Abstract
Sarcoplasmic reticulum (SR) calcium pump function requires a high local ATP/ADP ratio, which can be maintained by direct nucleotide channelling from mitochondria, and by SR-bound creatine kinase (CK)-catalysed phosphate-transfer from phosphocreatine. We hypothesized that SR calcium uptake supported by mitochondrial direct nucleotide channelling, but not bound CK, depends on the juxtaposition of these organelles. To test this, we studied a well-described model of cytoarchitectural disorganization, the muscle LIM protein (MLP)-null mouse heart. Subcellular organization was characterized using electron microscopy, and mitochondrial, SR and myofibrillar function were assessed in saponin-permeabilized fibres by measuring respiration rates and caffeine-induced tension transients. MLP-null hearts had fewer, less-tightly packed intermyofibrillar mitochondria, and more subsarcolemmal mitochondria. The apparent mitochondrial Km for ADP was significantly lower in the MLP-null heart than in control (175 ± 15 and 270 ± 33 μm, respectively), indicating greater ADP accessibility, although maximal respiration rate, mitochondrial content and total CK activity were unaltered. Active tension in the myofibres of MLP-null mice was 54% lower than in controls (39 ± 3 and 18 ± 1 mN mm−2, respectively), consistent with cytoarchitectural disorganization. SR calcium loading in the myofibres of MLP-null mice was similar to that in control myofibres when energy support was provided via Bound CK, but ∼36% lower than controls when energy support was provided by mitochondrial (P < 0.05). Mitochondrial support for SR calcium uptake was also specifically decreased in the desmin-null heart, which is another model of cytoarchitectural perturbation. Thus, despite normal oxidative capacity, direct nucleotide channelling to the SR was impaired in MLP deficiency, concomitant with looser mitochondrial packing and increased nucleotide accessibility to this organelle. Changes in cytoarchitecture may therefore impair subcellular energy transfer and contribute to energetic and contractile dysfunction.
Cardiomyocytes are a paradigm of highly compartmentalized cells. They possess a sophisticated subcellular architecture, in which repeated arrays of sarcomeres, T-tubules, sarcoplasmic reticulum (SR) and mitochondria interact, causing muscle contraction and relaxation. The coordinated production of microdomains of calcium triggers contraction (Wang et al. 2004), while myofibril-/SR-associated ATP pools provide the energy for cardiac function (Andrienko et al. 2003). Helping maintain this cytoarchitecture is the cytoskeleton, which holds sarcomeres in lateral register, mechanically couples myofibrils of adjacent myocytes, and transduces mechanical stress signals (Fatkin & Graham, 2002). Cytoarchitectural perturbation may result from mutation or deletion of cytoskeletal genes such as muscle LIM protein (MLP) or desmin (Arber et al. 1997; Kay et al. 1997; Fatkin & Graham, 2002) and from haemodynamic overload, in which cardiac hypertrophy, changes in mitochondrial size, number and function, and cytoskeletal protein accumulation occur (Tagawa et al. 1998; Ventura-Clapier et al. 2004).
Cardiac muscle cytoarchitecture is also characterized by the spatial separation of energy-utilizing and producing sites (Dzeja et al. 2000). The efficiency of contraction and relaxation depend on myofibrillar function and SR calcium uptake, respectively, and also on a high local ATP/ADP ratio maintained by a constant ATP supply to, and ADP withdrawal from, the myofibrillar and SR calcium ATPases. Mitochondria produce most of the ATP in cardiac cells, but the simple diffusion of nucleotides between them and myofibrils/SR via the bulk cytosol cannot on its own sustain cardiac function, due to thermodynamic and kinetic limitations (Dzeja & Terzic, 2003). To solve this problem, cardiomyocytes use specialized systems of energy transfer such as creatine kinase (CK), which is bound to the mitochondrial inner membrane, myofibrils and SR and which efficiently transfers the high-energy phosphate group of mitochondrial ATP to ADP in the vicinity of the myofibrils and SR. Recently, we showed that mitochondria can directly support myofibrillar/SR calcium ATPase function as efficiently as the bound CK system, and much better than cytosolic ATP alone (Kaasik et al. 2001). Consistent with this direct ATP channelling, others have shown that mitochondria can directly withdraw ADP from myofibrils/SR (Saks et al. 2001).
As direct channelling of ATP from mitochondria to the SR depends on the interaction of these two organelles, we hypothesized that the process would be altered by perturbation of the arrangement of mitochondria with SR. To test this, we characterized energy transfer in MLP-null mouse hearts, which show myofibre disarray, disorganized mitochondria and cytoskeletal remodelling (Arber et al. 1997; van den Bosch et al. 2005; Wilding et al. 2005).
We found fewer, less-tightly packed intermyofibrillar mitochondria in this model, with more subsarcolemmal mitochondria. Concomitant with these changes, the accessibility of exogenous ADP to mitochondria was higher in MLP-null hearts. Using a method that has been thoroughly tested in our laboratory, based on the use of tension measurements after SR calcium release induced by addition of caffeine, we found that SR calcium loading by mitochondrial ATP channelling was reduced, whereas loading supported by bound CK was similar to that in controls. To see whether our findings were specific to the MLP-deficient heart, we used a second model of cytoarchitectural perturbation, the desmin-null mouse heart, in which we also found impaired SR calcium loading supported by mitochondrial direct ATP channelling. We conclude that nucleotide channelling may depend on mitochondrial packing with the SR, such that cytoarchitectural perturbation could promote energetic dysfunction.
Methods
Animals
Two- to three-month-old C57BL/6 mice (control), MLP-null mice (kind gift from Professor Kieran Clarke, University of Oxford, UK) and desmin-null mice (kind gift from Dr D. Paulin, Institut Pasteur, France) were anaesthetized by intraperitoneal injection of sodium thiopental (150 mg kg−1). Hearts were excised and rinsed in ice-cold calcium-free Krebs solution equilibrated with 95% O2–5% CO2. For measurements of mitochondrial, SR and myofibrillar function, papillary muscle fibres were dissected and permeabilized for 30 min in saponin (50 μg ml−1) or Triton X-100 (1%), as previously described (De Sousa et al. 1999; Kaasik et al. 2001). This study conformed to the guidelines of the animal welfare committee of INSERM.
