Abstract
The present study was designed to assess the effects of dietary leucine supplementation on muscle protein synthesis and whole body protein kinetics in elderly individuals. Twenty healthy male subjects (70 ± 1 years) were studied before and after continuous ingestion of a complete balanced diet supplemented or not with leucine. A primed (3.6 μmol kg−1) constant infusion (0.06 μmol kg−1 min−1) of l-[1-13C]phenylalanine was used to determine whole body phenylalanine kinetics as well as fractional synthesis rate (FSR) in the myofibrillar fraction of muscle proteins from vastus lateralis biopsies. Whole body protein kinetics were not affected by leucine supplementation. In contrast, muscle FSR, measured over the 5-h period of feeding, was significantly greater in the volunteers given the leucine-supplemented meals compared with the control group (0.083 ± 0.008 versus 0.053 ± 0.009% h−1, respectively, P < 0.05). This effect was due only to increased leucine availability because only plasma free leucine concentration significantly differed between the control and leucine-supplemented groups. We conclude that leucine supplementation during feeding improves muscle protein synthesis in the elderly independently of an overall increase of other amino acids. Whether increasing leucine intake in old people may limit muscle protein loss during ageing remains to be determined.
During ageing, a decline in skeletal muscle mass occurs in both humans (Forbes & Halloran, 1976; Dutta & Hadley, 1995) and rodents (Holloszy et al. 1991). This atrophy is associated with a loss of muscle strength, which directly affects the mobility and health of elderly people. The mechanisms leading to sarcopenia are still unclear but result from an imbalance between rates of protein synthesis and degradation. This imbalance is not obvious when basal rates of protein turnover are measured (Dardevet et al. 1994; Mosoni et al. 1995; Volpi et al. 2001) but is detected in the postprandial state. An apparent defect in the stimulation of muscle protein synthesis has been shown in old rats (Mosoni et al. 1995) and elderly humans (Arnal et al. 1999) after the ingestion of a normal protein meal. Moreover, muscle protein breakdown has been shown to be unresponsive to food intake in aged rats (Combaret et al. 2005). This defect results in a small daily muscle protein loss, leading in the long term to muscle wasting in the elderly. The origin of this alteration remains obscure because muscle protein synthesis responded normally if large amounts of amino acids were continuously infused in old rats (Mosoni et al. 1993) or given orally in aged volunteers (Volpi et al. 1999). Nevertheless, the studies agreed in that aged muscle is less sensitive to the stimulatory effects of amino acids at physiological concentrations but is still able to respond if the increase of aminoacidaemia is large enough (Volpi et al. 1999; Arnal et al. 1999; Paddon-Jones et al. 2004). Recently, the same conclusion was drawn with essential amino acids (EAAs) by Katsanos et al. (2005) who showed in the elderly, that a small bolus of EAAs (∼7 g) was unable to stimulate muscle protein synthesis whereas an increase in protein synthesis occurred normally when EAA intake was doubled (Paddon-Jones et al. 2004).
Among the amino acids, leucine seems to play the major role. Indeed, Anthony et al. (2000a,b) showed that orally administered leucine stimulated muscle protein synthesis by itself in vivo and this was partly independent of insulin. Furthermore, leucine has been shown to act as a true mediator by specifically modulating the activities of intracellular kinases linked to the translation of proteins such as mammalian target of rapamycin (mTOR)/70 kDa ribosomal protein S6 (p70S6K) kinases (Kimball et al. 1999; Anthony et al. 2000b; Dardevet et al. 2000). We recently demonstrated in vitro that protein synthesis in old rat muscles becomes resistant to the stimulatory effect of leucine at its physiological concentration range (Dardevet et al. 2000). However, when leucine concentration was increased greatly above its postprandial level, protein synthesis was stimulated normally (Dardevet et al. 2002; Rieu et al. 2003) and the inhibition of muscle protein breakdown was restored in old rats (Combaret et al. 2005). Based on our observations, dietary leucine supplementation may represent a useful nutritional tool for the maintenance of muscle mass and the prevention of sarcopenia in the elderly. To our knowledge, the beneficial effect of a specific leucine supplementation in aged humans has only been shown by Katsanos et al. (2006) in combination with a bolus of EAAs. Whether a specific leucine effect on muscle protein synthesis can be obtained with leucine-supplemented meals under normal postprandial conditions (i.e. in the presence of carbohydrates and lipids) remains to be demonstrated. Indeed, Volpi et al. (2000) showed that the response of muscle protein anabolism to a large amino acid intake was blunted in combination with other nutrients (especially glucose) in the elderly. The aim of the present study was to evaluate the effect of complete meals (containing protein, carbohydrates and lipids) enriched or not with leucine on whole body protein metabolism and muscle protein synthesis in elderly volunteers.
Methods
Subjects
Twenty healthy elderly male subjects (69.6 ± 0.8 years) participated in the study. The physical characteristics of the subjects are indicated in Table 1. A history of clinical events was recorded for all subjects and a physical examination was performed before recruitment. All subjects recruited had normal blood biochemical profiles and appeared normal in physical examinations, without any chronic diseases. The experimental protocol was approved by the local ethical commitee (Comité Consultatif pour la Protection des Personnes en Recherche Biomédicale de Clermont Ferrand) and was conducted according to the Declaration of Helsink. The nature and potential risks of the study were fully explained to each volunteer and written informed consent was obtained before the study from each participant. To avoid marked differences between protein metabolism between individuals (linked to heterogeneity in their habitual dietary intakes), the volunteers were asked to follow a controlled protein intake adjusted to their body weight providing 0.8 g protein kg−1 day−1 during a 4 day period before the study.
Table 1.
Subject characteristics
| Control | Leucine | |
|---|---|---|
| Age (years) | 69.7 ± 1.5 | 69.5 ± 0.8 |
| Height (cm) | 172.8 ± 2.2 | 170.8 ± 1.4 |
| Weight (kg) | 75.4 ± 3.2 | 73.8 ± 2.4 |
| BMI (kg m−2) | 25.2 ± 0.7 | 25.3 ± 0.7 |
| Fasting glucose (mmol l−1) | 4.7 ± 0.1 | 4.7 ± 0.2 |
| Fasting albumin (g l−1) | 38.7 ± 0.8 | 38.2 ± 0.8 |
| Fasting creatinine (μmol l−1) | 87.9 ± 4.0 | 90.3 ± 3.8 |
Values are expressed as means ± s.e.m.
Materials
l-[1-13C]Phenylalanine (99 mole per cent excess, MPE) was obtained from Cambridge Isotope Laboratories Inc. (Andover, MA, USA). The isotopic and chemical purity was checked by gas chromatography–mass spectrometry. Solutions of the tracer were tested for sterility and pyrogenicity before use and were prepared in sterile non-pyrogenic saline. During each experiment, the tracer was filtered through 0.22 μm filters.
