Abstract
Maternal nutrient restriction (NR) affects fetal development with long-term consequences on postnatal health of offspring, including predisposition to obesity and diabetes. Most studies have been conducted in fetuses in late gestation, and little information is available on the persistent impact of NR from early to mid-gestation on properties of offspring skeletal muscle, which was the aim of this study. Pregnant ewes were subjected to 50% NR from day 28–78 of gestation and allowed to deliver. The longissimus dorsi muscle was sampled from 8-month-old offspring. Maternal NR during early to mid-gestation decreased the number of myofibres in the offspring and increased the ratio of myosin IIb to other isoforms by 17.6 ± 4.9% (P < 0.05) compared with offspring of ad libitum fed ewes. Activity of carnitine palmitoyltransferase-1, a key enzyme controlling fatty acid oxidation, was reduced by 24.7 ± 4.5% (P < 0.05) in skeletal muscle of offspring of NR ewes and would contribute to increased fat accumulation observed in offspring of NR ewes. Intramuscular triglyceride content (IMTG) was increased in skeletal muscle of NR lambs, a finding which may be linked to predisposition to diabetes in offspring of NR mothers, since enhanced IMTG predisposes to insulin resistance in skeletal muscle. Proteomic analysis by two-dimensional gel electrophoresis demonstrated downregulation of several catabolic enzymes in 8-month-old offspring of NR ewes. These data demonstrate that the early to mid-gestation period is important for skeletal muscle development. Impaired muscle development during this stage of gestation affects the number and composition of fibres in offspring which may lead to long-term physiological consequences, including predisposition to obesity and diabetes.
Failure of the fetus to achieve its optimal growth potential remains a major unsolved obstetric problem. Compromised growth in utero is associated with increased risk of neonatal death and long-term morbidity (Redmer et al. 2004). Many clinical disorders during gestation, including those of genetic, metabolic, vascular, coagulative, autoimmune and infective origins, result in poor fetal growth and development (Cetin et al. 2004). Whereas the primary causes are diverse, reduced fetal growth is by definition generally secondary to inadequate nutrition. Reduced oxygen delivery arising from reduced uterine and umbilical blood flow is often an additional insult in the total picture of intrauterine growth restriction (IUGR).
Sub-optimal nutrition during fetal development has long-term consequences in postnatal life, including predisposition of offspring to obesity and diabetes (Selak et al. 2003; Ozanne & Nicholas Hales, 2005). However, most studies of fetal nutrient restriction (NR) have been conducted at a late stage in gestation, and little information is available about the impact on offspring musculature of maternal NR in the early stages of gestation.
The fetal period is crucial for skeletal muscle development, because no net increase in the number of muscle fibres occurs after birth (Glore & Layman, 1983; Greenwood et al. 2000; Nissen et al. 2003). Skeletal muscle has a lower priority in nutrient partitioning compared with the brain and heart in response to the challenges the fetus faces during development, rendering it particularly vulnerable to nutrient deficiency (Bauman et al. 1982; Close & Pettigrew, 1990).We previously showed that NR from early to mid-gestation resulted in a reduction in the number of fetal skeletal muscle fibres, which might be related to a downregulation of mTOR signalling (Zhu et al. 2004). Skeletal muscle is the main site for the utilization of fatty acids and glucose (Petersen & Shulman, 2002). Impairment of skeletal muscle development due to NR from early to mid-gestation is likely to contribute significantly to the well-described predisposition of offspring of nutrient restricted mothers to obesity and diabetes (Ozanne & Nicholas Hales, 2005; Zambrano et al. 2005). The objective of this study was to determine if maternal NR from early to mid-gestation alters the muscle fibre composition and lipid metabolism of offspring skeletal muscle.
Methods
Animals
All animal procedures were approved by the University of Wyoming Animal Care and Use Committee. A detailed description of animal maintenance procedures has been published previously (Vonnahme et al. 2003). Briefly, white face ewes of mixed breeding were weighed on day 20 of gestation to enable calculation of individual diets on a metabolic body weight basis (weight0.75). The diet consisted of a pelleted beet pulp (79.7% total digestible nutrients (TDN), 93.5% dry matter (DM), and 10.0% crude protein). Rations were delivered on a DM basis to meet the total TDN required for maintenance for an early pregnant ewe (NRC requirements) (NRC, 1985). A mineral/vitamin mixture: 51.43% sodium triphosphate, 47.62% potassium chloride, 0.39% zinc oxide, 0.06% cobalt acetate, and 0.50% ADE vitamin premix (3629 000 IU vitamin A, 3629 000 IU vitamin D3, and 181 000 IU vitamin E per kg) was included with the beet pulp pellets to meet requirements.
On day 21 of gestation, all ewes were placed in individual pens and fed control rations. On day 28, half of the ewes were randomly assigned to a control-fed group (100% NRC requirements, CF) and the other half to a nutrient-restricted group (50% NRC requirements, NR). The two groups were balanced for weight and body condition score. Body condition score can be used to estimate the energy reserve available to ewes (Sanson et al. 1993). Beginning on day 28 of gestation, and continuing at 7 day intervals, ewes were weighed and rations adjusted up for weight gain and down for weight loss. The average weights of ewes on day 28 gestation were 78.27 ± 3.36 and 72.47 ± 2.89 kg, respectively, for CF and NR treatments which were similar, and were 80.00 ± 3.37 and 64.86 ± 1.33 kg, respectively, on day 78 gestation which differed (P < 0.01). This was due to a 6.86 ± 0.68% body weight gain in the CF ewes and a 10.50 ± 0.39% body weight loss in the NR ewes by day 78 gestation.
