Abstract
The relationships between blood lead (PbB) and saliva lead (PbSa) concentrations and the determinants of PbB and PbSa status in 970 low-income adults in the city of Detroit, Michigan were explored. Average PbB and PbSa values in the sample population were found to be 2.7 ± 0.1 μg/dl and 2.4 ± 0.13 μg/l (equivalent to 0.24 ± 0.13 μg/dl), respectively, and a weak but statistically significant association was found between the lead levels in the two types of body fluid samples. The average PbB level for men (4.0 ± 0.56 μg/dl) was higher than that for women (2.7 ± 0.11 μg/dl); other significant predictors of PbB included age, level of education, being employed, income level, the presence of peeling paint on the wall at home and smoking. There was no gender- or age-dependent difference in blood saliva values but statistically significant correlations were found between PbSa and level of education, employment, income level and smoking. Dental caries was severe in this population. Only 0.5% of the participants had no clinical signs of caries, over 80% had cavitated carious lesions (i.e., lesions that had progressed into dentin), and the number of lost teeth and carious lesions averaged 3.4 and 30, respectively. Weak but significant associations were found between PbB as well as PbSa and measures of dental caries in the study population. The positive associations are believed to be a reflection of the fact that the risk factors for dental caries, especially in low-income populations of the US, overlap extensively with those of lead poisoning and may not have a causal significance.
Keywords: Blood lead, Saliva lead, Dental caries, Lead exposure, Biomonitoring, Lead poisoning
Introduction
Blood and saliva are useful biofluids for monitoring the exposure and effects of lead in human population. Lead levels in blood (PbB) are widely accepted as the standard currency for estimating lead exposure and benchmarking its health effects (CDC, 1991). Because of cultural beliefs, plus growing apprehension about unintended use of blood samples (e.g., genetic screening for crime and drug enforcement), many at-risk members of urban communities are increasingly reluctant to volunteer blood samples for research. The use of other non-invasive methods for assessing environmental lead exposure has become a necessity if the magnitude of this well-documented problem in America’s inner cities is to be ascertained.
Saliva represents an easily accessible and useful body fluid for biomonitoring human exposure to environmental contaminants. Sialochemists point to several advantages of saliva over blood collection: it is non-invasive, so patients are spared the discomfiture of repeated venipunctures; it is the technique of choice for children and patients with limited coping abilities; costs are lower since there is no special training for staff and patients can collect samples themselves; there is no risk of infection, anemia or thrombosis; samples do not require special handling or preservation if they are to be used for trace metal analysis (Mandel, 1993; Jusko and Milsap, 1993). More importantly, the lead concentration in saliva (PbSa) is closely related to the unbound plasma fraction and intracellular level and thus reflects the internal lead level that can exert effects on human organs (Mandel, 1990; Wilson, 1993).
But despite recent growth in the application of sialochemistry to the clinical diagnosis of diseases, therapeutic and forensic drug monitoring, and the study of diseases of salivary glands (Mandel, 1993; Bardow et al., 2001; Sonesson et al., 2003; Moller et al., 2004), the use of saliva for environmental health purposes has been limited (Gonzalez et al., 1997; Koh et al., 2003; Timchalk et al., 2004). Lead is present in the saliva of lead-exposed and unexposed individuals, and the significant correlations that have been reported with PbB suggest that saliva may be a valuable tool for assessing lead exposure (DiGregorio et al., 1974; Fung et al., 1975; P’an, 1981; Brodeur et al., 1983).
In the oral cavity, high levels of lead can damage the acinar cells of the parotid gland, resulting in altered salivary secretion of protein, calcium and lysosomal enzyme N-acetyl-β-D-glucosaminidase (NAG) (Abdollahi et al., 2001). Lead poisoning can impair normal salivary function with xerostomia (dry mouth) being one of the first recognized clinical symptoms of lead poisoning (Pouchet, 1879; Nriagu, 1983). A number of lead-induced effects (including changes in emotional status, hormonal status, neurological status, immunological status and responsiveness as well as metabolic imbalances) can result in changes in the quality and quantity of saliva (Mandel, 1993). Although lead levels in saliva have not been directly associated with caries experience, the effects of lead on the oral ecology, and hence on the quality of life of many individuals, may be more significant than is generally recognized.
This study (a) compares the use of blood and saliva for biomonitoring the exposure to lead among a low-income adult population in Detroit, Michigan; (b) explores the relationships between PbSa and key demographic, lifestyle and environmental risk factors for lead exposure; and (c) assesses the relationships between PbB, PbSa and dental caries experience in a low-income African-American population in Detroit, Michigan.
Materials and methods
Study population
Blood and saliva samples were obtained from adult participants in a longitudinal study designed to collect three waves of data on principal determinants of oral health in a representative sample of low-income families in Detroit. Data in this report are taken from the first wave, and so are cross-sectional in design.
The participants in this study consisted of African-Americans in Detroit with household income below the 200th percentile of the federal poverty level. Participants were selected using a multistage area probability sample design based on data from Census 2000. The 39 census tracts within Wayne County with the highest proportion of African American households with income below 200% of poverty were selected, and a random sample of 565 blocks divided into 118 neighborhood segments was drawn from them. A household listing of these blocks was conducted from June to August 2002. From that household list, a number of housing units were chosen to yield the target sample of 1000 persons for the first wave of data collection. Between September 2002 and August 2003, a field team visited the selected housing units and successfully screened and recruited 1024 families to take part in the study. A family was defined as a primary caregiver and one child who had not yet attained his/her sixth birthday. When a family had more than one child under six, one of the children was designated as an ‘‘index child’’ for the study.
Interviews and collection of biofluids
All interviews, clinical examinations, and collection of blood and saliva samples were conducted at the same facility near downtown Detroit. Trained interviewers administered several face-to-face questionnaires to participating adult caregivers. The questions were designed to explore each caregiver’s general and oral health history, sociodemographic characteristics, and potential exposure to risk factors for lead. Dietary histories were completed by means of food frequency questionnaires (Block Dietary Systems, Berkeley, CA) completed through interviews with adults.
After all interviews and clinical examinations were completed, participating caregivers were asked to provide a sample of fingerstick blood and saliva. For collecting saliva, the participant first rinsed the mouth thoroughly with distilled water. The participant was then given a 15 ml LDPE bottle (Nalgene®) with a 6-inch Teflon-lined tube (Nalgene®) through which saliva dribbled down into the bottle. This procedure was timed for no more than 5 min, enough to yield a 5 ml sample. The minimum sample required was 3 ml. The bottle was then capped, placed in a zip lock bag, and stored in a freezer until transported to the laboratory in an icebox for analysis.
For blood samples, the participant first washed his/her hands thoroughly with low-metal soap, and then the middle finger was wiped thoroughly with acetone and dried. The puncture area, slightly off the ball of the fingertip on the middle finger, was cleaned with Milli-Q and again wiped dry, and then punctured with a B-D green Genie microtainer safety-flow lancet (Becton-Dickenson®, New Jersey). The first drop of blood was mopped up; subsequent blood drops were allowed to well up at the puncture site while holding the finger in a downward-pointing position. Two samples, each of 16 μl, were collected from the blood drops with a pipette (Brinkman Eppendorf series 2000®) and squirted into a LDPE tube containing 5 ml of 0.1% aqueous Triton X-100, 0.1% ammonium phosphate (NH4H2PO4), and 1 mg/ml of sodium heparin (diluent). The LDPE tube with the blood sample was capped, placed in a zip lock bag and stored in the refrigerator until transported to the laboratory in an icebox at the end of each day. After the draw of blood, the participant’s finger was wiped dry and an adhesive bandage applied.
