Abstract
In skeletal muscle, sarcoplasmic reticulum (SR) Ca2+ depletion is suspected to trigger a calcium entry across the plasma membrane and recent studies also suggest that the opening of channels spontaneously active at rest and possibly involved in Duchenne dystrophy may be regulated by SR Ca2+ depletion. Here we simultaneously used the cell-attached and whole-cell voltage-clamp techniques as well as intracellular Ca2+ measurements on single isolated mouse skeletal muscle fibres to unravel any possible change in membrane conductance that would depend upon SR Ca2+ release and/or SR Ca2+ depletion. Delayed rectifier K+ single channel activity was routinely detected during whole-cell depolarizing pulses. In addition the activity of channels carrying unitary inward currents of ∼1.5 pA at −80 mV was detected in 17 out of 127 and in 21 out of 59 patches in control and mdx dystrophic fibres, respectively. In both populations of fibres, large whole-cell depolarizing pulses did not reproducibly increase this channel activity. This was also true when, repeated application of the whole-cell pulses led to exhaustion of the Ca2+ transient. SR Ca2+ depletion produced by the SR Ca2+ pump inhibitor cyclopiazonic acid (CPA) also failed to induce any increase in the resting whole-cell conductance and in the inward single channel activity. Overall results indicate that voltage-activated SR Ca2+ release and/or SR Ca2+ depletion are not sufficient to activate the opening of channels carrying inward currents at negative voltages and challenge the physiological relevance of a store-operated membrane conductance in adult skeletal muscle.
The main source of calcium that activates contractile proteins in skeletal muscle is intracellular. Depolarization of the skeletal muscle fibre triggers a conformational change of the dihydropyridine receptor (DHPR) in the transverse-tubule membrane which in turn activates the ryanodine receptor (RyR) calcium channel in charge of the massive release of calcium from the sarcoplasmic reticulum. On the other hand, although there are several lines of evidence indicating that calcium does enter from the extracellular medium in resting as well as in working skeletal muscle, the possible role of this calcium entry is a matter of controversy. The first, most obvious trans-plasma membrane calcium entry pathway corresponds to the DHPR itself which acts as a voltage sensor transducing voltage changes into the opening of the RyR, but also as a voltage-activated calcium channel that contributes to a calcium entry of unclear role, during prolonged muscle activation (see Melzer et al. 1995). A second calcium entry pathway corresponds to voltage-independent calcium channels active at negative membrane potentials which have been described at the single channel level in adult mouse skeletal muscle and in cultured myotubes (Fong et al. 1990; Franco & Lansman, 1990; Turner et al. 1991; Franco-Obregon & Lansman, 1994, 2002; Hopf et al. 1996; Vandebrouck et al. 2002; Yeung et al. 2005). This type of channel was shown to be spontaneously active, sensitive to membrane stretch or to the intracellular Ca2+ store content.
Interestingly a calcium entry depending on the calcium content of the intracellular store was described in intact adult and fetal mammalian skeletal muscle fibres using fluorescence measurements from a Ca2+ indicator (Kurebayashi & Ogawa, 2001; Collet & Ma, 2004). However, evidence for the operating of this store-operated calcium entry was obtained under rather drastic non-physiological conditions including the absence of extracellular calcium and the use of SR Ca2+-ATPase inhibitors. Still this calcium influx was suggested to be of functional relevance for the refilling of a diminished intracellular calcium store content, a situation that may occur upon sustained muscle activation. Interestingly Launikonis et al. (2003) observed a store-operated calcium loss in the sealed tubular system of mechanically skinned skeletal muscle fibres, which appeared to be mediated by the inositol trisphosphate receptor. Finally and along the same line, electrical stimulation of skeletal myotubes was also shown to activate a Ca2+ entry pathway with properties similar to the ones of the store-dependent Ca2+ entry, but operating under conditions that did not produce substantial store depletion (Cherednichenko et al. 2004). This whole trend of data thus suggests that SR Ca2+ release and/or SR Ca2+ depletion could control the gating of a plasma membrane calcium conductance.
The physiological relevance of this store-operated calcium entry as well as the identification of a corresponding ion channel activity remain poorly documented. Generally, recording of ion currents through presumed store-operated calcium channels was not available until openings of channels spontaneously active at resting membrane potentials were found to be increased in fibres treated with caffeine or with the SR Ca2+-ATPase inhibitor thapsigargin (Hopf et al. 1996; Vandebrouck et al. 2002). This suggested that these channels described in the early 1990s could correspond to the store-operated calcium channels. Interestingly, in numerous studies, the activity of these channels has been demonstrated to be chronically increased in dystrophin-deficient muscle fibres and it was thus postulated that a dysfunction of store-operated calcium channels might be responsible for the exacerbated calcium influx that characterizes dystrophin-deficient muscle (Vandebrouck et al. 2002).
However, to date, there is a lack of experimental data showing real-time acute changes in membrane conductance at the macroscopic or single channel level in a skeletal muscle fibre, that would correlate with voltage-elicited SR calcium release or depletion, as seen for instance in smooth muscle (Wayman et al. 1996; Albert & Large, 2002). Yet such an approach would provide the most reliable and convincing proof for the existence and physiological relevance of an SR function-dependent transmembrane Ca2+ entry in skeletal muscle. The present study aimed at determining if a store-dependent ion influx develops in response to voltage-activated Ca2+ release in experimental conditions that allowed simultaneous measurement of the macroscopic membrane conductance, the intracellular [Ca2+] and the single channel activity on the same skeletal muscle fibre under control conditions and after depleting the SR. Results seriously question the physiological relevance of a store-operated calcium influx mechanism in skeletal muscle.
Methods
Preparation of the muscle fibres
Experiments were performed on single skeletal fibres enzymatically isolated from the flexor digitorum brevis muscles from adult Swiss OF1 and C57BL/10ScSn-mdx/J male mice. All experiments were performed in accordance with the guidelines of the French Ministry of Agriculture (87/848) and of the European Community (86/609/EEC).
Mice were killed by cervical dislocation before removal of the muscles. Procedures for enzymatic isolation of single muscle fibres, partial insulation of the fibres with silicone grease and intracellular microinjection were as previously described (Jacquemond, 1997; Collet et al. 1999, 2004; Collet & Jacquemond, 2002). In brief, the major part of a single fibre was electrically insulated with silicone grease so that whole-cell voltage-clamp could be achieved on a short portion of the fibre extremity. Prior to voltage clamp, fibres were pressure microinjected with a solution containing millimolar concentrations of EGTA and CaCl2 in a 10 : 4 ratio. The pH of this solution was buffered at 7.20 and the corresponding calculated free calcium concentration was 130 nm, within the range of commonly measured levels for resting free calcium under our conditions (see for instance Pouvreau et al. 2004). Under these calcium buffering conditions, the fibre integrity was well preserved during the experiments even when large amounts of calcium remained outside of the SR (as for instance in the presence of SR Ca2+ uptake inhibitors); this also allowed the tip of a cell-attached patch pipette to remain sealed on the surface membrane when whole-cell depolarizing pulses were applied. The concentration of EGTA in the microinjection pipette was set to 10 mm for the experiments described in relation to Fig. 5 and to 90 mm for all other experiments.
