Abstract
Troponin T (TnT) mutations that cause familial hypertrophic cardiomyopathy (FHC) and sudden cardiac death frequently increase myofilament Ca2+ sensitivity, suggesting that their Ca2+-sensitizing effect contributes importantly to the FHC pathogenesis. To test this hypothesis, we compared transgenic mice expressing the Ca2+-sensitizing TnT-I79N mutant (I79N), which causes a high rate of sudden cardiac death in patients, with mice expressing the more benign TnT-R278C mutant (R278C) that does not affect myofilament Ca2+ sensitivity. Acutely increasing myofilament Ca2+ sensitivity with EMD57033 served as a positive control. Isovolumically contracting hearts were compared over a range of loading conditions (Frank-Starling curve). Consistent with their increased myofilament Ca2+ sensitivity, I79N-Tg hearts demonstrated significantly higher systolic performance at low perfusate [Ca2+] compared with R278C-Tg hearts, which were not statistically different from control hearts expressing either human wild-type TnT or no transgene (CON). Diastolic function was impaired in both FHC mutants (time to 90% relaxation: I79N 48 ± 1.0 ms, n = 10 or R278C 47 ± 0.4 ms, n = 7, versus CON 44 ± 1.0 ms, n = 20, P < 0.05). In the presence of isoproterenol, almost all contractile parameters of R278C hearts became indistinguishable from control hearts, whereas both systolic and diastolic function of I79N hearts significantly worsened (end-diastolic pressure: I79N 20 ± 4 mmHg versus CON 13 ± 2 mmHg or R278C 11 ± 2 mmHg, P < 0.05). The Ca2+ sensitizer EMD57033 produced an even greater contractile dysfunction than the I79N mutation at fast pacing rates. In vivo, maximal exercise tolerance was significantly impaired only in I79N mice. Pretreatment with β-adrenergic receptor antagonists abolished differences in exercise tolerance. In conclusion, the Ca2+-sensitizing effects of TnT mutations may reduce the responsiveness of mouse hearts to inotropic stimuli.
Familial hypertrophic cardiomyopathy (FHC) is an autosomal-dominant disease resulting from mutations in genes encoding cardiac contractile proteins, and is an important cause of sudden cardiac death (Marian & Roberts, 2001). Mutations in cardiac Troponin T (TnT) are responsible for approximately 7% of FHC cases (Richard et al. 2003). Based on data from in vitro studies where mutant TnT was reconstituted in skinned fibres or where skinned fibres were obtained from transgenic mice, FHC-linked TnT mutations can have a variety of effects on regulation of contraction (i.e. alterations of actin–myosin ATPase activity, of maximally developed force, or of crossbridge cycling rate) depending on the mutation studied and the in vitro assay used. Interestingly, almost all TnT mutations appear to increase the apparent Ca2+ sensitivity of contraction (Knollmann & Potter, 2001), which suggests that the Ca2+-sensitizing effect of TnT mutations may play an important role in the pathogenesis of hypertrophic cardiomyopathy. Consistent with this idea, we recently discovered that the TnT-R278C mutation, which appears to carry a better prognosis of mild to moderate hypertrophy late in life (Elliott et al. 1999; Garcia-Castro et al. 2003; Van Driest et al. 2003), is different from other FHC-linked TnT mutations, in that it does not increase myofilament Ca2+ sensitivity when expressed in mice, or reconstituted in human cardiac fibres (Hernandez et al. 2005). At the same time, the clinical phenotype can be quite variable, and even the TnT-R278C mutation can produce vastly different phenotypes in different families, suggesting that modifier genes importantly contribute to the human phenotype (Theopistou et al. 2004). Several recent epidemiological studies also failed to identify a clear phenotype–genotype correlation (Van Driest et al. 2003). Given the limited data, small numbers per genotype and large genetic variability in clinical studies, the use of transgenic animals may provide an opportunity to determine the direct consequences of an individual mutation on cardiac function versus the results of modifier genes present in the afflicted family.
Our previous studies demonstrated that mice expressing TnT mutants that sensitize myofilaments to the effects of Ca2+ such as TnT-I79N and TnT-F110I had a significantly impaired maximum exercise tolerance in vivo, whereas mice expressing the non-sensitizing TnT-R278C mutant did not (Hernandez et al. 2005). Furthermore, transgenic mice expressing the sensitizing TnT-I79N mutant (I79N-Tg mice) develop cardiac dysfunction (Knollmann et al. 2001), diastolic heart failure (Westermann et al. 2006) and ventricular arrhythmias (Knollmann et al. 2003) in the presence of β-adrenergic stimulation. Thus, we hypothesize that the Ca2+-sensitizing effect may contribute to the contractile dysfunction caused by FHC-linked TnT mutations. To test this hypothesis, we compared the isovolumic function of hearts expressing the sensitizing TnT-I79N mutant with that of hearts expressing the non-sensitizing TnT-R278C mutant over a range of loading conditions (Frank-Starling properties). Acutely increasing Ca2+-myofilament sensitivity with EMD 57033 served as a positive control. Our results suggest that the Ca2+-sensitizing effects of either TnT mutations or drugs significantly impair the positive inotropic response to β-adrenergic receptor agonists, particularly at fast pacing rates.
Methods
Experimental protocol
All studies were carried out according to National Institutes of Health guidelines and were approved by the institutional animal care and use committee. To examine the effect of the TnT mutations that have differential effects on myofilament Ca2+ sensitivity on cardiac performance, we compared 3 to 4 month old mice of the following genotypes: transgenic mice expressing either human wild-type (WT-Tg) or mutant cardiac TnT (I79N-Tg, R278C-Tg) and non-transgenic littermates (NTG). The generation and in vitro characterization of these transgenic models has been described (Miller et al. 2001; Hernandez et al. 2005). Briefly, in skinned papillary muscle fibres, replacement of endogenous TnT with TnT-I79N resulted in an increased Ca2+ sensitivity of force development (average pCa50 increase of 0.2) and an average decrease in maximal developed force of 30%. These results were confirmed in two independently generated transgenic lines with relative expression levels of 35% and 52% TnT-I79N, respectively (Miller et al. 2001). In contrast, replacement of endogenous mouse TnT with TnT-R278C (four independently generated transgenic lines with relative expression levels ranging from 35% to 55%) had no significant effect on myofilament Ca2+ sensitivity of force, while decreasing maximal developed force, on average, by 42% (Hernandez et al. 2005). For the current study we examined mice from one transgenic line per genotype (WT-Tg line 3, I79N-Tg line 8 and R278C-Tg line 1, with approximately 46%, 52% and 35% relative expression of transgenic TnT protein, respectively). EMD 57033 was generously provided by Merck KGaA, Darmstadt, Germany. All other chemicals were obtained from Sigma-Aldrich Inc., St Louis, MO, USA).
Isolated perfused heart preparation
Mice were anaesthetized with 20 ml kg−1 2.5% tribromoethanol (Avertin) via intraperitoneal injection. After a surgical level of anaesthesia was confirmed, a thoracotomy was performed, the heart was removed, and the animal was killed by exsanguination. Hearts of 14 NTG, 12 Tg-WT, 14 Tg-I79N and 12 Tg-R278C mice were successfully isolated and perfused in the Langendorff mode as we previously described for mouse hearts (Knollmann et al. 2001). In brief, the chest was opened, the heart was rapidly excised, and the aorta was cannulated. Retrograde perfusion via the aorta was carried out at a constant perfusion pressure of 80 mmHg at 37°C. The flow of thebesian veins was drained via a thin polyethylene tube (PE-10) pierced through the apex of the left ventricle. Krebs-Henseleit (KH) buffer containing (mmol l−1) 118 NaCl, 4.7 KCl, 1.2 NaH2PO4, 1.2 MgCl2, 2.5 CaCl2, 0.5 Na-EDTA, 25 NaHCO3 and 11 glucose was prepared at the time of the experiment. To maintain a constant heart rate, the atrioventricular node was thermally ablated, and the hearts were epicardially paced at different pacing rates via a bipolar platinum hook electrode placed on the apex of the right ventricle.
