Abstract
This study was designed to examine the neuronal mechanisms of ethanol sensitivity by utilizing inbred short sleep (ISS) and inbred long sleep (ILS) mouse strains that display large differences in sensitivity to the behavioural effects of ethanol. Comparisons of whole-cell electrophysiological recordings from CA1 pyramidal neurons in hippocampal slices of ISS and ILS mice indicate that ethanol enhances GABAA receptor-mediated inhibitory postsynaptic currents (GABAA IPSCs) and reduces NMDA receptor-mediated excitatory postsynaptic currents (NMDA EPSCs) in a concentration- and strain-dependent manner. In ILS neurons, these receptor systems are significantly more sensitive to ethanol than those in ISS neurons. To further examine the underlying mechanisms of differential ethanol sensitivities in these mice, GABAB activity and presynaptic and postsynaptic actions of ethanol were investigated. Inhibition of GABAB receptor function enhances ethanol-mediated potentiation of distal GABAA IPSCs in ILS but not ISS mice, and this blockade of GABAB receptor function has no effect on the action of ethanol on NMDA EPSCs in either mouse strain. Thus, subregional differences in GABAB activity may contribute to the differential ethanol sensitivity of ISS and ILS mice. Moreover, analysis of the effects of ethanol on paired-pulse stimulation, spontaneous IPSC events, and brief local GABA or glutamate application suggest that postsynaptic rather than presynaptic mechanisms underlie the differential ethanol sensitivity of these mice. Furthermore, these results provide essential information to focus better on appropriate target sites for more effective drug development for the treatment of alcohol abuse.
Individuals show differential sensitivity to central effects of ethanol, but the mechanisms that mediate these actions of ethanol in the brain are not well understood. It is known, however, that γ-aminobutyric acidA (GABAA) and N-methyl-D-aspartate (NMDA) neurotransmitter receptors are two major targets for ethanol (Harris, 1999). Still, the relationships between the actions of ethanol on these receptors and their effects on behaviour remain elusive.
Selective breeding of rodents for ethanol sensitivity has been carried out by numerous researchers to aid in the investigation of the mechanisms of action of ethanol in the CNS (Deitrich et al. 1989; Collins et al. 1993; Crabbe, 2002; for review see Deitrich, 1993). Two of these strains of animals, long sleep (LS) and short sleep (SS) mice, were selectively bred based on the relative sensitivity to the hypnotic effects of systemic ethanol. To fix the genes that control these phenotypes, inbred strains of SS (ISS) and LS (ILS) mice were developed, and they retain the behavioural differences in sensitivity to the hypnotic effects of ethanol (Bennett & Johnson, 1998; Bennett, 2000; Bennett et al. 2002). Differences in expression or regulation of molecular targets of ethanol, such as those involving neurotransmitter systems, are likely contributors to the observed phenotypes.
Differential effects of ethanol on GABA function have been observed in LS and SS mice (Allan & Harris, 1986; Davies & Alkana, 2001). We also found that ethanol (80 mm) enhanced GABAA inhibitory postsynaptic currents (GABAA IPSCs) in hippocampal CA1 pyramidal neurons of ILS mice, but had little effect on GABAA IPSCs in ISS mice (Poelchen et al. 2000). As a result of these and other studies, it has been postulated that differences in responses of GABAA receptors to ethanol might be responsible for the behavioural differences in ethanol sensitivity of ILS and ISS mice. However, the precise neuronal mechanisms that underlie differences in ethanol sensitivity in intact tissue remained unknown. Therefore, in the present investigation we focused on identifying neuronal mechanisms that may underlie ethanol sensitivity.
In intact hippocampal slices, there are distinct subregional differences in modulation by ethanol of hippocampal GABAA responses in rats (Weiner et al. 1997). The effects of ethanol on hippocampal GABAA IPSCs at distal (dendritic) sites were negatively modulated by GABAB receptor activity (Wu et al. 2005). Thus, the difference in modulation by GABAB of GABAA IPSC activity between ISS and ILS mice may potentially explain the observed difference in ethanol sensitivity of GABAA function. In addition, NMDA receptor activity may also contribute to the observed individual phenotypes in these mice. Wilson & Collins (1996) reported that the hippocampus and striatum of SS mice have more NMDA receptors compared with those brain areas of LS mice, and other studies showed differences in NMDA receptor activity as well as effects of ethanol on NMDA receptor activity between these two strains of mice using receptor-binding studies (Wilson et al. 1990; Daniell & Phillips, 1994; Musleh et al. 1996; Velardo et al. 1998) and extracellular recordings (Hanania et al. 2000). However, it is not known whether ethanol can differentially affect NMDA receptor function in a manner that correlates with the behavioural phenotypes of ISS and ILS mice. The neuronal mechanisms that underlie the ethanol sensitivity of these receptors are also not known.
In this study we have focused on the mechanism(s) of action of ethanol on synaptic GABAA and NMDA neurotransmitter receptor complexes in ILS and ISS mice. Therefore we examined a number of potential mechanisms that could underlie ethanol sensitivity of GABAA and NMDA receptor-mediated activity in CA1 hippocampal pyramidal neurons in ISS and ILS mice. Our results show that: (a) ethanol sensitivity of functional GABAA IPSCs and NMDA excitatory postsynaptic currents (EPSCs) paralleled behavioural sensitivity in these mice; (b) ethanol exerts concentration-dependent effects on these synaptic currents in both strains of mice; (c) synaptic ethanol sensitivity is subregion-dependent for GABAergic but not glutamatergic function; (d) differential GABAB influences may contribute to the subregional differences in ethanol sensitivity; and (e) the presynaptic action of ethanol on GABAergic terminals did not differ in the two strains of mice. Taken together, these data support the hypothesis that postsynaptic modulation of ethanol at both GABAergic and glutamatergic neurotransmitter receptor–channel complexes underlie, at least in part, the differences in behavioural ethanol sensitivity between ISS and ILS mice.
Methods
Animals
Male young adult (6–12 weeks old) ISS and ILS mice were used in this study. The mice were housed five per cage with light from 07.00 to 19.00 h and with free access to food and water. The animals were maintained in an National Institutes of Health (NIH)-accredited facility at University of Colorado at Denver and Health Sciences Center (UCDHSC), and the animal procedures were performed in accordance with the NIH Guide for the Care and Use of Laboratory Animals and were approved by the Animal Use and Care Committee at UCDHSC.
Slice preparation, storage and recording bath conditions
Mice were quickly killed by cervical dislocation, and their brains were rapidly removed and immersed in ice-cold, sucrose buffer for 40–60 s to cool the interior of the brain. The sucrose buffer contained (mm): NaCl 87, KCl 2.5, MgCl2 7, CaCl2 0.5, NaH2PO4 1.25, d-glucose 25, sucrose 75 and NaHCO3 25 (Geiger & Jonas, 2000). After removing one or both hippocampi from the brain, transverse slices (400 μm) were made using a tissue chopper/slicer (TC-2 tissue sectioner, Sorvall) and the slices were transferred to individual compartments in a storage system (Proctor & Dunwiddie, 1999; Weiner, 2002), where the slices were constantly perfused with a 1:1 mixture of sucrose-containing artificial cerebrospinal fluid (aCSF) and normal aCSF with 95% O2–5% CO2 at 32–33°C. The normal aCSF contained (mm): NaCl 126, KCl 3.0, MgCl2 1.5, CaCl2 2.4, NaH2PO4 1.2, d-glucose 11 and NaHCO3 25.9. This storage procedure seemed to preserve cellular integrity so that synaptic GABAergic and glutamatergic NMDA function remained robust throughout the day, following slice preparation. This method has been in use in our laboratory for the past few years, and we obtain consistent effects of ethanol on GABAA IPSCs and NMDA EPSCs which are comparable effects reported by others.
Electrophysiological recording
Whole-cell recordings were made at 32–33°C while constantly superfusing the slice with oxygenated aCSF at 2 ml min−1. A Flaming/Brown electrode puller (Sutter Instruments, Novato, CA, USA) was used to make microelectrodes for whole-cell recording that have resistances of 6–9 MΩ when filled with a potassium gluconate-containing internal solution containing (mm): K+-gluconate 130, EGTA 1, MgCl2 2, CaCl2 0.5, disodium ATP 2.54 and Hepes 10; pH adjusted to 7.3 with KOH. In a subset of experiments, CsCl-filled electrodes were used; these electrodes contained (mm): CsCl 140, MgCl2 2, CaCl2 1, EGTA 10, Hepes 10, adenosine triphosphate (disodium salt) 2 and lidocaine N-ethyl bromide(QX-314) 5; pH adjusted to 7.3 with CsOH as described by Sanna et al. (2004). CA1 pyramidal neurons were recorded within the stratum pyramidale layer. Electrically evoked synaptic responses were obtained by stimulation in the stratum pyramidale layer (proximal stimulation) or stratum radiatum (distal stimulation) with twisted bipolar stimulating electrodes made from 0.0026-in Formvar-coated nichrome wire (Dunwiddie, 1986; Proctor et al. 2004) to activate presynaptic fibres on or near the pyramidal cell soma (proximal) or in the stratum radiatum dendrites (distal), respectively. Drugs were applied at 100-fold concentration to the bath aCSF ((flow rate: 2 ml min−1) via calibrated syringe pumps (Razel Scientific Instruments Inc., Stamford, CT, USA) to achieve the desired concentrations in the bath perfusate.