Electron microscopy
Left ventricular tissue was excised from control and MLP-null mouse hearts (n = 5, both groups), and fixed with 2% glutaraldehyde in cacodylate buffer (150 mmol l−1 sodium cacodylate and 2 mmol l−1CaCl2; pH 7.3). Tissue samples were postfixed in cacodylate buffer with 1% osmium tetroxide, contrasted with 1% uranyl acetate in H2O, dehydrated, and embedded in Durcupan (Fluka Chemie AG, Sweden). Ultrathin longitudinal sections were stained with lead citrate and viewed using a JEM 1200 (JEOL, Japan) electron microscope. Images were recorded using a Gatan DualVision 300 W CCD camera (Gatan Inc., Warrendale, PA, USA).
Mitochondrial function
Respiration rate and its sensitivity to ADP and creatine were measured in saponin-permeabilized control and MLP-null cardiac fibres using a Clark electrode (Strathkelvin Instruments), as previously described (Veksler et al. 1995). We determined the basal rate of mitochondrial oxygen consumption in the absence of ADP (V0), the maximal rate of oxygen consumption stimulated with 2 mmol l−1 ADP (Vmax), the acceptor control ratio (Vmax/V0) (control, n = 11; MLP-null, n = 29) and the apparent Michaelis-Menten constant for ADP, Km(ADP) (control, n = 8; MLP-null, n = 20).
Myofibrillar intrinsic properties and estimation of SR calcium uptake
The intrinsic sensitivity of control and MLP-null (n = 8, both groups) myofibrils to calcium and ATP was measured in Triton X-100-permeabilized cardiac fibres, as previously described (Ventura-Clapier et al. 1995). SR calcium loading for 30, 60, 180 and 300 s was measured in saponin-permeabilized control (n = 7–10) and MLP-null (n = 5–11) mouse cardiac fibres, and for 180 s in desmin-null fibres (n = 10) as previously described (Minajeva et al. 1996; Kaasik et al. 2001). Briefly, calcium uptake at pCa 6.5 (pCa is the negative logarithm of the free calcium concentration) occurred in the presence of exogenous ATP, with or without endogenous mitochondrial ATP production and/or activation of the CK system. SR calcium release was elicited with 5 mmol l−1 caffeine, and was detected using the resulting contractile force transient. Despite using different energetic loading conditions, calcium release was always measured using the same solution, which provided exogenous ATP, mitochondrial and CK-system activation together. By relating the subsequent contractile force elicited with caffeine to the calcium–force curve for each fibre, the [Ca2+]–time integral was calculated as an index of SR calcium load (for further details and discussion see Minajeva et al. 1996; Kaasik et al. 2001). The ATP channelling solution (ATP + Mito) contained (mmol l−1): EGTA 10 (0.2 during calcium release), Bes 40 (pH 7.1), free Mg2+ 1, taurine 10, glutamic acid 5, malic acid 2, K2HPO4 3, dithiothreitol 0.5, P1,P5 diadenosine pentaphosphate 0.04 (to inhibit adenylate kinase activity), ruthenium red 0.005 (to block mitochondrial calcium uptake), MgATP 3.16; ionic strength was adjusted to 160 mmol l−1 with potassium methanesulphonate, and 6% dextran was added to maintain normal cell volume. Addition of 12 mmol l−1 phosphocreatine (PCr) to this solution caused SR loading by both ATP channelling and the bound CK system (ATP + Mito + CK, optimal energetic conditions), and when 2 mm sodium azide was also present, then mitochondrial respiration was blocked so that loading was by the CK system alone (ATP + CK). Addition of 2 mmol l−1 azide in the absence of PCr caused SR loading by exogenous ATP alone (ATP).
Biochemical analysis
Activities of total CK, citrate synthase and mitochondrial complex I were measured using standard assays (Wharton & Tzagoloff, 1967; Estornell et al. 1993; Ventura-Clapier et al. 1995; Veksler et al. 1995).
Statistics
Data are expressed as means ±s.e.m. Statistical differences between two or more groups were determined by analysis of variance (ANOVA). Differences in SR loading by the same fibre under different energetic conditions were assessed by one-way ANOVA with repeated measures, using a Newman–Keuls test. Significance was defined by P < 0.05 (95% confidence).
Results
Anatomy and cardiac cytoarchitecture in MLP-null mice
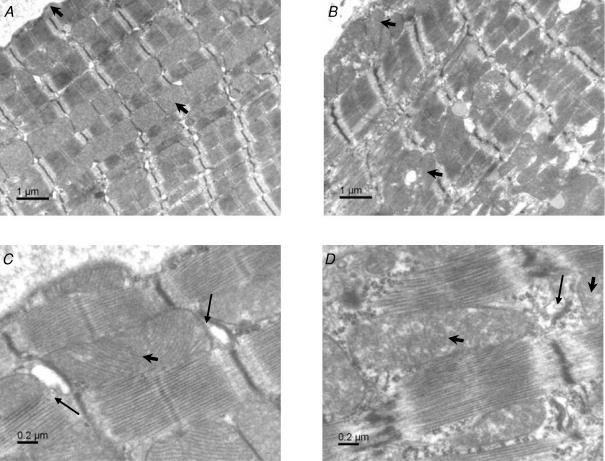
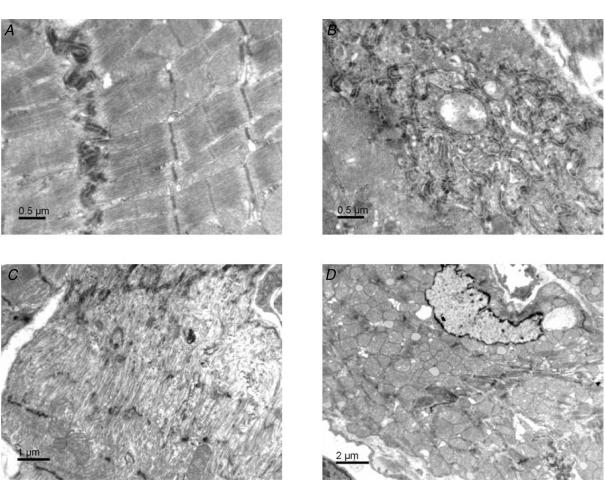
Compared with controls, heart and lung weights normalized to tibia length were increased in MLP-null mice by 36% and 20%, respectively (Table 1). Using electron microscopy, we confirmed that cardiac cytoarchitecture was greatly perturbed in MLP-null mice (Figs 1 and 2). In control heart, there was an orderly arrangement of myofibrils, interspersed by tightly packed rows of mitochondria (Fig. 1A and C). By contrast, MLP-null hearts had disorganized myofibrils with widened Z-lines, and intermyofibrillar mitochondria that were irregularly dispersed and not arranged in longitudinal columns (Fig. 1B and D). Mitochondria, myofibrils and SR appeared to be less tightly packed in MLP-null tissue, with more cytosol visible, and the content of subsarcolemmal mitochondria was increased (Fig. 1B and D). At intercalated disks, membrane folding was more elaborate in MLP-null heart than in controls, often with myofibrillar degeneration and an absence of mitochondria (Fig. 2A–C). However, large mitochondrial aggregates were sometimes visible in other regions of MLP-null myocytes (Fig. 2D), so that no obvious changes in total mitochondrial content were apparent.