Hearts were allowed to equilibrate for 15 min before baseline left ventricular pressure recordings were obtained. To examine the effects of different inotropic states, the heart was then perfused with KH buffer containing free [Ca2+] of 0.6, 1.2, 2, 3.5 and 5 mmol l−1. Free [Ca2+]o was calculated using Fabiato's program (Fabiato, 1988). All perfusion buffers were equilibrated with 95% O2 plus 5% CO2 for at least 1 h prior to the experiment, yielding a pH of 7.4.
To examine the effect of β-adrenergic receptor stimulation, isoproterenol was used at a concentration of 0.1 μmol l−1, based on its EC50 value of 0.05 μmol l−1 in the isolated perfused mouse heart (Zhai et al. 2000). It should be recognized that in the intact organism, the action of isoproterenol on the heart is amplified severalfold (Majewski, 1983; Ludwig et al. 1989). Hence, the isoproterenol concentration used in our study cannot be compared to isoproterenol concentrations used in vivo.
Measurement of isovolumic cardiac performance
After a 10 min baseline perfusion, a bubble-free, fluid-filled balloon, custom-made of polyvinylchloride film, was inserted through the mitral valve into the left ventricle (LV) via an incision in the left atrium, as we have previously described (Knollmann et al. 2001). For continuous LV pressure recordings, a 1.4-French microtip transducer (Millar, Inc, Houston, TX, USA) was guided through the polyethylene tube and positioned inside the balloon.
After 10–15 min of baseline recordings, systolic and diastolic pressure–volume relationships were determined by increasing the LV balloon volume in 3–5 μl increments using a gas-tight glass syringe (100 μl). The balloon volume was increased until peak LV developed pressure was reached and a further increase led to a decrease in LV developed pressure. A new steady state for each increment was reached usually in 30–60 s, and only this LV pressure recording was used for further data analysis. Left ventricular developed pressure was plotted against the corresponding preloads.
The left ventricular pressure was digitized at 1000 samples s−1 with the use of a commercially available data acquisition system (PowerLab, ADInstruments, Inc., Colorado Springs, CO, USA). The fast sampling rate, coupled with the use of a pressure catheter with a flat frequency response up to 10 kHz, allowed detailed kinetic analyses of pressure recordings. The following indices of cardiac performance were measured off-line using custom-built software (National Instruments, Inc., Austin, TX, USA) and averaged from three consecutive beats: left ventricular systolic pressure, end-diastolic pressure (EDP), developed pressure (the difference between systolic pressure and EDP), the minimum and maximum values of the first derivative of left ventricular pressure (peak +d P dt−1 and −dP dt−1), peak +dP dt−1 and −dP dt−1 normalized for developed pressure, ratio of +dP dt−1 and −dP dt−1, and time from peak systolic pressure to reach 90% relaxation.
Because mutations differently affected the LV volume and compliance (see Table 1), cardiac contractile parameters were compared at LV loading volumes that produced the maximum developed pressure (= peak of the Frank-Starling relationship) for each heart.
Table 1.
Baseline contractile parameters of isovolumetrically contracting mouse hearts paced at 10 Hz. For each heart, parameters were obtained at LV balloon volume that produced the maximum developed pressure
| ANOVA P-value | |||||||
|---|---|---|---|---|---|---|---|
| Parameters | NTG (n = 15) | WT-Tg (n = 5) | Control (NTG + WT-Tg) (n = 20) | I79N-Tg (n = 10) | R278C-Tg (n = 6) | NTG, WT, I79N, R278C | Control, 179N, R278C |
| End-diastolic pressure (mmHg) | 19.39 ± 2.12 | 24.3 ± 7.1 | 20.7 ± 2.3 | 22.7 ± 1.9 | 23.8 ± 1 | 0.5620 | 0.6784 |
| Developed pressure (mmHg) | 76.16 ± 2.35 | 74.73 ± 5.57 | 76 ± 2.18 | 68 ± 2.2 | 71 ± 2.76 | 0.1478 | 0.0618 |
| Peak +dP dt−1 (mmHg s−1) | 3293 ± 125 | 3199 ± 339.7 | 3268 ± 122 | 2739 ± 88‡* | 2847 ± 138.5 | 0.0283 | 0.0113 |
| Normalized +dP dt−1 | 43.19 ± 0.68 | 42.51 ± 1.59 | 43 ± 0.63 | 41 ± 1.0* | 40 ± 0.95* | 0.0987 | 0.0486 |
| Peak −dP dt−1 (mmHg s−1) | 2461 ± 106 | 2503 ± 297.44 | 2472 ± 105 | 1749 ± 112‡* | 1990 ± 77.4‡* | 0.0007 | 0.0002 |
| Normalized −dP dt−1 | 32.19 ± 0.61 | 33.12 ± 1.68 | 32 ± 0.61 | 26 ± 1.6‡§* | 28 ± 0.38‡+* | 0.0002 | 0.0002 |
| Ratio +dP dt−1 | |||||||
| to −dP dt−1 | 1.35 ± 0.03 | 1.29 ± 0.04 | 1.33 ± 0.02 | 1.6 ± 0.11‡§* | 1.43 ± 0.04 | 0.0198 | 0.0051 |
| TTR 90% (ms) | 44 ± 0.7 | 44 ± 1.8 | 44 ± 0.7 | 48 ± 1.0‡§* | 47.4 ± 0.4‡* | 0.0049 | 0.0020 |
| Balloon volume (μl) | 34.2 ± 1.54 | 40.4 ± 3.56 | 35.8 ± 1.53 | 25.9 ± 1.21‡§*† | 32.4 ± 1 | 0.0002 | 0.0003 |
Data are mean ±s.e.m.; control = combined data from NTG and WT-Tg
P < 0.05 versus NTG
P < 0.05 versus WT
P < 0.05 versus control
P < 0.05 I79N-Tg versus R278C-Tg.
Treadmill exercise protocol
Treadmill exercise was conducted as previously described (Hernandez et al. 2005). Briefly, mice were randomly assigned to groups of five and placed individually into special chambers of the motorized rodent treadmill (Exer-6m, Columbus Instruments, Columbus, OH, USA). The electrical shock grid located behind the belt of the treadmill was activated with every experimental session. The slope of the treadmill was kept constant at 15 deg inclination at a starting speed 16 m min−1, with incremental increases in treadmill belt speed by 2 m min−1 every 2 min, until the mouse exhibited signs of exhaustion. Exhaustion was defined as the mouse spending > 50% of the time or > 15 s consecutively on the shock grid (Desai et al. 1997). The total running distance was recorded and used as an indicator of maximum exercise tolerance. To avoid complications from psychological stress, animals were trained for 2 days prior to experimental trials to run on the treadmill two times a day, for 20 min at a fixed speed of 16 m min−1, for a total of 640 m day−1. Each running session was separated by 3 h. These training sessions reduced the amount of electrical stimulation necessary to maintain running behaviour during the experimental trial.
Statistical analysis
All experiments were done in random sequence with respect to the genotype, and measurements were taken by a single observer who was blinded to the genotype. Differences among the four groups were assessed using a one-way analysis of variance. If statistically significant differences were found, individual groups were compared with Student's t test. Results were considered statistically significant if the P-value was less than 0.05. Unless otherwise indicated, results are expressed as means and s.e.m. Consistent with our previous report (Knollmann et al. 2001), none of the contractile parameters examined were significantly different between the NTG and the WT-Tg control group (Tables 1 and 2). To further validate this finding, data from the NTG and the WT-Tg group were combined into a single control group and the ANOVA analysis repeated with only three groups (control, I79N-Tg, R278C-Tg). The ANOVA P-values consistently increased in the validation analysis (compare last two columns of Tables 1 and 2), suggesting that the approach of pooling the two control groups was valid. For clarity, only the data from the combined control group are reported below.
Table 2.
