Abstract
Modified Hb solutions have been developed as O2 carrier transfusion fluids, but of concern is the possibility that increased scavenging of nitric oxide (NO) within the plasma will alter vascular reactivity even if the Hb does not readily extravasate. The effect of decreasing hematocrit from ~30% to 18% by an exchange transfusion of a 6% sebacyl cross-linked tetrameric Hb solution on the diameter of pial arterioles possessing tight endothelial junctions was examined through a cranial window in anesthetized cats with and without a NO synthase (NOS) inhibitor. Superfusion of a NOS inhibitor decreased diameter, and subsequent Hb transfusion produced additional constriction that was not different from Hb transfusion alone but was different from the dilation observed by exchange transfusion of an albumin solution after NOS inhibition. In contrast, abluminal application of the cross-linked Hb produced constriction that was attenuated by the NOS inhibitor. Neither abluminal nor intraluminal cross-linked Hb interfered with pial arteriolar dilation to cromakalim, an activator of ATP-sensitive potassium channels. Pial vascular reactivity to hypocapnia and hypercapnia was unaffected by Hb transfusion. Microsphere-determined regional blood flow indicated selective decreases in perfusion after Hb transfusion in the kidney, small intestine, and neurohypophysis, which does not have tight endothelial junctions. Administration of a NOS inhibitor to reduce the basal level of NO available for scavenging before Hb transfusion prevented further decreases in blood flow to these regions compared with NOS inhibition alone. In contrast, blood flow to skeletal and left ventricular muscle increased, and cerebral blood flow was unchanged after Hb transfusion. This cross-linked Hb tetramer is known to appear in renal lymph but not in urine. We conclude that cell-free tetrameric Hb does not scavenge sufficient NO in the plasma space to significantly affect baseline tone in vascular beds with tight endothelial junctions but does produce substantial constriction in beds with porous endothelium. The data support increasing the molecular size of Hb by polymerization or conjugation to limit extravasation in all vascular beds to preserve normal vascular reactivity.
Keywords: blood substitute, cat, cerebral blood flow, ATP-sensitive potassium channel, pial artery
Cell-free Hb solutions have been explored for use as transfusion fluids capable of carrying O2. Cross-linking of the Hb tetramer with bis-(3,5-dibromosalicyl)-fumarate was used as a strategy to prevent formation of dimers that would be readily filtered by renal glomeruli (25). However, some clinical trials with cross-linked tetrameric Hb could not demonstrate efficacy, probably because improvements in oxygenation were offset by peripheral vasoconstriction and arterial hypertension (15, 21, 22). Peripheral vasoconstriction has been attributed largely to scavenging of nitric oxide (NO) by Hb (5, 23, 35) and possibly to increased precapillary O2 release (19, 30). Excessive vasoconstriction can limit the use of cell-free Hb as an O2 carrier and has led to the design of Hb molecules with reduced NO scavenging properties (14, 18) and with high O2 affinity to reduce precapillary O2 loss (30, 38).
Plasma-based Hb can provide a more effective sink for NO than red blood cell-based Hb can because of diffusion-limitation of NO into the red blood cell (11, 36). However, much of the vasoconstrictive effect seen with cell-free Hb transfusion may be due to scavenging of NO in the perivascular space by a fraction of the Hb that extravasates. For example, cross-linked tetrameric Hb that did not appear in the urine nevertheless appeared in renal lymph, thereby indicating extravasation of Hb selectively in peritubular capillaries (12). In contrast, large polymers of cross-linked Hb that did not appear in renal lymph did not produce arterial hypertension. Others (7, 20) have shown that increasing the molecular weight of Hb by polymerization or conjugation with polyethylene glycol results in less hypertension than occurs with cross-linked tetrameric Hb. However, still in question are whether Hb in the plasma scavenges sufficient NO to alter vascular tone, as suggested by mathematical models (8, 9), and, hence, whether the design of Hb molecules with reduced NO affinity is necessary when conjugated or polymerized Hb can be made to limit extravasation.
The brain, with its tight endothelial junctions, provides a model with which to assess vascular reactivity in vivo without local extravasation of Hb. Previous work (1) has demonstrated that NO-dependent dilation of pial arterioles to acetylcholine and ADP was intact after exchange transfusion of cross-linked tetrameric Hb. These results suggested that plasma-based Hb did not appreciably scavenge NO evoked by agonist stimulation. However, the exchange transfusion with Hb resulted in a decrease in arteriolar diameter in contrast to the dilation seen with exchange transfusion of an albumin solution. If agonist stimulation results in intermittent bursts of NO production, a sufficient amount of NO may escape scavenging by plasma-based Hb to effect arteriolar dilation (31). Thus it is possible that intraluminal Hb increases baseline vascular tone by scavenging NO without affecting vascular responsivity to endothelial-dependent dilators.
The purpose of the present study was to determine the role of NO in the vascular response of the cerebral circulation to exchange transfusion of cell-free Hb by inhibiting NO synthase (NOS) before performing the transfusion. Measurements were made of cerebral blood flow (CBF) after systemic NOS inhibition and of pial arteriolar diameter after local NOS inhibition. Blood flow measurements in the brain were contrasted with blood flow in neurohypophysis, which does not have tight endothelial junctions, and to major peripheral vascular beds, such as kidneys, intestines, skeletal muscle, and cardiac muscle. If Hb reduces blood flow to a particular region by scavenging NO, then reducing NO availability by prior NOS inhibition should prevent Hb transfusion from decreasing blood flow. Cross-linked tetrameric Hb was used because it has been demonstrated to extravasate in renal lymph (12) and thereby to provide a positive control for effects of extravasation. Because changes in vascular resistance after exchange transfusion of cell-free Hb are influenced by changes in hematocrit and blood viscosity as well as by arteriolar tone, pial arteriolar diameter was measured after Hb transfusion with and without local NOS inhibition. In addition, the direct effect of abluminal cross-linked Hb on cerebral vascular tone was assessed by topically applying the cross-linked Hb on the pial surface with and without NOS inhibition. Specificity of NO effects on vascular tone was determined by coapplication of Hb and cromakalim, an agonist of ATP-sensitive K+ (KATP) channels, with either local application of cross-linked Hb or systemic transfusion of Hb. A KATP channel agonist was used because of its ability to produce large dilations for testing potential inhibition by Hb and because of the clinical importance of knowing whether cell-free Hb is capable of blocking responses to the activation of these channels that are known to be important in hypoxic-ischemic conditions (27, 28).
METHODS
All procedures were approved by The Johns Hopkins University Animal Care and Use Committee and conformed to the principles of laboratory animal research outlined by the American Physiological Society and the National Institutes of Health guidelines. Cats were anesthetized with pentobarbital sodium (40 mg/kg ip). Catheters were advanced into the inferior vena cava via femoral veins for administration of fluids and drugs. Anesthesia was maintained with continuous infusion of pentobarbital sodium (6 mg · kg−1 · h−1 iv). Both femoral arteries were catheterized for measuring arterial blood pressure, sampling arterial blood, and withdrawing blood during the exchange transfusion. The lungs were mechanically ventilated to maintain arterial partial pressure of CO2 (PaCO2) at 35–40 mmHg. The arterial partial pressure of O2 (PaO2) was maintained >100 mmHg with enriched inspired O2. Rectal temperature was maintained at ~38°C with a heating lamp and warming blanket. Arterial blood samples were analyzed for pH, PCO2, PO2, Hb concentration, and O2 saturation.
The Hb solution contained 6–7% sebacyl cross-linked tetrameric Hb in lactated Ringer solution. Human deoxyhemoglobin was treated with bis-(3,5-dibromosalicyl)-sebacate to produce covalent cross-links between the β-82 lysine residues and between the α-99 lysine residues, as described previously (2, 3). The sebacyl cross-linked Hb has a 50% oxyhemoglobin saturation at PO2 of 34 mmHg (P50) and a cooperativity of 2.2. Exchange transfusion was performed isovolumetrically by infusing the solution intravenously while withdrawing blood from a femoral artery at a rate of 1.7 ml/min for ~40 min. The transfusion was stopped when hematocrit was reduced to ~18%. Both transfused and nontransfused control groups received intravenous hydration with lactated Ringer solution (4 ml · kg−1 · h−1).