At day 78 of gestation, all ewes were fed control diets meeting 100% of NRC requirements. At term, body weight of CF and NR-realimented ewes were similar. Ewes in each group were allowed to lamb. All lambs were given free access to a standard commercially available creep feed (Lamb Creep B30 w/Bovatec; Ranch-Way Feeds, Ft Collins, CO, USA) from birth to weaning. At 120 ± 2 days of age, wether lambs were weaned, and pairs of lambs of the same treatment group were placed in pens containing a feeder and waterer. Lambs were weighed at weekly intervals thereafter and transitioned to a high-concentrate feed (All-American Show Lamb Grower; Ranch-Way Feeds) with partial rations of hay until the concentrate could be utilized as their sole diet. Lambs were fed this concentrate ad libitum until they were slaughtered (Ford et al. 2006). Lambs were then slaughtered at the University of Wyoming abattoir using captive bolt stunning and exsanguination, a procedure recommended by Federation of Animal Science Societies, USA (FASS, 1999). Longissimus dorsi (Ld) and semitendenosus muscles were weighed and sampled. Two small pieces of muscle (approximately 1 g) were cut out from the centre of the Ld muscle; one piece was snap-frozen in liquid nitrogen and was used for biochemical analysis, and one piece was placed in fresh paraformaldehyde before being embedded in paraffin. In addition, the kidney and pelvic fat were collected and weighed. The carcass weight was obtained after removing the hide, head and viscera.
Immunoblotting
Ld muscle (0.1 g) was powdered in liquid nitrogen and homogenized in a polytron homogenizer (7 mm diameter generator) with 400 μl of ice-cold buffer containing 137 mm NaCl, 50 mm Hepes, 2% SDS, 1% NP-40, 10% glycerol, 2 mm PMSF, 10 mm sodium pyrophosphate, 10 μg ml−1 aprotinin, 10 μg ml−1 leupeptin, 2 mm Na3VO4, 100 mm NaF, pH 7.4. The protein content of lysates was determined by the Bradford method (Bio-Rad Laboratories, Hercules, CA, USA) (Du et al. 2004).
Each Ld muscle homogenate was mixed with an equal volume of 2× standard SDS sample loading buffer. A Hoefer mini-gel system was used for casting gels and running electrophoresis. Gradient gels of 5–20% were used for SDS-PAGE separation of proteins. After electrophoresis, the proteins on the gels were transferred to nitrocellulose membranes in a transfer buffer containing 20 mm Tris-base, 192 mm glycine, 0.1% SDS and 20% methanol (Du et al. 2004). Membranes were incubated in a blocking solution consisting of 5% non-fat dry milk in TBS/T (0.1% Tween-20, 50 mm Tris-HCl, pH 7.6, and 150 mm NaCl) for 1 h. Membranes were incubated overnight in primary antibodies with 1:200 dilution for anti-Glut 1 and –Glut 4 antibodies, and 1:1000 dilution for anti-actin antibody in TBS/T with 1% non-fat dry milk. Polyclonal anti-Glut 1 and –Glut 4 antibodies were purchased from Santa Cruz Biotechnology, Inc. (CA, USA; catalogue numbers sc-7903 and sc-1607, respectively) and monoclonal anti-actin antibody (JLA20) was purchased from Developmental Studies Hybridoma Bank (Iowa City, IA, USA). After the primary antibody incubation, membranes were washed three times for 5 min each with 20 ml of TBS/T. Membranes were then incubated with horseradish-peroxidase-conjugated secondary antibody at proper dilution for 1 h in TBS/T with gentle agitation. After three 10 min washes, membranes were visualized using ECL Western blotting reagents (Amersham Biosciences) and exposure to film (MR; Kodak, Rochester, NY, USA). Density of bands was quantified by using an Imager Scanner II and ImageQuant TL software (Zhu et al. 2004).
Histochemical examination
Ld muscle samples were fixed in 4% (w/v) paraformaldehyde in phosphate buffer (0.12 m, pH 7.4), embedded in paraffin, and cut into 10 μm sections. Sections were rehydrated by using a series of incubations in xylene and ethanol solutions, and then stained with haematoxylin and eosin for standard light microscopy. Ten fields were randomly selected for quantification of muscle fibre diameters. The majority of muscle fibres were circular and, thus, diameter was easily measured. For irregular muscle fibres, however, a maximum and a minimum distance of the two opposite sides of the muscle fibre circle were measured, and the average value was regarded as the diameter of the fibre. The diameter of 10 muscle fibres was measured per field and 100 muscle fibres for each muscle sample were quantified using the Image J 1.30v software (National Institutes of Health, USA). Muscle fibre diameter was measured in a blind fashion. Averaged data were used for calculations. The percentage of muscle fibre numbers were calculated based on the total fibre number per examined area, where the number of CF lambs was regarded as 100% (Greenwood et al. 1999).
Identification of myofibre isoforms
Ld muscle samples (0.1 g) were homogenized in 500 μl of buffer containing 250 mm sucrose, 100 mm KCl, 5 mm EDTA and 20 mm Tris/HCl, pH 6.8. The homogenate was filtered through nylon cloth to remove debris and centrifuged at 10 000 g for 10 min. The pellet obtained was resuspended in 500 μl of washing buffer containing 200 mm KCl, 5 mm EDTA, 0.5% Triton X-100 and 20 mm Tris/HCl pH 6.8. The suspension was centrifuged at 10 000 g for 10 min. The pellet containing purified myofibrillar proteins was resuspended in 200 μl water and 300 μl of standard 2× sample loading buffer, and then boiled for 5 min. After centrifugation at 12 000 g for 5 min, the supernatant was used for electrophoresis.
The stacking gels consisted of 4% acrylamide (acrylamide:bis, 50:1), 5% (v/v) glycerol, 70 mm Tris/HCl, pH 6.7, 0.4% (w/v) SDS, 4 mm EDTA, 0.1% (w/v) APS and 0.01% (v/v) TEMED. The separation gel contained 5% (w/v) glycerol, acrylamide:bis (50:1) at a concentration 8%, 200 mm Tris, pH 8.8, 4 mm EDTA, 0.4% (w/v) SDS, 0.01% (v/v) TEMED, and 0.1% (w/v) ammonium persulphate. The upper running buffer consisted of 0.1 m Tris/HCl, pH 8.8, 0.1% (w/v) SDS, 150 mm glycine, and 10 mm mercaptoethanol, and the lower running buffer consisted of 50 mm Tris/HCl, pH 8.8, 0.05% (w/v) SDS, and 75 mm glycine. Gels were run at 4°C in a Hoefer SE 600 (Hoefer Scientific, San Francisco, CA, USA) unit, at constant 100 V for 24 h (Bamman et al. 1999). After electrophoresis, gels were silver stained, and scanned with a densitometer to determine the amount of each myosin isoforms, and percentage composition was reported. Silver staining was conducted using a kit from Bio-Rad Laboratories.