Contractile parameters of isovolumetrically contracting mouse hearts after administration of 0.1 μmol l −1of ISO paced at 10 Hz. For each heart, parameters were obtained at LV balloon volume that produced the maximum developed pressure
| ANOVA P-value | |||||||
|---|---|---|---|---|---|---|---|
| Parameters | NTG (n = 16) | WT-Tg (n = 6) | Control (NTG + WT-Tg) (n = 22) | I79N-Tg (n = 10) | R278C-Tg (n = 6) | NTG, WT, I79N, R278C | Control, I79N, R278C |
| End-diastolic pressure (mmHg) | 14.8 ± 1.6 | 10.1 ± 2.9 | 13.5 ± 1.5 | 20.2 ± 3.22* | 11.2 ± 1.7 | 0.0434 | 0.0428 |
| Developed pressure (mmHg) | 108 ± 3.98 | 106 ± 8.13 | 107 ± 3.55 | 90.74 ± 5.3‡*† | 110 ± 2.08 | 0.0435 | 0.0192 |
| Peak +dP dt−1 (mmHg s−1) | 6664 ± 317 | 6636 ± 583 | 6656 ± 272 | 5572 ± 460 | 6757 ± 478 | 0.1842 | 0.0857 |
| Normalized +dP dt−1 | 61.54 ± 0.91 | 62.4 ± 1.16 | 61.8 ± 0.72 | 60.7 ± 2 | 61 ± 3.17 | 0.9272 | 0.8344 |
| Peak −dP dt−1 (mmHg s−1) | 5270 ± 221 | 5209 ± 426 | 5254 ± 193 | 3942 ± 290‡§*† | 5236 ± 324 | 0.0049 | 0.0015 |
| Normalized −dP dt−1 | 49 ± 0.85 | 49 ± 1.69 | 49 ± 0.75 | 43 ± 1.56‡§* | 47.5 ± 2.11 | 0.0083 | 0.0026 |
| Ratio +dP dt−1 | |||||||
| to −dP dt−1 | 1.27 ± 0.03 | 1.27 ± 0.04 | 1.27 ± 0.02 | 1.41 ± 0.06‡* | 1.29 ± 0.02 | 0.0682 | 0.0165 |
| TTR 90% (ms) | 29 ± 0.5 | 29 ± 1.1 | 29 ± 0.4 | 34 ± 1.4‡§*† | 32 ± 1.0‡* | 0.0010 | 0.0002 |
| Balloon volume (μl) | 28.5 ± 1.17 | 28.3 ± 1.05 | 28.45 ± 0.88 | 23.7 ± 0.72‡§*† | 27.7 ± 1.58 | 0.0209 | 0.0069 |
Data are mean ±s.e.m.; control = combined data from NTG and WT-Tg
P < 0.05 versus NTG
P < 0.05 versus WT
P < 0.05 versus control
P < 0.05 I79N-Tg versus R278C-Tg.
Results
Contractile function of isovolumically beating hearts
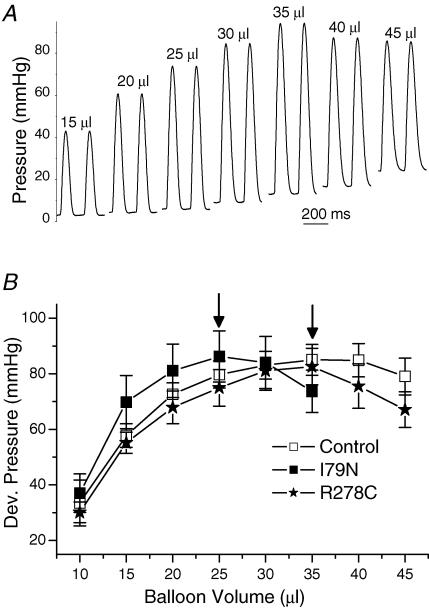
Data from skinned cardiac muscle fibres demonstrated that TnT-I79N increased Ca2+ sensitivity of force development (Miller et al. 2001), whereas TnT-R278C did not (Hernandez et al. 2005). To test the hypothesis that the differential Ca2+-sensitizing effect of these two TnT mutations significantly contributes to the contractile function of intact hearts, contractile parameters were measured in isolated, isovolumically beating hearts. This technique allows determining heart function at corresponding points of the Frank-Starling curve when examining hearts that have different diastolic stiffness or heart size (Stromer et al. 1997). This is particularly important as we have previously shown that I79N-Tg hearts are smaller and have an increased diastolic stiffness (Knollmann et al. 2001). Figure 1A illustrates the intraventricular pressure recordings that were obtained from an isolated perfused control heart paced at a rate of 5 Hz after stepwise increases in intraventricular balloon volume. In this fashion, pressure–volume relationships of developed pressure (Frank-Starling curve) were constructed for each heart and averaged for each group (Fig. 1B). Maximum developed pressure was not statistically different among the three groups of hearts (I79N-Tg 89 ± 8 mmHg, n = 4, versus control 87 ± 7 mmHg, n = 6, versus R278C-Tg 83 ± 6 mmHg, n = 6, not significant (n.s.)), but, as anticipated, the Frank-Starling curve was shifted leftward in I79N-Tg hearts (Fig. 1B), with peak developed pressure generated at significantly lower balloon volumes in I79N-Tg (26 ± 1.3 μl) versus control (37 ± 1.3 μl) and R278C-Tg hearts (34 ± 1.5 μl, P < 0.01 versus I79N-Tg). To correct for this leftward shift, contractile parameters were measured for each heart at the balloon volume that generated peak developed pressure. Parameters of systolic function (developed pressure, peak +dP dt−1) were not statistically different among the three groups. In contrast, peak rate of pressure decay was significantly slower in I79N-Tg (−1617 ± 223 mmHg s−1) and R278C-Tg (−1599 ± 175 mmHg s−1) compared with that of control hearts (−2121 ± 129 mmHg s−1, P < 0.05). Other parameters of diastolic function (peak +dP dt−1 to peak −dP dt−1 ratio, time to 90% relaxation) were also significantly impaired in both I79N-Tg and R278C-Tg hearts, with no statistically significant differences between the two TnT mutants (compare also Table 1 for results at a faster pacing rates). These results suggest that although the two TnT mutants have different effects of myofilament Ca2+ sensitivity of developed force based on fibre studies, this does not translate into changes of contractile parameters in the intact heart, at least under baseline conditions.
Figure 1. Isovolumic pressure recordings (A) used to construct Frank-Starling curves (B).
A, representative examples of isovolumic pressure recordings over a range of loading conditions (LV intraventricular balloon volumes) recorded in a non-transgenic control heart. B, comparison of average developed pressure at different intraventricular volumes (Frank-Starling curve). Note that I79N-Tg hearts reached maximum developed pressure (arrow) at significantly lower LV balloon volume compared to control and R278C-Tg hearts.