Experimental design
All subjects were studied in a postabsorptive state after a 12 h overnight fast. A sampling catheter (Venflon 2*, 20G; Viggo, Helsingborg, Sweden) was inserted retrogradely into a dorsal hand vein. A second catheter was inserted in a contralateral forearm vein for tracer infusion.
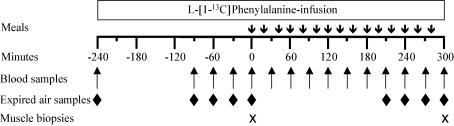
Each infusion protocol (Fig. 1) consisted of a 540 min study period throughout which l-[1-13C]phenylalanine was infused at a constant rate (0.063 ± 0.001 μmol kg−1 min−1) after a priming dose of 3.6 μmol kg−1. Each subject was first studied during a 240 min basal period (−240 to 0) to determine the postabsorptive whole body phenylalanine kinetics. After 240 min, a semiliquid diet was administered for the five remaining hours (from 0 to 300 min). The composition of the diets is indicated in Table 2. The diet provided 10.2 kcal, 0.4 g protein (in the form of casein), 1.3 g carbohydrate (dextrine maltose) and 0.36 g fat (vegetable oil, Isio 4™ Lesieur) per kg body weight, which corresponds to a normal meal at lunch. The leucine diet was supplemented with leucine (0.052 g kg−1) to increase plasma leucine to twice the normal postprandial plasma leucine concentrations. The leucine diet was also supplemented with isoleucine (0.0116 g kg−1) and valine (0.0068 g kg−1) to maintain their plasma levels at normal postprandial values. These amounts were established during pilot experiments. The control diet was supplemented with alanine (0.071 g kg−1), which did not affect protein metabolism, in order to supply the same amount of nitrogen as the leucine diet. The diets were prepared on the day of protocol and were ingested as 15 small meals (aliquots of 50 ml) given every 20 min.
Figure 1. Experimental design of the study.
Twenty normal elderly men were studied after an overnight fast. After basal samples were taken, a priming dose of l-[1-13C]phenylalanine (3.6 μmol kg−1) was injected followed by a continuous infusion at a constant rate (0.06 μmol kg−1 min−1) for 540 min. The study was divided into a basal postabsorptive period (−240 to 0 min) and a feeding period (0–300 min). During the feeding period, the subjects ingested a repeated bolus given every 20 min of either a control diet or a leucine supplemented diet (see Table 2 for diet composition). Blood samples were taken every 30 min between −90 and 300 min. Expired air samples were collected every 30 min at the end of the basal and feeding periods (between −90 and 0 min and between 210 and 300 min). Muscle biopsies were taken just before ingestion of the first bolus at time 0 and at the end of the feeding period (time 300 min).
Table 2.
Composition of the experimental meals
| Control | Leucine supplemented | |
|---|---|---|
| Casein | 0.400 | 0.400 |
| Leucine | — | 0.052 |
| Valine | — | 0.0068 |
| Isoleucine | — | 0.0116 |
| Alanine | 0.071 | — |
| Maltodextrin | 1.3 | 1.3 |
| Oil | 0.36 | 0.36 |
| Glycerol monostearate | 0.036 | 0.036 |
| Raspberry aroma | 0.005 | 0.005 |
Values are expressed in g (kg body weight)−1
Arterialized blood (obtained by placing the forearm and hand in a heating box at 60°C) was taken before starting the infusion of the tracer and every 30 min during the last 1.5 h of the basal period and every 30 min during the entire feeding period. Blood samples were centrifuged at 4°C and the resulting plasma was stored at −20°C for subsequent analyses. Breath samples were collected before infusion and every 30 min during the last 1.5 h of the basal and feeding periods and kept in 10 ml evacuated containers (Vacutainer Becton Dickinson, Grenoble, France). Total CO2 production rates were measured at isotopic plateau during the last hour of the basal and feeding periods by open-circuit indirect calorimetry (Deltatrac; Datex, Geneva).
Muscle biopsies were taken from the vastus lateralis after local anaesthesia at the end of basal and feeding periods using a percutaneous needle. Muscle samples were immediately frozen in liquid nitrogen and stored at −80°C until analysis.
Analytical procedures
Whole body phenylalanine kinetics were determined from plasma l-[1-13C]phenylalanine enrichments and breath 13CO2 enrichments. Plasma l-[1-13C]phenylalanine enrichments were determined as its ter-butyldimethylsilyl derivatives under electron impact ionization by gas chromatography–mass spectrometry (GC-MS) (mass selective detector 5972, coupled with a gas chromatograph 5890 series II; Hewlett Packard, Les Ullis, France) and by monitoring the ions with m/z 336 and 337. Breath 13CO2 enrichments were measured directly by isotopic ratio mass spectrometry using a VG Isochrom (Micromass HK, Manchester, UK).
For plasma l-[1-13C]phenylalanine enrichments, 1 ml of plasma was homogenized in 8 volumes of ice-cold 10% (0.6 m) trichloroacetic acid (TCA) and then centrifuged at 5000 g for 15 min at 4°C. The resultant pellets (TCA- insoluble materials) were washed 2 times in 4 volumes of cold 10% TCA. The combined supernatants, which contained free amino acids, were desalted by cation-exchange chromatography (AG 50 × 8, 100–200 mesh, H+ form, Bio-Rad, Richmond, CA, USA) in minidisposal columns. Phenylalanine and other amino acids were eluted with 4 m NH4OH. After evaporation of NH4OH under vacuum, free amino acids were resuspended in 0.01 m HCl for subsequent derivatization and enrichment measurements.
l-[1-13C]Phenylalanine enrichment in the free amino acid pool and myofibrillar muscle proteins were determined according to the method previously described (Guillet et al. 2004). A 50–100 mg piece of muscle biopsy was homogenized in a 5% ice-cold buffer containing 0.25 m sucrose, 2 mm EDTA and 10 mm Tris-HCl (pH 7.4) using a Potter-Elvehjem homogenizer. The homogenate was centrifuged at low speed (600 g) and the pellet containing myofibrillar proteins was collected. Myofibrillar muscle proteins were then hydrolysed using 6 m HCl (110°C for 24 h). HCl was removed by evaporation and amino acids purified by cation-exchange chromatography as described above. Amino acids were then derivatized as their N-acetyl-propyl residues and [1-13C]phenylalanine enrichment was performed using GC-MS. Free muscle amino acids were extracted from muscle tissue by using 10% TCA, purified by cation-exchange chromatography as described above, and derivatized as their ter-butyldimethylsilyl residues.