Measurement of GABAA IPSCs
CA1 pyramidal neurons were voltage clamped to −55 mV (corrected for the liquid junction potential) from the normal resting membrane potential of −65 to −70 mV. GABAA receptor-mediated IPSC responses were evoked (200 μs, 4–10 V) at 60-s intervals with a bipolar stimulating electrode placed either at distal and/or proximal sites in Schaffer collateral–commissural fibres 200–300 μm from the recorded cell. 6-cyano-7-nitroquinoxaline-2,3-dione disodium salt (CNQX, 20 μm) and d(−)-2-amino-5-phosphonovaleric acid (D-APV, 50 μm), were added to the superfused aCSF to block α-amino-3-hydroxy-5-methyl-4-isoxalone propionic acid (AMPA) and NMDA receptor-mediated EPSCs, respectively. This stimulation–recording protocol evokes synaptic responses predominantly from either proximal inputs (i.e. GABAA responses from interneurons that synapse on or near the soma of the recorded pyramidal cell in stratum pyramidale) or from distal inputs (i.e. GABAA responses from interneurons that synapse on the distal dendrites of the recorded cell located in stratum radiatum). To determine whether GABAB activity modulates the action of ethanol on the GABAA response, pretreatment with the GABAB antagonist, 3-[[(3,4-dichlorophenyl)methyl]amino] propyl] diethoxymethyl) phosphinic acid (CGP-52432, 0.5 μm) was included in a subgroup of experiments.
Measurement of NMDA EPSCs
CA1 pyramidal neurons were voltage clamped at −55 to −60 mV (corrected for the liquid junction potential) from the normal resting membrane potential of −65 to −70 mV. The NMDA receptor-mediated EPSCs were isolated pharmacologically using CNQX (20 μm) and bicuculline (BMI, 30 μm) to block AMPA and GABAA receptor-mediated currents, respectively. NMDA receptor-mediated EPSC responses were evoked at proximal and/or distal positions as described for recording GABAA responses (i.e. NMDA responses from glutamatergic terminals that synapse on or near the soma of the recorded pyramidal cell or at dendritic locations). In an experimental subgroup, as described above for the GABAA experiments, the possible interaction of GABAB activity on the NMDA EPSCs was tested in the presence of the GABAB antagonist, CGP-52432 (0.5 μm).
Paired-pulse stimulation and measurement of presynaptic actions of ethanol
A paired-pulse stimulation–recording protocol and measurements of spontaneous activity were used to determine the effects of ethanol at the GABAergic and glutamatergic synapses. The paired-pulse stimuli were delivered through a stimulating electrode which was located either on the proximal (soma) or distal (dendrites) subfield of CA1 pyramidal neurons as described above. The evoked responses were determined at an interpulse interval of 50 ms. Amplitudes of the evoked responses were measured using two different internal solutions in the recording electrodes: potassium gluconate (Wu et al. 2005) or CsCl (Sanna et al. 2004). The spontaneous activity of GABAergic terminals was measured with CsCl-filled electrodes and analysed using Mini Analysis software (SynaptoSoft, Decatur, GA, USA).
Local application of neurotransmitters
Brief, local pressure ejection (3–30 ms, 20 psi) of GABA (10 mm) or glutamate (0.3 mm plus 3.0 μm glycine) was applied onto CA1 pyramidal cell dendritic processes in stratum radiatum to directly activate postsynaptic GABA and NMDA receptor-mediated responses. The drugs were delivered via glass capillary tubing, that had been heated and pulled to produce a tip opening of approximately 2.0 μm. The GABAB antagonist, CGP-52432 (0.5 μm) was bath perfused during the GABA applications, and CNQX (20 μm) was added to block AMPA/kainate receptors during glutamate applications. Glycine (3 μm) was also added to ensure that the NMDA current was not compromised by diluting extracellular glycine at the NMDA receptor site during local application of glutamate. All recordings were carried out using the standard potassium gluconate intracellular filling solution, and the applications were made at 1-min intervals to allow for complete recovery between successive drug-evoked responses.
Statistical analysis
Drug effects were quantified as the percentage change in amplitude of the GABAA IPSCs and NMDA EPSCs following drug application relative to the mean of control and washout values. Statistical analyses were carried out with the use of SigmaStat (SPSS, Chicago, IL, USA) for Student's t test and ANOVA analyses. All pairwise multiple comparison procedure (Tukey's test) was used for post hoc analysis. The minimal significance level was set at P < 0.05.
Chemicals
All drugs used to make up the aCSF and internal recording solutions were purchased under the Fluka brand (Sigma Chemical Company, St Louis, MO, USA). Glutamatergic receptor antagonists, D-APV and CNQX, and the GABAA receptor antagonist, bicuculline methiodide (BMI) were also purchased from Sigma. The GABAB receptor antagonist, CGP-52432 was purchased from Tocris (Tocris Cookson Inc., Ellisville, MO, USA). An 8.0-m ethanol solution (in deionized water) was prepared immediately before each experiment from a 95% stock solution (Aaper, Shelbyville, KY, USA) and kept in a glass storage bottle at 4°C.
Results
Ethanol differentially enhances GABAA IPSCs in ISS and ILS mice
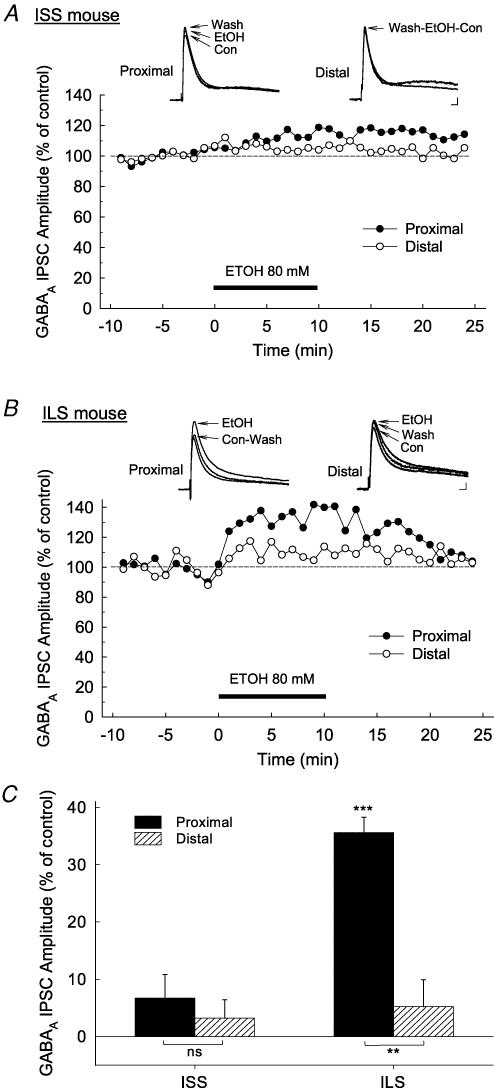
Proximal and distal stimulation of the CA1 neurons of the ISS mice produced robust GABAA IPSCs using potassium gluconate-filled electrodes. Control evoked responses were set by adjusting the stimulus voltage to approximately 30% of the maximum response (Wu et al. 2005). Bath superfusion of 80 mm ethanol had no significant effect on either the proximal or distal GABAA IPSCs recorded in neurons from ISS mice (Fig. 1A). In contrast, ethanol markedly enhanced the proximal GABAA IPSCs (38.1 ± 2.67%; n = 7) in ILS neurons, but had only a small effect on the distal GABAA IPSCs (5.96 ± 4.69%, n = 8; t = 5.724, P < 0.001, Student t test; Fig. 1B). Ethanol did not affect the holding current and membrane resistance (ISS, 9.1 ± 2.53 pA, 4 ± 2.46% change; ILS, 4.8 ± 4.22 pA, −0.2 ± 2.18% change). The enhancement by ethanol of GABAA IPSCs in ILS mice was maximal 3–5 min after initiating drug superfusion (Fig. 1B), and the effect was reversed upon 15–30 min of washout. A two-way ANOVA comparison of the effects of ethanol on GABAA IPSCs of both mouse strains (ISS versus ILS) and the hippocampal subregions (proximal versus distal) showed that ethanol (80 mm) produces (a) strong differential effects in these two strains of mice (F1,25= 34.826, P < 0.001), as well as (b) differential responses with respect to hippocampal subregions (proximal versus distal; F1,25= 34.332, P < 0.001). Thus, ethanol (80 mm) shows a greater enhancement of GABAA IPSCs in ILS than in ISS mice, and it also has a greater effect on GABAA IPSCs in the proximal (somal) than the distal (dendritic) subregions of the hippocampal CA1 neurons of ILS mice (Fig. 1C). This result leads one to question whether the ISS mouse strain is actually insensitive to ethanol, or is just less-sensitive to the effects of ethanol. To explore this question we next examined the effects of various concentrations of ethanol on proximal GABAA IPSCs in ISS and ILS mice.
Figure 1. Acute ethanol superfusion produced differential increases in proximal (somal) and distal (dendritic) GABAA IPSCs in ISS and ILS mice.
Responses were pharmacologically isolated using the competitive NMDA receptor antagonist, D-APV (50 μm) and the AMPA receptor antagonist, CNQX (30 μm) and are shown as insets above time courses. A, representative time course of the effect of 80 mm ethanol on the amplitude of proximal and distal GABAA IPSCs recorded from an ISS mouse. Ethanol (80 mm) did not significantly alter GABAA IPSCs in either the proximal or distal subregions of hippocampal pyramidal cells from ISS mice. B, representative time course of the effect of 80 mm ethanol on the amplitude of proximal and distal GABAA IPSCs recorded from an ILS mouse. Significant increases in proximal GABAA IPSCs were seen in ILS mice following ethanol treatment. C, summary of data showing the effect of ethanol (80 mm) on proximal and distal GABAA IPSCs from ISS and ILS mice. Number of neurons for each measurement: ISS proximal, n = 22; ISS distal, n = 15; ILS proximal, n = 21; ILS distal, n = 16. Scale for insets in A and B, 15 ms, 20 pA. ***P < 0.001.