Ca2+ dependence of contractile function
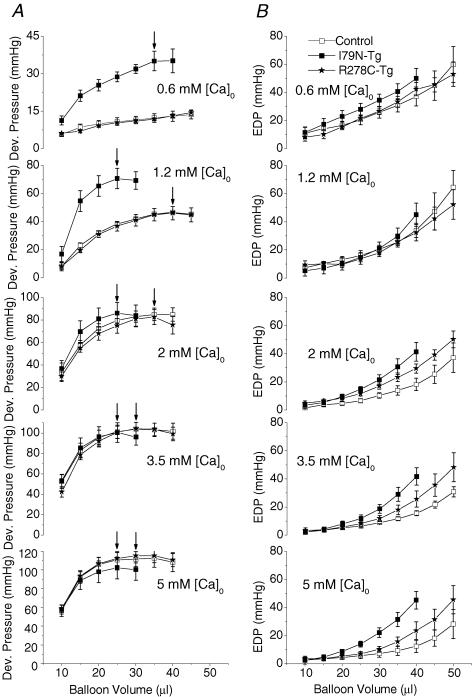
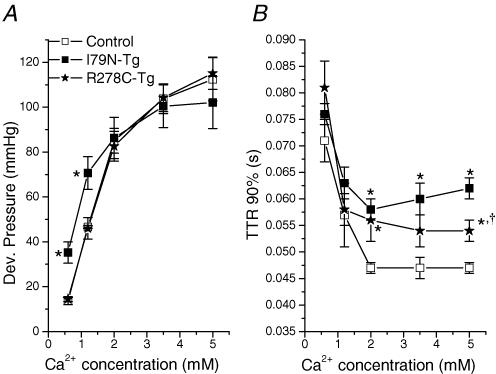
The next set of experiments were designed to try to corroborate the fibre data in the intact heart by measuring the effect of increasing perfusate Ca2+-concentration on the pressure-volume relationship of control and TnT-mutant hearts. Consistent with the fibre data, at low perfusate [Ca2+] (0.6 and 1.2 mmol l−1), peak developed pressure was significantly higher in I79N-Tg hearts, whereas R278C-Tg hearts were indistinguishable from control hearts (Fig. 2A, top two panels). Compared with control or R278C-Rg hearts, peak developed pressure (arrows, Fig. 2A) occurred at significantly lower LV balloon volumes in I79N-Tg hearts. When perfusate [Ca2+] was increased further (i.e. from 1.2 to 5 mmol l−1), the contractility of I79N-Tg hearts increased significantly less than that of either control or R278C-Tg hearts (increase in developed pressure: I79N-Tg 31 ± 6 mmHg versus control 71 ± 7 mmHg versus R278C-Tg 70 ± 8 mmHg, P < 0.01). As a result, peak developed pressures were not statistically different among the three groups of transgenic mice at perfusate [Ca2+] of 2 mmol l−1 or higher (Fig. 3A). I79N-Tg hearts also did not exhibit the physiological leftward shift of the Frank-Starling relationship observed in R278C-Tg or control hearts in response to higher perfusate [Ca2+] (Fig. 2, arrows). These results suggest that I79N-Tg hearts have a reduced response to positive inotropic stimulation.
Figure 2. Pressure–volume relationships of developed (A) and end-diastolic (B) pressure measured in isolated, perfused control (n = 6), R278C-Tg (n = 6) and I79N-Tg (n = 4) hearts at increasing perfusate [Ca2+].
Arrows indicate maximum developed pressure.
Figure 3. Ca2+ dependence of maximum developed pressure (A) and time to 90% relaxation (B) of control, R278C-Tg and I79N-Tg hearts.
For each heart, time to 90% relaxation was determined at balloon volumes that produced maximum developed pressure, and average values plotted for control (n = 6), R278C-Tg (n = 6) and I79N-Tg (n = 4) hearts. *P < 0.05 versus control; †P < 0.05 I79N-Tg versus R278C-Tg.
Diastolic function as measured by the end-diastolic pressure–volume relationship (Fig. 2B) was not significantly different among the three groups at low perfusate [Ca2+] (0.6 and 1.2 mmol l−1). At higher perfusate [Ca2+] there was an increasing separation of the end-diastolic pressure volume relationships of I79N-Tg, R278C-Tg and control hearts. These results suggest that compliance of TnT-mutant hearts was decreased compared to that of control hearts at higher perfusate [Ca2+]. Figure 3 summarizes the Ca2+-dependence of systolic (Fig. 3A) and diastolic function (Fig. 3B) of control, I79N-Tg and R278C-Tg hearts. Note that at the highest perfusate [Ca2+] examined (5 mmol l−1), diastolic function was significantly worse in I79N-Tg compared to R287C-Tg hearts (Fig. 3B).
Contractile function at fast heart rates and in presence of isoproterenol
We have previously demonstrated that anaesthetized I79N-Tg mice develop cardiac dysfunction (Knollmann & Potter, 2001; Westermann et al. 2006) and ventricular arrhythmias after administration of isoproterenol (Knollmann et al. 2003). However, in vivo it is difficult to distinguish between the effect of the isoproterenol-induced increase in heart rate and direct effects of isoproterenol on cardiac contractility. Thus, to further explore the mechanism of the isoproterenol-induced cardiac dysfunction, contractile parameters were first measured at a fast pacing rate of 600 beats min−1. To correct for the leftward shift of the Frank-Starling curve of I79N-Tg mice (see also Figs 2 and 3), contractile parameters were again measured for each heart at the balloon volume that generated peak developed pressure. Average values for each group are given in Table 1. In contrast to measurements at lower pacing rates (compare Fig. 1), parameters of systolic function (peak +dP dt−1, normalized +dP dt−1) were now significantly depressed in I79N-Tg hearts compared to control hearts (Table 1 and Fig. 4, baseline data). Systolic parameters of R278C-Tg hearts were also modestly suppressed, but only the rate of pressure rise normalized for developed pressure (+dP dt−1/−dP dt−1) was significantly different from control (Table 1). As already observed at slower pacing rates, diastolic parameters of both mutants were significantly different from those obtained from control hearts (Table 1).
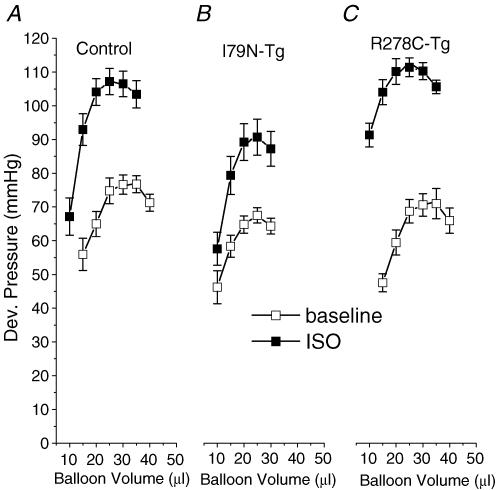
Figure 4. Effect of isoproterenol (ISO, 0.1 μmol l−1) on the pressure–volume relationship of control (A, n = 20), R278C-Tg (B, n = 9) and I79N-Tg (C, n = 6) hearts.
Application of isoproterenol (0.1 μmol l−1) in hearts paced at 10 Hz resulted in a dramatic increase in developed pressure and leftward shift of the Frank-Starling curve in control hearts (Fig. 4A). In contrast, I79N-Tg hearts had a significantly smaller increase in developed pressure, and no significant shift in the Frank-Starling curve (Fig. 4B and Table 2). Surprisingly, R278C-Tg hearts had an isoproterenol response that was much greater than that of I79N-Tg, and similar to that of control hearts (compare also Fig. 4B and C). Even adjusting for the lower absolute developed pressure of I79N-Tg mice under basal conditions did not explain their depressed isoproterenol response: isoproterenol induced average developed pressure increases of 35 ± 7.6% in I79N-Tg mice and 55 ± 5.2% in R278C-Tg mice, respectively (P < 0.05). Furthermore, parameters of diastolic function almost completely normalized in R278C-Tg hearts (with the exception of a slight prolongation in time to 90% relaxation, Table 2), whereas diastolic function was significantly impaired in I79N-Tg compared to both control and R278C-Tg hearts (Table 2). These data suggest that the Ca2+-sensitizing effect of the I79N mutation may contribute to the impaired contractile response and diastolic dysfunction, observed both at fast pacing rates and in the presence of β-adrenergic receptor stimulation with isoproterenol.