Plasma insulin concentrations were determined using a commercial human RIA kit (Insulin CT Cis Bio International).
The concentrations of free plasma amino acids were measured by ion-exchange chromatography after protein precipitation. Five hundred microlitres of plasma was added to 125 μl of a sulphosalicylic acid solution (1 m dissolved in ethanol with 0.5 m thiodiglycol) previously evaporated to dryness. Samples were incubated on ice for 1 h and centrifuged for 1 h at 3500 g at 4°C. An aliquot (250 μl) of the supernatant was added to 125 μl of 0.1 m lithium acetate buffer, pH 2.2. Amino acid concentrations were determined using an automated amino acid analyser with BTC 2410 resin (Biotronic LC 3000, Roucaire, Velizy, France).
Calculations
Whole body phenylalanine kinetics were calculated using samples taken during the last 1.5 h of the basal period (at times −90, −60, −30 and 0) and feeding period (at times 210, 240, 270 and 300 min). After checking the isotopic steady state for the last hour of each period, mean plateau enrichment values were used to calculate phenylalanine kinetics.
Total whole body phenylalanine flux (Q) (μmol kg−1 min−1) was determined using the equation:
where F is the l-[1-13C]phenylalanine infusion rate (μmol kg−1 min−1), IEinf is the isotopic enrichment of the infusate (i.e. 99 mol% excess), and IEa (also in mol% excess) is the plasma l-[1-13C]leucine enrichment.
Whole body phenylalanine oxidation flux (Ox) (μmol kg−1 min−1) was calculated using plasma l-[1-13C]phenylalanine enrichment from the following equation:
where IECO2 (mol per cent excess) is the expired 13CO2 enrichment, VCO2(μmol of CO2 min−1) is the expired CO2 volume and R is a factor correcting for incomplete recovery of labelled bicarbonate (R = 0.71 during basal period and 0.82 during feeding period) (Hoerr et al. 1989).
According to the model, the following equation applies:
where I is the rate of ingested unlabelled phenylalanine (I = 0 in the basal period), Ra is the endogenous phenylalanine appearance rate (an index of protein breakdown), and Rd the non-oxidative phenylalanine disposal rate (an index of protein synthesis) from plasma (all in μmol kg−1 min−1).
Knowing Q, I and Ox
The net phenylalanine balance, an index of protein deposition, is calculated as Rd−Ra.
Fractional synthesis rate (FSR) of myofibrillar muscle proteins was calculated at the end of feeding period by measuring the incorporation rate of l-[1-13C]phenylalanine into proteins according to the equation:
where ΔIEpb is the increment of protein bound phenylalanine enrichment between the two biopsies, t (h) is the time interval between the two biopsies, and Ief1 and Ief2 are the phenylalanine enrichments in the free muscle pool in the two subsequent biopsies. The results are expressed as per cent per hour.
Statistical analysis
All data are expressed as means ± s.e.m. A two-way ANOVA with repeated measures was used to compare whole body kinetics (Leucine versus Control groups and Feeding versus Basal periods) and Student's unpaired t test was used to compare FSR in Leucine and Control groups. P < 0.05 was considered to be significant.
Results
Characteristics of the subjects
The two groups of volunteers did not differ with respect to age, height, body weight and body mass index (Table 1). The values of fasting plasma glucose, albumin and creatinine were normal and did not differ between the two groups. The basal insulin: glucose ratio, which is an index of insulin sensitivity, was not different between the control and leucine groups (2.82 ± 0.41 versus 3.00 ± 0.28 mIU l−1, respectively, P = 0.7).
Plasma insulin concentrations
Plasma insulin levels were similar in the basal period and increased in both groups after the ingestion of the first meal. Insulin concentrations increased during the first 2 h and plateaued for the last 3 h (Fig. 2). The insulin increment after feeding tended to be higher in the group supplemented with leucine but the insulin response, expressed as the area under the curve (above baseline) during the 5 h feeding period was not different between the two groups (P = 0.5) (Fig. 2, inset).
Figure 2. Plasma insulin concentrations (expressed in μU ml−1) in elderly volunteers fed a control diet or a leucine-supplemented diet as repeated bolus between 0 and 300 min.
The inset represents the plasma insulin response expressed as the area under the curve minus baseline values. Values are means ± s.e.m.
Plasma amino acid concentrations
As shown in Table 3, plasma concentrations of both essential and non-essential amino acids were similar in both groups at the postabsorptive state.
Table 3.
Mean plasma amino acid concentrations before and after continuous ingestion of control or leucine-supplemented meals in elderly volunteers
| Control | Leucine | |||
|---|---|---|---|---|
| Basal | Fed | Basal | Fed | |
| Essential amino acids | ||||
| Histidine | 68 ± 3 | 72 ± 3 | 66 ± 2 | 67 ± 2 |
| Isoleucine | 63 ± 4 | 82 ± 4† | 55 ± 3 | 93 ± 5† |
| Lysine | 173 ± 10 | 203 ± 12† | 157 ± 6 | 190 ± 7† |
| Methionine | 7 ± 1 | 8 ± 1† | 7 ± 1 | 8 ± 1† |
| Phenylalanine | 61 ± 2 | 71 ± 2† | 60 ± 2 | 66 ± 3† |
| Threonine | 115 ± 7 | 124 ± 10† | 110 ± 7 | 108 ± 8† |
| Leucine | 130 ± 5 | 156 ± 6† | 111 ± 5 | 282 ± 14†* |
| Valine | 251 ± 13 | 280 ± 12† | 216 ± 12 | 270 ± 13† |
| Non-essential amino acids | ||||
| Alanine | 232 ± 13 | 351 ± 23† | 288 ± 22 | 300 ± 20 |
| Arginine | 70 ± 4 | 71 ± 4 | 67 ± 4 | 65 ± 5 |
| Asparagine | 88 ± 5 | 104 ± 8† | 86 ± 4 | 92 ± 6† |
| Aspartate | 11 ± 1 | 12 ± 1 | 10 ± 1 | 10 ± 1 |
| Glutamate | 212 ± 41 | 207 ± 37 | 197 ± 24 | 198 ± 24 |
| Glutamine | 215 ± 30 | 223 ± 32 | 223 ± 19 | 204 ± 20 |
| Glycine | 173 ± 9 | 166 ± 12† | 180 ± 15 | 164 ± 14† |
| Proline | 164 ± 14 | 244 ± 17† | 189 ± 9 | 248 ± 11† |
| Serine | 107 ± 6 | 111 ± 7 | 94 ± 3 | 91 ± 4* |
| Tyrosine | 52 ± 3 | 65 ± 3† | 52 ± 3 | 61 ± 3 † |
Values are expressed in μm as means ± s.e.m.