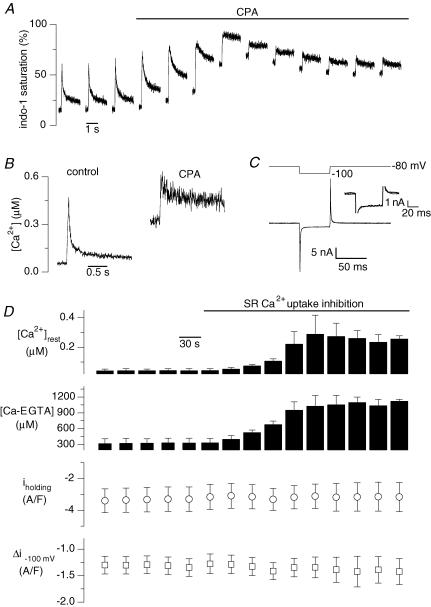
Figure 5. Effect of cyclopiazonic acid (CPA) on macroscopic background current and Ca2+ transients induced by depolarizations of the fibre.
The trace in A shows the indo-1 transients induced by 50 ms pulses to +10 mV applied every 30 s in a control fibre. The horizontal bar indicates the period during which CPA at a concentration of 50 μm was present in the external solution. The left and the right panels in B show the first indo-1 transient obtained in the presence of the control external solution and the last indo-1 transient obtained in the presence of the CPA-containing external solution, respectively. In C, the first membrane current record measured in response to a 20 mV hyperpolarization in the presence of the control external solution is shown superimposed on the last membrane current record obtained in response to the same pulse in the presence of the CPA-containing external solution. The corresponding voltage protocol is shown on top. The inset shows an enlarged view of the same two current traces; the thin trace corresponds to the control current whereas the thick trace corresponds to the one measured in CPA. In D, mean results from 4 experiments similar to the one shown in A are presented. It shows the mean values for resting [Ca2+], corresponding mean resting concentration of Ca2+–EGTA complex, background current at −80 mV and change in membrane current induced by the 20 mV hyperpolarization, along the course of the experiments. For these experiments the intracellular EGTA concentration was estimated to be ∼2 mm (see Methods).
Electrophysiology
Unless otherwise specified the silicone-clamp technique was combined with simultaneous measurements of the single channel activity of the surface membrane using the cell-attached configuration of the patch-clamp technique, as previously described (Jacquemond & Allard, 1998). For the silicone clamp an RK-400 patch-clamp amplifier (Bio-Logic, Claix, France) was used in whole-cell configuration. Command voltage pulse generation and data acquisition were done using the WinWCP software (John Dempster, University of Strathclyde, Glasgow, UK) driving an A/D, D/A converter (BNC-2090 or BNC-2120, National Instruments, Austin, TX, USA). Analog compensation was systematically used to decrease the effective series resistance. Voltage clamp was performed with a microelectrode filled with the whole-cell silicone-clamp intrapipette solution (see Solutions) and having a typical resistance of 2 MΩ. The tip of the microelectrode was inserted through the silicone, within the insulated part of the fibre. Analog compensation was systematically used to decrease the effective series resistance. Values for the capacitance of the whole-cell voltage-clamped cell portion ranged between 400 and 900 pF. The tip of the patch pipette was sealed on the silicone-free end portion of the fibre in order to record single channel activity in the cell-attached configuration at a pipette potential of 0 mV, with an additional RK400 patch-clamp amplifier. The resistance of the cell-attached pipettes were between 2 and 3 MΩ. Currents flowing into the pipette were considered to be positive. Acquisition of the whole-cell and unitary currents was synchronized with the command voltage pulse generation and was done using pCLAMP 9 software (Axon Instruments Inc.) driving an A/D converter (Digidata 1322A, Axon Instruments Inc.). The average single channel current was measured after filtering at 300 Hz and sampling at 1 kHz. Holding whole-cell voltage was always set to −80 mV. In the experiments described in Fig. 8A, fibres were voltage clamped using the whole-cell configuration of the patch-clamp technique using pipettes of low resistance (ranging from 0.4 to 0.6 MΩ) filled with the whole-cell patch-clamp intrapipette solution (see Solutions). Analog compensation was systematically used to decrease the effective series resistance. In Fig. 8B, the single channel activity was recorded in the cell-attached configuration with the pipette potential held at 0 mV.
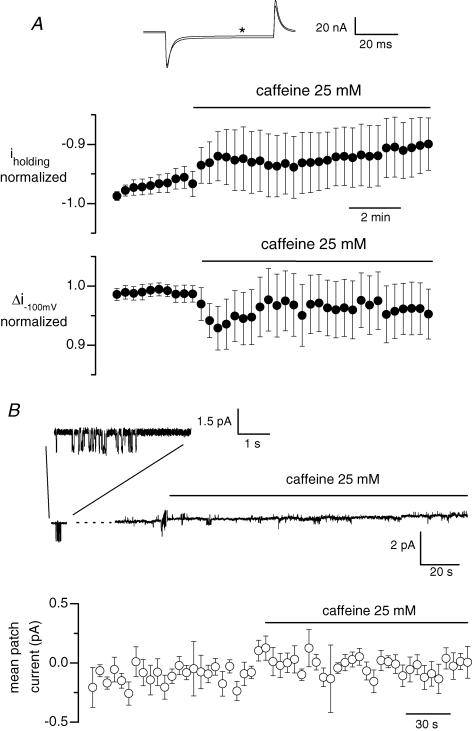
Figure 8. Effect of caffeine on whole-cell background current and single channel activity in fibres that were free of silicone.
In A, fibres were voltage-clamped using the whole-cell configuration of the patch-clamp technique with the pipette solution containing 20 mm internal EGTA. The holding voltage was set to −80 mV and 50 ms pulses to −100 mV were applied every 20 s. The upper panel shows the last membrane current record measured in response to a 20 mV hyperpolarization in the presence of the control external solution, superimposed on the membrane current record obtained in response to the same pulse applied 8 min and 40 s after addition of caffeine (record marked by a star). The middle and lower panels show the mean normalized value of the whole-cell background current at −80 mV and the mean change in membrane current induced by the 20 mV hyperpolarization, respectively, along the course of such experiments. Data are from 12 cells. In B, the upper panel shows the single channel activity recorded in a cell-attached patch established on a fibre previously bathed for 30 min in a Tyrode solution containing 250 μm EGTA-AM. The holding pipette potential was 0 mV. Activity of channels carrying inward current, illustrated on an expanded scale in the inset shown on top, was detected 2 min 30 s before the beginning of the continuous recording. The lower panel shows mean values for the patch current from 8 identical experiments. In each single channel record, the closed channel state was set to zero and the current value was averaged over successive intervals of 5 s; the graph shows the corresponding mean current versus time.