High concentrations of ethanol enhance GABAA IPSCs in ISS mice
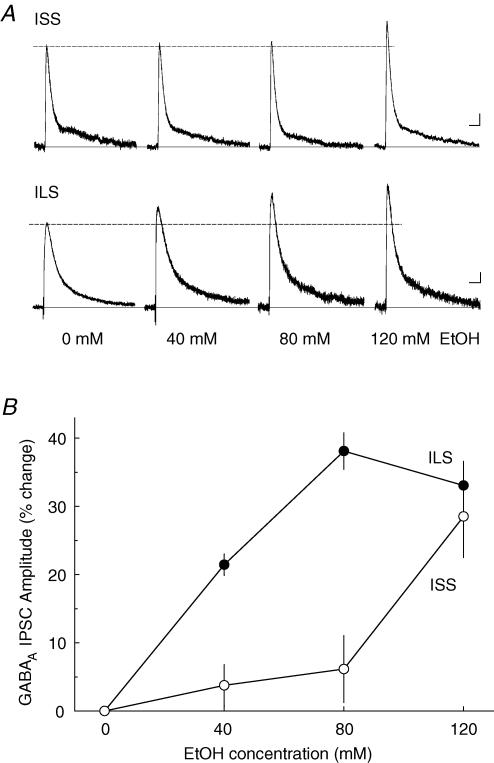
Ethanol, at a concentration of 40 mm, produced a small but significant enhancement of proximal GABAA IPSCs in ILS mice and no effect, as expected, in ISS mice (Fig. 2). When a higher concentration of ethanol (120 mm) was tested in ILS mice, the enhancement of GABAA IPSCs by ethanol was not significantly different from that seen with 80 mm ethanol. However, at 120 mm, ethanol significantly enhanced the GABAA IPSCs in ISS mice. Two-way ANOVA analysis of the effect of ethanol revealed that ethanol produced differential effects in these mice (F1,34= 9.915, P < 0.005), and this effect of ethanol was concentration dependent (F2,34= 11.282, P < 0.001). In addition, there was no significant strain–concentration interaction (F2,34= 2.260, P < 0.1). In the ILS mice, the estimated EC50 was 40 mm, whereas for ISS mice, an EC50 cannot be obtained because the maximal response was not achieved. However, this value was estimated to be greater than 100 mm.
Figure 2. Acute ethanol administration increases GABAA IPSCs from ISS and ILS mice in a concentration-dependent manner.
A, superfusion of ethanol (40, 80 or 120 mm) for 10 min increased evoked GABAA IPSC amplitude in both representative ISS and ILS mice. B, mean percentage increase in GABAA IPSC amplitudes showed differences in ethanol responses: ILS IPSCs were more sensitive to ethanol than those from ISS mice. Number of neurons for each ethanol concentration: 40 mm, n = 5; 80 mm, n = 6; 120 mm, n = 7, in ISS mice; and 40 mm, n = 5; 80 mm, n = 7; 120 mm, n = 6 in ILS mice. Scale for A, 50 ms, 25 pA.
GABAB blockade has differential effects on GABAA IPSCs in ISS and ILS mice
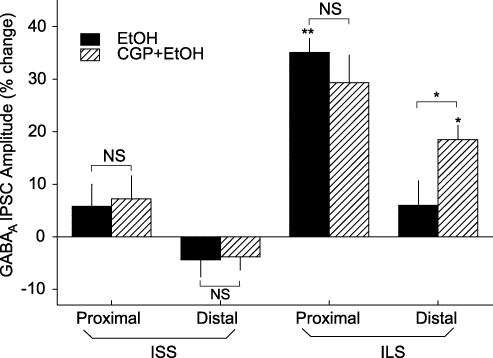
To evaluate the contribution of GABAB receptors to ethanol sensitivity of GABAA IPSCs, the selective GABAB receptor antagonist, CGP-52432 (0.5 μm), was used to block GABAB receptor activity in a subset of slices. Pretreatment with CGP-52432 in ILS mice did not alter the effect of ethanol on proximal GABAA IPSCs, but it did potentiate the effects of ethanol at distal GABAA IPSCs (t = 2.313, P < 0.05; Fig. 3). In both ISS and ILS cells, CGP-52432 alone did not significantly affect GABAA IPSCs at either proximal (ISS, −0.62 ± 4.1%, n = 5; ILS, 2.42 ± 4.81%, n = 7) or distal (ISS, −4.10 ± 2.9%, n = 5; ILS, −1.60 ± 3.12%, n = 6) locations. Taken together, these results show that the proximal GABAA IPSCs of ILS mice were more sensitive to the effect of ethanol than distal responses, but that blocking GABAB activity caused distal GABAA IPSCs from ILS mice to become more sensitive to ethanol treatment (Fig. 3). Three separate two-way ANOVA analyses were performed to assess these effects, including those of the GABAB antagonist, with respect to the strains and subregions (proximal and distal). Two-way ANOVA revealed that there were significant strain differences (F1,93= 37.821, P < 0.001) and subregion differences (F1,93= 20.960, P < 0.001), but there was no significant strain–subregion interaction (F1,93= 1.093, P < 0.1). When the results of drug treatments were analysed according to strain, two-way ANOVA revealed that there were significant differential effects of treatments on the strains (F1,91= 31.313, P < 0.001). ILS mice were significantly more affected by ethanol plus CGP-52432 than ISS mice (P < 0.05). Determining the effects of drugs on GABAA IPSCs in the subregions of the hippocampal CA1 area, also showed that ethanol and ethanol plus CGP-52432 showed greater enhancement of the proximal GABAA IPSCs than the distal GABAA IPSCs (F1,91= 12.790, P < 0.001) and that there was a significant differential effect of CGP-52432 on enhancement by ethanol of GABAA IPSCs at distal compared with proximal subregions (P < 0.01).
Figure 3. Pretreatment with a GABAB receptor antagonist enhanced distal GABAA IPSCs from ILS mice but not from ISS mice.
Ethanol (80 mm) did not significantly affect GABAA IPSCs for either proximal or distal GABAA IPSCs from ISS mice, whereas ethanol (80 mm) alone enhanced proximal, but not distal GABAA IPSCs from ILS mice. Pretreatment with CGP-52432 did not significantly alter the effect of ethanol on these responses from ISS mice. However in ILS mice, although CGP-52432 pretreatment did not further enhance the effect of ethanol (80 mm) on proximal responses, it significantly enhanced distal GABAA IPSCs. Number of cells for these experiments ranged from 6 to 12 for each individual determination. *P < 0.05; **P < 0.01.
Effects of ethanol on NMDA EPSCs in ISS and ILS mice
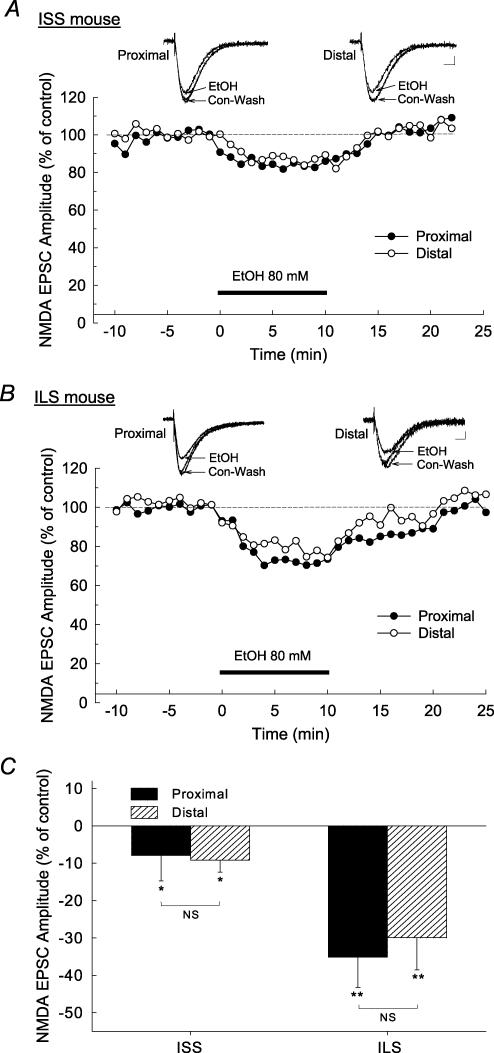
Both proximal and distal stimulation of Schaffer collateral–commissural fibres produced robust synaptic NMDA EPSCs which, like GABAA IPSCs, were differentially affected by 80 mm ethanol in the two strains of mice (Fig. 4A and B). The maximal inhibition by ethanol of NMDA EPSCs occurred approximately 3–5 min after the start of bath superfusion of ethanol, and this inhibition was completely reversed after washout with aCSF for 15–30 min. In ISS mice, ethanol (80 mm) produced a small (∼8%) inhibition in both proximally and distally stimulated NMDA EPSCs (P < 0.05, Fig. 4C). In contrast, ethanol produced a large (35%) inhibition in both proximal and distal NMDA EPSCs in cells from ILS mice (Fig. 4B). As in the GABAA responses, 80 mm ethanol did not significantly affect the holding current or membrane resistance while recording NMDA responses in these cells (ISS, −0.8 ± 4.38 pA, 0.6 ± 1.50% change; ILS: −1.9 ± 3.53 pA, −0.1 ± 2.50% change). This concentration of ethanol (80 mm) produced similar levels of inhibition of both proximal and distal NMDA EPSCs in the ILS mice (F1,28= 0.490, P > 0.1, two-way ANOVA comparing the effect of ethanol on proximal versus distal NMDA EPSCs), which was greater than that observed for either site in ISS mice (F1,28= 12.924, P < 0.001, two-way ANOVA comparing strain differences). There also was no significant strain–subregion interaction (F1,28= 0.0002, P > 0.1). Therefore, these results indicate that the proximal and distal NMDA EPSCs of ILS mice were equally sensitive to the effect of ethanol, whereas these responses were less sensitive in ISS mice (Fig. 4C).
Figure 4. Acute ethanol superfusion produced differential inhibition in NMDA EPSCs in ISS and ILS mice.
Responses were pharmacologically isolated using the competitive GABAA receptor antagonist, BMI (30 μm) and the AMPA receptor antagonist, CNQX (30 μm), and representative signals are indicated as insets above time courses in A and B. A, representative time course of the effect of 80 mm ethanol on the amplitude of proximally and distally evoked NMDA EPSCs recorded in pyramidal neurons from ISS mice. Ethanol produced only a small inhibition of NMDA EPSCs in the proximal and distal subregions of the hippocampus in ISS mice. B, representative time course of the effect of 80 mm ethanol on the amplitude of proximally and distally evoked NMDA EPSCs recorded in pyramidal neurons from ILS mice. In this mouse strain, 80 mm ethanol causes marked reductions in proximal and distal NMDA EPSCs. C, composite data for the overall effects of ethanol (80 mm) on proximal and distal NMDA EPSCs from ISS and ILS mice. Scale for insets in A and B, 15 ms, 10 pA. *P < 0.05; **P < 0.01.