Significantly different from control group at P < 0.05.
Significantly different from the basal values in the same group at P < 0.05.
Plasma leucine levels increased after ingestion of the first meal in both groups (P < 0.05). Whereas plasma leucine levels did not further increase in the control group, it strongly increased in the leucine group (Fig. 3A). As shown in Fig. 3B, the plasma leucine response over the entire feeding period was 6-fold higher in the leucine group than in the control group (P < 0.0001).
Figure 3. A, plasma leucine concentrations (expressed in μmol l−1). B, plasma leucine response (expressed as the area under the curve minus baseline values) in elderly volunteers fed a control diet or a leucine-supplemented diet as a repeated bolus between 0 and 300 min.
Values are means ± s.e.m. *Significantly different from control group at P < 0.05.
Compared with the postabsorptive state (basal state), control or leucine meal intake slightly increased most plasma essential amino acid concentrations, except histidine, which did not significantly change, and threonine, which decreased in the leucine group (Table 3). When plasma leucine was omitted, the mean plasma essential amino acid concentrations were not significantly different between control and leucine groups in the fed period (838 ± 24 and 803 ± 25 μm, respectively). It is important to note that plasma isoleucine and valine concentrations were not decreased in the volunteers fed the leucine-supplemented meals compared with the control group. Moreover, plasma isoleucine and valine concentrations were not significantly different between control and leucine groups (Table 3). Feeding the control meals significantly increased plasma alanine concentration in volunteers whereas the leucine meals did not. Other non-essential plasma amino acid levels were either unchanged (arginine, aspartate, glutamate, glutamine, serine), increased (asparagine, proline, tyrosine) or decreased (glycine) similarly in the control and leucine groups during feeding (Table 3).
Whole body phenylalanine kinetics
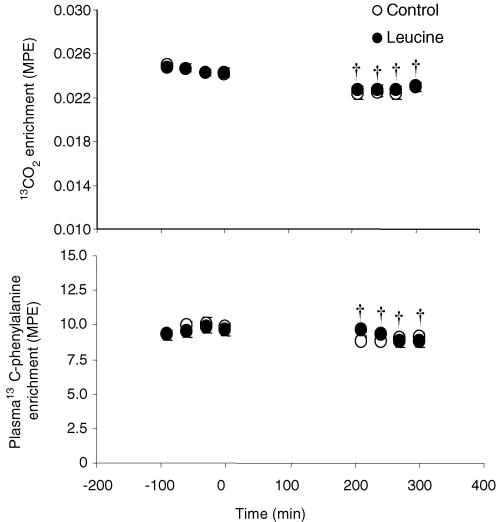
A steady state of 13CO2 expired and plasma 13C-phenylalanine enrichments was achieved during the final hours of basal and fed periods in each group of volunteers (Fig. 4). The rate of expired CO2 was similar in both groups during the basal period (99.7 ± 2.9 versus 100.7 ± 2.9 μmol min−1 kg−1 in control and leucine groups, respectively). Feeding increased the rate of expired CO2 (P < 0.0001) to the same extent in the control and leucine groups (117.9 ± 4.2 and 116.5 ± 2.9 μmol min−1 kg−1, respectively, P < 0.005). Consequently, expired CO2 enrichments decreased in both groups during the feeding period (P < 0.0001) (Fig. 4). Similarly, plasma 13C-phenylalanine enrichments decreased during the feeding period (P < 0.005) in both the control and leucine groups.
Figure 4. Expired 13CO2 enrichments (A) and plasma 13C-phenylalanine enrichments (B) in mol per cent excess (MPE) as a function of time in elderly volunteers during the basal postabsorptive period and during ingestion of a control diet or a leucine-supplemented diet given as a repeated bolus between 0 and 300 min.
Values are means ± s.e.m. †Significantly different from basal period at P < 0.05.
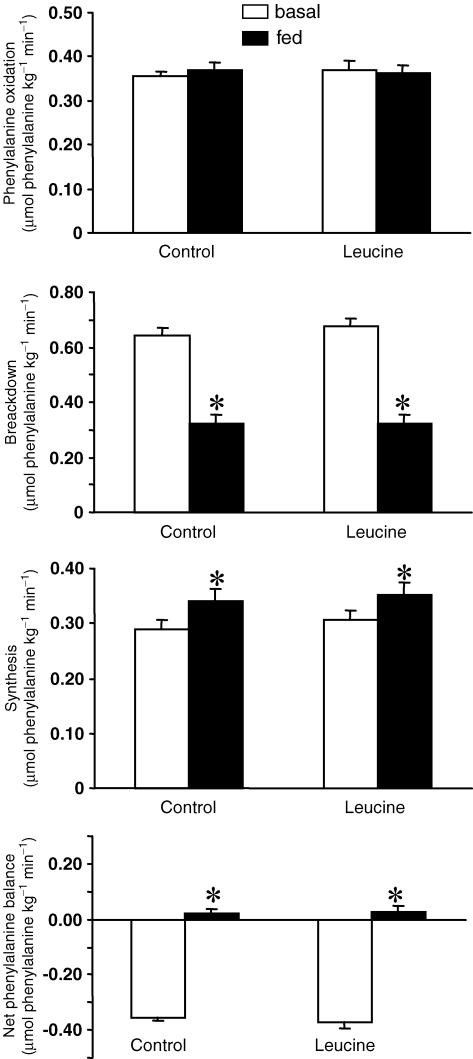
Whole body phenylalanine kinetics were similar in the control and leucine groups during the basal period (Fig. 5). The effect of feeding on whole body phenylalanine kinetics was similar whatever the composition of the meals given to the volunteers. The rate of whole body phenylalanine oxidation was not changed by feeding whereas whole body protein synthesis was increased (P < 0.05) in control and leucine groups. As expected, whole body protein breakdown was decreased during feeding and whole body phenylalanine balance was improved (Fig. 5). The increase in plasma leucine observed with the leucine-supplemented meals had no effect on whole body protein metabolism.
Figure 5. Whole body phenylalanine kinetics (expressed as μmol phenylalanine kg−1 min−1) in elderly volunteers during the basal postabsorptive period and during ingestion of a control diet or a leucine-supplemented diet given as a repeated bolus between 0 and 300 min.
Values are means ± s.e.m. *Significantly different from control group at P < 0.05.
Muscle protein synthesis
As shown in Fig. 6, leucine supplementation improved myofibrillar muscle protein fractional synthesis rate (FSR) measured at the end of the feeding period (0.083 ± 0.008 and 0.053 ± 0.009% h−1 in the leucine and control groups, respectively, P < 0.05).
Figure 6. Fractional synthesis rates (FSR) of myofibrillar muscle proteins in elderly volunteers fed a control diet or a leucine-supplemented diet as a repeated bolus between 0 and 300 min.