Intracellular [Ca2+] measurements
For these, the solution that was microinjected in the fibres also contained 1 mm of either indo-1 or fluo-3. The optical set-up and the procedures used for the indo-1 fluorescence measurements were as previously described (Jacquemond, 1997; Collet et al. 1999; Collet & Jacquemond, 2002; Pouvreau et al. 2004). In brief, a Nikon Diaphot epifluorescence microscope was used in diafluorescence mode. The beam of light from a high-pressure mercury bulb set on the top of the microscope was passed through a 335 nm interference filter and focused onto the preparation. The emitted indo-1 fluorescence light was collected by a 40 × objective and simultaneously detected at 405 ± 5 nm (F405) and 470 ± 5 nm (F470) by two photomultipliers. The fluorescence measurement field was 40 μm in diameter and the silicone-free extremity of each tested fibre was placed in the middle of the field. Background fluorescence at both emission wavelengths was measured next to each tested fibre and was then subtracted from all measurements. The standard ratio method was used with the parameters: R=F405/F470, and Rmin, Rmax, KD and β having their usual definition. Results were either expressed in terms of indo-1 percentage saturation or in actual free calcium concentration (for details of calculation, see Jacquemond, 1997). In vivo values for Rmin, Rmax and β were measured using procedures previously described (Collet et al. 1999; Collet & Jacquemond, 2002). For the results described in relation to Fig. 5D the concentration of the Ca–EGTA complex was calculated assuming the intracellular EGTA concentration to be one fifth of the concentration present in the injected solution (for details concerning microinjections see Csernoch et al. 1998) and a KD value for calcium binding to EGTA of 0.2 μm.
The optical set-up and procedures used for the fluo-3 fluorescence measurements were as previously described (Jospin et al. 2002). The set-up was used for all experiments combining whole-cell and cell-attached voltage clamp with intracellular Ca2+ detection. In brief, we used an inverted microscope (Olympus IMT2) equipped for epifluorescence. Cells were imaged using a 20 × objective. Fluo-3 fluorescence was produced and collected by excitation from a 100 W mercury-vapor lamp, using an appropriate filter set combination (excitation, 450–480 nm; emission, above 515 nm; dichroic mirror, 500 nm). Images from a circular region of interest centred on the silicone-free extremity of the cell under study and close to the tip of the cell-attached patch pipette, were captured with a Coolsnapfx charge-coupled device camera (Roper Scientific, Evry, France) at a frequency of 20 Hz. Although this sampling rate precluded accurate detection of the actual peak and time course of the voltage-activated transients, these measurements allowed us to assess the voltage-induced calcium release activity and how stable it was upon successive membrane depolarizations in a given fibre. For assessing the effect of 4-chloro-m-cresol on the fluo-3 fluorescence (see Fig. 7) a sampling rate of 1 Hz was used. Image acquisition and processing were performed using the MetaVue imaging workbench (Universal Imaging Corporation, Downingtown, PA, USA). Fluorescence values were expressed as F / F0, F being the background corrected intensity of fluorescence from the portion of cell under study and F0 the corresponding baseline fluorescence. No attempt was done to calibrate the fluo-3 signal in terms of actual [Ca2+].
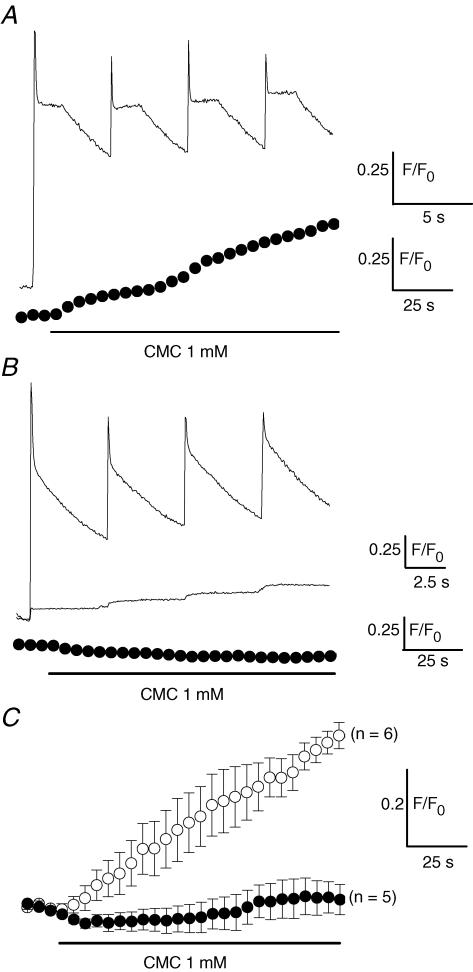
Figure 7. Effect of 4-chloro-m-cresol (CMC) on intracellular fluo-3 Ca2+ signals in fibres exposed to the control solution and in fibres treated with CPA.
The upper trace in A shows the fluo-3 Ca2+ transients induced by four successive voltage pulses given from −80 to 0 mV in the presence of control external solution. The lower trace presents, in the same fibre, the change in the fluo-3 Ca2+ signal produced by CMC at −80 mV. In B, the upper trace corresponds to the Ca2+ transients induced by a first series of four depolarizing pulses given from −80 to 0 mV in the presence of control external solution in another fibre. The middle trace shows, in this same fibre, the Ca2+ transients elicited in response to the same voltage pulses after 2 min CPA treatment during which 4 other same pulses were delivered. The lower trace shows, in the same fibre, the fluo-3 fluorescence signal produced upon application of CMC at −80 mV. CMC was applied 30 s after the above shown Ca2+ transients were recorded in the presence of CPA. In C are presented the mean changes in intracellular [Ca2+] induced by CMC at −80 mV in 6 fibres solely challenged with one series of depolarizing pulses and bathed throughout with the control external solution (○) and in 5 fibres first challenged with several series of depolarizing pulses in the presence of CPA until there was exhaustion of the voltage-activated Ca2+ transients (•). Each fluo-3 fluorescence data point presented for CMC experiments corresponds to the mean change in fluorescence measured in 5 consecutive images captured every second. In all fibres the intracellular EGTA concentration was estimated to be ∼20 mm.
