Abstract
The fluorescent styryl dyes FM1-43 and FM2-10 have been used to visualize the endocytic and exocytic processes involved in neurotransmission in a variety of central and peripheral nerve preparations. Their utility is limited to some extent by a poorly understood vesicular-independent labelling of cells and tissues. We show here that one likely cause of this troublesome background labelling is that FM1-43 and FM2-10 are selective and competitive antagonists at both cloned and endogenously expressed muscarinic acetylcholine receptors. In radioligand binding studies, FM1-43 and FM2-10 bound with moderate affinity (23–220 nm) to membranes of Chinese hamster ovary (CHO) cells expressing cloned human muscarinic receptors (M1–M5). In functional studies in vitro, FM1-43 and FM2-10 inhibited electrical field stimulation (EFS) and acetylcholine-induced cholinergic contractions of guinea-pig tracheal strips (IC50: FM1-43, 0.4 ± 0.1; FM2-10, 1.6 ± 0.1 μm; concentration of antagonist producing a 2-fold leftward shift in the acetylcholine concentration–response curve (Kb): FM1-43, 0.3 ± 0.1; FM2-10, 15.8 ± 10.1 μm). Neither compound inhibited EFS-evoked, non-adrenergic non-cholinergic nerve-mediated relaxations or contractions of the airways, or contractions mediated by histamine H1 receptor or tachykinin NK2 receptor activation. Incubating freshly excised tracheal whole-mount preparations with 5 μm FM1-43 resulted in intense fluorescence labelling of the smooth muscle that was reduced by up to 90% in the presence of selective M2 and M3 receptor antagonists. The potency of the FM dyes as muscarinic receptor antagonists is within the concentration range used to study vesicular cycling at nerve terminals. Given that muscarinic receptors play a key role in the regulation of neurotransmitter release from a variety of neurones, the anticholinergic properties of FM dyes may have important implications when studying vesicular events in the nervous system. In addition, these dyes may provide a novel tool for visualizing muscarinic receptor occupancy in living tissue or cell preparations.
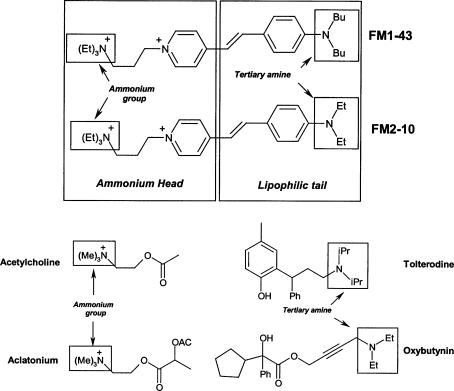
The fluorescent styryl dyes FM1-43 and FM2-10 are commonly used to monitor neuronal vesicular recycling (Ryan et al. 1993; Ribchester et al. 1994; Bewick & Betz, 1994; Kirchgessner et al. 1996; Hay & Hasser, 1998; Kay et al. 1999; Gale et al. 2001; Rizzoli et al. 2003; Dawkins et al. 2005). The chemical structure of these dyes consists of a positively charged ammonium head and a lipophilic tail (Fig. 1). It is thought that the lipophilic tail anchors the dye molecule into cellular membranes while the ammonium head prevents the molecule from permeating the cell. These chemical properties allow FM dyes to be employed for activity-dependent staining and destaining of nerve terminals during vesicular cycling.
Figure 1. Chemical structure of FM1-43 and FM2-10, and homology with other known muscarinic receptor ligands.
Structural similarities exist between both the head and tail regions of FM styryl dyes and the likely functional moieties of known muscarinic receptor ligands. See discussion for further details.
The utility of FM styryl dyes for monitoring vesicular recycling is limited to some extent by a poorly understood propensity for non-specific background labelling of tissues and sections (Pyle et al. 1999). Another potential issue lies within the implicit assumption that these styryl dyes are relatively inert and have little if any influence on the system under study. Interpreting the results of such studies could be confounded if FM styryl dyes exhibit pharmacological properties that alter neurotransmission or vesicular cycling. Indeed, previous studies have identified a curare-like action of FM1-43 in vertebrate skeletal muscle preparations, and FM1-43 has been shown to block mechanotransduction in mammalian auditory sensory cells (Bewick & Betz, 1994; Gale et al. 2001).
While attempting to visualize autonomic nerve endings innervating the guinea-pig trachealis during nerve-evoked contractions, we found that both FM1-43 and FM2-10 markedly inhibited parasympathetic–cholinergic nerve-mediated contractions of the smooth muscle. We report here that both FM1-43 and FM2-10 are modestly potent but selective antagonists at all five cloned human muscarinic acetylcholine receptor (mAchR) subtypes (M1–M5) as well as M2 and M3 receptors endogenously expressed in the guinea-pig airways. The affinity of these dyes for muscarinic receptors falls within the concentration range at which they have been used to study vesicular cycling at nerve terminals. As muscarinic receptors regulate neurotransmitter release from a variety of neuronal subtypes in the CNS and in the periphery, the anticholinergic properties of FM styryl dyes may have important implications when studying vesicular events in the nervous system. In addition, these dyes may provide a novel method for visualizing muscarinic receptor occupancy in real time in living tissues or cell preparations.
Methods
Radioligand binding experiments
The human M1–M5 receptors were cloned and stably expressed in Chinese hamster ovary (CHO) cell lines as previously described (Buckley et al. 1989). M2 and M4 mAChRs were co-expressed with the chimeric G protein, Gqi5 in CHO cells. Competition for [3H]-N-methyl scopolamine (0.5 nm) binding was performed using crude CHO cell membranes. Radioligand binding assays were conducted using a scintillation proximity assay (SPA) for M1, M2 and M3 and filtration assay for M4 and M5.
In the SPA assay, membranes were preincubated with wheatgerm agglutinin beads (Amersham) in 50 mm Hepes buffer (Sigma, St Louis, MO, USA) (pH 7.4) at 4°C for 30 min, and then incubated with the radioligand in a 96-well Optiplate (PerkinElmer) for a further 2 h in the presence of vehicle (0.1% DMSO), FM1-43 (3–3000 nm), FM2-10 (3–3000 nm) or atropine (0.3–300 nm) in a final volume of 0.2 ml, at room temperature (22°C). At the end of the incubation, the plates were spun in a centrifuge (Beckman CS-6R) for 5 min at 800 g and counted in a TopCount, microplate scintillation counter (model A9912; Packard, Meriden, CT, USA).
In the filtration assay, membranes were similarly incubated with the radioligand for 2 h at room temperature in the presence of vehicle (0.1% DMSO), FM1-43 (3–3000 nm), FM2-10 (3–3000 nm) or atropine (0.3–300 nm) in a final volume of 0.5 ml. Reactions were terminated by rapid filtration (Brandel Cell Harvester, Gaithersburg, MD, USA) through GF/C (Glass microfiber grade C) filters. Membranes were washed with ice-cold 50 mm Hepes and transferred to scintillation vials containing Beckman Ready Safe. Radioactivity was counted in a scintillation counter (model LS6500; Beckman, Fullerton, CA, USA).
Concentration–response curves for each compound were run using duplicate samples in three independent experiments. Specific binding was determined by subtracting non-specific binding (defined in the presence of 0.3 μm atropine) from total binding. Kd values were estimated from concentration–response curves and used to determine the inhibition constant (Ki) of each inhibitor using the Cheng and Prusoff equation (Cheng & Prusoff, 1973).
