. [DOI] [PubMed] [Google Scholar]
- 36.Zheng X X, Steele A W, Nickerson P W, Steurer W, Steiger J, Strom T B. J Immunol. 1995;154:5590–5600. [PubMed] [Google Scholar]
- 37.Reimann K A, Li J T, Voss G, Lekutis C, Tenner-Racz K, Racz P, Lin W, Montefiori D C, Lee-Parritz D E, Lu Y, et al. J Virol. 1996;70:3198–3206. doi: 10.1128/jvi.70.5.3198-3206.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reimann K A, Li J T, Veazey R, Halloran M, Park I-W, Karlsson G B, Sodroski J, Letvin N L. J Virol. 1996;70:6922–6928. doi: 10.1128/jvi.70.10.6922-6928.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xiang Z, Ertl H C J. Immunity. 1995;2:129–135. doi: 10.1016/s1074-7613(95)80001-8. [DOI] [PubMed] [Google Scholar]