Solutions
The whole-cell silicone-clamp intrapipette solution contained (mm): 120 potassium glutamate, 5 Na2-ATP, 5 Na2-phosphocreatine, 5.5 MgCl2, 5 glucose, 5 Hepes adjusted to pH 7.20 with KOH. The whole-cell patch-clamp intrapipette solution contained (mm): 145 caesium aspartate, 5 MgCl2, 20 EGTA, 10 Hepes adjusted to pH 7.20 with CsOH. Except for the experiments described in Fig. 8B, the standard extracellular solution contained (mm): 140 TEA-methanesulphonate, 2.5 CaCl2, 2 MgCl2, 10 TEA-Hepes and 0.002 tetrodotoxin, pH 7.20. For the experiments described in Fig. 8B, the extracellular solution was Tyrode solution, containing (mm): 140 NaCl, 5 KCl, 2.5 CaCl2, 2 MgCl2, 10 Hepes, pH 7.2; 250 μm EGTA-AM (acetoxymethyl ester) was also added to this solution. In the experiments depicted in Fig. 8B, the cell-attached pipette was filled with Tyrode solution. Cyclopiazonic acid and 4-chloro-m-cresol were dissolved in DMSO at 50 mm and 1 m, respectively, and diluted to the required concentration in the extracellular solution. Caffeine was dissolved at 25 mm in the extracellular solution. Cells were exposed to different solutions by placing them in the mouth of a perfusion tube from which flowed by gravity the rapidly exchanged solutions. Experiments were carried out at room temperature, ranging from 20 to 23°C.
Results
In order to determine whether changes in membrane conductance could be induced at the macroscopic or at the single channel level as a consequence of voltage-activated calcium release, we endeavoured to simultaneously record single channel activity, macroscopic ion currents and intracellular [Ca2+] changes on a same voltage-clamped muscle fibre. Although quite challenging, these conditions could be achieved by combining a whole-cell voltage-clamp technique and the cell-attached patch-clamp technique on a muscle fibre pressure microinjected with a solution containing a fluorescent calcium indicator. The microinjected solution contained a large amount of EGTA and calcium in such a ratio that fibre movement was prevented to preserve the integrity of the cell-attached patch, while also maintaining an effective SR Ca2+ release function.
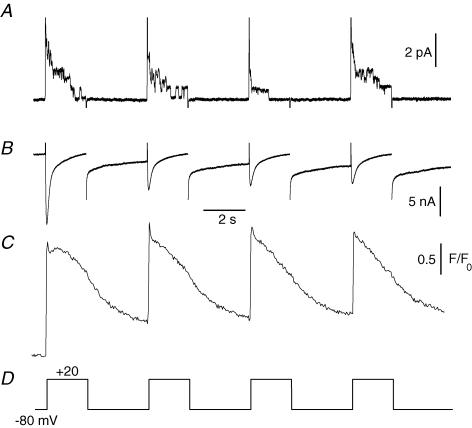
Figure 1 shows the recordings of these three parameters in response to four consecutive 2 s-long voltage-clamp pulses to +20 mV. The cell-attached pipette contained Tyrode solution while the bath contained a 140 mm TEA-MeSO3 and TTX-containing solution to optimize voltage control of the fibre portion under study. At the single channel level, cell depolarization elicited opening of delayed rectifier K+ channels which inactivated with time during pulses as already described under similar experimental conditions in a previous study (Jacquemond & Allard, 1998). At the macroscopic level an inward current through L-type voltage-dependent calcium channels was detected, somewhat contaminated by residual voltage-activated K+ conductances. The peak amplitude of the calcium current progressively decreased as the successive voltage pulses were applied, probably due to voltage-dependent inactivation and/or t-tubule calcium depletion (see for instance Collet et al. 2003; Friedrich et al. 2001). In the same fibre, the simultaneously recorded fluo-3 fluorescence showed that calcium was released by the SR in response to the depolarizing pulses. The 3 s interval between the pulses was insufficient to allow a complete return of the signal to its basal level, indicating that some of the released calcium remained outside the SR in between the pulses. We focused on the single channel activity between voltage pulses because, should that arise, currents through putative store-operated calcium channels would be expected to develop in response to, and as a consequence of, calcium release. To ascertain that the patch pipette remained sealed on the surface membrane all along the experiments, all patches that did not display delayed rectifier K+ channel activity upon whole-cell depolarization were discarded. In 110 cell-attached patches exhibiting delayed rectifier K+ channel activity during whole-cell pulses, activity of single channels carrying inward currents at −80 mV was never observed. In 17 other cell-attached patches, the unitary activity of a channel carrying an inward current of about 1.5 pA amplitude at −80 mV was detected before depolarizing pulses were given (Fig. 2A). The conductance properties of this channel indicated that it corresponds to the channel open at rest described by Haws & Lansman (1991), Hopf et al. (1996) and Mallouk & Allard (2002) under similar ionic conditions. As shown in Fig. 2B, while channels carrying inward currents at −80 mV were present in the patch, the single channel activity was not increased in response to the repetitive whole-cell depolarizing pulses given to 0 mV.
Figure 1. Combined electrophysiological and fluorescence measurements in a muscle fibre.
Simultaneous recordings of single channel activity (A), macroscopic current (B) and intracellular [Ca2+] changes (C) in the same voltage-clamped skeletal muscle fibre. Four consecutive depolarizing pulses of 2 s duration with 3 s intervals were applied to the fibre as indicated in D. [Ca2+] was detected with fluo-3. The fibre had been injected with an EGTA-containing solution (see Methods); following equilibration, the final intracellular EGTA concentration was estimated to be ∼20 mm.
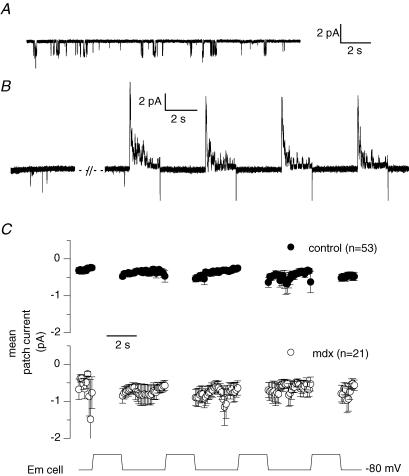
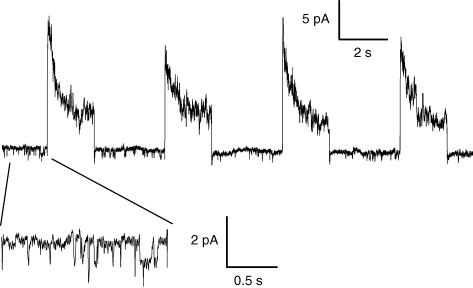
Figure 2. Single channel activity before and after whole-cell depolarizing pulses in control and in mdx muscle fibres.
A, current trace showing single channel activity in a cell-attached patch established on a voltage-clamped control fibre maintained at −80 mV. B, current trace corresponding to the single channel activity in another cell-attached patch obtained from a control voltage-clamped cell maintained at −80 mV (left panel) and depolarized by four consecutive whole-cell pulses of 2 s duration to 0 mV (right panel). C, average single channel activity detected at the holding whole-cell voltage of −80 mV in cell-attached patches experiencing the whole-cell protocol consisting of four 2 s-long depolarizing pulses. Depolarization levels were between −10 and +30 mV. Single channel current traces were exclusively obtained from cell-attached patches exhibiting the inward channel activity at −80 mV. For each record the current level corresponding to the closed state of the channel carrying inward current was set to zero. The single channel current was then averaged over successive intervals of 100 ms. The graph presents the corresponding mean cell-attached patch current obtained from 53 records taken from 17 patches established on control fibres (•) and from 21 records taken from 9 patches established on mdx fibres (○). In all fibres the intracellular EGTA concentration was estimated to be ∼20 mm.