In vitro functional experiments
The Johns Hopkins Medical Institutional Animal Care and Use Committee (JHMI ACUC) approved all in vitro pharmacological experiments described in this study. The majority of the studies were performed using airway tissues harvested from male Hartley guinea-pigs (300–400 g; Hilltop, Scottdale, PA, USA). For these studies, guinea-pigs were killed by 100% CO2 inhalation followed by rapid exsanguination. A limited number of experiments were repeated using discarded airway segments harvested from rats and cats (which had been killed for other experiments) in order to assess potential species differences in the antimuscarinic effects of FM styryl dyes.
Effect of FM1-43 and FM2-10 on contractions and relaxations of airway smooth muscle in vitro
Tracheal strips or intact bronchial segments (two rings each) were suspended with 1.5 g (trachea) or 1 g (bronchi) passive tension in 10-ml organ baths. Tissues were equilibrated for 1 h with the buffer in the organ bath changed at 15-min intervals with warmed (37°C), oxygenated (95% O2–5% C02) Krebs solution containing (mm): NaCl 118, KCl 5.4, NaHPO4 1, MgSO4 1.2, CaCl2 1.9, NaHCO3 25 and dextrose 11.1. In all experiments, 3 μm indomethacin, 2 μm propranolol and 1 μm phentolamine were added to the Krebs solution to prevent any effects of prostaglandins or catecholamines on the airway segments under study. Contractions and relaxations were measured isometrically (model FT03; Grass) and displayed on a polygraph (Model 7D; Grass).
Cholinergic-mediated contractions and non-adrenergic non-cholinergic-mediated relaxations of the guinea-pig trachea were evoked by electric field stimulation (EFS) at optimal stimulus intensities (8 V, 200–300 mA, 1-ms pulse duration, 10-s trains) using a Grass model S44 stimulator connected in series to a Stimusplitter (MedLab Instruments, Fort Collins, CO, USA) and platinum stimulating electrodes. Relaxations were evoked by the addition of atropine and contraction of the trachealis with 10 μm histamine. Frequency–response curves (2–32 Hz) were constructed in a paired fashion in the absence and presence of FM1-43 or FM2-10. Tissues were preincubated for 10 min with vehicle (DMSO), 5 μm FM1-43 or 50 μm FM2-10 prior to EFS stimulation. The concentrations of FM1-43 and FM2-10 employed were chosen based on the effective concentrations reportedly required to visualize autonomic nerve terminals in other tissues (Kirchgessner et al. 1996). The cholinergic nature of EFS-evoked contractions was confirmed by blocking tracheal contractions with 1 μm atropine. Neuronal involvement in the EFS-evoked relaxations was shown by inhibiting relaxations with the fast sodium channel blocker tetrodotoxin (TTX, 1 μm) or by administration of the N-type calcium channel blocker ω-conotoxin GVIA (0.1 μm). The concentrations of FM1-43 and FM2-10 required to inhibit maximum EFS-evoked cholinergic contractions by 50% (IC50) and the time required to restore cholinergic contractions following washout of FM1-43 and FM2-10 were assessed in additional experiments. The antimuscarinic properties of FM1-43 and FM2-10 were compared to those of known muscarinic receptor antagonists (atropine, pirenzepine, methoctramine and 4-diphenylacetoxy-N-methylpiperidine methiodide (4-DAMP)) in similar experiments.
To further characterize the pharmacology of the FM styryl dyes, we assessed the effect of 5 μm FM1-43 and 50 μm FM2-10 on EFS-evoked non-cholinergic (tachykinin-dependent) contractions of the guinea-pig bronchus (Martin et al. 1992). For these experiments, atropine (1 μm) was first added to the incubation buffer to prevent cholinergic nerve-mediated responses. The effect of FM1-43 or FM2-10 on non-cholinergic contractions evoked by EFS (200–300 mA, 16 Hz, 1-ms pulses for 10-s trains) was assessed in a paired fashion. In subsequent experiments we determined the effect of the FM styryl dyes on guinea-pig airway smooth muscle contractions evoked by exogenously administered acetylcholine (10 nm–1 mm), histamine (10 nm–1 mm) and [β-Ala8]-neurokinin A(4–10) (0.1 nm–1 μm). The optimum incubation times (10–120 min) for both FM1-43 and FM2-10 were determined in additional experiments with acetylcholine.
Visualization of FM1-43 labelling of muscarinic receptors in guinea-pig trachealis
Tracheal segments (6–10 rings in length) were preincubated in Krebs solution (30 min at 37°C) with vehicle (control) or 1 μm atropine. Tissues were then incubated with 5 μm FM1-43 for an additional 15 min, washed in fresh Krebs solution for 15 min, cut open longitudinally along the ventral surface and pinned flat to the bottom of a sylgard-filled dissecting dish with the muscosal surface facing upwards. The mucosa was gently rubbed with a cotton swab to remove the overlying epithelium and expose the underlying smooth muscle. FM1-43 labelling of tracheal whole-mount preparations was visualized using an Olympus BX60 fluorescence microscope. Digital images of trachea were captured at identical exposure times using a Pixera Penguin 600 CL (5.8 million pixel) cooled CCD digital camera for Macintosh, and stored for later quantification of FM1-43 fluorescence intensity (see below). In some experiments we attempted to visualize in real time the dissociation of FM1-43 from airway smooth muscle muscarinic receptors. In these studies, tracheal segments were mounted in Sylgard-filled 35-mm Petri dishes, continuously superfused with Krebs solution (37°C, 10 ml min−1) and viewed microscopically. Segments were incubated for 10 min with 5 μm FM1-43 followed by a 30-min wash with Krebs solution in the absence or presence of 10 μm atropine. Digital images were captured at 1- to 5-min intervals as described above.
Data analysis and statistics
For functional assays, the magnitudes of EFS- and agonist-evoked airway smooth muscle contractions were expressed as a percentage of the maximum attainable smooth muscle contraction evoked by adding 300 mm BaCl2 to the organ bath. Relaxations were defined as a reversal of the histamine-evoked contraction and expressed as a percentage of the maximum attainable reversal (denoted percentage reversal). IC50 values for FM1-43 and FM2-10 against maximum EFS-evoked tracheal contractions were estimated from individual experiments and used to calculate the average for each group. Kb for FM1-43 and FM2-10 were likewise estimated from the dye-evoked shift in individual agonist dose–response curves. Quantification of the fluorescence intensity of FM1-43-labelled smooth muscle preparations was performed using National Institutes of Health (NIH)-image analysis software (version 1.62). Images from time-matched control and treated smooth muscle preparations were viewed at × 40 magnification, captured digitally (tiff file format) at identical exposure times and imported into NIH image. The intensity of FM1-43 fluorescence was determined by assessing the average density of the image pixels (inversely proportional to the intensity of the FM1-43 signal) across the entire field of view. All data are presented as means ±s.e.m. Differences between groups were assessed using analysis of variance (ANOVA) on Statview for Macintosh (Berkely, CA, USA). P < 0.05 was considered significant. When significant variation between groups was detected, treatment group means were compared using Scheffe's F-test for unplanned comparisons.