Laboratory methods
All samples were analyzed using an Agilent 7500c series inductively coupled plasma/mass spectrometer (ICP-MS) equipped with a collision cell. Instrumental operating conditions recommended by the manufacturer were used. The blood samples diluted with the chemical cocktail in the sample tubes were injected into the instrument without further modification. Each saliva sample was diluted three-fold with trace metal free nitric acid before being analyzed. Each batch of 12 blood or saliva samples included a reagent blank (diluent with no blood), a duplicate sample and a standard reference blood sample (NIST 1640) mixed with the diluent. Detection limits were calculated as three times the standard deviation for the reagent blanks.
Dental caries evaluation
Each adult received a thorough dental clinical examination from a dentist. Findings were recorded according to a strict, well-tested protocol used in other dental epidemiological studies. Teeth were cleaned if necessary prior to the examination, and missing teeth recorded. Assessment of caries was primarily visual, with a ball-tipped explorer used only when necessary to confirm visual assessments. Each surface of each tooth was dried prior to examination and then categorized as sound, with incipient caries, with caries confined to enamel, early dentinal involvement, distinct dentinal caries, or open cavitation. Data for caries and other dental conditions were recorded in a laptop computer and later loaded into the study’s main database.
Data analysis
All data were encoded and compiled into a computer database. Values below the analytical detection limits were treated as if they were half the detection limits. The SAS software system was used to analyze the data and determine associations between PbB and PbSa and the various risk factors. Frequency plots show that PbB and PbSa approximated log normal distributions and transformed data was not employed in the statistical analysis. Multiple regression models were used to explore the relationships between PbB and PbSa levels with variables such as gender, age, socioeconomic indicators and risk behaviors (smoking, flushing the tap before drawing the water, etc.). In assessing the effects of lead on tooth decay, the lead levels were stratified into four categories for the multiple regression analysis.
Protection of human subjects
The study was approved by the Institutional Review Board (IRB) of the University of Michigan, Ann Arbor.
Results
Blood lead levels (PbB)
Complete questionnaire data and usable blood and saliva samples were provided by 934 adults, 49 (5%) male and 885 (95%) female. This predominance of females is not uncommon in poor African-American communities where households are mostly headed by women. The age of the caregivers ranged from 14 years to over 55 years with the largest group (45% of participants) being 25–34 years old (Table 1). About 44% of the study population had ≤12 years of education (12 years is completion of high school) and 32% had 13–14 years of education (Table 1). About one-third of the participants were employed at the time of the study.
Table 1.
Analysis of blood lead (μg/dl) with demographic categoriesa
| Demographic characteristics | Sample size, n | Blood lead level mean ± s.e.b | p-value |
|
| |||
| Sex | |||
| Male | 49 | 4.04 ± 0.56 | 0.03 |
| Female | 885 | 2.68 ± 0.11 | |
| Age | |||
| 14–24 yr | 325 | 2.60 ± 0.16 | <0.001 |
| 25–34 yr | 419 | 2.54 ± 0.14 | |
| 35–44 yr | 131 | 3.16 ± 0.26 | |
| 45–54 yr | 43 | 4.37 ± 0.65 | |
| 55+ yr | 16 | 4.29 ± 0.56 | |
| Education | |||
| 7–12 yr | 413 | 2.92 ± 0.15 | <0.001 |
| 13–14 yr | 302 | 2.59 ± 0.14 | |
| 15 yr | 181 | 2.49 ± 0.20 | |
| 16–19 yr | 28 | 2.66 ± 0.67 | |
| Employment | |||
| Yes | 350 | 2.56 ± 0.12 | 0.04 |
| No | 580 | 2.88 ± 0.14 | |
| Income | |||
| < $ 10,000 | 392 | 3.00 ± 0.19 | <0.001 |
| $ 10,000–$ 19,000 | 242 | 2.53 ± 0.16 | |
| $ 20,000–$ 29,000 | 139 | 2.66 ± 0.19 | |
| $ 30,000–$ 39,000 | 73 | 2.62 ± 0.40 | |
| $ 40,000 or more | 34 | 2.11 ± 0.31 | |
|
| |||
| Dental question | Sample size, n | Blood lead level mean ± s.e. | p-value |
|
| |||
| Smoked 100 cigarettes in life? | |||
| Yes | 426 | 3.19 ± 0.13 | <0.001 |
| No | 507 | 2.37 ± 0.10 | |
| Smoking now? | |||
| Yes | 391 | 3.26 ± 0.14 | 0.02 |
| No | 35 | 2.44 ± 0.34 | |
| How many years have you been smoking? | |||
| Correlation Pearson: 0.143 | 387 | 0.05 | |
| How many cigarettes a day? | |||
| Correlation Pearson: 0.10 | 385 | 0.15 | |
| How many years did you smoke? | |||
| Correlation Pearson: −0.05 | 28 | 0.96 | |
| How many cigarettes a day? | |||
| Correlation Pearson: −0.25 | 29 | 0.07 | |
| Ever used chewing tobacco or snuff? | |||
| Yes | 4 | 2.25 ± 0.24 | 0.04 |
| No | 927 | 2.74 ± 0.10 | |
| Previously tested for lead? | |||
| Yes | 257 | 2.92 ± 0.24 | 0.43 |
| No | 585 | 2.73 ± 0.10 | |
| Cracks on wall or peeling paint from walls or pipes? | |||
| Yes | 343 | 2.96 ± 0.13 | 0.03 |
| No | 582 | 2.63 ± 0.12 | |
| Remodeling or renovation of current living place in the last 5 years? | |||
| Yes | 280 | 2.81 ± 0.12 | 0.69 |
| No | 625 | 2.74 ± 0.13 | |
| Replacing of pipes inside current living place in the last 2 years? | |||
| Yes | 250 | 2.84 ± 0.20 | 0.47 |
| No | 645 | 2.67 ± 0.11 | |
|
| |||
| Biological question | Sample size, n | Blood lead level mean ± s.e. | p-value |
|
| |||
| Have water filter/purifier on kitchen tap? | |||
| Yes | 130 | 2.50 ± 0.28 | 0.30 |
| No | 796 | 2.77 ± 0.09 | |
| When taking water from tap, do you. . . | |||
| Catch first water | 33 | 2.36 ± 0.29 | 0.36 |
| Let tap run for few seconds | 248 | 2.64 ± 0.17 | |
| Let tap run for a minute | 641 | 2.80 ± 0.13 | |
| Is home close to factory, incarnation or industrial plant? | |||
| Yes | 115 | 2.72 ± 0.35 | 0.97 |
| No | 804 | 2.73 ± 0.10 | |
| Is home on the same block as car repair shop or gas station? | |||
| Yes | 183 | 2.84 ± 0.30 | 0.68 |
| No | 748 | 2.71 ± 0.09 | |
All analysis was done using a median value of 0.073 μg/dl for including blood lead values that were below the instrumental detection limit of 0.147 μg/dl.
s.e. = standard error.
The concentrations of lead in blood (PbB) of the study population ranged from non-detectable values (<0.15 μg/dl; n = 46) to over 20 μg/dl (n = 5). The distribution of the PbB values is close to national norms with most (about 72%) being in the 1.0–5 μg/dl range (Fig. 1). The average PbB value was 2.7 ± 0.1 μg/dl, a figure that is consistent with the results of the 1999–2000 National Health and Nutrition Examination Survey (NHANES 99-00). NHANES 99-00 reported a geometric mean PbB value of 1.66 μg/dl for the US population aged 1 year and older (CDC, 2003). The average PbB value for men (4.0 ± 0.56 μg/dl) was higher than that for women (2.7 ± 0.11 μg/dl) (Table 1), and other significant determinants of lead exposure seen in Table 1 include level of education, being employed, income level, and the presence of peeling paint on the wall at home.