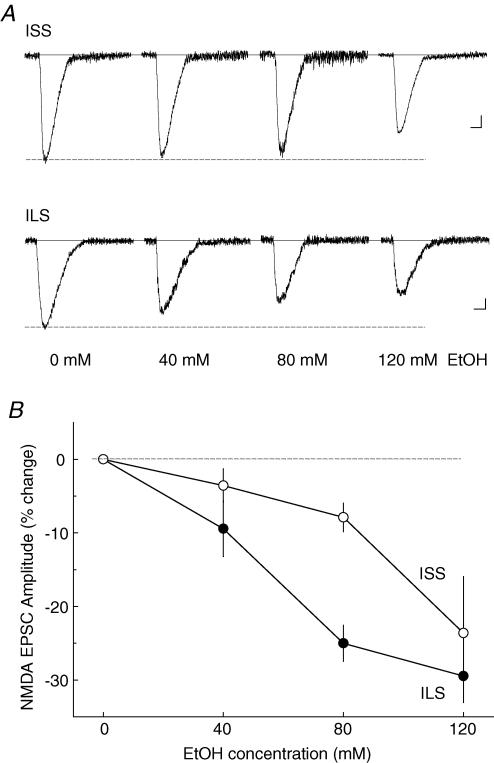
High concentrations of ethanol inhibited NMDA EPSCs in ISS mice
Ethanol concentrations of 40, 80 and 120 mm were used to test inhibition by ethanol of proximal NMDA EPSCs in hippocampal CA1 pyramidal neurons in ISS and ILS mouse strains. In ISS mice, ethanol had no significant effect at 40 mm and only ∼8% inhibition (P < 0.05) at 80 mm. However, at the highest concentration tested (120 mm), ethanol produced 23.6 ± 7.6% inhibition of the NMDA EPSC in these mice (Fig. 5A). Two-way ANOVA analysis of these data showed that there was a significant strain difference in the effect of ethanol (F1,34= 9.915, P < 0.005) and a significant ethanol-dose effect (F2,34= 11.282, P < 0.001). However, there was not a significant strain–dose interaction (F2,34= 2.260, P > 0.1). The approximate EC50 for this effect of ethanol in ISS mice is likely to be at least 100 mm, but this is an imprecise estimate because the maximum effect of ethanol may not have been attained at the highest concentration (120 mm) tested. In ILS mice, these ethanol concentrations produced a dose-dependent inhibition of the proximal NMDA EPSCs with maximal effects at about 80 mm ethanol (Fig. 5A), giving an estimated EC50 of approximately 50 mm ethanol (Fig. 5B).
Figure 5. Acute ethanol inhibits NMDA EPSCs from ISS and ILS mice in a concentration-dependent manner.
A, superfusion of ethanol (40, 80 or 120 mm) for 10 min decreased evoked NMDA EPSC amplitudes in both representative ISS and ILS mice. B, mean percentage inhibition of NMDA EPSC amplitudes shows differences in ethanol responses: ILS EPSCs are more sensitive to ethanol than those from ISS mice. Number of neurons for each ethanol concentration: 40 mm, n = 5; 80 mm, n = 6; 120 mm, n = 7, in ISS mice; and 40 mm, n = 5; 80 mm, n = 7; 120 mm, n = 6 in ILS mice. Scale for A: ISS, 50 ms, 20 pA; ILS, 50 ms, 15 pA.
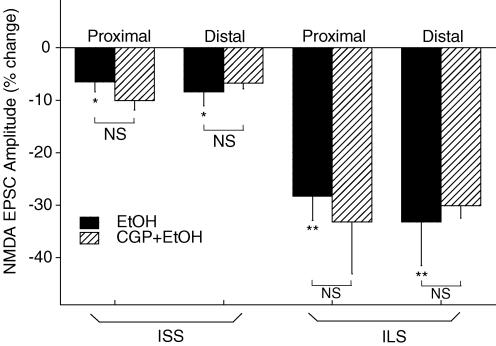
Effects of GABAB receptor blockade on inhibition by ethanol of NMDA EPSCs in ISS and ILS mice
The GABAB receptor antagonist, CGP-52432 (0.5 μm), alone did not have significant effects on either proximal or distal NMDA EPSCs in either ISS or ILS mice, nor did it alter any of the effects of ethanol on these NMDA responses from ISS and ILS mice (Fig. 6). Two-way ANOVA showed that there was a significant difference in the effects of ethanol and ethanol plus CGP-52432 treatments (F1,82= 15.746, P < 0.001), but that ethanol plus CGP-52432 did not further alter the effect of ethanol (F1,82= 1.209, P > 0.1). When the effect of ethanol plus CGP-52432 was compared to that of ethanol alone in the proximal and distal subregions of ISS and ILS mice, two-way ANOVA revealed that ethanol plus CGP-52432 did not have differential effects on subregions (F1,82= 0.922, P > 0.1), nor did CGP-52432 change the effect of ethanol on NMDA EPSCs in these two subregions (F1,82= 0.0475, P > 0.1). These data demonstrate that under these recording conditions, inhibition by ethanol of NMDA EPSCs in the hippocampal CA1 pyramidal neurons of ISS and ILS mice is not influenced by GABAB receptor activity.
Figure 6. Pretreatment with a GABAB receptor antagonist failed to alter the effects of ethanol on either proximal or distal NMDA EPSCs from ISS or ILS mice.
Ethanol (80 mm) produced only a small, but significant decrease in proximal and distal NMDA EPSCs from ISS mice, whereas ethanol alone produced large reductions of both proximal and distal NMDA EPSCs from ILS mice. Pretreatment with CGP-52432 did not significantly alter the effect of ethanol on these NMDA responses from either ISS or ILS mice. Therefore, these results indicate that there were no subregional differences in the effect of ethanol on NMDA EPSCs, and CGP-52432 treatment did not change the effect of ethanol in these two hippocampal subregions. Number of cells for these experiments ranged from 6 to 12 for each individual determination. *P < 0.05; **P < 0.01.
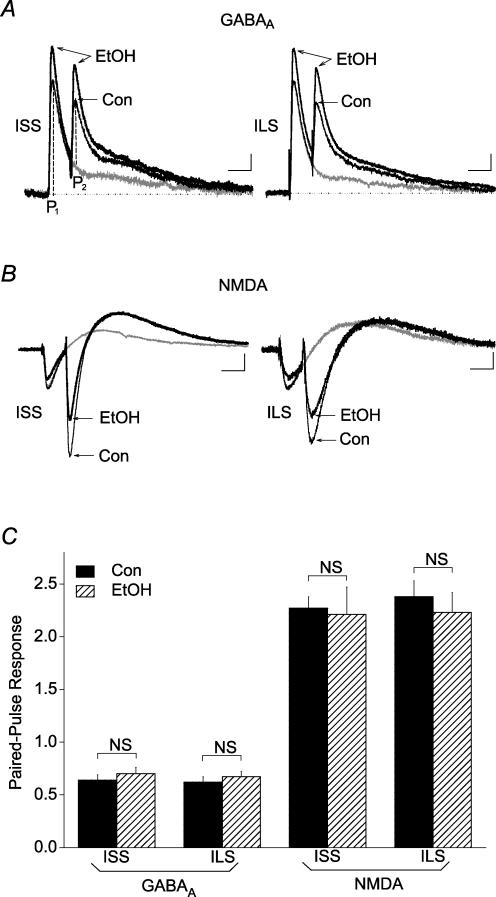
Effects of ethanol on paired-pulse ratios of GABAA IPSCs and NMDA EPSCs in the hippocampus of ISS and ILS mice
The potential presynaptic effects of ethanol on GABAergic GABAA and glutamatergic NMDA transmissions in the hippocampal CA1 pyramidal neurons were studied indirectly using a paired-pulse stimulation protocol. As 120 mm ethanol produced significant effects on GABAA IPSCs and NMDA EPSCs in both ISS and ILS mice, this concentration was used to test the effect of ethanol on the paired-pulse ratios (PPRs). Proximal paired-pulse stimulation with an interpulse interval of 50 ms produced PPRs of 0.62 ± 0.05 and 0.64 ± 0.05 (indicating paired-pulse depression, PPD) at the GABAergic terminals as calculated by the ratio of the peak responses from each stimulus) in ISS and ILS mice, respectively (Fig. 7A). Ethanol (120 mm) did not significantly affect PPRs at these synapses. The effect of GABAB receptor blockade on GABA release was also measured by paired-pulse stimulation of proximal and distal subregions of CA1 neurons using potassium gluconate-filled recording electrodes. In both subregions of CA1 neurons, paired-pulse stimulation produced PPD of GABAA IPSCs with no significant differences in PPRs between proximal and distal subregions (F1,113= 0.238, P > 0.05, two-way ANOVA comparing subregions and strains) or between ISS and ILS mice (F1,113= 0.373, P > 0.05, two-way ANOVA comparing subregions and strains) (Table 1). Ethanol alone or in combination with CGP-52432 did not significantly alter the PPR. Thus, these results indicate no difference in GABAB receptor-mediated GABA release between ISS and ILS mice.
Figure 7. Ethanol did not significantly alter the PPR recorded with potassium gluconate-filled electrodes at GABAergic or glutamatergic terminals from ISS or ILS mice.