Table 1.
Anatomical parameters in MLP-null mice
| Control | MLP-null | |
|---|---|---|
| Age (days) | 77 ± 3 | 74 ± 2 |
| Body wt (g) | 26.3 ± 0.4 | 26.1 ± 0.5 |
| Tibia length (mm) | 17.7 ± 0.1 | 17.4 ± 0.4 |
| Heart wt/tibia length (mg mm−1) | 7.4 ± 0.2 | 10.1 ± 0.5** |
| Lung wt/tibia length (mg mm−1) | 7.9 ± 0.2 | 9.5 ± 0.6* |
P < 0.05
P < 0.0001 versus Control.
Figure 1. Electron microscopic images of left ventricle from control and MLP-null mice.
A, overview of a control myocyte in longitudinal section, with mitochondria arranged in longitudinal columns. B, overview of an MLP-null cardiomyocyte, showing myofibrillar disorganization, an irregular arrangement of intermyofibrillar mitochondria, and increased content of subsarcolemmal mitochondria. C, detail of sarcomeres in a control myocyte, showing mitochondria tightly packed with SR. D, detail of sarcomeres in an MLP-null myocyte, showing looser packing of mitochondria with SR, irregular and widened Z-lines, and cytoplasm in the intermyofibrillar space. Arrowheads show mitochondria, arrows show SR.
Figure 2. Electron microscopic images of intercalated disks in control and MLP-null mouse heart, and of mitochondrial aggregates in MLP-null myocytes.
A, longitudinal section of control myocyte showing myofibrils attached to the intercalated disc. B, an oblique section through the widely spread intercalated disc of an MLP-null myocyte. C, a longitudinal section through a region of intercalated disc lacking myofibrils in an MLP-null myocyte. D, mitochondrial aggregates in MLP-null myocytes.
Myofibrillar, mitochondrial and CK function
Myofibrillar function was assessed in permeabilized cardiac fibres from MLP-null and control mice (Table 2). Maximal active tension at pCa 4.5 was 54% lower in MLP-null mouse heart than in controls, consistent with myofibrillar disarray, whereas passive tension, calcium sensitivity and ATP sensitivity were unchanged. In order to determine the intrinsic activity of ATP-regenerating systems in MLP-null cardiac muscle (i.e. independently of SR- or myosin-ATPase function), we measured mitochondrial function in situ and total CK activity in heart tissue extracts (Table 2). The maximal rate of ADP-stimulated mitochondrial respiration with glutamate and malate as electron donors, and total ADP-stimulated CK activity, were similar in control and MLP-null mice, suggesting that the intrinsic function of these two systems was normal. However, mitochondrial apparent Km(ADP) was 35% lower in MLP-null cardiac fibres than in controls (P < 0.05), indicating increased sensitivity to ADP. The basal, uncoupled respiration rate, V0, was 28% lower in MLP-null fibres, and consequently the acceptor control ratio was 49% higher. Total citrate synthase and the activity of complex I of the respiratory chain were normal in MLP-null heart (Table 2), consistent with normal mitochondrial content and maximal respiration rate.
Table 2.
Organellar function and enzyme activities
| Con | MLP-null | |
|---|---|---|
| Myofibrillar function | ||
| Active tension (mN mm−2) | 39 ± 3 | 18 ± 1** |
| Resting tension (mN mm−2) | 2.6 ± 0.4 | 3.1 ± 0.3 |
| pCa50 | 5.71 ± 0.02 | 5.67 ± 0.01 |
| pATP50 | 3.24 ± 0.04 | 3.29 ± 0.04 |
| Mitochondrial function | ||
| V0 (μmol O2 min−1 (g dry wt−1)) | 6.0 ± 0.4 | 4.3 ± 0.2* |
| Vmax (μmol O2 min−1 (g dry wt−1)) | 22.5 ± 1.1 | 23.3 ± 1.0 |
| Acceptor control ratio | 3.9 ± 0.3 | 5.8 ± 0.3** |
| Apparent Km(ADP) | 270 ± 34 | 175 ± 15* |
| Enzyme activities | ||
| Total CK (IU (g protein)−1) | 4226 ± 385 | 3134 ± 365 |
| CS (IU (g protein)−1) | 942 ± 112 | 923 ± 67 |
| Complex I (IU (g protein)−1) | 933 ± 47 | 898 ± 88 |
P < 0.05
P < 0.005 versus Control. pCa50, (pCa for half-maximal active tension); pATP50, (pATP for half-maximal rigor tension); CK, creatine kinase; CS, citrate synthase.