Effect of myofilament Ca2+ sensitizers on cardiac performance
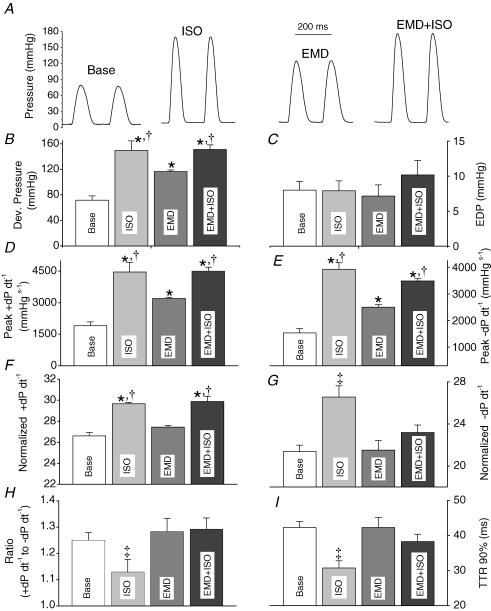
To more directly test the hypothesis that the Ca2+-sensitizing effect of the I79N mutation was responsible for the cardiac dysfunction at fast pacing rates and in the presence of isoproterenol, we measured cardiac contractile parameters in the presence of the myofilament sensitizer, EMD 57033. This compound has been reported to be relatively specific for its action on myofilaments (White et al. 1993) and increases myofilament Ca2+ sensitivity slightly more (i.e. ∼0.3 pCa units) (Solaro et al. 1993) than the I79N mutation (i.e. ∼0.2 pCa units) (Miller et al. 2001). As illustrated in Figs 5 and 6, the effect of EMD (3 μm) on cardiac contractility of control hearts was significantly dependent on the pacing rate: at slow pacing rates (5 Hz, Fig. 5), EMD significantly increased parameters of systolic function (developed pressure, peak +dP dt−1, normalized +dP dt−1), although its effect was significantly less than that of 0.1 μmol l−1 isoproterenol (Fig. 5B, D and F). Interestingly, applying EMD in the presence of isoproterenol did not further enhance systolic function compared with the effects of isoproterenol alone (Fig. 5B, D and F). Application of EMD alone had no effect on parameters of diastolic function (i.e. EDP, peak −dP dt−1, normalized −dP dt−1, dP dt−1 ratio, time to 90% relaxation, Fig. 5C, E, G, H and I). However, applying EMD in the presence of isoproterenol significantly worsened most parameters of diastolic function compared to application of isoproterenol alone (Fig. 5G, H and I).
Figure 5. Effect of the myofilament Ca2+ sensitizer EMD 57033 on cardiac contractile function at a slow pacing rate (5 Hz).
A, representative pressure tracings recorded from hearts paced at 5 Hz at baseline, in the presence of isoproterenol (ISO, 0.1 μmol l−1), in the presence of EMD 57033 (3 μmol l−1), and in the presence of ISO and EMD. Comparison of average maximum developed pressure (B), end-diastolic pressure (EDP, C), peak +dP dt−1 (D), peak −dP dt−1 (E), normalized peak +dP dt−1 (F), normalized peak −dP dt−1 (G), ratio of peak −dP dt−1 over peak +dP dt−1 (H), and time to 90% relaxation (I). Data are means ±s.e.m.; *P < 0.05 versus baseline; †P < 0.0.5 versus EMD, ‡P < versus all other.
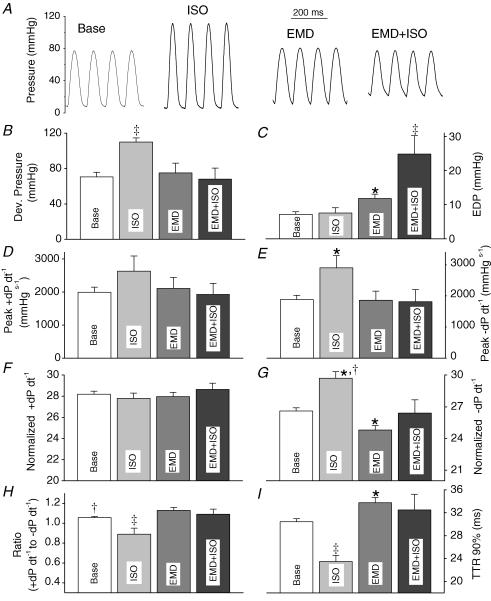
Figure 6. Effect of the myofilament Ca2+ sensitizer EMD 57033 on cardiac contractile function at a fast pacing rate (10 Hz).
A, representative pressure tracings recorded from hearts at baseline, in presence of isoproterenol (ISO, 0.1 μmol l−1), in presence of EMD 57033 (3 μmol l−1), and in presence of ISO and EMD. Comparison of average maximum developed pressure (B), end-diastolic pressure (EDP, C), peak +dP dt−1 (D), peak −dP dt−1 (E), normalized peak +dP dt−1 (F), normalized peak −dP dt−1 (G), ratio of peak −dP dt−1 over peak +dP dt−1 (H), and time to 90% relaxation (I). Data are means ±s.e.m.; *P < 0.05 versus baseline; †P < 0.0.5 versus EMD, ‡P < versus all other.
Unlike at slow pacing rates, at fast pacing rates myofilament sensitization with EMD had no measurable effect on systolic function (10 Hz, Fig. 6B, D and F), and significantly worsened parameters of diastolic function (Fig. 6C and G). Furthermore, applying EMD in presence of isoproterenol significantly decreased developed pressure compared to application of isoproterenol alone (Fig. 6B). Parameters of diastolic function significantly and reversibly worsened compared to measurements obtained either at baseline or in the presence of isoproterenol or EMD alone (Fig. 6C, E, G, H and I). These data indicate that the Ca2+ sensitization of myofilaments, either pharmacologically or by a TnT mutation, may lead to impaired cardiac performance at fast heart rates and/or in presence of β-adrenergic stimulation.
Effect of β-adrenergic receptor blockade on maximum exercise tolerance
Maximum exercise tolerance provides a measure of overall cardiopulmonary function. We previously showed that maximum exercise tolerance of R278C-Tg mice was not statistically different from that of WT-Tg mice, whereas I79N-Tg mice had a significantly reduced exercise tolerance (Hernandez et al. 2005). Based on the in vitro data presented here (Figs 1–6), it is intriguing to speculate that the detrimental effects of a Ca2+ sensitivity increase was responsible for decreased exercise tolerance of I79N-Tg mice. To test the hypothesis, we repeated the exercise tolerance test after intraperitoneal administration of the β-adrenergic inhibitor propranolol (1 mg kg−1). This dose has previously been shown to prevent the exercise-induced increase in heart rate in mice and will inhibit effects of β-adrenergic stimulation (Desai et al. 1997). Indeed, after pretreatment with propranolol, there were no statistically significant differences in acute exercise tolerance among all three groups of mice (average running distance: WT-Tg 518 ± 54 m, n = 13, I79N-Tg 457 ± 95 m, n = 14, R278C-Tg 494 ± 47 m, n = 11, n.s). It should be noted that all groups showed a reduced exercise tolerance after propranolol.
Heart size
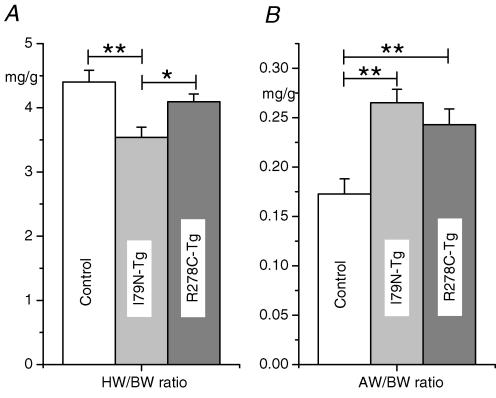
Average heart weight to body weight ratio of R278C-Tg mice was not significantly different from control, whereas heart weight to body weight ratio of I79N-Tg hearts was on average smaller than that of control mice (Fig. 7A). As discussed earlier, this finding may partially explain why I79N-Tg hearts reached peak developed pressure at lower LV volumes and demonstrated a leftward shift of the Frank-Starling curves (compare also Figs 1, 2 and 4). On the other hand, there was significant atrial hypertrophy in both mutant TnT groups (Fig. 7B), which may represent a consequence of the impaired diastolic function induced by the TnT mutants under basal conditions (compare Table 1).
Figure 7. Comparison of atrial weight (AW) and total heart weight (HW) to body weight (BW) ratio.
Hearts from control (n = 10), I79N-Tg (n = 9) and R278C-Tg (n = 11) mice were removed, rinsed and blotted dry before weighing. Weights were normalized by body weight. Data are means ±s.e.m., *P < 0.05.