Fig. 1.
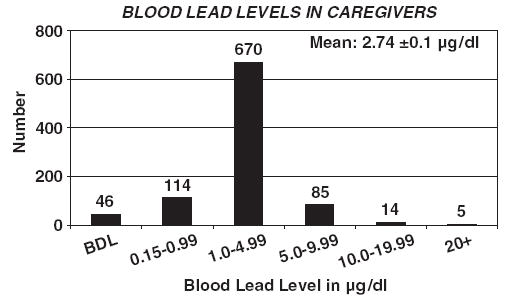
Distribution of lead in blood samples of participating adults (caregivers) involved in the study.
A number of lifestyle factors were associated with increased risk of lead exposure. Smokers, for example, had significantly higher PbB (3.3 ± 0.13 μg/dl) than did non-smokers (2.4 ± 0.10 μg/d). Smoking over 100 cigarettes in a lifetime and the length of time that a participant smoked were significantly associated with higher PbB (Table 1). Only a small number of participants chewed tobacco or used snuff and the effect of this exposure route on PbB could not be determined. Remodeling or renovation of participant’s living space in the last 5 years (before the study) was apparently not a risk factor for lead exposure. Contrary to what one would have expected, there was no significant association between PbB and living in a house near a factory, incinerator or industrial plant or in the same block as a repair shop or a gas station (Table 1). It would appear that the disappearance of such outdoor sources of lead contamination from the Detroit landscape might have some benefits in that not enough lead is being released into the ambient environment to affect the PbB burden of local inhabitants.
It is estimated that 80% of the homes in Detroit have lead pipes (MDCH, 2004). Drinking water, however, did not appear to be a risk factor for lead exposure. No significant association was found between PbB and recent replacement of pipes in the residence, the use of water filter/purifier, or running the tap for several seconds before collecting water for household use (Table 1). As the relative contributions of other sources to the body burden of lead decrease, however, one would expect the fraction from drinking water to become increasingly important. In the adult population of Detroit, the threshold where the drinking water becomes a major source of lead in blood has apparently not been reached.
Saliva lead levels (PbSa)
Average concentration of lead in saliva was 2.4 ± 0.13 μg/l (equivalent to 0.24 ± 0.13 μg/dl) for 969 individuals, among the lowest PbSa levels reported for non-occupationally exposed individuals. Other such values reported include 34 μg/l for parotid samples by DiGregorio et al. (1974), 55 μg/l for whole saliva by P’an (1981) and 31 μg/l for whole saliva by Gonzalez et al. (1997). Trace metal measurements made before the late 1980s carried high risk of being compromised by contamination artifacts (Settle and Patterson, 1980) and most of the PbSa values published before this period are suspect. A study by Wilhem et al. (2002) found that 89% of the lead content of saliva samples was below the limit of 1.5 μg/l, consistent with our observation that the lead concentration in saliva is lower than has generally been presumed. Reported PbSa levels in occupationally exposed individuals tend to be higher than our values: 129 μg/l by Brodeur et al. (1983), 4.8 μg/l by Omokhodion and Crawford (1991) and 7.7 μg/l by Koh et al. (2003).
There was no significant gender difference in PbSa values (Table 2). There was, however, a statistically significant correlation between PbSa and level of education, though the somewhat higher PbSa concentration in people with higher educational attainment may be biased by the small sample size. A weak correlation (p = 0.06) was found between employment and PbSa, with more lead found in blood of unemployed participants (2.6 ± 0.19 μg/l) than those who were employed (2.1 ± 0.14 μg/l). The PbSa concentrations had an inverse correlation with income; higher PbSa values were associated with lower incomes (Table 2). However, the small sample size (n = 37) of people earning over $ 40,000 per year is believed to be biasing their PbSa on the high side. The PbSa for current smokers (2.5 ± 0.16 μg/l) were significantly higher than those for non-smokers (1.8 ± 0.29 μg/l). The following variables were not found to be associated with lead levels in saliva: cracks on wall and peeling paint, remodeling of current living place, use of water filter/purifier on kitchen tap, using first draw water from the tap, or living near some potential point sources for lead (Table 2).
Table 2.
Analysis of saliva lead (μg/l) with demographic categories
| Demographic characteristics | Sample size, n | Saliva lead level mean ± s.e. | p-value |
|---|---|---|---|
| Sex | |||
| Male | 53 | 3.08 ± 0.50 | 0.14 |
| Female | 916 | 2.34 ± 0.13 | |
| Age | |||
| 14–24 yr | 324 | 2.45 ± 0.25 | 0.00 |
| 25–34 yr | 449 | 2.21 ± 0.13 | |
| 35–44 yr | 136 | 2.72 ± 0.27 | |
| 45–54 yr | 44 | 2.29 ± 0.51 | |
| 55+ yr | 16 | 3.55 ± 1.39 | |
| Education | |||
| 7–12 yr | 436 | 2.43 ± 0.15 | 0.00 |
| 13–14 yr | 310 | 2.37 ± 0.28 | |
| 15 yr | 184 | 2.27 ± 0.31 | |
| 16–19 yr | 28 | 2.74 ± 0.77 | |
| Employment | |||
| Yes | 370 | 2.05 ± 0.14 | 0.06 |
| No | 595 | 2.57 ± 0.19 | |
| Income | |||
| < $ 10,000 | 405 | 2.43 ± 0.17 | 0.00 |
| $ 10,000–$ 19,000 | 253 | 2.57 ± 0.35 | |
| $ 20,000–$ 29,000 | 148 | 2.28 ± 0.27 | |
| $ 30,000–$ 39,000 | 72 | 1.60 ± 0.23 | |
| $ 40,000 or more | 37 | 2.99 ± 1.04 | |
| Smoked 100 cigarettes in life? | |||
| Yes | 446 | 2.48 ± 0.16 | 0.50 |
| No | 516 | 2.31 ± 0.19 | |
| Smoking now? | |||
| Yes | 391 | 2.53 ± 0.16 | 0.01 |
| No | 35 | 1.80 ± 0.29 | |
| How many years have you been smoking? | |||
| Correlation Pearson: −.02 | 405 | 0.50 | |
| How many cigarettes a day? | |||
| Correlation Pearson: −0.01 | 403 | 0.06 | |
| How many years did you smoke? | |||
| Correlation Pearson: 0.17 | 29 | 0.24 | |
| How many cigarettes a day? | |||
| Correlation Pearson: −0.04 | 32 | 0.74 | |
| Ever used chewing tobacco or snuff? | |||
| Yes | 4 | 2.65 ± 0.59 | 0.66 |
| No | 962 | 2.39 ± 0.13 | |
| Previously tested for lead? | |||
| Yes | 263 | 2.41 ± 0.28 | 0.99 |
| No | 611 | 2.41 ± 0.14 | |
| Cracks on wall or peeling paint from walls or pipes? | |||
| Yes | 348 | 2.19 ± 0.17 | 0.15 |
| No | 612 | 2.51 ± 0.16 | |
| Remodeling or renovation of current living place in the last 5 years? | |||
| Yes | 291 | 2.42 ± 0.24 | 0.84 |
| No | 645 | 2.36 ± 0.17 | |
| Replacing of pipes inside current living place in the last 2 years? | |||
| Yes | 263 | 2.58 ± 0.23 | 0.31 |
| No | 661 | 2.30 ± 0.15 | |
| BQQ20: Have water filter/purifier on kitchen tap? | |||
| Yes | 135 | 2.23 ± 0.27 | 0.59 |
| No | 826 | 2.40 ± 0.14 | |
| BQQ21: what taking water from tap, do you. . . | |||
| Catch first water | 33 | 4.64 ± 1.98 | 0.23 |
| Let tap run for few seconds | 265 | 2.23 ± 0.22 | |
| Let tap run for a minute | 659 | 2.33 ± 0.14 | |
| BQQ23: Is home close to factory, incarnation or industrial plant? | |||
| Yes | 120 | 2.17 ± 0.29 | 0.46 |
| No | 832 | 2.39 ± 0.13 | |
| BQQ24: Is home on the same block as car repair shop or gas station? | |||
| Yes | 193 | 2.51 ± 0.22 | 0.49 |
| No | 773 | 2.34 ± 0.14 | |
These analyses were done using 0.0424 for all values below the instrumental detection limit of 0.0848 μg/l.