SR calcium uptake
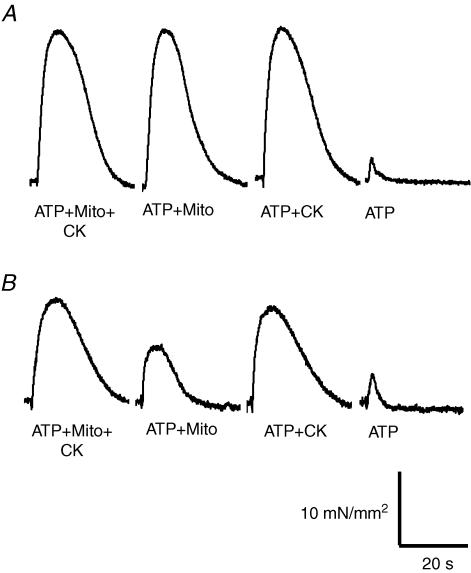
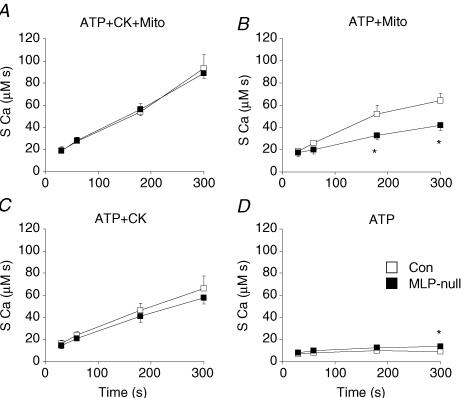
In saponin-permeabilized control and MLP-null mouse cardiac fibres, we measured SR calcium loading over time, supported by mitochondrial direct ATP channelling and bound CK (Figs 3–5). The SR was loaded with calcium using different solutions that controlled the ATP source, and SR calcium content was assessed using the force transient induced by stimulation with caffeine. Caffeine-induced tension transients had decreased amplitude in MLP-null cardiac fibres compared to controls (Fig. 3), consistent with reduced maximal active tension (Table 2). Using the force–calcium relationship for each fibre, developed tension was converted into calcium concentration units, and the resulting calcium concentration–time integral (SCa) was used as an index of SR calcium content. By this method, we found similar SR calcium content in control and MLP-null fibres after loading under optimal energetic conditions (ATP + Mito + CK, Fig. 4A). It was clear that mitochondrial ATP channelling occurred in both control and MLP-null mouse fibres, because calcium uptake was much greater with ATP + Mito solution than with exogenous ATP alone (Figs 4 and 5). Compared with controls, SR calcium load supported by mitochondrial direct ATP channelling (ATP + Mito) was decreased by 37% and 35% in MLP-null fibres after loading for 180 and 300 s, respectively (P < 0.05, Fig. 4B). Relative to controls, calcium loading supported by bound CK (ATP + CK) was normal in MLP-null fibres (Fig. 4C), whereas loading supported by exogenous ATP alone was 54% higher after loading for 300 s (9.2 ± 0.9 and 14.2 ± 1.7 μMs, respectively) (Fig. 4C and D). Note that after calcium loading for 300 s in control mice, there was a tendency towards lower calcium release in the presence of mitochondrial substrates relative to maximum loading capacity (ATP + MITO + CK), but this was not statistically significant (P = 0.19, n = 5; one-way ANOVA with repeated measures (Newman–Keuls test).
Figure 3. Representative tension transients during SR calcium release in control (A) and MLP-null (B) cardiac fibres.
The SR was loaded with calcium for 3 min in permeabilized fibres under different energetic conditions (ATP + Mito + CK, ATP + Mito, ATP + CK, ATP, see Methods), and then calcium load was estimated by relating these tension transients, elicited by caffeine stimulation, to the calcium–tension curve for each fibre.
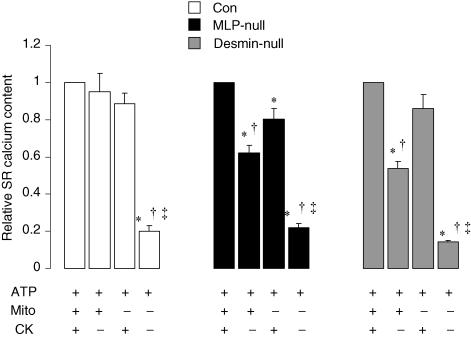
Figure 5. Relative SR calcium content after loading for 180 s, as a proportion of the load effected by ATP + Mito + CK in the same fibre, under different energetic conditions in control, MLP-null and desmin-null cardiac fibres.
*P < 0.05 versus ATP + Mito + CK, †P < 0.05 versus ATP + CK, ‡P < 0.05 versus ATP + Mito. See Results for precise P values.
Figure 4. SR calcium loading over time under different energetic conditions in control and MLP-null mouse cardiac fibres.
SR calcium load was estimated using the surface of the tension transient caused by caffeine stimulation (SCa). Energy supplied by exogenous ATP, mitochondria and the creatine kinase reaction (ATP + CK + Mitro, panel A), by exogenous ATP and mitochondria (ATP + Mitro, panel B), by exogenous ATP and creatine kinase (ATP + CK, panel C) and by exogenous ATP alone (ATP, panel D). *P < 0.05 versus control (Con).
In order to determine the relative ability of different energetic systems to support SR calcium loading, we compared the size of caffeine-induced calcium transients in the same fibre after loading for 180 s under each of the four conditions (Fig. 5). In controls, SR calcium content was similar whether loading was supported by mitochondrial direct ATP channelling, by bound CK, or by both systems together, whereas loading with exogenous ATP alone was significantly lower compared with these conditions.
In MLP-null fibres, loading by mitochondrial direct ATP channelling (ATP + Mito) was 23% lower than loading by ATP + CK (P = 0.005), and 38% lower than loading by ATP + Mito + CK (P = 0.0001). Loading by bound CK (ATP + CK) was 20% lower than loading by ATP + Mito + CK in MLP-null fibres (P = 0.01), and loading with exogenous ATP alone was significantly lower than all other conditions. Similar results were obtained with both groups after 300 s of loading (data not shown).