Carnitine palmitoyltransferase-1 (CPT-1) activity measurement
Ld muscle samples were homogenized in 10 vols buffer (w/v) containing 0.25 m sucrose, 0.2 mm EDTA, and 50 mm Tris/HCl, pH 7.5, at 9500 r.p.m. by a polytron homogenizer (7 mm diameter generator) for 10 s. The homogenate was centrifuged at 500 g for 10 min at 4°C to precipitate debris, and the supernatant was centrifuged at 13 000 g to pellet mitochondria. The mitochondria were resuspended in homogenization buffer, and mitochondrial preparation protein content was determined by the Bradford method (Bio-Rad Laboratories). The CPT-1 activity was measured in a buffer containing 100 mm Tris/HCl, pH 8.0, 0.1% Triton X-100, 1 mm EDTA, 0.01 mm palmitoyl CoA, 0.5 mm DTNB, with/without 1.25 mm l-carnitine. The reaction was monitored at 412 nm with a spectrophotometer (Bieber et al. 1972). The CPT-1 activity was calculated as the difference between the rates in the presence and absence of l-carnitine, and expressed either as micromoles of CoA released per minute per gram of muscle, or as nanomoles of CoA released per minute per milligram of mitochondrial protein.
Intramuscular triglyceride (IMTG)
After removing all visible fats, Ld muscle samples (0.5 g) were homogenized in 10 ml of 2:1 chloroform/methanol. Triglycerides were extracted and saponified in 4% ethanolic KOH. Free glycerol concentration in these samples was then determined spectrophotometrically as previously described (Schenk et al. 2005). Pure glycerol was used as the standard for quantification. The content of IMTG was expressed as millimoles of glycerol per kilogram of muscle.
Two-dimensional electrophoresis
Ld muscle samples (0.1 g) were homogenized in a polytron homogenizer (7 mm diameter generator) with 400 μl of ice-cold buffer containing 137 mm NaCl, 50 mm Hepes, 1 mm MgCl2, 1 mm CaCl2, 1% NP-40, 10% glycerol, 2 mm PMSF, 10 mm sodium pyrophosphate, 2.5 mm EDTA, 10 μg ml−1 aprotinin, 10 μg ml−1 leupeptin, 2 mm Na3VO4, and 100 mm NaF, pH 7.4 (Zhu et al. 2004).
Muscle homogenate (40 μl) was transferred to 600 μl of lysis buffer containing 8 m urea, 4% CHAPS, and 20 mm Tris/HCl, pH 7.4. The mixture was shaken at 4°C for 1 h and centrifuged at 12 000 g for 10 min at 4°C. The supernatant containing the soluble protein was collected and used to rehydrate commercially available immobilized-pH-gradient (IPG) strips (pH 3–10, 1 × 17 cm, Bio-Rad Laboratories). Rehydration of strips was conducted under manufacturer's instructions, followed by isoelectric focusing with a maximal voltage of 8000 V to reach 100 000 voltage hours. The strip was equilibrated in solution 1 (6 m urea, 2% SDS, 30% glycerol, 50 mm Tris/HCl, pH 8.8, 1% DTT) and solution 2 (6 m urea, 2% SDS, 30% glycerol, 50 mm Tris/HCl, pH 8.8, 2% iodoacetamide with 0.01% bromophenol blue) for 15 min each. The strip was loaded onto a precast 5–20% 200 × 200 mm gradient gel for second-dimension separation. To fix the strip on top of gradient gel, low melt agarose solution (0.188 m Tris/HCl, pH 8.8, 0.1% SDS, 9% glycerol, 1% agarose, 0.01% bromophenol blue) was heated to 65°C and then pipetted onto the IPG strip, followed by second-dimension SDS electrophoresis. Electrophoresis was conducted in electrophoresis solution (25 mm Tris base, 192 mm glycine, 1 g l−1 SDS) at 5°C, with 10 W per gel. After the front dye line arrived at the end of the gel, the gels were removed and fixed (40% ethanol, 10% acetic acid) before being stained with Coomassie blue. After staining, gels were scanned by an Image Scanner II (Amersham Biosciences) (Arthur et al. 2002).
Quantitative analysis of protein expression and in-gel trypsin digestion
Image Master (Amershan Biosciences) software was used for matching and quantitative analysis of the protein spots on the gels according to manufacturer's instructions. Proteins with differential expression (P ≤ 0.05) were selected for identification by a matrix assisted laser desorption ionization time of flight (MALDI-TOF) mass spectrometer. Briefly, protein spots with differential expression were excised, cut into 1 mm3 pieces, and washed two more times with 50% acetonitrile, 50 mm ammonium bicarbonate for 15 min, with gentle shaking, or until the Coomassie dye had been completely removed. Gel pieces were dehydrated in 100% acetonitrile for 5 min and incubated in a solution containing 50 mm ammonium bicarbonate and 10 mm dithiothreitol for 30 min at 56°C, and then incubated in another solution containing 50 mm ammonium bicarbonate and 55 mm iodoacetamide (freshly made) for 30 min in the dark. The gel pieces were washed with acetonitrile, air dried, and rehydrated with 20 μg ml−1 trypsin (sequencing grade; Promega, Madison, WI, USA) in 50 mm ammonium bicarbonate. Then, gel pieces rehydrated with trypsin were incubated at 37°C overnight. After centrifugation at 12 000 g for 5 min, supernatant (containing tryptic peptides) was transferred to a sterile centrifuge tube. An aliquot of extraction solution (25–50 μl, comprising 60% acetonitrile and 1% TFA) was added to the gel pieces and agitated gently by vortexing at the lowest setting for 10 min. The above step was repeated once and supernatants were pooled together. Supernatants were dried under vacuum, and 5 μl of resuspension solution (50% acetonitrile, 0.1% TFA) was added to each tube to resolve peptides (Arthur et al. 2002).
Mass spectrometry and protein identification
Peptides were mixed with an equal volume of 10 mg ml−1 α-cyano-4-hydroxycinnamic acid in 65% acetonitrile/0.3% trifluoroacetic acid, and applied to the steel plate for MALDI-TOF peptide fingerprinting analysis. The instrument (Voyager DE-STR; Applied Biosystems, Foster City, CA, USA) was set at a positive ion reflector mode, and the laser strength and voltage were optimized to obtain the greatest signal:noise ratio.