Discussion
The major finding of this study is that increasing myofilament Ca2+ sensitivity either acutely with the Ca2+ sensitizer EMD 57033, or chronically by cardiac-targeted expression of Ca2+-sensitizing TnT mutants, significantly limited the cardiac inotropic response to isoproterenol, whereas the relatively benign TnT-R278C mutation, which has no effect on myofilament Ca2+ sensitivity, did not (compare Figs 3, 4, 5 and 6). This suggests that the Ca2+ sensitization caused by most TnT mutations may contribute to the pathogenesis of the hypertrophic cardiomyopathy. The findings that only the mice expressing the Ca2+-sensitizing TnT-I79N mutation exhibit impaired exercise tolerance, which can be prevented by β-adrenergic receptor blockade, and that these mice die after isoproterenol administration (Knollmann et al. 2001), further confirms the significance of the reduced inotropic response to β-adrenergic stimulation in vivo.
Consistent with our data, several investigators have previously documented a reduced contractile response to inotropic agents in other murine models expressing FHC-linked mutant sarcomeric proteins that increase myofilament Ca2+ sensitivity: isovolumically contracting hearts of transgenic mice expressing the TnT-R92Q mutant, which sensitizes myofilaments to a similar degree as the TnT-I79N mutant, failed to increase developed pressure upon increase in perfusate Ca2+ from 2.5 to 4 mmol l−1 (Javadpour et al. 2003). Transgenic mice harbouring the α-tropomyosin D175N mutation demonstrated a decreased relaxation rate and blunted contractile response to β-adrenergic stimulation (Evans et al. 2000). Similarly, myocytes expressing slow skeletal TnI, which significantly increases myofilament Ca2+ sensitivity, also demonstrate a blunted contractile response to isoproterenol (Fentzke et al. 1999; Layland et al. 2004). In contrast, the contractile response remained intact in transgenic mice with decreased myofilament Ca2+ sensitivity induced by overexpressing a proteolytic degradation fragment of troponin I found in stunned myocardium (Kogler et al. 2001). Together with the data reported here, these results suggest that increasing myofilament Ca2+ sensitivity may affect the positive inotropic response to isoproterenol, whereas decreasing myofilament Ca2+ sensitivity does not.
The positive inotropic response to isoproterenol is thought to be largely a function of β-adrenergically mediated increases in intracellular Ca2+ transients and Ca2+ stores, since myofilament Ca2+ sensitivity actually decreases in response to β-adrenergic agonist via protein kinase A (PKA) phosphorylation of troponin I (Robertson et al. 1982). Maximum tension of intact mammalian cardiac cells is limited by the amount of Ca2+ that the sarcoplasmic reticulum can accumulate and release (Fabiato, 1981). Hence, impaired Ca2+ cycling and/or disruption of the β-adrenergic signalling cascade are commonly found in humans and animal models with ischaemic or dilated cardiomyopathy (Ayobe & Tarazi, 1983; Beuckelmann et al. 1992; Yao et al. 1998; Ito et al. 2000). However, previous experimental results suggest that β-adrenergic signalling may not be affected in I79N-Tg and other transgenic mice with increased myofilament Ca2+ sensitivity: (1) I79N-Tg mice demonstrate a normal heart rate response to isoproterenol (Knollmann & Potter, 2001); (2) in the presence of isoproterenol, intracellular Ca2+ transients and SR Ca2+ stores increased to an even greater degree in I79N-Tg myocytes compared to control myocytes (Knollmann et al. 2003); and (3) β-adrenergic receptor density of transgenic mice expressing the Ca2+-sensitizing α-tropomyosin D175N mutation were increased despite a significant reduction in isoproterenol-recruitable contractile response (Evans et al. 2000). More recently, an essential role of troponin I for the positive inotropic response to β-adrenergic receptor has been demonstrated both in isovolumically and auxotonically contracting hearts (Layland et al. 2004). Interestingly, these experiments utilized transgenic mice cardiac targeted expression of slow-skeletal TnI, which causes a myofilament Ca2+ sensitivity increase similar to that observed in the I79N-Tg mice examined here. Together with our data from acute application of the Ca2+ sensitizer EMD 57033 (Figs 5 and 6), these results suggest that the Ca2+ sensitization of myofilaments alone may be sufficient to reduce the inotropic response to isoproterenol.
There are several possible mechanisms that may contribute to this phenomenon.
(1) Muscle relaxation is impaired with interventions that increase myofilament Ca2+ sensitivity such as EMD (Fig. 6, TR90% and White et al. 1993). Thus, incomplete relaxation could result in decreased diastolic filling especially at fast pacing rates, accompanied by decreased end-diastolic sarcomere length, and decreased cardiac stroke volume. While this mechanism could clearly contribute to a decrease in systolic function in ejecting hearts and in vivo, it is less likely to explain the decrease in maximum contractility in the isovolumic preparation examined here.
(2) Interventions that increase myofilament Ca2+ sensitivity decrease the amplitude and slow the decay kinetics of cytosolic free [Ca2+] (Ca2+ transient) during the cardiac cycle. This has been shown experimentally with EMD 57033 (Kawai et al. 2000) or during isometric versus unloaded muscle contractions (Janssen & de Tombe, 1997). These data suggests that the apparent binding affinity of troponin C to Ca2+ is increased, either directly, or as is the case for EMD, via increasing the rate of interaction of myosin with actin resulting in more strongly bound crossbridges (Solaro et al. 1993). As a result, more Ca2+ may be bound to troponin C during systole, and dissociate more slowly during muscle relaxation. While not tested directly, this idea is supported by the observation that myocytes expressing the Ca2+-sensitizing TnT-I79N mutant also had significantly slower decay kinetics and elevated end-diastolic [Ca2+] at fast pacing rates and in the presence of isoproterenol (Knollmann et al. 2003), whereas myocytes expressing the non-sensitizing TnT-R287N mutant did not (Sirenko et al. 2003). Since the major SR Ca2+ transport enzyme SERCA2 has to compete against an increased cytoplasmic buffering capacity provided by the myofilaments, less Ca2+ may be taken up into the sarcoplasmic reticulum at fast pacing rates. As a result, less Ca2+ would be available for release for the next, and therefore weaker, contractions. Future experiments will have to test this hypothesis directly.
(3) It is intriguing to speculate that the physiological Ca2+ desensitization of troponin I by PKA phosphorylation (Robertson et al. 1982) serves to enhance Ca2+ reuptake at fast heart rates. In this regard, we cannot exclude that the TnT-I79N mutation interferes with the PKA regulation of troponin I. However, this was found not to be the case in mice expressing a Ca2+-sensitizing α-Tm mutation, which also have decreased contractile response to isoproterenol and closely resemble the phenotype of I79N-Tg mice. We also have preliminary data suggesting that PKA phosphorylation of TnI right-shifts the pCa–force relationship of control and I79N-Tg mutant fibres to a similar degree.
(4) Finally, decreased energetics has recently been postulated as the cause of impaired inotropic response of transgenic hearts expressing the Ca2+-sensitizing TnT-R92Q mutant (Javadpour et al. 2003). The TnT-R92Q mutation shares many characteristics with the TnT-I79N mutation studied here, both in skinned fibres (Szczesna et al. 2000; Chandra et al. 2001) and in transgenic mice (Tardiff et al. 1999). However, that the other mutation examined here, TnT-R278C-Tg, also reduced the force produced per mole of ATP used (i.e. energy cost of contraction) in skinned muscle fibre experiments (Hernandez et al. 2005) suggests that an increased energy cost alone is not sufficient to explain the reduced positive inotropic response.
It should be noted that in R278C-Tg hearts 35% of endogenous TnT was replaced with human TnT-R278C, whereas the replacement level was 52% in the I79N-Tg hearts. Arguably, higher TnT-R278C expression levels could have resulted in a different phenotype. However, previous studies found no difference in myofilament Ca2+ sensitivity, exercise tolerance and other phenotypic parameters between transgenic lines that expressed TnT-R278C at levels ranging from 35% to 55% (Hernandez et al. 2005). Similarly, no phenotypic differences were observed between transgenic lines expressing either 39% or 52% TnT-I79N (Knollmann et al. 2001; Miller et al. 2001). This contrasts with reports that higher expression levels of another TnT mutant (R92Q) caused a more severe phenotype (Tardiff et al. 1999). Possibly, the much larger range of expression levels (from 30% to 92%) in the latter study may have uncovered a protein-dosage effect that we were unable to detect. Alternatively, not all TnT mutants demonstrate a significant protein dosage effect. It should also be recognized that the expression level of mutant TnT in human hearts is not known.