Regional blood flow with NOS inhibition and Hb transfusion
Regional blood flow was measured by the radiolabeled microsphere technique as previously described (33, 35). A left thoracotomy was performed, and a catheter was inserted into the left atrium for injection of microspheres. Pancuronium bromide (0.1 mg/kg iv) was used as a muscle relaxant to facilitate electrocauterization. For each blood flow determination, a dose of ~1.5 × 106 microspheres (15 ± 0.5 μm diameter) was injected followed by a 5-ml flush of saline over a 20-s period. A reference sample was collected from the femoral artery at a rate of 1.94 ml/min during injection and continuing for 90 s after saline injection flush. Isotope labels used for each injection included 153Gd, 114mIn, 113Sn, 103Ru, 95Nb, and 46Sc. At the end of the experiment, samples of the kidney, small intestine, skeletal muscle, and left ventricular muscle containing at least 400 microspheres of each isotope were obtained. The brain was fixed in 10% buffered formalin to permit dissection of cerebrum and neurohypophysis. For each tissue and arterial blood sample, the amount of radioactivity for each isotope was counted after correcting for spectral overlap. Regional blood flow was calculated as the product of the radioactive counts in the tissue and the arterial reference withdrawal rate (1.94 ml/min) divided by the radioactive counts in the reference sample. Blood samples were also obtained from a sagittal sinus catheter for measurement of O2 content and calculation of cerebral O2 consumption as previously described (33). Cerebrovascular resistance (CVR) was calculated by using sagittal sinus pressure as the downstream pressure. Vascular resistance in peripheral organs was calculated as mean arterial blood pressure (MABP) divided by blood flow.
Measurements of blood flow, vascular pressures, arterial blood gases, total Hb concentration, and O2 saturation were made at baseline and at 50, 120, and 160 min after baseline measurements in three groups of cats (Fig. 1). In the first group (n = 12 cats), an exchange transfusion of cross-linked Hb was performed over a 40-min period starting 60 min after baseline measurements to determine the effect of the transfusion alone. In the second group (n = 9 cats), Nω-nitro-l-arginine methyl ester (l-NAME) was infused over a 5-min period starting 20 min after baseline measurements to determine the effect of NOS inhibition alone. A dose of l-NAME (20 mg/kg iv) was chosen based on previous work (29) showing >90% inhibition of NOS activity in cats. In the third group (n = 8 cats), l-NAME was infused at 20 min, and an exchange transfusion of Hb was performed at 60–100 min after baseline measurements to determine the effect of NOS inhibition on the subsequent response to Hb transfusion. Data within each group were analyzed by ANOVA with repeated measures (SPSS, Chicago, IL). If significant, comparisons between individual time points were performed by paired t-test with Bonferroni correction for three comparisons (between baseline and 50-min values for the effect of l-NAME and between the 50-min values and the 120-and 160-min values for the effect of Hb transfusion). In this and all remaining experiments below, the level of significance was set at P < 0.05, and values are means ± SE.
Fig. 1.
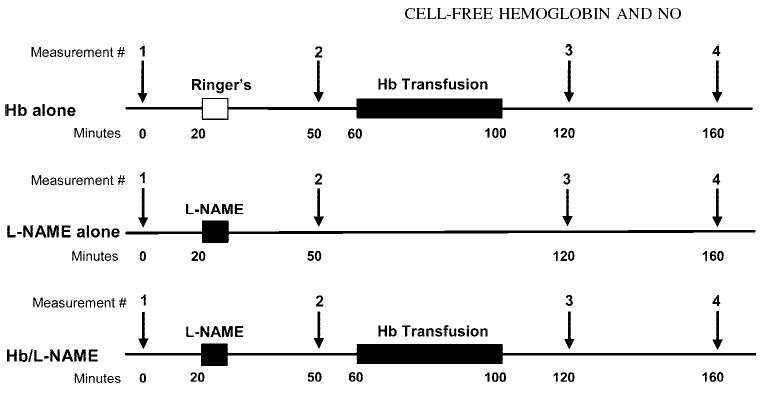
Schematic diagram of experimental design involving regional blood flow measurements after administration of Ringer lactate solution or Nω-nitro-l-arginine methyl ester (l-NAME) and after exchange transfusion with Hb solution in three groups of cats.
Pial arteriolar response to Hb transfusion after NOS inhibition
To test whether the pial arteriolar response to exchange transfusion is influenced by prior NOS inhibition, Nω-nitro-l-arginine (l-NNA) was superfused over the cortical surface through a closed cranial window before exchange transfusion of Hb. Four groups were studied (Fig. 2). In the first group (n = 9 cats), l-NNA was superfused without subsequent Hb transfusion to control for the effect of time after NOS inhibition. In the second group (n = 6 cats), l-NNA was superfused followed by an exchange transfusion with a solution of 5% human serum albumin to control for effects of reduced hematocrit. In the third group (n = 8 cats), artificial cerebrospinal fluid (CSF) was superfused followed by an exhange transfusion with the Hb solution. In the fourth group (n = 7 cats), l-NNA was superfused followed by an exchange transfusion with the Hb solution. A closed cranial window was constructed as described (1) by drilling a 12-mm diameter craniotomy over the parietal cortex, securing a plastic ring to the skull with acrylic cement, gently cutting and retracting the dura mater, and sealing the window with a glass coverslip glued to the plastic ring. The plastic ring was fitted with inlet and outlet ports and a port for measuring pressure. The window was filled with artificial CSF (10), bubbled with 6% O2-6% CO2-88% N2, and the fluid temperature in the window was monitored with a thermistor and regulated at 37–38°C. The diameters of 10 to 15 arteriolar segments were measured at various time points in each cat by a video microscopy system (1). Responses were segregated by the initial inner diameter into small (<50 μm), medium (50–100 μm), and large (>100 μm) vessels. Within each vessel-size grouping, the percent changes in diameter of multiple arteriolar segments were averaged for each intervention in individual cats. For statistical analysis, a single average value per cat was used for each size grouping, such that n = number of cats. The experimental protocol (Fig. 2) involved measuring the diameter 1) at baseline ~45 min after completion of the surgery, 2) at 5 min of hypocapnia (PaCO2, 20–25 mmHg) to demonstrate the ability of the arterioles to constrict, 3) after 30 min of superfusion of either artificial CSF or 300 μM l-NNA, 4) 30 min after completion of an exchange transfusion with the Hb or albumin solution or an equivalent time in a time-control group with no transfusion, and 5) when hypocapnia was repeated after transfusion in the continued presence of l-NNA or CSF. With each intervention, the percent change in diameter was compared among the four groups by using one-way ANOVA and the Newman-Keuls multiple range test. Within each group, changes in MABP, PaCO2, arterial Hb concentration, and hematocrit after the transfusion were analyzed by paired t-test.
Fig. 2.
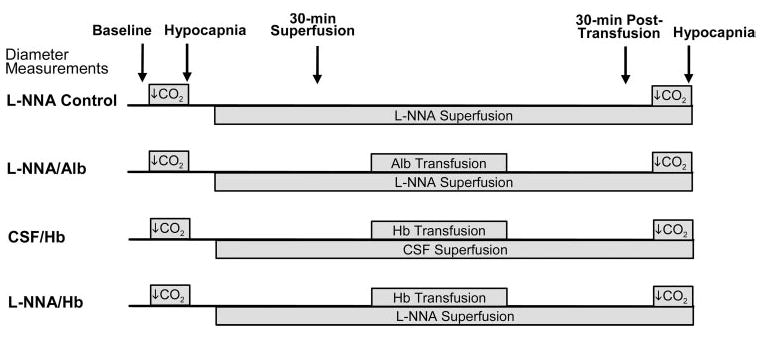
Schematic diagram of experimental design involving pial arteriolar diameter measurements after superfusion of cerebro-spinal fluid (CSF) or Nω-nitro-l-arginine (l-NNA) and after exchange transfusion with albumin (Alb) or Hb solution in four groups of cats.
Pial arteriolar response to Hb superfusion after NOS inhibition
To determine whether cross-linked Hb is capable of directly constricting pial arterioles by a NOS-dependent mechanism, the cranial window was superfused with the cross-linked Hb before and after local application of a NOS inhibitor in eight cats. Baseline diameter measurements were obtained after the window was superfused with artificial CSF. Diameter measurements were repeated at 5 min of superfusing 1 μM of cross-linked Hb, at 15 min of washout with CSF, and at 5 min of superfusing 10 μM of cross-linked Hb. After a 15-min washout period with CSF, the window was superfused with 300 μM l-NNA for 30 min. Vascular responses to Hb in the presence of the NOS inhibitor were then tested by superfusing the window with l-NNA plus 1 μM of cross-linked Hb for 5 min, l-NNA for 15 min, and l-NNA plus 10 μM of cross-linked Hb for 5 min. For each Hb concentration, the percent change in diameter was calculated from the baseline, measured immediately before Hb was increased in the window CSF. For each size grouping of vessels, the percent change in diameter was analyzed by two-way repeated measures ANOVA with NOS inhibition as one factor and Hb concentration as a second factor. If an overall effect of NOS inhibition was detected, responses at individual Hb concentrations were compared with and without NOS inhibition by paired t-test.