Representative paired-pulse responses from proximal GABAA IPSCs (A) and NMDA EPSCs (B) from ISS and ILS mice before and during application of 120 mm ethanol. The PPR was calculated as the ratio of the peak amplitude (P2) generated by a second stimulus pulse, subtracting for any overlapping component due to the first response, divided by the amplitude of the response (P1) generated from the first pulse, as shown in A. C, summary data shows that although ethanol (120 mm) had significant effects on GABAA IPSCs and NMDA EPSCs from both ISS and ILS mice, as indicated by changes in P1, this concentration of ethanol did not significantly alter PPR values for GABAA IPSCs or NMDA EPSCs from either ISS or ILS mice. Number of cells in these experiments: ISS, GABAA, n = 6; ISS, NMDA, n = 7; ILS, GABAA, n = 7; ILS, NMDA, n = 6. Scales for A: ISS, 50 ms, 15 pA; ILS, 50 ms, 20 pA. Scales for B: ISS, 50 ms, 25 pA; ILS, 50 ms, 15 pA.
Table 1.
Effects of CGP-52432 and ethanol on GABAA IPSC paired-pulse ratios recorded from proximal and distal subregions of CA1 pyramidal neurons in ISS and ILS mice
| ISS | ILS | |||
|---|---|---|---|---|
| Proximal | Distal | Proximal | Distal | |
| Control | 0.64 ± 0.04 (8) | 0.56 ± 0.04 (6) | 0.63 ± 0.05 (8) | 0.72 ± 0.06 (11) |
| EtOH | 0.61 ± 0.05 (5) | 0.51 ± 0.04 (6) | 0.68 ± 0.04 (4) | 0.63 ± 0.04 (6) |
| CGP | 0.74 ± 0.06 (7) | 0.73 ± 0.05 (5) | 0.67 ± 0.08 (5) | 0.72 ± 0.02 (8) |
| CGP + EtOH | 0.73 ± 0.03 (8) | 0.72 ± 0.09 (8) | 0.64 ± 0.07 (8) | 0.77 ± 0.04 (10) |
Paired-pulse GABAA IPSCs were recorded with potassium gluconate-filled electrodes in the absence or the presence of ethanol (80 mm), CGP-52432 (CGP, 0.5 μm) or ethanol (EtOH) plus CGP-52432. The GABAA IPSC amplitude evoked by the second pulse was divided by the amplitude evoked by the first pulse to obtain a paired-pulse ratio. Paired-pulse depression was found in all GABAergic terminals recorded with these electrodes. The results are means ±s.e.m. of the number of neurons indicated in parentheses. No significant effect of treatment with respect to CA1 subregion or strain was indicated.
When PPRs for NMDA EPSCs at proximal glutamatergic terminals were measured, paired-pulse stimulation produced PPRs of 2.27 ± 0.08 and 2.38 ± 0.15 in ISS and ILS mice, respectively, indicating paired-pulse facilitation (PPF) at these glutamatergic terminals (Fig. 7B). Ethanol (120 mm) also did not significantly alter the PPRs. As there were no subregional differences in NMDA EPSCs and no effects of GABAB blockade on NMDA EPSCs, PPRs were not determined for these EPSC responses. Therefore, although ethanol (120 mm) had significant effects on GABAA IPSCs and NMDA EPSCs, it did not significantly affect the measured PPRs at these nerve terminals (Fig. 7C). Therefore, ethanol appeared to affect sensitivity measures via postsynaptic mechanisms.
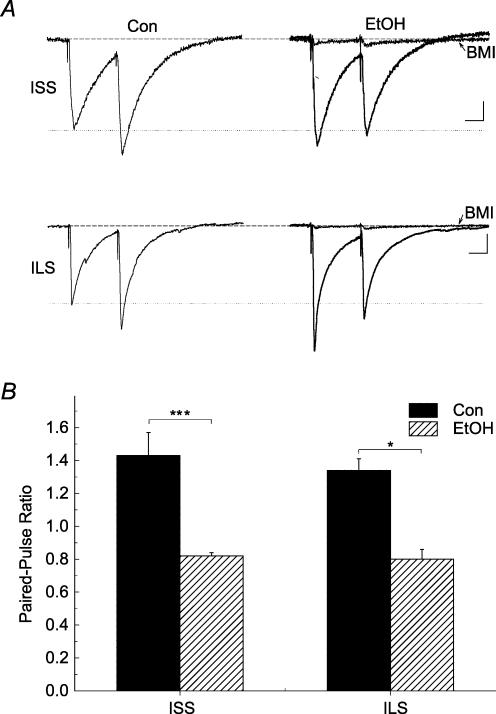
Modulation by ethanol of PPR determinations at GABAergic terminals has been reported previously, therefore we further investigated this result using the CsCl recording conditions reported by Sanna et al. (2004). In both mouse strains, recording with CsCl-filled electrodes produced outward currents and revealed significantly more robust effects of enhancement by ethanol (80 mm) of GABAA IPSCs (Table 2). While paired-pulse stimulation of proximal pathways to CA1 pyramidal neurons recorded with the standard potassium gluconate-filled electrodes resulted in inward currents and PPD, recording with CsCl-filled electrodes produced outward currents and PPF in 5 of 7 cells recorded from ILS mice and in 5 of 6 cells from ISS mice. Under these recording conditions, we measured the effect of ethanol on the PPR at these GABAergic synapses that showed PPF using CsCl-filled electrodes. Figure 8A shows that ethanol had significant overall effects on PPRs (F4,22= 16.992, P < 0.001, one-way ANOVA). Under control conditions, PPRs were similar between ISS and ILS mice (P > 0.1). Ethanol (80 mm) significantly decreased PPRs in ISS (P < 0.001) and ILS (P < 0.001) mice. In ISS mice, ethanol (120 mm) also decreased PPRs compared with those of controls (P < 0.001), but it did not decrease PPRs further than those seen in the presence of 80 mm ethanol (P > 0.1). When spontaneous GABAA IPSCs were measured with CsCl-filled electrodes in ISS and ILS mice, the data showed no significant difference in the effect of ethanol on the frequency or amplitude of spontaneous events (Table 3). Thus, our results showed that ethanol (80 mm) decreased PPRs and increased spontaneous GABAA IPSC frequency by 40–50% in both ISS and ILS mice, when these responses were recorded with CsCl-filled electrodes. This is consistent with an enhanced probability of release of GABA by ethanol. However, we saw no strain differences in this effect.
Table 2.
Ethanol enhancement of the proximal GABAA IPSC amplitudes recorded with electrodes filled with potassium gluconate or CsCl
| ISS (120 mm ethanol) | ILS (80 mm ethanol) | ||
|---|---|---|---|
| Potassium gluconate | CsCl | Potassium gluconate | CsCl |
| 28.53 ± 6.03 (6) | 59.78 ± 13.38 (4)* | 38.11 ± 2.67 (7) | 65.55 ± 12.43 (6)* |
Synaptically evoked GABAA IPSCs in CA1 pyramidal neurons were recorded from ISS and ILS mice using potassium gluconate- or CsCl-filled electrodes in the absence or presence of ethanol (80 or 120 mm, respectively). The results (% enhancement from control) are means ±s.e.m. for the number of neurons recorded (in parentheses).
P < 0.05.
Figure 8. Recording with CsCl-filled electrodes showed presynaptic effects of ethanol on GABAergic terminals from ISS and ILS mice.
Representative paired-pulse responses from proximal GABAA IPSCs from ISS and ILS mice (A) before and during application of 80 mm ethanol recorded using CsCl-filled electrodes. The PPR was determined as described in Figure 7A. The effect of BMI on ethanol-treated GABAA IPSCs shows almost complete inhibition for both ISS and ILS mice. B, summary data showing that ethanol significantly decreased PPR of GABAA IPSCs from both ISS and ILS mice. Number of cells recorded: ISS, GABAA, n = 5; ILS, GABAA, n = 5. Scales for A: ISS, 20 ms, 20 pA; ILS, 20 ms, 25 pA.
Table 3.
Effects of ethanol on frequency and amplitude of spontaneous IPSCs
| Frequency | Amplitude | ||
|---|---|---|---|
| ISS | ILS | ISS | ILS |
| 52.9 ± 3.85 (5) | 53.5 ± 8.86 (5) | 7.1 ± 3.98 (5) | 2.5 ± 3.35 (5) |
Spontaneous GABAA IPSCs in CA1 pyramidal neurons were recorded from ISS and ILS mice using CsCl-filled electrodes in the absence or presence of ethanol (80 mm). The results (% change from control) are means ±s.e.m. for the number of neurons recorded (in parentheses). No significant difference was measured between ISS and ILS mice.
Local application of GABA or glutamate
To further examine the effects of ethanol on postsynaptic GABAA and NMDA receptor–ion channel complexes, application of GABA or glutamate was made via a glass micropipette to the CA1 pyramidal cell dendrites in the stratum radiatum. Under these conditions, receptor agonist-evoked GABAA and NMDA receptor-mediated responses were obtained (Fig. 9). Application of GABA (10 mm) in the presence of the GABAB antagonist, CGP-52432 (0.5 μm), evoked GABAA responses that could be completely blocked by BMI (20 μm). Ethanol (80 mm) differentially enhanced these GABAA responses from ISS and ILS neurons (t = 2.389, P < 0.05], indicating that enhancement by ethanol of the postsynaptic GABAA receptor-mediated responses could account for the major differences in the alcohol sensitivity of the GABAergic interneuronal pathway between ISS and ILS mice.
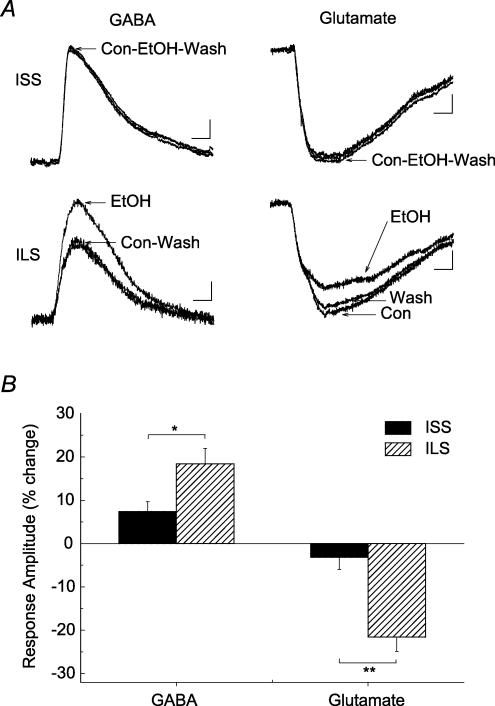
Figure 9. Ethanol enhances the GABA-evoked GABAA receptor-mediated response and inhibits the glutamate-evoked NMDA receptor-mediated response in hippocampal CA1 neurons from ILS but not ISS mice.
