Abstract
Coupling between pyramidal tract (PT) neurones and ipsilateral hindlimb motoneurones was investigated by recording from commissural interneurones interposed between them. Near maximal stimulation of either the left or right PT induced short latency EPSPs in more than 80% of 20 commissural interneurones that were monosynaptically excited by reticulospinal tract fibres in the medial longitudinal fascicle (MLF). The EPSPs were evoked at latencies that were only 1–2 ms longer than those of EPSPs evoked from the MLF, compatible with a disynaptic coupling between PT fibres and these commissural interneurones. EPSPs evoked by PT stimulation were frequently associated with IPSPs which either followed or preceded the EPSPs. The latencies of the IPSPs (on average about 1 ms longer than latencies of the earliest EPSPs) indicated that they were mediated via single additional inhibitory interneurones. Records from a sample of nine commissural interneurones from a different population (with monosynaptic input from group I and/or II muscle afferents, and disynaptically excited from the MLF) suggest that actions of PT fibres on such interneurones are weaker because only four of them were excited by PT stimuli and at longer latencies. By demonstrating disynaptic coupling between PT neurones and commissural interneurones via reticulospinal fibres, the results provide a direct demonstration of trisynaptic coupling in the most direct pathways between PT neurones and ipsilateral motoneurones, and thereby strengthen the proposal that the double crossed pathways between PT neurones and ipsilateral motoneurones might be used to replace crossed actions of damaged PT neurones.
We recently demonstrated that some actions of pyramidal tract (PT) neurones on ipsilateral hindlimb motoneurones are evoked via pathways that cross the midline twice. The first crossing occurs in the brainstem where PT neurones contact reticulospinal (RS) neurones with axons that descend in the contralateral medial longitudinal fascicle (MLF), as indicated in Fig. 1A. The second crossing occurs at a spinal level; it involves axons of contralaterally located midlumbar commissural interneurones activated by RS neurones which contact motoneurones on the side of location of the PT neurones (Bannatyne et al. 2003; Jankowska et al. 2003a, 2005; Edgley et al. 2004). We proposed that the minimal coupling in these pathways is trisynaptic, with the first synapse between the PT and RS neurones, the second between the RS neurones and midlumbar commissural interneurones, and the third between the commissural interneurones and motoneurones. However, this proposal was based on indirect measurements – particularly the time course of facilitation of the actions of RS neurones on motoneurones by PT stimulation which can only be assessed with a relatively low time resolution (Edgley et al. 2004). Indirect rather than direct estimates of PT actions on ipsilateral motoneurones were used because postsynaptic potentials were only exceptionally evoked in motoneurones following stimulation of the PT alone and it precluded reliable measurements of latencies. It was subsequently found that a K+ channel blocker, 4-aminopyridine (4-AP) enhanced synaptic transmission from PT neurones, and under these conditions PT stimuli evoked distinct EPSPs and IPSPs (Jankowska et al. 2005a). However, only about one-third of these PSPs were evoked at latencies compatible with a trisynaptic linkage. The aims of the present study were therefore twofold. The first aim was to analyse the coupling between PT neurones and commissural interneurones interposed between them and hindlimb motoneurones, and verify that these interneurones may be disynaptically activated by PT neurones. The second aim was to investigate whether all of the effects of PT stimulation on commissural interneurones are replicated by MLF stimulation and vice versa. If not, this might indicate that PT neurones may act on commissural interneurones via both RS and other relay neurones.
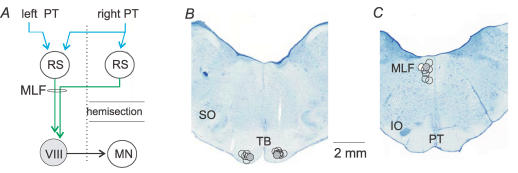
Figure 1. Diagram of the most direct double crossed pathways between pyramidal tract (PT) neurones and ipsilateral motoneurones investigated in this study and the location of the stimulating electrodes.
A, proposed disynaptic pathway between the PT fibres and lamina VIII commissural interneurones with direct actions on motoneurones on the opposite side of the spinal cord. Reticulospinal neurones descending through the left medial longitudinal fascicle (MLF) that relay PT actions could be located on either the right side or on the left side (Mitani et al. 1988a, b, c). RS, reticulospinal neurones; MLF, medial longitudinal fascicle; MN, motor nucleus; VIII, a commissural interneurone in the lamina VIII of Rexed (Rexed, 1954). B and C, reconstruction of the stimulation sites in the medullary pyramids at the level of the trapezoid body (TB) and the superior olive (SO), and in the MLF at the level of the inferior olive (IO). The location of small electrolytic lesions made at the stimulation sites is plotted on the representative sections of the brainstem from one of the experiments. Locations indicated by grey circles were used for data in Fig. 2A–E.
Methods
Preparation
The experiments were performed on eight deeply anaesthetized cats weighing 2.9–4.7 kg. All experimental procedures were as previously described (Edgley et al. 2004; Jankowska et al. 2005a), and were approved by the local Ethics Committee (Göteborgs djurförsöksetiska nämnd) and followed NIH and EU guidelines for animal care. Briefly, anaesthesia was induced with sodium pentobarbital (40–44 mg kg−1, i.p.) and maintained with intermittent doses of α-chloralose (Rhône-Poulenc Santé, France; 5 mg kg−1; administered every 1–2 h, up to 55 mg kg−1, i.v.). Additional doses of α-chloralose were given when increases in continuously monitored blood pressure or heart rate were evoked by peripheral or central stimulation, or if the pupils dilated. During recordings, neuromuscular transmission was blocked by pancuronium bromide (Pavulon, Organon, Sweden; about 0.2 mg kg−1 h−1, i.v.) and the animals were artificially ventilated. The effectiveness of synaptic transmission was increased by intravenous application of 4-AP in doses 0.2–0.4 mg kg−1, i.v. The experiments were terminated by a lethal dose of pentobarbital resulting in cardiac arrest.
A laminectomy exposed the third to seventh lumbar (L3–L7), low thoracic (Th11–Th13) and the third cervical (C3) segments, and the spinal cord was hemisected on the right side at low thoracic level. A number of peripheral nerves were dissected free and mounted on stimulating electrodes. They included the quadriceps (Q) and sartorius (Sart) branches of the left and right femoral nerve and of the right gastrocnemius–soleus (GS) nerve (mounted in subcutaneous cuff electrodes), and sometimes branches of the left sciatic nerve: posterior biceps and semitendinosus (PBST), anterior biceps and semimembranosus (ABSM), GS, plantaris (PL), flexor digitorum and hallucis longus (FDL), and deep peroneal (DP) including extensor digitorum longus and anterior tibial nerves.
Stimulation
Axons of commissural interneurones located on the left side of the spinal cord were stimulated using tungsten electrodes placed within the right GS motor nuclei. The electrodes were introduced through a hole in the dura overlying the dorsal columns at the level of the caudal part of the L7 segment, and left at the depth at which the field potential evoked by stimulation of the GS nerve was maximal. Projections of the interneurones to these motor nuclei were demonstrated by their antidromic activation in response to stimuli of 10–100 μA. Stimulation of the lateral funiculi at the thoracic level (up to 1 mA) was used to identify and exclude any neurones projecting rostral to the lumbar enlargement. The peripheral nerves were stimulated at intensities up to five times threshold (5T) for group I afferents; the threshold was defined as stimulus intensity at which just visible afferent volleys appeared in records from the cord dorsum.
Tungsten electrodes were placed in the left MLF (ipsilateral with respect to commissural interneurones) at the level of the inferior olive (Horsley-Clarke coordinates posterior 8–9, lateral 0.6–1.0 and horizontal −5 to −7) and either in both, or only in the right (contralateral) PT at the level of the superior olive (Horsley-Clarke coordinates posterior 5–6, lateral 0.7–1.2 and horizontal about −10.5).The electrodes were inserted through the cerebellum (at an angle of 35 deg) and left at sites from which maximal descending volleys were evoked at threshold stimulus intensities of 20 μA or less. The descending volleys were recorded monopolarly with a ball electrode in contact with the dura from the C3 and Th12 segments caudal to the hemisection. The stimulation sites were marked with electrolytic lesions at the end of the experiment and verified histologically (Fig. 1B and C). For activation of reticulospinal and corticospinal tract fibres, constant current cathodal stimuli (0.2 ms, 50–200 μA) were used. Near maximal stimuli applied in MLF were expected to activate a large proportion of ponto- and medullary reticulospinal tract fibres (see Jankowska et al. 2003). These stimuli could also activate the medial vestibulospinal tract fibres (which do not project caudally as far as the lumbar segments) but would not activate fibres of the lateral vestibular tract (Aoyama et al. 1971; Hongo et al. 1975). Effects of MLF stimuli in the lumbar segments could thus be attributed to reticulospinal fibres.