Drugs
Reagents used in this study were purchased from the following suppliers: acetylcholine, atropine sulphate, BaCl2, 4-DAMP, histamine, indomethacin, phentolamine hydrochloride, pirenzepine and dl-propranolol hydrochloride were from Sigma; N-(3-triethylammoniumpropyl)-4-(4-(dibutylamino) styryl) pyridinium dibromide (FM1-43) and N-(3-triethylammoniumpropyl)-4-(4-(diethylamino)styryl) pyridinium dibromide (FM2-10) from Molecular Probes (Eugene OR, USA); methoctramine hydrochloride from RBI (Natick, MA, USA); tetrodotoxin (TTX from Ballwin (MO, USA); [β-Ala8]-neurokinin A(4–10) from Peninsula Laboratories (Belmont, CA, USA); ω-conotoxin GVIA from Tocris (Ellisville, MO, USA); wheatgerm agglutinin SPA beads from Amersham Pharmacia Biotech; and [3H]-N-methyl scopolamine (or [3H]-NMS) methyl chloride (PerkinElmer). All drugs were dissolved in distilled water except indomethacin which was dissolved (30 mm stock) in 100% ethanol and diluted 1: 10 000 in Krebs solution, and FM1-43 (10 mm stock) and FM2-10 (100 mm stock) which were dissolved in DMSO and diluted to final concentrations in Krebs solution.
Results
FM1-43 and FM2-10 binding to cloned human muscarinic receptor subtypes
FM1-43 competed for [3H]-N-methyl scopolamine binding sites, displaying moderate affinity for each receptor subtype. FM2-10 displayed similar affinity to FM1-43 for M1–M4 receptors. However, FM2-10 was approximately 4-fold less potent at binding to M5 receptors compared to FM1-43. Both FM styryl dyes had much lower affinity for the muscarinic receptors than atropine (Table 1).
Table 1.
Competition by FM1-43, FM2-10 and atropine for [3H]-N-methyl scopolamine (0.5 nm) binding to cloned human muscarinic receptors expressed in CHO cells
| Ligand | Receptor subtype | ||||
|---|---|---|---|---|---|
| M1 | M2 | M3 | M4 | M5 | |
| FM1-43 | 43.8 ± 7.1 | 54.6 ± 6.9 | 30.0 ± 3.3 | 103.2 ± 0.9 | 82.7 ± 11.2 |
| FM2-10 | 33.5 ± 8.5 | 37.5 ± 6.6 | 23.6 ± 5.8 | 120.7 ± 10.2 | 227.8 ± 13.3 |
| Atropine | 0.34 ± 0.08 | 0.53 ± 0.09 | 0.29 ± 0.03 | 0.31 ± 0.03 | 0.26 ± 0.02 |
Values represent the mean Kd±s.e.m. (nm) obtained from duplicate assays in three independent experiments.
Effect of FM1-43 and FM2-10 on cholinergic contractions of guinea-pig trachea
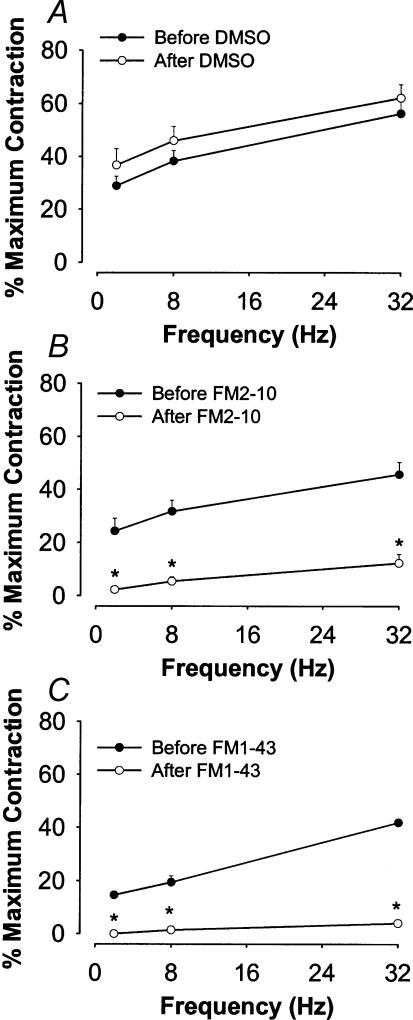
EFS of guinea-pig tracheal strips in vitro evoked frequency-dependent contractions of the trachealis. In the absence of FM styryl dyes, peak tracheal contractions evoked by EFS approached 50–60% of the maximum contraction produced by 300 mm BaCl2 (Fig. 2A). EFS-evoked contractions were abolished by atropine, confirming their dependency on acetylcholine-induced activation of muscarinic receptors. Neither FM1-43 nor FM2-10 had any direct contractile or relaxant effects on the trachealis. However, both FM1-43 (5 μm) and FM2-10 (50 μm) nearly abolished the EFS-induced cholinergic contractions of the trachealis (Figs 2B and C).
Figure 2. Electric field stimulation (EFS)-evoked contractions of guinea-pig tracheal strips in vitro before and after pretreatment with vehicle (DMSO; A) 50 μm FM2-10 (B) or 5 μm FM1-43 (C).
Data represent the means ±s.e.m. of n = 3–4 experiments and are expressed as a percentage of the maximum attainable contraction evoked by 300 mm BaCl2. See Table 1 for estimated IC50 values. *P < 0.05, significantly different compared to before the treatment.
Repeated EFS at optimum stimulation intensities (8 V, 32 Hz, 1-ms pulses, 10-s trains every 100 s) evoked cholinergic contractions of guinea-pig tracheal strips that were stable and reproducible for the duration of control (vehicle) experiments (up to 5 h). The addition of increasing concentrations of FM styryl dyes or other known muscarinic receptor antagonists resulted in a dose-dependent reduction in the magnitude of EFS-evoked contractions. The IC50 of FM1-43 and FM2-10 for the EFS-induced contractions were estimated to be 0.4 ± 0.1 and 1.6 ± 0.1 μm, respectively (Table 2), roughly equipotent or more potent than the M1- and M2-selective antagonists pirenzepine and methoctramine, respectively, but considerably less potent than the M3 selective antagonist 4-DAMP or the non-selective muscarinic receptor antagonist atropine (Table 2). The time to 50% recovery of EFS-evoked contractions following washout of the dyes was significantly longer in the tissues treated with FM1-43 (111 ± 18 min) than in tissues pretreated with FM2-10 (12 ± 3 min; n = 3, P < 0.05).
Table 2.
Inhibition of EFS-induced and acetylcholine-induced cholinergic contractions of guinea-pig trachealis
| Compound | IC50 (μm) | Kb (μm) |
|---|---|---|
| FM2-10 | 1.6 ± 0.1 | 15.8 ± 10 |
| FM1-43 | 0.4 ± 0.1 | 0.33 ± 0.15 |
| Atropine | 0.0006 ± 0.0003 | 0.0017 ± 0.0009 |
| Pirenzepine | 1.6 ± 0.6 | 0.13 ± 0.02 |
| Methoctramine | 12.9 ± 4.6 | >3 μm |
| 4-DAMP | 0.0021 ± 0.0002 | 0.00077 ± 0.00009 |
Data represent the means ±s.e.m. of n = 3–4 experiments. The IC50 is defined as the concentration of antagonist that inhibits the maximum EFS-evoked contractions by 50%. The Kb is the concentration of antagonist producing a 2-fold leftward shift in the acetylcholine concentration–response curve. The absolute potency and rank order of potency for the reference compounds is comparable to that reported previously in studies of muscarinic receptor agonist-induced contractions of guinea-pig trachealis (Eglen et al. 1999).