The concentrations of lead in saliva were much lower than those in blood, the average PbSa:PbB ratio being only 0.09. Except for Koh et al. (2003) who found mean PbSa to be only 3% of the PbB, other investigations have reported higher values that ranged from 13% to 56% (P’an, 1981; Omokhodion and Crawford, 1991; DiGregorio et al., 1974). The large variation in the previously reported values has been attributed largely to sample contamination (during collection, handling and analysis) and low detection limit for the analytical methods of the time (Koh et al., 2003).
A weak association (r = 0.156) was found between PbB and PbSa concentrations, although this may not seem obvious from graph in Fig. 2. The large clustering of data at low concentrations apparently has averaged out the variations in PbB data below about 7.5 μg/dl. The plot of log-transformed data shows more variation (Fig. 3) but does not result in a significant improvement in r-value (0.135). The weak association shows that PbSa may not be a surrogate for PbB but represents an important biomarker on its merit.
Fig. 2.
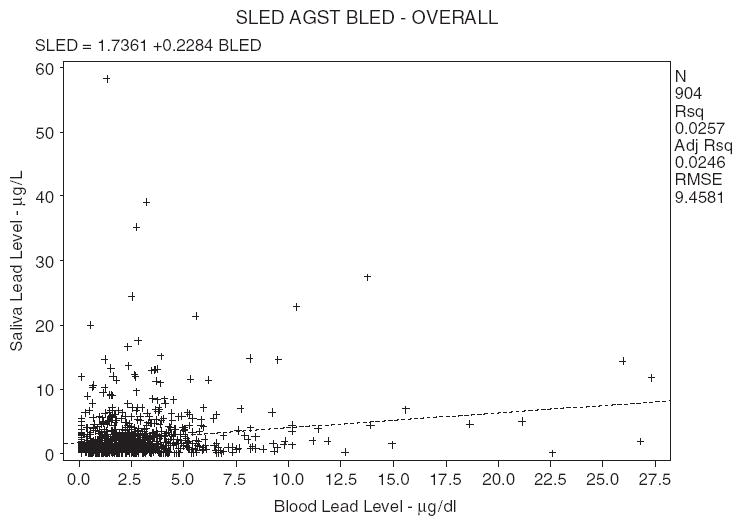
Saliva lead (SLED) versus blood lead (BLED) using all the data (samples below detection limit were set half the value of the BDL). Concentration of blood lead is in μg/dl and concentration of saliva lead is in μg/dl.
Fig. 3.
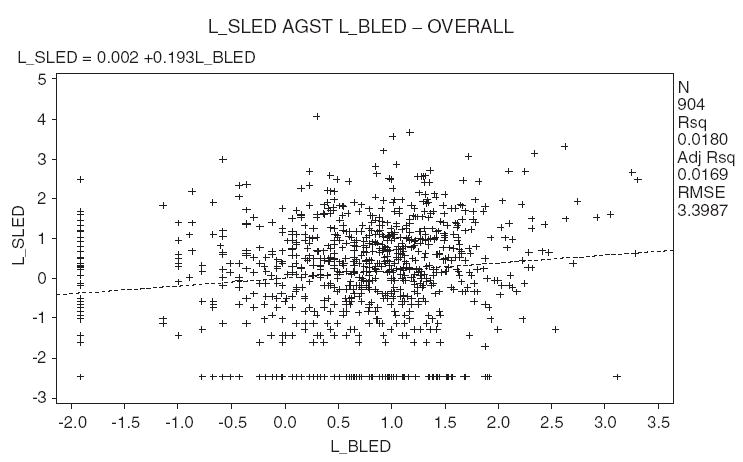
Log-transformed saliva lead (L_SLED) versus blood lead (L_BLED) for all the data. Concentration of blood lead is in μg/dl and concentration of saliva lead is in μg/dl.
Dental caries
The study population suffered extensively from dental caries, with only 0.5% showing no clinical signs of caries. There were nearly 83% of the adult participants with cavitated carious lesions (i.e., lesions that had progressed into dentin) and 16% with the less-extensive lesions confined to enamel. The adults on average had lost 3.4 teeth, and they averaged 9.4 untreated cavitated lesions each. When all lesions were included, cavitated and non-cavitated, this number ballooned to 29.9. By any measure, this population has severe problems with dental caries.
The relation between PbSa level and the number of carious teeth are shown in Table 5. There did not appear to be systematic relationships between PbSa and the number of decayed, missing and filled surfaces at either level 1 (D1MFS) or level 2 (D2MFS). On the hand, an increase in PbSa is associated with a decrease in D1S as well as D2S (Table 5).
Table 5.
Saliva lead and dental caries
| Means of decayed surface
| |||
|---|---|---|---|
| (Group)/lead levels | Sample size, n | D1Sa | D2Sb |
| (1) 0.1<Lead level≤0.99 | 265 | 30.22 ± 1.26 | 9.98 ± 1.11 |
| (2) 1.0≤Lead level≤4.99 | 483 | 31.49 ± 1.13 | 10.36 ± 0.77 |
| (3) 5.0≤Lead level≤9.99 | 74 | 27.26 ± 1.40 | 7.28 ± 0.87 |
| (4) Lead level≥10.0 | 36 | 26.06 ± 2.49 | 8.52 ± 1.54 |
| p-value = 0.081 | p-value = 0.087 | ||
| Mean number of DMF surface
| |||
| (Group)/lead levels | Sample size, n | D1MFSc | D2MFSd |
|
| |||
| (1) 0.1<Lead level≤0.99 | 265 | 45.69 ± 1.97 | 27.12 ± 2.12 |
| (2) 1.0 ≤ Lead level≤4.99 | 483 | 48.36 ± 1.61 | 28.87 ± 1.49 |
| (3) 5.0 ≤ Lead level≤9.99 | 74 | 44.76 ± 3.19 | 26.58 ± 3.87 |
| (4) Lead level≥10.0 | 36 | 45.60 ± 4.10 | 29.76 ± 3.73 |
| p-value = 0.564 | p-value = 0.892 | ||
D1S: Number of decayed surfaces at level 1 (including non-cavitated enamel lesions).
D2S: Number of decayed surfaces at level 2 (including only dentinal or cavitated lesions).
D1MFS: Weighted number of decayed, missing and filled surfaces at level 1 (including non-cavitated enamel lesions).
D2MFS: Weighted number of decayed, missing and filled surfaces at level 2 (including only dentinal or cavitated lesions).
Discussion
The average PbB value was 2.7 ± 0.1 μg/dl, a value that is low compared to previously reported values and reflects the general national decline in PbB concentrations associated with the phase-out of leaded gasoline and other measures aimed at reducing environmental exposures to lead (CDC, 1991; Pirkle et al., 1998).