Descending volleys evoked by PT stimuli could be differentiated from those evoked by stimulation of the MLF by their longer latency in records from the C3 cervical segment and their considerable asynchrony at the Th12 level. By utilizing these differences it was possible to ensure that no spread of current occurred from the final position of the stimulating electrode in the PT to the MLF, even when stimulus intensity was 200 μA. As shown in Fig. 2A and B, when the electrode was correctly placed, neither short latency C3 volleys, nor any volleys at the Th12 level, were evoked from within the PT or from the area at least 1.8 mm above the dorsal border of the PT.
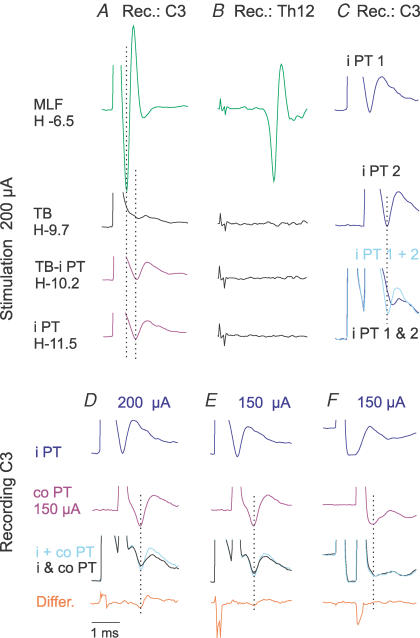
Figure 2. Estimation of current spread from the ipsilateral PT stimulation site.
A and B, descending volleys evoked by stimulation at the indicated depths along an electrode track, above and within the ipsilateral PT (i PT). The depths are in the Horsley-Clarke horizontal coordinates. The volleys were recorded from the cord dorsum at the third cervical (C3) segment and from the left lateral funiculus at the 12th thoracic (Th12) segment. Note that stimuli applied within and up to 1.8 mm above the final position of the electrode in the PT (in the trapezoid body, TB) failed to evoke short latency volleys that are induced from the MLF (upper traces, H-6.5 mm), both in records from the Th12 and from the C3 where the positive peaks of MLF and PT volleys are indicated by two dotted lines. C–F, examples of paired-pulse tests used to define the critical stimulus intensity at which stimuli applied in the i PT might coexcite fibres in the contralateral pyramidal tract (co PT), as indicated by smaller C3 volleys following co PT stimuli applied ≤ 0.8 ms after i PT stimuli (due to their refractoriness). As shown in C, the volley evoked by two 200 μA stimuli applied in the same PT 0.6 ms apart (bottom records; i PT 1 & 2) was much smaller than the sum of volleys (top and middle records) evoked by same stimuli (i PT 1 + 2) when they were applied separately. Records in D show that 200 μA stimuli showed some spread of current from the i PT to co PT, but the spread of current became negligible when the intensity of PT stimuli was reduced to 150 μA because only marginal difference was then seen between volleys evoked by separate (i + co PT) and joint (i & co PT) stimulation of the two PTs in some experiments (E) and no differences were detected in other experiments (F). In all these tests co PT was stimulated at the same intensity of 150 μA. In all panels, averages of 20 single records are shown, with the negativity upwards.
In order to estimate the degree of spread of current from one of the PTs to the other, stimuli of different intensities were applied in the two PTs at intervals (0.6 ms) so that any fibres stimulated twice would be refractory at the time of the second stimulus and would only respond to the first stimulus. As shown in the superimposed records in Fig. 2C, a second stimulus applied to the same PT on one side failed to evoke the triphasic volley with similarly timed positive peak. The volleys following two stimuli (black trace) were smaller than the sum of the volleys evoked when these stimuli were applied separately (grey trace) due to the refractoriness of the fibres. Using the same interval between two shocks, but with one of the stimuli delivered to the contralateral PT (at 150 μA) and the other to the ipsilateral PT at varied strengths, only negligible differences were found (Fig. 2E; in three out of four experiments in which this was verified) or no differences were detected (Fig. 2F; in one experiment when stimulus intensity was 150 μA or less). When stimuli of 200 μA were used, the resulting volleys were smaller than the sum of volleys evoked by individual stimuli (Fig. 2D). No differences were found in any experiments when the intensity was reduced to 100 μA. Thus stimuli of 150 μA or less were used for comparison of actions evoked from the two PTs and stimuli of 200 μA for defining maximal PT actions.
Recording and analysis
Glass micropipettes filled with 2 m solution of potassium citrate (2–5 MΩ) were used for intracellular or extracellular recording, except in one experiment in which they were obtained with pipettes filled with a mixture of rhodamine-dextran and Neurobiotin, used for labelling and subsequent morphological and immunocytochemical analyses (Bannatyne et al. 2003). Some extracellular records were also obtained with glass micropipettes filled with a 2 m solution of sodium chloride (1–2 MΩ). Both the original data and averages of 10–20 single records (with the time resolution of 20 or 30 μs per address) were stored on-line using a software sampling and analysis system designed by E. Eide, T. Holmström and N. Pihlgren (Göteborg University). Differences between samples of neurones were assessed for statistical significance using Student's t test (for paired and/or unpaired, normally distributed data).
Sampling
The sample of commissural interneurones analysed in this study included 29 intracellularly recorded interneurones located in the L3–L5 segments. Nine of these and nine other interneurones were also recorded extracellularly. They were concluded to be activated antidromically from the contralateral motor nuclei on the basis of a constant response latency (0.5–1.4 ms), most of which (in particular those < 1 ms) were in addition too short to allow a synaptic delay. Action potentials classified as evoked antidromically were collided by preceding synaptically evoked responses in extracellular records and appeared in an all-or-none fashion in intracellular records. The longest latencies of antidromic activation (1.2–1.4 ms) corresponded to conduction velocities of about 25–30 m s−1 and the shortest to velocities of 50 m s−1. The search was made for commissural interneurones with monosynaptic input from either the MLF or group II afferents, both ipsilateral with respect to the interneurones, at locations at which largest field potentials from both sources were recorded. At some of these locations field potentials were also evoked from either one or both PTs.
Results
Coupling in excitatory pathways between PT neurones and commissural interneurones with monosynaptic input from the MLF
EPSPs were evoked by PT stimulation in the majority of commissural interneurones that had monosynaptic input from the MLF. When short trains of stimuli of 150 μA were used they were evoked from both the contralateral and the ipsilateral PT in more than 75% of these interneurones. As shown in Figs 3 and 4, distinct EPSPs followed successive PT stimuli, and the first step in the analysis was linking them to the individual stimuli. When an EPSP appeared after a second or third stimulus, the number of stimuli in the train was reduced to define which stimulus was responsible for it. The double-headed arrows in Fig. 3A indicate which of the stimuli evoked the successive EPSPs.
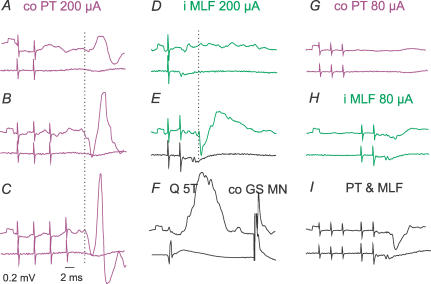
Figure 3. Examples of EPSPs evoked by successive PT and MLF stimuli in a commissural interneurone.
A, the three upper traces are averaged (n = 10) intracellular records and the two lower traces are extracellular field potentials and records from the cord dorsum, evoked by 150 μA stimuli applied within the co PT at 400 Hz. Double-headed arrows indicate the latencies of EPSPs with respect to the stimuli that induced them. Note the temporal facilitation of EPSPs evoked by successive stimuli, and that the larger EPSPs coincided with larger field potentials in the extracellular recording. B, averaged antidromic potentials evoked by stimuli applied in the contralateral GS motor nucleus, recorded intracellularly. C, records as in A, but following stimulation of the ipsilateral MLF. In this and in the following figures, the negativity is downwards in microelectrode recordings and upwards in the records from the cord dorsum. Square pulses at the beginning of each trace are 0.2 mV voltage calibration pulses. The time calibration in B applies to all records. The largest stimulus artefacts in this and in the following figures are truncated.