Pial arteriolar responses to KATP channel agonist after Hb transfusion
To test whether circulating cell-free Hb interfered with pial arteriolar dilation to an activator of KATP channels, responses to cromakalim were compared between a group of five cats transfused with Hb and a group of five cats with no transfusion. Thirty minutes after completion of the exchange transfusion, or an equivalent time in the control group, the cranial window was superfused at a rate of 1 ml/min for 5 min with the vehicle for cromakalim (0.03% dimethyl sulfoxide in artificial CSF). The window was then superfused with 1 μM cromakalim for 5 min, followed by a 5-min washout period with vehicle. After an additional 5 min to allow recovery of baseline diameter, 10 μM cromakalim was superfused for 5 min, followed by washout with vehicle. After an additional 10-min recovery period, the KATP channel antagonist glibenclamide (1 μM) was superfused for 5 min. An additional 5 min was allowed to establish a new baseline diameter. Responses to 1 and 10 μM cromakalim were repeated in the presence of 1 μM glibenclamide added to the superfusate. After the window was washed out with artificial CSF, dilatory capacity to hypercapnia was tested by adding 5% CO2 to the inspired gas mixture. For each size vessel, percent changes in diameter were analyzed by two-way ANOVA with repeated measures, where Hb transfusion was a between-subject factor and responses to the various drugs were a within-subject factor. For each dose of cromakalim, comparisons before and after glibenclamide were made by paired t-test.
Pial arteriolar responses to KATP channel agonist after Hb superfusion
To test whether abluminal application of cross-linked Hb tetramer interfered with dilation to a KATP channel activator, the dilator response to 1 and 10 μM cromakalim was tested in the presence of 0.1, 1, and 10 μM cross-linked Hb. After we obtained a control response to superfusion of the cranial window with either 1 μM cromakalim (n = 3 cats) or 10 μM cromakalim (n = 4 cats), the window was superfused with 0.1 μM cross-linked Hb for 5 min, and a new baseline diameter was recorded. The window was then superfused for 5 min with 0.1 μM Hb plus the corresponding dose of cromakalim. The procedure was repeated for 1 and then 10 μM Hb superfusion. The percent change in diameter in response to cromakalim at each Hb concentration was analyzed by repeated measures ANOVA.
RESULTS
Regional blood flow with NOS inhibition and Hb transfusion
Exchange transfusion with the 6% sebacyl cross-linked Hb solution, occurring over 60–100 min after baseline measurements, reduced arterial hematocrit by one-third from ~30% to 20%. Total arterial Hb concentration was reduced by ~17% (Table 1). Reductions were similar in groups pretreated with vehicle (Ringer lactate) or l-NAME administration at 20 min after baseline measurements. No major changes were observed in arterial pH or PaCO2 after administration of l-NAME, Hb transfusion, or Hb transfusion after l-NAME. In all groups, PaO2 was maintained in the range of 100–150 mmHg to keep oxyhemoglobin saturation >95%.
Table 1.
Physiological variables in regional blood flow experiment with no transfusion or Hb transfusion after Ringer lactate or l-NAME infusion
| Time, min
|
||||
|---|---|---|---|---|
| 0 | 50 | 120 | 160 | |
| Hematocrit, % | ||||
| Ringer/Hb | 30 ± 1 | 30 ± 1 | 20 ± 1* | 20 ± 1* |
| l-NAME alone | 28 ± 1 | 29 ± 1 | 29 ± 1 | 28 ± 1 |
| l-NAME/Hb | 31 ± 1 | 31 ± 1 | 20 ± 1* | 20 ± 1* |
| Arterial Hb, g/dl | ||||
| Ringer/Hb | 10.8 ± 0.2 | 11.1 ± 0.2 | 9.2 ± 0.2* | 9.0 ± 0.1* |
| l-NAME alone | 10.0 ± 0.2 | 10.4 ± 0.3 | 10.5 ± 0.4 | 9.8 ± 0.3 |
| l-NAME/Hb | 10.8 ± 0.5 | 11.6 ± 0.6 | 9.4 ± 0.3* | 9.7 ± 0.4* |
| Arterial pH | ||||
| Ringer/Hb | 7.42 ± 0.01 | 7.41 ± 0.01 | 7.40 ± 0.01 | 7.39 ± 0.01 |
| l-NAME alone | 7.42 ± 0.01 | 7.39 ± 0.01 | 7.40 ± 0.01 | 7.39 ± 0.01 |
| l-NAME/Hb | 7.42 ± 0.01 | 7.40 ± 0.01 | 7.38 ± 0.02 | 7.37 ± 0.01 |
| PaCO2, mmHg | ||||
| Ringer/Hb | 38 ± 1 | 39 ± 1 | 41 ± 1 | 41 ± 1 |
| l-NAME alone | 39 ± 1 | 40 ± 1 | 40 ± 1 | 41 ± 1 |
| l-NAME/Hb | 39 ± 1 | 40 ± 1 | 40 ± 2 | 41 ± 1 |
| CMRO2, ml O2·min−1·100 g−1 | ||||
| Ringer/Hb | 3.6 ± 0.2 | 3.7 ± 0.7 | 3.4 ± 0.2 | 3.5 ± 0.5 |
| l-NAME alone | 3.0 ± 0.2 | 3.0 ± 0.1 | 2.9 ± 0.1 | 3.0 ± 0.2 |
| l-NAME/Hb | 3.2 ± 0.3 | 2.5 ± 0.2 | 2.6 ± 0.3 | 2.9 ± 0.3 |
Values are means ± SE. Times correspond to microsphere-determined blood flow with Ringer lactate or Nω-nitro-l-arginine methyl ester (l-NAME) infused at 20 min and Hb transfusion performed between 60 and 100 min. Ringer/Hb, Ringer lactate infusion followed by Hb transfusion (n = 12); l-NAME alone, l-NAME infusion followed by no transfusion (n = 9); l-NAME/Hb, l-NAME infusion followed by Hb transfusion (n = 8). PaCO2, arterial partial pressure of CO2; CMRO2, cerebral metabolic rate of O2.
P < 0.05 from 50-min value by repeated measures ANOVA and paired t-test with Bonferroni correction.
In the group pretreated with Ringer vehicle, Hb transfusion increased MABP (Fig. 3). In the l-NAME time-control group with no Hb transfusion, MABP also increased. In the group with Hb transfusion after l-NAME pretreatment, MABP increased after l-NAME but did not increase further after Hb transfusion. Values in the latter group remained similar to those in the l-NAME control group over a 60-min period after completion of the transfusion.
Fig. 3.

Mean arterial blood pressure (means ± SE) in a group (n = 12 cats) exchange transfused with cross-linked Hb between 60 and 100 min (left), in a group (n = 9 cats) infused with l-NAME at 20 min (middle), and in a group (n = 8 cats) infused with l-NAME at 20 min and exchange transfused with cross-linked Hb between 60 and 100 min (right). *P < 0.05 between 0- and 50-min values; +P < 0.05 from 50-min value (repeated measures ANOVA and paired t-test with Bonferroni correction).
Blood flow to kidneys decreased after Hb transfusion alone and after l-NAME infusion alone (Fig. 4). However, Hb transfusion after l-NAME pretreatment did not cause a further reduction in renal blood flow. The pattern was similar in the small intestine where blood flow decreased significantly 1 h after completion of the Hb transfusion. Treatment with l-NAME also decreased intestinal blood flow, and no further reduction occurred with subsequent Hb transfusion. Blood flow to skeletal muscle and the left ventricular free wall exhibited a different pattern in that the flow increased after Hb transfusion. Administration of l-NAME produced a modest decrease in blood flow to skeletal muscle but not in the left ventricle, possibly because of the offsetting effect of increased afterload and increased myocardial work. Transfusion of Hb after l-NAME treatment produced no significant change in skeletal muscle blood flow and a transient increase in coronary blood flow.
Fig. 4.
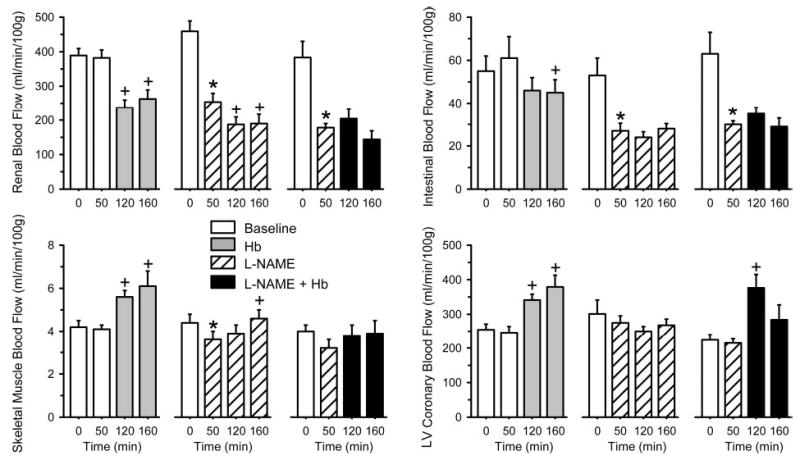
Microsphere-determined blood flow (means ± SE) to kidney, small intestine, skeletal muscle, and left ventricular (LV) free wall. Each tissue is presented from a group (n = 12 cats; left) exchange transfused with cross-linked Hb between 60 and 100 min, a group (n = 9 cats; middle) infused with l-NAME at 20 min, and a group (n = 8 cats; right) infused with l-NAME at 20 min and exchange transfused with cross-linked Hb between 60 and 100 min. *P < 0.05 between 0- and 50-min values; +P < 0.05 from 50-min value (repeated measures ANOVA and paired t-test with Bonferroni correction).