Our result is in agreement with data from other recent national surveys of adults in countries where leaded gasoline is no longer being used; for example, 1.9 μg/dl for Japanese women in (Shimbo et al., 2000), 3.9 μg/dl in different parts of the UK (White and Sabbioni, 1998), 4.5 and 3.1 μg/dl for Italian men and women, respectively, in several urban areas (Apostoli et al., 2002), 3.1 μg/dl in Dusseldorf, Germany (Wilhem et al., 2002; Becker et al., 2002), 4.7 μg/dl in the city of Badajoz, Spain (Moreno et al., 1999), 1.6 μg/dl for adolescents in the cities of Uppsala and Trollhattan, Sweden (Barany et al., 2002). Countries where leaded gasoline is still being used or has only recently been banned report higher PbB values: 8.2 μg/dl in Taiwan (Liou et al., 1996), 6.4 μg/dl in Korea (Yang et al., 1996), 12 μg/dl in Mansoura City, Egypt (Mortada et al., 2002) and 7.4 μg/dl for pregnant mother in the middle part of China (Wang et al., 2004).
Some demographic characteristics of the study population are risk factors for lead exposure. The disparity between men and women (Table 1) may be attributed to the different body stores of lead in the two sexes which is related to the increased skeletal mass of men (White and Sabbioni, 1998). The lower concentration of red blood cells (where a large percentage of the lead is bound) in females compared to males may also be a contributing factor (Iyengar, 1987). A gradual increase in PbB with age should be expected for an element with a large body burden and long systemic and whole-body half-life. The correlation of PbB with age suggests that the body burden of lead is relatively dynamic and that remobilization of lead stored in the bones is continuously contributing to the lead content of the blood stream (White and Sabbioni, 1998).
Associations between PbB and PbSa reported in the literature have been discordant. P’an (1981) found a good correlation between PbB and PbSa (r = 0.72) for adult male occupationally exposed to lead. Koh et al. (2003) also reported a strong correlation (r = 0.41) among occupationally exposed individuals with high PbB values. For PbB concentrations <30 μg/dl, DiGregorio et al. (1974) showed poor correlation with PbSa (r = 0.137) while a negative correlation was found between the two parameters by Omokhodion and Crawford (1991).
The effects of demographic characteristics on the correlation between PbB and PbSa are shown in Table 3. The highest Pearson correlation coefficient was found for participants older than 46 years (r = 0.49 compared to r = <0.15 for younger participants). This observation suggests that saliva may be an important route for excretion of lead by elderly adults. The correlation was stronger for male (r = 0.33) compared to female (r = 0.15), somewhat surprising since one would have expected lower levels of diffusible lead in females with lower count of red blood cells. Weak but significant differences in correlation coefficients (r = −0.18 to 0.16) were found when the lead concentrations were stratified into four categories; the correlation increased with lead concentration (Table 3).
Table 3.
Correlation between blood lead and saliva lead levels
| All dataa | N | Pearson correlation | No BDL in both blood and salivab | N | Pearson correlation |
|---|---|---|---|---|---|
| Untransformed data | |||||
| Overall | 904 | 0.16 | Overall | 770 | 0.16 |
| Male | 48 | 0.33 | Male | 44 | 0.34 |
| Female | 856 | 0.15 | Female | 726 | 0.14 |
| Age ≤25 | 373 | 0.11 | Age ≤25 | 323 | 0.09 |
| Age 26–45 | 487 | 0.15 | Age 26–45 | 407 | 0.16 |
| Age 46 and older | 44 | 0.49 | Age 46 and older | 40 | 0.48 |
| PbB (0.1–0.99 μg/dl) | 156 | −0.07 | PbB (0.1–0.99 μg/dl) | 97 | −0.18 |
| PbB (1.0–4.99 μg/dl) | 649 | 0.04 | PbB (1.0–4.99 μg/dl) | 580 | 0.03 |
| PbB (5.0–9.99 μg/dl) | 81 | 0.13 | PbB (5.0–9.99 μg/dl) | 76 | 0.11 |
| PbB (≥10.0 μg/dl) | 18 | 0.08 | PbB (≥10.0 μg/dl) | 17 | 0.16 |
| Data log-transformed | |||||
| Overall | 904 | 0.13 | Overall | 770 | 0.14 |
| Male | 48 | 0.05 | Male | 44 | 0.20 |
| Female | 856 | 0.13 | Female | 726 | 0.13 |
| Age ≤25 | 373 | 0.12 | Age ≤25 | 323 | 0.04 |
| Age 26–45 | 487 | 0.14 | Age 26–45 | 407 | 0.18 |
| Age 46 and older | 44 | 0.36 | Age 46 and older | 40 | 0.44 |
| Log PbB (−2.31–−0.01) | 156 | 0.01 | Log PbB (−2.31–−0.01) | 97 | −0.18 |
| Log PbB (0–1.61) | 649 | 0.10 | Log PbB (0–1.61) | 580 | 0.08 |
| Log PbB (1.61–2.30) | 81 | −0.01 | Log PbB (1.61–2.30) | 76 | 0.09 |
| Log PbB (>2.30) | 18 | −0.04 | Log PbB (>2.30) | 17 | 0.22 |
All data means that adults with lead levels equal to BDL are given values which are half that of the BDL values ( = 0.14733 μg/dl) which is a half of minimum detectable value.
No BDL means that adults with lead levels equal to BDL are considered to be missing values.
The close similarity in the isotopic composition of lead in saliva and blood (Fig. 4) points to a close genetic affinity of lead in the two body fluids. Average 208Pb/206P for PbB was 2.236 compared to 2.237 for saliva while average 207Pb/206Pb was 0.9036 for blood and 0.9040 for saliva. The fact that the isotopic signatures are nearly identical is consistent with previous reports that lead in saliva is derived primarily from lead in the blood stream. The large scatter in isotopic ratios at PbB<10 μg/dl suggests that the study participants get their lead from many local sources. By contrast, the near uniformity of lead isotopic ratios at elevated PbB values suggests that elevated exposure to lead is due to one dominant source, presumably house paint. Although the release of lead into the environment has been curtailed with recent introduction of lead-free gasoline and legal restrictions on industrial emissions in most countries, human exposure to lead remains a public health concern. Pathways of exposure in urban areas of the United States are still numerous and include leaded water pipes and plumbing systems with lead-soldered joints and leaded brass water meters; deteriorated lead-based paint on house walls; contaminated dusts and soils, improperly glazed ceramic pottery; leaded pewter drinking vessels and glasses; emissions from a host of industrial operations such as mining and smelting, incineration, coal combustion, iron and steel works and dump sites; occupational and recreational sources; contaminated foods; etc. Our results show that the risk of elevated exposure to lead from many of these sources common in many inner cities remains high.
Fig. 4.
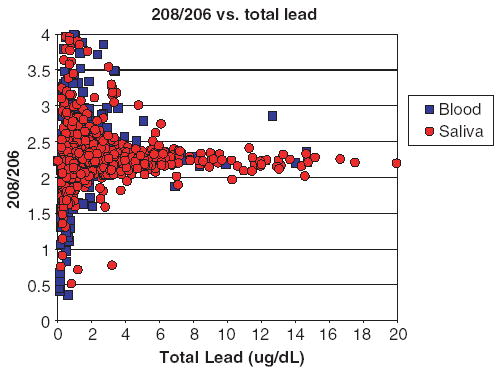
Ratios of 208Pb/206Pb in saliva (red) and blood (blue) samples.