Figure 4. Examples of postsynaptic potentials evoked by PT and MLF stimuli of different intensities.
Intracellular records from a commissural interneurone (upper traces) and records from the cord dorsum (lower traces); averages of 20 successive records. A and B, EPSPs evoked by stimulation of the co PT and i PT at decreasing stimulus intensities. Double-headed arrows indicate the latencies of these potentials with respect to the effective stimuli, following the procedure illustrated in Fig. 3. Note that when the intensity of PT stimuli was decreased, the third but not the second 150 μA stimulus in A, and 50 μA in B, evoked early EPSPs, and that the 50 μA stimulus in A evoked only late EPSPs. C, similar series of records of EPSPs and EPSP/IPSP sequences evoked by stimulation of the MLF. Note that the weakest MLF stimuli evoked only IPSPs in this interneurone. Blocked antidromic spike potentials evoked from the contralateral GS motor nucleus (co GS MN; at threshold of 9 μA) are shown only in A.
When this procedure was used, single stimuli were found to evoke short-latency EPSPs in several interneurones (Table 1, row 1); these occurred more often when the stimuli were applied to the ipsilateral than to the contralateral PT, and only when the intensity was 150–200 μA. In other interneurones, only the second, third or fourth stimuli of a train evoked the EPSPs, especially when the stimulus intensity was lowered (cf. the top and middle panels in Fig. 4A; Table 1, rows 2 and 3). Furthermore, weaker stimuli were sometimes followed by much longer latency EPSPs (see Fig. 4A bottom). The characteristic feature of the early EPSPs was their marked temporal facilitation, as EPSPs evoked by the second or third stimuli were always larger than those evoked by the first stimulus (see, e.g. Fig. 3A). The temporal facilitation of EPSPs of PT origin contrasted with the features of the monosynaptic EPSPs evoked in the same neurones by stimulation of the MLF, which were evoked as effectively by the first as by later stimuli in a train and were not temporally facilitated (Fig. 3C). EPSPs evoked by later MLF stimuli were larger only when monosynaptic components of these EPSPs were followed by disynaptic components (Fig. 4C).
Table 1.
Comparison of synaptic actions evoked from the contralateral and ipsilateral PTs
| Ipsilateral PT | Contralateral PT | ||||
|---|---|---|---|---|---|
| 1 | EPSPs evoked by single 150–200 μA PT stimuli | 14/19 | 74% | 6/14 | 43% |
| 2 | EPSPs evoked by trains of 150 μA PT stimuli | 17/17 | 100% | 10/13 | 77% |
| 3 | EPSPs evoked by trains of 100 μA PT stimuli | 11/12 | 92% | 8/12 | 67% |
| 4 | EPSPs at latencies < 1.6 ms longer than from the MLF | 9/11 | 90% | 6/11 | 55% |
| 5 | Extracellular spikes at latencies < 1.8 ms longer than from the MLF | 6/10 | 60% | 2/8 | 25% |
| 6 | Mean amplitudes of EPSPs evoked by third PT stimuli | 0.47 ± 0.11 mV** | 0.35 ± 0.04 mV | ||
Differences between Mean amplitudes of EPSPs evoked by ipsilateral and contralateral PT stimuli were significant at P < 0.001. The comparison is only for commissural interneurones in which effects of various stimulus parameters could be tested. Data in rows 4–6 are for stimuli of ≤ 150 μA. It will be noted that weaker effects from the contralateral than from the ipsilateral PT stimulation manifested themselves when 150–200 μA stimuli (with the risk for current spread between the PTs) and weaker stimuli were used.
Amplitudes of the EPSPs evoked by the first and second PT stimuli of ≤ 150 μA were on average 33 and 51% of those evoked by the third contralateral PT stimuli, and 42 and 61% of those evoked by the third ipsilateral PT stimuli. The smaller amplitudes of EPSPs evoked by the first two stimuli would explain why extracellularly recorded action potentials (see below) usually appeared only to the third or fourth stimulus.
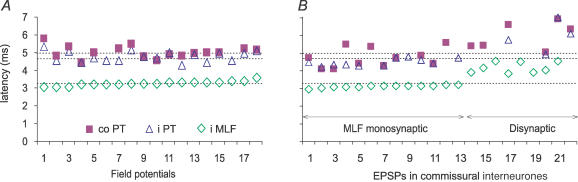
Extracellular field potentials (reflecting intracellular EPSPs) evoked by PT stimulation were recorded at 36 different locations. Like EPSPs evoked in individual commissural interneurones, distinct temporally facilitated field potentials were evoked by successive PT stimuli. They were usually detectable only after the second and third stimuli of a train (Fig. 3A), even at maximal stimulus intensity. This contrasted with field potentials evoked by MLF stimulation which were evoked by the first as well as the successive stimuli and were of similar amplitude in response to each stimulus (Fig. 3C). When evoked by stimuli not exceeding 150 μA, field potentials were induced from both the contralateral and ipsilateral PT at latencies 1.4–1.7 ms longer than latencies of field potentials evoked from the MLF (Fig. 5A; Table 2B, columns 5 and 6).
Figure 5. Latencies of EPSPs evoked from the contralateral and ipsilateral PTs in relation to EPSPs evoked from the MLF and to field potentials of PT and MLF origin.
A, shortest latencies of extracellular field potentials evoked by the third or fourth contralateral PT (filled squares) and ipsilateral PT (open triangles) and by the first or second MLF (open diamonds) stimuli. Stimuli were 150 μA for the PT, and 100 or 150 μA for the MLF. B, similar data for latencies of intracellularly recorded EPSPs of PT and MLF origin in interneurones with monosynaptic input from the MLF (interneurones 1–13, as indicated by the left double-headed arrow) and with disynaptic or longer latency input (interneurones 14–22). Dotted horizontal lines indicate the mean latency of field potentials evoked by co PT, i PT and MLF stimuli from the top down, respectively.
Table 2.
Minimal latencies of synaptic actions evoked from the ipsilateral and contralateral PT and from the ipsilateral MLF
| 1 Latency (in ms) of: | 2 i PT | 3 co PT | 4 i MLF | 5 i PT-MLF | 6 co PT-MLF | ||||
|---|---|---|---|---|---|---|---|---|---|
| A | Extracellular spikes | 5.52 ± 0.18 | n.s. | 5.74 ± 0.17 | ** | 3.63 ± 0.13 | 1.81 ± 0.10 | * | 2.16 ± 0.22 |
| n = 10 | n = 8 | n = 18 | |||||||
| B | Field potentials | 4.76 ± 0.07 | * | 5.00 ± 0.08 | ** | 3.21 ± 0.15 | 1.49 ± 0.07 | * | 1.74 ± 0.09 |
| n = 18 | n = 16 | n = 36 | |||||||
| C | Monosynaptic EPSPs from MLF | (n = 20) | |||||||
| EPSPs | 4.48 ± 0.06 | n.s. | 4.74 ± 0.12 | ** | 3.17 ± 0.05 | 1.38 ± 0.05 | n.s. | 1.97 ± 0.36 | |
| n = 11 | n = 11 | n = 20 | |||||||
| IPSPs | 5.65 ± 0.17 | n.s. | 5.52 ± 0.10 | ** | 4.02 ± 0.13 | 1.97 ± 0.36 | n.s. | 1.67 ± 0.13 | |
| n = 11 | n = 10 | n = 16 | |||||||
| EPSPs–field potentials | −0.17 | −0.06 | −0.10 | — | — | ||||
| IPSPs–EPSPs | 1.17 ± 0.17 | n.s. | 0.94 ± 0.17 | n.s. | 0.91 ± 0.12 | — | — | ||
| D | Disynaptic EPSPs or no EPSPs from MLF | (n = 9) | |||||||
| EPSPs | 5.95 ± 0.43 | n.s. | 5.96 ± 0.31 | ** | 4.18 ± 0.11 | 1.77 ± 0.46 | n.s. | 1.79 ± 0.33 | |
| n = 4 | n = 6 | n = 8 | |||||||
| IPSPs | 5.97 ± 0.31 | n.s. | 5.29 ± 0.24* | * | 4.53 ± 0.30 | 1.73 ± 0.13 | n.s. | 0.93 ± 0.58 | |
| n = 4 | n = 5 | n = 7 | |||||||
| EPSPs–field potentials | 1.30 | 1.16 | 0.97 | — | — | ||||
| IPSPs–EPSPs | −0.04 ± 0.52 | n.s. | −0.22 ± 0.44 | n.s. | 0.17 ± 0.26 | — | — |
All the data are for stimulus intensities of 100–150 μA. i, ipsilateral; co, contralateral; MLF, medial longitudinal fascicle. Data in A are for extracellularly recorded spike potentials of 18 commissural interneurones antidromically activated from the contralateral GS motor nucleus; in B for field potentials recorded at 36 locations; in C for PSPs recorded in 20 commissural interneurones with monosynaptic EPSPs from the MLF; in D for PSPs recorded in 9 commissural interneurones in which MLF stimuli evoked only disynaptic EPSPs (n= 8) or IPSPs, or had no effect (n= 1). The data show effects evoked by the third or fourth PT stimuli and by the first or second MLF stimuli. Means and s.e.m. of minimal latencies are for events with latencies of less than 7 ms, measured from averages of 10–20 individual records. The numbers of measurements on which these means are based are shown. However, these numbers do not reflect the number of neurones in which PT stimuli evoked spikes, EPSPs or IPSPs because the neurones sometimes deteriorated before effects of different intensities or numbers of stimuli applied to the PTs on both sides and the MLF could be tested. The proportions of neurones in which stimuli with various parameters were effective are given in Table 1. Since the time resolution in the averaged records was 30 μs per address, any differences of the order of ±0.03 ms are considered as being within measurement errors. Statistically significant differences between data in columns 2 and 3, 3 and 4 and 5 and 6 are indicated between the pairs of columns (**P < 0.001; *P < 0.05; n.s., not statistically significant). In addition to those indicated, there was a statistically significant difference between latencies of EPSPs (at P < 0.001) but not of IPSPs of PT origin in C and D.