Values are means ± s.e.m. *Significantly different from control group at P < 0.05.
Discussion
The present study was designed to assess the impact of specific dietary leucine-supplemented meals on protein metabolism in elderly volunteers. For this, we compared the effects induced by mixed semi-liquid meals containing either casein (control group) or casein plus free leucine (leucine group) as the protein source on whole body and muscle protein synthesis in old men. We showed that leucine supplementation during feeding improves muscle protein synthesis in elderly subjects. This beneficial effect was due only to increased plasma leucine concentrations because only plasma free leucine concentration significantly differed between the two groups of volunteers during the feeding period. By contrast, whole body turnover was not affected by leucine supplementation.
Over the past several years, it has become clear that muscle protein loss during ageing may be partly explained by a decreased ability of old muscle to respond appropriately to food intake (Mosoni et al. 1995; Arnal et al. 1999, 2002; Dardevet et al. 2002; Rieu et al. 2003). Food intake normally increases muscle protein synthesis and most of this effect results from the stimulatory effect of amino acids (Bennet et al. 1989; Fryburg et al. 1995; Svanberg et al. 1996), whereas insulin has only a permissive effect. We demonstrated in a previous study that the effect of dietary amino acids was blunted in old rats whereas insulin effect was not significantly altered (Prod'homme et al. 2005). Wolfe and coworkers have extensively investigated the effect of amino acids on muscle protein synthesis in the elderly. Surprisingly, they showed that amino acids given orally (Volpi et al. 1999; Rasmussen et al. 2002; Paddon-Jones et al. 2004) or intravenously (Volpi et al. 1998; Rasmussen et al. 2002) were able to stimulate muscle protein synthesis in old as in young adult volunteers. They also demonstrated that essential amino acids are primarily responsible for the amino acid-induced stimulation of muscle protein anabolism (Volpi et al. 2003). However it is important to note that the amount of amino acids infused or orally ingested in these experiments led to a sustained large hyperaminoacidaemia. Besides, a more recent study from this group (Katsanos et al. 2005) and from Cuthbertson et al. (2005) confirmed our observations and showed a decreased sensitivity and responsiveness of muscle protein synthesis to essential amino acids in elderly subjects compared to adults in the case of a milder and more physiological rise of aminoacidaemia.
Among amino acids, branched chain amino acids and especially leucine (Fulks et al. 1975; Buse & Reid, 1975; Li & Jefferson, 1978) are the most efficient for protein synthesis stimulation. Indeed, we and others clearly demonstrated that leucine alone is able to stimulate muscle protein synthesis to the same extent as all amino acids (Anthony et al. 2000a,b; Dardevet et al. 2000; Lynch et al. 2002; Crozier et al. 2005). In a previous experiment, we clearly showed a decreased sensitivity of protein synthesis to leucine in muscles from old rats compared to adults (Dardevet et al. 2000). Indeed, muscle protein synthesis in old rats required greater leucine concentration than young or adult rats to be stimulated. This suggested that at postprandial amino acid levels, muscle protein synthesis was maximally stimulated in adult rats but poorly in old animals. Accordingly, we previously showed that a leucine-supplemented meal corrected the defect of postprandial protein synthesis stimulation in muscle from old rats, suggesting that increased leucine intake in the elderly would be beneficial for maintaining muscle protein mass (Dardevet et al. 2002; Dardevet et al. 2003; Rieu et al. 2003). Recently, Katsanos et al. (2006) showed that an enriched leucine bolus of EAAs also stimulated muscle protein accretion in the elderly. However, it has been previously shown that, when given in combination with glucose, which further increased plasma insulin levels, the effect of amino acids on muscle protein accretion was blunted in humans (Volpi et al. 2000). This raised the question whether the beneficial effect of leucine supplementation recorded by Katsanos et al. (2006) could be maintained if leucine was added in a complex meal associated with proteins, carbohydrates and lipids. Our data clearly demonstrated that the supplementation was still efficient under such conditions despite the fact that plasma insulin was increased to the same extent as in the study of Volpi et al. (2000). The reasons for such differences are unclear. It may be related with methodological differences such as ingestion of complete meals versus intravenous infusion of an amino acid–glucose mixture. Leucine infusion has been reported to have a transient effect on muscle protein metabolism if infused alone (Abumrad et al. 1982; Escobar et al. 2005), probably because of a reduction in other plasma amino acid availability. Indeed, it was suggested that the presence of all amino acids or essential amino acids might be required to sustain protein synthesis stimulated by leucine (Abumrad et al. 1982; Frexes-Steed et al. 1992; Escobar et al. 2005). When leucine was infused alone, the resulting increase in circulating leucine induced a decline of most plasma essential amino acids (Hagenfeldt et al. 1980; Nair et al. 1992; Tom & Nair, 2006), the effect being more pronounced for isoleucine and valine because of the well-described phenomenon of branched-chain amino acid antagonism (Calvert et al. 1982; Harper et al. 1984). In the present study, in order to prevent a fall of plasma valine and isoleucine concentrations, which can become rate-limiting for protein synthesis, the leucine-supplemented meals were also supplemented with valine and isoleucine. As in our previous experiment in rats (Dardevet et al. 2002; Rieu et al. 2003), postprandial plasma valine and isoleucine were maintained at normal postprandial concentrations and were not different in the leucine and control groups. The major relevance of our protocol design was the fact that only plasma leucine concentration was dramatically increased in the leucine group whereas other plasma EAAs were similar between leucine and control volunteers and only slightly increased. Indeed, in our experiment, total plasma EAAs (minus leucine) was only 14–19% increased during feeding whereas it was 2.2-fold increased after a bolus ingestion of EAAs (Volpi et al. 1999, 2003; Katsanos et al. 2005). Actually, our protocol design reproduced the plasma amino acid pattern occurring after ingestion of a normal single mixed meal in humans (Elia et al. 1989). The present experiment demonstrated for the first time that increasing plasma leucine availability alone may favour muscle protein synthesis in old humans and does not require a large increase of other amino acids.