The four successive 2 s-long whole-cell depolarizing pulses protocol could be applied several times in the same fibre with the cell-attached patch recording maintained. Under these conditions we collected 53 records from the 17 control patches that exhibited the inward single channel activity, with the whole-cell depolarizing voltage being equal to, or more positive than, −10 mV. The top panel in Fig. 2C presents the corresponding mean value of the cell-attached patch current measured at −80 mV during the protocol. In each single channel record, the closed channel state was set to zero and the current value was averaged over successive intervals of 100 ms. There was obviously no trend for an increase in single channel activity as the successive four whole-cell pulses were applied.
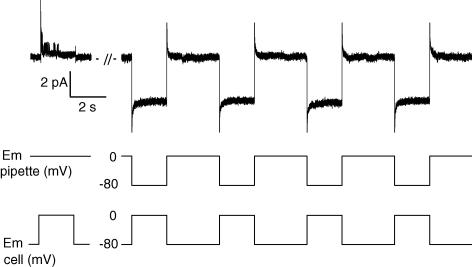
In another set of experiments, we tested the possibility that single channels carrying inward currents would activate at negative potentials during calcium release as described by Cherednichenko et al. (2004). For this purpose, we constrained the cell-attached patch potential to remain at −80 mV during the whole-cell depolarizing pulses by simultaneously applying in the patch pipette a hyperpolarizing pulse of the same amplitude as the whole-cell depolarization. Whereas delayed rectifier K+ channels activated when the cell-attached patch potential was driven by the whole-cell depolarizing pulse (as shown in Fig. 1), channel opening was not observed during whole-cell voltage pulses with the patch potential held at −80 mV (Fig. 3). In four patches tested under these conditions, activation of channels carrying inward currents was never detected during the whole-cell depolarizations.
Figure 3. Single channel activity recorded during depolarizing pulses applied to the fibre while the cell-attached patch is maintained at −80 mV.
The left part of the current trace (upper panel) shows single channel activity recorded in a cell-attached patch in response to a single pulse of 2 s duration from −80 to 0 mV in a control fibre. The right part of the current trace shows single channel activity recorded in the same cell-attached patch in response to four consecutive pulses of 2 s duration given from −80 to 0 mV in the fibre and simultaneously from 0 to +80 mV in the patch pipette (which results in holding the membrane patch at −80 mV). The middle and lower panels show the voltage protocols applied in the pipette and in the fibre, respectively. The intracellular EGTA concentration was estimated to be ∼20 mm.
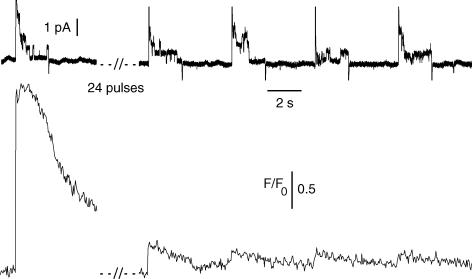
As illustrated in Fig. 1, within a series of four consecutive 2 s-long voltage pulses given to +20 mV, the amplitude of the calcium transient did not profoundly drop, indicating that the SR did not get dramatically depleted during a single run of such pulses. In four separate experiments we challenged fibres with several series of such four consecutive whole-cell pulses to values more positive than −20 mV. Runs were applied until a strong decrease in the resulting fluo-3 peak transient amplitude was observed. Although perhaps not entirely true we believe this drop in the peak calcium transient to result from, or at least to be associated with, SR Ca2+ depletion. In these four fibres the cell-attached pipette remained sealed on the muscle fibre throughout the experiment. Figure 4 shows the single channel activity recorded at the surface of a fibre depolarized by 24 consecutive whole-cell depolarizing pulses to +20 mV that ended up producing an almost complete exhaustion of the calcium transient. While delayed rectifier K+ channels reproducibly activated during the whole-cell depolarizing pulses, single channels carrying inward currents never opened in between the pulses. On average in these four fibres the amplitude of the fluo-3 transient dropped to 12 ± 7% of its initial value but single channels carrying inward currents never activated at −80 mV following exhaustion of the calcium signal.
Figure 4. Single channel activity recorded in response to series of depolarizing pulses leading to Ca2+ signal exhaustion.
The upper trace shows the current recorded in the cell-attached patch pipette in response to a first depolarizing pulse to +20 mV applied in a control fibre and to four consecutive pulses of the same amplitude applied in the same fibre after 25 such pulses had been given. The lower trace shows the corresponding fluo-3 Ca2+ transients induced by the voltage pulses. The intracellular EGTA concentration was estimated to be ∼20 mm.
In order to ensure conditions of SR Ca2+ depletion, we tested the effect of inhibiting intracellular store Ca2+ uptake, as classically done to provide evidence for store-operated plasma membrane processes. The effect of cyclopiazonic acid (CPA), an inhibitor of SR Ca2+ uptake, was first tested on membrane current and intracellular calcium on cells that were depolarized by short (50 ms long) pulses to +10 mV delivered every 30 s. An illustrative example of indo-1 calcium transients recorded under such conditions is shown in Fig. 5A. CPA produced a slowing of the decay phase of the transients and a progressive increase in the resting level indicating that a larger amount of Ca2+ remained outside of the SR as further voltage pulses elicited calcium release. A reduction in the peak change in [Ca2+] elicited by the pulse was also indicative of SR depletion (Fig. 5B). The superposition of the first (control) and the last (CPA) membrane currents measured in response to a 20 mV hyperpolarization applied just before the depolarizing pulse shows that there was hardly any detectable change in the macroscopic membrane conductance of the fibre (Fig. 5C). Figure 5D shows that in four cells tested under these conditions, while the increase in free calcium and in the fraction of calcium bound to EGTA gave evidence for SR Ca2+ depletion in the presence of CPA, the holding background current as well as the current change induced by a 20 mV hyperpolarizing step were not significantly modified. Identical results were obtained in two fibres from an mdx mouse.