We demonstrated previously that the earliest synaptic actions of RS neurones on commissural interneurones are evoked monosynaptically (Krutki et al. 2003). In order to be compatible with disynaptic coupling between PT neurones and commissural interneurones, the latencies of EPSPs evoked by PT stimulation should be longer, but only by a time appropriate to conduction time along collaterals of PT fibres plus one synaptic delay, which is estimated to about 1–1.5 ms. As shown in Table 2, mean minimal latencies of both field potentials and EPSPs evoked from the ipsilateral PT were within this range while those from the contralateral PT were somewhat longer (Table 2B and C, columns 2 and 3). However, plots in Fig. 5A and B show that the latencies of many individual field potentials and EPSPs evoked from the contralateral PT when compared to those from the ipsilateral PT were similarly short.
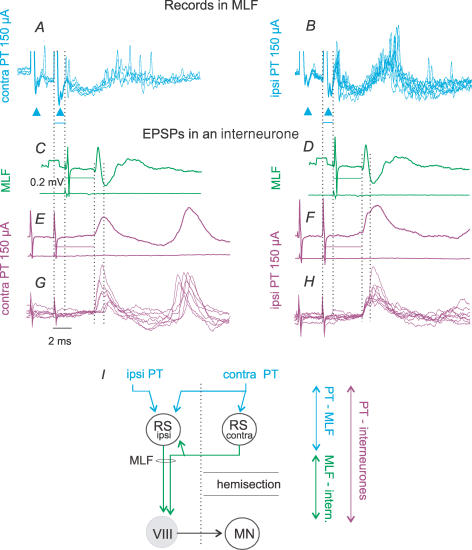
In order to relate EPSPs evoked in commissural interneurones to either monosynaptic, disynaptic or polysynaptic activation of RS neurones by PT stimuli, we used recordings from the MLF to monitor activity in RS neurones. The records were obtained using the same electrode with which RS fibres were stimulated. In all five experiments in which this was done, the effects of PT stimuli were similar. The stimuli evoked first small triphasic volleys at about 0.5 ms latency (upward arrowheads in Fig. 6A and B), most probably reflecting action potentials in collaterals of PT fibres. These were followed by population potentials (at about 0.9 ms from the stimulus) on which asynchronous spike discharges were superimposed, especially after the second or third stimuli. The earliest discharges appeared at latencies of 1.1–1.6 ms from the stimuli, but less than 1 ms from the triphasic volleys, and could therefore have been due to monosynaptically evoked activation of RS neurones. These were followed by discharges evoked at about 2–3 ms and 5–10 ms latencies from the stimuli and attributable to disynaptically and polysynaptically evoked activation of RS neurones. PT stimuli could thus activate RS neurones monosynaptically, disynaptically and polysynaptically.
Figure 6. Time relationship between EPSPs of PT and MLF origin and responses of RS neurones to PT stimuli.
A and B, single sweep records from the MLF. Only records with discharges following either the first or the second stimulus have been superimposed. C–F, averages (n = 20) of PSPs from commissural interneurones (upper traces) and cord dorsum potentials aligned so that the onsets of the EPSPs evoked from the MLF and PT, indicated by the third dotted lines, coincided. Note that the difference between the stimuli is very similar to the latency of MLF responses to PT stimuli. G and H, superimposed single sweep records of the largest EPSPs used for these averages. Records are from the same interneurone as in Fig. 4. The time intervals between the first two dotted lines in C–F indicate differences in latencies of EPSPs evoked from the MLF and PT. The same lines in A and B indicate a delay in discharges recorded in MLF with respect to PT stimuli. Arrowheads in A and B indicate triphasic presynaptic volleys induced by PT stimuli. I, interconnections between RS neurones which would allow excitation of RS neurones (e.g. that to the left of the midline) by axon collaterals of other RS neurones (e.g. that to the right) and explain additional synaptic delays in actions of PT neurones on RS neurones. Double-headed arrows indicate sums of synaptic delays and conduction times as indicated. Voltage calibration of 0.2 mV in C and time calibration of 2 ms in G are for all records.
The simplified diagram in Fig. 6I provides an explanation of the monosynaptically and disynaptically evoked discharges following PT stimulation, by direct actions of PT fibres (represented by the ipsilateral RS neurone) and by actions relayed through other RS neurones (represented by the contralateral RS neurone), or by reticular neurones that do not project down to the spinal cord. The later discharges could likewise be due to indirect activation of RS neurones, but via a larger number of interposed neurones and/or by much slower conducting PT fibres. Some other alternative explanations are mentioned in the Discussion.
A closer inspection of EPSPs evoked by PT stimulation revealed that the earliest components were often followed by components with onsets 1–2 ms later; such later components can be seen both in individual and averaged records (indicated by the fourth dotted line in Fig. 6E–H). We related, therefore, the two components of EPSPs evoked by PT stimuli to the earliest and most likely disynaptic discharges of RS neurones evoked by PT stimuli and compared their timing.
The earliest components of EPSPs evoked by PT stimuli had latencies (Table 2C, columns 2 and 3) that nearly equalled the sums of latencies of the earliest discharges recorded in the MLF and of latencies of EPSPs evoked from the MLF, as indicated to the right of the diagram in Fig. 6I. This is illustrated by the close correspondence between the delays of EPSPs of PT origin with respect to those evoked from the MLF in a commissural interneurone and the latencies of discharges of RS neurones in MLF (first two dotted lines in Fig 6A and B). The data in Table 2B, columns 5 and 6, show further that the longer latencies of EPSPs and field potentials of PT than of MLF origin (1.4–1.7 ms) match the 1.1–1.6 ms latencies of the earliest discharges of RS neurones recorded in the MLF. We propose, therefore, that the earliest EPSPs were induced via monosynaptically excited RS neurones.
The later components of EPSPs following the monosynaptic EPSPs might in a similar way be related to the disynaptically evoked discharges of RS neurones, and reflect trisynaptic rather than disynaptic coupling between PT neurones and commissural interneurones. One could expect longer latency activation when RS neurones are less excitable and when their activation by PT neurones requires summation of mono- and disynaptically evoked EPSPs. Polysynaptically evoked excitation of RS neurones (the late discharges in Fig. 6A and B) might also summate with early effects of the third and fourth PT stimuli and increase the effectiveness of the successive PT stimuli. Using the above arguments we may thus set the borderline between latencies of disynaptic and trisynaptic actions of PT stimuli plotted in Fig. 5B at about 5 ms from the stimuli, even if this borderline is somewhat arbitrary.