Administration of l-NAME alone decreased CBF and blood flow to the neurohypophysis (Fig. 5). Transfusion of the Hb solution alone produced no change in CBF but did produce a large decrease in blood flow to the neurohypophysis, which does not have a blood-brain barrier. After pretreatment with l-NAME, no additional decrease in neurohypophyseal blood flow occurred with Hb transfusion. CBF transiently increased by 20% at 20 min after the Hb transfusion but returned to the post-l-NAME value at 60 min after the transfusion. Repeated measures ANOVA did not indicate a significant change in cerebral O2 consumption in any group over time (Table 1).
Fig. 5.
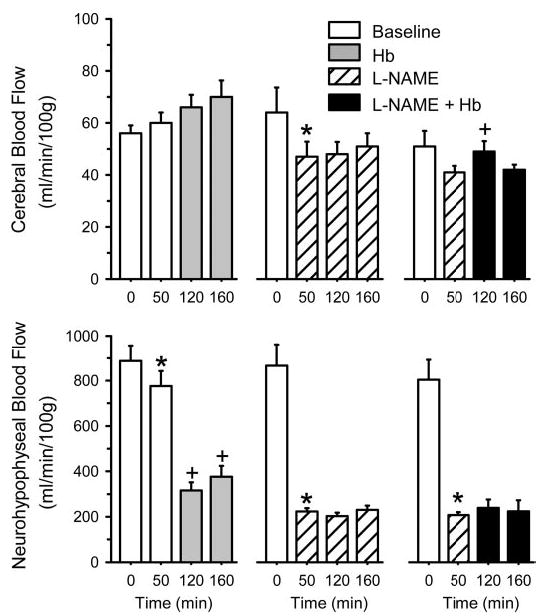
Microsphere-determined blood flow (means ± SE) to cerebrum and neurohypophysis. Each tissue is presented from a group (n = 12 cats; left) exchange transfused with cross-linked Hb between 60 and 100 min, a group (n = 9 cats; middle) infused with l-NAME at 20 min, and a group (n = 8 cats; right) infused with l-NAME at 20 min and exchange transfused with cross-linked Hb between 60 and 100 min. *P < 0.05 between 0- and 50-min values; +P < 0.05 from 50-min value (repeated measures ANOVA and paired t-test with Bonferroni correction).
Vascular resistances were analyzed for each organ, and percent changes were calculated to plot regional changes on the same scale in Fig. 6. Transfusion of Hb alone increased vascular resistance in the kidney and intestine, decreased resistance in the skeletal muscle and left ventricle, and produced no change in CVR. Infusion of l-NAME increased vascular resistance in all organs, with the kidney and intestine being most prominent on a percentage basis. Transfusion of Hb after l-NAME infusion did not produce an increase in resistance in any organ; resistance in the left ventricle and cerebrum decreased significantly.
Fig. 6.
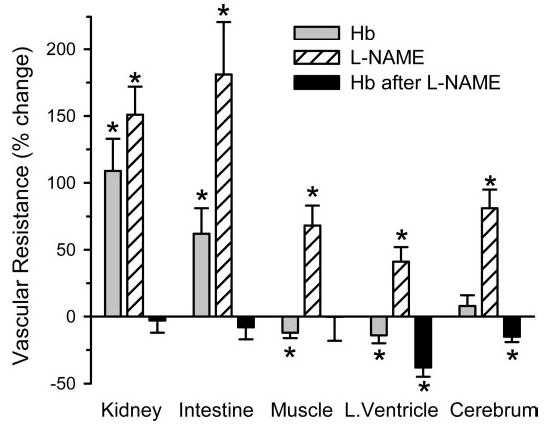
Percent change in vascular resistance (means ± SE) to kidney, small intestine, skeletal muscle, left ventricle (L. Ventricle), and cerebrum with exchange transfusion of cross-linked Hb alone (n = 12 cats), with infusion of l-NAME alone (n = 9 cats), and with exchange transfusion of cross-linked Hb after infusion of l-NAME (n = 8 cats; percent change calculated from post- l-NAME value). *P < 0.05 from previous resistance value by repeated measures ANOVA and paired t-test.
Pial arteriolar response to Hb transfusion after NOS inhibition
Superfusion of l-NNA through the cranial window produced constriction of pial arterioles of all sizes, whereas no changes in diameter occurred in the group superfused with CSF (Fig. 7A). The degree of constriction with l-NNA was similar in the time-control group and in the groups to be transfused subsequently with albumin and Hb. The initial constriction partially subsided over time, resulting in a modest dilation in the time-control group compared with the initial diameter after l-NNA superfusion (Fig. 7B). Exchange transfusion with the albumin solution after l-NNA superfusion produced dilation. Exchange transfusion with the Hb solution after CSF superfusion produced constriction. After l-NNA superfusion, Hb transfusion initially produced 12 ± 2%, 12 ± 2%, and 9 ± 1% constriction in small, medium, and large vessels, respectively, in addition to the constriction that occurred with l-NNA. In some cats, this additional constriction, measured at the completion of the exchange transfusion, subsided somewhat 30 min later. However, the response measured 30 min after the transfusion (Fig. 7B) was not significantly different from the time-matched response in the Hb-transfused group with CSF superfusion but was significantly less than the change in diameter observed in the l-NNA time-control group and the group transfused with albumin after l-NNA. The increase in MABP after transfusion was similar in the Hb groups superfused with CSF and l-NNA (Table 2). A slight increase in PaCO2 occurred in the Hb-transfused group after CSF superfusion. As expected, the decrease in arterial Hb concentration was greater in the albumin-transfused group than in the Hb-transfused groups at equivalent reductions in hematocrit (Table 2).
Fig. 7.
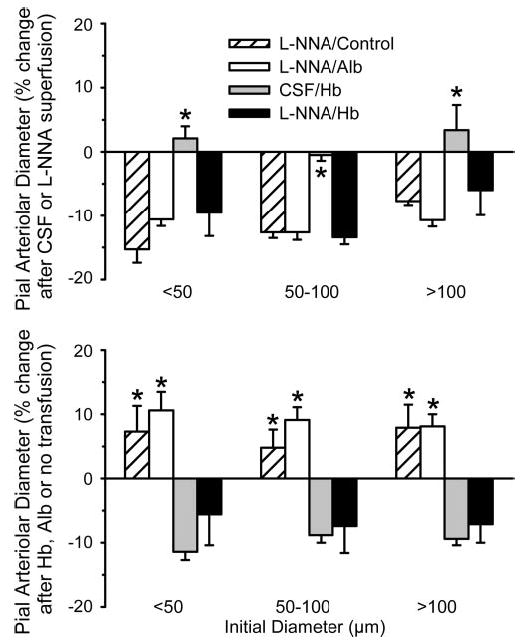
Top: percent change (means ± SE) in pial arteriolar diameter 1) after cranial window superfusion with l-NNA for 30 min in a time-control group (l-NNA/Control; n = 9 cats), 2) after superfusion with l-NNA for 30 min in a group with subsequent exchange transfusion of Alb solution (l-NNA/Alb; n = 6 cats), 3) after superfusion with artificial CSF for 30 min in a group with subsequent exchange transfusion of cross-linked Hb solution (CSF/Hb; n = 8 cats), and 4) after superfusion with l-NNA for 30 min in a group with subsequent exchange transfusion of cross-linked Hb solution (l-NNA/Hb; n = 7 cats). Bottom: percent change (means ± SE) in pial arteriolar diameter 1) over time with no further intervention from value measured after 30 min of l-NNA superfusion (l-NNA/Control), 2) after Alb transfusion from value measured after l-NNA superfusion (l-NNA/Alb), 3) after Hb transfusion from value measured after CSF superfusion (CSF/Hb), and 4) after Hb transfusion from value measured after l-NNA superfusion (l-NNA/Hb). Responses were segregated by vessels with initial diameters of <50 μm, 50–100 μm, and >100 μm. *P < 0.05 from l-NNA/Hb group by ANOVA and Newman-Keuls test.
Table 2.