The transport and pharmacokinetic relationships between lead in blood and saliva have been explored in a number of studies using laboratory animals. Repeated intraperitoneal injection of lead acetate (100 mg/l) into rats found the lead concentrations in submandibular and parotid saliva to be 50% and 22% of the whole PbB levels (Ghazi-Khansari et al., 1997). Whole PbB concentrations were highly correlated with submandibular PbSa (r = 0.78) and parotid PbSa (r = 0.93; Ghazi-Khansari et al., 1997). Craan et al. (1984) showed that the blood disappearance curve for lead injected intravenously into rats more closely resembled the appearance of lead in saliva than in urine. During the initial disappearance of lead from the blood stream following injection of rats with lead acetate, the concentration of lead in saliva was found to be two orders of magnitude lower than those of blood, but the ratio narrowed to one order of magnitude over time (Craan et al., 1984). The studies have led to the suggestion that lead in blood consists of two pools –protein bound fraction in red blood cells and diffusible lead in plasma. It is generally believed that the latter fraction is directed excreted into saliva although slow equilibration with the former pool may be maintained (Cleymaet et al., 1991; Craan et al., 1984).
Although PbSa concentration is generally considered to represent the free (pharmacologically active) lead fraction in blood (Brodeur et al., 1983; Cleymaet et al., 1991), the relationship is far from being simple. The weak correlation of PbSa with PbB may be indicative of many factors moderating the excretion of lead into saliva as well as the biokinetic relationships. The primary physiological factors that affect the PbSa/PbB ratio include saliva composition (especially the content of proteins that can complex lead), and flow rate of saliva pathophysiology of the oral cavity (Jusko and Milsap, 1993). The flow rate can affect the PbSa/PbB ratio because of its strong influence on saliva pH and membrane permeability (Haeckel, 1993). Variables that have been shown to influence saliva flow rate include age, medication, circadian rhythm, psychological stress and some disease states (Sonesson et al., 2003; Rotteveel et al., 2004). Time of sampling can affect the PbSa/PbB ratio. During resting conditions, about 70% of the saliva is supplied by submandibular glands, about 25% by parotid gland, despite their larger size, while only 5% is produced by sublingual and other minor glands (Paxton, 1979). During stimulation of saliva flow, the production by parotid glands can increase to over 40% (Paxton, 1979). The observation that lead concentration in saliva increases during periods of decreased salivary flow and that salivary glands are diffusive barriers for lead (Craan et al., 1984) support the suggestion that differences in binding capacities for salivary glands for lead can influence the PbSa/PbB ratio.
The saliva glands represent a clearance organ for lead in the blood stream. The half-life of lead in saliva is much less than that of blood (Brodeur et al., 1983; Craan et al., 1984), suggesting rapid removal of the PbSa by various organs in the oral cavity especially the teeth, gum and tongue. Lead acquired by the tongue via the saliva pathway may subsequently be transferred to teeth surfaces (Purchase and Fergusson, 1986). Bioaccumulation of PbSa in the oral cavity serves to increase the risk to oral health. In healthy individuals, the resting saliva flow rate typically is 0.3–0.7 ml/min and when stimulated by chewing or with chemicals such as citric acid, the flow rate can increase to about 2–6 ml/min with great individual variation (Heintze et al., 1983; Dawes et al., 1987; Rotteveel et al., 2004). Average saliva production has been estimated to be 1.6 l/day (Rotteveel et al., 2004). Assuming that 50% of the PbSa found in this study is transferred to teeth surfaces, the accumulation of lead on the tooth enamel through this pathway is estimated to be about 2 μg/day or about 730 μg/year. This figure suggests that saliva may be a significant route in the accumulation of lead on the enamel.
The susceptibility of victims of lead poisoning to dental decay and periodontal disease was first reported in early 19th century (Tanquerel des Planches, 1839). However, epidemiological and ecological studies of the association between lead exposure and caries prevalence (Gil et al., 1996; Moss et al., 1999; Gemmel et al., 2002) have not presented a coherent picture. In a cross-sectional study, Gil et al. (1996) reported significant associations between tooth lead levels and dental plaque, Salivalis lactobacilli number dental abrasion and dental color. An analysis of data from the Third National Health and Nutrition Examination Survey (NHANES III) found a significant association between PbB levels and the risk of caries lesions. Among children 5–17 years old, a doubling of the risk of dental caries was associated with an increase of PbB level by 5 μg/dl (Moss et al., 1999). On the other hand, Campbell et al. (2000) reported that the association between caries in deciduous teeth and PbB levels was only marginally significant and that the data failed to support any possible mechanisms for lead cariogenicity. Gemmel et al. (2002) could only find a positive association between PbB level and the number of various lesions among urban children but not among the rural subgroup. The meaning of the positive association between PbB and dental caries is not clear; a biochemical mechanism by which lead exerts its cariogenic effects is unknown.
Although previous studies have used PbB as a measure of lead exposure, none has explored the association between PbSa and the severity of dental caries in a human population. As noted above, saliva is an important pathway in the accumulation of lead on tooth surfaces. The PbB and PbSa data were divided into four categories: 0.1–0.99, 1.0–4.99, 5.0–9.99, and >10 μg/dl and 0.1–0.99, 1.0–4.99, 5.0–9.99, and >10 μg/l, respectively. The mean numbers of decayed tooth surfaces associated with each PbB category are shown in Table 4. The decayed surface (D1S – non-cavitated and cavitated lesions) scores varied between 29.0 and 30.9 and did not show a definite pattern with increase in PbB values (the p-value = 0.587). By comparison, D2S (cavitated lesions only) scores were much lower, the range being from 6.95 ± 1.86 to 11.6 ± 2.36. There was an increase in D2S scores with increasing PbB values and a marginal association (p = 0.1001) was found between PbB and D2S (Table 4). The slightly lower D2S at PbB>10 μg/dl may be related to small number of samples in this particular lead concentration range. By contrast, both D1MFS (p-value = 0.063) and D2MFS (p = 0.003) were significantly associated with PbB (Table 4). Our results are generally consistent with previous epidemiological and ecological studies that have reported significant associations between dental caries and PbB levels (Gil et al., 1996; Moss et al., 1999; Gemmel et al., 2002).
Table 4.
Blood lead and dental caries in the study population
| Means of decayed surface (μg/dl)
| |||
|---|---|---|---|
| (Group)/lead levels | Sample size, n | D1Sa | D2Sb |
| (1) 0.1<Lead level≤0.99 | 159 | 28.97 ± 1.86 | 6.95 ± 1.21 |
| (2) 1.0≤Lead level≤4.99 | 663 | 30.75 ± 0.75 | 9.92 ± 0.53 |
| (3) 5.0≤Lead level≤9.99 | 81 | 29.14 ± 2.66 | 11.63 ± 2.36 |
| (4) Lead level≥10.0 | 18 | 30.90 ± 3.05 | 9.68 ± 2.20 |
| p-value = 0.587 | p-value = 0.100 | ||
| Mean number of DMF surface
| |||
| (Group)/lead levels | Sample size, n | D1MFSc | D2MFSd |
|
| |||
| (1) 0.1<Lead level≤0.99 | 159 | 41.81 ± 2.41 | 22.02 ± 1.97 |
| (2) 1.0≤Lead level≤4.99 | 663 | 47.84 ± 1.52 | 28.63 ± 1.58 |
| (3) 5.0≤Lead level≤9.99 | 81 | 53.59 ± 3.66 | 37.76 ± 3.71 |
| (4) Lead level≥10.0 | 18 | 51.97 ± 6.18 | 31.58 ± 7.09 |
| p-value = 0.063 | p-value = 0.003 | ||
D1S: Weighted number of decayed surfaces at level 1 (including non-cavitated enamel lesions).
D2S: Weighted number of decayed surfaces at level 2 (including only dentinal or cavitated lesions).
D1FMS: Number of decayed, missing and filled surfaces at level 1 (including non-cavitated enamel lesions).
D2FMS: Number of decayed , missing and filled surfaces at level 2 (including only dentinal or cavitated lesions).
