Abstract
Key structural and functional properties of the skeletal muscle vasculature that underlie diminished vascular conductance with ageing remain obscure. The purpose of this investigation was to test the hypotheses that (1) reduced levels of spontaneous physical activity in old rats are associated with skeletal muscle vascular remodelling (e.g. arterial rarefaction), and (2) consequent to a vascular remodelling, calculated shear stress is maintained in feed arteries of aged muscle at levels commensurate with that in young. Activity during daily light and dark cycles (12–12 h) was measured at 30-s intervals for 2 weeks in young (6 months; n = 9) and old (24 months; n = 9) Fisher 344 rats via telemetry. Subsequently, the gastrocnemius complex and soleus muscles were excised and all feed arteries were counted, isolated, cannulated and maximally dilated for measurement of luminal diameter. Resting blood flow was also measured to estimate vessel wall shear-stress in the feed arteries perforating the soleus and gastrocnemius muscles. Overall, young rats were ∼1.6 times more active during dark periods and ∼4 times more active during light periods than old rats. In addition, young rats had approximately one additional feed artery perforating both the soleus (young, 3.3 ± 0.2; old, 2.6 ± 0.2 vessels; P < 0.05) and gastrocnemius (young, 8.8 ± 0.1; old, 7.5 ± 0.2 vessels, P < 0.05) muscles compared with old rats. However, average vessel wall shear stress at rest was similar between young and old rats (soleus: Y, 65 ± 5; O, 64 ± 5 dynes cm−2; gastrocnemius: Y, 329 ± 22; O, 327 ± 27 dynes cm−2) resulting from a larger vessel diameter in arteries from old rats. In conclusion, lower activity levels of old rats likely contribute to resistance artery rarefaction and, consequently, this provides a plausible mechanism for the altered blood flow patterns observed during exercise in aged skeletal muscle.
Ageing is associated with several muscular and cardiovascular perturbations which culminate in a reduced capacity to perform and sustain exercise (Fleg & Lakatta, 1988; Inbar et al. 1994). Key to this ageing-induced muscular dysfunction is a temporal and spatial mismatching of O2 delivery (blood flow) to O2 requirement in contracting myocytes (Behnke et al. 2005). Indeed, with submaximal treadmill exercise, the distribution pattern of blood flow among and within hindlimb muscles is altered in old versus young animals, despite similar total hindlimb blood flows (Musch et al. 2004). Specifically, in the old rat, there is a significant reduction in blood flow to highly oxidative muscles (e.g. red gastrocnemius muscle) and an elevation in blood flow to low oxidative muscles (e.g. white gastrocnemius muscle), producing a mismatch of oxygen delivery to muscle oxidative capacity. The precise mechanisms for the alteration in blood flow distribution associated with old age remain incompletely understood, although alterations in vasoreactivity of skeletal muscle resistance vessels (Muller-Delp et al. 2002a, b; Spier et al. 2004; Donato et al. 2005) and a possible structural remodelling of the individual vessels (e.g. change in lumen diameter), as well as the entire vascular network (e.g. arterial rarefaction) (Muller-Delp et al. 2002a) have been proposed to contribute to this phenomenon.
Shear stress, the frictional force acting at the interface of the vessel wall and flowing blood, is a crucial modulator of vessel luminal diameter. Specifically, with alterations in shear stress, vessels respond by altering lumen diameter transiently through the release of vasoactive agents such as nitric oxide and prostacyclin (Frangos et al. 1985; Olesen et al. 1988; Yoshizumi et al. 1989; Cooke et al. 1991) and, if the change in shear persists, by restructuring vessel morphology (Kamiya & Togawa, 1980; Langille & O'Donnell, 1986; Langille et al. 1989; Delp et al. 2000). Whether changes in vascular morphology and shear stress occur with ageing remain unknown. The purpose of this investigation was to test the hypothesis that arterial rarefaction occurs in the soleus and gastrocnemius muscles of old rats, but that increases in vessel diameter previously reported to occur with old age (Muller-Delp et al. 2002a; Spier et al. 2004) serve to maintain wall shear stress relative to that in young animals. Because age-related differences in physical activity and corresponding muscle hyperaemia may provide the stimulus for the putative arterial rarefaction in muscles of old rats, a secondary purpose of this investigation was to determine whether spontaneous physical activity differs between young and old rats. Findings from this investigation will thus provide a mechanistic link between ageing, physical activity and vascular remodelling, and show whether structural vascular changes contribute to altered blood flow distribution and exercise intolerance in senescent individuals.
Methods
For all experiments, 6- and 24- to 26-month-old, male Fisher-344 rats were used to represent young (n = 16) and old (n = 16) animals, respectively. All procedures were approved by the Institutional Animal Care and Use Committee at Texas A&M and West Virginia Universities. Rats were housed individually at 23°C and were maintained on a 12 h light–12 h dark cycle. All rats were fed rat chow and water ad libitum.
Activity monitoring
A telemetry system (VitalView 4000, Mini-Miter, Bend, OR, USA) was used to monitor heart rate (HR), core body temperature and activity in conscious, freely moving animals. Rats were anaesthetized (isoflurane 2.5% in 100% O2) and a small sterile transmitter (volume, 1.13 ml; Standard E-Mitter, Mini-Mitter, Bend) was implanted in the abdomen under aseptic conditions. Buprenorphine hydrochloride (0.05 mg kg−1s.c.) was administered as an analgesic after surgery. Animals were allowed a recovery period of ∼1 week before activity measurements were made. During the recovery period, rats were monitored daily by a veterinarian for signs of infection and discomfort.
For measures of physical activity (basic index of cage movement), HR and body temperature, animals were housed individually in custom-made cages (54 cm × 29 cm × 15 cm) placed upon TR-4000 receivers (Mini-Mitter Co., Bend). These cages were located in a room separate from animals involved in other studies to minimize possible influences which could affect telemetry measurements (e.g. noise). Only personnel involved in this investigation entered the room to monitor and supply food and water. These cages, which are considerably larger than standard rat cages, were designed to allow the maximum area for free movement. HR, core body temperature and activity were measured at 30-s intervals for 2 weeks, with mean HR, temperature and activity values assessed during active (dark cycle) and inactive (light cycle) periods of the day (12 h light–12 h dark). The Mini-Mitter transmitter records activity counts that are generated when the transmitter changes position and/or orientation relative to the TR-4000 receiver antenna. Position change can be in any plane (i.e. horizontal or vertical) to cause counts to be recorded. Each time there is a change in position or orientation of the transmitter relative to the receiver, an automatic gain circuit (AGC) is activated. Each time the AGC is activated, an activity count is generated. Counts are totalled during the sample epoch selected (i.e. 30-s period), and then reported as a whole number of activity counts for that epoch. The activity count, as well as data collected on HR and body temperature, is stored until the end of the 30-s period and then data are collected anew for the following period. These stored periods were then averaged for the light and dark daily cycles for the 2-week measurement session. Following successful measurements of daily activity levels, rats were anaesthetized with sodium pentobarbital (60 mg kg−1i.p..) and killed by decapitation before measuring vascular morphology (see below).