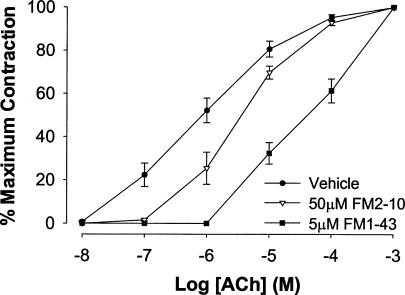
Unlike the M2 receptor selective antagonist methoctramine, which at low concentrations potentiated the EFS-evoked contractions (an effect attributed to antagonism of prejunctional M2 receptors), FM1-43 and FM2-10 and the other reference antagonists only inhibited the contractions evoked by EFS. FM1-43 (5 μm) and FM2-10 (50 μm) also antagonized contractions of the trachealis evoked by exogenously administered acetylcholine. The maximum contraction evoked by acetylcholine was approximately equal to the maximal attainable contractions evoked by BaCl2. Both compounds produced rightward shifts in the acetylcholine concentration–response curve. The effects of FM1-43 and FM2-10 on the exogenously administered acetylcholine were dose dependent (not shown) and surmountable. FM1-43 was more potent than FM2-10 at inhibiting the acetylcholine-induced contractions (estimated Kb values: FM1-43, 0.3 ± 0.2; FM2-10, 16 ± 10 μm; Fig. 3, Table 2). Both compounds were considerably less potent than atropine or 4-DAMP.
Figure 3. Log dose–response curves for acetylcholine-evoked contractions of guinea-pig tracheal strips in vitro in the presence of vehicle (DMSO), 50 μm FM2-10 or 5 μm FM1-43.
Tissues were pretreated with vehicle or FM styryl dyes for 10 min. Each curve represents the mean ±s.e.m. of n = 3–4 experiments and is expressed as a percentage of the maximum attainable contraction evoked by 300 mm BaCl2. See Table 2 for estimated Kb values.
Pre-incubation with the FM styryl dyes for longer time periods (up to 120 min) did not further shift the acetylcholine dose–response curve for FM2-10, and slightly reduced the potency of FM1-43 (not shown). In separate experiments, we found that FM1-43 inhibited EFS- and acetylcholine-mediated contractions of rat and cat airway preparations with a similar potency and efficacy to the inhibition seen in guinea-pigs (not shown).
Effect of FM1-43 and FM2-10 on non-cholinergic contractions and relaxations of the airways
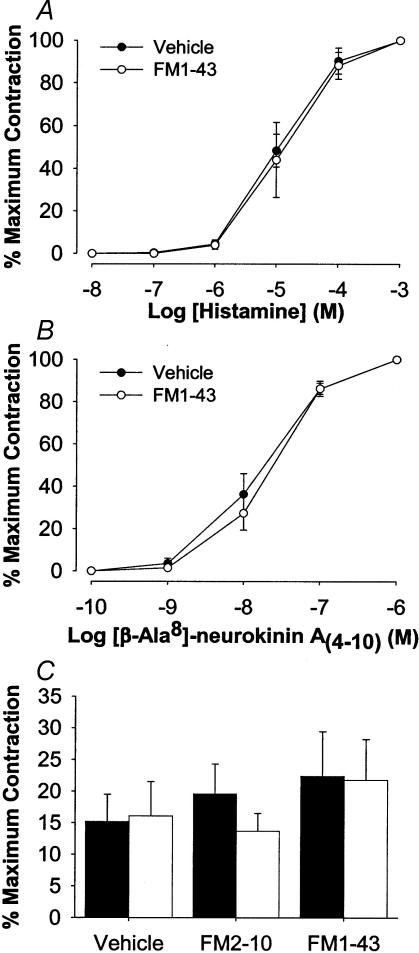
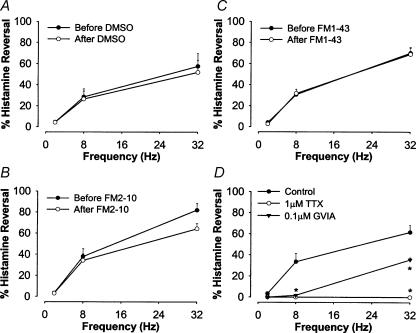
FM1-43 and FM2-10 selectively inhibit acetylcholine-mediated contractions of the airways. Neither compound inhibited non-cholinergic nerve-mediated (tachykinin-dependent; Martin et al. 1992) contractions of guinea-pig trachea and mainstem bronchus evoked by EFS or contractions evoked by bath application of histamine or [β-Ala8]-neurokinin A(4–10) (Fig. 4A–C). Likewise, following pretreatment with 1 μm atropine (to prevent cholinergic nerve-mediated contractions) and precontraction of the trachealis with 10 μm histamine, EFS produced frequency-dependent relaxations of the guinea-pig tracheal smooth muscle that were completely unaffected by pretreatment with either 5 μm FM1-43, 50 μm FM2-10 or vehicle (Fig. 5A–C). The EFS-induced relaxations were, however, significantly reduced by either TTX or ω-conotoxin GVIA, confirming the nerve-dependency of these responses (Fig. 5D).
Figure 4. Non-cholinergic evoked contractions of guinea-pig tracheal strips in vitro in the absence or presence of FM1-43 or FM2-10.
Concentration-response curves for contractions evoked by histamine (A) and [β-Ala8]-neurokinin A(4–10) (B) in guinea-pig tracheal strips in vitro in the presence of vehicle or 5 μm FM1-43. C, EFS-evoked non-cholinergic contractions of guinea-pig bronchial rings in vitro before (filled bars) and after (open bars) vehicle (DMSO), 50 μm FM2-10 or 5 μm FM1-43. Tissues were first treated with 1 μm atropine to prevent cholinergic responses. Contractions were abolished by 0.3 μm each of the neurokinin receptor antagonists CP-99 994 and SR48968 (not shown). Each data set represents the mean ±s.e.m. of n = 3–4 experiments and is expressed as a percentage of the maximum attainable contraction evoked by 300 mm BaCl2.
Figure 5. Electric field stimulation (EFS)-evoked non-adrenergic non-cholinergic-mediated relaxations of guinea-pig tracheal strips in vitro before and after vehicle (DMSO; A) 50 μm FM2-10 (B) 5 μm FM1-43 (C) 1 μm tetrodotoxin (TTX) (D) and 0.1 μm ω-conotoxin GVIA (E).
Tissues were first treated with 1 μm atropine to prevent cholinergic contractions and then precontracted with 10 μm histamine. Each curve represents the mean ±s.e.m. of n = 3–4 experiments and is expressed as a percentage reversal of the contraction evoked by 10 μm histamine. *P < 0.05, significantly different compared to before the treatment.