Physiological variables in cranial window experiment with transfusion after CSF or l-NNA superfusion
| Pretransfusion | Posttransfusion | |
|---|---|---|
| MABP, mmHg | ||
| l-NNA/Control | 106 ± 4 | 98 ± 5* |
| l-NNA/Alb | 96 ± 3 | 95 ± 4 |
| CSF/Hb | 111 ± 6 | 129 ± 9* |
| l-NNA/Hb | 104 ± 7 | 119 ± 6* |
| PaCO2, mmHg | ||
| l-NNA/Control | 39 ± 1 | 40 ± 1 |
| l-NNA/Alb | 37 ± 1 | 36 ± 2 |
| CSF/Hb | 38 ± 1 | 40 ± 1* |
| l-NNA/Hb | 39 ± 1 | 41 ± 1 |
| Arterial Hb, g/dl | ||
| l-NNA/Control | 8.9 ± 0.4 | 9.1 ± 0.4 |
| l-NNA/Alb | 9.4 ± 0.5 | 6.1 ± 0.1* |
| CSF/Hb | 9.4 ± 0.3 | 7.8 ± 0.1* |
| l-NNA/Hb | 8.3 ± 0.1 | 7.5 ± 0.1* |
| Hematocrit, % | ||
| l-NNA/Control | 29 ± 1 | 29 ± 1 |
| l-NNA/Alb | 28 ± 1 | 18 ± 1* |
| CSF/Hb | 29 ± 1 | 18 ± 1* |
| l-NNA/Hb | 28 ± 1 | 18 ± 1* |
Values are means ± SE. Pretransfusion after cerebrospinal fluid (CSF) or Nω-nitro-l-arginine (l-NNA) superfusion and 30-min posttransfusion. l-NNA/ control, l-NNA time-control group (no transfusion; n = 9); l-NNA/Alb, group transfused with albumin after l-NNA (n = 6); CSF/Hb, group transfused with Hb after CSF superfusion (n = 8); and l-NNA/Hb, group transfused with Hb after l-NNA superfusion (n = 7). MABP, mean arterial blood pressure.
P < 0.05 from pretransfusion by paired t-test.
To demonstrate whether pial arterioles were still capable of constriction after treatment with l-NNA and Hb transfusion, reactivity to hypocapnia was tested and compared with the percent constriction before l-NNA and Hb transfusion. Constriction occurred in all groups after either l-NNA, Hb transfusion, or the combination of both. The percent constriction was attenuated after l-NNA alone in large size vessels and after l-NNA plus albumin transfusion in small size arterioles compared with the baseline hypocapnic response by paired t-test (Fig. 8). No significant changes occurred in other sized vessels in these groups or in any sized vessel in the two groups receiving Hb.
Fig. 8.
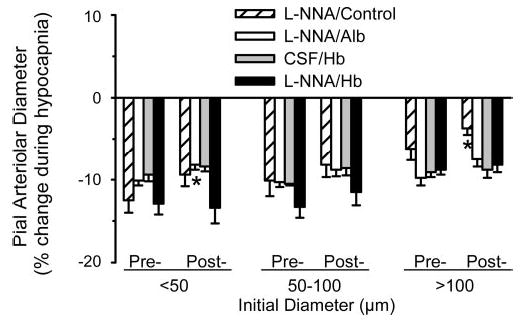
Percent change in pial arteriolar diameter (means ± SE) during hypocapnia at the beginning of the protocol before l-NNA superfusion or exchange transfusion (pre-) and after intervention (post-) with either superfu-sion of l-NNA and no transfusion (l-NNA/Control; n = 9 cats), with superfusion of l-NNA and subsequent exchange transfusion of Alb solution (l-NNA/Alb; n = 6 cats), with superfusion of artificial CSF and subsequent exchange transfusion of cross-linked Hb solution (CSF/Hb; n = 8 cats), and with superfusion of l-NNA and subsequent exchange transfusion of cross-linked Hb solution (l-NNA/Hb; n = 7 cats). Responses were segregated by vessels with initial diameters of <50 μm, 50–100 μm, and >100 μm. *P < 0.05 from corresponding pretransfusion response by paired t-test.
Pial arteriolar response to Hb superfusion after NOS inhibition
Abluminal application of sebacyl cross-linked Hb was previously found to constrict pial arterioles (1). To determine whether NOS inhibition could reduce the direct effects of abluminal cross-linked Hb on pial arterioles, the window was superfused with cross-linked Hb before and after exposure to 300 μM l-NNA in the CSF. Before superfusion of l-NNA, placement of 1 and 10 μM cross-linked Hb in the window CSF decreased the diameter of arterioles of all sizes (Fig. 9). Two-way repeated measures ANOVA indicated an overall effect of l-NNA on the response to the two Hb concentrations. Individual comparisons with paired t-test at each dose indicated that l-NNA significantly reduced the constrictor response to cross-linked Hb superfusion in all vessel sizes.
Fig. 9.
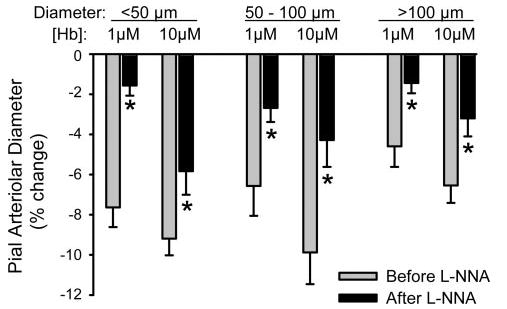
Percent change in pial arteriolar diameter (means ± SE) after 5-min superfusion of cranial window with CSF containing 1 and 10 μM sebacyl cross-linked Hb before and after cosuperfusion of 300 μM l-NNA (n = 8 cats). Responses were segregated by vessels with initial diameters of <50 μm, 50–100 μm, and >100 μm. *P < 0.05 from corresponding value before l-NNA by repeated measures ANOVA and paired t-test.
Pial arteriolar responses to KATP channel agonist after Hb transfusion
Opening of KATP channels is important in cerebrovascular dilation to various stimuli, including decreased oxygenation (27, 28). The ability of pial arterioles to dilate to the KATP channel activator cromakalim was tested after transfusion of Hb. As in previous groups, exchange transfusion produced a decrease in hematocrit (30 ± 1% to 18 ± 1%), a decrease in total Hb concentration (9.8 ± 0.2 to 7.8 ± 0.1 g/dl), and an increase in MABP (123 ± 4 to 143 ± 2 mmHg). Plasma Hb concentration was 2.2 ± 0.2 g/dl. PaCO2 after Hb transfusion (41 ± 1 mmHg) was similar to that in a time-control group with no transfusion (40 ± 1 mmHg). Superfusion of the 0.03% dimethyl sulfoxide vehicle alone had no significant effect on baseline arteriolar diameter in any size vessel (small, − 0.2 ± 1.0%; medium, − 0.5 ± 1.3%; large, 3.1 ± 1.3%). Responses to cromakalim were calculated as a percentage of the baseline diameter after exposure to vehicle. Superfusion of 1 and 10 μM cromakalim produced dose-dependent dilation in all sized pial arterioles (Fig. 10). The responses were similar in control and Hb-transfused groups. Application of the KATP channel antagonist glibenclamide (1 μM) blocked the dilator response to 1 μM cromakalim and attenuated the response to 10 μM cromakalim to the same extent in both groups. After washout of glibenclamide, reactivity to hypercapnia was tested by increasing PaCO2 from 40 ± 1 to 58 ± 2 mmHg in the control group and from 41 ± 1 to 59 ± 2 mmHg in the Hb-transfused group. For each vessel size, the percent increase in diameter during hypercapnia was similar in the control and Hb-transfused groups (Fig. 11).
Fig. 10.
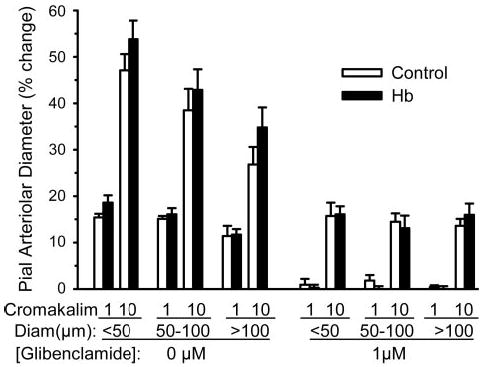
Percent change in pial arteriolar diameter (means ± SE) after 5-min superfusion of cranial window with CSF containing 1 and 10 μM of the ATP-sensitive K+ (KATP) channel activator cromakalim before and after cosuperfusion of 1 μM of the KATP channel antagonist glibenclamide in five control cats and in five cats that had undergone cross-linked Hb exchange transfusion. Responses were segregated by vessels with initial diameters of <50 μm, 50–100 μm, and >100 μm. No significant differences were observed between control and Hb-transfused groups. Glibenclamide significantly reduced the dose-dependent response to cromakalim.
Fig. 11.
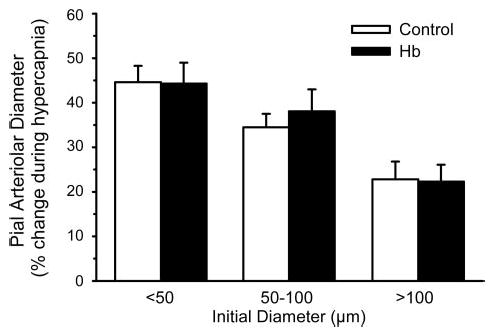
Percent change in pial arteriolar diameter (means ± SE) during hypercapnia in five control cats and in five cats that had undergone cross-linked Hb exchange transfusion (after washout of cromakalim and glibenclamide). Responses were segregated by vessels with initial diameters of <50 μm, 50–100 μm, and >100 μm. No significant differences were observed among groups.