Because leucine has been shown to stimulate insulin secretion, the increase in muscle protein synthesis could result indirectly from an increase in plasma insulin. Despite the fact that plasma insulin concentrations tended to increase more rapidly in the leucine-supplemented group than in the control group, the insulin response during the entire 5 h feeding period was not different between the two groups. Therefore, the increase in muscle protein synthesis that we observed in the leucine-supplemented group was independent of changes in insulin and cannot be attributed to an insulin effect. This was also the case in our previous studies in rats (Dardevet et al. 2002; Rieu et al. 2003). In agreement with this, Cuthbertson et al. (2005) demonstrated that EAAs stimulate muscle protein synthesis independently of increased insulin availability in both young and old men. It has also been shown that leucine supplementation increased protein synthesis in rabbit skin wound and muscle without changes in plasma insulin (Zhang et al. 2004). Whether insulin contributes to the leucine-induced stimulation of muscle protein synthesis remains under debate. Overall, the studies demonstrated that leucine activated protein synthesis through both insulin-independent (Anthony et al. 2002b) and insulin-dependent mechanisms (Anthony et al. 2002a). It is likely that insulin is required to cause stimulation of protein synthesis by leucine and that the role of insulin appears to be permissive. This could explain why it has been possible to maintain a stimulation of muscle protein synthesis in the leucine-supplemented group for over 5 h in the present experiment, whereas Bohé et al. (2001) could not stimulate muscle protein synthesis for longer than 2 h after the beginning of an amino acid infusion. Indeed, plasma insulin remained elevated during all the feeding period in our study whereas plasma insulin elevation was only transient in the study of Bohé et al. (2001). Because muscle protein breakdown was not determined in that experiment, it is unknown whether the improvement of muscle protein synthesis resulted in an anabolic response in volunteers fed the leucine-supplemented meals. We previously demonstrated that the normal fall in muscle protein breakdown induced by feeding was blunted in old rats and was restored by the leucine supplementation as for protein synthesis stimulation (Combaret et al. 2005), suggesting an anabolic effect of leucine on muscle proteins in old rats. Moreover, Katsanos et al. (2006) demonstrated that increasing the proportion of leucine in a mixture of EAAs (41% versus 25%) improved the muscle balance in old subjects.
Although we found that leucine supplementation improves muscle protein synthesis, we failed to observe an effect on whole body protein turnover in the present experiment. Indeed, both whole body protein synthesis and protein breakdown as well as net balance were similar in both groups whatever the nutritional conditions. This is not surprising because muscle protein synthesis only represents 27% of whole body protein synthesis (Nair et al. 1988). Moreover, our data are consistent with those of Koopman et al. (2005) who did not show any change in whole body turnover in young male subjects given either carbohydrate and protein or carbohydrate, protein and free leucine despite greatly increased plasma leucine concentrations.
In conclusion, the present experiment demonstrates that dietary leucine supplementation improves postprandial muscle protein synthesis in old humans. Recently, Katsanos et al. (2006) also demonstrated such a beneficial effect of leucine in the elderly. However, of most physiological relevance from our work is that the improvement of muscle protein synthesis induced by leucine supplementation (1) occurred after ingestion of a complete meal (i.e. containing proteins, carbohydrates and lipids) instead of a bolus of free amino acids, (2) was sustained for at least 5 h, and (3) was visible without the large increases of postprandial amino acid levels. Taken together, these data suggest that leucine supplementation may represent an effective nutritional strategy to limit muscle protein losses during ageing. It may be considered as a good alternative to high protein diets, which could have deleterious effects on renal function in the elderly (Fliser et al. 1993). However, further experiments will be necessary to determine the best conditions of leucine supplementation in human to obtain protein gain in muscle without negative side effects in old people.
Acknowledgments
We thank the volunteers for their participation. The authors thank C. Obled for helpful discussion and advice, F. Laporte and N. Mathieu for their participation in the recruitment of volunteers, M. Brandolini for dietary recall analysis, M. C. Ribeyre for mass spectrometry sample preparations, E. Debras and G. Bayle for amino acid analysis, and H. Lafarge for management of the bibliography. This work was supported by a grant from ESPEN – The European Society for Clinical Nutrition and Metabolism.
References
- Abumrad NN, Robinson RP, Gooch BR, Lacy WW. The effect of leucine infusion on substrate flux across the human forearm. J Surg Res. 1982;32:453–462. doi: 10.1016/0022-4804(82)90126-3. [DOI] [PubMed] [Google Scholar]
- Anthony JC, Anthony TG, Kimball SR, Vary TC, Jefferson LS. Orally administered leucine stimulates protein synthesis in skeletal muscle of postabsorptive rats in association with increased elF4F formation. J Nutr. 2000a;130:139–145. doi: 10.1093/jn/130.2.139. [DOI] [PubMed] [Google Scholar]
- Anthony JC, Lang CH, Crozier SJ, Anthony TG, MacLean DA, Kimball SR, et al. Contribution of insulin to the translational control of protein synthesis in skeletal muscle by leucine. Am J Physiol Endocrinol Metab. 2002a;282:E1092–E1101. doi: 10.1152/ajpendo.00208.2001. [DOI] [PubMed] [Google Scholar]
- Anthony JC, Reiter AK, Anthony TG, Crozier SJ, Lang CH, MacLean DA, et al. Orally administered leucine enhances protein synthesis in skeletal muscle of diabetic rats in the absence of increases in 4E-BP1 or S6K1 phosphorylation. Diabetes. 2002b;51:928–936. doi: 10.2337/diabetes.51.4.928. [DOI] [PubMed] [Google Scholar]