Visualization of FM1-43 binding in guinea-pig trachea
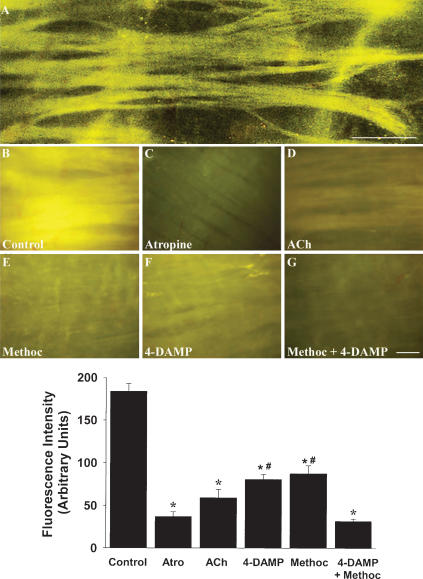
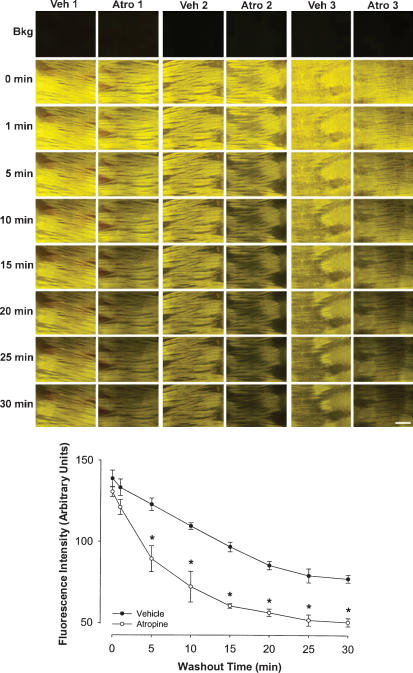
The results presented above provide conclusive evidence that FM1-43 and FM2-10 are muscarinic receptor antagonists. Taking advantage of the fluorescence inherent in these styryl dyes, we evaluated the ability of FM1-43 and FM2-10 to intravitally label muscarinic receptors in isolated whole-mount preparations of the airways and segments of the small intestine. Incubating freshly excised tracheal whole-mount preparations with either FM1-43 or FM2-10 resulted in an intense fluorescent labelling of the tracheal smooth muscle (Fig. 6A). Labeling of the muscle with the dyes was uniformly distributed over the bands of trachealis. Preincubation with 1 μm atropine reduced the fluorescence intensity within the tracheal smooth muscle by up to 90% (cf. Fig. 6B and C). Preincubation with 1 mm acetylcholine also essentially abolished FM1-43 labelling of the trachealis (Fig. 6D). Preincubation with either 3 μm methoctramine or 100 nm 4-DAMP reduced labelling intensity, but the reduction produced by these M2 and M3 selective antagonists administered alone was significantly less than that produced by either atropine or the combination of 3 μm methoctramine plus 100 nm 4-DAMP (Fig. 6). In a separate study, we evaluated the ability of atropine to reverse FM1-43 labelling of the trachealis. Tracheal whole-mount preparations first labelled with 5 μm FM1-43 were continuously superfused with Krebs solution not containing the styryl dye. This continuous superfusion resulted in a time-dependent reduction in fluorescence intensity over the smooth muscle (Fig. 7). Adding 10 μm atropine to the superfusion buffer significantly and visibly increased the rate of washout of the fluorescence (Fig. 7). Similar effects of atropine on FM2-10 labelling of smooth muscle were seen in whole-mount preparations of the guinea-pig trachea and ileum (see Fig. 8A–D).
Figure 6. Visual characterisation of FM1-43 labelling of guinea-pig airway smooth muscle.
A, high-contrast representative image showing FM1-43 labelling of guinea-pig airway smooth muscle. B–G, representative photomicrographs showing FM1-43 labelling of guinea-pig trachea whole-mount preparations in the absence (control, B) or presence of 1 μm atropine (C), 100 μm acetylcholine (D), 3 μm methoctramine (E), 100 nm 4-DAMP (F) or 3 μm methoctramine plus 100 nm 4-DAMP (G). Bar chart displays quantitative analysis (mean ±s.e.m., n = 4–8 for each) of FM1-43 labelling intensity assessed from digital photomicrographic images. *P < 0.05, significantly different from control; #P < 0.05, significantly different from atropine pretreatment. Atro, atropine; ACh, acetylcholine; Methoc, methoctramine. Scale bars, 50 μm.
Figure 7. Real-time monitoring of displacement of FM1-43 labelling of guinea-pig airway smooth muscle by atropine.
Photomicrographs show matched representative whole-mount preparations stained with 5 μm FM1-43 from three different animals (denoted 1–3). After labelling, each tissue was washed continuously for 30 min in the absence (veh) or presence (atro) of 10 μm atropine. Line graph displays quantitative analysis (mean ±s.e.m., n = 3 for each) of time-dependent alterations in FM1-43 staining intensity (assessed from digital photomicrographic images). *P < 0.05, significantly different from time = 0 min. Scale bar, 50 μm.
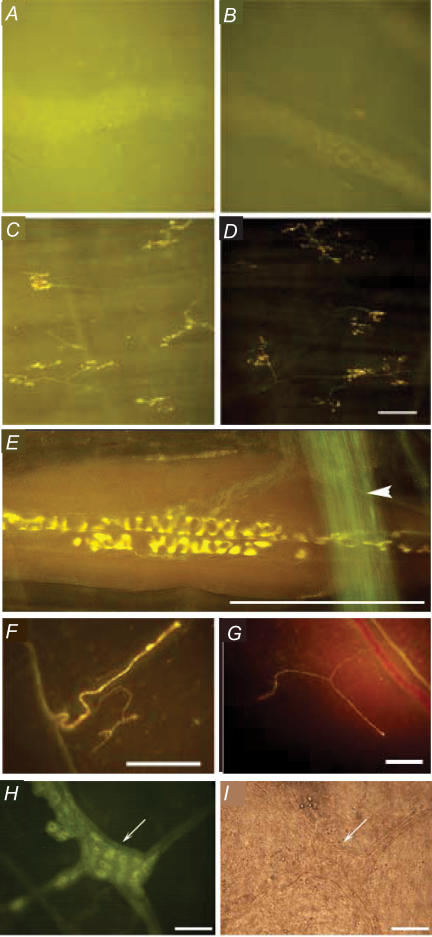
Figure 8. Stimulation- and muscarinic receptor-independent labelling of neuronal structures by the styryl dye FM2-10.
Representative photomicrographs showing FM2-10 labelling of the guinea-pig myenteric plexus (A and B) and mucosal afferent nerve terminals in the trachea (C and D) in the absence (A and C) and presence (B and D) of 1 μm atropine. Note that atropine substantially reduces the background fluorescence in both tissues without preventing staining of the nerves in either the myenteric plexus or the airway mucosa. E, intravital labelling of a sensory nerve terminal associated with the abdominal muscles in guinea-pigs. Labelling was carried out in the presence of atropine and occurred without any stimulation of the tissue. Also note the large nerve bundle stained green in the foreground of the image (arrowhead). Less complex neuronal structures were similarly labelled by FM2-10 in the presence of atropine in the guinea-pig (F) and rat membranous diaphragm (G). These structures did not appear to be motor nerve terminals and are presumably diaphragm stretch receptors. As with all structures labelled in this figure, labelling occurred independent of any overt attempt to stimulate these nerves. H, parasympathetic ganglia associated with the rat trachea were also labelled by FM2-10 in the presence of atropine; the same results were also seen with guinea-pig trachea (not shown). I, shows the ganglia in H when viewed in bright field. Scale bar, 50 μm (A–D, H and I) and 100 μm (E–G).
It is worth noting that muscarinic receptor ligands including atropine and acetylcholine did not prevent FM styryl dye labelling of neuronal structures in any tissues studied (Fig. 8). In both ileum and trachea, neuronal structures (myenteric plexus in the ileum, vagal afferent nerve terminals in the tracheal mucosa) could be clearly visualized following incubation with either FM1-43 or FM2-10. Although labelling of the surrounding muscle was decreased, the intensity of labelling of the neuronal structures was not obviously altered by adding atropine to the incubation medium. Comparable results were obtained in studies of afferent nerve terminals in the striated muscle of rat and guinea-pig diaphragm and in guinea-pig abdominal muscle. Labelling occurred in all tissues studied without any stimulus applied to the tissues.