Pial arteriolar responses to KATP channel agonist after Hb superfusion
The effect of increasing the CSF concentration of cross-linked Hb on the pial arteriolar response to cromakalim was investigated by superfusing the window stepwise with 0, 0.1, 1, and 10 μM cross-linked Hb and either 1 or 10 μM cromakalim. Dilation to either dose of cromakalim was not significantly affected by any dose of Hb in arterioles of any size (Fig. 12).
Fig. 12.
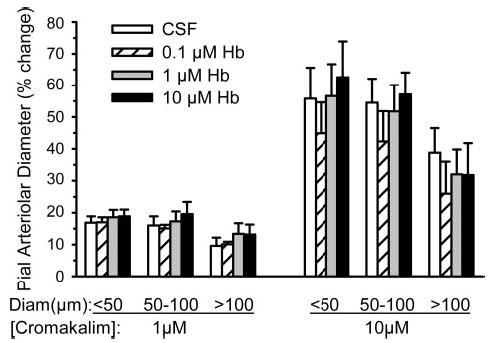
Percent change in pial arteriolar diameter (means ± SE) after 5-min superfusion of cranial window with CSF containing 1 μM cromakalim (n = 3 cats) or 10 μM cromakalim (n = 4 cats) and with cosuperfusion of 0, 0.1, 1, and 10 μM of sebacyl cross-linked Hb in the window. Responses were segregated by vessels with initial diameters of <50 μm, 50–100 μm, and >100 μm. For each cromakalim dose, there was no significant effect of CSF Hb concentration.
DISCUSSION
The major findings of this study are as follows: 1) exchange transfusion of cell-free tetrameric cross-linked Hb produced selective decreases in regional blood flow to tissues without tight endothelial junctions, such as the kidney, small intestine, and neurohypophysis; 2) these regional reductions in blood flow after Hb transfusion were blocked by treatment with a NOS inhibitor; 3) in the cerebrum where Hb exchange transfusion produced no change in blood flow and a decrease in pial arteriolar diameter, the pial arteriolar constrictor response to Hb transfusion was unaffected by local application of a NOS inhibitor; 4) abluminal application of the cross-linked Hb to pial arterioles in vivo can directly produce constriction that is NOS dependent; 5) plasma-based Hb does not exert a major effect on pial arteriolar reactivity to hypocapnia, hypercapnia, or KATP channel activation; and 6) abluminal Hb application does not interfere with pial arteriolar dilation to KATP channel activation.
Because adverse effects reported in clinical trials of a tetrameric cross-linked Hb solution in patients with hemorrhagic shock and stroke were thought to be attributed to NO scavenging and a resultant peripheral vasoconstriction, efforts have been directed at designing Hb molecules with decreased NO affinity (14, 18). Our data showing that increases in MABP after tetrameric Hb transfusion were blocked by prior l-NAME treatment support the concept that hypertension induced by transfusion of cell-free Hb tetramers is attributed to scavenging of NO. However, the percent increase in vascular resistance was heterogeneous among organs. The hypertensive response was primarily the result of increased vascular resistance in the kidney and small intestines. Vascular resistance was unchanged in the cerebrum and decreased in the skeletal and cardiac muscle. Cerebral, coronary, and skeletal muscle vascular beds have smaller capillary pores than kidney and intestines (17) and are less likely to permit extravasation of the 64-kDa cross-linked tetramer. Although cross-linked tetrameric Hb does not appear in urine, it does appear in renal lymph draining the peritubular capillary bed (12). In contrast, any extravasation into CSF is too small to functionally impair NO-dependent dilation to acetylcholine and ADP (1). The increase in vascular resistance in the kidney and intestines after Hb transfusion was blocked by prior NOS inhibition, thereby indicating that the increase in resistance required basal amounts of NO. It should be noted that this conclusion assumes that any vasoconstrictive property of cross-linked Hb not mediated by NO scavenging could still have exerted an additional vasoconstrictive effect when blood flow was already markedy reduced by l-NAME. Previous work (35) with a reverse protocol sequence in which l-NAME was infused after transfusion of a fumaryl cross-linked Hb failed to show a further reduction in renal and intestinal blood flow with NOS inhibition. The lack of an increase in vascular resistance in the skeletal and cardiac muscle and the cerebrum after Hb transfusion in the present study did not appear to be due to a lack of tonic NO production because NOS inhibition alone increased vascular resistance in these beds and decreased blood flow in skeletal muscle and cerebrum. (The lack of a significant decrease in coronary blood flow with l-NAME alone may have been due to the increase in afterload and consequent increase in O2 consumption.) Although part of the increase in vascular resistance after l-NAME administration may be the result of an autoregulatory response to the increase in MABP, the increase in MABP was equivalent to that occurring after Hb transfusion when vascular resistance failed to increase in these beds. Taken together, these data support the concept that an increase in vascular resistance after tetrameric cross-linked Hb transfusion is primarily attributed to scavenging of NO by extravasated Hb in selected vascular beds rather than by Hb in the plasma space. Thus these results suggest that increasing the size of the Hb molecule would be more effective in limiting vasoconstriction than would engineering modest decreases in NO affinity. The lack of a major hypertensive response with transfusion of Hb molecules polymerized by glutaraldehyde (7), together with our own data (12) of a zero-link Hb polymer that does not appear in renal lymph and does not increase MABP, supports the concept of limiting extravasation in the design of these oxygen carriers.
Blood flow results in the neurohypophysis, in which the blood vessels are highly permeable and NO exerts a tonic vasodilatory effect (37), offer a useful contrast with the rest of the brain. Whereas l-NAME decreased blood flow in both the cerebrum and neurohypophysis, Hb transfusion decreased blood flow only in neurohypophysis. Moreover, prior treatment with l-NAME prevented further reductions in neurohypophy-seal blood flow by Hb transfusion. This finding agrees with previous work (33) showing that l-NAME administration after Hb transfusion did not produce further reductions in neurohypophyseal blood flow. Therefore, results in the neurohypophysis also lend support to an abluminal site as the primary functional location of NO scavenging by tetrameric Hb.
Direct measurements of diameter of pial arteriolar resistance vessels on the brain surface revealed that abluminal application of the sebacyl cross-linked Hb produced constriction that was inhibited by l-NNA. Thus the chemical cross-linking of the Hb molecule did not block the ability of the tetramer to constrict arterioles by a NO-dependent mechanism. However, when the cross-linked Hb was transfused, the initial constrictor response in pial arterioles was not blocked by l-NNA, and the response remained significantly different from the responses observed in control groups with no transfusion or with albumin transfusion at equivalent hematocrit. The concentration of Hb in the plasma after the exchange transfusion was ~300 μM, which greatly exceeded the abluminal concentration of 10 μM used to generate similar constriction of pial arterioles. Therefore intraluminal NO scavenging is not the primary mechanism of the pial arteriolar constriction seen after an exchange transfusion of cross-linked Hb. The constriction is not related to a decrease in energy metabolism because Hb transfusion did not decrease cerebral O2 consumption. The constriction is more likely attributed to 1) an autoregulatory response to the increase in MABP or 2) the decrease in blood viscosity associated with the decrease in hematocrit. Blood viscosity is decreased ~25% after cross-linked Hb exchange transfusion to a hematocrit of 18% in the cat (33). Studies (16) with exchange transfusion of the large zero-link polymer of bovine Hb to the same hematocrit demonstrated that pial arterioles still constricted after the transfusion in the absence of an increase in MABP but that the constriction can be reversed to dilation when plasma viscosity is concurrently increased. Thus the constrictor response to cell-free Hb transfusion does not require arterial hypertension. Rather, the constriction of pial arterioles apparently offsets the decrease in blood viscosity associated with decreased hematocrit and results in no change in CBF when O2-carrying capacity is preserved by cell-free Hb.
Increased unloading of O2 by plasma-based Hb is also considered to contribute to vasoconstriction after Hb transfusion (19), particularly when the O2 affinity is low (30). The currently used cross-linked Hb with a P50 of 34 mmHg would be expected to enhance precapillary O2 loss and enhance constriction. Although the lack of further increases in vascular resistance when Hb was transfused after NOS inhibition argues against the importance of this mechanism, we cannot exclude that the increase in vascular resistance resulting from NOS inhibition may have mitigated the ability of increased oxygenation to produce further increases in vascular resistance. In addition, the pial arteriolar diameter measurements indicate that resistance vessels can, in fact, constrict in response to Hb transfusion after NOS inhibition but that the concurrent decrease in viscosity offsets an increase in vascular resistance.
Cell-free Hb can scavenge NO more effectively than red blood cell-based Hb (11, 36), and mathematical models of NO transport in arterioles predict that NO concentration in arteriolar smooth muscle will be markedly reduced by the presence of Hb in the plasma (8, 9). If Hb does scavenge NO in the plasma space, the present results indicate that the amount scavenged is inadequate to account for changes in basal diameter after Hb transfusion, and previous work (1) indicates that the amount scavenged is inadequate to impair NO-dependent dilation to the agonist stimulation.