Proteins were identified by searching against MSDB and NCBInr protein database through the Mascot Peptide Mass Fingerprint software, which is available free for academic use at http://www.matrixscience.com/. The search parameters were as follows: the fixed carbamidomethyl modification was chosen; up to one missed cleavage was allowed; and peptide tolerance was set at 1.0 Da (Kwon et al. 2003; Vitorino et al. 2004).
Statistical analysis
Data were analysed as a complete randomized design using GLM (General Linear Model of Statistical Analysis System, SAS, 2000). The differences in the mean values were compared by the Tukey's multiple comparison, and means ± s.e.m. are reported. Statistical significance was considered as P < 0.05.
Results
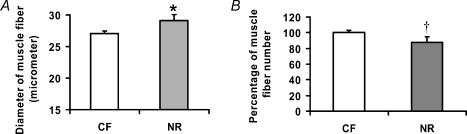
Live weight of offspring of NR ewes was increased and absolute and relative amount of kidney and pelvic fat was greater than that of CF lambs (P < 0.05, Table 1). Total and relative mass of the semitendinosus and Ld did not differ between offspring of NR and CF ewes (Table 1). NR increased Ld muscle fibre diameter (P < 0.05, Fig. 1). However, the number of muscle fibres was not reduced, though there was a trend of reduction in NR offspring (P < 0.10, Fig. 1).
Table 1.
Selected fat and muscle weight of 8-month-old wether lambs
| Category | CF | NR |
|---|---|---|
| Live weight (kg) | 56.80 ± 1.23 | 61.72 ± 1.62* |
| Carcass weight (kg) | 28.83 ± 0.92 | 31.62 ± 1.01† |
| Kidney and pelvic fat (kg) | 0.46 ± 0.05 | 0.68 ± 0.06* |
| Left Ld muscle (g) | 776.5 ± 12.1 | 775.7 ± 30.0 |
| Left semitendinosus muscle (g) | 166.4 ± 4.4 | 169.3 ± 3.9 |
| Kidney and pelvic fat (% carcass weight) | 1.66 ± 0.12 | 2.18 ± 0.20* |
| Left Ld muscle (% carcass weight) | 2.71 ± 0.11 | 2.46 ± 0.08† |
| Left semitendinosus muscle (% carcass weight) | 0.58 ± 0.02 | 0.54 ± 0.02† |
CF, control fed; NR, nutrient restricted; Ld, longissimus dorsi. Means ± s.e.m. within a measurement differ
P < 0.05 and
P < 0.10.
Figure 1. Muscle fibre number and diameter of 8-month-old offspring of control fed and nutrient-restricted ewes.
A, average diameter of muscle fibres; B, muscle fibre number. (n = 8 per group). CF, control fed; NR, nutrient restricted. *P < 0.05; †P < 0.10. Data are means + s.e.m.
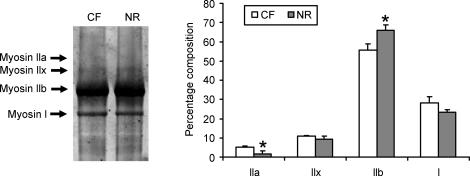
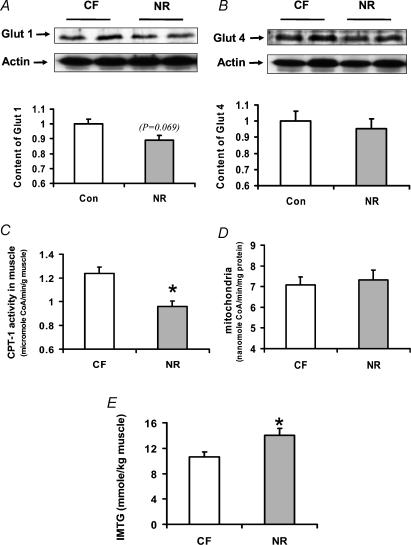
Each muscle fibre type has its corresponding myosin isoform, which was analysed by SDS-PAGE electrophoresis. The ratio of myosin IIb was increased by 17.6 ± 4.9% (P < 0.05) in skeletal muscle of offspring NR ewes (Fig. 2), with a corresponding reduction in the ratio of type IIa muscle fibres by 69.1 ± 21.9% (P < 0.05) compared with offspring of CF ewes. No significant difference was detected in Glut 1 and Glut 4 contents in skeletal muscle of offspring of NR ewes (Fig 3A and B). The activity of CPT-1, a key enzyme controlling fatty acid oxidation, was decreased (24.7 ± 4.5%, P < 0.05) in Ld of offspring of NR ewes when expressed as muscle weight (Fig. 3C). However, no difference in CPT-1 activity was evident when expressed relative to mitochondrial protein concentration (Fig. 3D). The IMTG was higher (31.8 ± 10.7%, P < 0.05) in muscle of offspring of NR ewes compared with muscle of offspring of CF ewes (Fig. 3E).
Figure 2. Composition of myosin isoforms in longissimus dorsi muscle of 8-month-old offspring of CF and NR ewes.
*P < 0.05. Data are means + s.e.m.; n = 8 per group.
Figure 3. Glut 1 and Glut 4 protein abundance, activity of carnitine palmitoyltransferase-1 (CPT-1) and intramuscular triglyceride content in skeletal muscle of 8-month-old offspring of CF and NR ewes.
A, Glut 1 immunoblot and statistical data; B, Glut 4 immunoblot and statistical data; C, CPT-1 activity based on muscle weight; D, CPT-1 activity based on mitochondrial protein; E, intramuscular triglyceride content. *P ≤ 0.05. Data are means + s.e.m.; n = 8 per group.
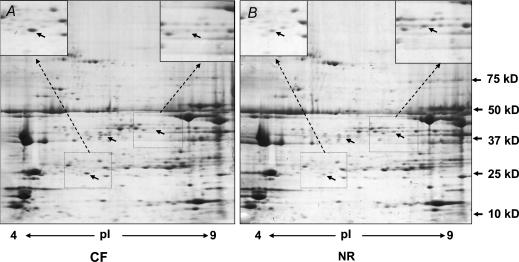
Two-dimensional gel electrophoresis showed a total of 27 protein spots with differential expression (P < 0.05, Fig. 4). Of these, 11 protein spots were tentatively identified by peptide finger printing using MALDI-TOF mass spectrometry (Table 2). Of proteins identified to be down-regulated in skeletal muscle of NR lambs, most of them were enzymes either in mitochondria or associated with glucose metabolism.