Discussion
In the present study, we employed receptor binding assays and functional bioassays to show that both FM1-43 and FM2-10 bind to and antagonize muscarinic receptors. Binding and antagonistic effects occurred at dye concentrations typically used to label nerve terminals (Ryan et al. 1993; Ribchester et al. 1994; Kirchgessner et al. 1996; Kay et al. 1999). Based on our results, it seems likely that muscarinic receptor binding contributes to background labelling produced by the styryl dyes and may also modulate vesicular recycling at synapses, particularly at cholinergic synapses, where autoreceptors have been frequently found. These naturally fluorescent compounds may prove useful for monitoring muscarinic receptor occupancy in living tissues.
Anticholinergic properties of FM1-43 and FM2-10
While the precise mechanism whereby the styryl dyes or other anticholinergic compounds interact with and cause blockade of muscarinic receptors is not known, it is interesting to note that comparing FM1-43 and FM2-10 with other known muscarinic receptor ligands, both agonists and antagonists, reveals significant structural homology (Fig. 1). The quaternary ammonium functionality found in the head of FM1-43 and FM2-10 is quite common in compounds displaying activity at the muscarinic receptors. This quaternary ammonium moiety can be found in the endogenous muscarinic agonist, acetylcholine, as well as in the synthetic cholinergic agonist aclatonium (Fig. 1). It has been hypothesized that the positive charge on this moiety interacts with the negatively charged extracellular regions common to all mAChR subtypes located between extracellular loops. Indeed, given that the only structural differences between FM1-43 and FM2-10 is the length of the tail portions of the molecules (Fig. 1), the similarities observed in binding affinities between these dyes may suggest that the functional regions are primarily dependent on the quaternary ammonium head. Nevertheless, the tertiary amine found in the tail portion of FM1-43 and FM2-10 is also found in several muscarinic receptor antagonists, including tolterodine and oxybutynin (Fig. 1). A closer approximation of the putative structural components necessary for interaction of the styryl dyes discussed here and muscarinic receptors could be achieved through theoretical models using X-ray crystals of rhodopsin (e.g. see Tanczos et al. 2004) or by using non-selective agents such as N-methyl-scopolamine and atropine with muscarinic receptors containing point mutations made at putative binding sites to identify key amino acid residues (e.g. see Krejci & Tucek, 2001). Such experiments were outside the scope of this study.
The muscarinic receptor binding profiles of FM1-43 and FM2-10 were established by radioligand binding studies using cloned human M1–M5 receptors. These studies indicate that both FM1-43 and FM2-10 bind with moderate affinity to all muscarinic receptor subtypes. Our functional studies using isolated airway preparations also suggest that these styryl dyes selectively interact with muscarinic receptors. Contractions evoked by exogenously administered histamine or [β-Ala8]-neurokinin A(4–10) were unaffected by either compound. Likewise, neither the contractions nor relaxations evoked by stimulation (EFS) of the non-adrenergic, non-cholinergic nerves innervating the airways were inhibited by the dyes. This suggests that FM1-43 and FM2-10, at concentrations that inhibit acetylcholine-induced contractions of airway smooth muscle, have no effect on smooth muscle contractions evoked by activation of histamine H1 receptors, neurokinin (NK) 1 or NK2 receptors, or relaxations mediated by either nitric oxide or vasoactive intestinal peptide and related peptides (Canning & Fischer, 2001). Moreover, as the non-cholinergic nerve-mediated contractions and relaxations evoked by EFS are dependent upon voltage-sensitive sodium (TTX-sensitive) and calcium (ω-conotoxin GVIA-sensitive) channels, the styryl dyes are also unlikely to functionally alter gating of these channels or other ion channels regulating smooth muscle and/or nerve excitability.
We did not evaluate the receptor selectivity of FM1-43 or FM2-10 in functional studies, but used only the guinea-pig tracheal strip preparation, which is commonly used to evaluate M3 receptor pharmacology (Eglen et al. 1999). Autoradiographic and receptor-binding studies indicate that both M2 and M3 receptors are expressed by airway smooth muscle in most species, including guinea-pigs, with M2 receptors outnumbering M3 receptors by as much as 3: 1 (Zaagsma et al. 1997; Haddad et al. 1991; Torneke et al. 2002). Despite this ratio of M2/M3 receptors, acetylcholine-induced contraction of airway smooth muscle is mediated primarily by M3 receptor activation with little if any contribution from M2 receptors in most species (Zaagsma et al. 1997; Fryer & Jacoby, 1998; Struckmann et al. 2003; Fisher et al. 2004). The results of pharmacological analyses using receptor-selective antagonists reported here and elsewhere are consistent with this hypothesis. Our functional studies indicate that both FM1-43 and FM2-10 are M3 receptor antagonists. We also evaluated FM1-43 and FM2-10 staining of M2 and M3 receptors in whole-mount preparations of the guinea-pig trachea. The results of these studies show that FM1-43 labelling of the airway smooth muscle is reduced in the presence of either a selective M2- or M3-receptor antagonist (methoctramine and 4-DAMP, respectively), whereas labelling was almost abolished by combined blockade of both M2 and M3 receptors. This would strongly suggest that FM1-43 interacts with both receptor subtypes expressed by the airway smooth muscle.
It is interesting that, unlike in functional studies using guinea-pig, rat and cat tracheal preparations where FM1-43 was 10-fold more potent then FM2-10 at inhibiting M3-dependent contractions of the trachealis, there appears to be no significant difference in the binding affinity of these two molecules for the cloned human M3 receptor. It is difficult to explain this discrepancy in potency between functional and binding studies. The fact that the same quaternary ammonium moiety is found in both FM1-43 and FM2-10 indicates that the tertiary amine functionality found in the tail of these compounds may be driving the difference in activity. One explanation may be that the amine in FM1-43 is slightly more basic than the amine in FM2-10 and therefore displays a stronger interaction with the active site of the M3 receptor. Alternatively, it may reflect a difference in the guinea-pig and human M3 receptor, or simply relate to the nature of the binding experiments in which muscarinic receptors were singularly highly over-expressed, or the stability of the compounds in the various buffer and tissue systems. It is also worth noting, however, that while FM1-43 and FM2-10 displayed comparable binding affinity for human M1–M4 receptors, FM2-10 was significantly less potent at the human M5 receptor compared to FM1-43. This would suggest that the slightly differing chemical structures of FM1-43 and FM2-10 probably influence their binding properties.
Other pharmacological activities of styryl dyes have been reported. FM1-43 may interact with several non-selective cation channels and may block mechanotransduction in a subset of auditory sensory cells (Gale et al. 2001; Meyers et al. 2003). Another styryl dye, RH414, evokes constriction of the cerebral artery, although the mechanism underlying this response is unclear (Grinvald et al. 1986). Similar to the results of the present study, Bewick & Betz (1994) found evidence for styryl dye-mediated inhibition of nerve- and acetylcholine-evoked skeletal muscle contractions in frogs, suggesting that some styryl dyes interact with postjunctional nicotinic acetylcholine receptors. It is interesting to note that inhibition of skeletal muscle contraction was readily resolved following washing or illumination of the tissue (Bewick & Betz, 1994). We did not attempt to directly study the specific effects of illumination in our experiments; however, all studies were carried out in ambient light and under these conditions antagonist activities persisted for several hours, even following thorough washing to remove excess dye. An early study by Smith et al. (1967) reported that a number of styryl-based molecules (with structures similar to the FM dyes) inhibit choline acetyltransferase activity. The fact that both FM1-43 and FM2-10 inhibited contractions evoked by exogenous acetylcholine suggests that the results of the present study are at least in part independent of dye-mediated inhibition of acetylcholine synthesis.