Vascular geometry and morphology
Once the animal had been killed, the soleus–plantaris–gastrocnemius muscle group was carefully dissected free and placed in a filtered calcium-free physiological saline solution (PSS) buffer at 4°C containing in (mm): NaCl 147, KCl 4.7, NaH2PO2 1.2, MgSO4 1.17, glucose 5.0, pyruvate 2.0 and Mops 3.0; pH 7.4. The feed arteries perforating the soleus muscle and all portions of the gastrocnemius muscle (i.e. red, mixed and white portions) were identified and isolated with the use of a stereomicroscope, removed from the muscle and transferred to a Lucite tissue viewing chamber. Subsequently, both ends of the resistance vessel were cannulated with glass micropipettes filled with the filtered PSS and secured to the pipettes with 11-O ophthalmic suture as previously described (McCurdy et al. 2000; Muller-Delp et al. 2002a, b). After cannulation, each vessel in the tissue chamber was transferred to the stage of an inverted microscope (Olympus IX70) coupled to a video camera (Panasonic BP310) and video caliper (Microcirculation Research Institute, Texas A&M University). Intraluminal pressure in the isolated resistance vessels was set at 60 cmH2O, and 10−4m sodium nitroprusside (SNP) was added to the vessel chamber to prevent development of spontaneous tone and ensure maximal dilatation. Leaks were detected by pressurizing the vessel and then closing the valves to the reservoirs and verifying that intraluminal pressure remained constant. Vessels that were free of leaks were equilibrated for at least 1 h at 37°C before their maximal diameter and wall thickness were measured; the bathing solution was replaced every 20 min during this period.
Bulk blood flow measurements
To prevent possible radioactive contamination of instruments used for vascular morphology measurements, a separate group of rats was utilized for quantifying resting blood flow to the soleus and gastrocnemius muscles. Blood flow was determined in a separate cohort of 14 Fisher-344 rats (n = 7 per group) using the radionuclide-tagged microsphere technique (Laughlin et al. 1982) in the conscious standing condition. Initially, rats were anaesthetized with isoflurane (2.5% in 100% O2). Polyethlene catheters (PE-10 connected to PE-50) were implanted in the right carotid and caudal (tail) arteries. The carotid artery catheter was advanced 2–3 mm rostral to the aortic valve and secured. The tail artery catheter was advanced towards the bifurcation of the descending aorta and secured. After the incisions were closed (4-O silk), anaesthesia was terminated and the animal was given ≥ 2 h to recover as Flaim et al. (1984) demonstrated that cardiac or circulatory dynamics, regional blood flow, arterial blood gases and acid–base status are stable in the awake unrestrained rat 1–6 h after gas anaesthesia. The tail artery catheter was connected to a 5-ml glass syringe, which was attached to a Harvard withdrawal pump (model 907, Cambridge, MA, USA). The carotid artery catheter was connected to a pressure transducer (BP100, ADInstruments) to monitor mean arterial pressure.
Radiolabelled (46Sc or 85Sr) microspheres (15 μm diameter; DuPont/NEN, Boston, MA, USA) were used for blood flow measurements as previously described (Laughlin et al. 1982; Delp et al. 1991). Prior to infusion, the microspheres were agitated by sonication to suspend the beads and prevent clumping. At 30 s prior to initiating infusion, blood withdrawal from the caudal artery at 0.25 ml min−1 was begun. The right carotid artery catheter was disconnected from the pressure transducer and a specified microsphere (∼250000 microspheres) was infused into the ascending aorta, which was then flushed with warmed saline to ensure clearance of the beads. Blood withdrawal from the caudal artery continued for 45 s after microsphere infusion.
Following the microsphere infusion, the rats were killed with an overdose of sodium pentobarbital (>80 mg kg−1) via the right carotid artery catheter. After verifying correct placement of the carotid catheter, the left and right soleus muscle, gastrocnemius muscle and kidneys were removed. The gastrocnemius muscle was then sectioned into red (RG), mixed (MG) and white (WG) portions of the muscle, which correspond to the predominately high oxidative type I and IIA fibres (RG), a mixed fast fibre-type population (MG) and the predominately low oxidative type IIB fibres (WG) (Delp & Duan, 1996). The radioactivity level of the tissues was determined by a three-channel gamma scintillation counter (Packard Auto Gamma Spectrometer, model 5230) set to record the peak energy activity of each isotope for 5 min. Total blood flow to each tissue was calculated by the reference sample method (Ishise et al. 1980; Musch & Terrell, 1992) and expressed in ml min−1 (100 g tissue)−1. Adequate mixing of the microspheres was verified by demonstrating a <15% difference in blood flow to the right and left kidneys.
Estimated shear stress
Shear stress determination
The maximum internal luminal radius (i.e. rmax) of the feed arteries was calculated from the measured inner perimeters of the media via a video caliper (Microcirculation Research Institute). Luminal cross sectional area (CSA) was calculated as:
Wall shear stress (τ) was then calculated using the equation:
Where η is the apparent blood viscosity (0.035 Poise) (Liposky, 1995) and Q is the blood flow rate through the vessel. Q in the conscious standing condition was derived using the following equation:
Where Qvessel is blood flow through an individual vessel, luminal CSAvessel is the cross-sectional area of lumen of the individual vessel, total luminal CSA is the summed total of all individual lumen CSAs for each muscle group, and Qmuscle is the measured total blood flow (ml min−1) to the soleus and gastrocnemius muscles, as well as the RG, MG and WG muscle portions of the gastrocnemius muscle. To calculate shear stress, it was necessary to consider the relative state of the artery in vivo during standing in the young and old animals. The r for soleus feed artery during standing was considered to be equivalent to rmax because the soleus muscle is actively recruited for maintaining posture and, correspondingly, the soleus muscle vascular conductance can approach near maximal levels during standing (Laughlin & Armstrong, 1982). However, because the gastrocnemius muscle is relatively inactive during standing (Laughlin & Armstrong, 1982), r was therefore adjusted to reflect the amount of intrinsic tone these vessels develop in vitro (Muller-Delp et al. 2002a, b). The r for the gastrocnemius feed arteries during standing was estimated to be ∼65% of rmax.
Statistical analysis
Unpaired student's t tests were used to determine the significance of differences in the morphological parameters of resistance arteries, body mass, soleus and gastrocnemius muscle mass, and the soleus and gastrocnemius muscle to body mass ratios between young and old groups. A one-way ANOVA was used to compare wall shear stress, feed artery number and muscle blood flow between young and old animals. The Student–Newman–Keuls method was used as a post hoc test to determine the significance of differences among means. All values are presented as means ± s.e.m.P < 0.05 was required for significance.
Results
Body and muscle masses
The body mass of old rats was significantly greater than that of young animals (Table 1). There was a significant atrophy observed in the gastrocnemius muscle with ageing (P < 0.05), and a tendency for atrophy in the soleus muscle (P = 0.06; Table 1).
Table 1.