A, representative current traces from hippocampal CA1 neurons from ILS and ISS mice. Brief local applications of GABA (10 mm) in the presence of CGP-52432 (0.5 μM) or glutamate (0.3 mm, plus 3 μm glycine) in the presence of CNQX (20 μm) produced robust GABAA receptor-mediated or NMDA receptor-mediated responses, respectively. Ethanol (80 mm) (20 μm) perfusion enhanced GABAA responses and inhibited NMDA responses in neurons from ILS mice. This concentration of ethanol did not significantly alter these responses in neurons from ISS mice. B, summary data showing that ethanol significantly affected these agonist-evoked responses in neurons from ILS but not ISS mice. Number of cells in these experiments: ISS, GABAA, n = 6; ISS, NMDA, n = 6; ILS, GABAA, n = 8; ILS, NMDA, n = 6. Scales for GABA-evoked responses: ISS, 50 ms, 20 pA; ILS, 50 ms, 10 pA. Scales for glutamate-evoked responses: ISS, 50 ms, 15 pA; ILS, 50 ms, 15 pA.
Pressure ejection of glutamate (0.3 mm) near pyramidal cell dendrites, in the presence of CNQX (20 μm) in the superfusion, produced NMDA responses that were blocked by the NMDA antagonist, D-APV (25 μm). These glutamate-evoked NMDA responses were attenuated differentially by ethanol (80 mm) in the two mice strains. Ethanol inhibited the response by 21.6 ± 3.32% (n = 6) in ILS neurons, whereas it only decreased the response by 3.2 ± 2.81% (n = 6) in cells from ISS mice (t = 4.229, P < 0.005). Therefore, the behaviourally ethanol-sensitive ILS mice were also more sensitive to the inhibitory effect of ethanol on postsynaptic and possibly extrasynaptic NMDA responses.
Discussion
The data presented here significantly increase our knowledge of the mechanisms of acute alcohol sensitivity at the molecular level. We have shown that ISS and ILS mouse strains that display large differences in sensitivity to the behavioural effects of ethanol also show very marked differences in sensitivity to the effects of ethanol on the NMDA and the GABAA receptor complexes. We have previously demonstrated that GABAergic neurotransmission was a likely molecular target underlying the differential behavioural sensitivity to ethanol in ILS and ISS mice (Poelchen et al. 2000; Proctor et al. 2004). However it was not known whether the differential effects of ethanol on GABA responses in these mice was due to effects on GABA release or on the GABAA or GABAB receptor systems. We now clearly show, using local application of exogenous GABA, that ethanol has differential effects on the GABAA receptor itself in ILS and ISS mice. These data also provide the first conclusive demonstration in situ that ethanol does in fact potentiate the GABAA receptor-mediated current. The data also show that the NMDA receptor–ion channel complex is differentially sensitivity to ethanol in the ILS and ISS mice. These new findings could provide important insights into the molecular basis of the genetic predisposition to alcoholism, because individuals with low initial sensitivity to the effects of ethanol on cognition and motor behaviours have a higher risk for alcoholism later in life than those with a high initial ethanol sensitivity (Schuckit, 1994; Schuckit et al. 2004).
As the effects of ethanol on GABAA IPSCs were inversely related to the extent of GABAB receptor influences (Wu et al. 2005), strain-related differences in GABAB receptors may explain the differential ethanol sensitivity between ISS and ILS mice. However, the fact that GABAB receptor blockade affected only the GABAA IPSCs in ILS but not in ISS mice suggests that under control conditions (in the absence of GABAB receptor blockade) GABAB receptors most probably do not contribute to the observed differences in ethanol sensitivity between these two strains of mice. One of the known functions of GABAB receptors is the ability to regulate release of GABA (i.e. autoreceptors: Waldmeier et al. 1988; Raiteri et al. 1989; Baumann et al. 1990; Dutar & Nicoll, 1993), and ethanol was shown to affect GABA release by GABAB receptor-mediated mechanisms (Ariwodola & Weiner, 2004). Our data show that, recording with potassium gluconate-filled electrodes, GABAB receptor blockade did not significantly alter the PPRs of GABAA IPSCs in the proximal or the distal subregions of CA1 neurons from ISS and ILS mice, suggesting that presynaptic GABAB receptors may not be responsible for differential ethanol sensitivity. Therefore, differences in ethanol sensitivity are most probably due to postsynaptic GABAA receptor mechanisms. To investigate this possibility, we measured the effect of ethanol on GABAA receptor responses which were evoked by brief local application of GABA in ISS and ILS mice. The results showed that ethanol potentiated GABA-evoked responses in ILS but not in ISS mice, suggesting that differences in ethanol sensitivity of GABAergic responses may be mediated by postsynaptic GABAA receptor-mediated mechanisms. Detailed analysis of the differences in GABAA receptor subunit composition (Wallner et al. 2003), GABAA receptor ethanol binding sites (Foster et al. 2004) and/or modulation by protein kinase of this receptor may be required to fully explain the difference in ethanol sensitivity of GABAA IPSCs between ISS and ILS mice. As Zahniser et al. (1992) found no significant differences in GABAA receptor subunit mRNAs in LS and SS mouse hippocampus, it appears unlikely that differences in GABAA receptor sequence could explain the differential sensitivity of GABAA IPSCs to ethanol in these mice. However, Zahniser et al. (1992) used an mRNA preparation from mouse whole hippocampus, and therefore any subtle differences in CA1 pyramidal neuron expression of GABAA receptor subunits may not be readily detected. Therefore, strain-related differences in GABAA receptor subunit composition remain the most likely cause of differential ethanol sensitivity.
Ethanol is known to inhibit NMDA receptor function (Lovinger, 1997; Allgaier, 2002), and so differences in inhibition by ethanol of NMDA receptors may also contribute to differential behavioural sensitivity in these mice. Ethanol produced greater inhibition of the binding of MK-801 (an NMDA receptor channel blocker) in LS than in SS mice (Musleh et al. 1996). Therefore, ethanol may differentially affect NMDA EPSCs in these mice. Indeed, ethanol inhibited NMDA EPSCs more in ILS than in ISS mice, suggesting that glutamate–NMDA systems in hippocampal CA1 pyramidal neurons from highly ethanol sensitive ILS mice are more sensitive to ethanol than those from poorly ethanol sensitive ISS mice. Evidence from studies in rats indicates that CA1 pyramidal cells receive two major excitatory inputs: the perforant path that terminates primarily in the distal dendrites, and the Schaffer collaterals that terminate more proximally (Otmakhova & Lisman, 2004). Also, GABAB receptors exert more inhibitory influences on the distal NMDA EPSCs (Morrisett et al. 1991; Otmakhova & Lisman, 2004) and if ethanol sensitivity of NMDA EPSCs was also regulated by GABAB receptors, one would anticipate that ethanol sensitivity may be altered during GABAB receptor blockade. However, CGP-52432 did not significantly alter the effect of ethanol on NMDA EPSCs in these two strains of mice, nor did it change the effect of ethanol on the proximal and distal NMDA EPSCs, suggesting that GABAB receptor activity is not involved in the differential inhibition by ethanol of NMDA EPSCs in these mice. As inhibition by ethanol of NMDA responses evoked by local application of glutamate during AMPA blockade occurred in neurons from ILS but not ISS mice, the data suggest that ethanol exerts its actions mainly via inhibition of the postsynaptic NMDA responses.
There appears to be a discrepancy between our data and those of others with respect to the effects of ethanol on presynaptic release of GABA in the hippocampus (Ariwodola & Weiner, 2004; Sanna et al. 2004) and amygdala (Roberto et al. 2003; Siggins et al. 2005) in rats. Whether this presynaptic effect of ethanol on GABAA IPSCs is unique to an animal strain or species was investigated. One notable technical difference between our experiments and those of other investigators was the use of CsCl-filled electrodes (Jiang et al. 2000; Sanna et al. 2004; Ariwodola & Weiner, 2004) or KCl-filled electrodes (Roberto et al. 2003) to record from pyramidal or amygdala neurons. Others demonstrated PPF and a significant effect of ethanol on paired-pulse measurements at GABAergic terminals (Roberto et al. 2003; Sanna et al. 2004; Ariwodola & Weiner, 2004) in contrast to our results of showing PPD and lack of an effect of ethanol on paired-pulse measures. Therefore, we repeated our earlier experiments, but recording with CsCl-filled electrodes from ISS and ILS mice. The majority of cells recorded showed GABAergic PPF, and the effect of ethanol on GABAA IPSCs was now increased in both strains of mice. It is important to note that under this recording condition, ethanol now showed presynaptic enhancement, consistent with previous reports, yet the presynaptic effect of 80 mm ethanol on the GABAergic terminals that show PPF, was not different between ISS and ILS mice (Fig. 8B), suggesting that, at the majority of the GABAergic terminals, presynaptic effects of ethanol do not account for the differential ethanol sensitivity between these two strains of mice. Interestingly, these results also suggest that CsCl loading of CA1 pyramidal neurons sensitized GABAA IPSCs to the effect of ethanol. As this effect of ethanol was completely blocked by BMI (20 μm; Fig. 8A), the measured increase in GABAA IPSCs (using CsCl-filled electrodes) was unlikely to be due to modulation by ethanol of other non-specific chloride currents. High intracellular Cl− concentrations have been shown to depress G-protein-modulated ionic conductances (Lenz et al. 1997), suppress K+ currents in astrocytes (Bekar & Walz, 1999; Bekar et al. 2005), and alter various electrophysiological characteristics of excitable cells (Baker et al. 1962; Adams & Oxford, 1983; Nakajima et al. 1992), suggesting that increased intracellular Cl− concentrations (using CsCl-filled or KCl-filled electrodes) could alter GABA-mediated cellular responses. It is difficult to speculate on the mechanisms by which CsCl loading of the neurons could enhance GABA presynaptic transmission. However, a GABA self-signalling mechanism has been described, such that auto-transmission between adjacent GABAergic hippocampal neurons was dependent on the chloride gradient, and this mechanism of synaptic transmission occurred on both sides of the same synaptic cleft and could function as a form of immediate presynaptic action (Vautrin et al. 1994). Therefore, if ethanol enhances this form of self-signalling, CsCl loading of neurons may increase GABA presynaptic transmission. Similarly, our paired-pulse study of the effect of ethanol on NMDA EPSCs, which were recorded with potassium gluconate-filled electrodes, also did not show any involvement of presynaptic glutamate mechanisms. Although Hendricson et al. (2004) showed that ethanol reduced glutamate release in rat accumbens neurons under certain recording conditions (CsF-filled electrodes and in the presence of 4 mm Sr2+), our present data suggest that differential ethanol sensitivity of ISS and ILS mice is most probably mediated by the effect of ethanol on postsynaptic GABAA and NMDA receptors.