- Anthony JC, Yoshizawa F, Anthony TG, Vary TC, Jefferson LS, Kimball SR. Leucine stimulates translation initiation in skeletal muscle of postabsorptive rats via a rapamycin-sensitive pathway. J Nutr. 2000b;130:2413–2419. doi: 10.1093/jn/130.10.2413. [DOI] [PubMed] [Google Scholar]
- Arnal MA, Mosoni L, Boirie Y, Houlier ML, Morin L, Verdier E, et al. Protein pulse feeding improves protein retention in elderly women. Am J Clin Nutr. 1999;69:1202–1208. doi: 10.1093/ajcn/69.6.1202. [DOI] [PubMed] [Google Scholar]
- Arnal MA, Mosoni L, Dardevet D, Ribeyre MC, Bayle G, Prugnaud J, et al. Pulse protein feeding pattern restores stimulation of muscle protein synthesis during the feeding period in old rats. J Nutr. 2002;132:1002–1008. doi: 10.1093/jn/132.5.1002. [DOI] [PubMed] [Google Scholar]
- Bennet WM, Connacher AA, Scrimgeour CM, Smith K, Rennie MJ. Increase in anterior tibialis muscle protein synthesis in healthy man during mixed amino acid infusion: studies of incorporation of [1-13C] leucine. Clin Sci. 1989;76:447–454. doi: 10.1042/cs0760447. [DOI] [PubMed] [Google Scholar]
- Bohé J, Low JFA, Wolfe RR, Rennie MJ. Latency and duration of stimulation of human muscle protein synthesis during continuous infusion of amino acids. J Physiol. 2001;532:575–579. doi: 10.1111/j.1469-7793.2001.0575f.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buse MG, Reid SS. Leucine: A possible regulator of protein turnover in muscle. J Clin Invest. 1975;56:1250–1261. doi: 10.1172/JCI108201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvert CC, Klasing KC, Austic RE. Involvement of food intake and amino acid catabolism in the branched-chain amino acid antagonism in chicks. J Nutr. 1982;112:627–635. doi: 10.1093/jn/112.4.627. [DOI] [PubMed] [Google Scholar]
- Combaret L, Dardevet D, Rieu I, Pouch MN, Bechet D, Taillandier D, et al. A leucine-supplemented diet restores the defective postprandial inhibition of proteasome-dependent proteolysis in aged rat skeletal muscle. J Physiol. 2005;569:489–499. doi: 10.1113/jphysiol.2005.098004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crozier SJ, Kimball SR, Emmert SW, Anthony JC, Jefferson LS. Oral leucine administration stimulates protein synthesis in rat skeletal muscle. J Nutr. 2005;135:376–382. doi: 10.1093/jn/135.3.376. [DOI] [PubMed] [Google Scholar]
- Cuthbertson D, Smith K, Babraj J, Leese G, Waddell T, Atherton P, et al. Anabolic signaling deficits underlie amino acid resistance of wasting, aging muscle. FASEB J. 2005;19:422–424. doi: 10.1096/fj.04-2640fje. [DOI] [PubMed] [Google Scholar]
- Dardevet D, Sornet C, Attaix D, Baracos VE, Grizard J. Insulin-like growth factor-1 and insulin resistance in skeletal muscles of adult and old rats. Endocrinology. 1994;134:1475–1484. doi: 10.1210/endo.134.3.8119189. [DOI] [PubMed] [Google Scholar]
- Dardevet D, Sornet C, Balage M, Grizard J. Stimulation of in vitro rat muscle protein synthesis by leucine decreases with age. J Nutr. 2000;130:2630–2635. doi: 10.1093/jn/130.11.2630. [DOI] [PubMed] [Google Scholar]
- Dardevet D, Sornet C, Bayle G, Prugnaud J, Pouyet C, Grizard J. Postprandial stimulation of muscle protein synthesis in old rats can be restored by a leucine-supplemented meal. J Nutr. 2002;132:95–100. doi: 10.1093/jn/132.1.95. [DOI] [PubMed] [Google Scholar]
- Dardevet D, Rieu I, Fafournoux P, Sornet C, Combaret L, Bruhat A, Mordier S, Mosoni L, Grizard J. Leucine: a key amino acid in ageing-associated sarcopenia? J Nutr. 2003;16:61–70. doi: 10.1079/NRR200252. [DOI] [PubMed] [Google Scholar]
- Dutta C, Hadley EC. The significance of sarcopenia in old age. J Gerontol A Biol Sci Med Sci. 1995;50A:1–4. doi: 10.1093/gerona/50a.special_issue.1. [DOI] [PubMed] [Google Scholar]
- Elia M, Folmer P, Schlatmann A, Goren A, Austin S. Amino acid metabolism in muscle and in the whole body of man before and after ingestion of a single mixed meal. Am J Clin Nutr. 1989;49:1203–1210. doi: 10.1093/ajcn/49.6.1203. [DOI] [PubMed] [Google Scholar]
- Escobar J, Frank JW, Suryawan A, Nguyen HV, Kimball SR, Jefferson LS, et al. Physiological rise in plasma leucine stimulates muscle protein synthesis in neonatal pigs by enhancing translation initiation factor activation. Am J Physiol Endocrinol Metab. 2005;288:E914–E921. doi: 10.1152/ajpendo.00510.2004. [DOI] [PubMed] [Google Scholar]
- Fliser D, Zeier M, Nowack R, Ritz E. Renal functional reserve in healthy elderly subjects. J Am Soc Nephrol. 1993;3:1371–1377. doi: 10.1681/ASN.V371371. [DOI] [PubMed] [Google Scholar]
- Forbes GB, Halloran E. The adult decline in lean body mass. Hum Biol. 1976;48:162–173. [PubMed] [Google Scholar]
- Frexes-Steed M, Lacy DB, Collins J, Abumrad NN. Role of leucine and other amino acids in regulating protein metabolism in vivo. Am J Physiol. 1992;262:E925–E935. doi: 10.1152/ajpendo.1992.262.6.E925. [DOI] [PubMed] [Google Scholar]
- Fryburg DA, Jahn LA, Hill SA, Oliveras DM, Barrett EJ. Insulin and insulin-like growth factor-I enhance human skeletal muscle protein anabolism during hyperaminoacidemia by different mechanisms. J Clin Invest. 1995;96:1722–1729. doi: 10.1172/JCI118217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulks R, Li JB, Goldberg AL. Effects of insulin, glucose and aminoacids on protein turnover in rat diaphragm. J Biol Chem. 1975;250:290–298. [PubMed] [Google Scholar]
- Guillet C, Zangarelli A, Mishellany A, Rousset P, Sornet C, Dardevet D, et al. Mitochondrial and sarcoplasmic proteins, but not myosin heavy chain, are sensitive to leucine supplementation in old rat skeletal muscle. Exp Gerontol. 2004;39:745–751. doi: 10.1016/j.exger.2004.02.011. [DOI] [PubMed] [Google Scholar]
- Hagenfeldt L, Eriksson S, Wahren J. Influence of leucine on arterial concentrations and regional exchange of amino acids in healthy subjects. Clin Sci. 1980;59:173–181. doi: 10.1042/cs0590173. [DOI] [PubMed] [Google Scholar]
- Harper AE, Miller RH, Block KP. Branched-chain amino acid metabolism. Annu Rev Nutr. 1984;4:409–454. doi: 10.1146/annurev.nu.04.070184.002205. [DOI] [PubMed] [Google Scholar]
- Hoerr RA, Yu YM, Wagner DA, Burke JF, Young VR. Recovery of 13C in breath from NaH13CO2 infused by gut and vein: effect of feeding. Am J Physiol. 1989;257:E426–E438. doi: 10.1152/ajpendo.1989.257.3.E426. [DOI] [PubMed] [Google Scholar]