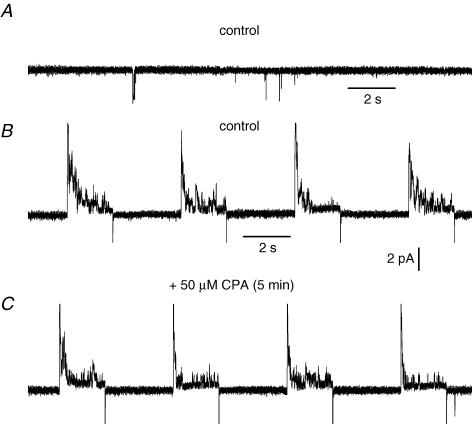
The effect of CPA was then tested on single channel activity recorded on fibres repetitively depolarized by voltage pulses. Figure 6A shows the activity of a channel carrying inward currents at −80 mV, present in a cell-attached patch on a whole-cell voltage-clamped portion of fibre. As observed previously, four consecutive depolarizing pulses to 0 mV did not lead to any increase in the single channel activity in between pulses in the presence of the control bath solution. A subsequent 5 min treatment of the cell with CPA, during which 16 depolarizing pulses were given, also did not lead to opening of the inward current-carrying channels. Similar results were obtained in three other fibres. It should be mentioned that, also under these conditions of long-lasting depolarizations, CPA had no effect on the macroscopic holding current measured in between the pulses (not illustrated).
Figure 6. Effect of cyclopiazonic acid on single channel activity recorded on fibres stimulated by repetitive depolarizations.
The current trace in A shows single channel activity in a cell-attached patch obtained on a voltage-clamped control fibre maintained at −80 mV. The current trace in B shows single channel activity in the same cell-attached patch in response to four consecutive depolarizing pulses to 0 mV in the presence of a control external solution. The current trace in C shows single channel activity in the same cell-attached patch in response to four consecutive depolarizing pulses to 0 mV after a 5 min treatment with cyclopiazonic acid during which 16 depolarizing pulses to 0 mV were delivered. The intracellular EGTA concentration was estimated to be ∼20 mm.
In order to obtain further evidence for SR Ca2+ depletion after CPA treatment, we challenged fibres with 4-chloro-m-cresol (CMC), a potent activator of SR Ca2+ release in skeletal muscle (Zorzato et al. 1993). Figure 7A shows that CMC produced a slow increase in intracellular [Ca2+] probed with fluo-3 (lower trace) at −80 mV in a fibre with a filled SR as shown by the robust Ca2+ transients which were beforehand produced in this fibre by successive depolarizing pulses (upper trace). In contrast, CMC failed to induce an increase in intracellular [Ca2+] (Fig. 7B, lower trace) in a fibre that was first repetitively stimulated in the presence of CPA until exhaustion of the Ca2+ transients (Fig. 7B, middle trace). Figure 7C presents the mean changes in intracellular fluo-3 Ca2+ signals induced by CMC in fibres challenged with a single series of four depolarizing pulses (open symbols) and in fibres challenged with several series of depolarizing pulses in the presence of CPA that led, on average, to a 97 ± 0.5% reduction of the voltage-evoked fluo-3 Ca2+ transients (filled symbols). While CMC gave rise to a substantial increase in intracellular [Ca2+] in fibres bathed with control external solution, CMC failed to produce a change in intracellular Ca2+ in CPA-treated fibres, thus confirming that the combination of repetitive depolarizations together with CPA treatment led to a drastic depletion of the SR.
In our voltage-clamp experiments, the SR compartment within the silicone-covered main portion of fibre did not release Ca2+ in response to depolarization and it is also likely to be poorly accessible to CMC applied externally. It could then be argued that this part of the SR, which is not Ca2+ depleted, may influence the membrane conductance in the silicone-free portion of the fibre under voltage control so as to prevent the occurrence of store-operated channel activity. We thus tested the effect of the SR Ca2+ releasing agent caffeine on the whole-cell membrane current recorded from entire fibres with the conventional whole-cell configuration of the patch-clamp technique following the procedure described by Wang et al. (1999). The intrapipette solution contained a high concentration of EGTA (20 mm) in order to prevent caffeine-induced contracture. The fibres were held at −80 mV and a 20 mV hyperpolarizing pulse of 50 ms duration was applied every 20 s. The superposition of the corresponding last membrane current record obtained in control conditions and of the one taken 8 min 40 s after exposition of the whole fibre to 25 mm caffeine shows that caffeine only induced a small reduction in the amplitude of the inward background current associated with a somewhat decrease in membrane conductance (Fig. 8A, upper panel). The middle and lower panels of Fig. 8A show that in 12 cells tested under these conditions, the holding inward background current as well as the current change induced by a 20 mV hyperpolarizing step were only slightly reduced in response to caffeine exposition. Furthermore, we also checked whether exposition of the whole cell to caffeine could induce opening of channels recorded at the unitary level. For that series of experiments, fibres were bathed 30 min prior to recording in a Tyrode solution containing 250 μm of the membrane-permeant Ca2+ chelator EGTA-AM. Single channel activity was recorded in the cell-attached configuration with Tyrode solution in the bath and in the pipette, at a holding pipette potential of 0 mV. All patches that did not display delayed rectifier K+ channel activity upon depolarization of the patch membrane were discarded. In Fig. 8B the upper panel shows that channels carrying inward currents were present in the patch (see inset) in control conditions, but that the single channel activity was not increased in response to the exposure of the whole fibre to caffeine. On average, in eight cells tested under these experimental conditions and in which opening of channels carrying inward currents was detected in control conditions, exposition of the whole fibre to caffeine failed to induce any change in single channel activity. These data thus exclude the possibility that non-uniform depletion of the SR in the entire fibre may be the reason for the absence of depletion-induced changes in membrane conductance in the silicone-clamped fibres.
Since a possible dysregulation of store-operated calcium channels in dystrophin-deficient muscle cells has been put forward, we performed a series of experiments on mdx mouse skeletal muscle fibres. Out of 59 patches tested on mdx muscle fibres, 21 exhibited spontaneous activity of the channels carrying inward currents at negative voltages. Figure 9 shows that in an mdx muscle patch where the channels opened before whole-cell depolarizations were applied, the activity of the channel was clearly not altered by repetitive whole-cell depolarizations of the fibre. On average, as observed in control fibres, there was no tendency for an increase in inward single channel activity as the depolarizing pulses were given (see Fig. 2C).
Figure 9. Single channel activity recorded in response to series of depolarizing pulses in mdx muscle fibres.
The main trace shows the single channel activity in the cell-attached patch in response to 4 depolarizing pulses given to 0 mV. Inset shows a short segment of the current trace on an expanded scale. The intracellular EGTA concentration was estimated to be ∼20 mm.