It has been shown previously that differences in GABAA receptor subunit assembly may underlie differences in behavioural ethanol sensitivity (Blednov et al. 2003; Hanchar et al. 2004; Harris & Mihic, 2004). As ethanol sensitivity of NMDA receptors was also different between ISS and ILS mice, the question of whether other ethanol-sensitive ligand-gated ion channels (e.g. 5-HT3 and nicotinic receptor ion channel complexes, de Fiebre et al. 1987) may also be differentially altered by ethanol remains to be answered. Alternatively, it is possible that ISS and ILS mice differ in their effects of ethanol at common modulatory systems, such as different protein kinase C (PKC) isozymes, which may underlie the ethanol modulation at GABAA IPSCs, NMDA EPSCs and perhaps other ligand-gated ion channels in the brain. The fact that the PKC isozyme knockout mice showed differential ethanol sensitivity of GABAA IPSCs (Proctor et al. 2003) and NMDA EPSCs (PH Wu, AT Moritz, RK Freund, RO Messing, BJ Bowessi, JM Wehner & WR Proctor et al. unpublished data) suggests that effects of ethanol on common modulatory mechanisms may underlie differential ethanol sensitivity between ISS and ILS mice, and we are currently investigating this possibility.
In summary, the data presented here offer very striking examples of differences in molecular sensitivity to ethanol in strains of mice selected for their behavioural differences in ethanol sensitivity. Given the crucial role played by these molecular targets (i.e. the GABAA and NMDA receptor complexes) in virtually all brain regions, it is likely that these molecular differences in ethanol sensitivity are responsible, at least in part, for the behavioural differences in ethanol sensitivity seen in the ILS and ISS mice. This new body of data thus provides important new information about the mechanisms that underlie alcohol sensitivity, a parameter which has strong predictive value for the vulnerability to alcoholism in humans (Schuckit, 1994; Schuckit et al. 2004). Moreover, these results may also provide clues about the likely mechanism(s) by which ethanol exerts it effects on these molecular targets. It is possible that the behaviour-based selection of these mice leads to alterations in ethanol binding pockets in both NMDA and GABAA receptors. However, we favour a more parsimonious explanation that the selection leads to alterations in a single cellular target of ethanol that in turn influences both the NMDA and GABAA receptor–channel complexes. Given that phosphorylation enhances the NMDA receptor while it often leads to inhibition of the GABAA receptor, an inhibitory effect of ethanol on phosphorylation signalling could explain inhibition of the NMDA receptor and potentiation of the GABAA receptor systems by ethanol. Whatever, the ultimate explanation of these effects, these two strains of mice should prove invaluable for studies designed to probe the mechanism of action of ethanol on the NMDA and GABAA receptor–ion channel neurotransmitter systems.
Acknowledgments
This research is supported by the funds made available to us by a Veteran Affairs merit review grant, NIH/National Institute on Alcohol Abuse and Alcoholism grants (AA015086 and AA014691) and a Colorado Tobacco Research Program grant (4I-007).
References
- Adams DJ, Oxford GS. Interaction of internal anions with potassium channels of the squid giant axon. J Gen Physiol. 1983;82:429–448. doi: 10.1085/jgp.82.4.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allan AM, Harris RA. Gamma-aminobutyric acid and alcohol actions: neurochemical studies of long sleep and short sleep mice. Life Sci. 1986;39:2005–2015. doi: 10.1016/0024-3205(86)90324-3. [DOI] [PubMed] [Google Scholar]
- Allgaier C. Ethanol sensitivity of NMDA receptors. Neurochem Int. 2002;41:377–382. doi: 10.1016/s0197-0186(02)00046-3. [DOI] [PubMed] [Google Scholar]
- Ariwodola OJ, Weiner JL. Ethanol potentiation of GABAergic synaptic transmission may be self-limiting: role of presynaptic GABAB receptors. J Neurosci. 2004;24:10679–10686. doi: 10.1523/JNEUROSCI.1768-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker PF, Hodgkin AL, Shaw TI. The effects of changes in internal ionic concentrations on the electrical properties of perfused giant axons. J Physiol. 1962;164:355–374. doi: 10.1113/jphysiol.1962.sp007026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann PA, Wicki P, Stierlin C, Waldmeier PC. Investigations on GABAB receptor-mediated autoinhibition of GABA release. Naunyn Schmiedebergs Arch Pharmacol. 1990;341:88–93. doi: 10.1007/BF00195063. [DOI] [PubMed] [Google Scholar]
- Bekar LK, Loewen ME, Forsyth GW, Walz W. Chloride concentration affects Kv channel voltage-gating kinetics: Importance of experimental anion concentrations. Brain Res Bull. 2005;67:142–146. doi: 10.1016/j.brainresbull.2005.06.023. [DOI] [PubMed] [Google Scholar]
- Bekar LK, Walz W. Evidence for chloride ions as intracellular messenger substances in astrocytes. J Neurophysiol. 1999;82:248–254. doi: 10.1152/jn.1999.82.1.248. [DOI] [PubMed] [Google Scholar]
- Bennett B. Congenic strains developed for alcohol- and drug-related phenotypes. Pharmacol Biochem Behav. 2000;67:671–681. doi: 10.1016/s0091-3057(00)00412-3. [DOI] [PubMed] [Google Scholar]
- Bennett B, Beeson M, Gordon L, Carosone-Link P, Johnson TE. Genetic dissection of quantitative trait loci specifying sedative/hypnotic sensitivity to ethanol: mapping with interval-specific congenic recombinant lines. Alcohol Clin Exp Res. 2002;26:1615–1624. doi: 10.1097/01.ALC.0000037136.49550.B3. [DOI] [PubMed] [Google Scholar]
- Bennett B, Johnson TE. Development of congenics for hypnotic sensitivity to ethanol by QTL-marker-assisted counter selection. Mamm Genome. 1998;9:969–974. doi: 10.1007/s003359900908. [DOI] [PubMed] [Google Scholar]
- Blednov YA, Walker D, Alva H, Creech K, Findlay G, Harris RA. GABAA receptor α1 and β2 subunit null mutant mice: behavioral responses to ethanol. J Pharmacol Exp Ther. 2003;305:854–863. doi: 10.1124/jpet.103.049478. [DOI] [PubMed] [Google Scholar]
- Collins AC, Wehner JM, Wilson WR. Animal models of alcoholism: genetic strategies and neurochemical mechanisms. Biochem Soc Symp. 1993;59:173–191. [PubMed] [Google Scholar]
- Crabbe JC. Genetic contributions to addiction. Annu Rev Psychol. 2002;53:435–462. doi: 10.1146/annurev.psych.53.100901.135142. [DOI] [PubMed] [Google Scholar]
- Daniell LC, Phillips TJ. Differences in ethanol sensitivity of brain NMDA receptors of long-sleep and short-sleep mice. Alcohol Clin Exp Res. 1994;18:1482–1490. doi: 10.1111/j.1530-0277.1994.tb01454.x. [DOI] [PubMed] [Google Scholar]
- Davies DL, Alkana RL. Ethanol enhances GABAA receptor function in short sleep and long sleep mouse brain membranes. Alcohol Clin Exp Res. 2001;25:478–483. [PubMed] [Google Scholar]
- de Fiebre CM, Medhurst LJ, Collins AC. Nicotine response and nicotinic receptors in long-sleep and short-sleep mice. Alcohol. 1987;4:493–501. doi: 10.1016/0741-8329(87)90092-9. [DOI] [PubMed] [Google Scholar]
- Deitrich RA. Selective breeding for initial sensitivity to ethanol. Behav Genet. 1993;23:153–162. doi: 10.1007/BF01067420. [DOI] [PubMed] [Google Scholar]
- Deitrich RA, Dunwiddie TV, Harris RA, Erwin VG. Mechanism of action of ethanol: initial central nervous system actions. Pharmacol Rev. 1989;41:489–537. [PubMed] [Google Scholar]
- Dunwiddie TV. The use of in vitro brain slices in neuropharmacology. In: Geller HM, editor. Electrophysiological Techniques in Pharmacology. New York: Alan R. Liss; 1986. pp. 65–90. [Google Scholar]
- Dutar P, Nicoll RA. GABA-B receptor-mediated inhibition of synaptic transmission in the hippocampus: pharmacology and intracellular mechanisms. In: Dunwiddie T V, Lovinger DM, editors. Presynaptic Receptors in the Mammalian Brain. Boston: Birkhauser; 1993. pp. 14–26. [Google Scholar]
- Foster KL, McKay PF, Seyoum R, Milbourne D, Yin WY, Sarma PVVS, Cook JM, June HL. GABAA and opioid receptors of the central nucleus of the amygdala selectively regulate ethanol-maintained behaviors. Neuropsychopharmacology. 2004;29:269–284. doi: 10.1038/sj.npp.1300306. [DOI] [PubMed] [Google Scholar]
- Geiger JR, Jonas P. Dynamic control of presynaptic Ca2+ inflow by fast-inactivating K+ channels in hippocampal mossy fiber boutons. Neuron. 2000;28:927–939. doi: 10.1016/s0896-6273(00)00164-1. [DOI] [PubMed] [Google Scholar]