Relative SR calcium content after loading for 180 s was also measured in desmin-null mouse cardiac fibres (Fig. 5). Loading supported by ATP + Mito was 37% lower than loading by ATP + CK (P = 0.03), and 46% lower than loading by ATP + Mito + CK (P = 0.004), whereas loading by bound CK (ATP + CK) was not significantly different from loading by ATP + Mito + CK. Therefore, as with MLP-null hearts, desmin-null fibres had reduced SR calcium content supported by mitochondrial direct ATP channelling.
These data reveal that in cardiac fibres lacking the cytoskeletal proteins MLP or desmin, energetic coupling of mitochondria with the sarcoplasmic/endoplasmic reticulum Ca2+-ATPase (SERCA) by direct nucleotide channelling was impaired, whereas energetic support by bound CK was nearly normal.
Discussion
In this study, we used a model of subcellular disorganization, the MLP-null mouse heart, to determine the role cytoarchitecture plays in the direct transfer of energy from mitochondria to the SR calcium pump. Relative to controls, MLP-null hearts were characterized by: (1) myofibrillar disorganization, looser packing of mitochondria with myofibrils and SR, and increased accessibility of exogenous ADP to mitochondria; (2) normal mitochondrial content and oxidative capacity and normal total CK activity; and (3) impaired calcium uptake with mitochondrial direct ATP channelling, but near-normal calcium uptake when bound CK was active. Similarly impaired direct mitochondrial energy channelling was also observed in hearts lacking the cytoskeletal protein desmin. Therefore, perturbation of cardiac cytoarchitecture may impair direct energy transfer between mitochondria and SR.
MLP-null mice develop dilated cardiomyopathy between 4 and 8 weeks of age (Hoshijima et al. 2002). In addition, rodent cardiomyocyte cytoarchitecture is not fully developed until 6–8 weeks of age, when physiological cardiomyocyte hypertrophy and energy flux compartmentation are complete (Hoerter et al. 1994). Use of 2- to 3-month-old MLP-null mice was therefore advantageous in that developmental changes in cytoarchitecture were complete and heart failure was not long established, so that oxidative capacity and CK activity were not yet decreased, and therefore changes in mitochondrial direct ATP channelling could be related to cytoarchitecural perturbation. In addition, to show that changes were not specifically related to MLP loss, but were most probably a consequence of cytoarchitectural disorganization, we used another genetic model of cytoarchitectural perturbation, the desmin-null mouse, and found similarly impaired mitochondrial direct ATP channelling.
Myofibrillar disarray with intercalated disk remodelling is a hallmark of MLP-null mouse cardiomyopathy (Arber et al. 1997; Minamisawa et al. 1999; Ehler et al. 2001), as we confirmed using electron microscopy. Consistent with this phenotype, and shown here for the first time, we found lower maximal active tension in Triton X-100-skinned cardiac fibres lacking MLP, although myofibrillar calcium and ATP sensitivities were unchanged. We also found increased cytosolic space in MLP-null hearts, as previously described (Arber et al. 1997), concomitant with looser packing of mitochondria with SR and myofibrils. van den Bosch et al. (2005) similarly reported misalignment of mitochondria with myofibrils in MLP-null mouse hearts, with reduced mitochondrial size and some regions lacking mitochondria altogether. Although we saw regions lacking mitochondria, and fewer intermyofibrilar mitochondria, we also observed areas with mitochondrial accumulation, and more subsarcolemmal mitochondria in MLP-null heart. This may reflect differences between MLP-null mouse colonies in the two studies, or different stages of cardiomyopathy, which is progressive in this model (Hongo et al. 2000). Maximal glutamate-/malate-stimulated respiration rate, complex I activity and citrate synthase levels were normal in our MLP-null hearts, therefore we conclude that at this stage, mitochondrial content and tissue oxidative capacity were normal, whereas the juxtaposition of mitochondria with SR and myofibrils was perturbed. It should be noted that we took care to assess mitochondrial function without disrupting the cellular structure so that our results reflect the true mitochondrial reorganization/disorganization. Thus, some rearrangement between both populations of mitochondria may have occurred. Such redistribution, despite maintained mitochondrial function within fibres, could result in diminished local mitochondria–SR crosstalk. Whatever the case, it emphasizes that subcellular organization is important for energetic regulation of SERCA, because a change in the distribution of functional mitochondria within cells alters local ATP/ADP ratio in the vicinity of SERCA.
MLP-null mouse heart mitochondria had a lower apparent Km(ADP) in situ, indicating increased sensitivity to stimulation by exogenous ADP. This is entirely consistent with looser mitochondrial packing and increased cytosolic space in these hearts, which would provide greater access of exogenous ADP to mitochondria. Conversely, we would expect the Km(ADP) for endogenous ADP produced by SERCA or myosin ATPase to be increased, because nucleotide channelling was impaired and mitochondria and SR appeared to be less tightly packed together in MLP-null hearts. This change in ADP sensitivity was therefore probably a consequence of cytoarchitectural perturbation. Alternatively, increased mitochondrial outer membrane permeability may underlie a decreased Km(ADP) (Saks et al. 2003). As with MLP-deficient animals, mice lacking desmin had cardiomyopathy with a reduced mitochondrial Km(ADP) in situ and no change in oxidative capacity (Kay et al. 1997), suggesting a similar pathophysiology in other models of cytoskeletal protein loss. Compared to controls, basal respiration rate was lower in MLP-null mouse cardiac fibres, causing a significant increase in the acceptor control ratio and suggesting reduced proton leak across the inner mitochondrial membrane. As MLP may promote expression of uncoupling protein UCP3 (de Lange et al. 2004), which is associated with dissipation of the proton gradient (Boehm et al. 2001), it is possible that this was due to decreased UCP3 levels. Total ADP-stimulated CK activity was not significantly lower in MLP-null and control hearts, although it approached significance (P = 0.07). CK and mitochondrial function are decreased in other models of heart failure (De Sousa et al. 1999), and may have decreased in our MLP-null hearts as the cardiomyopathy progressed.