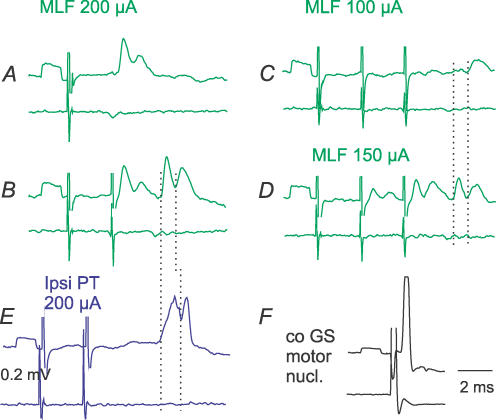
One of the consequences of the postulated collateral actions of RS neurones on other RS neurones would be that stimuli applied in the MLF should not only give rise to descending volleys but also to synaptically evoked activation of RS neurones. Monosynaptic actions of fibres stimulated in MLF on commissural interneurones should thus be followed by disynaptically evoked PSPs, as has indeed been found and is illustrated in Fig. 7. Records in Fig. 7A and B show the expected temporal facilitation of the second components of these EPSPs. Comparison of the timing of these components in Fig. 7B with the timing of the disynaptic and trisynaptic components of EPSPs evoked by PT stimuli in Fig. 7E shows a good match. Finally, comparison of the timing of EPSPs evoked by MLF stimuli that were too weak to activate fibres responsible for monosynaptic EPSPs in the interneurone illustrated in Fig. 7C, with the two components of EPSPs evoked by stronger stimuli in Fig. 7D, shows that the EPSP in Fig. 7C and the second component of the EPSP in Fig. 7D coincided. Following the same reasoning, one may conclude that even if PT stimuli are too weak to evoke monosynaptic activation of RS neurones, they may activate RS neurones disynaptically, via other brainstem neurones, and induce trisynaptic EPSPs in commissural interneurones via the same RS neurones.
Figure 7. Disynaptic and trisynaptic components of EPSPs evoked in commissural interneurones from the MLF and PTs.
A–F, intracellular records from a commissural interneurone (upper traces) and cord dorsum potentials (lower traces); averages of 20 successive records. A and B, monosynaptic and disynaptic components of EPSPs evoked by strong MLF stimuli. Note constant amplitudes of the first components and temporal facilitation of the later components, after the first and the second stimuli. C, 100 μA MLF stimuli only evoked the later disynaptic EPSP. D, 150 μA MLF stimuli evoked the later components as well as smaller early EPSPs. E, two components of EPSPs evoked by PT stimuli. The dotted lines indicate the onset of the mono- and disynaptic EPSPs in B, C and D, and of those classified as evoked di- and trisynaptically in E. F, an antidromic action potential in the interneurone evoked from the GS motor nucleus (stimulus 40 μA).
Coupling in inhibitory pathways between PT neurones and commissural interneurones
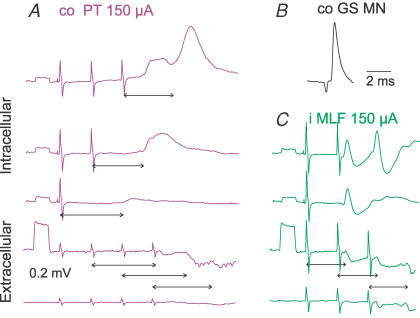
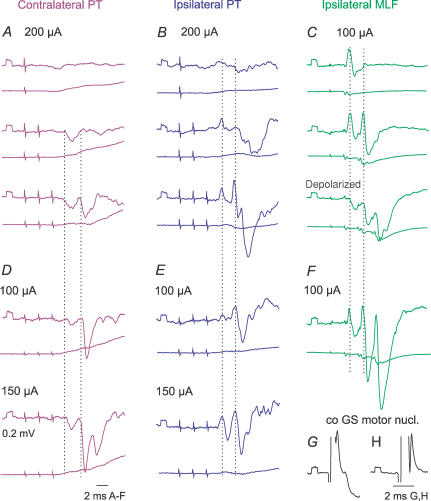
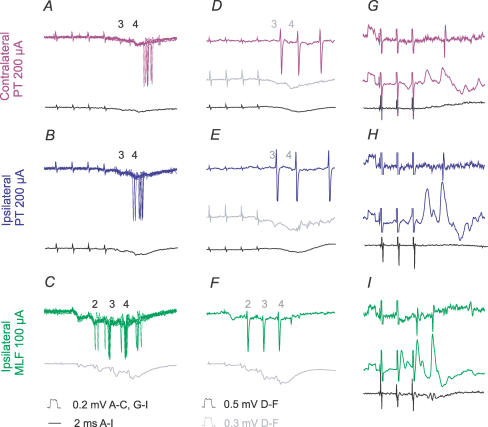
PT stimuli evoked IPSPs in practically all of the commissural interneurones in which IPSPs were evoked by MLF stimulation. However, in several interneurones the IPSPs of PT origin were less prominent than the IPSPs evoked from the MLF, as in interneurones illustrated in Figs 4 and 8, and often required at least two maximal PT stimuli to appear (Fig 8A and B). As in the case of the EPSPs, when stronger PT stimuli were used, IPSPs were evoked by earlier stimuli, and/or were larger (Fig. 8D and E).
Figure 8. Examples of IPSPs evoked from contralateral and ipsilateral PT.
Intracellular records of IPSPs (upper traces) evoked in two commissural interneurones (A–C, and D–F) and records from the cord dorsum (lower traces); averages of 20 records. Records of blocked spikes that were antidromically evoked in these interneurones from the contralateral motor nuclei are in G and H. Dotted lines indicate the onset of IPSPs evoked by the second and third stimuli. C, the lowest panel shows records taken when the cell was depolarized with 15 nA current. Other indications are as in Fig. 3.
Measurements of the latencies of IPSPs were most reliable when IPSPs were evoked without preceding EPSPs (as in the interneurones in Fig. 8A and D and Fig. 9B and C), or were preceded by only small EPSPs (as in Fig. 8B and E). When the IPSPs overlapped with the EPSPs, their onset was defined by comparing the declining phase of EPSPs followed by the IPSPs with that of EPSPs that did not evoke the IPSPs, the former being much steeper (cf. EPSPs in Fig. 4A and B and EPSPs evoked by single and double stimuli in Fig. 8B), especially when the IPSPs increased after depolarization of the neurones. All the measurements showed consistently that IPSPs of PT origin were delayed with respect to EPSPs evoked by the same stimuli by about 1 ms (Table 2C, columns 2 and 3); with similar delays to those of IPSPs evoked from the MLF (column 4). Given that the EPSPs were evoked disynaptically, as argued above, the latencies of the IPSPs are thus compatible with a minimal trisynaptic coupling between PT neurones and commissural interneurones. Figure 8A–C illustrates temporal facilitation of the IPSPs and Fig. 8D and E their dependence on stimulus intensity.
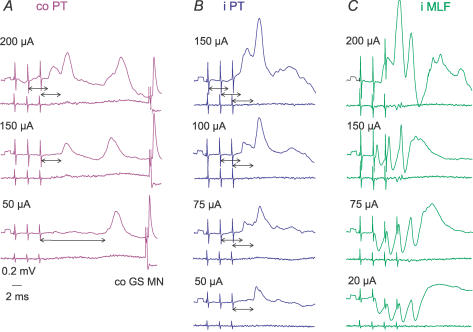
Figure 9. Mutual facilitation of IPSPs of PT and MLF origin.
Intracellular records from an interneurone (upper traces) and from the cord dorsum (lower traces); averages of 20 records. A–C, and D–E, effects of increasing numbers of PT and MLF stimuli, respectively. G–I, facilitation of IPSPs on combining subthreshold PT and MLF stimuli. The neurone is from the subpopulation with monosynaptic excitation from group II afferents (F) but not from the MLF (D and E).
Potent mutual facilitation of IPSPs evoked in commissural interneurones by PT and the MLF stimulation illustrated in Fig. 9G–I supports the conclusion that they are mediated by the same interneurones. Note that in this case, similar IPSPs were evoked by strong third and fourth PT stimuli, by the second MLF stimulus, and upon joint application of much weaker PT and MLF stimuli which were not effective when applied separately.