Figure 4. Two-dimensional gel electrophoresis of longissimus dorsi.
A, skeletal muscle from an offspring of a CF ewe; B, skeletal muscle from an offspring of a NR ewe. Arrows show protein spots with visible differential expression (n = 5 per group).
Table 2.
Identified proteins with differential expression (P < 0.05) between skeletal muscle of CF and NR lambs
| Protein | Fold increase or decrease | pI | Molecular mass (kDa) |
|---|---|---|---|
| Protein upregulated due to NR | |||
| p21 | 1.85 ± 0.20 | 4.81 | 24.0 |
| Fatty acid binding protein | 2.01 ± 0.41 | 5.27 | 13.1 |
| Histidine-tRNA ligase | 1.64 ± 0.20 | 5.72 | 57.9 |
| Protein downregulated due to NR | |||
| ATP synthase, H+ transporting | −3.45 ± 1.25 | 5.10 | 56.3 |
| Aldehyde dehydrogenase | −2.18 ± 0.37 | 6.15 | 50.5 |
| NADPH-cytochrome reductase | −1.34 ± 0.07 | 5.28 | 71.5 |
| Tropomyosin beta | −1.79 ± 0.20 | 4.66 | 32.8 |
| Glutathione S-transferase | −2.00 ± 0.32 | 6.24 | 25.9 |
| Triosephosphate isomerase | −1.41 ± 0.11 | 6.87 | 6.9 |
| Muscle-specific enolase | −1.43 ± 0.10 | 7.60 | 47.0 |
| Glucose-regulated protein ERp57 | −4.76 ± 1.06 | 6.23 | 57.3 |
Discussion
Fetal NR occurs frequently in human pregnancy (Wu et al. 2004). The causes vary from hyperemesis gravidarum to conditions in which maternal nutrition and/or uterine nutrient delivery is reduced by voluntary or imposed maternal nutrient intake or uterine vascular disease. The average loss of initial body weight of ewes during NR at the level used in our study was about 7.5% (Vonnahme et al. 2003), very similar to the 5–10% initial body weight loss observed in patients with hyperemesis gravidarum (Philip, 2003). We observed that NR confined to the period from early to mid-gestation caused metabolic damage to skeletal muscle development of offspring.
During prenatal muscle development, primary myofibres are first formed, followed by the formation of secondary myofibres (Beermann et al. 1978). The secondary myofibres are derived from muscle precursor cells which are initially maintained in a proliferating undifferentiated state (Swatland, 1973). Those precursor cells differentiate into myoblasts and fuse to form secondary myofibres parallel to primary myofibres (Beermann, 1978). Limited availability of nutrients influences the proliferation of precursor cells and may reduce the number of secondary and total myofibres in fetuses. We previously observed that when measured at 78 days of gestation fetal muscle fiber number was reduced due to NR in the period day 28 to day 78 of gestation (Zhu et al. 2004). This reduction in muscle fibre number is mainly due to reduction in secondary myofibre number (Zhu et al. 2004). In another report, peri-conceptional 50% NR of pregnant sheep reduced the total muscle fibre number of fetal muscle by about 20% (Quigley et al. 2005). Muscle fibre number at birth is of critical importance because the number of muscle fibres is fixed at this point (Greenwood et al. 2000; Nissen et al. 2003). Skeletal muscle is the main site for the utilization of glucose and fatty acids (Guo & Zhou, 2004; Krebs & Roden, 2004) and is the primary tissue responsible for insulin resistance in obese and type 2 diabetic subjects (Kemp et al. 2003; Lowell & Shulman, 2005).The reduction in skeletal muscle mass during fetal development may therefore have long-lasting irreversible negative physiological consequences for offspring, including predisposing offspring to obesity, diabetes and muscle weakness (Stannard & Johnson, 2004; Zambrano et al. 2005). Human infants who are small at birth are at greater risk for type 2 diabetes and obesity (Forsen et al. 2000; Harding, 2003; Eriksson et al. 2004). Decreased muscle mass is a major factor in low birth weight (Hediger et al. 1998; Eriksson et al. 2004). On the other hand, mice with enhanced fetal skeletal muscle growth and hyperplasia due to a muscle-specific-myostatin knockout are resistant to diabetes and obesity induced by high glucose and fat diets (J. Yang, University of Hawaii, personal communication). Therefore, the reduced muscle fibre number and the ratio of muscle mass to body weight observed in this study may have contributed to the glucose intolerance observed in these lambs, as reported elsewhere (Burt et al. 2005).
In addition to muscle mass, muscle fibre type composition affects the oxidative capacity of muscle. The oxidative capacity of muscle fibres generally follows the order of type I ≥ type IIa ≥ type IIb (He et al. 2001). Muscle fibre type composition also affects insulin sensitivity. GLUT4 is found in larger amounts in type I and IIa muscle fibres than in type IIb muscle fibres (Daugaard & Richter, 2001; Gaster et al. 2001). The insulin sensitivity of muscle fibres follows the order of type I ≥ type IIa ≥ type IIb (He et al. 2001). Insulin-resistance in skeletal muscle is associated with an increased number of type IIb muscle fibres (Nyholm et al. 1997). Thus, the altered fibre distribution in NR offspring we have observed would be expected to reduce the insulin sensitivity and glucose utilization of skeletal muscle. Indeed, less efficient glucose utilization was observed in these lambs at 8 months of age (Burt et al. 2005). In support of this, the content of Glut 4 was lower in skeletal muscle of NR lambs. These data strongly support the concept that the impairment of fetal skeletal muscle development as a result of early maternal NR predisposes skeletal muscle of offspring to insulin resistance. In keeping with this conclusion, rats whose mothers are fed a low protein diet during pregnancy and lactation show reduced skeletal muscle expression of various components of the insulin signalling cascade (Ozanne et al. 2005).
Our observation that the activity of CPT-1, a key enzyme controlling fatty acid oxidation, was decreased in muscles of lambs exposed to NR is consistent with the increased proportion of type IIb myofibres. In rats, surgically induced IUGR at late gestation impairs mitochondrial function and oxidative capacity of skeletal muscle (Selak et al. 2003). It is of importance in this regard that CPT-1 is located on the mitochondrial membrane. Further, our data suggested that this reduction in CPT-1 activity in NR muscle was due to a reduction in mitochondria density rather than their function, since no difference in CPT-1 activity was observed between CF and NR muscle after adjusting for the difference in mitochondrial density.