To investigate a direct interaction between organelles, we used a method that we initially developed 10 years ago (Minajeva et al. 1996). It is based on the use of cardiac bundles permeabilized with saponin in order to preserve the integrity of the cellular architecture. It uses myofilaments as an internal probe for calcium released by SR. Maximal SR calcium uptake capacity is assessed by recording the tension–time integral of the tension transient induced by caffeine at optimal and constant energy supply (ATP + Mito + CK). An advantage is that myofilaments respond to ‘the functional calcium’. To circumvent possible confounding effects due to intrinsic myofibrillar changes in MLP (or desmin) deficiency and sensitization of myofilaments by caffeine, the calcium sensitivity of myofilaments was assessed for each fibre and released calcium was calculated from tension changes and calcium sensitivity (in the presence of caffeine, i.e. the same condition as during calcium release). Released calcium was thus calculated from tension changes and calcium sensitivity, which are equivalent to a calibration curve for each fibre (Minajeva et al. 1996). An alternative possibility would have been to use a recently developed SR-localized calcium indicator in order to monitor calcium loading. We tried to use two such indicators, Mag-Fluo4 and fluo-5N in mouse heart, but had great difficultly with the protocol. Both dyes appeared to saturate too soon during loading, and Mag-Fluo4 revealed itself to be highly susceptible to bleaching. Others have also reported specific difficulties with SR-loaded fluorescent dyes in rodent myocytes (Venetucci et al. 2003). Another alternative to our approach would have been to measure SR calcium release using fluorescent indicators located in the external medium. It is clear that there are also technical problems to overcome and that it requires expertise. In addition, most of the fluorescent dyes also interact with caffeine thus altering calcium measurements (Muschol et al. 1999). Nevertheless, we believe that both approaches would provide similar results. Indeed, using permeabilized fibres, we could show the importance of local ATP/ADP ratios for optimal functioning of ATPases either by bound CK (Minajeva et al. 1996) or by close energetic interactions between subcellular organelles (Kaasik et al. 2001). These results have been confirmed and extended by others using fluorescent dyes (Duke & Steele, 1999; Yang & Steele, 2002) and biochemical (Saks et al. 2001) approaches.
To determine the effects of cytoarchitectural perturbation on mitochondrial direct ATP channelling, we compared SR calcium uptake supported by mitochondria, bound CK and exogenous ATP in permeabilized control and MLP-null mouse cardiac fibres. Previous studies have shown normal SERCA2 and phospholamban levels in MLP deficiency (Minamisawa et al. 1999; Lorenzen-Schmidt et al. 2005) and consistent with this, we now report normal SR calcium loading under optimal energetic conditions (ATP + Mito + CK). However, we found a defect in mitochondrial direct ATP supply (ATP + Mito) to the SR in MLP deficiency, which was not related to changes in the intrinsic oxidative capacity of mitochondria or a specific limitation in the SERCA activity. In desmin-null hearts, we found a specific defect in the support for SR calcium uptake by mitochondrial direct ATP channelling, suggesting that this process may be impaired in many models of cytoskeletal protein loss and cytoarchitectural perturbation. Loading supported by bound CK (ATP + CK) was slightly depressed in MLP-null fibres relative to loading under optimal energetic conditions, consistent with the tendency towards lower total CK activity in this model. With exogenous ATP alone, SR calcium content in MLP-null fibres was significantly higher than in controls after loading for 5 min, consistent with increased nucleotide accessibility to intracellular organelles.
The main finding of this work is that muscle cytoarchitecture may play an important role in myofibrillar, SR and mitochondrial function and in direct channelling of ADP from the SR to mitochondria. For example, it has already been shown that myofibrillar disarray in cardiomyopathic hearts, is associated with reduced contractile function (Arber et al. 1997; McConnell et al. 1999), while remodelling of the SR–T-tubule junction may underlie decreased excitation–contraction coupling gain in heart failure (Gomez et al. 2001). Others have shown that cytoskeletal proteolysis increased the rate of ADP diffusion and the sensitivity of mitochondrial respiration to ADP stimulation, concomitant with reduced ADP channelling from myofibrils/SR to mitochondria (Saks et al. 2003). Previously, we showed that loss of mitochondrial and cytosolic CK caused compensatory remodelling of the cytoarchitecture in cardiac and skeletal muscle, characterized by greater packing of mitochondria with myofibrils and SR, concomitant with maintained calcium uptake supported by mitochondrial direct ATP channelling (Kaasik et al. 2001, 2003). Recently it was suggested that muscle cytoarchitecture is organized around the ‘intracellular energetic unit’ (ICEU), which consists of a sarcomere coupled with a mitochondrion, SR and two T-tubules, and which promotes nucleotide channelling and efficient communication between mitochondria, SR and sarcomeres (Saks et al. 2001). Our electron microscopy images reveal disrupted ICEUs in MLP deficiency, which were associated with impaired mitochondrial direct ATP channelling to the SR, thus underlining the importance of muscle cytoarchitecture in energy transfer.
In this study we have shown that independent of changes in the energy-producing systems, cytoarchitectural changes can also affect the energetic support for calcium uptake. The amplitude of the calcium transient is decreased in MLP-deficient mouse cardiomyocytes and relaxation is slower in MLP-null mouse heart (Minamisawa et al. 1999; Esposito et al. 2000), perhaps partly as a results of impaired direct nucleotide channelling between mitochondria and SR (van den Bosch et al. 2005). This may be especially important under conditions of increased workload, in which energy supply could become limiting. It is interesting that correction of the calcium cycling defect in MLP-null mice by phospholamban gene deletion also corrected the cytoarchitectural changes, suggesting that impaired calcium handling and the resulting haemodynamic stress may themselves engender cytoarchitectural disorganization (Minamisawa et al. 1999).
In summary, we have shown that in two models of cytoarchitectural perturbation and heart failure, MLP-null and desmin-null mouse hearts, there was a specific decrease in SR calcium loading supported by mitochondrial direct ATP channelling, with near-normal loading supported by bound CK. Cardiac cytoarchitecture is therefore optimized both for force production and efficient energy transfer, such that cytoarchitectural perturbation may impair mitochondrial direct ATP channelling and contribute to energy wastage and contractile dysfunction.