Mean body mass and muscle mass for young and old rats
| Young (n = 7) | Old (n = 7) | ||
|---|---|---|---|
| Body wt (g) | 379 ± 8 | 438 ± 9* | |
| Soleus wt (mg) | 141 ± 6 | 148 ± 6 | |
| Soleus wt/body wt (mg g−1) | 0.37 ± 0.01 | 0.34 ± 0.02† | (P = 0.06) |
| Gastroc. wt (mg) | 1579 ± 57 | 1482 ± 43† | (P = 0.09) |
| Gastroc. wt/body wt (mg g−1) | 4.17 ± 0.10 | 3.39 ± 0.10* |
P < 0.05 versus young
P < 0.10 versus young. Gastroc., gastrocnemius; wt, weight.
Vascular geometry and morphology
Soleus muscle
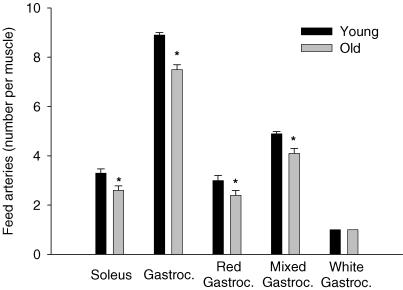
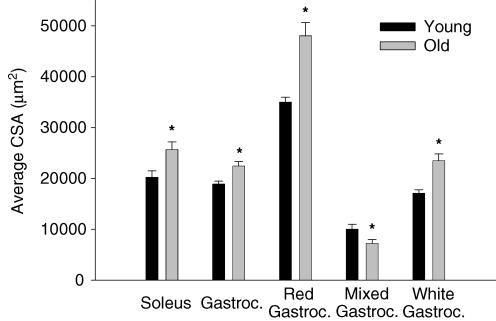
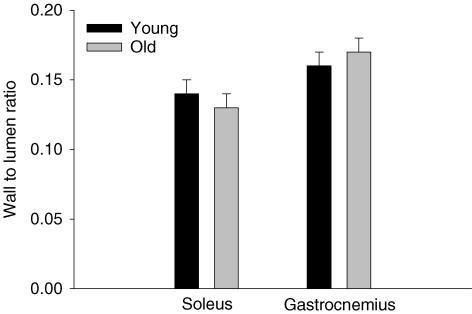
As illustrated in Fig. 1, on average there was approximately one less feed artery perforating the soleus muscle of old (2.6 ± 0.2 vessels muscle−1) versus young (3.4 ± 0.2 vessels muscle−1; P < 0.05) animals. The average luminal diameter (young, 158 ± 5 μm; old, 177 ± 5 μm; P < 0.05) and luminal CSA (Fig. 2) of the individual feed arteries perforating the soleus muscle were significantly greater in the old versus young group. Average wall thickness (young, 23.5 ± 2.3 μm; old, 23.2 ± 2.2 μm) was not different between the young and old soleus muscle arteries, nor was the wall to lumen ratio (Fig. 3).
Figure 1. The number of feed vessels perforating the soleus and gastrocnemius muscle, as well as the major constituents of the gastrocnemius muscle.
There was ∼0.7 less arteries perforating the soleus and ∼1.3 less arteries in the gastrocnemius (Gastroc.). *P < 0.05 versus young.
Figure 2. Average lumen cross-sectional area (CSA) of individual vessels perforating the soleus and gastrocnemius muscle, and the different sections of the gastrocnemius muscle.
Old animals demonstrated a greater average lumen CSA than young in arteries of the soleus and gastrocnemius muscle, as well as larger CSA in the red and white sections of the gastrocnemius (Gastroc.). Only in the mixed portion of the gastrocnemius muscle was the average lumen CSA greater in young than in old. *P < 0.05 versus young.
Figure 3. Average vessel wall to lumen ratio derived from video caliper measurements of feed arteries in the soleus and gastrocnemius muscles of young and old rats.
No differences were observed in the wall to lumen ratio between age groups. However, in the old group the wall to lumen ratio in the gastrocnemius was significantly greater than that of the soleus.
Gastrocnemius muscle
There was also approximately one less feed artery perforating the gastrocnemius muscle of old versus young animals (Fig. 1). This artery rarefaction was most evident in the red and mixed portions of the muscle, with no change in the number of feed arteries perforating the white portion (Fig. 1). In old versus young animals, arteries in the red and white muscle had greater luminal diameters (red: old, 242 ± 7 μm; young, 214 ± 11 μm; white: old, 172 ± 5 μm; young, 147 ± 3 μm; P < 0.05) as well as average luminal CSA (Fig. 2). There were no differences in average wall thickness for gastrocnemius muscle feed arteries between young and old animals (young, 22.0 ± 1.7 μm; old, 24.0 ± 1.3 μm), or in the wall to lumen ratio (Fig. 3).
Blood flow and mean arterial pressure
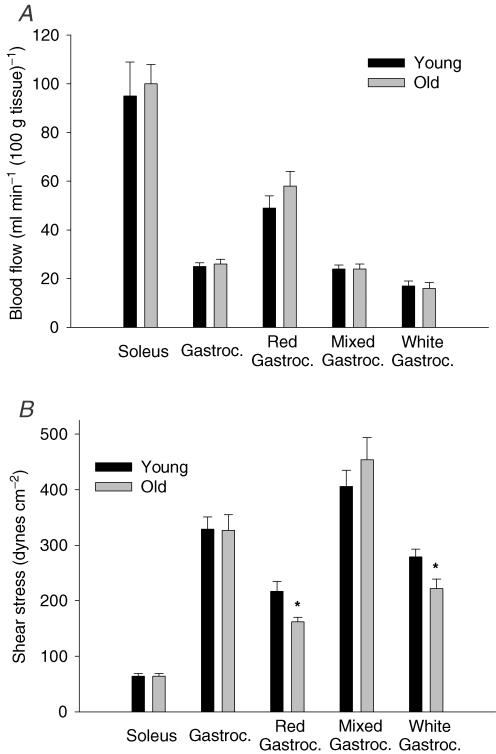
There were no age-related differences in mean arterial pressure (young, 112 ± 7 mmHg; old, 117 ± 6 mmHg) or in blood flow to soleus or gastrocnemius muscles or gastrocnemius parts (Fig. 4A). For both age groups, conscious resting blood flow was greater in the soleus than in the gastrocnemius muscle. In addition, resting blood flow was greatest in the red portion, followed by the mixed and white portions of the gastrocnemius muscle (Fig. 4A).
Figure 4. Haemodynamic measures in the soleus and gastrocnemius muscles measured in conscious, resting young and old rats.
A, resting haemodynamic measures for the soleus and gastrocnemius (Gastroc.) muscles, as well as the separate sections of the gastrocnemius muscle. There were no age-related differences in blood flow to the soleus or gastrocnemius muscle, or the different sections of the gastrocnemius. B, calculated resting shear stress for the soleus and gastrocnemius muscles, as well as for the red, mixed and white fibre-type sections of the gastrocnemius muscle. There were no age-related differences in shear stress to the soleus or gastrocnemius muscles; however, the old animals demonstrated lower shear stress in feed arteries of the red and white portions of the gastrocnemius muscle than in corresponding arteries from the younger animals. *P < 0.05 versus young.