Reduction in CPT-1 activity would tend to reduce the fatty acid oxidation in NR muscle, leading to an accumulation of IMTG. Indeed, the IMTG content in muscle of offspring of NR ewes was higher than in muscle of offspring of CF ewes. Accumulation of intracellular lipids due to insufficient fatty acid oxidation by mitochondria leads to the suppression of insulin signalling (Lowell & Shulman, 2005), and insulin resistance (Krebs & Roden, 2004). These results are also in line with our observation that lambs of ewes exposed to maternal NR had 48% more renal and pelvic fat than control lambs (Table 1). This increased level of obesity would be compatible with decreased fatty acid oxidation.
To further demonstrate the mechanisms responsible for the alteration of skeletal muscle properties in NR lambs, we compared the protein profiles of CF and NR skeletal muscle by two-dimensional electrophoresis. We observed that several enzymes which are either located in mitochondria or are involved in energy metabolism are downregulated in skeletal muscle of offspring of NR compared with CF animals. This finding would also suggest that the ability of glucose and fatty acid utilization in skeletal muscle of NR offspring was impaired. Decreased glucose and fatty acid oxidation predisposes offspring to obesity and diabetes. Indeed, offspring delivered by dams that experienced maternal NR during pregnancy were fatter than control animals, in agreement with previous reports (Bispham et al. 2003; Symonds et al. 2004; Desai et al. 2005).
We previously observed that maternal NR downregulates mTOR signalling in fetal skeletal muscle at day 78 gestation (Zhu et al. 2004). Because mTOR signalling controls protein synthesis (Du et al. 2005; Latres et al. 2005), downregulation of mTOR signalling in fetal muscle would tend to decrease the rate of protein synthesis, reducing muscle fibre number and muscle mass as observed in fetuses exposed to nutrient restriction (Zhu et al. 2004). In our previous studies, we found that the serum amino acids and glucose content were reduced in NR fetuses (Kwon et al. 2004). Amino acids, especially branched-chain amino acids, are known activators of mTOR signalling. The reduction in branched-chain amino acids in fetal serum after nutrient deficiency would potentially downregulate mTOR signalling in fetal muscle. These observations need to be evaluated in any attempt to develop nutrient supplementation strategies for IUGR. We hypothesize that nutrient supplementation by effectively boosting fetal serum amino acids and glucose levels would upregulate mTOR signalling and promote protein synthesis in skeletal muscle of NR ewes. Further studies are needed to address this hypothesis.
In summary, we have demonstrated that maternal NR during early to mid-gestation negatively affects skeletal muscle development of offspring with long-term consequences. Changes induced by maternal NR include an increase in type IIb myofibres, decreased GLUT 4 transporter, decreased activity of CPT-1, accumulation of IMTG, and downregulation of key enzymes involved in mitochondrial function. Together these changes may at least partially explain the observed insulin resistance in NR offspring and the predisposition of offspring of NR ewes to obesity and diabetes.
Acknowledgments
The authors thank Justin Jones at the Macromolecular Core Equipment Facility, University of Wyoming, for MALDI-TOF analysis of peptides for protein identification. The authors thank Gunnar Olson, an undergraduate student in biology, University of Wyoming, for conducting the tissue section, myofibre counting and diameter measurements. This work was supported by University of Wyoming INBRE P20 RR016474-04, NIH-HD21350, and National Research Initiative Competitive grant 2006 55618-16914 from the USDA Cooperative State Research, Education, and Extension Service.
References
- Arthur JM, Thongboonkerd V, Scherzer JA, Cai J, Pierce WM, Klein JB. Differential expression of proteins in renal cortex and medulla: a proteomic approach. Kidney Int. 2002;62:1314–1321. doi: 10.1111/j.1523-1755.2002.kid588.x. [DOI] [PubMed] [Google Scholar]
- Bamman MM, Clarke MS, Talmadge RJ, Feeback DL. Enhanced protein electrophoresis technique for separating human skeletal muscle myosin heavy chain isoforms. Electrophoresis. 1999;20:466–468. doi: 10.1002/(SICI)1522-2683(19990301)20:3<466::AID-ELPS466>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Bauman DE, Eisemann JH, Currie WB. Hormonal effects on partitioning of nutrients for tissue growth: role of growth hormone and prolactin. Fed Proc. 1982;41:2538–2544. [PubMed] [Google Scholar]
- Beermann DH, Cassens RG, Hausman GJ. A second look at fiber type differentiation in porcine skeletal muscle. J Anim Sci. 1978;46:125–132. doi: 10.2527/jas1978.461125x. [DOI] [PubMed] [Google Scholar]
- Bieber LL, Abraham T, Helmrath T. A rapid spectrophotometric assay for carnitine palmitoyltransferase. Anal Biochem. 1972;50:509–518. doi: 10.1016/0003-2697(72)90061-9. [DOI] [PubMed] [Google Scholar]
- Bispham J, Gopalakrishnan GS, Dandrea J, Wilson V, Budge H, Keisler DH, Broughton Pipkin F, Stephenson T, Symonds ME. Maternal endocrine adaptation throughout pregnancy to nutritional manipulation: consequences for maternal plasma leptin and cortisol and the programming of fetal adipose tissue development. Endocrinology. 2003;144:3575–3585. doi: 10.1210/en.2003-0320. [DOI] [PubMed] [Google Scholar]