- Holloszy JO, Chen M, Cartee GD, Young JC. Skeletal muscle atrophy in old rats: differential changes in the three fiber types. Mech Ageing Dev. 1991;60:199–213. doi: 10.1016/0047-6374(91)90131-i. [DOI] [PubMed] [Google Scholar]
- Katsanos CS, Kobayashi H, Sheffield-Moore M, Aarsland A, Wolfe RR. Aging is associated with diminished accretion of muscle proteins after the ingestion of a small bolus of essential amino acids. Am J Clin Nutr. 2005;82:1065–1073. doi: 10.1093/ajcn/82.5.1065. [DOI] [PubMed] [Google Scholar]
- Katsanos CS, Kobayashi H, Sheffield-Moore M, Aarsland A, Wolfe RR. A high proportion of leucine is required for optimal stimulation of the rate of muscle protein synthesis by essential amino acids in the elderly. Am J Physiol Endocrinol Metab. 2006 doi: 10.1152/ajpendo.00488.2005. in press. [DOI] [PubMed] [Google Scholar]
- Kimball SR, Shantz LM, Horetsky RL, Jefferson LS. Leucine regulates translation of specific mRNAs in L6 myoblasts through mTOR-mediated changes in availability of eIF4E and phosphorylation of ribosomal protein S6. J Biol Chem. 1999;274:11647–11652. doi: 10.1074/jbc.274.17.11647. [DOI] [PubMed] [Google Scholar]
- Koopman R, Wagenmakers AJM, Manders RJF, Zorenc AHG, Senden JMG, Gorselink M, et al. Combined ingestion of protein and free leucine with carbohydrate increases postexercise muscle protein synthesis in vivo in male subjects. Am J Physiol Endocrinol Metab. 2005;288:E645–E653. doi: 10.1152/ajpendo.00413.2004. [DOI] [PubMed] [Google Scholar]
- Li JB, Jefferson LS. Influence of amino acid availability on protein turnover in perfused skeletal muscle. Biochim Biophys Acta. 1978;544:351–359. doi: 10.1016/0304-4165(78)90103-4. [DOI] [PubMed] [Google Scholar]
- Lynch CJ, Patson BJ, Anthony J, Vaval A, Jefferson LS, Vary TC. Leucine is a direct-acting nutrient signal that regulates protein synthesis in adipose tissue. Am J Physiol Endocrinol Metab. 2002;283:E503–E513. doi: 10.1152/ajpendo.00084.2002. [DOI] [PubMed] [Google Scholar]
- Mosoni L, Houlier ML, Patureau Mirand P, Bayle G, Grizard J. Effect of amino acids alone or with insulin on muscle and liver protein synthesis in adult and old rats. Am J Physiol. 1993;264:E614–E620. doi: 10.1152/ajpendo.1993.264.4.E614. [DOI] [PubMed] [Google Scholar]
- Mosoni L, Valluy MC, Serrurier B, Prugnaud J, Obled C, Guezennec CY, et al. Altered response of protein synthesis to nutritional state and endurance training in old rats. Am J Physiol. 1995;268:E328–E335. doi: 10.1152/ajpendo.1995.268.2.E328. [DOI] [PubMed] [Google Scholar]
- Nair KS, Halliday D, Griggs RC. Leucine incorporation into mixed skeletal muscle protein in humans. Am J Physiol. 1988;254:E208–E213. doi: 10.1152/ajpendo.1988.254.2.E208. [DOI] [PubMed] [Google Scholar]
- Nair KS, Schwartz RG, Welle S. Leucine as a regulator of whole body and skeletal muscle protein metabolism in humans. Am J Physiol. 1992;263:E928–E934. doi: 10.1152/ajpendo.1992.263.5.E928. [DOI] [PubMed] [Google Scholar]
- Paddon-Jones D, Sheffield-Moore M, Zhang XJ, Volpi E, Wolf SE, Aarsland A, et al. Amino acid ingestion improves muscle protein synthesis in the young and elderly. Am J Physiol Endocrinol Metab. 2004;286:E321–E328. doi: 10.1152/ajpendo.00368.2003. [DOI] [PubMed] [Google Scholar]
- Prod'homme M, Balage M, Debras E, Farges MC, Kimball S, Jefferson L, et al. Differential effects of insulin and dietary amino acids on muscle protein synthesis in adult and old rats. J Physiol. 2005;563:235–248. doi: 10.1113/jphysiol.2004.068841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen BB, Wolfe RR, Volpi E. Oral and intravenously administered amino acids produce similar effects on muscle protein synthesis in the elderly. J Nutr Health Aging. 2002;6:358–362. [PMC free article] [PubMed] [Google Scholar]
- Rieu I, Sornet C, Bayle G, Prugnaud J, Pouyet C, Balage M, et al. Leucine-supplemented meal feeding for ten days beneficially affects postprandial muscle protein synthesis in old rats. J Nutr. 2003;133:1198–1205. doi: 10.1093/jn/133.4.1198. [DOI] [PubMed] [Google Scholar]
- Svanberg E, Möller-Loswick AC, Matthews DE, Korner U, Andersson M, Lundholm K. Effects of amino acids on synthesis and degradation of skeletal muscle proteins in humans. Am J Physiol Endocrinol Metab. 1996;271:E718–E724. doi: 10.1152/ajpendo.1996.271.4.E718. [DOI] [PubMed] [Google Scholar]
- Tom A, Nair KS. Assessment of branched-chain amino acid status and potential for biomarkers. J Nutr. 2006;136:324S–330S. doi: 10.1093/jn/136.1.324S. [DOI] [PubMed] [Google Scholar]
- Volpi E, Ferrando AA, Yeckel CW, Tipton KD, Wolfe RR. Exogenous amino acids stimulate net muscle protein synthesis in the elderly. J Clin Invest. 1998;101:2000–2007. doi: 10.1172/JCI939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpi E, Kobayashi H, Sheffield-Moore M, Mittendorfer B, Wolfe RR. Essential amino acids are primarily responsible for the amino acid stimulation of muscle protein anabolism in healthy elderly adults. Am J Clin Nutr. 2003;78:250–258. doi: 10.1093/ajcn/78.2.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpi E, Mittendorfer B, Rasmussen BB, Wolfe RR. The response of muscle protein anabolism to combined hyperaminoacidemia and glucose-induced hyperinsulinemia is impaired in the elderly. J Clin Endocrinol Metab. 2000;85:4481–4490. doi: 10.1210/jcem.85.12.7021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpi E, Mittendorfer B, Wolf SE, Wolfe RR. Oral amino acids stimulate muscle protein anabolism in the elderly despite higher first-pass splanchnic extraction. Am J Physiol Endocrinol Metab. 1999;277:E513–E520. doi: 10.1152/ajpendo.1999.277.3.E513. [DOI] [PubMed] [Google Scholar]
- Volpi E, Sheffield Moore M, Rasmussen BB, Wolfe RR. Basal muscle amino acid kinetics and protein synthesis in healthy young and older men. JAMA. 2001;286:1206–1212. doi: 10.1001/jama.286.10.1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang XJ, Chinkes DL, Wolfe RR. Leucine supplementation has an anabolic effect on proteins in rabbit skin wound and muscle. J Nutr. 2004;134:3313–3318. doi: 10.1093/jn/134.12.3313. [DOI] [PubMed] [Google Scholar]