Estimated shear stress
As illustrated in Fig. 4B, there were no age-related differences in calculated feed artery shear stress in the soleus or gastrocnemius muscles during standing, although shear stress was greater in the gastrocnemius than in the soleus muscle for both age groups. Within the gastrocnemius muscle, estimated shear stress was less in the old than in the young arteries from the red and white gastrocnemius, but was not significantly different in the mixed portion of gastrocnemius muscle (Fig. 4B).
Physical activity
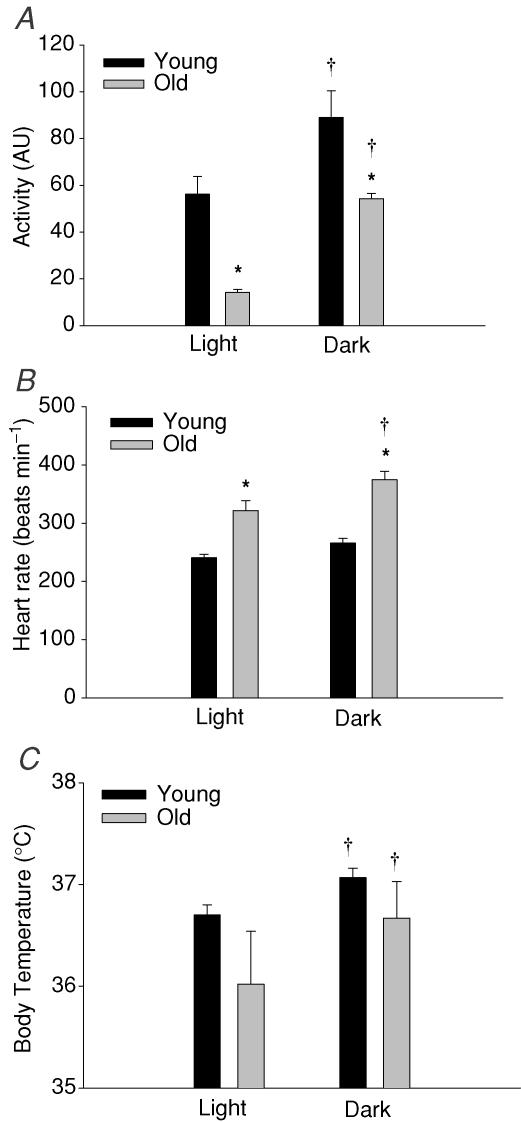
Young animals demonstrated greater spontaneous activity levels (i.e. gross index of cage movement) during daily light and dark periods than old animals (Fig. 5A). However, the greatest difference in activity was observed during the light period where young animals were ∼4 times more active than the old group (versus∼1.6 times more active during the dark period). Both young and old animals demonstrated an elevated HR (Fig. 5B) and body temperature (Fig. 5C) during the more active nocturnal period.
Figure 5. Mean measures of heart rate, core body temperature, and activity from telemetry in young and old rats during diurnal (light, 12 h) and nocturnal (dark, 12 h) daily cycles.
A, young and old rats were both significantly more active during the dark period. In addition, young animals were more active than their older counterparts during both activity cycles. Young rats were ∼4 times more active during the light (inactive) period of the day than old animals. B, heart rate was significantly elevated in old versus young rats during both light and dark periods. Both young and old rats demonstrated an elevated mean heart rate during the dark, active period. C, body temperature increased significantly for both groups during the dark versus light period. There was no difference in mean body temperature values between age groups during either period. †P < 0.05 versus light period; *P < 0.05 versus young. AU, arbitary units.
Discussion
With ageing, there is a reduction in the blood-flow capacity of skeletal muscle (Wahren et al. 1974; Irion et al. 1987; Proctor et al. 1998; Lawrenson et al. 2003; Donato et al. 2006), as well as an altered distribution of blood flow within and among muscles (Musch et al. 2004). The primary purpose of this investigation was to determine whether changes in vascular geometry might contribute to alterations in skeletal muscle blood flow associated with old age. The data demonstrate that ageing results in fewer arterial vessels leading to both the soleus and gastrocnemius muscles. Within the gastrocnemius muscle, the arterial rarefaction was only evident in the highly oxidative red and mixed fibre portions of the muscle, with no change in the number of feed arteries perforating the low oxidative white portion of the muscle (Fig. 1). There was also a corresponding enlargement of luminal diameter of the remaining feed arteries from the soleus and gastrocnemius muscles of old rats. These structural changes, coupled with similar resting muscle blood flows (Fig. 4A), resulted in the estimated shear stress being maintained within the feed arteries (Fig. 4B). These results are fundamental to understanding skeletal muscle haemodynamics during exercise as they set the boundary conditions in which to interpret the age-related alterations in vascular conductance (Musch et al. 2004) and the O2 delivery to O2 uptake 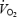 mismatching in aged skeletal muscle (Behnke et al. 2005).
mismatching in aged skeletal muscle (Behnke et al. 2005).
A secondary purpose of this investigation was to determine whether old rats exhibited reduced levels of spontaneous activity in order to ascertain whether different activity patterns are associated with vascular alterations induced by old age. The results demonstrate that during both the light and dark periods of the day, old rats are more sedentary than young rats (Fig. 5). As skeletal muscle perfusion increases during nocturnal periods of the day in young rats (Delp et al. 1991), the reduced levels of activity and muscle metabolism in old rats would attenuate skeletal muscle perfusion, and over time could act to alter the morphology of feed arteries perforating these muscles. It is worth noting that the study design utilized herein does not allow the effects of ageing and reduced spontaneous activity to be investigated separately. Therefore, further studies investigating whether enhancing physical activity (e.g. exercise training) can reduce the age-related differences in vascular structure observed in this study are warranted.
Ageing and vascular remodelling
Previous work has shown that the luminal diameter of single isolated arterioles from the gastrocnemius muscle of old rats is larger than that of young animals (Muller-Delp et al. 2002a). Based on established patterns of arterial remodelling (Kamiya & Togawa, 1980; Langille, 1993), such a change in vascular structure suggests that these vessels from old rats are exposed to a chronically higher blood flow to the muscle. However, several studies (as well as the present study) have shown that muscle blood flow is not greater at rest in old animals (Delp et al. 1998; Musch et al. 2004). This apparent paradox between vascular structure and direct measures of muscle perfusion could result from ageing-induced changes in the vascular network, which analysis of vessel diameter from the muscle would not address. Data from the present study demonstrate that in addition to enlarged luminal diameters, there are also fewer feed arteries supplying blood to the muscle (Fig. 1). Therefore, even though resting muscle blood flow does not differ between young and old rats (Fig. 4A), the blood flow rate through the remaining feed arteries to the muscle must be greater with ageing, which is supported by greater red blood cell velocities in capillaries from old skeletal muscle (Russell et al. 2003). Such an increase in blood flow would elevate the intravascular shear stress in the remaining arteries and provide a stimulus for a structural increase in maximal diameter over time. Notwithstanding structural changes in individual feed arteries, the decrease of the vascular network at the level of the feed arteries in skeletal muscle of old rats would severely perturb the ability of the vasculature to modulate blood flow to active motor units within exercising muscle, which appears to be the case (Musch et al. 2004).