Possible relevance of the antimuscarinic activity of FM styryl dyes
It seems unlikely that either of these styryl dyes in their current form would be useful therapeutic agents, as neither compound is potent or selective and FM1-43 at least appears to be unstable during incubation with airway tissue from either guinea-pigs or humans (authors' unpublished observations). However, the ability of FM1-43 and FM2-10 to label and antagonize muscarinic receptors (at dye concentrations used for monitoring endocytosis) may need to be considered when interpreting results from experiments using these dyes to study vesicular events at neuronal synapses and/or nerve–muscle junctions. Muscarinic receptors are not only expressed postjunctionally on effector tissues such as muscle and glands, but can also be found on many neurones throughout the central and peripheral nervous systems (Levey, 1993; Jaarsma et al. 1997; Van der Zee & Luiten, 1999; Belmonte et al. 2000; Khan et al. 2002). Presynaptic muscarinic receptors have a clearly defined role in regulating neurotransmitter release from a variety of neurones, including cholinergic, dopaminergic and adrenergic neurones (Myers & Undem, 1996; Fryer & Jacoby, 1998; Iannazzo & Majewski, 2000; Zhang & Warren, 2002; Zhang et al. 2002; Slutsky et al. 2003; Trendelenburg et al. 2003; Santafe et al. 2003). In addition, both pre- and postganglionic muscarinic receptors modulate neuronal excitation and synaptic potential (Ashe & Yarosh, 1984; Yang & Biggs, 1991; Bernheim et al. 1992). The potential for non-specific labelling and dye-evoked alterations in vesicular transport should be considered when using FM styryl dyes, particularly when studying cholinergic synapses. However, it should be noted that FM dye labelling of neuronal structures is not likely to be a direct result of binding to muscarinic receptors on nerve terminals as in the present study we were still able to visualize FM2-10-labelled neuronal profiles in several tissues in the presence of atropine.
The results of the present study may also suggest a novel use for FM styryl dyes. As demonstrated, these molecules are naturally fluorescent and readily interact with all muscarinic receptor subtypes in a species-independent fashion. With the appropriate study design, it may be possible to use FM1-43 and FM2-10 to monitor in real time the innervation and occupancy of muscarinic receptors in living tissues. When combined with non-fluorescent, receptor-selective ligands, it may also be possible to evaluate the relative density and occupancy of receptor subtypes in living tissues.
Acknowledgments
This study was funded by grants from the National Institutes of Health (Bethesda, MD, USA). S.B.M. is an NH and MRC of Australia C.J. Martin Fellow (#007188).
References
- Ashe JH, Yarosh CA. Differential and selective antagonism of the slow inhibitory postsynaptic potential and slow-excitatory postsynaptic potential by gallamine and pirenzepine in the superior cervical ganglion of the rabbit. Neuropharmacology. 1984;23:1321–1329. doi: 10.1016/0028-3908(84)90053-4. [DOI] [PubMed] [Google Scholar]
- Belmonte KE, McKinnon LA, Nathanson NM. Developmental expression of muscarinic acetylcholine receptors in chick retina: selective induction of M2 muscarinic receptor expression in ovo by a factor secreted by muller glial cells. J Neurosci. 2000;20:8417–8425. doi: 10.1523/JNEUROSCI.20-22-08417.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernheim L, Mathie A, Hille B. Characterization of muscarinic receptor subtypes inhibiting Ca2+ current and M current in rat sympathetic neurons. Proc Natl Acad Sci U S A. 1992;89:9544–9548. doi: 10.1073/pnas.89.20.9544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bewick GS, Betz WJ. Illumination partly reverses the postsynaptic blockade of the frog neuromuscular junction by the styryl pyridinium dye RH414. Proc R Soc Lond B Biol Sci. 1994;258:201–207. doi: 10.1098/rspb.1994.0163. [DOI] [PubMed] [Google Scholar]
- Buckley NJ, Bonner TI, Buckley CM, Brann MR. Antagonist binding properties of five cloned muscarinic receptors expressed in CHO-K1 cells. Mol Pharmacol. 1989;35:469–476. [PubMed] [Google Scholar]
- Canning BJ, Fischer A. Neural regulation of airway smooth muscle tone. Respir Physiol. 2001;25:113–127. doi: 10.1016/s0034-5687(00)00208-5. [DOI] [PubMed] [Google Scholar]
- Cheng Y-C, Prusoff WH. Relationship between the inhibition constant (Ki) and the concentration of inhibitor which cause 50 percent inhibition (IC50) of an enzymatic reaction. Biochem Pharmacol. 1973;22:3099–3108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- Dawkins R, Keller SL, Sewell WF. Pharmacology of acetylcholine-mediated cell signaling in the lateral line organ following efferent stimulation. J Neurophysiol. 2005;93:2541–2545. doi: 10.1152/jn.01283.2004.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eglen RM, Choppin A, Watson N, Hegde SS. Muscarinic receptor assays. In: Enna SJ, William M, Barrett JF, Ferkany JW, Kenakin T, Porsolt RD, editors. Current Protocols in Pharmacology. USA: John Wiley and Sons; 1999. pp. 4.15.1–4.15.20. [Google Scholar]
- Fisher JT, Vincent SG, Gomeza J, Yamada M, Wess J. Loss of vagally mediated bradycardia and bronchoconstriction in mice lacking M2 or M3 muscarinic acetylcholine receptors. FASEB J. 2004;18:711–713. doi: 10.1096/fj.03-0648fje. [DOI] [PubMed] [Google Scholar]
- Fryer AD, Jacoby DB. Muscarinic receptors and control of airway smooth muscle. Am J Respir Crit Care Med. 1998;158:S154–S160. doi: 10.1164/ajrccm.158.supplement_2.13tac120. [DOI] [PubMed] [Google Scholar]
- Gale JE, Marcotti W, Kennedy HJ, Kros CJ, Richardson GP. FM1-43 dye behaves as a permeant blocker of the hair-cell mechanotransducer channel. J Neurosci. 2001;21:7025. doi: 10.1523/JNEUROSCI.21-18-07013.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinvald A, Lieke E, Frostig RD, Gilbert CD, Weisel TN. Functional architecture of cortex revealed by optical imaging of intrinsic signals. Nature. 1986;324:361–364. doi: 10.1038/324361a0. [DOI] [PubMed] [Google Scholar]
- Haddad EB, Landry Y, Gies JP. Muscarinic receptor subtypes in guinea-pig airways. Am J Physiol. 1991;261:L327–L333. doi: 10.1152/ajplung.1991.261.4.L327. [DOI] [PubMed] [Google Scholar]
- Hay M, Hasser EM. Measurement of synaptic vesicle exocytosis in aortic baroreceptor neurons. Am J Physiol. 1998;275:H710–H716. doi: 10.1152/ajpheart.1998.275.2.H710. [DOI] [PubMed] [Google Scholar]