Coupling between PT neurones and commissural interneurones without monosynaptic input from the MLF
The subpopulation of nine commissural interneurones that were not monosynaptically excited from the MLF (Table 2D) appeared to include interneurones of two categories: interneurones with monosynaptic input from group II afferents (Jankowska et al. 2005b), and interneurones with monosynaptic input from group I and II afferents (Jankowska & Noga, 1990). However, group I input was tested from only a small selection of peripheral nerves and could not be excluded in the four interneurones in which only group II was found. All these interneurones will therefore be considered jointly.
Table 2 shows that stimulation of both the contralateral and ipsilateral PT evoked EPSPs and IPSPs in this subpopulation of commissural interneurones. However, two main differences have been found in PT actions on commissural interneurones lacking monosynaptic input from the MLF and those described above. Firstly, EPSPs evoked in these commissural interneurones appeared at latencies more than 1 ms longer than those in interneurones with monosynaptic MLF EPSPs (Table 2D). However, these latencies exceeded latencies of EPSPs evoked from the MLF to the same extent as in interneurones with monosynaptic input from the MLF.
Secondly, latencies of IPSPs evoked in them did not exceed latencies of the EPSPs, latencies of the IPSPs being similar to the latencies of IPSPs evoked in interneurones with monosynaptic EPSPs from the MLF. It appears thus that IPSPs evoked in the two subpopulations of commissural interneurones might be mediated by the same interneurones and be related to monosynaptically rather than disynaptically evoked PT excitatory actions on RS neurones.
Extracellularly recorded responses
Extracellular records were obtained from 18 commissural interneurones, nine of which were subsequently penetrated. They were activated more effectively by MLF stimuli (first to third stimulus) than by PT stimuli (third to fifth stimulus). Activation of these interneurones thus required temporal facilitation to a greater extent when they were evoked from PTs than from the MLF.
The spikes had a tendency to coincide with the peak or the declining phases of field potentials evoked by the same stimuli, but their latencies varied greatly, as illustrated in Fig. 10A–C. However, the minimal latencies exceeded latencies of EPSPs evoked by the same stimuli in the subsequently penetrated neurones by 0.99 ± 0.30 ms for those from the contralateral PT, 0.53 ± 0.20 ms from the ipsilateral PT, and 0.29 ± 0.03 ms from the ipsilateral MLF, as illustrated in Fig. 10G–I. The earliest action potentials are thus compatible with monosynaptic activation of commissural interneurones from the MLF and disynaptic from the PTs.
Figure 10. Timing of extracellularly recorded responses evoked by PT and MLF stimuli in three commissural interneurones.
A–C, superimposed extracellular records from a commissural interneurone obtained at the same location as the records of field potentials in Fig. 3A and B. D–F, extracellular records from another commissural interneurone and averaged records of the corresponding field potentials (grey traces, at a higher amplification). G–I, extracellular (top) and intracellular (middle) records from the third interneurone. Bottom traces in all panels are from the cord dorsum. The numbers above records in A–F indicate by which stimuli the field potentials and the spikes were evoked.
The minimal latencies of activation of commissural interneurones of the present sample from the MLF (about 3 ms, see Fig. 5B and Table 2A) would allow about 1 ms for the generation of disynaptic EPSPs and/or IPSPs in motoneurones (that are evoked at latencies of about 4 ms). Interneurones activated from the contralateral and ipsilateral PTs at 5–6 ms latencies should likewise be able to mediate PSPs compatible with trisynaptic actions evoked at latencies 6–7 ms (see Fig. 5A–C in Jankowska et al. 2005a). However, in order to contribute to the very earliest EPSPs of PT origin (at latencies 5–6 ms; see Fig. 5D and E in Jankowska et al. 2005a) they should be activated more effectively, e.g. by longer trains of PT stimuli than those used in the present study or by combined actions of the ipsilateral and contralateral PT neurones.
Discussion
The results of this study substantiate previous proposals regarding the coupling between PT neurones and ipsilateral hindlimb motoneurones by showing that the earliest PT actions on these motoneurones may be evoked trisynaptically (Edgley et al. 2004; Jankowska et al. 2005a), by disynaptic activation of commissural interneurones which monosynaptically excite or inhibit motoneurones. This conclusion took into account previously demonstrated direct coupling between PT neurones and reticulospinal neurones (Peterson et al. 1974; He & Wu, 1985; Canedo & Lamas, 1993; Matsuyama & Drew, 1997), between reticulospinal neurones and commissural interneurones (Jankowska et al. 2003, 2005b) and between commissural interneurones and contralateral α-motoneurones (Bannatyne et al. 2003; Butt & Kiehn, 2003; Matsuyama et al. 2004). However, not all PSPs evoked by PT stimulation in hindlimb motoneurones were found to be evoked at latencies compatible with trisynaptic coupling (Jankowska et al. 2005a) and the longer latencies of other PSPs might involve additional neuronal relays at either brainstem or spinal levels. Additional brainstem relays would be in keeping with the finding that reticulospinal neurones are excited by PT neurones not only directly but also indirectly (Peterson et al. 1974; Ito & McCarley, 1987; Canedo & Lamas, 1993) and also with disynaptic excitation of commissural interneurones by MLF stimuli (Krutki et al. 2003) which could be explained either by re-excitation of RS neurones via their brainstem target neurones or by involvement of additional spinal relays. Longer latency PSPs evoked by PT stimuli could also reflect actions of slower conducting corticoreticular (Peterson et al. 1974; Matsuyama & Drew, 1997) and/or reticulospinal neurones (He & Wu, 1985; Mitani et al. 1988a), or of a relatively inefficient synaptic activation of RS neurones and commissural interneurones. Whether any other commissural interneurones, in addition to those with input from RS neurones and/or from group II muscle afferents analysed in the present study, mediate PT actions to motoneurones remains still an open question. However, as indicated in the next section they would be unlikely to contribute to the shortest latency PT actions.
Mode of excitation of commissural interneurones by PT stimuli
Our estimates of the timing of PT actions on commissural interneurones required first finding which particular stimulus in a train was responsible for the PT actions, as illustrated in Figs 3, 8 and 9. These were usually the third or fourth stimuli for action potentials, and the first or second stimuli for EPSPs and IPSPs. The minimal latencies of these PSPs of PT origin were then measured and compared with minimal latencies of PSPs evoked from the MLF and related to the timing of effects of PT neurones on reticulospinal neurones (monitored by recording from fibres running in the MLF). Taken together, the results led to the conclusion that latencies of PSPs evoked by PT stimuli that were less than 2 ms longer than those of PSPs evoked by MLF stimuli are compatible with actions mediated by RS neurones that were monosynaptically activated by PT stimuli. In turn, this led to the conclusion that contralateral as well as ipsilateral PT fibres may provide disynaptic input to commissural interneurones with monosynaptic input from the MLF. However, the earliest components of EPSPs evoked from either PT were often followed by later components, especially after the third or fourth stimuli, which are more effective in indirectly activating reticulospinal neurones. It cannot therefore be resolved whether disynaptic EPSPs of PT origin are sufficient to induce action potentials in commissural interneurones or whether summation of di- and trisynaptically evoked EPSPs and/or of late actions of the earlier PT stimuli is needed for the EPSPs to reach action potential threshold. The most reasonable conclusion might be that disynaptically mediated excitation of commissural interneurones by PT neurones contributes to trisynaptic PT actions on motoneurones on a background of PT effects mediated by additional supraspinal relay neurones.
This conclusion is in agreement with several sets of previous data, e.g. the range of latencies (2.4–30 ms) of spike activation in RS neurones by cortical stimuli reported by Peterson et al. (1974) shows that the earliest of these latencies were only slightly longer than latencies of indirect volleys recorded in MLF in our study (2 ms), even though they involved a longer conduction distance. The ranges of latencies of EPSPs evoked in reticulospinal neurones by single stimuli (0.8–1.6 and 1.7–2.4 ms) in the study of Peterson et al. (1974) show an even closer correspondence. Of particular interest for identifying the reticular relays of corticospinal actions is that EPSPs likely to be evoked mono- and disynaptically were found in RS neurones in both pontine and medullary nuclei (Peterson et al. 1974), that EPSPs evoked at shortest latencies (< 2 ms) were found primarily in the fastest conducting RS neurones (He & Wu, 1985). It is also of relevance that both corticospinal neurones and other PT neurones were found to evoke monosynaptic EPSPs in RS neurones, while disynaptic EPSPs followed activity of the latter, but not of the former (Canedo & Lamas, 1993).