Given that resting muscle blood flow does not differ between young and old rats, the nature of the stimulus that induces the rarefaction of the arterial network associated with old age in muscle is still unknown. Several possible mechanisms may exist including: (1) a reduction in vascular endothelial growth factor (VEGF) protein and mRNA expression in skeletal muscle (Croley et al. 2005) and VEGF promoter activity in smooth muscle cells (Rivard et al. 1999); (2) an increased vascular oxidative stress (Van Der Loo et al. 2000; Francia et al. 2004; Sullivan et al. 2004) contributing to endothelial cell apoptosis (Asai et al. 2000); and (3) a chronically reduced muscular perfusion and shear stress associated with diminished physical activity and muscle metabolism. With regard to this latter possibility, Pries et al. (1998; 2003) postulated that, in addition to wall shear stress, the metabolic condition of the muscle is key to structural adaptation and the stability of microvascular networks. Specifically, if flow increases preferentially through one arterial segment, and correspondingly decreases through another, over time one would expect a shrinking and eventual recession of the low-flow arterial branch. However, a ‘metabolic response’ (i.e. the release of metabolites from downstream, poorly perfused tissue) would act to stabilize vascular segments and preserve low-flow pathways (Pries et al. 1998, 2001, 2003). Therefore, in old rats, the reduction in spontaneous locomotion and muscle activity may fail to provide a sufficiently robust local metabolic stimulus for vascular stability. This scenario, coupled with reduced pro-angiogenic agents in aged blood vessels (e.g. reduced bioavailability of nitric oxide) (Muller-Delp et al. 2002a; Woodman et al. 2002; Spier et al. 2004), could result in an obligatory increase in the luminal diameter of those arteries supporting higher flows, and the eventual recession and disappearance of the low-flow vessels.
Ageing, activity and blood flow
As evidenced from cage movement patterns (Fig. 5), the old rats were less active than the young animals during both the light and dark daily periods. We speculate that the reduced spontaneous activity in the old rats is key to understanding the altered vascular morphology observed in this study. Briefly, when an animal is standing at rest the soleus muscle, which is composed mainly of type I fibres, is recruited, resulting in a high resting blood flow (Laughlin & Armstrong, 1982; Delp & Duan, 1996). In addition, the predominant fibre type recruited with low-intensity exercise is that of the highly oxidative type IIA fibres (Armstrong et al. 1977, 1987; Laughlin & Armstrong, 1982; Delp & Duan, 1996), which is the primary fibre type within the red portion of the gastrocnemius muscle. With treadmill walking (15 m min−1), for example, the largest absolute increase in blood flow within the hindlimb musculature is to the red gastrocnemius (increase of 235%), with a smaller increase in the mixed portion of the muscle (138%), and no change in blood flow to the white portion (Laughlin & Armstrong, 1982). Moreover, only during high-intensity exercise does blood flow increase to the white muscle (Laughlin & Armstrong, 1982). Therefore, with less-spontaneous activity (as cage activity is within the low-intensity domain, based upon comparisons of heart rates measured here in and those reported as a function of exercise intensity (Laughlin & Armstrong, 1982)), the daily hyperaemic responses to the soleus and high-oxidative portions of the gastrocnemius muscle of old rats would be lower over the 24-h period. The consequent diminution in shear stress within the muscle feed arteries, which as detailed above, would be likely to lead to less metabolic stabilization and fewer arterial networks in high oxidative muscle. Furthermore, it is likely that reduced daily blood flow patterns also attenuate the overall cyclic stretch that the vessel would be subjected to during that period. Indeed, reduced exposure to cyclic stretch is one possible mechanism contributing to age-related increases in arterial stiffness (Cox, 1983).
It is interesting that, although old rats demonstrated lower levels of spontaneous physical activity compared with young rats, HR values were significantly elevated in the old animals. This probably reflects a cardiovascular deconditioning effect (McDonald et al. 1992; Mujika & Padilla, 2001; Hansen et al. 2004), which may include elevations in sympathetic nerve activity (Ebert et al. 1992) in the old animals, which would result in a higher HR for a given level of activity.
Functional ramifications of altered vascular structure
Ageing causes a profound redistribution of skeletal muscle blood flow during submaximal exercise (Musch et al. 2004). Specifically, blood flow decreases to highly oxidative muscles (e.g. soleus and red gastrocnemius) while increasing to low oxidative muscle (e.g. white gastrocnemius), which is a similar perfusion pattern to that in which inactivity is chronically imposed with hindlimb unloading (McDonald et al. 1992), and the inverse of that occurring with exercise training (Armstrong & Laughlin, 1984). Given the multifaceted effects of ageing on the functional and structural vascular properties, the delineation of precise mechanisms responsible for the reduction in vascular conductance associated with old age (Wahren et al. 1974; Koch et al. 2003; Lawrenson et al. 2003; Musch et al. 2004; Donato et al. 2006) is challenging. From the present study, as well as others (Muller-Delp et al. 2002b), it appears that ageing-induced alterations in vascular structure and function are fibre-type specific. Within the soleus muscle, the culmination of fewer feed arteries (Fig. 1) and an impairment of endothelium-dependent vasodilatation associated with old age (Muller-Delp et al. 2002a; Spier et al. 2004) would act to attenuate muscle blood flow during exercise (Musch et al. 2004). Within the gastrocnemius muscle, the largest reduction in perforating feed arteries occurred in the red portion of gastrocnemius muscle ( > 20% fewer vessels; Fig. 1). Functionally, it could be assumed that feed arteries from the red gastrocnemius muscle demonstrate similar endothelial dysfunction associated with old age as that observed in the soleus muscle (Muller-Delp et al. 2002a; Woodman et al. 2003), because arteries from both respond similarly to endothelium-dependent vasodilator agents (McAllister, 2003). Therefore, the same mechanisms as detailed for the soleus are likely to be responsible for the diminished blood flow in the red gastrocnemius of old rats during exercise, although specific functional studies of the effects of ageing on vascular reactivity within the red gastrocnemius have not been conducted.
The white gastrocnemius was the only muscle part that did not demonstrate an age-associated reduction in the number of feed arteries. The maintenance of feed artery number along with the enlargement of luminal diameter resulted in a 38% increase in feed artery luminal CSA within this low oxidative muscle of old rats (Fig. 2). Furthermore, arteries from the white gastrocnemius do not show any decrement in responsiveness to endothelium-dependent vasodilators such as acetylcholine with ageing (Muller-Delp et al. 2002a; Woodman et al. 2002). Therefore, unlike that occurring in high oxidative muscle, structural and functional vascular alterations associated with old age do not appear to limit blood flow and vascular conductance in low oxidative skeletal muscles. The combined effects of ageing on vascular structure and function in high and low oxidative muscles could therefore underlie the decrease in blood flow to high oxidative muscle and increased perfusion of low oxidative muscle during submaximal exercise, despite total hindlimb muscle blood flow being unchanged by old age (Musch et al. 2004). Changes in muscle perfusion patterns associated with old age therefore result in a mismatch of O2 delivery to muscle oxidative capacity. Such a conclusion is further supported by the observations that microvascular O2 pressure is lower in muscles from old animals at rest and during contractions, indicating an O2 delivery to 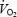 mismatching (Behnke et al. 2005). This mismatch could alter the metabolic milieu (Wilson et al. 1977) and contribute to the increased end-exercise ADPfree concentrations demonstrated in the elderly (Taylor et al. 1997).
mismatching (Behnke et al. 2005). This mismatch could alter the metabolic milieu (Wilson et al. 1977) and contribute to the increased end-exercise ADPfree concentrations demonstrated in the elderly (Taylor et al. 1997).