- Iannazzo L, Majewski H. M2/M4-muscarinic receptors mediate automodulation of acetylcholine outflow from the mouse cortex. Neurosci Lett. 2000;287:129–132. doi: 10.1016/s0304-3940(00)01163-0. [DOI] [PubMed] [Google Scholar]
- Jaarsma D, Ruigrok TJ, Caffe R, Cozzari C, Levey AI, Mugnaini E, Voogd J. Cholinergic innervation and receptors in the cerebellum. Prog Brain Res. 1997;114:67–96. doi: 10.1016/s0079-6123(08)63359-2. [DOI] [PubMed] [Google Scholar]
- Kay AR, Alfonso A, Alford S, Cline HT, Holgado AM, Sakmann B, Snitsarev VA, Stricker TP, Takahashi M, Wu L-G. Imaging synaptic activity in intact brain and slices with FM1-43 in C. elegans, lamprey and rat. Neuron. 1999;24:809–817. doi: 10.1016/s0896-6273(00)81029-6. [DOI] [PubMed] [Google Scholar]
- Khan KM, Drescher MJ, Hatfield JS, Khan AM, Drescher DG. Muscarinic receptor subtypes are differentially distributed in the rat cochlea. Neuroscience. 2002;111:291–302. doi: 10.1016/s0306-4522(02)00020-9. [DOI] [PubMed] [Google Scholar]
- Kirchgessner AL, Liu M-T, Gershon MD. In situ identification and visualization of neurons that mediate enteric and enteropancreatic reflexes. J Comp Neurol. 1996;371:270–286. doi: 10.1002/(SICI)1096-9861(19960722)371:2<270::AID-CNE7>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Krejci A, Tucek S. Changes of cooperativity between N-methylscopolamine and allosteric modulators alcuronium and gallamine induced by mutations of external loops of muscarinic M3 receptors. Mol Pharm. 2001;60:761–767. [PubMed] [Google Scholar]
- Levey AI. Immunological localization of m1-m5 muscarinic acetylcholine receptors in peripheral tissues and brain. Life Sci. 1993;52:441–448. doi: 10.1016/0024-3205(93)90300-r. [DOI] [PubMed] [Google Scholar]
- Martin CA, Naline E, Emonds-Alt X, Advenier C. Influence of (+/-) -CP-96,345 and SR 48968 on electrical field stimulation of the isolated guinea-pig main bronchus. Eur J Pharmacol. 1992;224:137–143. doi: 10.1016/0014-2999(92)90797-8. [DOI] [PubMed] [Google Scholar]
- Meyers JR, MacDonald RB, Duggan A, Lenzi D, Standaert DG, Corwin JT, Corey DP. Lighting up the senses: FM1-43 loading of sensory cells through nonselective ion channels. J Neurosci. 2003;23:4054–4065. doi: 10.1523/JNEUROSCI.23-10-04054.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers AC, Undem BJ. Muscarinic receptor regulation of synaptic transmission in airway parasympathetic ganglia. Am J Physiol. 1996;270:L630–L636. doi: 10.1152/ajplung.1996.270.4.L630. [DOI] [PubMed] [Google Scholar]
- Pyle JL, Kavalali ET, Choi S, Tsien RW. Visualization of synaptic activity in hippocampal slices with FM1-43 enabled by fluorescence quenching. Neuron. 1999;24:803–808. doi: 10.1016/s0896-6273(00)81028-4. [DOI] [PubMed] [Google Scholar]
- Ribchester RR, Mao F, Betz WJ. Optical measurements of activity-dependent membrane recycling in motor nerve terminals of mammalian skeletal muscle. Proc R Soc Lond B Biol Sci. 1994;255:61–66. doi: 10.1098/rspb.1994.0009. [DOI] [PubMed] [Google Scholar]
- Rizzoli SO, Richards DA, Betz WJ. Monitoring synaptic vesicle recycling in frog motor nerve terminals with FM dyes. J Neurocytol. 2003;32:539–549. doi: 10.1023/B:NEUR.0000020609.19873.e8. [DOI] [PubMed] [Google Scholar]
- Ryan TA, Reuter H, Wendland B, Schweizer FE, Tsien RW, Smith SJ. The kinetics of synaptic vesicle recycling measured at single presynaptic boutons. Neuron. 1993;11:713–724. doi: 10.1016/0896-6273(93)90081-2. [DOI] [PubMed] [Google Scholar]
- Santafe MM, Salon I, Garcia N, Lanuza MA, Uchitel OD, Tomas J. Modulation of ACh release by presynaptic muscarinic autoreceptors at the neuromuscular junction of the newborn and adult rat. Eur J Neurosci. 2003;17:119–127. doi: 10.1046/j.1460-9568.2003.02428.x. [DOI] [PubMed] [Google Scholar]
- Slutsky I, Wess J, Gomeza J, Dudel J, Parnas I, Parnas H. Use of knockout mice reveals involvement of M2-muscarinic receptor control of the kinetics of acetylcholine release. J Neurophysiol. 2003;89:1954–1967. doi: 10.1152/jn.00668.2002. [DOI] [PubMed] [Google Scholar]
- Smith JC, Cavallito CJ, Foldes FF. Choline acetyltransferase inhibitors: a group of styryl-pyridine analogs. Biochem Pharmacol. 1967;16:2438–2441. doi: 10.1016/0006-2952(67)90231-6. [DOI] [PubMed] [Google Scholar]
- Struckmann N, Schwering S, Wiegand S, Gschnell A, Yamada M, Kummer W, Wess J, Haberberger RV. Role of muscarinic receptor subtypes in the constriction of peripheral airways: studies on receptor-deficient mice. Mol Pharmacol. 2003;64:1444–1451. doi: 10.1124/mol.64.6.1444. [DOI] [PubMed] [Google Scholar]
- Tanczos AC, Palmer RA, Potter BS, Saldanha JW, Howlin BJ. Antagonist binding in the rat muscarinic receptor A study by docking and X-ray crystallography. Comput Biol Chem. 2004;28:375–385. doi: 10.1016/j.compbiolchem.2004.09.009. [DOI] [PubMed] [Google Scholar]
- Torneke K, Ingvast-Larsson C, Bostrom A, Appelgren LE. Muscarinic receptors in equine airways. Vet Res Commun. 2002;26:637–650. doi: 10.1023/a:1020924921676. [DOI] [PubMed] [Google Scholar]
- Trendelenburg AU, Gomeza J, Klebroff W, Zhou H, Wess J. Heterogeneity of presynaptic muscarinic receptors mediating inhibition of sympathetic transmitter release: a study with M2- and M4-receptor deficient mice. Br J Pharmacol. 2003;138:469–480. doi: 10.1038/sj.bjp.0705053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Zee EA, Luiten PG. Muscarinic receptors in the hippocampus, neocortex and amygdale: a review of immunocytochemical localization in relation to learning and memory. Prog Neurobiol. 1999;58:409–471. doi: 10.1016/s0301-0082(98)00092-6. [DOI] [PubMed] [Google Scholar]
- Yang Z-J, Biggs DF. Muscarinic receptors and parasympathetic neurotransmission in guinea-pig trachea. Eur J Pharmacol. 1991;193:301–308. doi: 10.1016/0014-2999(91)90143-e. [DOI] [PubMed] [Google Scholar]
- Zaagsma J, Roffel AF, Meurs H. Muscarinic control of airway function. Life Sci. 1997;60:1061–1068. doi: 10.1016/s0024-3205(97)00048-9. [DOI] [PubMed] [Google Scholar]
- Zhang W, Basile AS, Gomeza J, Volpicelli LA, Levey AI, Wess J. Characterization of central inhibitory muscarinic autoreceptors by the use of muscarinic acetylcholine receptor knockout mice. J Neurosci. 2002;22:1709–1717. doi: 10.1523/JNEUROSCI.22-05-01709.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Warren RA. Muscarinic and nicotinic presynaptic modulation of EPSCs in the nucleus accumbens during postnatal development. J Neurophysiol. 2002;88:3315–3330. doi: 10.1152/jn.01025.2001. [DOI] [PubMed] [Google Scholar]