Considering that all commissural interneurones with monosynaptic input from RS neurones (but not those with disynaptic input) were disynaptically excited from PTs suggests that the earliest (trisynaptic) PT actions on hindlimb motoneurones are mediated primarily via the former. However, commissural interneurones without monosynaptic input from RS neurones (e.g. commissural interneurones which mediate crossed reflexes from group I and II muscle afferents; see Jankowska & Noga, 1990; Jankowska et al. 2005b) could contribute to later PT actions and these might be further enhanced by nerve impulses induced during muscle stretches and/or contractions as sensory feedback. Involvement of all these mutually enhancing sources of input to reticulospinal neurones and commissural interneurones should be of particular use for recovery of functions after central injuries when the effectiveness of activation of PT neurones and of their actions on RS neurones is reduced.
Even though all of the reported results support the mediation of the excitatory actions of PT neurones on commissural interneurones by RS neurones, it should be considered that RS neurones are not the only neurones via which PT neurones may excite commissural interneurones.
One alternative route of PT actions might be via vestibulospinal neurones in view of the demonstration that neurones in the lateral vestibular nucleus provide both monosynaptic and disynaptic input to commissural interneurones (Krutki et al. 2003) as well as the evidence for direct cortico-vestibular projections from the areas 6, 3 and 2a (Wilson et al. 1999). However, Wilson et al. (1999) suggested that cortical neurones activate vestibulospinal neurones polysynaptically rather than monosynaptically and, if so, vestibulospinal neurones might contribute to the later but not the earliest PT actions on commissural interneurones.
Other alternative relay neurones mediating PT actions on commissural interneurones might be spinal interneurones that are monosynaptically excited by PT neurones and have commissural neurones as their target cells. So far we have no direct evidence either for or against this possibility. However, we might consider that conduction velocity of the PT fibres is lower than of the RS tract fibres. For the fastest conducting PT neurones (with conduction velocity of about 60 m s−1; Lloyd, 1941) the conduction time to midlumbar segments would thus be about 1.5 times longer than for RS neurones (conducting at 90–100 m s−1). For slower conducting PT neurones (e.g. conducting at about 30 or 20 m s−1) it would be about 3–4 times longer. After subtracting about 0.5 ms for the latent period of generation of action potentials in the stimulated axons and one synaptic delay from the latencies of the PSPs, the conduction time along axons of RS neurones would amount to about 2.5 ms. By multiplying it by 1.5 and 3 and adding 0.5 ms, the fastest and slower conducting PT fibres might be predicted to act monosynaptically at latencies of 4.25 and 8 ms, respectively, and disynaptically at latencies of about 5.25 and 9 ms (making allowance for about 1 ms for conduction time along axons of the interposed interneurones and one additional synaptic delay). The earliest pyramidal volleys might thus reach the lumbosacral enlargement and exert monosynaptic actions at latencies of about 4 ms, which is in keeping with the original observations of Lloyd (1941). Disynaptic actions via spinal interneurones would then be expected after an additional millisecond (about 5 ms), and later actions over several milliseconds. Latencies of the earliest disynaptic excitatory PT actions (about 5 ms) would thus be within the range of latencies of later components of EPSPs evoked in commissural interneurones by PT stimuli and would allow these components to be evoked disynaptically via spinal neurones, rather than trisynaptically via RS neurones. However, this would require that some interneurones exciting commissural interneurones are activated by PT fibres at latencies of 4–5 ms, whilst the shortest reported latencies of monosynaptic EPSPs evoked from the contralateral motor cortex in spinal interneurones were of 6–8 ms (Lundberg et al. 1962) and latencies of responses of unspecified dorsal horn and intermediate zone interneurones much longer (9–20 ms; Lloyd, 1941). Until any suitable spinal relay neurones are found, the most plausible explanation of the di- and trisynaptic actions of PT neurones on commissural interneurones will remain that they are mediated primarily via RS neurones.
Inhibition of commissural interneurones
In the majority of commissural interneurones, inhibition was evoked in parallel by contralateral PT and ipsilateral MLF stimuli, indicating that PT neurones activate reticulospinal neurones with inhibitory as well as excitatory actions. Since there are no indications for projections of inhibitory reticulospinal neurones to the lumbar segments (Grillner et al. 1968; Wilson & Yoshida, 1968, 1969; Peterson, 1979) and the minimal latencies of IPSPs are about 1 ms longer than of EPSPs, these IPSPs should be mediated by spinal inhibitory neurones activated by reticulospinal neurones. Theoretically, these inhibitory interneurones might include inhibitory commissural interneurones (Bannatyne et al. 2003) and the inhibition be considered as an expression of inhibitory interactions between commissural interneurones. However, this is unlikely because commissural interneurones with input from RS neurones appear to lack local axon collaterals (Bannatyne et al. 2003; Matsuyama et al. 2004) via which they might act before they cross. Inhibition should thus be mediated by other, so far unidentified, inhibitory interneurones, possibly including both intermediate zone and ventral horn interneurones contacted by reticulospinal fibres (Takakusaki et al. 1989, 2001; Davies & Edgley, 1994).
The sequences of IPSPs preceding EPSPs would be particularly well suited for feed-forward modulation of actions of commissural interneurones, especially when the IPSPs are evoked at lower thresholds than the EPSPs. They could thus set the balance between descending and peripheral inputs to commissural interneurones and increase the relative importance of the excitatory input from muscle afferents. Small amplitude IPSPs of PT origin would more effectively interfere with similarly small EPSPs of PT origin than with much larger EPSPs from group II afferents. The IPSPs might nevertheless add to other means of weakening reflex actions from muscle afferents by reticulospinal neurones while assisting in movements induced by descending commands (see Lundberg, 1982; Noga et al. 1992; Riddell et al. 1993).
Relative importance of contralateral and ipsilateral PT neurones for PT actions mediated by commissural interneurones
As summarized in Tables 1 and 2, EPSPs evoked from the contralateral PT were found in a smaller proportion of commissural interneurones than from the ipsilateral PT when either single maximal stimuli or trains of submaximal stimuli were used. Furthermore, these EPSPs were smaller and were more often evoked at latencies exceeding those evoked from the MLF by more than 1.6 ms, i.e. more likely trisynaptically than disynaptically. These differences were noted when the stimuli were near maximal but did not exceed (unless stated otherwise) 150 μA and therefore were likely to affect primarily, if not exclusively, fibres in only one PT, and were even more marked when weaker stimuli were tested. However, even though excitatory actions from the contralateral PT were evoked less readily, effects of stimuli applied in the ipsilateral and contralateral PT were generally similar and were enhanced when stimuli likely to encroach over the other PT (200 μA) were used. These results are thus compatible with the mediation of PT actions by RS neurones coexcited by fibres from the left and the right PT. They are also in support of the proposal that RS neurones and commissural interneurones might mediate movements that are initiated by contralateral as well as by ipsilateral PT neurones and that they provide a means for replacing the actions of PT neurones damaged on one side of the body by the actions of intact PT neurones. However, since the actions of PT neurones from one hemisphere would be weaker than normal when not assisted by the actions of PT neurones from the other hemisphere, enhancement of synaptic actions from the remaining PT fibres either pharmacologically (Jankowska et al. 2005a), or by other procedures, might be of critical importance for the recovery of motor functions. The probability of recovery will also depend on the integrity of connections between intact PT fibres and RS neurones, and between the RS neurones and commissural interneurones.
Acknowledgments
We wish to thank Mrs Rauni Larsson for her invaluable assistance, and Dr P. Krutki for permission to include records from two neurones analysed in another series of experiments. The study was supported by grants from NIH (NS 40863) and the Swedish Research Council (15393-01A).