Inhibition of neuronal NOS attenuates cerebrovascular dilation during hypercapnia (6). However, cross-linked Hb transfusion did not attenuate hypercapnic dilation of pial arterioles of any size, in agreement with previous work (16) in which polymerized Hb was transfused. The constrictor response to hypocapnia was also intact after cross-linked Hb transfusion. These results indirectly suggest that plasma-based Hb does not scavenge neuronally derived NO in large quantities.
One potential limitation of studying pial arterioles is that intraparenchymal arterioles may behave differently. Because of their large surface area, capillary endothelia represent a significant source of NO that could be scavenged by plasma-based Hb and could influence the concentration of NO in intraparenchymal arteriolar smooth muscle (9, 32). Measurements of CBF, which depends on both intraparenchymal and extraparenchymal resistance, showed a transient increase from a reduced baseline value when Hb was transfused after NOS inhibition, whereas CBF was not significantly changed by Hb transfusion in the absence of NOS inhibition. Perhaps the scavenging of NO in capillary endothelium by Hb limited the increase in CBF that might be anticipated at reduced blood viscosity and selectively enhanced intraparenchymal arteriolar tone.
The present work demonstrates that pial arteriolar dilation to cromakalim was not impaired by the presence of Hb in either the plasma space or in the CSF. Basilar artery dilation to a KATP channel activator has been reported to remain intact 2 days after cisternal injection of blood (26). Because the release of Hb from red blood cells after subarachnoid hemorrhage plays a role in vasospasm, our results with an acute application of Hb in the CSF also support the potential of KATP channel activators as a therapeutic target for subarachnoid hemorrhage. Pharmacological evidence indicates that KATP channels play an important role in cerebrovascular regulation in a variety of conditions, such as hypoxia (27, 28). Previous work (34) with sebacyl cross-linked Hb transfusion demonstrated that the increase in CBF during graded hypoxic hypoxia was equivalent to that in controls when one adjusted for differences in arterial O2 content. With the assumption that the vasodilatory response to hypoxia remained dependent on the activation of KATP channels after Hb transfusion, these results are consistent with the present finding of intact pial arteriolar dilation to cromakalim after Hb transfusion. It has also been proposed that the release of NO bound to cysteine residues on red blood cell-based Hb during deoxygenation may contribute to hypoxic vasodilation (13, 24). However, free NO released from red blood cells would be readily scavenged by Hb in the plasma. Thus the normal CBF response to hypoxia previously observed in the presence of cell-free Hb implies that the release of free NO into the plasma by red blood cells is not essential for hypoxic vasodilation in the brain. An alternative mechanism involving NO storage in a nitrite pool, which can be converted to NO in the presence of deoxyhemoglobin (4), might still be operative in the presence of plasma-based deoxyhemoglobin, which could release NO at the plasma-endothelial wall interface.
In summary, our results with exchange transfusion of a cross-linked tetrameric Hb indicate that peripheral vasoconstriction that was blocked by prior NOS inhibition is most likely attributed to scavenging of NO by extravasated Hb because the effect was most prominent in vascular beds with more porous endothelium. In contrast, in pial arterioles with tight endothelial junctions, the constriction that occurred after exchange transfusion of the Hb solution was not blocked by NOS inhibition. This constrictor response apparently offsets the decrease in blood viscosity to keep CBF constant and to possibly limit overoxygenation of the brain. Furthermore, cell-free Hb transfusion did not interfere with pial arteriolar responses to hypercapnia, hypocapnia, or KATP channel activation. These findings, together with previous data showing intact dilatory responses to hypoxia (34) and to abluminal application of acetylcholine, ADP and NO donors (1), indicate that the presence of cross-linked Hb in the plasma does not adversely affect vascular reactivity to diverse stimuli in a vascular bed with tight endothelial junctions. These results imply that the design of large Hb molecules that do not extravasate should help preserve arteriolar reactivity in other vascular beds with more porous endothelium.
Acknowledgments
The authors thank Tzipora Sofare for editorial assistance.
Footnotes
GRANTS
This work was supported by National Institutes of Health Grants NS-38684 and HL-48517.
References
- 1.Asano Y, Koehler RC, Ulatowski JA, Traystman RJ, Bucci E. Effect of crosslinked hemoglobin transfusion on endothelial dependent dilation in feline pial arterioles. Am J Physiol Heart Circ Physiol. 1998;275:H1313–H1321. doi: 10.1152/ajpheart.1998.275.4.H1313. [DOI] [PubMed] [Google Scholar]
- 2.Bucci E, Razynska A, Kwansa H, Gryczynski Z, Collins JH, Fronticelli C, Unger R, Braxenthaler M, Moult J, Ji X, Gilliland G. Positive and negative cooperativities at subsequent steps of oxygenation regulate the allosteric behavior of multistate sebacylhemoglobin. Biochemistry. 1996;35:3418–3425. doi: 10.1021/bi952446b. [DOI] [PubMed] [Google Scholar]
- 3.Bucci E, Razynska A, Kwansa H, Matheson-Urbaitis B, O’Hearne M, Ulatowski JA, Koehler RC. Production and characteristics of an infusible oxygen-carrying fluid based on hemoglobin intramolecularly cross-linked with sebacic acid. J Lab Clin Med. 1996;128:146–153. doi: 10.1016/s0022-2143(96)90006-2. [DOI] [PubMed] [Google Scholar]
- 4.Cosby K, Partovi KS, Crawford JH, Patel RP, Reiter CD, Martyr S, Yang BK, Waclawiw MA, Zalos G, Xu X, Huang KT, Shields H, Kim-Shapiro DB, Schechter AN, Cannon RO, III, Gladwin MT. Nitrite reduction to nitric oxide by deoxyhemoglobin vasodilates the human circulation. Nat Med. 2003;9:1498–1505. doi: 10.1038/nm954. [DOI] [PubMed] [Google Scholar]
- 5.Doherty DH, Doyle MP, Curry SR, Vali RJ, Fattor TJ, Olson JS, Lemon DD. Rate of reaction with nitric oxide determines the hypertensive effect of cell-free hemoglobin. Nat Biotechnol. 1998;16:672–676. doi: 10.1038/nbt0798-672. [DOI] [PubMed] [Google Scholar]
- 6.Iadecola C, Zhang F. Permissive and obligatory roles of NO in cerebrovascular responses to hypercapnia and acetylcholine. Am J Physiol Regul Integr Comp Physiol. 1996;271:R990–R1001. doi: 10.1152/ajpregu.1996.271.4.R990. [DOI] [PubMed] [Google Scholar]
- 7.Johnson JL, Moore EE, Offner PJ, Haenel JB, Hides GA, Tamura DY. Resuscitation of the injured patient with polymerized stroma-free hemoglobin does not produce systemic or pulmonary hypertension. Am J Surg. 1998;176:612–617. doi: 10.1016/s0002-9610(98)00275-x. [DOI] [PubMed] [Google Scholar]
- 8.Kavdia M, Popel AS. Wall shear stress differentially affects NO level in arterioles for volume expanders and Hb-based O2 carriers. Microvasc Res. 2003;66:49–58. doi: 10.1016/s0026-2862(03)00008-6. [DOI] [PubMed] [Google Scholar]
- 9.Kavdia M, Tsoukias NM, Popel AS. Model of nitric oxide diffusion in an arteriole: impact of hemoglobin-based blood substitutes. Am J Physiol Heart Circ Physiol. 2002;282:H2245–H2253. doi: 10.1152/ajpheart.00972.2001. [DOI] [PubMed] [Google Scholar]
- 10.Levasseur JE, Wei EP, Raper AJ, Kontos HA, Patterson JL. Detailed description of a cranial window technique for acute and chronic experiments. Stroke. 1975;6:308–317. doi: 10.1161/01.str.6.3.308. [DOI] [PubMed] [Google Scholar]