The associations of dental caries and salivary lead levels (Table 5) are different from the relationships found with PbB (Table 4). The association between PbSa and either D1MFS or D2MFS was not statistically significant (Table 5). On the other hand, the association of PbSa was found to be marginally significant with D1S (p = 0.0814) and D2S (p = 0.0871). Interestingly, higher PbSa levels were associated with both lower D1S and D2S values (Table 5), unlike PbB where higher values were positively associated with the D2S, D1MFS and D2MFS (Table 4). The disparity in relationships between dental caries and PbSa and PbB serves to emphasize the fact that our understanding of the mechanisms for the cariogenic effects of lead is very limited. Lead is a well-known bactericide. At high enough concentration, lead can be expected to reduce the caries activity by reducing the number of cariogenic microorganisms in the oral environment such as Salivalis lactobacilli and Streptococcus mutans. Dental caries is an infectious disease with various steps in its pathogenesis (Larmas, 1993). The multicausal nature of this disease on the one hand and the interactions between the host and cariogenic microorganisms on the other hand make the interpretation of statistical relationship between PbB and caries prevalence difficult. Our saliva results suggest that the effect of lead on cariogenicity is indirect, presumably mediated through its bactericidal action. The positive associations reported in this and previous studies may well be a reflection of the fact that the risk factors for dental caries especially in children overlap extensively with those of lead poisoning. Both diseases disproportionately affect inner-city poor, are related to defects in calcium metabolism and are dependent on nutritional as well as a number of lifestyle factors (Rabinowitz et al., 1993; Bowen, 1998; Gemmel et al., 2002). Whether lead is a direct cariogenic agent is debatable.
References
- Abdollahi M, Dehpour AR, Fooladgar M. Alterations of rat submandibulary gland secretion of proteins, calcium and N-acetyl-β-D-glucosaminidase activity by lead. Gen Pharmacol. 2001;29:675–680. doi: 10.1016/s0306-3623(96)00560-5. [DOI] [PubMed] [Google Scholar]
- Apostoli P, Baj A, Bavazzano P, Ganzi A, Neri G, Ronchi A, Soleo L, Di Lorenzo L, Spinelli P, Valente T, Minoia C. Blood lead reference values: the results of an Italian polycentric study. Sci Total Environ. 2002;287:1–11. doi: 10.1016/s0048-9697(01)00975-5. [DOI] [PubMed] [Google Scholar]
- Barany E, Bergdahl IA, Bratteby LE, Lundh T, Samuelson G, Schutz A, Skerfving S, Oskarsson A. Trace element levels in whole blood and serum from Swedish adolescents. Sci Total Environ. 2002;286:129–141. doi: 10.1016/s0048-9697(01)00970-6. [DOI] [PubMed] [Google Scholar]
- Bardow A, Nyvad B, Nauntofte B. Relationships between medication intake, complaints of dry mouth, salivary flow rate and composition and the rate of tooth demineralization in situ. Arch Oral Biol. 2001;46:413–423. doi: 10.1016/s0003-9969(01)00003-6. [DOI] [PubMed] [Google Scholar]
- Becker K, Kaus S, Krause C, Lepm P, Schulz C, Seiwert M, Seifert B. German environmental survey 1998 (GerES III): environmental pollutants in blood of German population. Int J Hyg Environ Health. 2002;205:297–308. doi: 10.1078/1438-4639-00155. [DOI] [PubMed] [Google Scholar]
- Bowen WH. Biological mechanisms of childhood caries. Community Dent Oral Epidemiol. 1998;26:28–31. doi: 10.1111/j.1600-0528.1998.tb02091.x. Suppl 1. [DOI] [PubMed] [Google Scholar]
- Brodeur J, Lacasse Y, Talbot D. Influence of removal from occupational lead exposure on blood and saliva lead concentrations. Toxicol Lett. 1983;19:195–199. doi: 10.1016/0378-4274(83)90282-5. [DOI] [PubMed] [Google Scholar]
- Campbell JR, Moss ME, Raubertas RF. The association between caries and childhood lead exposure. Environ Health Perspect. 2000;108:1099–1102. doi: 10.1289/ehp.001081099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC. Preventing Lead Poisoning in Young Children. US Department of Health & Human Services, Centers for Disease Control; Atlanta, GA: 1991. [Google Scholar]
- CDC. Second National Report on Human Exposure to Environmental Chemicals. US Department of Health & Human Services, Centers for Disease Control and Prevention; Atlanta, GA: 2003. [Google Scholar]
- Cleymaet R, Collys K, Retief DH, Michotte Y, Slop D, Taghon E, Coomans D. Relation between lead in surface tooth enamel, blood and saliva from children residing in the vicinity of a non-ferrous metal plant in Belgiu. Br J Ind Med. 1991;48:702–709. doi: 10.1136/oem.48.10.702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craan AG, Nadon G, P’an AYS. Lead flux through the kidney and salivary glands of rats. Am J Physiol. 1984;247(16):773–783. doi: 10.1152/ajprenal.1984.247.5.F773. [DOI] [PubMed] [Google Scholar]
- Dawes C, Cross HG, Baker CG. The influence of gland size on the flow rate and composition of human parotid saliva. Dent J. 1987;44:21–25. [PubMed] [Google Scholar]
- DiGregorio GJ, Ferko AP, Sample RG, Bobycock E, McMichael R, Chernick WS. Lead and δ-aminodeluvinic acid concentrations in human parotid saliva. Toxicol Appl Pharmacol. 1974;27:491–493. doi: 10.1016/0041-008x(74)90028-3. [DOI] [PubMed] [Google Scholar]
- Fung HL, Jaffe SJ, Mattar ME, Lanighan MC. Blood and salivary lead levels in children. Clin Chim Acta. 1975;61:423–424. doi: 10.1016/0009-8981(75)90436-2. [DOI] [PubMed] [Google Scholar]
- Gemmel A, Tavares M, Alperin S, Soncini J, Daniel D, Dunn J, Crawford S, Braveam N, Clarkson TW, McKinlay S, Bellinger DC. Blood lead level and dental caries in school-age children. Environ Health Perspect. 2002;110:A625–A630. doi: 10.1289/ehp.021100625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghazi-Khansari M, Mortazapour Z, Shayaganfar N, Abdollahi M, Dehpour AR. Lead determination in parotid and submandibular salivas and whole blood in rat. Toxic Subst Mech. 1997;16:327–335. [Google Scholar]
- Gil F, Facio A, Villanueva E, Perez ML, Tojo R, Gil A. The association of tooth lead content with dental health factors. Sci Total Environ. 1996;192:183–191. doi: 10.1016/s0048-9697(96)05313-2. [DOI] [PubMed] [Google Scholar]
- Gonzalez M, Banderas JA, Baez A, Belmont R. Salivary lead and cadmium in a young population residing in Mexico City. Toxicol Lett. 1997;93:55–64. doi: 10.1016/s0378-4274(97)00067-2. [DOI] [PubMed] [Google Scholar]
- Haeckel R. Factors influencing the saliva/plasma ratio of drugs. In: Malamud D, Tabak L, editors. Saliva as a Diagnostic Tool. 1993. pp. 128–142. [Google Scholar]