Acknowledgments
This work was supported by La Fondation de France, The Leverhulme Trust (J.R.W.), the Centre National de la Recherche Scientifique (F.J., R.V-C.), APVT(Agency for Research and Development)-51-31104 and VEGA(Grant Agency for Science) 2/3189/25 (M.N.). The Franco-Slovak collaboration was funded by an integrated program Stefanik grant. We are grateful to Allen Kaasik and Rodolphe Fischmeister for their support.
References
- Andrienko T, Kuznetsov AV, Kaambre T, Usson Y, Orosco A, Appaix F, Tiivel T, Sikk P, Vendelin M, Margreiter R, Saks VA. Metabolic consequences of functional complexes of mitochondria, myofibrils and sarcoplasmic reticulum in muscle cells. J Exp Biol. 2003;206:2059–2072. doi: 10.1242/jeb.00242. [DOI] [PubMed] [Google Scholar]
- Arber S, Hunter JJ, Ross J, Jr, Hongo M, Sansig G, Borg J, Perriard JC, Chien KR, Caroni P. MLP-deficient mice exhibit a disruption of cardiac cytoarchitectural organization, dilated cardiomyopathy, and heart failure. Cell. 1997;88:393–403. doi: 10.1016/s0092-8674(00)81878-4. [DOI] [PubMed] [Google Scholar]
- Boehm EA, Jones BE, Radda GK, Veech RL, Clarke K. Increased uncoupling proteins and decreased efficiency in palmitate-perfused hyperthyroid rat heart. Am J Physiol Heart Circ Physiol. 2001;280:H977–H983. doi: 10.1152/ajpheart.2001.280.3.H977. [DOI] [PubMed] [Google Scholar]
- de Lange P, Ragni M, Silvestri E, Moreno M, Schiavo L, Lombardi A, Farina P, Feola A, Goglia F, Lanni A. Combined cDNA array/RT-PCR analysis of gene expression profile in rat gastrocnemius muscle: relation to its adaptive function in energy metabolism during fasting. FASEB J. 2004;18:350–352. doi: 10.1096/fj.03-0342fje. [DOI] [PubMed] [Google Scholar]
- De Sousa E, Veksler V, Minajeva A, Kaasik A, Mateo P, Mayoux E, Hoerter J, Bigard X, Serrurier B, Ventura-Clapier R. Subcellular creatine kinase alterations. Implications in heart failure. Circ Res. 1999;85:68–76. doi: 10.1161/01.res.85.1.68. [DOI] [PubMed] [Google Scholar]
- Duke AM, Steele DS. Effects of creatine phosphate on Ca2+ regulation by the sarcoplasmic reticulum in mechanically skinned rat skeletal muscle fibres. J Physiol. 1999;517:447–458. doi: 10.1111/j.1469-7793.1999.0447t.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dzeja PP, Redfield MM, Burnett JC, Terzic A. Failing energetics in failing hearts. Curr Cardiol Rep. 2000;2:212–217. doi: 10.1007/s11886-000-0071-9. [DOI] [PubMed] [Google Scholar]
- Dzeja PP, Terzic A. Phosphotransfer networks and cellular energetics. J Exp Biol. 2003;206:2039–2047. doi: 10.1242/jeb.00426. [DOI] [PubMed] [Google Scholar]
- Ehler E, Horowits R, Zuppinger C, Price RL, Perriard E, Leu M, Caroni P, Sussman M, Eppenberger HM, Perriard JC. Alterations at the intercalated disk associated with the absence of muscle LIM protein. J Cell Biol. 2001;153:763–772. doi: 10.1083/jcb.153.4.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito G, Santana LF, Dilly K, Cruz JD, Mao L, Lederer WJ, Rockman HA. Cellular and functional defects in a mouse model of heart failure. Am J Physiol Heart Circ Physiol. 2000;279:H3101–H3112. doi: 10.1152/ajpheart.2000.279.6.H3101. [DOI] [PubMed] [Google Scholar]
- Estornell E, Fato R, Pallotti F, Lenaz G. Assay conditions for the mitochondrial NADH: coenzyme Q oxidoreductase. FEBS Lett. 1993;332:127–131. doi: 10.1016/0014-5793(93)80498-j. [DOI] [PubMed] [Google Scholar]
- Fatkin D, Graham RM. Molecular mechanisms of inherited cardiomyopathies. Physiol Rev. 2002;82:945–980. doi: 10.1152/physrev.00012.2002. [DOI] [PubMed] [Google Scholar]
- Gomez AM, Guatimosim S, Dilly KW, Vassort G, Lederer WJ. Heart failure after myocardial infarction: altered excitation-contraction coupling. Circulation. 2001;104:688–693. doi: 10.1161/hc3201.092285. [DOI] [PubMed] [Google Scholar]
- Hoerter JA, Ventura-Clapier R, Kuznetsov A. Compartmentation of creatine kinases during perinatal development of mammalian heart. Mol Cell Biochem. 1994;133–134:277–286. doi: 10.1007/BF01267960. [DOI] [PubMed] [Google Scholar]
- Hongo M, Ryoke T, Schoenfeld J, Hunter J, Dalton N, Clark R, Lowe D, Chien K, Ross J., Jr Effects of growth hormone on cardiac dysfunction and gene expression in genetic murine dilated cardiomyopathy. Basic Res Cardiol. 2000;95:431–441. doi: 10.1007/s003950070018. [DOI] [PubMed] [Google Scholar]
- Hoshijima M, Pashmforoush M, Knoll R, Chien KR. The MLP family of cytoskeletal Z disc proteins and dilated cardiomyopathy: a stress pathway model for heart failure progression. Cold Spring Harb Symp Quant Biol. 2002;67:399–408. doi: 10.1101/sqb.2002.67.399. [DOI] [PubMed] [Google Scholar]
- Kaasik A, Veksler V, Boehm E, Novotova M, Minajeva A, Ventura-Clapier R. Energetic crosstalk between organelles: architectural integration of energy production and utilization. Circ Res. 2001;89:153–159. doi: 10.1161/hh1401.093440. [DOI] [PubMed] [Google Scholar]