- 11.Liu X, Samouilov A, Lancaster JR, Jr, Zweier JL. Nitric oxide uptake by erythrocytes is primarily limited by extracellular diffusion not membrane resistance. J Biol Chem. 2002;277:26194–26199. doi: 10.1074/jbc.M201939200. [DOI] [PubMed] [Google Scholar]
- 12.Matheson B, Kwansa HE, Bucci E, Rebel A, Koehler RC. Vascular response to infusions of a nonextravasating hemoglobin polymer. J Appl Physiol. 2002;93:1479–1486. doi: 10.1152/japplphysiol.00191.2002. [DOI] [PubMed] [Google Scholar]
- 13.McMahon TJ, Moon RE, Luschinger BP, Carraway MS, Stone AE, Stolp BW, Gow AJ, Pawloski JR, Watke P, Singel DJ, Piantadosi CA, Stamler JS. Nitric oxide in the human respiratory cycle. Nat Med. 2002;8:711–717. doi: 10.1038/nm718. [DOI] [PubMed] [Google Scholar]
- 14.Olson JS, Foley EW, Rogge C, Tsai AL, Doyle MP, Lemon DD. NO scavenging and the hypertensive effect of hemoglobin-based blood substitutes. Free Radic Biol Med. 2004;36:685–697. doi: 10.1016/j.freeradbiomed.2003.11.030. [DOI] [PubMed] [Google Scholar]
- 15.Przybelski RJ, Daily EK, Kisicki JC, Mattia-Goldberg C, Bounds MJ, Colburn WA. Phase I study of the safety and pharmacologic effects of diaspirin cross-linked hemoglobin solution. Crit Care Med. 1996;24:1993–2000. doi: 10.1097/00003246-199612000-00011. [DOI] [PubMed] [Google Scholar]
- 16.Rebel A, Ulatowski JA, Kwansa H, Bucci E, Koehler RC. Cerebrovascular response to decreased hematocrit: effect of cell-free hemoglobin, plasma viscosity, and CO2. Am J Physiol Heart Circ Physiol. 2003;285:H1600–H1608. doi: 10.1152/ajpheart.00077.2003. [DOI] [PubMed] [Google Scholar]
- 17.Renkin EM, Rew K, Wong M, O’Loughlin D, Sibley L. Influence of saline infusion on blood-tissue albumin transport. Am J Physiol Heart Circ Physiol. 1989;257:H525–H533. doi: 10.1152/ajpheart.1989.257.2.H525. [DOI] [PubMed] [Google Scholar]
- 18.Resta TC, Walker BR, Eichinger MR, Doyle MP. Rate of NO scavenging alters effects of recombinant hemoglobin solutions on pulmonary vasoreactivity. J Appl Physiol. 2002;93:1327–1336. doi: 10.1152/japplphysiol.00175.2002. [DOI] [PubMed] [Google Scholar]
- 19.Rohlfs RJ, Bruner E, Chiu A, Gonzales A, Gonzales ML, Magde D, Magde MD, Jr, Vandegriff KD, Winslow RM. Arterial blood pressure responses to cell-free hemoglobin solutions and the reaction with nitric oxide. J Biol Chem. 1998;273:12128–12134. doi: 10.1074/jbc.273.20.12128. [DOI] [PubMed] [Google Scholar]
- 20.Sakai H, Hara H, Yuasa M, Tsai AG, Takeoka S, Tsuchida E, Intaglietta M. Molecular dimensions of Hb-based O2 carriers determine constriction of resistance arteries and hypertension. Am J Physiol Heart Circ Physiol. 2000;279:H908–H915. doi: 10.1152/ajpheart.2000.279.3.H908. [DOI] [PubMed] [Google Scholar]
- 21.Saxena R, Wijnhoud AD, Carton H, Hacke W, Kaste M, Przybelski RJ, Stern KN, Koudstaal PJ. Controlled safety study of a hemoglobin-based oxygen carrier, DCLHb, in acute ischemic stroke. Stroke. 1999;30:993–996. doi: 10.1161/01.str.30.5.993. [DOI] [PubMed] [Google Scholar]
- 22.Saxena R, Wijnhoud AD, Man in ’t Veld AJ, van den Meiracker AH, Boomsma F, Przybelski RJ, Koudstaal PJ. Effect of diaspirin cross-linked hemoglobin on endothelin-1 and blood pressure in acute ischemic stroke in man. J Hypertens. 1998;16:1459–1465. doi: 10.1097/00004872-199816100-00009. [DOI] [PubMed] [Google Scholar]
- 23.Sharma A, Singh G, Gulati A. Role of NO mechanism in cardiovascular effects of diaspirin cross-linked hemoglobin in anesthetized rats. Am J Physiol Heart Circ Physiol. 1995;269:H1379–H1388. doi: 10.1152/ajpheart.1995.269.4.H1379. [DOI] [PubMed] [Google Scholar]
- 24.Singel DJ, Stamler JS. Chemical physiology of blood flow regulation be red blood cells. Annu Rev Physiol. 2005;67:99–145. doi: 10.1146/annurev.physiol.67.060603.090918. [DOI] [PubMed] [Google Scholar]
- 25.Snyder SR, Welty EV, Walder RY, Williams LA, Walder JA. HbXL99α: a hemoglobin derivative that is cross-linked between the α subunits is useful as a blood substitute. Proc Natl Acad Sci USA. 1987;84:7280–7284. doi: 10.1073/pnas.84.20.7280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sobey CG, Heistad DD, Faraci FM. Effect of subarachnoid hemorrhage on cerebral vasodilatation in response to activation of ATP-sensitive K+ channels in chronically hypertensive rats. Stroke. 1997;28:392–396. doi: 10.1161/01.str.28.2.392. [DOI] [PubMed] [Google Scholar]
- 27.Taguchi H, Heistad DD, Kitazono T, Faraci FM. ATP-sensitive K+ channels mediate dilatation of cerebral arterioles during hypoxia. Circ Res. 1994;74:1005–1008. doi: 10.1161/01.res.74.5.1005. [DOI] [PubMed] [Google Scholar]
- 28.Tomiyama Y, Brian JE, Jr, Todd MM. Cerebral blood flow during hemodilution and hypoxia in rats: role of ATP-sensitive potassium channels. Stroke. 1999;30:1942–1947. doi: 10.1161/01.str.30.9.1942. [DOI] [PubMed] [Google Scholar]
- 29.Traystman RJ, Moore LE, Helfaer MA, Davis S, Banasiak K, Williams M, Hurn PD. Nitro-l-arginine analogues: Dose- and time-related nitric oxide synthase inhibition in brain. Stroke. 1995;26:864–869. doi: 10.1161/01.str.26.5.864. [DOI] [PubMed] [Google Scholar]
- 30.Tsai AG, Vandegriff KD, Intaglietta M, Winslow RM. Targeted O2 delivery by low-P50 hemoglobin: a new basis for O2 therapeutics. Am J Physiol Heart Circ Physiol. 2003;285:H1411–H1419. doi: 10.1152/ajpheart.00307.2003. [DOI] [PubMed] [Google Scholar]
- 31.Tsoukias NM, Kavdia M, Popel AS. A theoretical model of nitric oxide transport in arterioles: frequency- vs. amplitude-dependent control of cGMP formation. Am J Physiol Heart Circ Physiol. 2004;286:H1043–H1056. doi: 10.1152/ajpheart.00525.2003. [DOI] [PubMed] [Google Scholar]
- 32.Tsoukias NM, Popel AS. A model of nitric oxide capillary exchange. Microcirculation. 2003;10:479–495. doi: 10.1038/sj.mn.7800210. [DOI] [PubMed] [Google Scholar]
- 33.Ulatowski JA, Bucci E, Nishikawa T, Razynska A, Williams MA, Takeshima R, Traystman RJ, Koehler RC. Cerebral O2 transport with hematocrit reduced by cross-linked hemoglobin transfusion. Am J Physiol Heart Circ Physiol. 1996;270:H466–H475. doi: 10.1152/ajpheart.1996.270.2.H466. [DOI] [PubMed] [Google Scholar]
- 34.Ulatowski JA, Bucci E, Razynska A, Traystman RJ, Koehler RC. Cerebral blood flow during hypoxic hypoxia with plasma-based hemoglobin at reduced hematocrit. Am J Physiol Heart Circ Physiol. 1998;274:H1933–H1942. doi: 10.1152/ajpheart.1998.274.6.H1933. [DOI] [PubMed] [Google Scholar]
- 35.Ulatowski JA, Nishikawa T, Matheson-Urbaitis B, Bucci E, Traystman RJ, Koehler RC. Regional blood flow alterations after bovine fumaryl ββ-crosslinked hemoglobin transfusion and nitric oxide synthase inhibition. Crit Care Med. 1996;24:558–565. doi: 10.1097/00003246-199604000-00003. [DOI] [PubMed] [Google Scholar]
- 36.Vaughn MW, Huang KT, Kuo L, Liao JC. Erythrocytes possess an intrinsic barrier to nitric oxide consumption. J Biol Chem. 2000;275:2342–2348. doi: 10.1074/jbc.275.4.2342. [DOI] [PubMed] [Google Scholar]
- 37.Wagner BP, Stingele R, Williams MA, Wilson DA, Traystman RJ, Hanley DF. NO contributes to neurohypophysial but not other regional cerebral fluorocarbon-induced hyperemia in cats. Am J Physiol Heart Circ Physiol. 1997;273:H1994–H2000. doi: 10.1152/ajpheart.1997.273.4.H1994. [DOI] [PubMed] [Google Scholar]
- 38.Wettstein R, Tsai AG, Erni D, Winslow RM, Intaglietta M. Resuscitation with polyethylene glycol-modified human hemoglobin improves microcirculatory blood flow and tissue oxygenation after hemorrhagic shock in awake hamsters. Crit Care Med. 2003;31:1824–1830. doi: 10.1097/01.CCM.0000069340.16319.F2. [DOI] [PubMed] [Google Scholar]