- Heintze U, Birkhed D, Bjorn H. Secretion rate and buffer effect of resting and stimulated whole saliva as a function of age and sex. Swed Dent J. 1983;7:227–238. [PubMed] [Google Scholar]
- Iyengar GV. Reference values for concentrations of As, Cd, Co, Cr, Cu, Fe, I, Hg, Mn, Mo, Ni, Pn, Se and Zn in selected human tissues and body fluids. Biol Trace Element Res. 1987;12:263–295. doi: 10.1007/BF02796686. [DOI] [PubMed] [Google Scholar]
- Jusko WJ, Milsap RL. Pharmacokinetic principles of drug distribution in saliva. In: Malamud D, Tabak L, editors. Saliva as a Diagnostic Tool. 1993. pp. 36–47. [DOI] [PubMed] [Google Scholar]
- Koh D, Ng V, Chua LH, Yang Y, Ong HY, Chia SE. Can salivary lead be used for biological monitoring of lead exposed individuals? Occup Environ Med. 2003;60:696–698. doi: 10.1136/oem.60.9.696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larmas M. Plaque-mediated disease. In: Malamud D, Tabak L, editors. Saliva as a Diagnostic Tool. 1993. pp. 252–264. [Google Scholar]
- Liou SH, Wu TN, Chiang HC, Yang GY, Yang T, Wu YQ, Lai JS, Ho ST, Lee CC, Ko YC, Ko KN, Chiang PY. Blood lead levels in Taiwanese adults: distribution and influencing factors. Sci Total Environ. 1996;180:211–219. doi: 10.1016/0048-9697(96)80245-2. [DOI] [PubMed] [Google Scholar]
- Mandel ID. The diagnostic uses of saliva. J Oral Pathol Med. 1990;19:119–125. doi: 10.1111/j.1600-0714.1990.tb00809.x. [DOI] [PubMed] [Google Scholar]
- Mandel ID. Salivary diagnosis: promises, promises. In: Malamud D, Tabak L, editors. Saliva as a Diagnostic Tool. 1993. pp. 1–10. [DOI] [PubMed] [Google Scholar]
- MDCH. Childhood Lead Poisoning Prevention Task Force Report. Michigan Department of Community Health; Lansing, MI: 2004. [Google Scholar]
- Moller P, Perrier M, Ozsahin M, Monnier P. A prospective study of salivary gland function in patients undergoing radiotherapy for squamous cell carcinoma of the oropharynx. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2004;97:173–189. doi: 10.1016/s1079-2104(03)00473-6. [DOI] [PubMed] [Google Scholar]
- Moreno MA, Marin C, Vinagre F, Ostapczuk P. Trace element levels in whole blood samples from residents of the city Badajoz, Spain. Sci Total Environ. 1999;229:209–215. doi: 10.1016/s0048-9697(99)00074-1. [DOI] [PubMed] [Google Scholar]
- Mortada WI, Sobh MA, El-Defrawy MM, Farahat SE. Reference intervals of cadmium, lead and mercury in blood, urine, hair and nails among residents in Mansoura City, Nile Delta, Egypt. Environ Res. 2002;90:104–110. doi: 10.1006/enrs.2002.4396. [DOI] [PubMed] [Google Scholar]
- Moss ME, Lanphear BP, Auinger P. Association of dental caries and blood lead levels. J Am Med Assoc. 1999;281:2294–2298. doi: 10.1001/jama.281.24.2294. [DOI] [PubMed] [Google Scholar]
- Nriagu JO. Lead and Lead Poisoning in Antiquity. Wiley; New York: 1983. [Google Scholar]
- Omokhodion FO, Crawford GW. Lead in sweat and relationship to salivary and urinary levels in normal healthy subjects. Sci Total Environ. 1991;103:113–122. doi: 10.1016/0048-9697(91)90137-4. [DOI] [PubMed] [Google Scholar]
- P’an AYS. Lead levels in saliva and in blood. J Toxicol Environ Health. 1981;7:273–280. doi: 10.1080/15287398109529978. [DOI] [PubMed] [Google Scholar]
- Paxton JW. Measurement of drugs in saliva: a review. Exp Clin Pharmacol. 1979;1:11–21. [PubMed] [Google Scholar]
- Pirkle JL, Kaufman RB, Brody DJ, Hickman T, Gunter EW, Paschal DC. Exposure of the US population to lead. Environ Health Perspect. 1998;106:745–750. doi: 10.1289/ehp.98106745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouchet AG. Recherche des substances medicamenteuses et toxiques dans la salive. C R Acad Sci. 1879;84:244–245. [Google Scholar]
- Purchase NG, Fergusson JE. Lead in teeth: the influence of the tooth type and the sample within a tooth on lead levels. Sci Total Environ. 1986;52:239–250. doi: 10.1016/0048-9697(86)90124-5. [DOI] [PubMed] [Google Scholar]
- Rabinowitz MB, Leviton A, Bellinger D. Relationships between blood lead levels and exfoliated tooth dentine lead levels: models of tooth lead kinetics. Calcif Tissue Int. 1993;53:338–341. doi: 10.1007/BF01351840. [DOI] [PubMed] [Google Scholar]
- Rotteveel JC, Jongerius PH, van Limbeek J, van den Hoogen FJA. Salivation in health school children. Int J Pediat Otorhinolaryngol. 2004;68:767–774. doi: 10.1016/j.ijporl.2004.01.005. [DOI] [PubMed] [Google Scholar]
- Settle DM, Patterson CC. Lead in albacore: guide to lead pollution in Americans. Science. 1980;207:1167–1176. doi: 10.1126/science.6986654. [DOI] [PubMed] [Google Scholar]
- Shimbo S, Zhang ZW, Moon CS, Watanabe T, Nakatsuka H, Matsuda-Inoguchi N, Higashikawa K, Ikeda M. Correlation between urine and blood concentrations and dietary intake of cadmium and lead among women in the general population of Japan. Int Arch Occup Environ Health. 2000;73:163–170. doi: 10.1007/s004200050023. [DOI] [PubMed] [Google Scholar]
- Sonesson M, Eliasson L, Matsson L. Minor salivary gland secretion in children and adults. Arch Oral Biol. 2003;48:535–539. doi: 10.1016/s0003-9969(03)00086-4. [DOI] [PubMed] [Google Scholar]
- Tanquerel des Planches L. Traite des Maladies de Plom ou Saturnines. Fera; Paris: 1839. [Google Scholar]
- Timchalk C, Poet TS, Kousba AA, Campbell JA, Lin Y. Noninvasive biomonitoring approaches to determine dosimetry and risk following acute chemical exposure: analysis of lead or organophosphate insecticide in saliva. J Toxicol Environ Health A. 2004;67:635–650. doi: 10.1080/15287390490428035. [DOI] [PubMed] [Google Scholar]
- Wang C, Huang L, Zhou X, Xu G, Shi Q. Blood lead levels of both mothers and their newborn infants in the middle part of China. Int J Hyg Environ Health. 2004;207:431–436. doi: 10.1078/1438-4639-00311. [DOI] [PubMed] [Google Scholar]
- White MA, Sabbioni E. Trace element reference values in tissues from inhabitants of the European Union: X. A study of 13 elements in blood and urine of a United Kingdom population. Sci Total Environ. 1998;216:253–270. doi: 10.1016/s0048-9697(98)00156-9. [DOI] [PubMed] [Google Scholar]
- Wilhem M, Pesch A, Rostek U, Begerow J, Schmidtz N, Idel H, Ranft U. Concentrations of lead in blood, hair and saliva of German children living in three different areas of traffic density. Sci Total Environ. 2002;297:109–118. doi: 10.1016/s0048-9697(02)00101-8. [DOI] [PubMed] [Google Scholar]
- Wilson JT. Clinical correlates of drug in saliva. In: Malamud D, Tabak L, editors. Saliva as a Diagnostic Tool. 1993. pp. 48–57. [Google Scholar]
- Yang JS, Kang SK, Park IJ, Rhee KY, Moon YH, Sohn DH. Lead concentrations in blood among the general population of Korea. Int Arch Occup Environ Health. 1996;68:199–202. doi: 10.1007/BF00381632. [DOI] [PubMed] [Google Scholar]