While the analogous effects on cardiac performance of the Ca2+-sensitizing TnT-I79N mutation and EMD 57033 are intriguing, there are substantial differences between their effects on skinned fibres: EMD 57033 increases force even in the virtual absence of Ca2+ by promoting the actin–myosin interaction and development of strongly bound crossbridges, whereas TnT-I79N does not (Miller et al. 2001). This may explain why the effect of EMD on contractile function is more severe than that of the I79N mutation at fast pacing rates (compare Table 2 and Fig. 6). On the other hand, EMD 57033 increases maximal developed force (Gross et al. 1993), whereas the I79N mutation has the opposite effect (Miller et al. 2001). Accordingly, EMD increased developed pressure at slow pacing rates (Fig. 5B), whereas the TnT-I79N mutation did not (Fig. 2).
Another novel finding of our study is that the TnT-R278C impaired cardiac relaxation and diastolic function in the absence of any effects on Ca2+ sensitivity (see Table 1), resulting in significant atrial hypertrophy (Fig. 7). This suggests that the TnT-R278C mutation may alter crossbridge cycling and/or detachment without affecting Ca2+-sensitivity, and demonstrates that effects other than Ca2+ sensitivity are also involved in the poor relaxation of HCM-related troponin T mutants and contribute to the resulting phenotype. Interestingly, in the case of the TnT-R278C mutants, these effects can largely be compensated by adrenergic stimulation with isoproterenol (see Table 2) and did not result in an impaired exercise tolerance in vivo (Hernandez et al. 2005).
The finding of reduced maximal developed pressure in I79N-Tg mice compared to R278C-Tg mice (Fig. 4, Table 2) is surprising, because the maximal developed force per cross-al area of TnT-I79N mutant fibres is actually higher than that of TnT-R278C mutant fibres (compare Miller et al. 2001versusHernandez et al. 2005). Given that in intact cardiac myocytes intracellular Ca2+ never reaches concentrations high enough to activate more than 70% of available crossbridges (Fabiato, 1981), a maximal Ca2+ release should activate more crossbridges in hearts expressing the Ca2+-sensitizing TnT-I79N mutant, thereby producing considerably more force than hearts expressing the TnT-R278C mutant. In fact, the opposite effect was observed (Fig. 4, Table 2). This result supports the hypothesis that increasing myofilament Ca2+ sensitivity has additional effects on cardiac contractility, such as possibly interfering with intracellular Ca2+ cycling and SR Ca2+ reuptake at fast heart rates. Future experiments at the single cell level will have to test this hypothesis.
Acknowledgments
This work was supported by the National Institute of Health (HL07670 to B.C.K. and HL042325 and HL067415 to J.D.P.)
References
- Ayobe MH, Tarazi RC. β-Receptors and contractile reserve in left ventricular hypertrophy. Hypertension. 1983;5:I192–I197. doi: 10.1161/01.hyp.5.2_pt_2.i192. [DOI] [PubMed] [Google Scholar]
- Beuckelmann DJ, Nabauer M, Erdmann E. Intracellular calcium handling in isolated ventricular myocytes from patients with terminal heart failure [see comments] Circulation. 1992;85:1046–1055. doi: 10.1161/01.cir.85.3.1046. [DOI] [PubMed] [Google Scholar]
- Chandra M, Rundell VL, Tardiff JC, Leinwand LA, De Tombe PP, Solaro RJ. Ca2+ activation of myofilaments from transgenic mouse hearts expressing R92Q mutant cardiac troponin T. Am J Physiol Heart Circ Physiol. 2001;280:H705–H713. doi: 10.1152/ajpheart.2001.280.2.H705. [DOI] [PubMed] [Google Scholar]
- Desai KH, Sato R, Schauble E, Barsh GS, Kobilka BK, Bernstein D. Cardiovascular indexes in the mouse at rest and with exercise: new tools to study models of cardiac disease. Am J Physiol. 1997;272:H1053–H1061. doi: 10.1152/ajpheart.1997.272.2.H1053. [DOI] [PubMed] [Google Scholar]
- Elliott PM, D'Cruz L, McKenna WJ. Late-onset hypertrophic cardiomyopathy caused by a mutation in the cardiac troponin T gene. N Engl J Med. 1999;341:1855–1856. doi: 10.1056/NEJM199912093412416. [DOI] [PubMed] [Google Scholar]
- Evans CC, Pena JR, Phillips RM, Muthuchamy M, Wieczorek DF, Solaro RJ, Wolska BM. Altered hemodynamics in transgenic mice harboring mutant tropomyosin linked to hypertrophic cardiomyopathy. Am J Physiol Heart Circ Physiol. 2000;279:H2414–H2423. doi: 10.1152/ajpheart.2000.279.5.H2414. [DOI] [PubMed] [Google Scholar]
- Fabiato A. Myoplasmic free calcium concentration reached during the twitch of an intact isolated cardiac cell and during calcium-induced release of calcium from the sarcoplasmic reticulum of a skinned cardiac cell from the adult rat or rabbit ventricle. J General Physiol. 1981;78:457–497. doi: 10.1085/jgp.78.5.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabiato A. Computer programs for calculating total from specified free or free from specified total ionic concentrations in aqueous solutions containing multiple metals and ligands. Meth Enzymol. 1988;157:378–417. doi: 10.1016/0076-6879(88)57093-3. [DOI] [PubMed] [Google Scholar]
- Fentzke RC, Buck SH, Patel JR, Lin H, Wolska BM, Stojanovic MO, Martin AF, Solaro RJ, Moss RL, Leiden JM. Impaired cardiomyocyte relaxation and diastolic function in transgenic mice expressing slow skeletal troponin I in the heart. J Physiol. 1999;517:143–157. doi: 10.1111/j.1469-7793.1999.0143z.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Castro M, Reguero JR, Batalla A, Diaz-Molina B, Gonzalez P, Alvarez V, Cortina A, Cubero GI, Coto E. Hypertrophic cardiomyopathy: low frequency of mutations in the β-myosin heavy chain (MYH7) and cardiac troponin T (TNNT2) genes among Spanish patients. Clin Chem. 2003;49:1279–1285. doi: 10.1373/49.8.1279. [DOI] [PubMed] [Google Scholar]
- Gross T, Lues I, Daut J. A new cardiotonic drug reduces the energy cost of active tension in cardiac muscle. J Mol Cell Cardiol. 1993;25:239–244. doi: 10.1006/jmcc.1993.1030. [DOI] [PubMed] [Google Scholar]
- Hernandez O, Szczesna-Cordary D, Knollmann BC, Miller T, Bell M, Zhao J, Sirenko SG, Diaz Z, Guzman G, Xu Y, Wang Y, Kerrick WG, Potter JD. F110I and R278C troponin T mutations that cause familial hypertrophic cardiomyopathy affect muscle contraction in transgenic mice and reconstituted human cardiac fibers. J Biol Chem. 2005;280:37183–37194. doi: 10.1074/jbc.M508114200. [DOI] [PubMed] [Google Scholar]
- Ito K, Yan X, Tajima M, Su Z, Barry WH, Lorell BH. Contractile reserve and intracellular calcium regulation in mouse myocytes from normal and hypertrophied failing hearts. Circ Res. 2000;87:588–595. doi: 10.1161/01.res.87.7.588. [DOI] [PubMed] [Google Scholar]
- Janssen PM, De Tombe PP. Uncontrolled sarcomere shortening increases intracellular Ca2+ transient in rat cardiac trabeculae. Am J Physiol. 1997;272:H1892–H1897. doi: 10.1152/ajpheart.1997.272.4.H1892. [DOI] [PubMed] [Google Scholar]