References
- Aoyama M, Hongo T, Kudo N, Tanaka R. Convergent effects from bilateral vestibulospinal tracts on spinal interneurons. Brain Res. 1971;35:250–253. doi: 10.1016/0006-8993(71)90612-3. [DOI] [PubMed] [Google Scholar]
- Bannatyne BA, Edgley SA, Hammar I, Jankowska E, Maxwell DJ. Networks of inhibitory and excitatory commissural interneurons mediating crossed reticulospinal actions. Eur J Neurosci. 2003;18:2273–2284. doi: 10.1046/j.l460-9568.2003.02973.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butt SJ, Kiehn O. Functional identification of interneurons responsible for left–right coordination of hindlimbs in mammals. Neuron. 2003;38:953–963. doi: 10.1016/s0896-6273(03)00353-2. [DOI] [PubMed] [Google Scholar]
- Canedo A, Lamas JA. Pyramidal and corticospinal synaptic effects over reticulospinal neurones in the cat. J Physiol. 1993;463:475–489. doi: 10.1113/jphysiol.1993.sp019606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies HE, Edgley SA. Inputs to group II-activated midlumbar interneurones from descending motor pathways in the cat. J Physiol. 1994;479:463–473. doi: 10.1113/jphysiol.1994.sp020310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgley SA, Jankowska E, Hammar I. Ipsilateral actions of feline corticospinal tract neurons on limb motoneurons. J Neurosci. 2004;24:7804–7813. doi: 10.1523/JNEUROSCI.1941-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillner S, Hongo T, Lund S. Reciprocal effects between two descending bulbospinal systems with monosynaptic connections to spinal motoneurones. Brain Res. 1968;10:477–480. doi: 10.1016/0006-8993(68)90221-7. [DOI] [PubMed] [Google Scholar]
- He XW, Wu CP. Connections between pericruciate cortex and the medullary reticulospinal neurons in cat: an electrophysiological study. Exp Brain Res. 1985;61:109–116. doi: 10.1007/BF00235626. [DOI] [PubMed] [Google Scholar]
- Hongo T, Kudo N, Tanaka R. The vestibulospinal tract: crossed and uncrossed effects on hindlimb motoneurones in the cat. Exp Brain Res. 1975;24:37–55. doi: 10.1007/BF00236016. [DOI] [PubMed] [Google Scholar]
- Ito K, McCarley RW. Physiological studies of brainstem reticular connectivity. I. Responses of mPRF neurons to stimulation of bulbar reticular formation. Brain Res. 1987;409:97–110. doi: 10.1016/0006-8993(87)90745-1. [DOI] [PubMed] [Google Scholar]
- Jankowska E, Cabaj A, Pettersson LG. How to enhance ipsilateral actions of pyramidal tract neurons. J Neurosci. 2005a;25:7401–7405. doi: 10.1523/JNEUROSCI.1838-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowska E, Edgley SA, Krutki P, Hammar I. Functional differentiation and organization of feline midlumbar commissural interneurones. J Physiol. 2005b;565:645–658. doi: 10.1113/jphysiol.2005.083014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowska E, Hammar I, Slawinska U, Maleszak K, Edgley SA. Neuronal basis of crossed actions from the reticular formation upon feline hindlimb motoneurons. J Neurosci. 2003;23:1867–1878. doi: 10.1523/JNEUROSCI.23-05-01867.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowska E, Noga BR. Contralaterally projecting lamina VIII interneurones in middle lumbar segments in the cat. Brain Res. 1990;535:327–330. doi: 10.1016/0006-8993(90)91618-q. [DOI] [PubMed] [Google Scholar]
- Krutki P, Jankowska E, Edgley SA. Are crossed actions of reticulospinal and vestibulospinal neurons on feline motoneurons mediated by the same or separate commissural neurons? J Neurosci. 2003;23:8041–8050. doi: 10.1523/JNEUROSCI.23-22-08041.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd DPC. The spinal mechanism of the pyramidal system in cats. J Neurophysiol. 1941;4:525–546. [Google Scholar]
- Lundberg A. Inhibitory control from the brain stem of transmission from primary afferents to motoneurones, primary afferent terminals and ascending pathways. In: Sjölund B, Björklund A, editors. Brain Stem Control of Spinal Mechnisms. Amsterdam: Elsevier; 1982. pp. 179–225. [Google Scholar]
- Lundberg A, Norrsell U, Voorhoeve P. Pyramidal effects on lumbo-sacral interneurones activated by somatic afferents. Acta Physiol Scand. 1962;56:220–229. doi: 10.1111/j.1748-1716.1962.tb02497.x. [DOI] [PubMed] [Google Scholar]
- Matsuyama K, Drew T. Organization of the projections from the pericruciate cortex to the pontomedullary brainstem of the cat: a study using the anterograde tracer Phaseolus vulgaris-leucoagglutinin. J Comp Neurol. 1997;389:617–641. doi: 10.1002/(sici)1096-9861(19971229)389:4<617::aid-cne6>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Matsuyama K, Nakajima K, Mori F, Aoki M, Mori S. Lumbar commissural interneurons with reticulospinal inputs in the cat: Morphology and discharge patterns during fictive locomotion. J Comp Neurol. 2004;474:546–561. doi: 10.1002/cne.20131. [DOI] [PubMed] [Google Scholar]
- Mitani A, Ito K, Mitani Y, McCarley RW. Descending projections from the gigantocellular tegmental field in the cat: cells of origin and their brainstem and spinal cord trajectories. J Comp Neurol. 1988a;268:546–566. doi: 10.1002/cne.902680406. [DOI] [PubMed] [Google Scholar]
- Mitani A, Ito K, Mitani Y, McCarley RW. Morphological and electrophysiological identification of gigantocellular tegmental field neurons with descending projections in the cat. I. Pons. J Comp Neurol. 1988b;268:527–545. doi: 10.1002/cne.902680405. [DOI] [PubMed] [Google Scholar]
- Mitani A, Ito K, Mitani Y, McCarley RW. Morphological and electrophysiological identification of gigantocellular tegmental field neurons with descending projections in the cat. II. Bulb. J Comp Neurol. 1988c;274:371–386. doi: 10.1002/cne.902740307. [DOI] [PubMed] [Google Scholar]
- Noga BR, Bras H, Jankowska E. Transmission from group II muscle afferents is depressed by stimulation of locus coeruleus/subcoeruleus, Kolliker-Fuse and raphe nuclei in the cat. Exp Brain Res. 1992;88:502–516. doi: 10.1007/BF00228180. [DOI] [PubMed] [Google Scholar]
- Peterson BW. Reticulospinal projections to spinal motor nuclei. Annu Rev Physiol. 1979;41:127–140. doi: 10.1146/annurev.ph.41.030179.001015. [DOI] [PubMed] [Google Scholar]
- Peterson BW, Anderson ME, Filion M. Responses of ponto-medullary reticular neurons to cortical, tectal and cutaneous stimuli. Exp Brain Res. 1974;21:19–44. doi: 10.1007/BF00234256. [DOI] [PubMed] [Google Scholar]
- Rexed B. A cytoarchitectonic atlas of the spinal cord in the cat. J Comp Neurol. 1954;100:297–379. doi: 10.1002/cne.901000205. [DOI] [PubMed] [Google Scholar]
- Riddell JS, Jankowska E, Eide E. Depolarization of group II muscle afferents by stimuli applied in the locus coeruleus and raphe nuclei of the cat. J Physiol. 1993;461:723–741. doi: 10.1113/jphysiol.1993.sp019538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takakusaki K, Kohyama J, Matsuyama K, Mori S. Medullary reticulospinal tract mediating the generalized motor inhibition in cats: parallel inhibitory mechanisms acting on motoneurons and on interneuronal transmission in reflex pathways. Neuroscience. 2001;103:511–527. doi: 10.1016/s0306-4522(00)00586-8. [DOI] [PubMed] [Google Scholar]
- Takakusaki K, Ohta Y, Mori S. Single medullary reticulospinal neurons exert postsynaptic inhibitory effects via inhibitory interneurons upon alpha-motoneurons innervating cat hindlimb muscles. Exp Brain Res. 1989;74:11–23. doi: 10.1007/BF00248276. [DOI] [PubMed] [Google Scholar]
- Wilson VJ, Yoshida M. Vestibulospinal and reticulospinal effects on hindlimb, forelimb, and neck alpha motoneurons of the cat. Proc Natl Acad Sci U S A. 1968;60:836–840. doi: 10.1073/pnas.60.3.836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson VJ, Yoshida M. Comparison of effects of stimulation of Deiters' nucleus and medial longitudinal fasciculus on neck, forelimb, and hindlimb motoneurons. J Neurophysiol. 1969;32:743–758. doi: 10.1152/jn.1969.32.5.743. [DOI] [PubMed] [Google Scholar]
- Wilson VJ, Zarzecki P, Schor RH, Isu N, Rose PK, Sato H, et al. Cortical influences on the vestibular nuclei of the cat. Exp Brain Res. 1999;125:1–13. doi: 10.1007/s002210050651. [DOI] [PubMed] [Google Scholar]