Discussion
By combining whole-cell voltage-clamp, cell-attached patch-clamp and intracellular calcium measurements in the same muscle fibre, we were able to investigate whether plasma membrane single ion channel activity was associated with voltage-activated SR Ca2+ release in adult mouse skeletal muscle fibres. Our main goal was to determine if, under physiological conditions of activation of Ca2+ release, the partial or total emptying of the SR calcium content would activate store-operated sarcolemmal ion channels carrying inward currents at negative membrane voltages. Reproducible activation of delayed rectifier single K+ channels throughout the work guaranteed that the patch membrane under study was in functional continuity with the voltage-clamped portion of the cell and thus that the patch was experiencing the consequences of voltage-activated calcium release. We first investigated the effect of 2 s-long large whole-cell depolarizing pulses on single channel activity. Such long depolarizing pulses should induce substantial transient emptying of the SR since it has been estimated that a decrease by about 80% in lumenal SR Ca2+ occurs within 100 ms at maximal depolarizing levels in skeletal muscle (Ursu et al. 2005). Our data show that this transient SR calcium depletion did not elicit any opening of channels conducting inward current at negative resting potentials, either during or after the voltage pulses. In addition, when several series of such 2 s-long depolarizations of the cell led to exhaustion of the voltage-activated calcium signal, probably indicative of substantial SR Ca2+ depletion, there was again no increased activity of channels carrying inward currents at −80 mV.
In the majority of studies investigating store-operated Ca2+ influx, depletion of internal calcium stores was induced by the use of SR Ca2+-ATPase inhibitor. Exhaustion of the voltage-activated Ca2+ transients and of the CMC-induced Ca2+ rise confirmed that, under our experimental conditions, depolarizing pulses applied in the presence of CPA induced a strong SR Ca2+ depletion. However, even under such drastic conditions, we did not detect any activation of channels carrying inward currents at negative voltages. Moreover, whenever ion channel activity might have been missed at the single channel level, analysis of the macroscopic current at negative membrane potentials revealed that CPA-induced SR depletion did not lead to any change in the whole-cell conductance indicating that no net ion influx occurred.
Channels spontaneously active at negative membrane voltages in freshly dissociated mouse skeletal muscle fibres have been considered as possible candidates for store-dependent channels (Hopf et al. 1996; Vandebrouck et al. 2002). Indeed, Vandebrouck et al. (2002) observed that store depletion induced by thapsigargin or caffeine in the absence of external calcium significantly increased the open probability of these channels in control mouse muscle. We thus paid particular attention to membrane patches in which spontaneous activity of these channels was revealed before depolarizing pulses were applied. As previously demonstrated by others, in control muscle, a low fraction of patches (∼13%) displayed such an activity which, in addition, was very weak. We observed that the activity of these channels open at rest was not significantly modified by either a single or by repetitive bursts of voltage-activated calcium release. Moreover, in contrast to what Vandebrouck et al. (2002) described, we also found that repetitive series of depolarizations in the presence of CPA did not affect the activity of these channels.
As reported many times by others (Franco-Obregon & Lansman, 1994; Hopf et al. 1996; Vandebrouck et al. 2002), the occurrence as well as the open probability of the spontaneously active channels is significantly higher in adult skeletal muscle from the dystrophic mdx mouse. Vandebrouck et al. (2002) postulated that the lack of dystrophin in mdx muscle might induce an up-regulation of the channels which were shown to be activated by store depletion to a similar extent as control muscle. While we also found that the occurrence of these channels was higher in mdx than in control muscle although from a different mouse strain (∼36% of mdx patches), we did not observe any modification of their activity in response to repeated voltage-induced calcium release.
Taken together, our results show that neither a transient nor a chronic SR Ca2+ depletion elicits or increases opening of single channels carrying inward currents at negative voltages in control as well as in dystrophic mdx skeletal muscle. These results thus contrast with the data from Vandebrouck et al. (2002) and the discrepancy may be due to differences in the experimental conditions between the two studies. In the present work, we elicited SR Ca2+ release and depletion under whole-cell voltage clamp, a condition that ensures control of the Ca2+-release activating parameter (i.e. the whole-cell membrane voltage) as well as control of the plasma membrane integrity by monitoring the membrane current. Our experimental conditions further differ in that Vandebrouck et al. (2002) compared different subsets of patches from control and treated fibres whereas we could compare the membrane activity on the same fibre under different conditions. Finally, Vandebrouck et al. (2002) induced SR Ca2+ depletion by a pharmacological treatment associated with the presence of an external solution devoid of Ca2+. To our knowledge, in all the studies describing a store-operated Ca2+ influx in mammalian skeletal muscle, store depletion was induced in the presence of a free-Ca2+ external solution. It may be that the consecutive removal and re-addition of external Ca2+ together with SR depletion promotes a sarcolemmal Ca2+ permeability while SR depletion alone does not, as demonstrated here. In this respect the situation in skeletal muscle seems to differ from the one observed in smooth muscle; in smooth muscle a store-operated inward current characterized at the whole-cell as well as the single channel level was indeed found to develop in response to the emptying of the internal stores by treatment with SR Ca2+-ATPase inhibitors without the need to remove calcium in the external solution (Wayman et al. 1996; Albert & Large, 2002).
Our results do not exclude the possibility that the store-operated Ca2+ influx detected by others in skeletal muscle through either the Mn2+ quenching technique or by intracellular [Ca2+] measurements may flow across the sarcolemma via channels whose conductance is too low for the unitary currents to be resolved or via channels exclusively located in the t-tubule membrane. However, in that latter case, one would still expect changes in the whole-cell membrane conductance of the fibre upon Ca2+ store depletion, unless the amplitude of the corresponding current is too small to be detected. An alternative possibility is that Ca2+ may enter the cell through electrically silent transport or leak processes which, if true, would question the existence of store-operated channels in skeletal muscle.
In conclusion, this study shows that voltage-activated SR Ca2+ release and/or SR Ca2+ depletion are not sufficient to activate the opening of channels carrying inward currents at negative voltages. These results thus seriously challenge the physiological relevance of a store-operated membrane conductance in skeletal muscle, although it cannot be excluded that other conditions also need to be fulfilled for substantial activation of this calcium entry pathway.
Acknowledgments
This work was supported by the Centre National de la Recherche Scientifique, the Université Claude Bernard Lyon 1, and the Association Française contre les Myopathies.