- Javadpour MM, Tardiff JC, Pinz I, Ingwall JS. Decreased energetics in murine hearts bearing the R92Q mutation in cardiac troponin T. J Clin Invest. 2003;112:768–775. doi: 10.1172/JCI15967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai M, Lee JA, Orchard CH. Effects of the Ca2+ sensitizer EMD 57033 on intracellular Ca2+ in rat ventricular myocytes: relevance to arrhythmogenesis during positive inotropy. Clin Sci (Lond) 2000;99:547–554. [PubMed] [Google Scholar]
- Knollmann BC, Blatt SA, Horton K, De Freitas F, Miller T, Bell M, Housmans PR, Weissman NJ, Morad M, Potter JD. Inotropic stimulation induces cardiac dysfunction in transgenic mice expressing a troponin T (I79N) mutation linked to familial hypertrophic cardiomyopathy. J Biol Chem. 2001;276:10039–10048. doi: 10.1074/jbc.M006745200. [DOI] [PubMed] [Google Scholar]
- Knollmann BC, Kirchhof P, Sirenko SG, Degen H, Greene AE, Schober T, Mackow JC, Fabritz L, Potter JD, Morad M. Familial hypertrophic cardiomyopathy-linked mutant troponin T causes stress-induced ventricular tachycardia and Ca2+-dependent action potential remodeling. Circ Res. 2003;92:428–436. doi: 10.1161/01.RES.0000059562.91384.1A. [DOI] [PubMed] [Google Scholar]
- Knollmann BC, Potter JD. Altered regulation of cardiac muscle contraction by troponin T mutations that cause familial hypertrophic cardiomyopathy. Trends Cardiovasc Med. 2001;11:206–212. doi: 10.1016/s1050-1738(01)00115-3. [DOI] [PubMed] [Google Scholar]
- Kogler H, Soergel DG, Murphy AM, Marban E. Maintained contractile reserve in a transgenic mouse model of myocardial stunning. Am J Physiol Heart Circ Physiol. 2001;280:H2623–H2630. doi: 10.1152/ajpheart.2001.280.6.H2623. [DOI] [PubMed] [Google Scholar]
- Layland J, Grieve DJ, Cave AC, Sparks E, Solaro RJ, Shah AM. Essential role of troponin I in the positive inotropic response to isoprenaline in mouse hearts contracting auxotonically. J Physiol. 2004;556:835–847. doi: 10.1113/jphysiol.2004.061176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig J, Halbrugge T, Vey G, Walter J, Graefe KH. Haemodynamics as a determinant of the pharmacokinetics of and the plasma catecholamine responses to isoprenaline. Eur J Clin Pharmacol. 1989;37:493–500. doi: 10.1007/BF00558130. [DOI] [PubMed] [Google Scholar]
- Majewski H. Modulation of noradrenaline release through activation of presynaptic β-adrenoreceptors. J Auton Pharmacol. 1983;3:47–60. doi: 10.1111/j.1474-8673.1983.tb00496.x. [DOI] [PubMed] [Google Scholar]
- Marian AJ, Roberts R. The molecular genetic basis for hypertrophic cardiomyopathy. J Mol Cell Cardiol. 2001;33:655–670. doi: 10.1006/jmcc.2001.1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller T, Szczesna D, Housmans PR, Zhao J, DeFreitas F, Gomes AV, Culbreath L, McCue J, Wang Y, Xu Y, Kerrick WG, Potter JD. Abnormal contractile function in transgenic mice expressing an FHC-linked troponin T (I79N) mutation. J Biol Chem. 2001;276:3743–3755. doi: 10.1074/jbc.M006746200. [DOI] [PubMed] [Google Scholar]
- Richard P, Charron P, Carrier L, Ledeuil C, Cheav T, Pichereau C, Benaiche A, Isnard R, Dubourg O, Burban M, Gueffet JP, Millaire A, Desnos M, Schwartz K, Hainque B, Komajda M. Hypertrophic cardiomyopathy: distribution of disease genes, spectrum of mutations, and implications for a molecular diagnosis strategy. Circulation. 2003;107:2227–2232. doi: 10.1161/01.CIR.0000066323.15244.54. [DOI] [PubMed] [Google Scholar]
- Robertson SP, Johnson JD, Holroyde MJ, Kranias EG, Potter JD, Solaro RJ. The effect of troponin I phosphorylation on the Ca2+-binding properties of the Ca2+-regulatory site of bovine cardiac troponin. J Biol Chem. 1982;257:260–263. [PubMed] [Google Scholar]
- Sirenko SG, Potter JD, Knollmann BC. Transgenic expression of the FHC-linked troponin T (R278C) mutation results in a benign cardiac phenotype (Abstract) Biophys J. 2003;84:241a. [Google Scholar]
- Solaro RJ, Gambassi G, Warshaw DM, Keller MR, Spurgeon HA, Beier N, Lakatta EG. Stereoselective actions of thiadiazinones on canine cardiac myocytes and myofilaments. Circ Res. 1993;73:981–990. doi: 10.1161/01.res.73.6.981. [DOI] [PubMed] [Google Scholar]
- Stromer H, Cittadini A, Szymanska G, Apstein CS, Morgan JP. Validation of different methods to compare isovolumic cardiac function in isolated hearts of varying sizes. Am J Physiol. 1997;272:H501–H510. doi: 10.1152/ajpheart.1997.272.1.H501. [DOI] [PubMed] [Google Scholar]
- Szczesna D, Zhang R, Zhao J, Jones M, Guzman G, Potter JD. Altered regulation of cardiac muscle contraction by troponin T mutations that cause familial hypertrophic cardiomyopathy. J Biol Chem. 2000;275:624–630. doi: 10.1074/jbc.275.1.624. [DOI] [PubMed] [Google Scholar]
- Tardiff JC, Hewett TE, Palmer BM, Olsson C, Factor SM, Moore RL, Robbins J, Leinwand LA. Cardiac troponin T mutations result in allele-specific phenotypes in a mouse model for hypertrophic cardiomyopathy. J Clin Invest. 1999;104:469–481. doi: 10.1172/JCI6067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theopistou A, Anastasakis A, Miliou A, Rigopoulos A, Toutouzas P, Stefanadis C. Clinical features of hypertrophic cardiomyopathy caused by an Arg278Cys missense mutation in the cardiac troponin T gene. Am J Cardiol. 2004;94:246–249. doi: 10.1016/j.amjcard.2004.03.077. [DOI] [PubMed] [Google Scholar]
- Van Driest SL, Ellsworth EG, Ommen SR, Tajik AJ, Gersh BJ, Ackerman MJ. Prevalence and spectrum of thin filament mutations in an outpatient referral population with hypertrophic cardiomyopathy. Circulation. 2003;108:445–451. doi: 10.1161/01.CIR.0000080896.52003.DF. [DOI] [PubMed] [Google Scholar]
- Westermann D, Knollmann BC, Steendijk P, Rutschow S, Riad A, Pauschinger M, Potter JD, Schultheiss HP, Tschope C. Diltiazem treatment prevents diastolic heart failure in mice with familial hypertrophic cardiomyopathy. Eur J Heart Fail. 2006;8:115–121. doi: 10.1016/j.ejheart.2005.07.012. [DOI] [PubMed] [Google Scholar]
- White J, Lee JA, Shah N, Orchard CH. Differential effects of the optical isomers of EMD 53998 on contraction and cytoplasmic Ca2+ in isolated ferret cardiac muscle. Circ Res. 1993;73:61–70. doi: 10.1161/01.res.73.1.61. [DOI] [PubMed] [Google Scholar]
- Yao A, Su Z, Nonaka A, Zubair I, Spitzer KW, Bridge JH, Muelheims G, Ross J Jr, Barry WH. Abnormal myocyte Ca2+ homeostasis in rabbits with pacing-induced heart failure. Am J Physiol. 1998;275:H1441–H1448. doi: 10.1152/ajpheart.1998.275.4.H1441. [DOI] [PubMed] [Google Scholar]
- Zhai J, Schmidt AG, Hoit BD, Kimura Y, MacLennan DH, Kranias EG. Cardiac-specific overexpression of a superinhibitory pentameric phospholamban mutant enhances inhibition of cardiac function in vivo. J Biol Chem. 2000;275:10538–10544. doi: 10.1074/jbc.275.14.10538. [DOI] [PubMed] [Google Scholar]