- Burt BE, Hess BW, Nathanielsz PW, Nijland MJ, Ford SP. Impact of multi-generational selection on insulin resistance in offspring of undernourished ewes. J Soc Gynecol Investig. 2005;12:278A–278A. [Google Scholar]
- Cetin I, Foidart JM, Miozzo M, Raun T, Jansson T, Tsatsaris V, Reik W, Cross J, Hauguel-De-Mouzon S, Illsley N, Kingdom J, Huppertz B. Fetal growth restriction: a workshop report. Placenta. 2004;25:753–757. doi: 10.1016/j.placenta.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Close WH, Pettigrew JE. Mathematical models of sow reproduction. J Reprod Fertil Suppl. 1990;40:83–88. [PubMed] [Google Scholar]
- Daugaard JR, Richter EA. Relationship between muscle fibre composition, glucose transporter protein4 and exercise training: possible consequences in non-insulin-dependent diabetes mellitus. Acta Physiol Scand. 2001;171:267–276. doi: 10.1046/j.1365-201x.2001.00829.x. [DOI] [PubMed] [Google Scholar]
- Desai M, Gayle D, Babu J, Ross MG. Programmed obesity in intrauterine growth-restricted newborns: modulation by newborn nutrition. Am J Physiol Regul Integr Comp Physiol. 2005;288:R91–R96. doi: 10.1152/ajpregu.00340.2004. [DOI] [PubMed] [Google Scholar]
- Du M, Zhu MJ, Means WJ, Hess BW, Ford SP. Effect of nutrient restriction on calpain and calpastatin content of skeletal muscle from cows and fetuses. J Anim Sci. 2004;82:2541–2547. doi: 10.2527/2004.8292541x. [DOI] [PubMed] [Google Scholar]
- Du M, Zhu MJ, Means WJ, Hess BW, Ford SP. Nutrient restriction differentially modulates the mammalian target of rapamycin signaling and the ubiquitin–proteasome system in skeletal muscle of cows and their fetuses. J Anim Sci. 2005;83:117–123. doi: 10.2527/2005.831117x. [DOI] [PubMed] [Google Scholar]
- Eriksson JG, Yliharsila H, Forsen T, Osmond C, Barker DJ. Exercise protects against glucose intolerance in individuals with a small body size at birth. Prev Med. 2004;39:164–167. doi: 10.1016/j.ypmed.2004.01.035. [DOI] [PubMed] [Google Scholar]
- FASS. Guide for the Care and Use of Agricultural Animals in Agricultural Research and Teaching. Federation of Animal Science Societies Savoy IL: 1999. [Google Scholar]
- Ford SP, Hess BW, Schwope MM, Nijland MJ, Gilbert JS, Vonnahme KA, Means WJ, Han H, Nathanielsz PW. Maternal undernutrition during early gestation in the ewe results in altered growth, adiposity and glucose tolerance in male offspring. J Anim Sci. 2006 doi: 10.2527/jas.2005-624. in press. [DOI] [PubMed] [Google Scholar]
- Forsen T, Eriksson J, Tuomilehto J, Reunanen A, Osmond C, Barker D. The fetal and childhood growth of persons who develop type 2 diabetes. Ann Intern Med. 2000;133:176–182. doi: 10.7326/0003-4819-133-3-200008010-00008. [DOI] [PubMed] [Google Scholar]
- Gaster M, Staehr P, Beck-Nielsen H, Schroder HD, Handberg A. GLUT4 is reduced in slow muscle fibers of type 2 diabetic patients: is insulin resistance in type 2 diabetes a slow, type 1 fiber disease? Diabetes. 2001;50:1324–1329. doi: 10.2337/diabetes.50.6.1324. [DOI] [PubMed] [Google Scholar]
- Glore SR, Layman DK. Cellular growth of skeletal muscle in weanling rats during dietary restrictions. Growth. 1983;47:403–410. [PubMed] [Google Scholar]
- Greenwood PL, Hunt AS, Hermanson JW, Bell AW. Effects of birth weight and postnatal nutrition on neonatal sheep. II. Skeletal muscle growth and development. J Anim Sci. 2000;78:50–61. doi: 10.2527/2000.78150x. [DOI] [PubMed] [Google Scholar]
- Greenwood PL, Slepetis RM, Hermanson JW, Bell AW. Intrauterine growth retardation is associated with reduced cell cycle activity, but not myofibre number, in ovine fetal muscle. Reprod Fertil Dev. 1999;11:281–291. doi: 10.1071/rd99054. [DOI] [PubMed] [Google Scholar]
- Guo Z, Zhou L. Evidence for increased and insulin-resistant lipolysis in skeletal muscle of high-fat-fed rats. Metabolism. 2004;53:794–798. doi: 10.1016/j.metabol.2003.11.029. [DOI] [PubMed] [Google Scholar]
- Harding JE. Nutrition and growth before birth. Asia Pac J Clin Nutr. 2003;12(suppl):S28. [Google Scholar]
- He J, Watkins S, Kelley DE. Skeletal muscle lipid content and oxidative enzyme activity in relation to muscle fiber type in type 2 diabetes and obesity. Diabetes. 2001;50:817–823. doi: 10.2337/diabetes.50.4.817. [DOI] [PubMed] [Google Scholar]
- Hediger ML, Overpeck MD, Kuczmarski RJ, McGlynn A, Maurer KR, Davis WW. Muscularity and fatness of infants and young children born small- or large-for-gestational-age. Pediatrics. 1998;102:E60. doi: 10.1542/peds.102.5.e60. [DOI] [PubMed] [Google Scholar]
- Kemp BE, Stapleton D, Campbell DJ, Chen ZP, Murthy S, Walter M, Gupta A, Adams JJ, Katsis F, Van Denderen B, Jennings IG, Iseli T, Michell BJ, Witters LA. AMP-activated protein kinase, super metabolic regulator. Biochem Soc Trans. 2003;31:162–168. doi: 10.1042/bst0310162. [DOI] [PubMed] [Google Scholar]
- Krebs M, Roden M. Nutrient-induced insulin resistance in human skeletal muscle. Curr Med Chem. 2004;11:901–908. doi: 10.2174/0929867043455620. [DOI] [PubMed] [Google Scholar]
- Kwon H, Ford SP, Bazer FW, Spencer TE, Nathanielsz PW, Nijland MJ, Hess BW, Wu G. Maternal nutrient restriction reduces concentrations of amino acids and polyamines in ovine maternal and fetal plasma and fetal fluids. Biol Reprod. 2004;71:901–908. doi: 10.1095/biolreprod.104.029645. [DOI] [PubMed] [Google Scholar]