- Kaasik A, Veksler V, Boehm E, Novotova M, Ventura-Clapier R. From energy store to energy flux: a study in creatine kinase-deficient fast skeletal muscle. FASEB J. 2003;17:708–710. doi: 10.1096/fj.02-0684fje. [DOI] [PubMed] [Google Scholar]
- Kay L, Li Z, Mericskay M, Olivares J, Tranqui L, Fontaine E, et al. Study of regulation of mitochondrial respiration in vivo. An analysis of influence of ADP diffusion and possible role of cytoskeleton. Biochim Biophys Acta. 1997;1322:41–59. doi: 10.1016/s0005-2728(97)00071-6. [DOI] [PubMed] [Google Scholar]
- Lorenzen-Schmidt I, Stuyvers BD, Ter Keurs HE, Date MO, Hoshijima M, Chien KR, McCulloch AD, Omens JH. Young MLP deficient mice show diastolic dysfunction before the onset of dilated cardiomyopathy. J Mol Cell Cardiol. 2005;39:241–250. doi: 10.1016/j.yjmcc.2005.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConnell BK, Jones KA, Fatkin D, Arroyo LH, Lee RT, Aristizabal O, et al. Dilated cardiomyopathy in homozygous myosin-binding protein-C mutant mice. J Clin Invest. 1999;104:1235–1244. doi: 10.1172/JCI7377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minajeva A, Ventura-Clapier R, Veksler V. Ca2+ uptake by cardiac sarcoplasmic reticulum ATPase in situ strongly depends on bound creatine kinase. Pflugers Arch. 1996;432:904–912. doi: 10.1007/s004240050214. [DOI] [PubMed] [Google Scholar]
- Minamisawa S, Hoshijima M, Chu G, Ward CA, Frank K, Gu Y, Martone ME, Wang Y, Ross J, Jr, Kranias EG, Giles WR, Chien KR. Chronic phospholamban-sarcoplasmic reticulum calcium ATPase interaction is the critical calcium cycling defect in dilated cardiomyopathy. Cell. 1999;99:313–322. doi: 10.1016/s0092-8674(00)81662-1. [DOI] [PubMed] [Google Scholar]
- Muschol M, Dasgupta BR, Salzberg BM. Caffeine interaction with fluorescent calcium indicator dyes. Biophys J. 1999;77:577–586. doi: 10.1016/S0006-3495(99)76914-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saks V, Kaambre T, Sikk P, Eimre M, Orlova E, Paju K, Piirsoo A, Appaix F, Kay L, Regitz-Zagrosek V, Fleck E, Seppet E. Intracellular energetic units in red muscle cells. Biochem J. 2001;356:643–657. doi: 10.1042/0264-6021:3560643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saks V, Kuznetsov A, Andrienko T, Usson Y, Appaix F, Guerrero K, Kaambre T, Sikk P, Lemba M, Vendelin M. Heterogeneity of ADP diffusion and regulation of respiration in cardiac cells. Biophys J. 2003;84:3436–3456. doi: 10.1016/S0006-3495(03)70065-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagawa H, Koide M, Sato H, Zile MR, Carabello BA, Cooper G., 4th Cytoskeletal role in the transition from compensated to decompensated hypertrophy during adult canine left ventricular pressure overloading. Circ Res. 1998;82:751–761. doi: 10.1161/01.res.82.7.751. [DOI] [PubMed] [Google Scholar]
- van den Bosch BJ, van den Burg CM, Schoonderwoerd K, Lindsey PJ, Scholte HR, de Coo RF, et al. Regional absence of mitochondria causing energy depletion in the myocardium of muscle LIM protein knockout mice. Cardiovasc Res. 2005;65:411–418. doi: 10.1016/j.cardiores.2004.10.025. [DOI] [PubMed] [Google Scholar]
- Veksler VI, Kuznetsov AV, Anflous K, Mateo P, van Deursen J, Wieringa B, Ventura-Clapier R. Muscle creatine kinase-deficient mice. II. Cardiac and skeletal muscles exhibit tissue-specific adaptation of the mitochondrial function. J Biol Chem. 1995;270:19921–19929. doi: 10.1074/jbc.270.34.19921. [DOI] [PubMed] [Google Scholar]
- Venetucci L, Trafford AW, Eisner DA. Illuminating sarcoplasmic reticulum calcium. Circ Res. 2003;93:4–5. doi: 10.1161/01.RES.0000082768.74160.8D. [DOI] [PubMed] [Google Scholar]
- Ventura-Clapier R, Garnier A, Veksler V. Energy metabolism in heart failure. J Physiol. 2004;555:1–13. doi: 10.1113/jphysiol.2003.055095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventura-Clapier R, Kuznetsov AV, d'Albis A, van Deursen J, Wieringa B, Veksler VI. Muscle creatine kinase-deficient mice. I. Alterations in myofibrillar function. J Biol Chem. 1995;270:19914–19920. doi: 10.1074/jbc.270.34.19914. [DOI] [PubMed] [Google Scholar]
- Wang SQ, Wei C, Zhao G, Brochet DX, Shen J, Song LS, Wang W, Yang D, Cheng H. Imaging microdomain Ca2+ in muscle cells. Circ Res. 2004;94:1011–1022. doi: 10.1161/01.RES.0000125883.68447.A1. [DOI] [PubMed] [Google Scholar]
- Wharton DC, Tzagoloff A. Cytochrome oxidase from beef heart mitochondria. Methods Enymol. 1967;10:245–250. [Google Scholar]
- Wilding JR, Schneider JE, Sang AE, Davies KE, Neubauer S, Clarke K. Dystrophin- and MLP-deficient mouse hearts: marked differences in morphology and function, but similar accumulation of cytoskeletal proteins. FASEB J. 2005;19:79–81. doi: 10.1096/fj.04-1731fje. [DOI] [PubMed] [Google Scholar]
- Yang Z, Steele DS. Effects of phosphocreatine on SR Ca2+ regulation in isolated saponin-permeabilized rat cardiac myocytes. J Physiol. 2002;539:767–777. doi: 10.1113/jphysiol.2001.012987. [DOI] [PMC free article] [PubMed] [Google Scholar]