References
- Albert AP, Large WA. A Ca2+-permeable non-selective cation channel activated by depletion of internal Ca2+ stores in single rabbit portal vein myocytes. J Physiol. 2002;538:717–728. doi: 10.1113/jphysiol.2001.013101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherednichenko G, Hurne AM, Fessenden JD, Lee EH, Allen PD, Beam KG, Pessah IN. Conformational activation of Ca2+ entry by depolarization of skeletal myotubes. Proc Natl Acad Sci U S A. 2004;101:15793–15798. doi: 10.1073/pnas.0403485101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collet C, Allard B, Tourneur Y, Jacquemond V. Intracellular calcium signals measured with indo-1 in isolated skeletal muscle fibres from control and mdx mice. J Physiol. 1999;520:417–429. doi: 10.1111/j.1469-7793.1999.00417.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collet C, Csernoch L, Jacquemond V. Intramembrane charge movement and L-type calcium current in skeletal muscle fibers isolated from control and mdx mice. Biophys J. 2003;84:251–265. doi: 10.1016/S0006-3495(03)74846-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collet C, Jacquemond V. Sustained release of calcium elicited by membrane depolarization in ryanodine-injected mouse skeletal muscle fibers. Biophys J. 2002;82:1509–1523. doi: 10.1016/S0006-3495(02)75504-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collet C, Ma J. Calcium-dependent facilitation and graded deactivation of store-operated calcium entry in fetal skeletal muscle. Biophys J. 2004;87:268–275. doi: 10.1529/biophysj.103.039305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collet C, Pouvreau S, Csernoch L, Allard B, Jacquemond V. Calcium signaling in isolated skeletal muscle fibers investigated under ‘silicone voltage-clamp’ conditions. Cell Biochem Biophys. 2004;40:225–236. doi: 10.1385/CBB:40:2:225. [DOI] [PubMed] [Google Scholar]
- Csernoch L, Bernengo JC, Szentesi P, Jacquemond V. Measurements of intracellular Mg2+ concentration in mouse skeletal muscle fibers with the fluorescent indicator mag-indo-1. Biophys J. 1998;75:957–967. doi: 10.1016/S0006-3495(98)77584-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong PY, Turner PR, Denetclaw WF, Steinhardt RA. Increased activity of calcium leak channels in myotubes of Duchenne human and mdx mouse origin. Science. 1990;250:673–676. doi: 10.1126/science.2173137. [DOI] [PubMed] [Google Scholar]
- Franco A, Lansman JB. Calcium entry through stretch-inactivated ion channels in mdx myotubes. Nature. 1990;344:670–673. doi: 10.1038/344670a0. [DOI] [PubMed] [Google Scholar]
- Franco-Obregon A, Lansman JB. Mechanosensitive ion channels in skeletal muscle from normal and dystrophic mice. J Physiol. 1994;481:299–309. doi: 10.1113/jphysiol.1994.sp020440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco-Obregon A, Lansman JB. Changes in mechanosensitive channel gating following mechanical stimulation in skeletal muscle myotubes from the mdx mouse. J Physiol. 2002;539:391–407. doi: 10.1113/jphysiol.2001.013043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedrich O, Ehmer T, Uttenweiler D, Vogel M, Barry PH, Fink RH. Numerical analysis of Ca2+ depletion in the transverse tubular system of mammalian muscle. Biophys J. 2001;80:2046–2055. doi: 10.1016/S0006-3495(01)76178-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haws CM, Lansman JB. Developmental regulation of mechanosensitive calcium channels in skeletal muscle from normal and mdx mice. Proc Biol Sci. 1991;245:173–177. doi: 10.1098/rspb.1991.0105. [DOI] [PubMed] [Google Scholar]
- Hopf FW, Reddy P, Hong H, Steinhardt RA. A capacitative calcium current in cultured skeletal muscle cells is mediated by the calcium-specific leak channel and inhibited by dihydropyridine compounds. J Biol Chem. 1996;271:22358–22367. doi: 10.1074/jbc.271.37.22358. [DOI] [PubMed] [Google Scholar]
- Jacquemond V. Indo-1 fluorescence signals elicited by membrane depolarization in enzymatically isolated mouse skeletal muscle fibers. Biophys J. 1997;73:920–928. doi: 10.1016/S0006-3495(97)78124-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacquemond V, Allard B. Activation of Ca2+-activated K+ channels by an increase in intracellular Ca2+ induced by depolarization of mouse skeletal muscle fibres. J Physiol. 1998;509:93–102. doi: 10.1111/j.1469-7793.1998.093bo.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jospin M, Jacquemond V, Mariol MC, Segalat L, Allard B. The L-type voltage-dependent Ca2+ channel EGL-19 controls body wall muscle function in Caenorhabditis elegans. J Cell Biol. 2002;159:337–348. doi: 10.1083/jcb.200203055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurebayashi N, Ogawa Y. Depletion of Ca2+ in the sarcoplasmic reticulum stimulates Ca2+ entry into mouse skeletal muscle fibres. J Physiol. 2001;533:185–199. doi: 10.1111/j.1469-7793.2001.0185b.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Launikonis BS, Barnes M, Stephenson DG. Identification of the coupling between skeletal muscle store-operated Ca2+ entry and the inositol trisphosphate receptor. Proc Natl Acad Sci U S A. 2003;100:2941–2944. doi: 10.1073/pnas.0536227100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallouk N, Allard B. Ca2+ influx and opening of Ca2+-activated K+ channels in muscle fibers from control and mdx mice. Biophys J. 2002;82:3012–3021. doi: 10.1016/S0006-3495(02)75642-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melzer W, Herrmann-Frank A, Luttgau HC. The role of Ca2+ ions in excitation-contraction coupling of skeletal muscle fibres. Biochim Biophys Acta. 1995;8:59–116. doi: 10.1016/0304-4157(94)00014-5. [DOI] [PubMed] [Google Scholar]
- Pouvreau S, Allard B, Berthier C, Jacquemond V. Control of intracellular calcium in the presence of nitric oxide donors in isolated skeletal muscle fibres from mouse. J Physiol. 2004;560:779–794. doi: 10.1113/jphysiol.2004.072397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner PR, Fong P, Denetclaw WF, Steinhardt RA. Increased calcium influx in dystrophic muscle. J Cell Biol. 1991;115:1701–1712. doi: 10.1083/jcb.115.6.1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ursu D, Shuhmeier RP, Melzer W. Voltage-controlled Ca2+ release and entry flux in isolated adult muscle fibres of the mouse. J Physiol. 2005;562:347–365. doi: 10.1113/jphysiol.2004.073882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandebrouck C, Martin D, Colson-Van Shoor M, Debaix H, Gailly P. Involvement of TRPC in the abnormal calcium influx observed in dystrophic mdx mouse skeletal muscle fibers. J Cell Biol. 2002;158:1089–1096. doi: 10.1083/jcb.200203091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang ZM, Messi ML, Delbono O. Patch-clamp recording of charge movement, Ca2+ current, and Ca2+ transients in adult skeletal muscle fibers. Biophys J. 1999;77:2709–2716. doi: 10.1016/s0006-3495(99)77104-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wayman CP, McFadzean I, Gibson A, Tucker JF. Two distinct membrane currents activated by cyclopiazonic acid-induced calcium store depletion in single smooth muscle cells of the mouse anococcygeus. Br J Pharmacol. 1996;117:566–572. doi: 10.1111/j.1476-5381.1996.tb15228.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung EW, Whitehead NP, Suchyna TM, Gottlieb PA, Sachs F, Allen DG. Effects of stretch-activated channel blockers on [Ca2+]i and muscle damage in the mdx mouse. J Physiol. 2005;562:367–380. doi: 10.1113/jphysiol.2004.075275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorzato F, Scutari E, Tegazzin V, Clementi E, Treves S. Chlorocresol: an activator of ryanodine receptor-mediated Ca2+ release. Mol Pharmacol. 1993;44:1192–1201. [PubMed] [Google Scholar]