- Hanania T, Negri CA, Dunwiddie TV, Zahniser NR. N-methyl-D-aspartate receptor responses are differentially modulated by noncompetitive receptor antagonists and ethanol in inbred long-sleep and short-sleep mice: behavior and electrophysiology. Alcohol Clin Exp Res. 2000;24:1750–1758. [PubMed] [Google Scholar]
- Hanchar HJ, Wallner M, Olsen RW. Alcohol effects on gamma-aminobutyric acid type A receptors: are extrasynaptic receptors the answer? Life Sci. 2004;76:1–8. doi: 10.1016/j.lfs.2004.05.035. [DOI] [PubMed] [Google Scholar]
- Harris RA. Ethanol actions on multiple ion channels: which are important? Alcohol Clin Exp Res. 1999;23:1563–1570. [PubMed] [Google Scholar]
- Harris RA, Mihic SJ. Alcohol and inhibitory receptors: unexpected specificity from a nonspecific drug. Proc Natl Acad Sci U S A. 2004;101:2–3. doi: 10.1073/pnas.0307281101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendricson AW, Sibbald JR, Morrisett RA. Ethanol alters the frequency, amplitude, and decay kinetics of Sr2+-supported, asynchronous NMDAR mEPSCs in rat hippocampal slices. J Neurophysiol. 2004;91:2568–2577. doi: 10.1152/jn.00997.2003. [DOI] [PubMed] [Google Scholar]
- Jiang L, Sun SD, Nedergaard M, Kang J. Paired-pulse modulation at individual GABAergic synapses in rat hippocampus. J Physiol. 2000;523:425–439. doi: 10.1111/j.1469-7793.2000.t01-1-00425.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenz RA, Pitler TA, Alger BE. High intracellular Cl− concentrations depress G-protein-modulated ionic conductances. J Neurosci. 1997;17:6133–6141. doi: 10.1523/JNEUROSCI.17-16-06133.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovinger DM. Alcohols and neurotransmitter gated ion channels: past, present and future. Naunyn Schmiedebergs Arch Pharmacol. 1997;356:267–282. doi: 10.1007/pl00005051. [DOI] [PubMed] [Google Scholar]
- Morrisett RA, Mott DD, Lewis DV, Swartzwelder HS, Wilson WA. GABAB-receptor-mediated inhibition of the N-methyl-D-aspartate component of synaptic transmission in the rat hippocampus. J Neurosci. 1991;11:203–209. doi: 10.1523/JNEUROSCI.11-01-00203.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musleh W, Alvarez S, Baudry M, Alkana RL. Effects of ethanol and temperature on NMDA receptor function in different mouse genotypes. Alcohol Clin Exp Res. 1996;20:1299–1304. doi: 10.1111/j.1530-0277.1996.tb01126.x. [DOI] [PubMed] [Google Scholar]
- Nakajima T, Sugimoto T, Kurachi Y. Effects of anions on the G protein-mediated activation of the muscarinic K+ channel in the cardiac atrial cell membrane. Intracellular chloride inhibition of the GTPase activity of GK. J Gen Physiol. 1992;99:665–682. doi: 10.1085/jgp.99.5.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otmakhova NA, Lisman JE. Contribution of Ih and GABAB to synaptically induced afterhyperpolarizations in CA1: a brake on the NMDA response. J Neurophysiol. 2004;92:2027–2039. doi: 10.1152/jn.00427.2004. [DOI] [PubMed] [Google Scholar]
- Poelchen W, Proctor WR, Dunwiddie TV. The in vitro ethanol sensitivity of hippocampal synaptic gamma-aminobutyric acidA responses differs in lines of mice and rats genetically selected for behavioral sensitivity or insensitivity to ethanol. J Pharmacol Exp Ther. 2000;295:741–746. [PubMed] [Google Scholar]
- Proctor WR, Dunwiddie TV. Electrophysiological analysis of G protein-coupled receptors in mammalian neurons. In: Enna SJ, Williams M, editors. Current Protocols in Pharmacology. New York: John Wiley & Sons Inc.; 1999. pp. 11.2.1–11.2.22. [DOI] [PubMed] [Google Scholar]
- Proctor WR, Poelchen W, Bowers BJ, Wehner JM, Messing RO, Dunwiddie TV. Ethanol differentially enhances hippocampal GABAA receptor-mediated responses in protein kinase C gamma (PKC gamma) and PKC epsilon null mice. J Pharmacol Exp Ther. 2003;305:264–270. doi: 10.1124/jpet.102.045450. [DOI] [PubMed] [Google Scholar]
- Proctor WR, Wu PH, Bennett B, Johnson TE. Differential effects of ethanol on gamma-aminobutyric acid-A receptor-mediated synaptic currents in congenic strains of inbred long and short-sleep mice. Alcohol Clin Exp Res. 2004;28:1277–1283. doi: 10.1097/01.alc.0000139816.32706.f1. [DOI] [PubMed] [Google Scholar]
- Raiteri M, Bonanno G, Fedele E. Release of gamma-[3H]aminobutyric acid (GABA) from electrically stimulated rat cortical slices and its modulation by GABAB autoreceptors. J Pharmacol Exp Ther. 1989;250:648–653. [PubMed] [Google Scholar]
- Roberto M, Madamba SG, Moore SD, Tallent MK, Siggins GR. Ethanol increases GABAergic transmission at both pre- and postsynaptic sites in rat central amygdala neurons. Proc Natl Acad Sci U S A. 2003;100:2053–2058. doi: 10.1073/pnas.0437926100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanna E, Talani G, Busonero F, Pisu MG, Purdy RH, Serra M, Biggio G. Brain steroidogenesis mediates ethanol modulation of GABAA receptor activity in rat hippocampus. J Neurosci. 2004;24:6521–6530. doi: 10.1523/JNEUROSCI.0075-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuckit MA. Low level of response to alcohol as a predictor of future alcoholism. Am J Psychiatry. 1994;151:184–189. doi: 10.1176/ajp.151.2.184. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Smith TL, Kalmijn J. The search for genes contributing to the low level of response to alcohol: patterns of findings across studies. Alcohol Clin Exp Res. 2004;28:1449–1458. doi: 10.1097/01.alc.0000141637.01925.f6. [DOI] [PubMed] [Google Scholar]
- Siggins GR, Roberto M, Nie Z. The tipsy terminal: presynaptic effects of ethanol. Pharmacol Ther. 2005;107:80–98. doi: 10.1016/j.pharmthera.2005.01.006. [DOI] [PubMed] [Google Scholar]
- Vautrin J, Schaffner AE, Barker JL. Fast presynaptic GABAA receptor-mediated Cl− conductance in cultured rat hippocampal neurones. J Physiol. 1994;479:53–63. doi: 10.1113/jphysiol.1994.sp020277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velardo MJ, Simpson VJ, Zahniser NR. Differences in NMDA receptor antagonist-induced locomotor activity and [3H]MK-801 binding sites in short-sleep and long-sleep mice. Alcohol Clin Exp Res. 1998;22:1509–1515. [PubMed] [Google Scholar]
- Waldmeier PC, Wicki P, Feldtrauer JJ, Baumann PA. Potential involvement of a baclofen-sensitive autoreceptor in the modulation of the release of endogenous GABA from rat brain slices in vitro. Naunyn Schmiedebergs Arch Pharmacol. 1988;337:289–295. doi: 10.1007/BF00168841. [DOI] [PubMed] [Google Scholar]
- Wallner M, Hanchar HJ, Olsen RW. Ethanol enhances alpha 4 beta 3 delta and alpha 6 beta 3 delta gamma-aminobutyric acid type A receptors at low concentrations known to affect humans. Proc Natl Acad Sci U S A. 2003;100:15218–15223. doi: 10.1073/pnas.2435171100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner JL. Electrophysiological assessment of synaptic transmission in brain slices. In: Liu Y, Lovinger DM, editors. Methods in Alcohol-Related Neuroscience Research. Boca Raton FL: CRC Press LLC; 2002. pp. 191–218. [Google Scholar]
- Weiner JL, Gu C, Dunwiddie TV. Differential ethanol sensitivity of subpopulations of GABAA synapses onto rat hippocampal CA1 pyramidal neurons. J Neurophysiol. 1997;77:1306–1312. doi: 10.1152/jn.1997.77.3.1306. [DOI] [PubMed] [Google Scholar]
- Wilson WR, Bosy TZ, Ruth JA. NMDA agonists and antagonists alter the hypnotic response to ethanol in LS and SS mice. Alcohol. 1990;7:389–395. doi: 10.1016/0741-8329(90)90021-4. [DOI] [PubMed] [Google Scholar]
- Wilson WR, Collins AC. Different levels of [3H]MK-801 binding in long-sleep and short-sleep lines of mice. Alcohol. 1996;13:315–320. doi: 10.1016/0741-8329(95)02113-2. [DOI] [PubMed] [Google Scholar]
- Wu PH, Poelchen W, Proctor WR. Differential GABA-A receptor modulation of ethanol effects on GABA-A synaptic activity in hippocampal CA1 neurons. J Pharmacol Exp Ther. 2005;312:1082–1089. doi: 10.1124/jpet.104.075663. [DOI] [PubMed] [Google Scholar]
- Zahniser NR, Buck KJ, Curella P, McQuilkin SJ, Wilson-Shaw D, Miller CL, Klein RL, Heidenreich KA, Keir WJ, Sikela JM. GABAA receptor function and regional analysis of subunit mRNAs in long-sleep and short-sleep mouse brain. Brain Res Mol Brain Res. 1992;14:196–206. doi: 10.1016/0169-328x(92)90174-a. [DOI] [PubMed] [Google Scholar]