- Kwon KH, Kim M, Kim JY, Kim KW, Kim SI, Park YM, Yoo JS. Efficiency improvement of peptide identification for an organism without complete genome sequence, using expressed sequence tag database and tandem mass spectral data. Proteomics. 2003;3:2305–2309. doi: 10.1002/pmic.200300620. [DOI] [PubMed] [Google Scholar]
- Latres E, Amini AR, Amini AA, Griffiths J, Martin FJ, Wei Y, Lin HC, Yancopoulos GD, Glass DJ. Insulin-like growth factor-1 (IGF-1) inversely regulates atrophy-induced genes via the phosphatidylinositol 3-kinase/Akt/mammalian target of rapamycin (PI3K/Akt/mTOR) pathway. J Biol Chem. 2005;280:2737–2744. doi: 10.1074/jbc.M407517200. [DOI] [PubMed] [Google Scholar]
- Lowell BB, Shulman GI. Mitochondrial dysfunction and type 2 diabetes. Science. 2005;307:384–387. doi: 10.1126/science.1104343. [DOI] [PubMed] [Google Scholar]
- Nissen PM, Danielsen VO, Jorgensen PF, Oksbjerg N. Increased maternal nutrition of sows has no beneficial effects on muscle fiber number or postnatal growth and has no impact on the meat quality of the offspring. J Anim Sci. 2003;81:3018–3027. doi: 10.2527/2003.81123018x. [DOI] [PubMed] [Google Scholar]
- National Research Council. Nutrient Requirements of Sheep. 6. Washington DC: National Academy Press; 1985. [Google Scholar]
- Nyholm B, Qu Z, Kaal A, Pedersen SB, Gravholt CH, Andersen JL, Saltin B, Schmitz O. Evidence of an increased number of type IIb muscle fibers in insulin-resistant first-degree relatives of patients with NIDDM. Diabetes. 1997;46:1822–1828. doi: 10.2337/diab.46.11.1822. [DOI] [PubMed] [Google Scholar]
- Ozanne SE, Nicholas Hales C. Poor fetal growth followed by rapid postnatal catch-up growth leads to premature death. Mech Ageing Dev. 2005;126:852–854. doi: 10.1016/j.mad.2005.03.005. [DOI] [PubMed] [Google Scholar]
- Petersen KF, Shulman GI. Pathogenesis of skeletal muscle insulin resistance in type 2 diabetes mellitus. Am J Cardiol. 2002;90:11G–18G. doi: 10.1016/s0002-9149(02)02554-7. [DOI] [PubMed] [Google Scholar]
- Philip B. Hyperemesis gravidarum: literature review. WMJ. 2003;102:46–51. [PubMed] [Google Scholar]
- Quigley SP, Kleemann DO, Kakar MA, Owens JA, Nattrass GS, Maddocks S, Walker SK. Myogenesis in sheep is altered by maternal feed intake during the peri-conception period. Anim Reprod Sci. 2005;87:241–251. doi: 10.1016/j.anireprosci.2004.11.005. [DOI] [PubMed] [Google Scholar]
- Redmer DA, Wallace JM, Reynolds LP. Effect of nutrient intake during pregnancy on fetal and placental growth and vascular development. Domest Anim Endocrinol. 2004;27:199–217. doi: 10.1016/j.domaniend.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Sanson DW, West TR, Tatman WR, Riley ML, Judkins MB, Moss GE. Relationship of body composition of mature ewes with condition score and body weight. J Anim Sci. 1993;71:1112–1116. doi: 10.2527/1993.7151112x. [DOI] [PubMed] [Google Scholar]
- Schenk S, Cook JN, Kaufman AE, Horowitz JF. Postexercise insulin sensitivity is not impaired after an overnight lipid infusion. Am J Physiol Endocrinol Metab. 2005;288:E519–E525. doi: 10.1152/ajpendo.00401.2004. [DOI] [PubMed] [Google Scholar]
- Selak MA, Storey BT, Peterside I, Simmons RA. Impaired oxidative phosphorylation in skeletal muscle of intrauterine growth-retarded rats. Am J Physiol Endocrinol Metab. 2003;285:E130–E137. doi: 10.1152/ajpendo.00322.2002. [DOI] [PubMed] [Google Scholar]
- Stannard SR, Johnson NA. Insulin resistance and elevated triglyceride in muscle: more important for survival than ‘thrifty’ genes? J Physiol. 2004;554:595–607. doi: 10.1113/jphysiol.2003.053926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swatland HJ. Muscle growth in the fetal and neonatal pig. J Anim Sci. 1973;37:536–545. doi: 10.2527/jas1973.372536x. [DOI] [PubMed] [Google Scholar]
- Symonds ME, Pearce S, Bispham J, Gardner DS, Stephenson T. Timing of nutrient restriction and programming of fetal adipose tissue development. Proc Nutr Soc. 2004;63:397–403. doi: 10.1079/pns2004366. [DOI] [PubMed] [Google Scholar]
- Vitorino R, Lobo MJ, Ferrer-Correira AJ, Dubin JR, Tomer KB, Domingues PM, Amado FM. Identification of human whole saliva protein components using proteomics. Proteomics. 2004;4:1109–1115. doi: 10.1002/pmic.200300638. [DOI] [PubMed] [Google Scholar]
- Vonnahme KA, Hess BW, Hansen TR, McCormick RJ, Rule DC, Moss GE, Murdoch WJ, Nijland MJ, Skinner DC, Nathanielsz PW, Ford SP. Maternal undernutrition from early- to mid-gestation leads to growth retardation, cardiac ventricular hypertrophy, and increased liver weight in the fetal sheep. Biol Reprod. 2003;69:133–140. doi: 10.1095/biolreprod.102.012120. [DOI] [PubMed] [Google Scholar]
- Wu G, Bazer FW, Cudd TA, Meininger CJ, Spencer TE. Maternal nutrition and fetal development. J Nutr. 2004;134:2169–2172. doi: 10.1093/jn/134.9.2169. [DOI] [PubMed] [Google Scholar]
- Zambrano E, Martinez-Samayoa PM, Bautista CJ, Deas M, Guillen L, Rodriguez-Gonzalez GL, Guzman C, Larrea F, Nathanielsz PW. Sex differences in transgenerational alterations of growth and metabolism in progeny (F2) of female offspring (F1) of rats fed a low protein diet during pregnancy and lactation. J Physiol. 2005;566:225–236. doi: 10.1113/jphysiol.2005.086462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu MJ, Ford SP, Nathanielsz PW, Du M. Effect of maternal nutrient restriction in sheep on the development of fetal skeletal muscle. Biol Reprod. 2004;71:1968–1973. doi: 10.1095/biolreprod.104.034561. [DOI] [PubMed] [Google Scholar]