Significance and conclusions
In old rats there is a structural remodelling of the microcirculation (e.g. arterial rarefaction) in skeletal muscle (Fig. 1). The consequences of arterial rarefaction appear to include greater heterogeneities in Q to 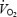 matching (Russell et al. 2003; Behnke et al. 2005), thus potentially limiting capillary O2 efflux (Honig & Odoroff, 1981), as well as restricting perfusion at a given workload (Donato et al. 2006). Moreover, the alterations in blood flow distribution associated with old age would supply an excess of O2 to less metabolically active regions of the muscle, with suboptimal O2 delivery to the more active muscles, which explains why aged muscle demonstrates a reduced O2 uptake at a matched convective O2 delivery (Hepple et al. 2003). These results are also consistent with the hypothesis that physical inactivity, and the corresponding reduction in muscle metabolism, is the stimulus for observed vascular rarefaction in the highly oxidative muscle and muscle parts. It is known that increased activity (e.g. exercise training) can reverse or reduce many of the detriments in vascular function related to old age (Spier et al. 2004; Donato et al. 2005). Although the angiogenic response to exercise training does not appear to be affected by age (Rossiter et al. 2005), it remains to be determined whether exercise training can result in arterial neogenesis in aged skeletal muscle.
matching (Russell et al. 2003; Behnke et al. 2005), thus potentially limiting capillary O2 efflux (Honig & Odoroff, 1981), as well as restricting perfusion at a given workload (Donato et al. 2006). Moreover, the alterations in blood flow distribution associated with old age would supply an excess of O2 to less metabolically active regions of the muscle, with suboptimal O2 delivery to the more active muscles, which explains why aged muscle demonstrates a reduced O2 uptake at a matched convective O2 delivery (Hepple et al. 2003). These results are also consistent with the hypothesis that physical inactivity, and the corresponding reduction in muscle metabolism, is the stimulus for observed vascular rarefaction in the highly oxidative muscle and muscle parts. It is known that increased activity (e.g. exercise training) can reverse or reduce many of the detriments in vascular function related to old age (Spier et al. 2004; Donato et al. 2005). Although the angiogenic response to exercise training does not appear to be affected by age (Rossiter et al. 2005), it remains to be determined whether exercise training can result in arterial neogenesis in aged skeletal muscle.
In summary, skeletal muscle from old rats has fewer feed arteries perforating the muscle, with a compensatory arterial luminal enlargement which maintains resting shear stress. These structural alterations associated with old age are presumed to contribute to the altered vascular conductance during submaximal exercise, resulting in reduced O2 delivery to the active musculature. Thus, the suboptimal O2 delivery to active muscles that occurs during exercise in old age, because of altered vascular structure and function (Muller-Delp et al. 2002a; Spier et al. 2004; Donato et al. 2005), would result in an increased O2 deficit and forced reliance upon substrate level phosphorylation, ultimately causing exercise intolerance in the aged population.
Acknowledgments
The authors gratefully acknowledge Dr Judy Muller-Delp for her contributions to this work. This study was supported, in part, by National Aeronautics and Space Administration Grants NAG2-1340 and NCC2-1166 (M.D.D.) and National Institutes of Health Grant F32 AG25622 (B.J.B.) and R21AG19248 (J. Muller-Delp).
References
- Armstrong RB, Delp MD, Goljan EF, Laughlin MH. Distribution of blood flow in muscles of miniature swine during exercise. J Appl Physiol. 1987;62:1285–1298. doi: 10.1152/jappl.1987.62.3.1285. [DOI] [PubMed] [Google Scholar]
- Armstrong RB, Laughlin MH. Exercise blood flow patterns within and among rat muscles after training. Am J Physiol. 1984;246:H59–H68. doi: 10.1152/ajpheart.1984.246.1.H59. [DOI] [PubMed] [Google Scholar]
- Armstrong RB, Marum P, Saubert CWT, Seeherman HJ, Taylor CR. Muscle fiber activity as a function of speed and gait. J Appl Physiol. 1977;43:672–677. doi: 10.1152/jappl.1977.43.4.672. [DOI] [PubMed] [Google Scholar]
- Asai K, Kudej RK, Shen YT, Yang GP, Takagi G, Kudej AB, et al. Peripheral vascular endothelial dysfunction and apoptosis in old monkeys. Arterioscler Thromb Vasc Biol. 2000;20:1493–1499. doi: 10.1161/01.atv.20.6.1493. [DOI] [PubMed] [Google Scholar]
- Behnke BJ, Delp MD, Dougherty PJ, Musch TI, Poole DC. Effects of aging on microvascular oxygen pressures in rat skeletal muscle. Respir Physiol Neurobiol. 2005;146:259–268. doi: 10.1016/j.resp.2004.12.009. [DOI] [PubMed] [Google Scholar]
- Cooke JP, Rossitch E, Jr, Andon NA, Loscalzo J, Dzau VJ. Flow activates an endothelial potassium channel to release an endogenous nitrovasodilator. J Clin Invest. 1991;88:1663–1671. doi: 10.1172/JCI115481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox RH. Age-related changes in arterial wall mechanics and composition of NIA Fischer rats. Mech Ageing Dev. 1983;23:21–36. doi: 10.1016/0047-6374(83)90096-9. [DOI] [PubMed] [Google Scholar]
- Croley AN, Zwetsloot KA, Westerkamp LM, Ryan NA, Pendergast AM, Hickner RC, Pofahl WE, Gavin TP. Lower capillarization, VEGF protein, and VEGF mRNA response to acute exercise in the vastus lateralis muscle of aged vs. young women. J Appl Physiol. 2005;99:1872–1879. doi: 10.1152/japplphysiol.00498.2005. [DOI] [PubMed] [Google Scholar]
- Delp MD, Colleran PN, Wilkerson MK, McCurdy MR, Muller-Delp J. Structural and functional remodeling of skeletal muscle microvasculature is induced by simulated microgravity. Am J Physiol Heart Circ Physiol. 2000;278:H1866–H1873. doi: 10.1152/ajpheart.2000.278.6.H1866. [DOI] [PubMed] [Google Scholar]
- Delp MD, Duan C. Composition and size of type I, IIA, IID/X, and IIB fibers and citrate synthase activity of rat muscle. J Appl Physiol. 1996;80:261–270. doi: 10.1152/jappl.1996.80.1.261. [DOI] [PubMed] [Google Scholar]
- Delp MD, Evans MV, Duan C. Effects of aging on cardiac output, regional blood flow, and body composition in Fischer-344 rats. J Appl Physiol. 1998;85:1813–1822. doi: 10.1152/jappl.1998.85.5.1813. [DOI] [PubMed] [Google Scholar]
- Delp MD, Manning RO, Bruckner JV, Armstrong RB. Distribution of cardiac output during diurnal changes of activity in rats. Am J Physiol. 1991;261:H1487–H1493. doi: 10.1152/ajpheart.1991.261.5.H1487. [DOI] [PubMed] [Google Scholar]
- Donato AJ, Lesniewski LA, Delp MD. The effects of aging and exercise training on endothelin-1 vasoconstrictor responses in rat skeletal muscle arterioles. Cardiovasc Res. 2005;66:393–401. doi: 10.1016/j.cardiores.2004.10.023. [DOI] [PubMed] [Google Scholar]
- Donato AJ, Uberoi A, Wray DW, Nishiyama S, Lawrenson L, Richardson RS. Differential effects of aging on limb blood flow in humans. Am J Physiol Heart Circ Physiol. 2006;290:H272–H278. doi: 10.1152/ajpheart.00405.2005. [DOI] [PubMed] [Google Scholar]
- Ebert TJ, Morgan BJ, Barney JA, Denahan T, Smith JJ. Effects of aging on baroreflex regulation of sympathetic activity in humans. Am J Physiol. 1992;263:H798–H803. doi: 10.1152/ajpheart.1992.263.3.H798. [DOI] [PubMed] [Google Scholar]
- Flaim SF, Nellis SH, Toggart EJ, Drexler H, Kanda K, Newman ED. Multiple simultaneous determinations of hemodynamics and flow distribution in conscious rat. J Pharmacol Methods. 1984;11:1–39. doi: 10.1016/0160-5402(84)90050-0. [DOI] [PubMed] [Google Scholar]
- Fleg JL, Lakatta EG. Role of muscle loss in the age-associated reduction in VO2 max. J Appl Physiol. 1988;65:1147–1151. doi: 10.1152/jappl.1988.65.3.1147. [DOI] [PubMed] [Google Scholar]
- Francia P, Delli Gatti C, Bachschmid M, Martin-Padura I, Savoia C, Migliaccio E, et al. Deletion of p66shc gene protects against age-related endothelial dysfunction. Circulation. 2004;110:2889–2895. doi: 10.1161/01.CIR.0000147731.24444.4D. [DOI] [PubMed] [Google Scholar]
- Frangos JA, Eskin SG, McIntire LV, Ives CL. Flow effects on prostacyclin production by cultured human endothelial cells. Science. 1985;227:1477–1479. doi: 10.1126/science.3883488. [DOI] [PubMed] [Google Scholar]
- Hansen AL, Johnsen BH, Sollers JJ, 3rd, Stenvik K, Thayer JF. Heart rate variability and its relation to prefrontal cognitive function: the effects of training and detraining. Eur J Appl Physiol. 2004;93:263–272. doi: 10.1007/s00421-004-1208-0. [DOI] [PubMed] [Google Scholar]
- Hepple RT, Hagen JL, Krause DJ, Jackson CC. Aerobic power declines with aging in rat skeletal muscles perfused at matched convective O2 delivery. J Appl Physiol. 2003;94:744–751. doi: 10.1152/japplphysiol.00737.2002. [DOI] [PubMed] [Google Scholar]
- Honig CR, Odoroff CL. Calculated dispersion of capillary transit times: significance for oxygen exchange. Am J Physiol. 1981;240:H199–H208. doi: 10.1152/ajpheart.1981.240.2.H199. [DOI] [PubMed] [Google Scholar]
- Inbar O, Oren A, Scheinowitz M, Rotstein A, Dlin R, Casaburi R. Normal cardiopulmonary responses during incremental exercise in 20- to 70-yr-old men. Med Sci Sports Exerc. 1994;26:538–546. [PubMed] [Google Scholar]
- Irion GL, Vasthare US, Tuma RF. Age-related change in skeletal muscle blood flow in the rat. J Gerontol. 1987;42:660–665. doi: 10.1093/geronj/42.6.660. [DOI] [PubMed] [Google Scholar]
- Ishise S, Pegram BL, Yamamoto J, Kitamura Y, Frohlich ED. Reference sample microsphere method: cardiac output and blood flows in conscious rat. Am J Physiol. 1980;239:H443–H449. doi: 10.1152/ajpheart.1980.239.4.H443. [DOI] [PubMed] [Google Scholar]
- Kamiya A, Togawa T. Adaptive regulation of wall shear stress to flow change in the canine carotid artery. Am J Physiol. 1980;239:H14–H21. doi: 10.1152/ajpheart.1980.239.1.H14. [DOI] [PubMed] [Google Scholar]
- Koch DW, Leuenberger UA, Proctor DN. Augmented leg vasoconstriction in dynamically exercising older men during acute sympathetic stimulation. J Physiol. 2003;551:337–344. doi: 10.1113/jphysiol.2003.042747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langille BL. Remodeling of developing and mature arteries: endothelium, smooth muscle, and matrix. J Cardiovasc Pharmacol. 1993;21(suppl. 1):S11–S17. doi: 10.1097/00005344-199321001-00003. [DOI] [PubMed] [Google Scholar]
- Langille BL, Bendeck MP, Keeley FW. Adaptations of carotid arteries of young and mature rabbits to reduced carotid blood flow. Am J Physiol. 1989;256:H931–H939. doi: 10.1152/ajpheart.1989.256.4.H931. [DOI] [PubMed] [Google Scholar]
- Langille BL, O'Donnell F. Reductions in arterial diameter produced by chronic decreases in blood flow are endothelium-dependent. Science. 1986;231:405–407. doi: 10.1126/science.3941904. [DOI] [PubMed] [Google Scholar]
- Laughlin MH, Armstrong RB. Muscular blood flow distribution patterns as a function of running speed in rats. Am J Physiol. 1982;243:H296–H306. doi: 10.1152/ajpheart.1982.243.2.H296. [DOI] [PubMed] [Google Scholar]
- Laughlin MH, Armstrong RB, White J, Rouk K. A method for using microspheres to measure muscle blood flow in exercising rats. J Appl Physiol. 1982;52:1629–1635. doi: 10.1152/jappl.1982.52.6.1629. [DOI] [PubMed] [Google Scholar]
- Lawrenson L, Poole JG, Kim J, Brown C, Patel P, Richardson RS. Vascular and metabolic response to isolated small muscle mass exercise: effect of age. Am J Physiol Heart Circ Physiol. 2003;285:H1023–H1031. doi: 10.1152/ajpheart.00135.2003. [DOI] [PubMed] [Google Scholar]
- Liposky H. Shear stress in the circulation. In: Bevan IA, Kaley G, Rubanyi GM, editors. Flow-Dependent Regulation of Vascular Function. New York: Oxford University Press; 1995. pp. 28–45. [Google Scholar]
- McAllister RM. Endothelium-dependent vasodilation in different rat hindlimb skeletal muscles. J Appl Physiol. 2003;94:1777–1784. doi: 10.1152/japplphysiol.00901.2002. [DOI] [PubMed] [Google Scholar]
- McCurdy MR, Colleran PN, Muller-Delp J, Delp MD. Effects of fiber composition and hindlimb unloading on the vasodilator properties of skeletal muscle arterioles. J Appl Physiol. 2000;89:398–405. doi: 10.1152/jappl.2000.89.1.398. [DOI] [PubMed] [Google Scholar]
- McDonald KS, Delp MD, Fitts RH. Effect of hindlimb unweighting on tissue blood flow in the rat. J Appl Physiol. 1992;72:2210–2218. doi: 10.1152/jappl.1992.72.6.2210. [DOI] [PubMed] [Google Scholar]
- Mujika I, Padilla S. Cardiorespiratory and metabolic characteristics of detraining in humans. Med Sci Sports Exerc. 2001;33:413–421. doi: 10.1097/00005768-200103000-00013. [DOI] [PubMed] [Google Scholar]
- Muller-Delp JM, Spier SA, Ramsey MW, Delp MD. Aging impairs endothelium-dependent vasodilation in rat skeletal muscle arterioles. Am J Physiol Heart Circ Physiol. 2002a;283:H1662–H1672. doi: 10.1152/ajpheart.00004.2002. [DOI] [PubMed] [Google Scholar]
- Muller-Delp JM, Spier SA, Ramsey MW, Lesniewski LA, Papadopoulos A, Humphrey JD, Delp MD. Effects of aging on vasoconstrictor and mechanical properties of rat skeletal muscle arterioles. Am J Physiol Heart Circ Physiol. 2002b;282:H1843–H1854. doi: 10.1152/ajpheart.00666.2001. [DOI] [PubMed] [Google Scholar]
- Musch TI, Eklund KE, Hageman KS, Poole DC. Altered regional blood flow responses to submaximal exercise in older rats. J Appl Physiol. 2004;96:81–88. doi: 10.1152/japplphysiol.00729.2003. [DOI] [PubMed] [Google Scholar]
- Musch TI, Terrell JA. Skeletal muscle blood flow abnormalities in rats with a chronic myocardial infarction: rest and exercise. Am J Physiol. 1992;262:H411–H419. doi: 10.1152/ajpheart.1992.262.2.H411. [DOI] [PubMed] [Google Scholar]
- Olesen SP, Clapham DE, Davies PF. Haemodynamic shear stress activates a K+ current in vascular endothelial cells. Nature. 1988;331:168–170. doi: 10.1038/331168a0. [DOI] [PubMed] [Google Scholar]
- Pries AR, Reglin B, Secomb TW. Structural adaptation of vascular networks: role of the pressure response. Hypertension. 2001;38:1476–1479. doi: 10.1161/hy1201.100592. [DOI] [PubMed] [Google Scholar]
- Pries AR, Reglin B, Secomb TW. Structural response of microcirculatory networks to changes in demand: information transfer by shear stress. Am J Physiol Heart Circ Physiol. 2003;284:H2204–H2212. doi: 10.1152/ajpheart.00757.2002. [DOI] [PubMed] [Google Scholar]
- Pries AR, Secomb TW, Gaehtgens P. Structural adaptation and stability of microvascular networks: theory and simulations. Am J Physiol. 1998;275:H349–H360. doi: 10.1152/ajpheart.1998.275.2.H349. [DOI] [PubMed] [Google Scholar]
- Proctor DN, Shen PH, Dietz NM, Eickhoff TJ, Lawler LA, Ebersold EJ, Loeffler DL, Joyner MJ. Reduced leg blood flow during dynamic exercise in older endurance-trained men. J Appl Physiol. 1998;85:68–75. doi: 10.1152/jappl.1998.85.1.68. [DOI] [PubMed] [Google Scholar]
- Rivard A, Fabre JE, Silver M, Chen D, Murohara T, Kearney M, Magner M, Asahara T, Isner JM. Age-dependent impairment of angiogenesis. Circulation. 1999;99:111–120. doi: 10.1161/01.cir.99.1.111. [DOI] [PubMed] [Google Scholar]
- Rossiter HB, Howlett RA, Holcombe HH, Entin PL, Wagner HE, Wagner PD. Age is no barrier to muscle structural, biochemical and angiogenic adaptations to training up to 24 months in female rats. J Physiol. 2005;565:993–1005. doi: 10.1113/jphysiol.2004.080663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell JA, Kindig CA, Behnke BJ, Poole DC, Musch TI. Effects of aging on capillary geometry and hemodynamics in rat spinotrapezius muscle. Am J Physiol Heart Circ Physiol. 2003;285:H251–H258. doi: 10.1152/ajpheart.01086.2002. [DOI] [PubMed] [Google Scholar]
- Spier SA, Delp MD, Meininger CJ, Donato AJ, Ramsey MW, Muller-Delp JM. Effects of ageing and exercise training on endothelium-dependent vasodilatation and structure of rat skeletal muscle arterioles. J Physiol. 2004;556:947–958. doi: 10.1113/jphysiol.2003.060301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan JC, Loomis ED, Collins M, Imig JD, Inscho EW, Pollock JS. Age-related alterations in NOS and oxidative stress in mesenteric arteries from male and female rats. J Appl Physiol. 2004;97:1268–1274. doi: 10.1152/japplphysiol.00242.2004. [DOI] [PubMed] [Google Scholar]
- Taylor DJ, Kemp GJ, Thompson CH, Radda GK. Ageing: effects on oxidative function of skeletal muscle in vivo. Mol Cell Biochem. 1997;174:321–324. [PubMed] [Google Scholar]
- Van Der Loo B, Labugger R, Skepper JN, Bachschmid M, Kilo J, Powell JM, et al. Enhanced peroxynitrite formation is associated with vascular aging. J Exp Med. 2000;192:1731–1744. doi: 10.1084/jem.192.12.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahren J, Saltin B, Jorfeldt L, Pernow B. Influence of age on the local circulatory adaptation to leg exercise. Scand J Clin Lab Invest. 1974;33:79–86. doi: 10.3109/00365517409114201. [DOI] [PubMed] [Google Scholar]
- Wilson DF, Erecinska M, Drown C, Silver IA. Effect of oxygen tension on cellular energetics. Am J Physiol. 1977;233:C135–C140. doi: 10.1152/ajpcell.1977.233.5.C135. [DOI] [PubMed] [Google Scholar]
- Woodman CR, Price EM, Laughlin MH. Aging induces muscle-specific impairment of endothelium-dependent dilation in skeletal muscle feed arteries. J Appl Physiol. 2002;93:1685–1690. doi: 10.1152/japplphysiol.00461.2002. [DOI] [PubMed] [Google Scholar]
- Woodman CR, Price EM, Laughlin MH. Aging impairs nitric oxide and prostacyclin mediation of endothelium-dependent dilation in soleus feed arteries. J Appl Physiol. 2003;95:2164–2170. doi: 10.1152/japplphysiol.01073.2002. [DOI] [PubMed] [Google Scholar]
- Yoshizumi M, Kurihara H, Sugiyama T, Takaku F, Yanagisawa M, Masaki T, Yazaki Y. Hemodynamic shear stress stimulates endothelin production by cultured endothelial cells. Biochem Biophys Res Commun. 1989;161:859–864. doi: 10.1016/0006-291x(89)92679-x. [DOI] [PubMed] [Google Scholar]